Abstract
The interaction between host immunity and infections in the context of a suppressed immune system presents an opportunity to study the interaction of colonization and infection with the development of acute and chronic pulmonary morbidity and mortality. This article summarizes presentations at the Pittsburgh International Lung Conference about comorbid consequences in two categories of immunosuppressed hosts: HIV-infected individuals and lung transplant recipients. Specifically, chronic obstructive pulmonary disease, pulmonary hypertension, and chronic lung rejection after transplant are three diseases that may be consequences of colonization or infection by viruses or fungi, whether HIV itself or the opportunistic infections Pneumocystis and cytomegalovirus. In the fourth section, we discuss unique aspects of infections after lung transplant as well as the battle against multidrug-resistant organisms in this population and theorize that the immunosuppressed population may provide a unique group of patients in which to study ways to overcome nosocomial pathogenic challenges. These host–pathogen interactions serve as models for developing new strategies to reduce acute and chronic morbidity due to colonization and subclinical infection, and potential therapeutic avenues, which are often overlooked in the clinical arena.
Keywords: immunosuppressed host, lung transplantation, Pneumocystis, cytomegalovirus, pulmonary hypertension
From HIV to lung transplantation, one of the most fascinating areas of infectious disease is that of the interaction of microorganisms and immunosuppressed hosts. Although it is well known that multiple opportunistic bacteria, fungi, viruses, and parasites cause acute disease, there has been less understanding of the potential role of infection and subclinical colonization on the host’s clinical course. There has also been a dearth of knowledge about the specific immunologic defects that cause host susceptibility to opportunistic pathogens. Different immunosuppressed hosts suffer different microbial complications. Even the same organism, for instance cytomegalovirus (CMV), can cause very different clinical manifestations in one immunosuppressed patient population compared with another. Hence, when we consider the immunosuppressed host, in this conference we explored both those people immunocompromised as a direct effect of HIV infection and those immunosuppressed due to transplant medications.
Before antiretroviral therapy (ART), the HIV/AIDS era increased physicians’ awareness of opportunistic infections. Even with the availability of highly effective ART, so many patients in the United States are unaware of their HIV infection, and so many patients do not have access to effective care, that AIDS-related opportunistic infections are still common. Additionally, there are still consequences of opportunistic fungal infections, specifically Pneumocystis, and the HIV-related comorbidity of pulmonary hypertension is also discussed.
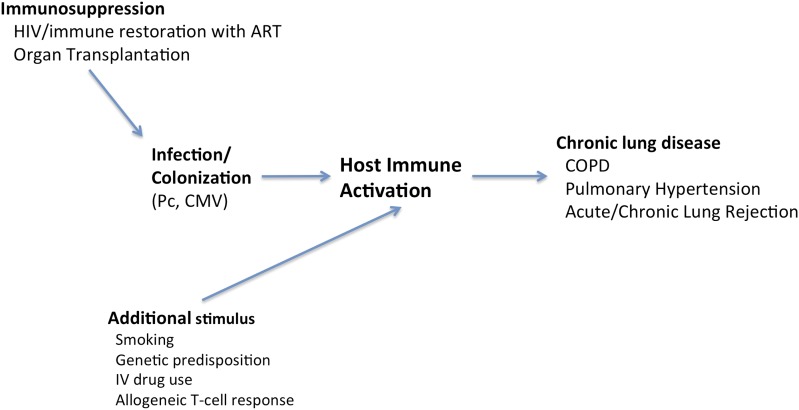
Immunosuppression necessary for organ transplantation or for controlling inflammatory disorders are also important causes of opportunistic infections. The impact of immunosuppression in lung transplantation may affect the host in terms of not only host susceptibility to the offending pathogen but also infections by certain pathogens that may in turn modulate immune function. For example, cytomegalovirus suppresses T-lymphocyte function and thus can enhance the predisposition to invasive fungal infections, including pneumocystosis (1). These complex interactions can have major impact on graft and patient survival (Figure 1).
Figure 1.
Infection and/or colonization in the immunosuppressed host may perpetuate chronic immune activation and disease. ART = antiretroviral therapy; CMV = cytomegalovirus; COPD = chronic obstructive pulmonary disease; IV = intravenous; Pc = Pneumocystis jiroveci.
The purpose of this symposium is to review new perspectives on host–organism interactions with novel impacts on human disease.
Pneumocystis jiroveci
Biology
Pneumocystis is a member of the Taphrinomycotina family. It is an extracellular fungus containing a cell wall β-glucan; does not form ascomata; and undergoes asexual reproduction by budding, conidia, and fission (2, 3). Pneumocystis species from different mammalian hosts are genetically different organisms. In an elegant analysis of Pneumocystis organisms from seven different host species, Ma and colleagues detailed the phylogeny based on the genetic sequence of dihydrofolate reductase and dihydropteroate synthase, the targets of trimethoprim and sulfamethoxazole (2).
Transmission
Species-specific transmission of Pneumocystis occurs via the respiratory route from host to host. There could conceivably be an environmental reservoir, although none has been definitively identified (4). Several rodent models have demonstrated animal-to-animal transmission in immunosuppressed and immunocompetent animals. Immunosuppressed rodents housed in a room with Pneumocystis carinii pneumonia (PCP)-infected rodents will develop PCP (5, 6). In addition, immunocompetent mice housed in a room with PCP-infected mice will transmit Pneumocystis to immunosuppressed mice, which in turn develop PCP (7, 8). Based on rodent models, a respiratory exposure of 1 to 2 days appears to be adequate inoculation for infection (9). In a host with an intact immune system there is no life-threatening manifestation, although mild clinical disease may occur at the time of primary infection. Latency has been reported to occur after infection, and asymptomatic carriage has been detected in individuals undergoing bronchoscopy (10, 11).
Infants become infected with Pneumocystis during the first few months of life. In a 1993 study, PCP was reported in more than one-third of 3,665 perinatally acquired AIDS cases, with more than half occurring between 3 and 6 months of age (8). These data support a high level of early exposure in infants generating latency with the potential to develop disease later in life in the setting of a compromised immune system. Presumably, these infants are infected by human-to-human spread from individuals in their immediate environment. Among adults, there is considerable evidence suggestive of person-to-person transmission. Clusters and outbreaks in hospitals have been sporadically reported over the years, suggesting either uniquely pathogenic strains of Pneumocystis or unusual transmission dynamics (12–16). There have been recent outbreaks of PCP of particular strains in Europe and Japan, supporting transmission directly from person to person (17–19). Multiple studies have shown that outbreaks can be due to a single strain of Pneumocystis, suggesting a common environmental or person-to-person route of spread (20, 21). In addition, it is possible that there are pathogenic variations of strains that predispose them to infection versus limited disease or colonization. It is likely that there have been more outbreaks of Pneumocystis than are reported in the literature, especially among immunocompetent individuals who do not manifest acute disease. Pneumocystis colonization/exposure is not routinely examined, nor is the impact of infection that may not manifest as acute PCP; therefore, we do not know the true incidence of disease. There are also reports in the literature of negative colonization of healthcare workers (defined as detection of Pneumocystis using in situ hybridization or nested polymerase chain reaction [PCR]) after exposure to PCP-infected patients (22). Several possible explanations exist, in addition to the authors’ discussion that healthcare workers are not carriers of Pneumocystis. The studies may not have allowed enough time for Pneumocystis exposure (median exposure, 5.6 h) or replication within the host before obtaining induced sputum (within 1 d of last exposure). These reports may not have allowed enough time for Pneumocystis replication.
