Introduction
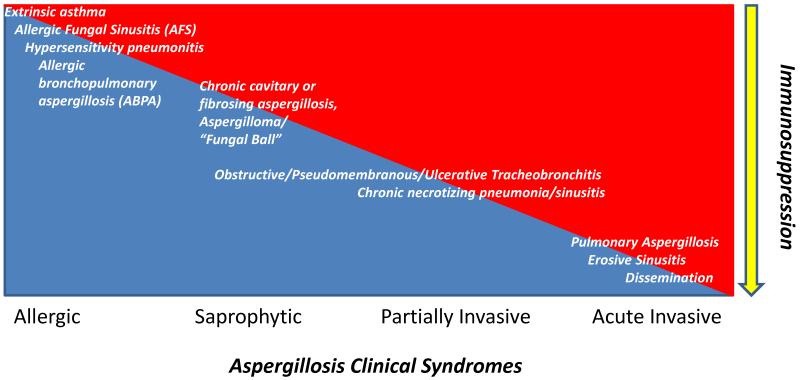
Aspergillus species are ubiquitous Ascomycetes that may cause a variety of syndromes depending on the degree of immunosuppression in the host. In ascending order of immunocompromise, these include: allergic bronchopulmonary aspergillosis (ABPA), saprophytic aspergillomas, chronic necrotizing aspergillosis, and invasive aspergillosis (IA) (Figure 1). Angioinvasion is the hallmark of IA, with sinopulmonary involvement being the most common manifestation while dissemination to the central nervous system, gastrointestinal tract, skin, or contiguously may occur amongst the most severely immune impaired. Definitions of proven and probable IA and complete or partial response to therapy have been previously published [1,2]. The most important species include A. fumigatus, A. flavus, A. niger, A. terreus, and A. nidulans which may have varying resistance patterns [3].
Figure 1.
Spectrum of aspergillosis disease as a function of immunosuppression. With decreasing cell-mediated immunity, the likelihood of invasive disease increases.
IA causes tremendous morbidity and mortality, particularly among those with prolonged and severe functional or quantitative (absolute neutrophil count [ANC] < 500 cells/μl) neutropenia. For instance, data from the Transplant Associated Infections Surveillance Network (TRANSNET) from 2001 to 2006 revealed a 25.4% and 59% one-year survival from IA among hematopoietic stem cell transplant (HSCT) recipients and solid organ transplant (SOT) recipients, respectively. While the IA one-year cumulative incidence may be 1-8% in such populations, the one-year overall cumulative mortality incidence is 18-42% [4,5]. More recently, the Prospective Antifungal Therapy (PATH) alliance registry reported a 12-week survival of 66.3% for IA among a variety of risk groups [6].
The timing of interventions – empiric, pre-emptive, or targeted – affects outcomes with early interventions generally ameliorating success endpoints and potentially lowering drug-related toxicities, costs, and resistance. For instance, two recent multi-center, randomized clinical trials compared pre-emptive approaches based on biomarkers such as galactomannan or PCR, radiographic signs (e.g., “halo”), and clinical symptoms in high-risk patients to: (1.) empirical based on persistent febrile neutropenia despite broad-spectrum antibacterials and (2) targeted approaches based on culture and/or histology. In the first study, the pre-emptive model was found to be non-inferior to empirical therapy with respect to survival assessed 14 days after neutrophil recovery and not statistically different 4 months after study inclusion among those with hematologic malignancies or autologous HSCT (lower 95%CI margin for mortality difference was −5.9%, which was within the non-inferiority margin of −8%). However, it was also associated with a 2.5-fold increased IA incidence, particularly during induction chemotherapy (p<0.01), yet there was a decreased antifungal costs by 35% [7]. In the second study comparing pre-emptive to directed modalities among allogeneic HSCT and those receiving chemotherapy for acute leukemia, use of biomarkers reduced empirical antifungal usage by 17% (p<0.002) at 26-weeks following randomization, but drug adverse events and all-cause or IA-related mortality did not differ between the groups – the latter perhaps due to the higher risk IA population included in this study [8]. Thus, empiric therapy may be advisable in high-risk patients whereas a pre-emptive approach may be supported in low-risk patients.
Treatment options in IA are an evolving with novel antifungals introduced within the past decade. The use of combination antifungals, immunotherapy, and surgery for refractory disease has become particularly appealing in certain situations. This review will give an overview of current practices in the management of IA.
Treatment
Pharmacologic Treatment
Antifungals (Table 1)
Table 1. Antifungal Use for Invasive Aspergillosis. Duration may be dependent on the level of immunosuppression and clinical/radiographic response. Please see the Infectious Diseases Society of America (IDSA) guidelines#[3].
| Antifungal | Dose | Approved Indication |
|---|---|---|
| Azoles | ||
| Itraconazole* | 200mg IV/PO BID-TID × 3-4d then 200mg IV/PO QD-BID |
Salvage (refractory or intolerant to OLAT****)# |
| Voriconazole** | 6mg/kg IV/PO q12h × 1 then 4mg/kg IV/PO q12h |
Primary therapy# |
| Posaconazole*** | 200-400mg PO QID-BID | Prolonged neutropenia prophylaxis (e.g., AML, MDS, GVHD, HSCT) |
| Polyenes | ||
| Amphotericin B deoxycholate (AmB-D) |
0.5-1.0 mg/kg IV QD to 1.5mg/kg IV QOD |
Primary Therapy# |
| Liposomal Amphotericin B (LAmB) |
3-5mg IV QD | Primary Therapy; empiric febrile neutropenia therapy# |
| Amphotericin B lipid complex (ABLC) |
5mg/kg IV QD | Salvage (refractory or intolerant to AmB-d)# |
| Amphotericin B colloid dispersion (ABCD) |
3-6 mg/kg IV QD | Salvage (refractory or intolerant to AmB-d) |
| Echinocandins | ||
| Caspofungin | 70mg IV × 1 then 50mg IV QD |
Salvage (refractory or intolerant to OLAT****);# empiric febrile neutropeniatherapy |
| Micafungin | 100-150mg IV QD | N/A |
| Anidulafungin | 200mg IV × 1 then 100mg IV QD |
N/A |
Oral solution with greater bioavailability than capsule (target trough > 250 ng/ml);
target trough 1 μg/mL and 5.5 μg/mL;
target trough > 0.5 μg/ml, a delayed-release tablet that has improved oral absorption with food is now available;
Other licensed antifungal therapy
Mold-active triazoles
Triazoles inhibit a step in fungal cell membrane ergosterol biosynthesis by blocking 14α-demethylase, a fungal cytochrome p450 enzyme. Fluconazole does not have mold activity. The intravenous (i.v.) formulation of itraconazole contains hydroxypropyl cyclodextrin while voriconazole i.v. contains sulfobutyl cyclodextrin, both which may cause renal toxicity. I.V. itraconazole is now no longer routinely available in the U.S. but may be available in other countries. In a study by Caillot et al, a complete or partial response was seen at the end of therapy (EOT) in 15 (48%) of 31 patients receiving 12 days of intravenous itraconazole followed by 12 weeks of oral itraconazole capsule. Itraconazole has erratic oral bioavailability, though the oral solution has improved upon the capsule form in this regard. In general, a trough plasma concentration of itraconazole >250 ng/mL is considered to be the minimum efficacy level [9]. Hydroxy-itraconazole is also antifungal and present in nearly equal concentrations to native drug. In a non-comparative study by Denning et al, oral itraconazole capsules (600 mg/d for 4 days followed by 400 mg/d) afforded 39% complete and partial response at the EOT, with failure rates varying according to site of infection (pulmonary and tracheobronchial disease: 14%; sinus disease: 50%; central nervous system disease: 63%) and degree of immunosuppression (SOT: 7%; allogeneic HSCT: 29%; neutropenia > 2 weeks: 14%; AIDS: 44%) [10]. In addition, it has been used in the prevention of fungal infections, including IA, among those with neutropenia and chronic granulomatous disease (CGD) – an entity that predisposes to infections with organisms such as Staphylococcus, Serratia, Burkholderia, Nocardia, and Aspergillus [11,12]. In CGD, A. nidulans may be more virulent than A. fumigatus, causing tissue plane invasion rather than true angioinvasion, but paradoxically are not killed by reactive oxygen species [13,14]. Based on these studies, itraconazole received indication for IA among those intolerant of or who are refractory to amphotericin B therapy.
