Abstract
The emergence of multi-drug-resistant gram negative bacillary infections has regained popularity of ancient drugs such as polymyxins. We report a case of acute respiratory failure induced by use of intravenous colistimethate, which is one of the forms of polymyxin. The patient is a 31 year old female with paraplegia due to spina bifida who underwent excisional debridement of large lumbosacral decubitus ulcer with osteomyelitis infected with pan-resistant Pseudomonas aeruginosa and MRSA. Six days after initiation of intravenous colistimethate and vancomycin, she developed acute respiratory failure requiring mechanical ventilation. Pan-culture was negative including a chest radiograph. V/Q scan showed low probability for pulmonary embolism. Echocardiogram showed normal right ventricle with no strain or pulmonary hypertension. Colistimethate was discontinued. Within 24 hours, she was extubated. In the early years after introduction of polymyxin, there were several reports of acute respiratory paralysis. The mechanism is thought to be noncompetitive myoneuronal presynaptic blockade of acetylcholine release. Though a direct causal relationship for respiratory failure is often difficult to establish in current era with multiple co morbidities, the timeframe of apnea, acuity of onset as well as rapid recovery in our case clearly point out the causal relationship. In addition, our patient also developed acute renal failure, presumably due to colistimethate induced nephrotoxicity, a possible contributing factor for her acute respiratory failure. In summary, colistimethate can induce acute neurotoxicity including respiratory muscular weakness and acute respiratory failure. Clinicians should consider its toxicity in the differential diagnosis of acute respiratory failure especially in critically ill patients.
Keywords: Acute respiratory failure, colistin, neuromuscular blockade
INTRODUCTION
Colistin belongs to the polymyxin class of antibiotics which is a group of cationic polypeptides.[1] Polymyxins were in clinical use during the 1960s after introduction in 1947. However, the popularity rapidly faded in 1970s because of significant renal and neurological toxicity and was replaced with less toxic antibiotics with a comparable or broader antibacterial spectrum such as aminoglycosides.[2] With the recent emergence of multi drug-resistant (MDR) gram-negative organisms, in particular Pseudomonas aeruginosa, Acinetobacter baumannii, and Klebsiella pneumonia, colistin has been reconsidered as a potential therapeutic agent for the past 10-15 years.[1] This has not only increased the clinical usage of this ancient drug, but has also increased the prevalence of all the toxicities related to colistin.
Neurotoxicity is the second most common toxicity after nephrotoxicity following colistin therapy. Among the various manifestations of its neurotoxicity, apnea and respiratory failure are of great concern. Several cases of respiratory failure due to colistin were reported in the previous era. However, this has not been seen commonly since its reintroduction.
We report a case of acute respiratory failure induced by intravenous use of colistin.
CASE REPORT
A 31-year-old white female with history of spina bifida, paraplegia, hydrocephalus status post ventriculo-peritoneal shunt, osteomyelitis of the left hip status post left leg amputation, and chronic sacral decubitus ulcer presented with foul smelling discharge from her sacral wound for 2 weeks. She underwent excisional debridement of her sacral wound. The culture from the wound grew two biotypes of Pseudomonas aeruginosa: One was resistant to all antibiotics except for colistin while the other was resistant to all antibiotics except for colistin and imipenem. In addition, methicillin-resistant Staphylococcus aureus (MRSA) grew from the intraoperative bone culture. She was started on colistimethate sodium (CMS) 200 mg intravenously every 12 hours for her MDR P. aeruginosa. Intravenous vancomycin was used to treat MRSA. She was then discharged to a sub-acute rehabilitation center on CMS and vancomycin.
After three days, she was noted to have sudden respiratory distress in the sub-acute rehabilitation center for which emergency medical service (EMS) was called. On arrival, she was noted to be unresponsive and having agonal breathing. She was immediately started on bag-valve mask ventilation (BVM) and was taken to the emergency room (ER). In the ER, she was noted to have oxygen saturation at 80s on BVM and was emergently intubated. Shortly after resuscitation, her arterial blood gas analysis revealed pH of 7.45, PaCO2 of 25, and PaO2 of 280 mmHg (on FiO2 of 100%). Her labs were significant for leukocytosis with white cell count of 20,000/mm3 and an acutely elevated creatinine of 2.2 mg/dL (baseline creatinine was 0.9 mg/dl at the time of discharge) with metabolic acidosis with bicarbonate of 18 mmol/L secondary to her renal failure. On further inquiry, there was no exposure to any narcotics or muscle relaxants that might have contributed to her respiratory depression. She was in a supervised unit prior to the presentation, which deterred the possibility of any toxic exposure to the patient. Two sets of blood cultures were negative while the wound culture revealed the same biotypes of MDR P. aeruginosa. Chest radiograph did not reveal any parenchymal abnormalities. Neurological workup was unrevealing and ventilation/perfusion scan (V/Q scan) for pulmonary embolism was low probability. Venous duplex of her lower extremity revealed a chronic posterior tibial vein thrombus. An echocardiogram showed normal right ventricle with no strain or pulmonary hypertension.