Rather than focus on detection of Pneumocystis itself, other studies have looked at the humoral responses of exposed healthcare workers as evidence of prior Pneumocystis exposure (22–24). Results have been variable, although some studies have shown healthcare workers previously exposed to patients with PCP demonstrate an increase in anti-Pneumocystis antibody titers or higher antibody levels than nonclinical individuals without patient exposure. Perhaps in some cases certain strains of Pneumocystis may be more transmissible. Perhaps these study differences are also a result of nonuniform sampling and test methods.
Diagnosis and Clinical Disease
Patients at the highest risk for PCP include those with HIV, human T-lymphotropic virus-1, stem cell and solid organ transplants, and congenital immunodeficiencies; recipients of antineoplastic therapy; and recipients of anti-tumor necrosis factor (TNF), anti-lymphocyte antibodies, and high-dose corticosteroids. Circulating CD4 lymphocyte counts are sensitive predictors for the occurrence of PCP in patients with HIV infection but not in other patients (25).
The diagnosis of PCP has evolved over time. The human organism has never successfully been cultured, and there is no useful serologic test to detect antigen or nucleic acid. Until the late 1970s, diagnosis was based on visualization of organisms in lung biopsy specimens (26). Although open lung biopsies were the standard approach initially, the development of bronchoscopy led to a period when transbronchial biopsy became the procedure of choice for obtaining a clinical sample for staining. The clinical standard has evolved to use less-invasive methods of bronchoalveolar lavage or induced sputum. In respiratory samples or tissue, organisms can be visualized using methenamine silver stain, immunofluorescence, or Giemsa/Diff-Quik stains. Some laboratories are now using molecular techniques to detect Pneumocystis in respiratory specimens (but not in serum). Real-time PCR is highly sensitive for the detection of Pneumocystis in bronchoalveolar lavage, sputum, and oral washes. The impressive negative predictive value makes these tests potentially useful for ruling out PCP. However, because many immunosuppressed patients appear to be colonized and to have low-level colonization by Pneumocystis in situations where a different process is the cause of the pulmonary dysfunction, the positive predictive value of this technique is disappointing to use as a method to help determine when to treat Pneumocystis as the cause of a patient’s respiratory decline (27–29). Therefore, at this time, real-time PCR testing for Pneumocystis remains a research-based assay.
CD4 count is the most widely used biomarker for risk stratification in making the diagnosis of PCP in HIV-infected individuals; other biomarkers have been sought in various populations to measure susceptibility to Pneumocystis pneumonia. To date, no individual biomarker has yet had sufficient positive or negative predictive value to be clinically useful. There has been literature on the usefulness of serum lactate dehydrogenase levels and (1-3)-β-d-glucan assays. However, both of these tests are insensitive, especially for mild disease, and very nonspecific (30, 31). Recently researchers have found been combining serum lactate dehydrogenase and (1-3)-β-d-glucan levels may provide a promising alternative approach in the diagnosis of PCP (30–32).
Pneumocystis infection could have a role in causing or exacerbating disease in immunologically normal patients. There has long been speculation about the possibility that primary Pneumocystis infection could be associated with mild and self-limited upper or lower respiratory manifestations. There was also conjecture, never convincingly substantiated, that primary Pneumocystis infection might be associated with sudden infant death syndrome (33).
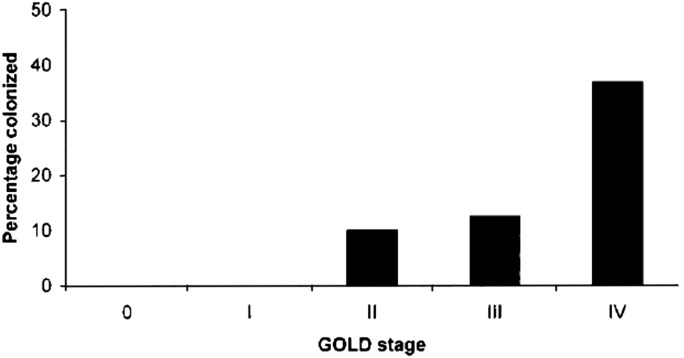
More data have emerged, however, about the potential role of Pneumocystis colonization in chronic obstructive lung disease (34, 35). In an HIV-infected population, Pneumocystis colonization was associated with increased risk of airway obstruction (36). Colonization is more prevalent among HIV-uninfected individuals with more severe chronic obstructive pulmonary disease (COPD) compared with less severe COPD (Figure 2) (35). Although it is difficult to assert causation from human studies, emphysema and airflow obstruction as a consequence of Pneumocystis colonization have been demonstrated in rodent and nonhuman primate models (36–38).
Figure 2.
Prevalence of HIV-uninfected subjects colonized with Pneumocystis, isolated from lung tissue, according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage. Number of subjects per group: 0 = 10, I = 10, II = 10, III = 8, IV = 30. Reprinted by permission from Reference 35.
Pneumocystis colonization has been found to lead to airway obstruction and development of emphysema in combined simian human immunodeficiency virus (SHIV)-infected macaques (38). Interestingly, cigarette smoke exposure in mice with Pneumocystis has been shown to lead to increased Pneumocystis burden as well as increased airspace enlargement, demonstrating a potential synergy between Pneumocystis colonization and cigarette smoking in the development of emphysema (37). Pneumocystis colonization in COPD has been associated with increased production of matrix metalloproteinase 12, a putative enzyme in COPD pathogenesis, as well as increased expression of inflammatory markers such as IL-6, IL-8, and TNF-α (39–41). Given these findings, it seems plausible that Pneumocystis colonization could serve as a nidus, stimulating chronic immune activation and downstream development or worsening of COPD. Future studies in animal models may help define mechanisms by which Pneumocystis may cause or perpetuate disease and help formulate potential treatment or prevention strategies related to chronic pulmonary disease in humans.
Conclusions
Pneumocystis is ubiquitous in the environment, and most humans are exposed to this pathogen, likely by human-to-human transmission, early in life for their primary infection. The organism continues to cause disease in immunosuppressed patients despite the availability of effective prophylactic drugs. The role of this organism in causing morbidity in immunologically normal hosts is emerging, giving hope that further research into understanding this role will lead to more effective management strategies for patients with chronic lung disease and other pulmonary disorders.
HIV-associated Pulmonary Hypertension
The association of HIV with pulmonary hypertension has been intriguing. Pulmonary arterial hypertension (PAH) is defined clinically as a mean pulmonary artery pressure at rest greater than 25 mm Hg without signs of left ventricular compromise (pulmonary capillary wedge pressure ≤15 mm Hg). It is a disease of endothelial dysfunction and smooth muscle cell proliferation, with many potential pathways. PAH develops when increase in vascular tone and chronic obstruction of small pulmonary arteries contribute to elevated pulmonary artery pressures and right ventricular dysfunction and failure (42). HIV-associated PAH is classified along with idiopathic PAH in the World Health Organization classification of pulmonary arterial hypertension Class I, with a reported prevalence of 0.5% in HIV-infected patients, much higher than the prevalence of idiopathic PAH in the general population (1–2 cases per 1 million people) (43). It has been associated with a 2-year survival of 60%, although whether this holds in the era of modern PAH therapy is not known (44). ART has improved the prognosis of HIV-infected individuals, yet the prevalence of HIV-PAH has remained unchanged in the ART era, and the impact of ART on PAH survival and outcome remains controversial (43, 45–47). Although there has been great progress in medical therapy, to date there is still no cure.
Pulmonary hypertensive changes may be much more common than the prevalence of PAH previously reported. A recent study by Morris and colleagues demonstrated that echocardiographic manifestations of pulmonary hypertension are common in HIV (48). In this study, 116 HIV-infected outpatients without evidence of acute cardiopulmonary disease underwent pulmonary function testing and echocardiography. Seventy-seven percent of subjects had a pulmonary artery systolic pressure (PASP) greater than 30 mm Hg, 15% had a PASP greater than 40 mm Hg, and 8% of subjects had a tricuspid regurgitant jet velocity greater than 3 cm/s. Elevated pressures were associated with respiratory symptoms, airway obstruction, decreased diffusing capacity of carbon monoxide, more advanced HIV disease (i.e., lower CD4 count and higher viral load), as well as increased markers of peripheral inflammation (48). Although echocardiographic findings of elevated pulmonary artery pressures do not necessarily correlate with right heart catheterization findings, these echocardiographic manifestations are clinically important.