Voriconazole received approval for primary therapy of IA in 2002 based on the pivotal RCT by Herbecht et al, which found it to be superior over AmB-d. A loading dose of 6mg/kg every 12 hours for the first day followed by 4mg/kg twice daily was given in order to achieve steady state more rapidly (i.e., 24 hours), that may otherwise take 5-7 days. Voriconazole achieved 52.8% 12-week global response rate vis à vis vs. 31.6% in the Amb-D arm and a 22% reduction in overall mortality (12-week survival: 70.8% vs. 57.9%; HR=0.59 [0.44-0.88], p=0.02) [15]. In the subsequent analysis, Patterson et al found that fewer patients receiving voriconazole switched to other antifungals than in the AmB-d arm due to disease progression or intolerance (24% vs. 70%, p < 0.001) and, despite the switch, success at 12-weeks was less common in the latter than former group (32% vs. 55%, p < 0.001) [16]. Ravuconazole, isavuconazole, and albaconazole have a structure similar to voriconazole with a fluorinated pyrimidine ring and may offer extended half-lives, but are still in the research stages of clinical development [17]. Clinical trials in Europe of isavuconazole are nearing completion.
Posaconazole is currently only available as an oral formulation and is structurally similar to itraconazole but has a broader spectrum of activity (e.g., certain Mucorales). It is indicated for prophylaxis during neutropenia, particularly in acute myelogenous leukemia (AML), myelodysplatic syndrome (MDS), and graft versus host disease (GVHD). Cornely et al demonstrated a significant reduction in IA (2 [1%] vs. 20 [7%], p<0.001) and prolonged survival (100-day survival, p=0.04) in those receiving posaconazole during neutropenia with AML or MDS compared with itraconazole or fluconazole [18]. Ullmann et al showed that those allogeneic HSCT who received posaconazole during GVHD had an 83% reduction in breakthrough IA infections compared with those receiving fluconazole (1.0% vs. 5.9%, p=0.001) and decreased fungal-related mortality (1% vs. 4%, p=0.046) [19]. Like itraconazole but unlike voriconazole, the oral absorption of poasaconazole suspension is dependent upon gastric acidity and gut integrity. Based on these data, posaconazole received indication for prophylaxis against IA among such patients at high risk in 2006. A new delayed-release tablet is now available with improved oral absorption with food (300mg twice daily loading dose on the first day followed by 300mg daily).
Unfortunately, azole drug interactions via the cytochrome P450 enzymes (CYP3A4 and 2C19) may lead to significant perturbations in levels of co-administered medications such as cyclosporine, proton pump inhibitors, HIV protease inhibitors, HMG-CoA reductase inhibitors, warfarin, and corticosteroids. Use with quinolones may lead to prolongation of the QTc interval. Concomitant use of voriconazole with sirolimus and efavirenz is contraindicated. The dose of calcineurin inhibitors such as tacrolimus and cyclosporine should be reduced by 25 to 75% when used with fluconazole and voriconazole or posaconazole, respectively. Liver function should be monitored, as a transaminitis five times the upper limit of normal in an asymptomatic individual would preclude its continued use. Voriconazole can cause a severe photosensitivity reaction; it can cause reversible visual changes, such as blurring, light sensitivity or abnormal color perception. Moreover, azole-resistant isolates of Aspergillus, via alterations of the target enzyme CYP51A, have been reported [20]. In the Netherlands, the TR/L98H mutation in the CYP51A gene was harbored by 64% of azole naïve patients’ A. fumigatus isolates which correlated with an 88% overall mortality and significant cross-azole resistance was observed [21]. This may have been due to the agriculture use of azoles in this particular region and its spread has been limited thus far [22]. The epidemiologic cut-off value (ECV) in this context may be valuable, as it can permit the tracing of where resistance changes have occurred. The ECV is the minimum inhibitory concentration (MIC) value identifying the upper limit of the wild type (WT) population, as defined as the predominant species strain without acquired resistance mutations to an antifungal, which enables discrimination of WT from acquired resistance phenotypes in population-based studies [23].
Azoles may also have immunomodulatory effects, but less so than lipid amphotericin formulations and echinocandins. For instance, transcriptional profiles of TLR-2 and TNF-α in human monocytes were upregulated in the presence of voriconazole -- even at sub-inhibitory concentrations (0.1 μg/ml) -- compared with hyphae alone and this correlated with hyphal damage. In addition, nuclear translocation of NF-κB – a key common event in the inflammatory response to cytokine via signal transduction and activator of transcription (STAT) pathways – was more pronounced in the presence of voriconazole [24]. However, other investigators have found these effects to be abrogated by conidia [25].
Polyenes
Amphotericin B formulations that disrupt the fungal ergosterol membrane by forming pores have been the “gold standard” antifungal for IA for over 50 years [26,27]. The deoxycholate version (AmB-d) can be prohibitively toxic (infusion and kidney-related) and has consequently been replaced largely by lipid formulations: liposomal amphotericin B [L-AmB] 3-5mg/kg/d and amphotericin B lipid complex [ABLC] 5mg/kg/d primarily, while amphotericin B colloid dispersion [ABCD] 3-5mg/kg/d is no longer used much [28-30]. In a double-blind, randomized, controlled trial (RCT) comparing ABCD 6mg/kg/d to AmB-d 1.0-1.5 mg/kg/d for the treatment of IA, those receiving ABCD had lower renal toxicity (25% vs. 49%; p=0.002), with a longer median time to onset of nephrotoxicity (301 vs. 22 days; p=0.001), but efficacy was equivalent (therapeutic response: 52% vs. 51%; p=1.0, and death due to IA: 32% vs. 26%; p=0.7) [30]. Infusion-related toxicities due to ABCD were much higher (30%), however, which has led to its disuse -- favoring L-AmB and ABLC. In the AmBiLoad trial, Cornely et al compared 3mg/kg/d to 10mg/kg/d of L-AmB and found a comparable overall favorable response for IA (50% vs. 46%, p=0.65) but greater nephrotoxicity and hypokalemia in the high-dose arm [31].
The different lipid formulations vary in their tissue distribution and toxicity, however, with L-AmB having a better therapeutic index while ABLC achieve higher lung and kidney tissue concentrations [32]. However, all three reach high levels in the liver, spleen, and other reticuloendothelial system organs including the bone marrow. Nonetheless, tissue penetration may be modified by site-specific inflammation. For instance, in a murine model of cerebral aspergillosis, investigators found L-AmB 3mg/kg IV daily to be most efficacious in reducing brain colony forming unit burden with high localization to the capillary endothelium [33]. The efficacy of these lipid formulations in IA treatment is comparable, with approximately 40-50% response rates, though head-to-head comparisons have been only done for febrile neutropenia. Nevertheless, certain species such as A. terreus, A. nidulans, and A. ustus may have intrinsic resistance to the polyenes [34-36].