Colistin was stopped as it was thought to be the cause of renal failure and respiratory failure and the patient was started on imipenem. She was extubated within 24 hours.
Her creatinine peaked at 3.7 mg/dl and she did not require hemodialysis. Her respiratory and neurological status remained stable during hospitalization and tolerated imipenem and vancomycin for her osteomyelitis and chronic decubitus ulcer infection. She was discharged after one week.
DISCUSSION
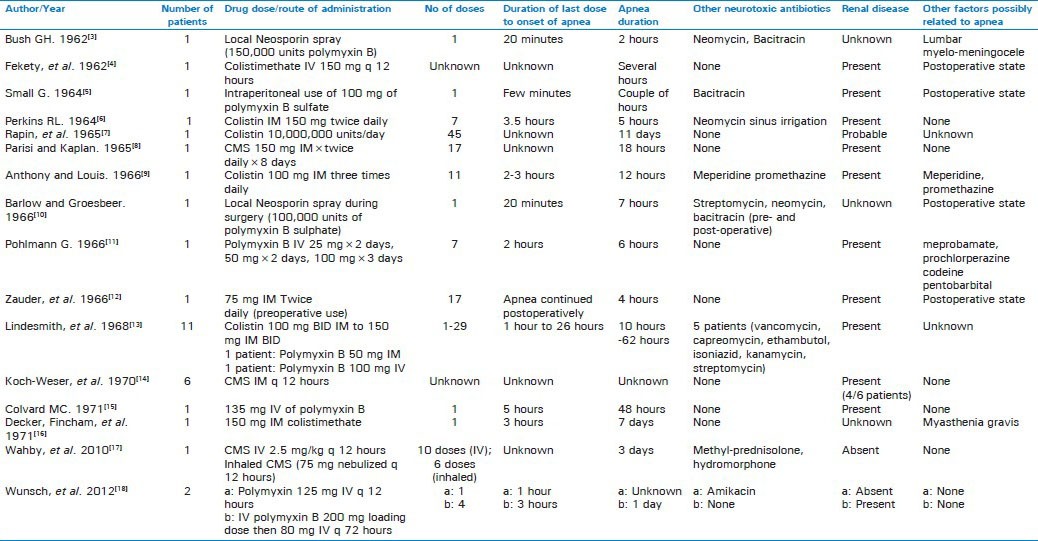
Our patient developed acute respiratory failure requiring intubation and mechanical ventilation 6 days after initiation of colistin. Respiratory muscle paralysis, as depicted, is one of the manifestations of the neurotoxic effect of colistin. The cases were reported mostly between 1964 and 1973 [Table 1]. We applied the Naranjo adverse drug reaction probability scale to our case, which indicated a probable relationship. The case is limited with the unavailability of arterial blood gas prior to the event as she was emergently intubated on presentation. Also, since colistin is reserved for severely ill patients with multiple co-morbidities, it may be difficult to establish direct causal relationship for acute respiratory failure and colistin. Colistin was most likely to be the culprit of her respiratory failure from the timing of apnea as well as rapid recovery after stopping colistin. Acute renal failure leading to presumed high concentration of serum colistin may be another contributory factor for her respiratory failure. She had a chronic deep vein thrombus which is unlikely to cause acute respiratory failure since low probability for pulmonary embolism by V/Q scan and she recovered within 24 hours without any intervention.
Table 1.