There are several unique aspects of HIV that may explain its involvement in PAH pathogenesis. Proposed mechanisms include the following: direct and indirect roles of the virus or its proteins, such as Nef or Tat; comorbid conditions, such as intravenous drug use and infection with other viruses such as human herpesvirus-8 or fungi, given the association of fungal wall polysaccharides in human serum and cardiopulmonary abnormalities in HIV-infected individuals; and the idea that HIV itself may fuel the host’s inflammatory response, driving pulmonary vascular remodeling and PAH development (49–54).
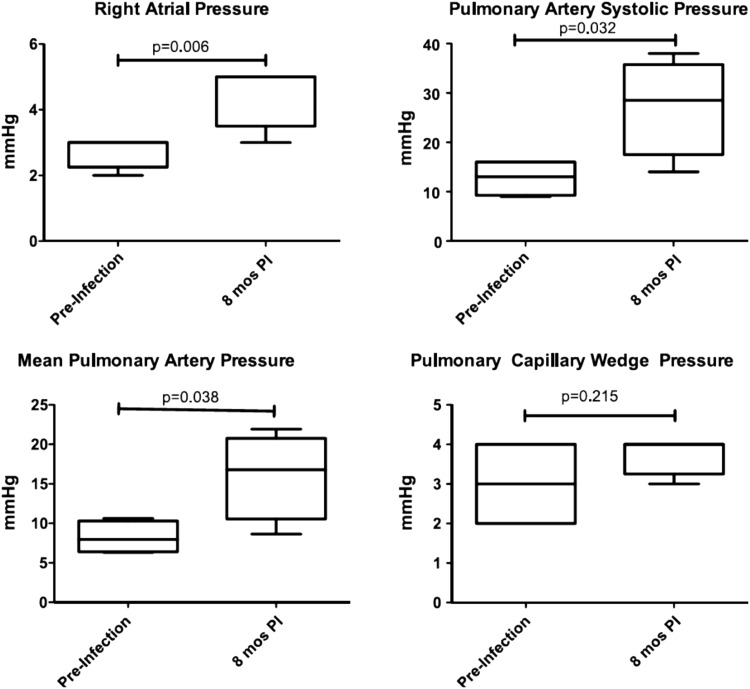
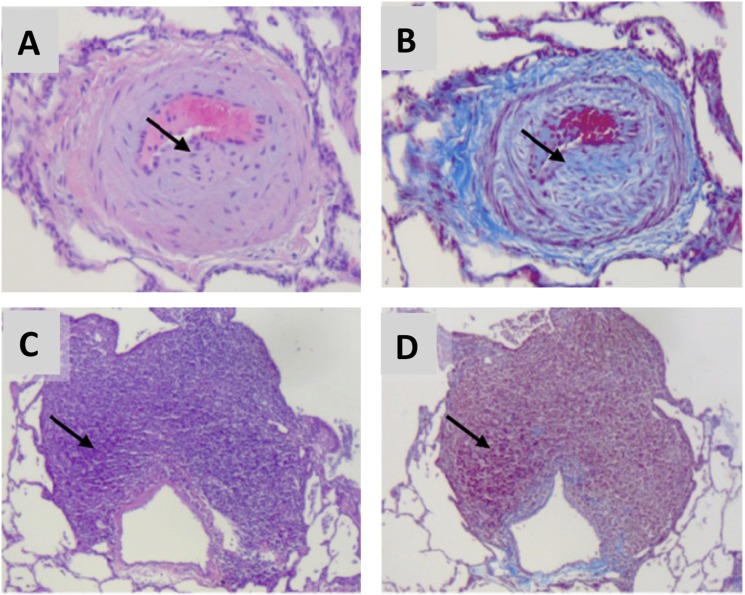
To explore these and other mechanisms, a novel nonhuman primate model of HIV-associated PAH was developed using simian immunodeficiency virus (SIV) deltaB670 infection of rhesus macaques (55). In this model, animals were infected via intravascular or intrarectal (mucosal) routes and had serial echocardiography and right heart catheterizations performed (55). In SIV-infected macaques, right heart catheterization showed that right atrial pressure, PASP, and mean pulmonary artery pressure were elevated, and pulmonary capillary wedge pressure remained unchanged (Figure 3). These pressures were associated with an increase in relative pulmonary vascular resistance but no change in cardiac output (55). Finally, pulmonary vascular remodeling was seen in the SIV macaque model, with prominent findings being subintimal collagen deposition and perivascular lymphocytic tissue as well as intimal and medial hyperplasia (Figure 4) (55). These findings of perivascular inflammatory lesions associated with animals that have developed pulmonary hypertension are intriguing within the context of the recent literature. It has long been believed that inflammation plays a role in PAH pathogenesis, but through mechanisms that are yet poorly understood (56, 57). Perivascular lymphoid follicles have been identified in patients with idiopathic PAH, and recent fascinating work in the monocrotaline rat model has demonstrated bronchus-associated lymphoid tissue that can generate pathologic autoantibodies (58, 59). Ongoing work in the SIV macaque model is currently investigating the role of chronic immune activation in HIV-PAH pathogenesis.
Figure 3.
Right atrial and pulmonary artery pressures are elevated in SIVΔB670-infected macaques without elevation of pulmonary capillary wedge pressure. PI = post infection. Reprinted by permission from Reference 55.
Figure 4.
Histologic findings in simian immunodeficiency virus–infected macaques are consistent with pulmonary hypertensive changes. Hematoxylin and eosin stain (A, C) and Masson trichrome stain (B, D). Arrows indicate medial hyperplasia and neointimal collagen deposition (A, B) and perivascular lymphoid tissue (C, D). Reprinted by permission from Reference 55.
The Role of CMV and Other Infections in Lung Transplant Outcomes
Since the 1990s, there has been tremendous growth in lung transplantation, with 3,519 performed worldwide in 2010 (60). Although survival has improved over time, the overall median survival after lung transplantation remains less than 6 years (60). Major complications include opportunistic infections, of which cytomegalovirus (CMV) is the most common (61). In addition, bronchiolitis obliterans syndrome (BOS), now known more broadly as chronic lung allograft dysfunction, is likely a manifestation of chronic graft rejection, although the precise mechanisms are poorly understood. Multiple clinical risk factors have been identified for BOS, including CMV and other community-acquired viral infections (62–64).
CMV, a member of the Herpesviridae family, is quite common. Approximately 60% of the general population is CMV seropositive (65). CMV can cause childhood illness and then persists as a latent virus. In patients receiving lung transplant, CMV infection can occur as reactivation in a recipient who had latent virus or it can be newly acquired from the donor. Patients who are primary mismatch for CMV (donor positive, recipient negative) are at highest risk for infection (66).
Reactivation of CMV infection is defined by asymptomatic viremia and is commonly diagnosed with highly sensitive PCR assays. CMV disease is defined as viremia and symptoms or signs (e.g., fever, malaise, hematological abnormalities, decline in pulmonary function tests), with tissue invasion seen on biopsy.