The liposomal carrier may produce immunomodulatory activity that attenuates a dysregulated inflammatory response to IA by promoting non-oxidative leukocyte killing mechanisms [37]. In addition, L-AmB may facilitate transition from TLR-2 to TLR-4 pattern recognition by neutrophils with consequent balance away from pro-inflammatory cytokines such as TNF-a towards anti-inflammatory IL-10, but preserved polymorphonuclear (PMN) leukocyte phagocytosis ability and degranulation rather than radical oxidative induction [38].
Echinocandins
Echinocandins (caspofungin, micafungin, and anidulafungin) work by inhibiting primarily fungal β-1,3-glucan synthase which makes a key component in the cell wall, but are fungistatic for Aspergillus – blunting hyphal growth with distended ballon-like tips [39]. In a non-comparative study by Maertens et al, caspofungin was found to have a 45% favorable response in salvage IA therapy – the majority with refractory pulmonary disease [40]. Based on data such as this, caspofungin received approval for salvage but not primary IA therapy. Echinocandins may differ in their fungicidal and post-antifungal effects, with micafungin and anidulafungin having a 2-10 fold lower minimum effective concentration (MEC) – “the lowest drug concentration at which small, rounded, and compact hyphal forms are observed” at 24-48 hours incubation [41]-- than caspofungin [42]. Among the TRANSNET cohort isolates, over 95% of isolates from proven or probable IA cases had echinocandin MECs less than the ECV, but there was inter-species variability; 17% of A. terreus isolates had an MEC greater than the ECV [41].
All three echinocandins may have a role in empirical febrile neutropenia therapy among those with hematologic malignancy and recipients of stem cell and solid organ transplantation, though only caspofungin is licensed for this indication with comparable 34% overall success to liposomal amphotericin B 3mg/kg/d [43,44]. Micafungin has been shown to be comparable to caspofungin among an adult febrile neutropenic cohort in terms of deaths, breakthrough fungal infections, and adverse events but the dose may need to be higher in the pediatric population [45,46]. However, breakthrough IA in patients receiving caspofungin is increasingly being reported -- 13% in one cohort compared with none among those receiving amphotericin B [47]. A fourth echinocandin – aminocandin – is in development and has potent fungicidal Aspergillus activity in a murine model of disseminated disease, reducing organ burden and improving survival over other echinocandins and itraconazole [48].
Echinocandins are metabolized in the liver with a half-life of 9-26 hours that permits once daily dosing. However, they are highly protein bound (>95%). Only intravenous formulations are made, permitting distribution to a wide variety of organs, including the brain, but low eye and CSF concentrations that preclude use in infections affecting these compartments [49]. Minor dose adjustments in hepatic insufficiency are only needed with caspofungin and micafungin but not anidulafungin and only micafungin does not require a loading dose. Caspofungin may interact with calcineurin inhibitors reducing the tacrolimus area-under-the-curve (AUC) levels by 20% but having 35% increased AUC levels in the presence of cyclosporine.
Micafungin was found to have a dose-dependent anti-inflammatory effect on human polymorphonuclear leukocytes in the presence of Aspergillus fumigatus conidia in vivo through decreasing TNF-α and increasing IL-10 via the TLR2/dectin-1 and TLR3/TRIF pathway signaling [50]. Echinocandins may unmask β-glucans to facilitate immune cell recognition by monocytes via pattern recognition receptors such as dectin-1 and modulate subsequent inflammatory response due to TNF and CXCL2 in a fungal growth stage specific manner over time [51]. In addition, fungal wall re-modeling through compensatory cytoskeletal synthesis mechanisms such as Rho-1p – the regulatory subunit of the beta glucan synthase complex – that are triggered during fungal stress may lead to epitope modification and subsequent fungal clearance through undetermined mechanisms [52].
Combination antifungal therapy
Based upon in vitro and animal study data, several investigators noted the potential for improved outcomes for IA through synergistic or additive effects of drugs with complimentary mechanisms of action such as combining mold active triazoles or an amphotericin B product with an echinocandin [53,54]. More recently, Elefanti et al performed serum inhibitory and fungicidal interaction studies against Aspergillus species by combining echinocandins with amphotericin B or voriconazole. The greatest additive inhibitory interactions were found with micafungin (3.6-fold) > anidulafungin (2.9-fold) > caspofungin (2-fold) and with A. flavus > A. fumigatus > A. terreus with the lowest serum interaction indices found with amphotericin B-caspofungin and A. terreus [55].
A few human observational studies and small-scale clinical trials have been published to support the use of such combination therapy for IA. In a retrospective cohort study, Kontoyiannis et al demonstrated that L-AmB combined with caspofungin resulted in no significant difference in composite response among those who received it for primary vis à vis refractory or intolerant to treat IA (53% vs. 35%, p=0.36) [56]. In a recently completed larger, randomized clinical trial comparing voriconazole and andidulfungin combination therapy to voriconazole alone for primary IA therapy, Marr et al noted a marginal 12-week survival effect of combination versus monotherapy (p=0.08, 95%CI: −21.4, 1.09). Notably, global success favored monotherapy in this trial (p=0.08, 95%CI: −21.6, 1.15) [57]. Given the difficulties (e.g., patient accrual) in completing this trial of primary IA treatment comparing modalities, the probability of a similar appropriately powered and comparative, double-blinded, multicenter trial for salvage therapy is low.
As a consequence, Panackal et al performed a recent comprehensive systematic review and meta-analysis looking at a combination of mold-active triazoles or lipid amphotericin B with echinocandins versus monotherapy with such a triazole or a lipid amphotericin B. The rationale was that the three drug classes have comparable efficacies in the salvage IA setting (i.e., primarily refractory disease). We demonstrated that the conglomerate evidence suggests improved 12-week survival (Peto OR=1.80 [1.08-3.01]) and success (Peto OR=2.17 [1.21-3.91]) with combination therapy by our fixed effects model. This remained significant after applying a random effects approach as a sensitivity analysis (12-week survival (Peto OR=1.90 [1.04-3.46] and unchanged value for success). Restriction to high quality studies and including echinocandins as the comparator for refractory IA revealed an adjusted OR=1.72[0.96-3.09], p=0.07 for global success while the survival endpoint remained unaltered. However, these findings should be interpreted with caution given the inherent limitations of meta-analyses and applied only in certain clinical settings. Moreover, time-varying parameters such as changes in conditioning regimens for HSCT resulting in improved patient care may make our conclusions based on past studies yield uncertain applicability with future studies (i.e., a cohort effect) [58].