Reported cases of respiratory paralysis associated with polymyxin/colistin use since 1960 to present
Colistin is also called polymyxin E and is a part of polymyxin family which is a group of polypeptide antibiotics. It exerts its bactericidal effect by displacing the divalent cations magnesium and calcium, which stabilize anionic lipopolysaccharide molecules in the outer membrane of gram-negative bacteria. This disrupts the cell permeability and ultimately leads to cell death.[1] It was initially introduced in 1949 and was in clinical use in 1960s. However, they were gradually abandoned in most parts of the world because of nephrotoxicity and neurotoxicity. The emergence of MDR gram negative bacteria led to the revival of polymyxins in more recent era as a valuable therapeutic option. In the last decade, intravenous polymyxins, particularly in the form of CMS, have been used to treat serious P. aeruginosa and A. baumannii infections of various types, including pneumonia, bacteremia, and urinary tract infections.
The most common adverse effects of colistin therapy are nephrotoxicity and neurotoxicity. The major manifestations of colistin induced neurotoxicity include dizziness, weakness, facial and peripheral paresthesias, vertigo, visual disturbances, confusion, ataxia, and neuromuscular blockade. Koch-Weser et al., reported the incidence of the neurotoxic manifestations to be about 7%, paresthesias being the most common.[14] The proposed mechanism is a non-competitive myoneuronal presynaptic blockade of acetylcholine release, in contrast to the competitive blockade by most other antibiotics like neomycin, kanamycin, and streptomycin.[19]
A total of 32 cases of colistin/polymyxin-induced respiratory failure were found in the literature.[3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18] All but three of those were reported before 1970s.[17,18] Recent studies have depicted the toxicities associated with colistin use to be less frequent than previously described. More purified formulations, close monitoring and better dosage might explain this finding.
The most important risk factor known to be associated with respiratory failure with polymyxin use is the renal disease, which was present in 24 out of 29 patients reported. Patients with preexisting renal insufficiency had a greater likelihood of developing nephrotoxicity during colistin therapy, compared to patients with normal baseline renal function.[14]
Concomitant drug therapies also play a major role in potentiating the effect of neuromuscular blockade, which was noted in 11 out of 29 patients. This includes use of other neurotoxic drugs (anesthetics, aminoglycosides, and paralytics), corticosteroids, narcotics and/or muscle relaxants. Most of the cases had other neuromuscular symptoms like circumoral tingling, paresthesias, and restlessness prior to the development of respiratory failure. The duration of apnea lasted from few hours up to several days, maximum noted to be 11 days and 20 out of 29 patients recovered, indicating reversibility of the blockade. The number of doses prior to the respiratory failure seems to be variable and range from a single dose to 45 doses [Table 1].
The management of patient with respiratory failure induced by colistin involves discontinuation of the drug and respiratory support. Intermittent monitoring of unassisted tidal volumes and forced vital capacity using a bedside spirometer will help determine recovery. Monitoring arterial blood gas helps determine the adequacy of ventilation during the period of ventilator support.[16] More importantly, one should be alert for the possibility of respiratory paralysis in any patients receiving this antibiotic and particularly in those patients with renal abnormality and with concomitant neurotoxic drug use. Careful observation, regular neurological monitoring, and early appreciation of the symptoms of neuromuscular toxicity especially of dyspnea and restlessness may prevent respiratory arrest.
Neostigmine is not indicated in polymyxin or colistin-induced neurotoxicity and may be contraindicated as seen in earlier experiments that the blocking action of polymyxin B was shown to be neostigmine resistant.[19] Intravenous calcium was reported to clinically reverse the respiratory paralysis associated with colistin. Thus, early administration of calcium gluconate IV may be helpful. Animal studies have noted the ability of polymyxin B to discharge histamine from tissue mast cells, which lead to deposition of chelated calcium along the nerve sheaths, leading to toxicity. Swinefor thus suggested the toxicity may be prevented with prior use of antihistamines.[20] Higginbotham also reported use of heparin prevents death from respiratory arrest in mice receiving colistin.[21] The use of antihistamines and heparin in polymyxin induced respiratory failure has not been studied in human subjects.