There have been conflicting studies in the literature as to whether CMV pneumonitis is a definite risk factor for BOS. In the past, studies were limited by small numbers of patients, older transplant eras, variable CMV prophylaxis and diagnosis, and inadequate statistical methods (67). An Australian study published in 2004 reported that treated CMV pneumonia was not a risk factor for BOS (68). It was the largest study of its kind, with 341 subjects in a single center registry. This study focused on disease rather than viremia. It is not known whether antiviral treatment changed the effect of CMV on BOS; however, interpretation of the findings is limited by the 14-year duration of the study, with variable CMV prevention and treatment over that time period. Additionally, the diagnosis of CMV pneumonitis was made subjectively by a pathologist, and CMV was classified as a time-independent predictor of BOS.
A subsequent study analyzed 231 consecutive patients receiving lung transplants from 2000 to 2004 (69). All received CMV prophylaxis with similar protocols, and prospective CMV immunohistochemical staining was performed on all biopsies. Mean follow-up was 6.7 years. Forty-nine patients (21%) had at least one episode of CMV pneumonitis. CMV pneumonitis was found to predict an increased risk for BOS (P value = 0.001; hazard ratio, 2.19; 95% confidence interval [CI], 1.36–3.51) and death after lung transplant (P value = 0.02; hazard ratio, 1.89; 95% CI, 1.11–3.23) (69). The association of CMV with BOS has been seen in other centers as well (62, 70). CMV can up-regulate the anti-allograft immune response through mechanisms such as up-regulation of donor human leukocyte antigens and the release of proinflammatory cytokines (71, 72).
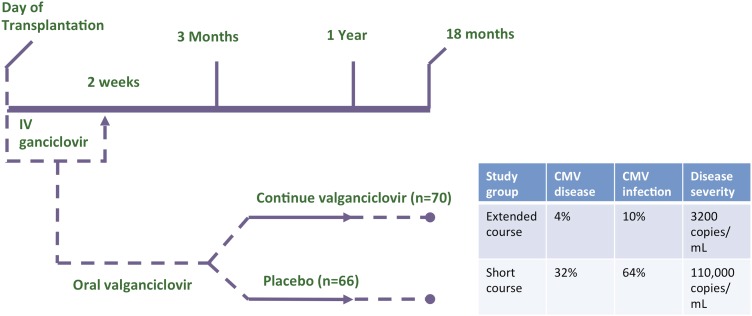
Given the potential impact of CMV infection on overall transplant outcomes, prevention is key, and until a recent randomized trial, there were no data guiding type or duration of CMV prophylaxis. In the early era of lung transplantation, most patients took intravenous or oral ganciclovir for 3 months. In 2001, valganciclovir, a highly bioavailable oral ganciclovir formulation, was approved; however, practice patterns varied due to concerns over drug toxicity, development of viral resistance, the concern over preventing versus merely delaying disease, and cost. In 2010, the VALGAN study, a prospective, randomized, double-blind, placebo-controlled trial of CMV prevention in lung transplant recipients compared valganciclovir prophylaxis for 1 year compared with 3 months (73). Subjects in the extended prophylaxis group had significant reductions in CMV disease (4 vs. 32%, P = 0.001), CMV infection (10 vs. 64%; P = 0.001), and disease severity (110,000 vs. 3,200 copies/ml, P = 0.009) (Figure 5). There were comparable safety results, and no ganciclovir resistance was reported. There also was a trend toward less acute rejection with extended therapy (73). In a long-term follow-up study of randomized subjects from a single center, patients in the extended prophylaxis group had significantly longer freedom from CMV (mean follow-up over 4 years in each group), supporting prevention rather than delay of CMV in most patients treated with extended prophylaxis (74).
Figure 5.
Prevention of cytomegalovirus (CMV) is better when valganciclovir is used for 12 months rather than 3 months. A significant reduction in CMV disease (P = 0.001), CMV infection (P = 0.001), and disease severity (P = 0.009) was seen with extended compared with short course of prophylaxis. IV = intravenous. Adapted by permission from Reference 73.
Although 12 months of CMV prophylaxis is better than 3 months in overall patient populations, use of this regimen overtreats some patients and fails to prevent CMV in others. To understand factors contributing to these differences and to better tailor therapy, the Duke research team has set forth to develop a personalized approach. A prospective study of CMV-specific immunity is underway using CMV-specific T-cell immunity to predict the risk for infection and/or disease in donor-positive/recipient-positive patients receiving lung transplant at risk for CMV and personalize treatment accordingly. Preliminary results demonstrate that transplant recipients can mount a CMV-specific CD8+ T-cell response, which can be polyfunctional but often is not (75). The hope of this patient-centered approach is that physicians will be able to improve transplant outcomes by identifying patients with impaired CMV-specific immunity who would most benefit from prolonged prophylaxis, thereby reducing CMV infection and disease. In the meantime, such strategies would potentially lower costs and drug toxicity by using a shortened course in patients who already mount an effective T cell–protective response.
The Banal and the Profound: Everyday Infectious Disease Issues after Lung Transplantation
Infections are major causes of morbidity and the leading cause of death in the first year after lung transplantation (60, 76). These infections range from the “banal” of surgical site infections (SSIs) to the “profound” of extensively drug-resistant and pan–drug-resistant (XDR/PDR) gram-negative bacterial infections. Caring for the entire range of infections is important for both early and long-term postoperative care of this patient population. In addition to the morbidity and mortality of the acute infections themselves, similar to the host immune response to pathogens described in previous sections, gram-negative infections have been associated with development of chronic rejection (77).
SSIs are infections within 90 days of transplant that include the following: deep incisional surgical wound infection, sternal osteomyelitis, empyema, and mediastinitis. In a comprehensive single-center review of a 5-year experience in the modern era, SSI occurred in 5% (31 of 586) of patients. These infections occurred early in the postoperative course (median time to SSI was 25 d) and involved a variety of pathogens (gram-positive and gram-negative bacteria, fungi, and mycobacterium). The most common SSI was empyema in 42% of cases, followed by surgical wound infection in 29% of cases and mediastinitis in 16%; 23% of SSIs were caused by microbes that had been colonizers of the recipient’s native lungs. Patient-related risk factors for SSI included prior thoracic surgery (odds ratio [OR], 4.16; 95% confidence interval [CI], 1.79–9.62; P < 0.001), and diabetes (OR, 3.03; 95% CI, 1.32–6.98; P < 0.009). Perioperative risk factors included female donor (OR, 2.40; 95% CI, 1.21–6.50; P < 0.009), units of blood transfused (OR, 1.04; 95% CI, 1.02–1.06; P < 0.0001), and prolonged ischemic time in minutes (OR, 1.005; 95% CI, 1.001–1.009; P < 0.01) (78). All patients received antibiotics directed against the pathogens, and 49% underwent surgical debridement with video-assisted thoracic surgery. SSI significantly prolonged length of stay and worsened all-cause mortality at 6 months. Subgroup analyses indicated that this effect was likely due to nonempyema chest wall infection (78). The site’s transplant program responded with a renewed focus on infection control measures, with particular attention to resistant bacteria, systematic use of aggressive antimicrobial irrigation intraoperatively, and tailored systemic antimicrobial therapy post-transplant. In addition, it was noted that potential seeding during transplant might have been reduced with minimally invasive surgical approach introduced in 2009 (78). Finally, aside from findings in this paper, there has also been increased emphasis on aggressive pretransplant antifungal management and Staphylococcus aureus screening and mupirocin/chlorhexidine decolonization protocol.