Adjuvant Immunotherapy/Immunoprophylaxis (Table 2)
Table 2. Adjunctive Immunotherapy/Immunoprophylaxis for Invasive Aspergillosis.
| Agent | Dose | Setting |
|---|---|---|
| G-CSF | 5-6mcg/kg/d SQ | high risk febrile neutropenia, especially cyclical/congential vs. cancer- related neutropenia; may prolong polymorphonuclear leukocyte survival |
| GM-CSF | 4-5mcg/kg/d SQ | high risk febrile neutropenia, stimulating myeloid lineages; may improve IFI survival; capillary leak syndrome may occur |
| IFN-γ | 50 mcg/m2 BSA SQ every other day |
chronic granulomatous disease prophylaxis; possiblly adjunctive refractory mold therapy in cancer chemotherapy and stem cell transplant recipients (graft versus host disease caution) |
| Granulocyte transfusions |
N/A | profound neutropenia as a bridge to engraftment in HSCT or natural neutrophil recovery in other conditions (CMV donor/recipient discordance and possible acute lung injury caution) |
| Dendritic cell infusions |
N/A | experimental prophylaxis model |
G-CSF=granulocyte colony stimulating factor; GM-CSF=granulocyte-macrophage colony stimulating factor; IFN-γ=recombinant interferon-gamma-1b; SQ=subcutaneous; BSA=body surface area; HSCT=hematopoietic stem cell transplant recipients; CMV=cytomegalovirus; N/A=not applicable
Growth factors and Cytokines
Apart from the immunomodulatory properties of antifungals (esp. L-AmB and echinocandins > triazoles), specific immunotherapy/immunoprophylaxis for IA has only been studied on a limited basis. For example, myeloid growth factors such as granulocyte-colony stimulating factor (G-CSF) and granulocyte macrophage colony-stimulating factor (GM-CSF) are mostly used to hasten cellular immune recovery in the susceptible host. Recombinant cytokines such as IFN-γ may facilitate cell-mediated Aspergillus killing. G-CSF and IFN-γ have been shown to augment neutrophil oxidative burst by 24-75% and 52-96%, respectively, regardless of serum opsonization of Aspergillus hyphae in vitro, and the effects may be additive; these cytokines caused 35-40% hyphal damage alone, but nearly 50% when combined [59].
Some animal studies combining amphotericin B, a mold-active triazole, or an echinocandin with G-CSF in murine models receiving immunosuppressive medications have shown differences in survival. For example, mice given intraperitoneal cyclophosphamide followed by intravenous Aspergllus conidia, which were subsequently given caspofungin and G-CSF with or without lipid amphotericin, had a 78.9% improved survival rate – prolonging life by 25 days [60]. In contrast, as corticosteroids can lead to demargination of neutrophils and impaired chemotaxis, G-CSF has been reported to worsen lung abscesses by increasing fungal tissue burden and polymorphonuclear leukocyte (PMN) infiltrates and shorten lives of mice treated with posaconazole with corticosteroid-associated IA [61]. Therefore, differences between cytotoxic and anti-inflammatory chemotherapies may lead to a differential response to G-CSF in murine models of IA.
However, in humans, two double-blinded randomized controlled trials of G-CSF demonstrated decreased duration of febrile neutropenia by approximately 15% but not decrease the risk of infections, including invasive fungal infections (IFI) in patients with AML [62,63]. Based on such data, the American Society of Clinical Oncology and European Organization for Research and Treatment of Cancer recommend myeloid growth factors when the risk of febrile neutropenia is greater than 20% [64]. Limited data exist favoring the use of G-CSF in IA. For instance, G-CSF was used in 5 neutropenic children with hematologic and solid tumor malignancies and IA along with liposomal amphotericin B, with 3 surviving with or without surgery [65]. In an open, randomized clinical trial of amphotericin B deoxycholate (1mg/kg/d) with G-CSF (3 to 5 μg/kg/d) compared to the polyene alone, among 29 neutropenic hematologic/BMT patients with IFI, 62% [95%: 43.3-80.9%] receiving both had a favorable response compared to 32% [95%CI: 15.4-51.2] with monotherapy (p=0.03) and the combination was found to be cost-effective [66].
Corticosteroids disrupt nuclear translocation and activation of NF-κB, which interferes with macrophage functionality – another defense in the clearance of pulmonary Aspergillus conidia. GM-CSF degrades inhibitors of NF-κB, thereby restoring macrophage ability to lead to a pro-inflammatory response via the JAK2-STAT5-NF-κB pathway [67]. Although pulmonary alveolar macrophages can phagocytose and kill Aspergillus conidia, GM-CSF (and IFN-γ) may also augment superoxide hyphal killing by monocytes [68]. Nevertheless, in a retrospective human study of 66 neutropenic patients who received GM-CSF, Safdar et al noted a 24 higher odds of antifungal treatment failure in the 15% receiving high-dose corticosteroids, but IFI-related deaths was significantly lower among GM-CSF responders (9%) compared with non-responders (94%) (p=0.0001), based on clinical and/or radiographic progression. No serious toxicities were reported, though increased capillary permeability issues had been suggested previously [69].
IFN-γ augments neutrophil oxidative hyphal killing – preventing corticosteroid suppression of such activity -- and improves monocyte conidiacidal ability [59,68]. In 1999, recombinant IFN-γ received approval by the U.S. FDA for prophylaxis against infections (not specifically IA) in CGD based on a randomized, double-blind, placebo-controlled study of 128 patients with CGD which showed a 67% relative risk reduction in infection rate among recombinant IFN-γ recipients but phagocyte function was not significantly altered [70]. The data for IFN-γ use in treating rather than preventing IFI, such as refractory IA, is scant. To illustrate, in a retrospective study of 32 HSCT patients, 26 with refractory IA received adjuvant recombinant interferon-γ -1b and antifungals (ABLC, voriconazole, posaconazole, and/or caspofungin) – the majority of whom had an ANC > 100 cells/μl. Of these, the attributable IA mortality was 54%, but 4 (36.3%) of 11 with proven/probable IA, 5 (50%) of 10 with possible IA, and 3 (60%) of 5 with disseminated IA responded. Although there have been concerns in using such pro-inflammatory cytokines in the setting of GVHD, only 2 (9.5%) of the 21 with acute or chronic GVHD in this cohort had persistent GVHD [71]. Similarly, sporadic case reports of the successful adjuvant use of IFN-γ for IA in the CGD and HIV population have been mentioned [72-74].
Based on such reports and expert opinion, the Infectious Diseases Society of America has given a category B-III recommendation for G-CSF and GM-CSF in high-risk neutropenic patients based on severity and duration, and IFN-γ in “non-neutropenic” patients with CGD as adjunctive therapy in IA [3]. Larger multicenter, randomized controlled trials taking such a multi-pronged approach to IA with G-CSF, GM-CSF, and IFN-γ are needed to determine their true therapeutic index among select patient populations with known immune deficits.
Granulocyte transfusions
Human donors primed with G-CSF have had subsequent granulocytes harvested and infused in the adjunctive management of a variety of refractory IFI, including fusariosis and IA. This has been investigated most often in profoundly neutropenic hosts, with favorable responses that extended 3 to 12 weeks after therapy [75,76]. Such studies suggest that granulocytes transfusions should be reserved for persistent IFI in the setting of severe neutropenia, as a bridge to neutrophil recovery and not as a long-term solution. Moreover, caution is advisable as cytomegalovirus (CMV) transmission and transfusion related acute lung injury (TRALI) has been noted to occur, particularly if the product is not strictly screened [77,78].
Dendritic cell infusions
Adoptive transfer of dendritic cells (DCs) pulsed ex vivo with Aspergillus conidia and RNA have shown promise as a vaccine in a murine allogeneic HSCT model at risk for IA. Bozza et al demonstrated that such DCs infused into the model produced antigen-specific T cells that produced IFN-γ (Th1 skewed). The efficacy of such pulsed DCs infused subcutaneously among mice subsequently administered intravenous A. fumigatus was significantly greater in terms of survival > 60 days (95-100% vs. 0%) and reduced organ burden compared with mice that did not receive the infusion, received unpulsed DCs, or received hyphal pulsed DCs. However, adoptive transfer of Aspergillus-specific Th1 cells did not produce the same survival effect. Interestingly, the immune response of similarly pulsed human DCs mirrored that of their murine counterpart [79]. Intriguing animal studies such as this suggest the need to study such therapy in primates prior to clinical investigation in humans [80].