CONCLUSION
As the use of colistin increased in recent years for treatment of life-threatening MDR infections, there are more reports of associated toxicities. Respiratory paralysis is the most serious side effect. Clinicians should be aware of colistin-related adverse reactions, especially nephrotoxicity and neurotoxicity. Since the formulation of the drug itself has changed, it has necessitated new data with the new formulation. Regular monitoring of renal function as well as development of a tool to monitor neurological toxicity is essential to prevent and manage the toxicities associated with colistin use.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Falagas ME, Kasiakou SK. Colistin: The revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis. 2005;40:1333–41. doi: 10.1086/429323. [DOI] [PubMed] [Google Scholar]
- 2.Nation RL, Li J. Colistin in the 21st century. Curr Opin Infect Dis. 2009;22:535–43. doi: 10.1097/QCO.0b013e328332e672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bush GH. Antibiotic Paralysis. BMJ. 1962;5311:1062. [Google Scholar]
- 4.Fekety FR, Jr, Norman PS, Cluff LE. The treatment of gram-negative bacillary infections with colistin. The toxicity and efficacy of large doses in forty-eight patients. Ann Intern Med. 1962;57:214–29. doi: 10.7326/0003-4819-57-2-214. [DOI] [PubMed] [Google Scholar]
- 5.Small GA. Report of a case. Respiratory paralysis after a large dose of intraperitoneal polymyxin B and bacitracin. Anesth Analg. 1964;43:137–9. [PubMed] [Google Scholar]
- 6.Perkins RL. Apnea with intramuscular colistin therapy. JAMA. 1964;190:421–4. doi: 10.1001/jama.1964.03070180019004. [DOI] [PubMed] [Google Scholar]
- 7.Rapin M, Bagros P, Amiel C, Barois A, Goulon M. Acute interstitial nephropathy and neurologic disorders during a massive and prolonged treatment with colistin methaesulfonate. Presse Med. 1965;73:1529–34. [PubMed] [Google Scholar]
- 8.Parisi AF, Kaplan MH. Apnea during treatment with sodium colistimethate. JAMA. 1965;194:298–9. [PubMed] [Google Scholar]
- 9.Anthony MA, Louis DL. Apnea due to intramuscular colistin therapy. Report of a case. Ohio State Med J. 1966;62:336–8. [PubMed] [Google Scholar]
- 10.Barlow MB, Groesbeek A. Apparent potentiation of neuromuscular block by antibiotics. S Afr Med J. 1966;40:135–6. [PubMed] [Google Scholar]
- 11.Pohlmann G. Respiratory arrest associated with intravenous administration of polymyxin B sulfate. JAMA. 1966;196:181–3. [PubMed] [Google Scholar]
- 12.Zauder HL, Barton N, Bennett EJ, Lore J. Colistimethate as a cause of postoperative apnoea. Can Anaesth Soc. 1966;13:607–10. doi: 10.1007/BF03002230. [DOI] [PubMed] [Google Scholar]
- 13.Lindesmith LA, Baines RD, Jr, Bigelow DB, Petty TL. Reversible respiratory paralysis associated with polymyxin therapy. Ann Intern Med. 1968;68:318–27. doi: 10.7326/0003-4819-68-2-318. [DOI] [PubMed] [Google Scholar]
- 14.Koch-Weser J, Sidel VW, Federman EB, Kanarek P, Finer DC, Eaton AE. Adverse effects of sodium colistimethate. Manifestations and specific reaction rates during 317 courses of therapy. Ann Intern Med. 1970;72:857–68. doi: 10.7326/0003-4819-72-6-857. [DOI] [PubMed] [Google Scholar]
- 15.Colvard MC., Jr Respiratory paralysis secondary to the use of polymyxin B. South Med J. 1971;64:652. doi: 10.1097/00007611-197106000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Decker DA, Fincham RW. Respiratory arrest in myasthenia gravis with colistimethate therapy. Arch Neurol. 1971;25:141–4. doi: 10.1001/archneur.1971.00490020059006. [DOI] [PubMed] [Google Scholar]
- 17.Wahby K, Chopra T, Chandrasekar P. Intravenous and inhalational colistin-induced respiratory failure. Clin Infect Dis. 2010;50:e38–40. doi: 10.1086/650582. [DOI] [PubMed] [Google Scholar]
- 18.Wunsch H, Moitra VK, Patel M, Dzierba AL. Polymyxin use associated with respiratory arrest. Chest. 2012;141:515–7. doi: 10.1378/chest.11-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubikowski P, Szreniawski Z. The mechanism of the neuromuscular blockade by antibiotics. Arch Int Pharmacodyn Ther. 1963;146:549–60. [PubMed] [Google Scholar]
- 20.Swinefor O. Respiratory arrest and polymyxin B. JAMA. 1966;196:1097. [PubMed] [Google Scholar]
- 21.Higginbotham RD. Effect of heparin on neomycin. Tex Rep Biol Med. 1960;18:408–17. [PubMed] [Google Scholar]