There has also been an emergence of XDR/PDR gram-negative bacterial infections in the lung transplant population. Two notable pathogens include XDR/PDR-Acinetobacter baumannii and carbapenemase (KPC)-producing Klebsiella pneumoniae. In 2009, an XDR/PDR-Acinetobacter outbreak in 11 solid organ transplant recipients resulted in 100% mortality (79, 80). In response, the medical center’s transplant infectious disease team collected isolates from patients undergoing transplant and performed antibiotic synergy testing by three methods against 17 isolates. Colistin combined with doripenem was superior to other combinations and thus recommended as standard therapy. Increased infection control practices were implemented as well. The impact of this antimicrobial protocol in patients was reviewed 2 years later. Of all Acinetobacter infections, 95% were respiratory tract infections, and of the 40 solid organ transplant recipients with respiratory diseases, 18 (45%) were lung transplant recipients. Ninety percent of patients were treated (four died before treatment), and 50% responded to their initial treatment regimen. The regimens included doripenem with colistin as well as other regimens. The sole predictor of 28-day survival was use of carbapenem and colistin combination therapy (P = 0.01); however, colistin resistance emerged in 36% of XDR-Acinetobacter isolates tested, occurring in 100% (3 of 3) patients treated with tigecycline plus colistin and in 18% (2 of 19) patients treated with carbapenem plus colistin. Recurrent infections also occurred in 44% of patients (8 of 18) who were initially treated successfully (79). With these new protocols, the number of Acinetobacter isolates decreased over time, and mortality declined as well.
Although the Acinetobacter epidemic seems to be controlled at least for now, another XDR organism is steadily rising to the forefront, KPC-producing K. pneumoniae. KPC-K. pneumoniae causes a wide range of diseases in solid organ transplant and other populations. Again, synergy testing at one medical center revealed that colistin and doripenem combination therapy achieved the highest rates of success (81).
Carbapenem resistance can occur via multiple mechanisms and/or mutations, with the contention being that not all KPC-K. pneumoniae are the same, leading to the hypothesis that optimal microbial regimens are likely to depend on specific antimicrobial resistance mechanisms. One mutation that seems to be a marker of resistance is Omp36, a mutation leading to porin loss or mutations, present in 70% of resistant organisms (82). Genotyping has revealed specific mutations in this gene that predict responsiveness of KPC-K. pneumoniae to combination therapy. Isolates that are resistant to doripenem but lack a specific mutation—either an AA134–135GD insertion or IS5 mutation—are significantly more likely to respond to doripenem plus colistin therapy at 24 hours (82). In isolates that do carry these mutations, gentamicin sensitivity is assessed, and doripenem plus gentamicin is indicated. In those KPC-K. pneumoniae that are resistant to gentamicin, however, new agents are needed.
These experiences have demonstrated the importance of synergy testing in MDR/XDR organisms. Furthermore, optimal drug treatment regimens at specific centers are likely to be determined by center-specific investigations into mechanisms of strain resistance, such as genotyping. As prevention of these infections is key, infection control and rational antibiotic use are important components of a plan to decrease MDR/XDR organisms. Finally, transplant recipients provide a powerful but underused population for studying a wide range of infectious disease issues germane to infectious disease practices and treatments in the general patient population.
Conclusions
Advances in the treatment of infections in the immunosuppressed patient have not only made a significant impact on mortality in this patient population but also provided powerful insights into the pathogenesis of chronic diseases. The HIV era has taught us much about the prevention and treatment of Pneumocystis, and now with improved HIV treatments and decreased frequency of PCP, the role of Pneumocystis has become redefined as a potential nidus promoting chronic immune activation and chronic lung disease. Similarly, chronic immune activation in some patients with HIV may be perpetuating endothelial dysfunction and immune activation leading to HIV-associated pulmonary hypertension. Models investigating the role of immune activation in HIV-associated PAH may lead to new understanding of PAH pathogenesis in idiopathic and other forms of PAH as well. In the lung transplant population, it has been shown that CMV is a risk factor for chronic lung rejection, quite possibly through the similar theme of chronic immune activation. Hence, preventing CMV infection and disease seems crucial in improving outcomes. Finally, in CMV prophylaxis, perioperative antimicrobial prophylaxis, and the treatment of XDR/MDR gram-negative infections, we are quickly learning that a patient-based approach may be beneficial in choosing the appropriate prophylaxis duration and most effective combination antimicrobial therapy. Many lessons learned in the treatment of complicated infections of the immunosuppressed host may well be applicable to other patient populations.
Footnotes
Supported by National Heart, Lung, and Blood Institute grants K23 HL096467 (M.P.G.), P01 HL103455 (K.A.N.), and K24-HL091140 (S.M.P.); Roche, Roche Organ Transplant Foundation, and the Biomarker Factory (S.M.P.); and National Institute of Allergy and Infectious Diseases grant R01 AI079175 (J.F.M.).
Author Contributions: Each author contributed substantial contributions to this work by providing the material for this review article, revising it critically, and providing final approval of the version to be published.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wang EH, Partovi N, Levy RD, Shapiro RJ, Yoshida EM, Greanya ED. Pneumocystis pneumonia in solid organ transplant recipients: not yet an infection of the past. Transpl Infect Dis. 2012;14:519–525. doi: 10.1111/j.1399-3062.2012.00740.x. [DOI] [PubMed] [Google Scholar]
- 2.Ma L, Imamichi H, Sukura A, Kovacs JA. Genetic divergence of the dihydrofolate reductase and dihydropteroate synthase genes in Pneumocystis carinii from 7 different host species. J Infect Dis. 2001;184:1358–1362. doi: 10.1086/324208. [DOI] [PubMed] [Google Scholar]
- 3.Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM, Lücking R, et al. A higher-level phylogenetic classification of the fungi. Mycol Res. 2007;111:509–547. doi: 10.1016/j.mycres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Kaneshiro ES, Maiorano JN. Survival and infectivity of Pneumocystis carinii outside the mammalian host. J Eukaryot Microbiol. 1996;43:35S. doi: 10.1111/j.1550-7408.1996.tb04971.x. [DOI] [PubMed] [Google Scholar]
- 5.Powles MA, McFadden DC, Pittarelli LA, Schmatz DM. Mouse model for Pneumocystis carinii pneumonia that uses natural transmission to initiate infection. Infect Immun. 1992;60:1397–1400. doi: 10.1128/iai.60.4.1397-1400.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolff L, Horch S, Gemsa D. The development of Pneumocystis carinii pneumonia in germ-free rats requires immunosuppression and exposure to the Pneumocystis carinii organism. Comp Immunol Microbiol Infect Dis. 1993;16:73–76. doi: 10.1016/0147-9571(93)90063-b. [DOI] [PubMed] [Google Scholar]