Adjunctive Surgery
In certain cases refractory to medical management, surgery may be necessary. For example, angioinvasion may lead to life threatening hemoptysis that endoscopic embolization procedures may solve [81,82]. In other situations, the extent of disease may be such that open surgical resection becomes necessary. To illustrate in a retrospective study of pediatric oncology patients with IA, 24 surgical interventions ranging from pulmonary wedge resection to lobectomy or neurosurgical and abdominal surgeries for extrapulmonary cases were performed. The 3-month overall survival was 94.4% (95%CI: 66.6–99.2) with minimal complications and half of these were still alive years later [83]. Similar results have been found in the hematologic malignancy adult population [84]. Based on such data, IDSA has given a category B-II recommendation for adjunctive surgery in cases of IA where lesions are contiguous with the great vessels or pericardium, infiltrating the chest wall or pleural space, causing hemoptysis (e.g., single cavity), or invading skin and soft tissues, bone, sinuses, or brain. In addition, surgery may be advised in single pulmonary lesion IA cases prior to HSCT or intensive chemotherapy, but clinical decision-making should be individualized [3].
To conclude, IA causes high mortality among mostly neutropenic hosts once infection is established. Early interventions such as empiric and pre-emptive strategies may assist in ameliorating outcomes when applied in a risk-stratified manner. Voriconazole is the drug of choice for primary IA, but other triazoles, lipid amphotericin B formulations, and echinocandins may have a role in empiric and salvage therapy for IA in certain situations. Combination therapy based on synergism between antifungals that work on the fungal cell membrane (i.e., mold active triazoles and amphotericin B preparations) and those that disrupt the fungal cell wall (i.e., echinocandins) may have a role in refractory IA, but improvements in practice over time -- such as less toxic but equally efficacious conditioning regimens in HSCT -- may impact this approach in the future. The role of immunotherapy for adjunctive management is less well defined and larger clinical trials are needed to determine its utility. Surgery is a last resort when disease progresses despite medical therapy, or if life-threatening invasive manifestations occur. Finally, recovery of neutrophil numbers and functioning ultimately dictates the likelihood for successful outcomes.
Opinion Statement.
Aspergilllus species are septated molds that cause a wide spectrum of clinical syndromes. Among these, invasive aspergillosis (IA) causes very high morbidity and mortality among the most severely immunosuppressed, especially those with profound qualitative or quantitative neutropenia. Empirical, pre-emptive, and targeted approaches have been attempted to blunt establishment of infection with variable success. The preferred treatment of primary IA is voriconazole, which has been found to be superior to amphotericin B. Azoles interfere with the synthesis of ergosterol found in the fungal cell membrane, whereas polyenes -- such as amphotericin B -- interfere with ergosterol function. An echinocandin that disrupts fungal cell wall synthesis – caspofungin -- and itraconazole have been approved for salvage therapy of IA. Lipid amphotericin B formulations are used in those intolerant to amphotericin B deoxycholate. Posaconazole is used in prophylaxis in high-risk groups such as those with acute myelogenous leukemia, myelodysplastic syndrome, and stem cell transplant recipients with graft versus host disease to reduce the incidence of invasive fungal infections (IFI) such as IA. Combining mold-active azoles or a lipid amphotericin B formulation with an echinocandin may have a role in refractory IA. Immunomodulatory properties of antifungals, growth factors, cytokines, and immune cell infusions may enhance host ability to facilitate adjunctive control of infection, but an uncontrolled, exhuberant inflammatory response can also cause significant pathology. Surgical resection may be a last resort when angioinvasion of critical structures places a patient at high risk for bleeding, thrombosis, or embolic phenomena, despite medical therapy. Nevertheless, immune reconstitution with myeloid lineage recovery is the key to successful outcomes.
Acknowledgements
This research was supported in part by the Intramural Research Program of the NIH/NIAID/DIR/LCID/TMU.
Footnotes
Disclosures: None
Disclaimer: The views herein do not reflect the official opinions of the Uniformed Services University or the Department of Defense.
References and Recommended Readings
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1*.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2*.Segal BH, Herbrecht R, Stevens DA, Ostrosky-Zeichner L, Sobel J, et al. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin Infect Dis. 2008;47:674–683. doi: 10.1086/590566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3**.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 4*.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50:1091–1100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 5*.Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Clin Infect Dis. 2010;50:1101–1111. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 6*.Azie N, Neofytos D, Pfaller M, Meier-Kriesche HU, Quan SP, et al. The PATH (Prospective Antifungal Therapy) Alliance(R) registry and invasive fungal infections: update 2012. Diagn Microbiol Infect Dis. 2012;73:293–300. doi: 10.1016/j.diagmicrobio.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Cordonnier C, Pautas C, Maury S, Vekhoff A, Farhat H, et al. Empirical versus preemptive antifungal therapy for high-risk, febrile, neutropenic patients: a randomized, controlled trial. Clin Infect Dis. 2009;48:1042–1051. doi: 10.1086/597395. [DOI] [PubMed] [Google Scholar]
- 8.Morrissey CO, Chen SC, Sorrell TC, Milliken S, Bardy PG, et al. Galactomannan and PCR versus culture and histology for directing use of antifungal treatment for invasive aspergillosis in high-risk haematology patients: a randomised controlled trial. Lancet Infect Dis. 2013;13:519–528. doi: 10.1016/S1473-3099(13)70076-8. [DOI] [PubMed] [Google Scholar]
- 9.Caillot D, Bassaris H, McGeer A, Arthur C, Prentice HG, et al. Intravenous itraconazole followed by oral itraconazole in the treatment of invasive pulmonary aspergillosis in patients with hematologic malignancies, chronic granulomatous disease, or AIDS. Clin Infect Dis. 2001;33:e83–90. doi: 10.1086/323020. [DOI] [PubMed] [Google Scholar]