- 7.An CL, Gigliotti F, Harmsen AG. Exposure of immunocompetent adult mice to Pneumocystis carinii f. sp. muris by cohousing: growth of P. carinii f. sp. muris and host immune response. Infect Immun. 2003;71:2065–2070. doi: 10.1128/IAI.71.4.2065-2070.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gigliotti F, Harmsen AG, Wright TW. Characterization of transmission of Pneumocystis carinii f. sp. muris through immunocompetent BALB/c mice. Infect Immun. 2003;71:3852–3856. doi: 10.1128/IAI.71.7.3852-3856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Icenhour CR, Rebholz SL, Collins MS, Cushion MT. Early acquisition of Pneumocystis carinii in neonatal rats as evidenced by PCR and oral swabs. Eukaryot Cell. 2002;1:414–419. doi: 10.1128/EC.1.3.414-419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maskell NA, Waine DJ, Lindley A, Pepperell JC, Wakefield AE, Miller RF, Davies RJ. Asymptomatic carriage of Pneumocystis jiroveci in subjects undergoing bronchoscopy: a prospective study. Thorax. 2003;58:594–597. doi: 10.1136/thorax.58.7.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wakefield AE, Lindley AR, Ambrose HE, Denis CM, Miller RF. Limited asymptomatic carriage of Pneumocystis jiroveci in human immunodeficiency virus-infected patients. J Infect Dis. 2003;187:901–908. doi: 10.1086/368165. [DOI] [PubMed] [Google Scholar]
- 12.Chave JP, David S, Wauters JP, Van Melle G, Francioli P. Transmission of Pneumocystis carinii from AIDS patients to other immunosuppressed patients: a cluster of Pneumocystis carinii pneumonia in renal transplant recipients. AIDS. 1991;5:927–932. doi: 10.1097/00002030-199108000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Cheung YF, Chan CF, Lee CW, Lau YL. An outbreak of Pneumocystis carinii pneumonia in children with malignancy. J Paediatr Child Health. 1994;30:173–175. doi: 10.1111/j.1440-1754.1994.tb00605.x. [DOI] [PubMed] [Google Scholar]
- 14.Ruebush TK, II, Weinstein RA, Baehner RL, Wolff D, Bartlett M, Gonzles-Crussi F, Sulzer AJ, Schultz MG. An outbreak of pneumocystis pneumonia in children with acute lymphocytic leukemia. Am J Dis Child. 1978;132:143–148. doi: 10.1001/archpedi.1978.02120270041009. [DOI] [PubMed] [Google Scholar]
- 15.Singer C, Armstrong D, Rosen PP, Schottenfeld D. Pneumocystis carinii pneumonia: a cluster of eleven cases. Ann Intern Med. 1975;82:772–777. doi: 10.7326/0003-4819-82-6-722. [DOI] [PubMed] [Google Scholar]
- 16.Choukri F, Menotti J, Sarfati C, Lucet JC, Nevez G, Garin YJ, Derouin F, Totet A. Quantification and spread of Pneumocystis jirovecii in the surrounding air of patients with Pneumocystis pneumonia. Clin Infect Dis. 2010;51:259–265. doi: 10.1086/653933. [DOI] [PubMed] [Google Scholar]
- 17.Sassi M, Ripamonti C, Mueller NJ, Yazaki H, Kutty G, Ma L, Huber C, Gogineni E, Oka S, Goto N, et al. Outbreaks of Pneumocystis pneumonia in 2 renal transplant centers linked to a single strain of Pneumocystis: implications for transmission and virulence. Clin Infect Dis. 2012;54:1437–1444. doi: 10.1093/cid/cis217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ripamonti C, Orenstein A, Kutty G, Huang L, Schuhegger R, Sing A, Fantoni G, Atzori C, Vinton C, Huber C, et al. Restriction fragment length polymorphism typing demonstrates substantial diversity among Pneumocystis jirovecii isolates. J Infect Dis. 2009;200:1616–1622. doi: 10.1086/644643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gianella S, Haeberli L, Joos B, Ledergerber B, Wüthrich RP, Weber R, Kuster H, Hauser PM, Fehr T, Mueller NJ. Molecular evidence of interhuman transmission in an outbreak of Pneumocystis jirovecii pneumonia among renal transplant recipients. Transpl Infect Dis. 2010;12:1–10. doi: 10.1111/j.1399-3062.2009.00447.x. [DOI] [PubMed] [Google Scholar]
- 20.Hauser PM, Nahimana A, Taffe P, Weber R, Francioli P, Bille J, Rabodonirina M. Interhuman transmission as a potential key parameter for geographical variation in the prevalence of Pneumocystis jirovecii dihydropteroate synthase mutations. Clin Infect Dis. 2010;51:e28–e33. doi: 10.1086/655145. [DOI] [PubMed] [Google Scholar]
- 21.Huang L, Beard CB, Creasman J, Levy D, Duchin JS, Lee S, Pieniazek N, Carter JL, del Rio C, Rimland D, et al. Sulfa or sulfone prophylaxis and geographic region predict mutations in the Pneumocystis carinii dihydropteroate synthase gene. J Infect Dis. 2000;182:1192–1198. doi: 10.1086/315824. [DOI] [PubMed] [Google Scholar]
- 22.Lidman C, Olsson M, Björkman A, Elvin K. No evidence of nosocomial Pneumocystis carinii infection via health care personnel. Scand J Infect Dis. 1997;29:63–64. doi: 10.3109/00365549709008666. [DOI] [PubMed] [Google Scholar]
- 23.Leigh TR, Millett MJ, Jameson B, Collins JV. Serum titres of Pneumocystis carinii antibody in health care workers caring for patients with AIDS. Thorax. 1993;48:619–621. doi: 10.1136/thx.48.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tipirneni R, Daly KR, Jarlsberg LG, Koch JV, Swartzman A, Roth BM, Walzer PD, Huang L. Healthcare worker occupation and immune response to Pneumocystis jirovecii. Emerg Infect Dis. 2009;15:1590–1597. doi: 10.3201/eid1510.090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stansell JD, Osmond DH, Charlebois E, LaVange L, Wallace JM, Alexander BV, Glassroth J, Kvale PA, Rosen MJ, Reichman LB, et al. Pulmonary Complications of HIV Infection Study Group. Predictors of Pneumocystis carinii pneumonia in HIV-infected persons. Am J Respir Crit Care Med. 1997;155:60–66. doi: 10.1164/ajrccm.155.1.9001290. [DOI] [PubMed] [Google Scholar]
- 26.Kovacs JA, Masur H. Evolving health effects of Pneumocystis: one hundred years of progress in diagnosis and treatment. JAMA. 2009;301:2578–2585. doi: 10.1001/jama.2009.880. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez-Martínez MJ, Miró JM, Valls ME, Moreno A, Rivas PV, Solé M, Benito N, Domingo P, Muñoz C, Rivera E, et al. Spanish PCP Working Group. Sensitivity and specificity of nested and real-time PCR for the detection of Pneumocystis jiroveci in clinical specimens. Diagn Microbiol Infect Dis. 2006;56:153–160. doi: 10.1016/j.diagmicrobio.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Wilson JW, Limper AH, Grys TE, Karre T, Wengenack NL, Binnicker MJ. Pneumocystis jirovecii testing by real-time polymerase chain reaction and direct examination among immunocompetent and immunosuppressed patient groups and correlation to disease specificity. Diagn Microbiol Infect Dis. 2011;69:145–152. doi: 10.1016/j.diagmicrobio.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris A, Norris KA. Colonization by Pneumocystis jirovecii and its role in disease. Clin Microbiol Rev. 2012;25:297–317. doi: 10.1128/CMR.00013-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogel M, Weissgerber P, Goeppert B, Hetzel J, Vatlach M, Claussen C, Horger M. Accuracy of serum LDH elevation for the diagnosis of Pneumocystis jiroveci pneumonia. Swiss Med Wkly. 2011;141:w13184. doi: 10.4414/smw.2011.13184. [DOI] [PubMed] [Google Scholar]
- 31.Morris AM, Masur H. A serologic test to diagnose pneumocystis pneumonia: are we there yet? Clin Infect Dis. 2011;53:203–204. doi: 10.1093/cid/cir348. [DOI] [PubMed] [Google Scholar]
- 32.Esteves F, Lee CH, de Sousa B, Badura R, Seringa M, Fernandes C, Gaspar JF, Antunes F, Matos O. (1-3)-Beta-D-glucan in association with lactate dehydrogenase as biomarkers of Pneumocystis pneumonia (PcP) in HIV-infected patients. Eur J Clin Microbiol Infect Dis. doi: 10.1007/s10096-014-2054-6. (In press) [DOI] [PubMed] [Google Scholar]