- 10.Denning DW, Lee JY, Hostetler JS, Pappas P, Kauffman CA, et al. NIAID Mycoses Study Group Multicenter Trial of Oral Itraconazole Therapy for Invasive Aspergillosis. Am J Med. 1994;97:135–144. doi: 10.1016/0002-9343(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 11.Nucci M, Biasoli I, Akiti T, Silveira F, Solza C, et al. A double-blind, randomized, placebo-controlled trial of itraconazole capsules as antifungal prophylaxis for neutropenic patients. Clin Infect Dis. 2000;30:300–305. doi: 10.1086/313654. [DOI] [PubMed] [Google Scholar]
- 12.Gallin JI, Alling DW, Malech HL, Wesley R, Koziol D, et al. Itraconazole to prevent fungal infections in chronic granulomatous disease. N Engl J Med. 2003;348:2416–2422. doi: 10.1056/NEJMoa021931. [DOI] [PubMed] [Google Scholar]
- 13.Segal BH, DeCarlo ES, Kwon-Chung KJ, Malech HL, Gallin JI, et al. Aspergillus nidulans infection in chronic granulomatous disease. Medicine (Baltimore) 1998;77:345–354. doi: 10.1097/00005792-199809000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Henriet SS, Hermans PW, Verweij PE, Simonetti E, Holland SM, et al. Human leukocytes kill Aspergillus nidulans by reactive oxygen species-independent mechanisms. Infect Immun. 2011;79:767–773. doi: 10.1128/IAI.00921-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15**.Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 16.Patterson TF, Boucher HW, Herbrecht R, Denning DW, Lortholary O, et al. Strategy of following voriconazole versus amphotericin B therapy with other licensed antifungal therapy for primary treatment of invasive aspergillosis: impact of other therapies on outcome. Clin Infect Dis. 2005;41:1448–1452. doi: 10.1086/497126. [DOI] [PubMed] [Google Scholar]
- 17.Pasqualotto AC, Denning DW. New and emerging treatments for fungal infections. J Antimicrob Chemother. 2008;61(Suppl 1):i19–30. doi: 10.1093/jac/dkm428. [DOI] [PubMed] [Google Scholar]
- 18.Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356:348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 19.Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356:335–347. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 20.Georgiadou SP, Kontoyiannis DP. The impact of azole resistance on aspergillosis guidelines. Ann N Y Acad Sci. 2012;1272:15–22. doi: 10.1111/j.1749-6632.2012.06795.x. [DOI] [PubMed] [Google Scholar]
- 21**.van der Linden JW, Snelders E, Kampinga GA, Rijnders BJ, Mattsson E, et al. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007-2009. Emerg Infect Dis. 2011;17:1846–1854. doi: 10.3201/eid1710.110226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verweij PE, Snelders E, Kema GH, Mellado E, Melchers WJ. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect Dis. 2009;9:789–795. doi: 10.1016/S1473-3099(09)70265-8. [DOI] [PubMed] [Google Scholar]
- 23.Pfaller MA, Diekema DJ, Ghannoum MA, Rex JH, Alexander BD, et al. Wild-type MIC distribution and epidemiological cutoff values for Aspergillus fumigatus and three triazoles as determined by the Clinical and Laboratory Standards Institute broth microdilution methods. J Clin Microbiol. 2009;47:3142–3146. doi: 10.1128/JCM.00940-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simitsopoulou M, Roilides E, Paliogianni F, Likartsis C, Ioannidis J, et al. Immunomodulatory effects of voriconazole on monocytes challenged with Aspergillus fumigatus: differential role of Toll-like receptors. Antimicrob Agents Chemother. 2008;52:3301–3306. doi: 10.1128/AAC.01018-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi JH, Kwon EY, Park CM, Choi SM, Lee DG, et al. Immunomodulatory effects of antifungal agents on the response of human monocytic cells to Aspergillus fumigatus conidia. Med Mycol. 2010;48:704–709. doi: 10.3109/13693780903471784. [DOI] [PubMed] [Google Scholar]
- 26.Gallis HA, Drew RH, Pickard WW. Amphotericin B: 30 years of clinical experience. Rev Infect Dis. 1990;12:308–329. doi: 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]
- 27**.Falagas ME, Karageorgopoulos DE, Tansarli GS. Continuous versus conventional infusion of amphotericin B deoxycholate: a meta-analysis. PLoS One. 2013;8:e77075. doi: 10.1371/journal.pone.0077075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leenders AC, Daenen S, Jansen RL, Hop WC, Lowenberg B, et al. Liposomal amphotericin B compared with amphotericin B deoxycholate in the treatment of documented and suspected neutropenia-associated invasive fungal infections. Br J Haematol. 1998;103:205–212. doi: 10.1046/j.1365-2141.1998.00944.x. [DOI] [PubMed] [Google Scholar]
- 29.Linden PK, Coley K, Fontes P, Fung JJ, Kusne S. Invasive aspergillosis in liver transplant recipients: outcome comparison of therapy with amphotericin B lipid complex and a historical cohort treated with conventional amphotericin B. Clin Infect Dis. 2003;37:17–25. doi: 10.1086/375219. [DOI] [PubMed] [Google Scholar]
- 30.Bowden R, Chandrasekar P, White MH, Li X, Pietrelli L, et al. A double-blind, randomized, controlled trial of amphotericin B colloidal dispersion versus amphotericin B for treatment of invasive aspergillosis in immunocompromised patients. Clin Infect Dis. 2002;35:359–366. doi: 10.1086/341401. [DOI] [PubMed] [Google Scholar]
- 31.Cornely OA, Maertens J, Bresnik M, Ebrahimi R, Ullmann AJ, et al. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial) Clin Infect Dis. 2007;44:1289–1297. doi: 10.1086/514341. [DOI] [PubMed] [Google Scholar]
- 32.Vogelsinger H, Weiler S, Djanani A, Kountchev J, Bellmann-Weiler R, et al. Amphotericin B tissue distribution in autopsy material after treatment with liposomal amphotericin B and amphotericin B colloidal dispersion. J Antimicrob Chemother. 2006;57:1153–1160. doi: 10.1093/jac/dkl141. [DOI] [PubMed] [Google Scholar]
- 33.Clemons KV, Schwartz JA, Stevens DA. Experimental central nervous system aspergillosis therapy: efficacy, drug levels and localization, immunohistopathology, and toxicity. Antimicrob Agents Chemother. 2012;56:4439–4449. doi: 10.1128/AAC.06015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinbach WJ, Benjamin DK, Jr., Kontoyiannis DP, Perfect JR, Lutsar I, et al. Infections due to Aspergillus terreus: a multicenter retrospective analysis of 83 cases. Clin Infect Dis. 2004;39:192–198. doi: 10.1086/421950. [DOI] [PubMed] [Google Scholar]
- 35.Kontoyiannis DP, Lewis RE, May GS, Osherov N, Rinaldi MG. Aspergillus nidulans is frequently resistant to amphotericin B. Mycoses. 2002;45:406–407. doi: 10.1046/j.1439-0507.2002.00797.x. [DOI] [PubMed] [Google Scholar]
- 36.Panackal AA, Imhof A, Hanley EW, Marr KA. Aspergillus ustus infections among transplant recipients. Emerg Infect Dis. 2006;12:403–408. doi: 10.3201/eid1203.050670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis RE, Chamilos G, Prince RA, Kontoyiannis DP. Pretreatment with empty liposomes attenuates the immunopathology of invasive pulmonary aspergillosis in corticosteroid-immunosuppressed mice. Antimicrob Agents Chemother. 2007;51:1078–1081. doi: 10.1128/AAC.01268-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bellocchio S, Gaziano R, Bozza S, Rossi G, Montagnoli C, et al. Liposomal amphotericin B activates antifungal resistance with reduced toxicity by diverting Toll-like receptor signalling from TLR-2 to TLR-4. J Antimicrob Chemother. 2005;55:214–222. doi: 10.1093/jac/dkh542. [DOI] [PubMed] [Google Scholar]