- 33.Vargas SL, Ponce CA, Gallo M, Pérez F, Astorga JF, Bustamante R, Chabé M, Durand-Joly I, Iturra P, Miller RF, et al. Near-universal prevalence of Pneumocystis and associated increase in mucus in the lungs of infants with sudden unexpected death. Clin Infect Dis. 2013;56:171–179. doi: 10.1093/cid/cis870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Probst M, Ries H, Schmidt-Wieland T, Serr A. Detection of Pneumocystis carinii DNA in patients with chronic lung diseases. Eur J Clin Microbiol Infect Dis. 2000;19:644–645. doi: 10.1007/s100960000329. [DOI] [PubMed] [Google Scholar]
- 35.Morris A, Sciurba FC, Lebedeva IP, Githaiga A, Elliott WM, Hogg JC, Huang L, Norris KA. Association of chronic obstructive pulmonary disease severity and Pneumocystis colonization. Am J Respir Crit Care Med. 2004;170:408–413. doi: 10.1164/rccm.200401-094OC. [DOI] [PubMed] [Google Scholar]
- 36.Morris A, Alexander T, Radhi S, Lucht L, Sciurba FC, Kolls JK, Srivastava R, Steele C, Norris KA. Airway obstruction is increased in pneumocystis-colonized human immunodeficiency virus-infected outpatients. J Clin Microbiol. 2009;47:3773–3776. doi: 10.1128/JCM.01712-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christensen PJ, Preston AM, Ling T, Du M, Fields WB, Curtis JL, Beck JM. Pneumocystis murina infection and cigarette smoke exposure interact to cause increased organism burden, development of airspace enlargement, and pulmonary inflammation in mice. Infect Immun. 2008;76:3481–3490. doi: 10.1128/IAI.00165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shipley TW, Kling HM, Morris A, Patil S, Kristoff J, Guyach SE, Murphy JE, Shao X, Sciurba FC, Rogers RM, et al. Persistent pneumocystis colonization leads to the development of chronic obstructive pulmonary disease in a nonhuman primate model of AIDS. J Infect Dis. 2010;202:302–312. doi: 10.1086/653485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson MP, Christmann BS, Dunaway CW, Morris A, Steele C. Experimental Pneumocystis lung infection promotes M2a alveolar macrophage-derived MMP12 production. Am J Physiol Lung Cell Mol Physiol. 2012;303:L469–L475. doi: 10.1152/ajplung.00158.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varela JM, Respaldiza N, Sánchez B, de la Horra C, Montes-Cano M, Rincón M, Dapena J, González-Becerra C, Medrano FJ, Calderón E. Lymphocyte response in subjects with chronic pulmonary disease colonized by Pneumocystis jirovecii. J Eukaryot Microbiol. 2003;50:672–673. doi: 10.1111/j.1550-7408.2003.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 41.Chou CW, Lin FC, Tsai HC, Chang SC. The importance of pro-inflammatory and anti-inflammatory cytokines in Pneumocystis jirovecii pneumonia. Med Mycol. 2013;51:704–712. doi: 10.3109/13693786.2013.772689. [DOI] [PubMed] [Google Scholar]
- 42.Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol. 2011;8:443–455. doi: 10.1038/nrcardio.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sitbon O, Lascoux-Combe C, Delfraissy JF, Yeni PG, Raffi F, De Zuttere D, Gressin V, Clerson P, Sereni D, Simonneau G. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177:108–113. doi: 10.1164/rccm.200704-541OC. [DOI] [PubMed] [Google Scholar]
- 44.Nunes H, Humbert M, Sitbon O, Morse JH, Deng Z, Knowles JA, Le Gall C, Parent F, Garcia G, Hervé P, et al. Prognostic factors for survival in human immunodeficiency virus-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2003;167:1433–1439. doi: 10.1164/rccm.200204-330OC. [DOI] [PubMed] [Google Scholar]
- 45.Sterne JA, Hernán MA, Ledergerber B, Tilling K, Weber R, Sendi P, Rickenbach M, Robins JM, Egger M Swiss HIV Cohort Study. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Lancet. 2005;366:378–384. doi: 10.1016/S0140-6736(05)67022-5. [DOI] [PubMed] [Google Scholar]
- 46.Barbaro G, Lucchini A, Pellicelli AM, Grisorio B, Giancaspro G, Barbarini G. Highly active antiretroviral therapy compared with HAART and bosentan in combination in patients with HIV-associated pulmonary hypertension. Heart. 2006;92:1164–1166. doi: 10.1136/hrt.2005.076794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Degano B, Guillaume M, Savale L, Montani D, Jaïs X, Yaici A, Le Pavec J, Humbert M, Simonneau G, Sitbon O. HIV-associated pulmonary arterial hypertension: survival and prognostic factors in the modern therapeutic era. AIDS. 2010;24:67–75. doi: 10.1097/QAD.0b013e328331c65e. [DOI] [PubMed] [Google Scholar]
- 48.Morris A, Gingo MR, George MP, Lucht L, Kessinger C, Singh V, Hillenbrand M, Busch M, McMahon D, Norris KA, et al. Cardiopulmonary function in individuals with HIV infection in the antiretroviral therapy era. AIDS. 2012;26:731–740. doi: 10.1097/QAD.0b013e32835099ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dalvi P, O’Brien-Ladner A, Dhillon NK. Downregulation of bone morphogenetic protein receptor axis during HIV-1 and cocaine-mediated pulmonary smooth muscle hyperplasia: implications for HIV-related pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2013;33:2585–2595. doi: 10.1161/ATVBAHA.113.302054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mermis J, Gu H, Xue B, Li F, Tawfik O, Buch S, Bartolome S, O’Brien-Ladner A, Dhillon NK. Hypoxia-inducible factor-1 α/platelet derived growth factor axis in HIV-associated pulmonary vascular remodeling. Respir Res. 2011;12:103. doi: 10.1186/1465-9921-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marecki JC, Cool CD, Parr JE, Beckey VE, Luciw PA, Tarantal AF, Carville A, Shannon RP, Cota-Gomez A, Tuder RM, et al. HIV-1 Nef is associated with complex pulmonary vascular lesions in SHIV-nef-infected macaques. Am J Respir Crit Care Med. 2006;174:437–445. doi: 10.1164/rccm.200601-005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bull TM, Meadows CA, Coldren CD, Moore M, Sotto-Santiago SM, Nana-Sinkam SP, Campbell TB, Geraci MW. Human herpesvirus-8 infection of primary pulmonary microvascular endothelial cells. Am J Respir Cell Mol Biol. 2008;39:706–716. doi: 10.1165/rcmb.2007-0368OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morris A, Hillenbrand M, Finkelman M, George MP, Singh V, Kessinger C, Lucht L, Busch M, McMahon D, Weinman R, et al. Serum (1→3)-β-D-glucan levels in HIV-infected individuals are associated with immunosuppression, inflammation, and cardiopulmonary function. J Acquir Immune Defic Syndr. 2012;61:462–468. doi: 10.1097/QAI.0b013e318271799b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dalvi P, Wang K, Mermis J, Zeng R, Sanderson M, Johnson S, Dai Y, Sharma G, Ladner AO, Dhillon NK. HIV-1/cocaine induced oxidative stress disrupts tight junction protein-1 in human pulmonary microvascular endothelial cells: role of Ras/ERK1/2 pathway. PLoS ONE. 2014;9:e85246. doi: 10.1371/journal.pone.0085246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.George MP, Champion HC, Simon M, Guyach S, Tarantelli R, Kling HM, Brower A, Janssen C, Murphy J, Carney JP, et al. Physiologic changes in a nonhuman primate model of HIV-associated pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2013;48:374–381. doi: 10.1165/rcmb.2011-0434OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol. 1994;144:275–285. [PMC free article] [PubMed] [Google Scholar]