- 39.Kurtz MB, Douglas CM. Lipopeptide inhibitors of fungal glucan synthase. J Med Vet Mycol. 1997;35:79–86. doi: 10.1080/02681219780000961. [DOI] [PubMed] [Google Scholar]
- 40.Maertens J, Raad I, Petrikkos G, Boogaerts M, Selleslag D, et al. Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin Infect Dis. 2004;39:1563–1571. doi: 10.1086/423381. [DOI] [PubMed] [Google Scholar]
- 41.Lockhart SR, Zimbeck AJ, Baddley JW, Marr KA, Andes DR, et al. In vitro echinocandin susceptibility of Aspergillus isolates from patients enrolled in the Transplant-Associated Infection Surveillance Network. Antimicrob Agents Chemother. 2011;55:3944–3946. doi: 10.1128/AAC.00428-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfaller MA, Boyken L, Hollis RJ, Kroeger J, Messer SA, et al. In vitro susceptibility of clinical isolates of Aspergillus spp. to anidulafungin, caspofungin, and micafungin: a head-to-head comparison using the CLSI M38-A2 broth microdilution method. J Clin Microbiol. 2009;47:3323–3325. doi: 10.1128/JCM.01155-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walsh TJ, Teppler H, Donowitz GR, Maertens JA, Baden LR, et al. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N Engl J Med. 2004;351:1391–1402. doi: 10.1056/NEJMoa040446. [DOI] [PubMed] [Google Scholar]
- 44.van Burik JA, Ratanatharathorn V, Stepan DE, Miller CB, Lipton JH, et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis. 2004;39:1407–1416. doi: 10.1086/422312. [DOI] [PubMed] [Google Scholar]
- 45.Kubiak DW, Bryar JM, McDonnell AM, Delgado-Flores JO, Mui E, et al. Evaluation of caspofungin or micafungin as empiric antifungal therapy in adult patients with persistent febrile neutropenia: a retrospective, observational, sequential cohort analysis. Clin Ther. 2010;32:637–648. doi: 10.1016/j.clinthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Seibel NL, Schwartz C, Arrieta A, Flynn P, Shad A, et al. Safety, tolerability, and pharmacokinetics of Micafungin (FK463) in febrile neutropenic pediatric patients. Antimicrob Agents Chemother. 2005;49:3317–3324. doi: 10.1128/AAC.49.8.3317-3324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lafaurie M, Lapalu J, Raffoux E, Breton B, Lacroix C, et al. High rate of breakthrough invasive aspergillosis among patients receiving caspofungin for persistent fever and neutropenia. Clin Microbiol Infect. 2010;16:1191–1196. doi: 10.1111/j.1469-0691.2009.03050.x. [DOI] [PubMed] [Google Scholar]
- 48.Warn PA, Sharp A, Morrissey G, Denning DW. Activity of aminocandin (IP960; HMR3270) compared with amphotericin B, itraconazole, caspofungin and micafungin in neutropenic murine models of disseminated infection caused by itraconazole-susceptible and -resistant strains of Aspergillus fumigatus. Int J Antimicrob Agents. 2010;35:146–151. doi: 10.1016/j.ijantimicag.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 49.Mikulska M, Viscoli C. Current role of echinocandins in the management of invasive aspergillosis. Curr Infect Dis Rep. 2011;13:517–527. doi: 10.1007/s11908-011-0216-6. [DOI] [PubMed] [Google Scholar]
- 50.Moretti S, Bozza S, Massi-Benedetti C, Prezioso L, Rossetti E, et al. An immunomodulatory activity of micafungin in preclinical aspergillosis. J Antimicrob Chemother. 2013 doi: 10.1093/jac/dkt457. [DOI] [PubMed] [Google Scholar]
- 51.Hohl TM, Feldmesser M, Perlin DS, Pamer EG. Caspofungin modulates inflammatory responses to Aspergillus fumigatus through stage-specific effects on fungal beta-glucan exposure. J Infect Dis. 2008;198:176–185. doi: 10.1086/589304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Safdar A. Fungal cytoskeleton dysfunction or immune activation triggered by beta-glucan synthase inhibitors: potential mechanisms for the prolonged antifungal activity of echinocandins. Cancer. 2009;115:2812–2815. doi: 10.1002/cncr.24323. [DOI] [PubMed] [Google Scholar]
- 53.Kirkpatrick WR, Perea S, Coco BJ, Patterson TF. Efficacy of caspofungin alone and in combination with voriconazole in a Guinea pig model of invasive aspergillosis. Antimicrob Agents Chemother. 2002;46:2564–2568. doi: 10.1128/AAC.46.8.2564-2568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagasaki Y, Eriguchi Y, Uchida Y, Miyake N, Maehara Y, et al. Combination therapy with micafungin and amphotericin B for invasive pulmonary aspergillosis in an immunocompromised mouse model. J Antimicrob Chemother. 2009;64:379–382. doi: 10.1093/jac/dkp175. [DOI] [PubMed] [Google Scholar]
- 55.Elefanti A, Mouton JW, Verweij PE, Tsakris A, Zerva L, et al. Amphotericin B- and voriconazole-echinocandin combinations against Aspergillus spp.: Effect of serum on inhibitory and fungicidal interactions. Antimicrob Agents Chemother. 2013;57:4656–4663. doi: 10.1128/AAC.00597-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kontoyiannis DP, Hachem R, Lewis RE, Rivero GA, Torres HA, et al. Efficacy and toxicity of caspofungin in combination with liposomal amphotericin B as primary or salvage treatment of invasive aspergillosis in patients with hematologic malignancies. Cancer. 2003;98:292–299. doi: 10.1002/cncr.11479. [DOI] [PubMed] [Google Scholar]
- 57**.Marr KA, Schlamm H, Rottinghaus ST, Jagannatha S, Bow EJ, et al. A randomised, double-blind study of combination antifungal therapy with voriconazole and anidulafungin versus voriconazole monotherapy for primary treatment of invasive aspergillosis; ECCMID; London, UK. 2012; p. 713. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. [Google Scholar]
- 58**.Panackal AA, Parisini E, Proschan M. Salvage combination antifungal therapy for acute invasive aspergillosis may improve outcomes: a systematic review and meta-analysis. Int J Infect Dis. 2014 Sep 18; doi: 10.1016/j.ijid.2014.07.007. pii: S1201-9712(14)01592-6. doi: 10.1016/j.ijid.2014.07.007. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roilides E, Uhlig K, Venzon D, Pizzo PA, Walsh TJ. Enhancement of oxidative response and damage caused by human neutrophils to Aspergillus fumigatus hyphae by granulocyte colony-stimulating factor and gamma interferon. Infect Immun. 1993;61:1185–1193. doi: 10.1128/iai.61.4.1185-1193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sionov E, Mendlovic S, Segal E. Experimental systemic murine aspergillosis: treatment with polyene and caspofungin combination and G-CSF. J Antimicrob Chemother. 2005;56:594–597. doi: 10.1093/jac/dki252. [DOI] [PubMed] [Google Scholar]