- 57.Hassoun PM, Mouthon L, Barberà JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54:S10–S19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 58.Perros F, Dorfmüller P, Montani D, Hammad H, Waelput W, Girerd B, Raymond N, Mercier O, Mussot S, Cohen-Kaminsky S, et al. Pulmonary lymphoid neogenesis in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;185:311–321. doi: 10.1164/rccm.201105-0927OC. [DOI] [PubMed] [Google Scholar]
- 59.Colvin KL, Cripe PJ, Ivy DD, Stenmark KR, Yeager ME. Bronchus-associated lymphoid tissue in pulmonary hypertension produces pathologic autoantibodies. Am J Respir Crit Care Med. 2013;188:1126–1136. doi: 10.1164/rccm.201302-0403OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Hertz MI International Society of Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant. 2012;31:1073–1086. doi: 10.1016/j.healun.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 61.Burguete SR, Maselli DJ, Fernandez JF, Levine SM. Lung transplant infection. Respirology. 2013;18:22–38. doi: 10.1111/j.1440-1843.2012.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duncan SR, Paradis IL, Yousem SA, Similo SL, Grgurich WF, Williams PA, Dauber JH, Griffith BP. Sequelae of cytomegalovirus pulmonary infections in lung allograft recipients. Am Rev Respir Dis. 1992;146:1419–1425. doi: 10.1164/ajrccm/146.6.1419. [DOI] [PubMed] [Google Scholar]
- 63.Gottlieb J, Schulz TF, Welte T, Fuehner T, Dierich M, Simon AR, Engelmann I. Community-acquired respiratory viral infections in lung transplant recipients: a single season cohort study. Transplantation. 2009;87:1530–1537. doi: 10.1097/TP.0b013e3181a4857d. [DOI] [PubMed] [Google Scholar]
- 64.Kumar D, Husain S, Chen MH, Moussa G, Himsworth D, Manuel O, Studer S, Pakstis D, McCurry K, Doucette K, et al. A prospective molecular surveillance study evaluating the clinical impact of community-acquired respiratory viruses in lung transplant recipients. Transplantation. 2010;89:1028–1033. doi: 10.1097/TP.0b013e3181d05a71. [DOI] [PubMed] [Google Scholar]
- 65.Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin Infect Dis. 2006;43:1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 66.Zamora MR. DNA viruses (CMV, EBV, and the herpesviruses) Semin Respir Crit Care Med. 2011;32:454–470. doi: 10.1055/s-0031-1283285. [DOI] [PubMed] [Google Scholar]
- 67.Sharples LD, McNeil K, Stewart S, Wallwork J. Risk factors for bronchiolitis obliterans: a systematic review of recent publications. J Heart Lung Transplant. 2002;21:271–281. doi: 10.1016/s1053-2498(01)00360-6. [DOI] [PubMed] [Google Scholar]
- 68.Tamm M, Aboyoun CL, Chhajed PN, Rainer S, Malouf MA, Glanville AR. Treated cytomegalovirus pneumonia is not associated with bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2004;170:1120–1123. doi: 10.1164/rccm.200310-1405OC. [DOI] [PubMed] [Google Scholar]
- 69.Snyder LD, Finlen-Copeland CA, Turbyfill WJ, Howell D, Willner DA, Palmer SM. Cytomegalovirus pneumonitis is a risk for bronchiolitis obliterans syndrome in lung transplantation. Am J Respir Crit Care Med. 2010;181:1391–1396. doi: 10.1164/rccm.200911-1786OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zamora MR. Use of cytomegalovirus immune globulin and ganciclovir for the prevention of cytomegalovirus disease in lung transplantation. Transpl Infect Dis. 2001;3:49–56. doi: 10.1034/j.1399-3062.2001.00010.x. [DOI] [PubMed] [Google Scholar]
- 71.Smith MA, Sundaresan S, Mohanakumar T, Trulock EP, Lynch JP, Phelan DL, Cooper JD, Patterson GA. Effect of development of antibodies to HLA and cytomegalovirus mismatch on lung transplantation survival and development of bronchiolitis obliterans syndrome. J Thorac Cardiovasc Surg. 1998;116:812–820. doi: 10.1016/S0022-5223(98)00444-9. [DOI] [PubMed] [Google Scholar]
- 72.Weigt SS, Elashoff RM, Keane MP, Strieter RM, Gomperts BN, Xue YY, Ardehali A, Gregson AL, Kubak B, Fishbein MC, et al. Altered levels of CC chemokines during pulmonary CMV predict BOS and mortality post-lung transplantation. Am J Transplant. 2008;8:1512–1522. doi: 10.1111/j.1600-6143.2008.02280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palmer SM, Limaye AP, Banks M, Gallup D, Chapman J, Lawrence EC, Dunitz J, Milstone A, Reynolds J, Yung GL, et al. Extended valganciclovir prophylaxis to prevent cytomegalovirus after lung transplantation: a randomized, controlled trial. Ann Intern Med. 2010;152:761–769. doi: 10.7326/0003-4819-152-12-201006150-00003. [DOI] [PubMed] [Google Scholar]
- 74.Finlen Copeland CA, Davis WA, Snyder LD, Banks M, Avery R, Davis RD, Palmer SM. Long-term efficacy and safety of 12 months of valganciclovir prophylaxis compared with 3 months after lung transplantation: a single-center, long-term follow-up analysis from a randomized, controlled cytomegalovirus prevention trial. J Heart Lung Transplant. 2011;30:990–996. doi: 10.1016/j.healun.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 75.Snyder LD, Medinas R, Chan C, Sparks S, Davis WA, Palmer SM, Weinhold KJ. Polyfunctional cytomegalovirus-specific immunity in lung transplant recipients receiving valganciclovir prophylaxis. Am J Transplant. 2011;11:553–560. doi: 10.1111/j.1600-6143.2010.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Speich R, van der Bij W. Epidemiology and management of infections after lung transplantation. Clin Infect Dis. 2001;33:S58–S65. doi: 10.1086/320906. [DOI] [PubMed] [Google Scholar]
- 77.Valentine VG, Gupta MR, Walker JE, Jr, Seoane L, Bonvillain RW, Lombard GA, Weill D, Dhillon GS. Effect of etiology and timing of respiratory tract infections on development of bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2009;28:163–169. doi: 10.1016/j.healun.2008.11.907. [DOI] [PubMed] [Google Scholar]
- 78.Shields RK, Clancy CJ, Minces LR, Shigemura N, Kwak EJ, Silveira FP, Abdel-Massih RC, Bhama JK, Bermudez CA, Pilewski JM, et al. Epidemiology and outcomes of deep surgical site infections following lung transplantation. Am J Transplant. 2013;13:2137–2145. doi: 10.1111/ajt.12292. [DOI] [PubMed] [Google Scholar]
- 79.Shields RK, Clancy CJ, Gillis LM, Kwak EJ, Silveira FP, Massih RC, Eschenauer GA, Potoski BA, Nguyen MH. Epidemiology, clinical characteristics and outcomes of extensively drug-resistant Acinetobacter baumannii infections among solid organ transplant recipients. PLoS ONE. 2012;7:e52349. doi: 10.1371/journal.pone.0052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shields RK, Kwak EJ, Potoski BA, Doi Y, Adams-Haduch JM, Silviera FP, Toyoda Y, Pilewski JM, Crespo M, Pasculle AW, et al. High mortality rates among solid organ transplant recipients infected with extensively drug-resistant Acinetobacter baumannii: using in vitro antibiotic combination testing to identify the combination of a carbapenem and colistin as an effective treatment regimen. Diagn Microbiol Infect Dis. 2011;70:246–252. doi: 10.1016/j.diagmicrobio.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 81.Jernigan MG, Press EG, Nguyen MH, Clancy CJ, Shields RK. The combination of doripenem and colistin is bactericidal and synergistic against colistin-resistant, carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2012;56:3395–3398. doi: 10.1128/AAC.06364-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Clancy CJ, Chen L, Shields RK, Zhao Y, Cheng S, Chavda KD, Hao B, Hong JH, Doi Y, Kwak EJ, et al. Epidemiology and molecular characterization of bacteremia due to carbapenem-resistant Klebsiella pneumoniae in transplant recipients. Am J Transplant. 2013;13:2619–2633. doi: 10.1111/ajt.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]