- 61.Graybill JR, Bocanegra R, Najvar LK, Loebenberg D, Luther MF. Granulocyte colony-stimulating factor and azole antifungal therapy in murine aspergillosis: role of immune suppression. Antimicrob Agents Chemother. 1998;42:2467–2473. doi: 10.1128/aac.42.10.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heil G, Hoelzer D, Sanz MA, Lechner K, Liu Yin JA, et al. The International Acute Myeloid Leukemia Study Group A randomized, double-blind, placebo-controlled, phase III study of filgrastim in remission induction and consolidation therapy for adults with de novo acute myeloid leukemia. Blood. 1997;90:4710–4718. [PubMed] [Google Scholar]
- 63.Godwin JE, Kopecky KJ, Head DR, Willman CL, Leith CP, et al. A double-blind placebo-controlled trial of granulocyte colony-stimulating factor in elderly patients with previously untreated acute myeloid leukemia: a Southwest oncology group study (9031) Blood. 1998;91:3607–3615. [PubMed] [Google Scholar]
- 64.Lyman GH, Kleiner JM. Summary and comparison of myeloid growth factor guidelines in patients receiving cancer chemotherapy. J Natl Compr Canc Netw. 2007;5:217–228. doi: 10.6004/jnccn.2007.0021. [DOI] [PubMed] [Google Scholar]
- 65.Dornbusch HJ, Urban CE, Pinter H, Ginter G, Fotter R, et al. Treatment of invasive pulmonary aspergillosis in severely neutropenic children with malignant disorders using liposomal amphotericin B (AmBisome), granulocyte colony-stimulating factor, and surgery: report of five cases. Pediatr Hematol Oncol. 1995;12:577–586. doi: 10.3109/08880019509030772. [DOI] [PubMed] [Google Scholar]
- 66.Flynn TN, Kelsey SM, Hazel DL, Guest JF. Cost effectiveness of amphotericin B plus G-CSF compared with amphotericin B monotherapy. Treatment of presumed deep-seated fungal infection in neutropenic patients in the UK. Pharmacoeconomics. 1999;16:543–550. doi: 10.2165/00019053-199916050-00010. [DOI] [PubMed] [Google Scholar]
- 67.Choi JH, Brummer E, Kang YJ, Jones PP, Stevens DA. Inhibitor kappaB and nuclear factor kappaB in granulocyte-macrophage colony-stimulating factor antagonism of dexamethasone suppression of the macrophage response to Aspergillus fumigatus conidia. J Infect Dis. 2006;193:1023–1028. doi: 10.1086/500948. [DOI] [PubMed] [Google Scholar]
- 68.Roilides E, Holmes A, Blake C, Venzon D, Pizzo PA, et al. Antifungal activity of elutriated human monocytes against Aspergillus fumigatus hyphae: enhancement by granulocyte-macrophage colony-stimulating factor and interferon-gamma. J Infect Dis. 1994;170:894–899. doi: 10.1093/infdis/170.4.894. [DOI] [PubMed] [Google Scholar]
- 69.Safdar A, Rodriguez G, Zuniga J, Al Akhrass F, Georgescu G, et al. Granulocyte macrophage colony-stimulating factor in 66 patients with myeloid or lymphoid neoplasms and recipients of hematopoietic stem cell transplantation with invasive fungal disease. Acta Haematol. 2013;129:26–34. doi: 10.1159/000342121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.The International Chronic Granulomatous Disease Cooperative Study Group A controlled trial of interferon gamma to prevent infection in chronic granulomatous disease. N Engl J Med. 1991;324:509–516. doi: 10.1056/NEJM199102213240801. [DOI] [PubMed] [Google Scholar]
- 71.Safdar A, Rodriguez G, Ohmagari N, Kontoyiannis DP, Rolston KV, et al. The safety of interferon-gamma-1b therapy for invasive fungal infections after hematopoietic stem cell transplantation. Cancer. 2005;103:731–739. doi: 10.1002/cncr.20883. [DOI] [PubMed] [Google Scholar]
- 72.Bernhisel-Broadbent J, Camargo EE, Jaffe HS, Lederman HM. Recombinant human interferon-gamma as adjunct therapy for Aspergillus infection in a patient with chronic granulomatous disease. J Infect Dis. 1991;163:908–911. doi: 10.1093/infdis/163.4.908. [DOI] [PubMed] [Google Scholar]
- 73.Yamashita K, Miyoshi T, Arai Y, Mizugishi K, Takaori-Kondo A, et al. Enhanced generation of reactive oxygen species by interferon-gamma may have contributed to successful treatment of invasive pulmonary aspergillosis in a patient with chronic granulomatous disease. Int J Hematol. 2013;97:505–510. doi: 10.1007/s12185-013-1315-y. [DOI] [PubMed] [Google Scholar]
- 74.Bandera A, Trabattoni D, Ferrario G, Cesari M, Franzetti F, et al. Interferon-gamma and granulocyte-macrophage colony stimulating factor therapy in three patients with pulmonary aspergillosis. Infection. 2008;36:368–373. doi: 10.1007/s15010-008-7378-7. [DOI] [PubMed] [Google Scholar]
- 75.Dignani MC, Anaissie EJ, Hester JP, O’Brien S, Vartivarian SE, et al. Treatment of neutropenia-related fungal infections with granulocyte colony-stimulating factor-elicited white blood cell transfusions: a pilot study. Leukemia. 1997;11:1621–1630. doi: 10.1038/sj.leu.2400811. [DOI] [PubMed] [Google Scholar]
- 76.Safdar A, Rodriguez GH, Lichtiger B, Dickey BF, Kontoyiannis DP, et al. Recombinant interferon gamma1b immune enhancement in 20 patients with hematologic malignancies and systemic opportunistic infections treated with donor granulocyte transfusions. Cancer. 2006;106:2664–2671. doi: 10.1002/cncr.21929. [DOI] [PubMed] [Google Scholar]
- 77.Nichols WG, Price T, Boeckh M. Cytomegalovirus infections in cancer patients receiving granulocyte transfusions. Blood. 2002;99:3483–3484. doi: 10.1182/blood.v99.9.3483. [DOI] [PubMed] [Google Scholar]
- 78.Danielson C, Benjamin RJ, Mangano MM, Mills CJ, Waxman DA. Pulmonary pathology of rapidly fatal transfusion-related acute lung injury reveals minimal evidence of diffuse alveolar damage or alveolar granulocyte infiltration. Transfusion. 2008;48:2401–2408. doi: 10.1111/j.1537-2995.2008.01879.x. [DOI] [PubMed] [Google Scholar]
- 79.Bozza S, Perruccio K, Montagnoli C, Gaziano R, Bellocchio S, et al. A dendritic cell vaccine against invasive aspergillosis in allogeneic hematopoietic transplantation. Blood. 2003;102:3807–3814. doi: 10.1182/blood-2003-03-0748. [DOI] [PubMed] [Google Scholar]
- 80.Shao C, Qu J, He L, Zhang Y, Wang J, et al. Dendritic cells transduced with an adenovirus vector encoding interleukin-12 are a potent vaccine for invasive pulmonary aspergillosis. Genes Immun. 2005;6:103–114. doi: 10.1038/sj.gene.6364167. [DOI] [PubMed] [Google Scholar]
- 81.Hot A, Mazighi M, Lecuit M, Poiree S, Viard JP, et al. Fungal internal carotid artery aneurysms: successful embolization of an Aspergillus-associated case and review. Clin Infect Dis. 2007;45:e156–161. doi: 10.1086/523005. [DOI] [PubMed] [Google Scholar]
- 82.Khalil A, Fartoukh M, Bazot M, Parrot A, Marsault C, et al. Systemic arterial embolization in patients with hemoptysis: initial experience with ethylene vinyl alcohol copolymer in 15 cases. AJR Am J Roentgenol. 2010;194:W104–110. doi: 10.2214/AJR.09.2379. [DOI] [PubMed] [Google Scholar]
- 83.Cesaro S, Pegoraro A, Tridello G, Pillon M, Cannata E, et al. The role of surgery in the treatment of invasive fungal infection in paediatric haematology patients: a retrospective single-centre survey. Mycoses. 2014 doi: 10.1111/myc.12172. [DOI] [PubMed] [Google Scholar]
- 84.Nebiker CA, Lardinois D, Junker L, Gambazzi F, Matt P, et al. Lung resection in hematologic patients with pulmonary invasive fungal disease. Chest. 2012;142:988–995. doi: 10.1378/chest.11-1964. [DOI] [PubMed] [Google Scholar]