Abstract
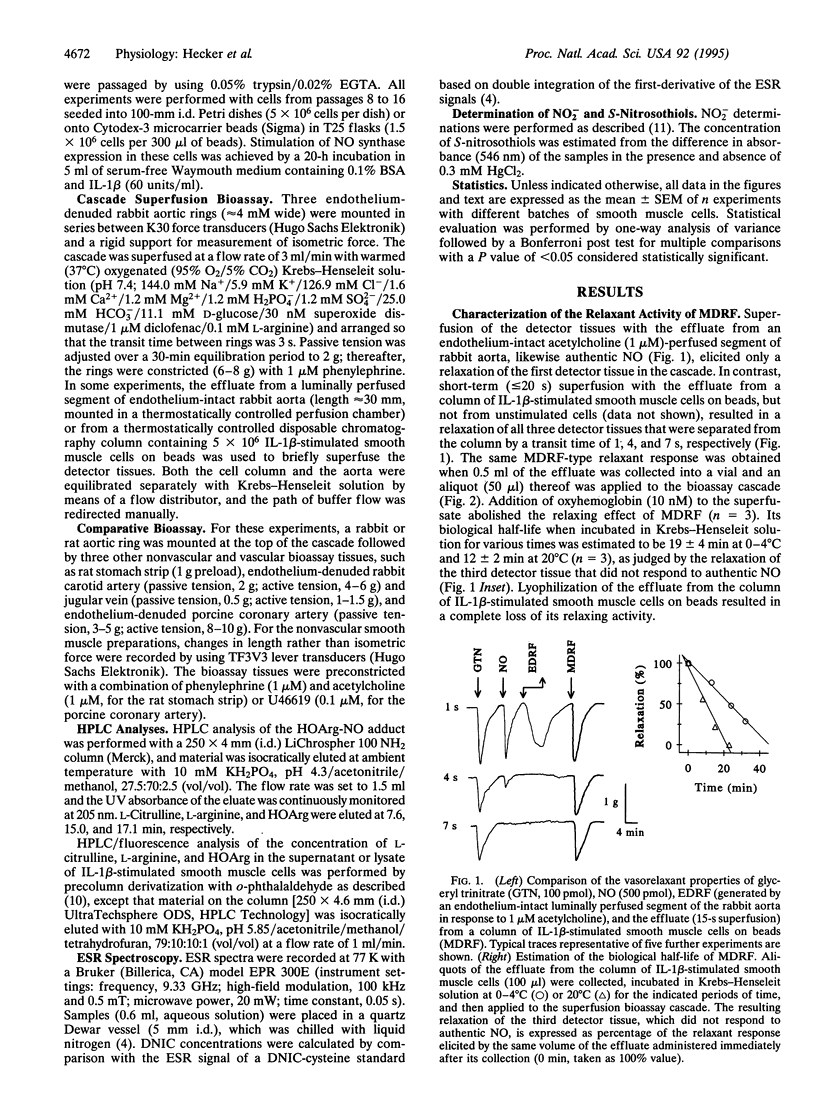
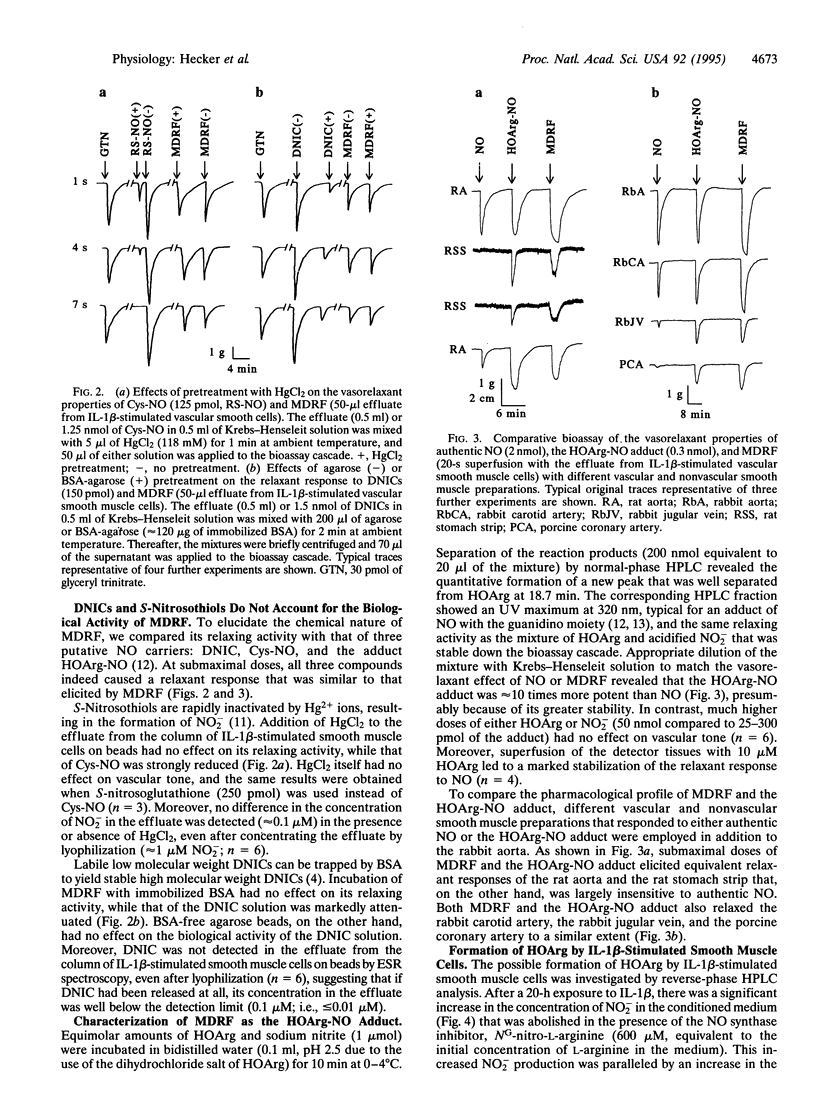
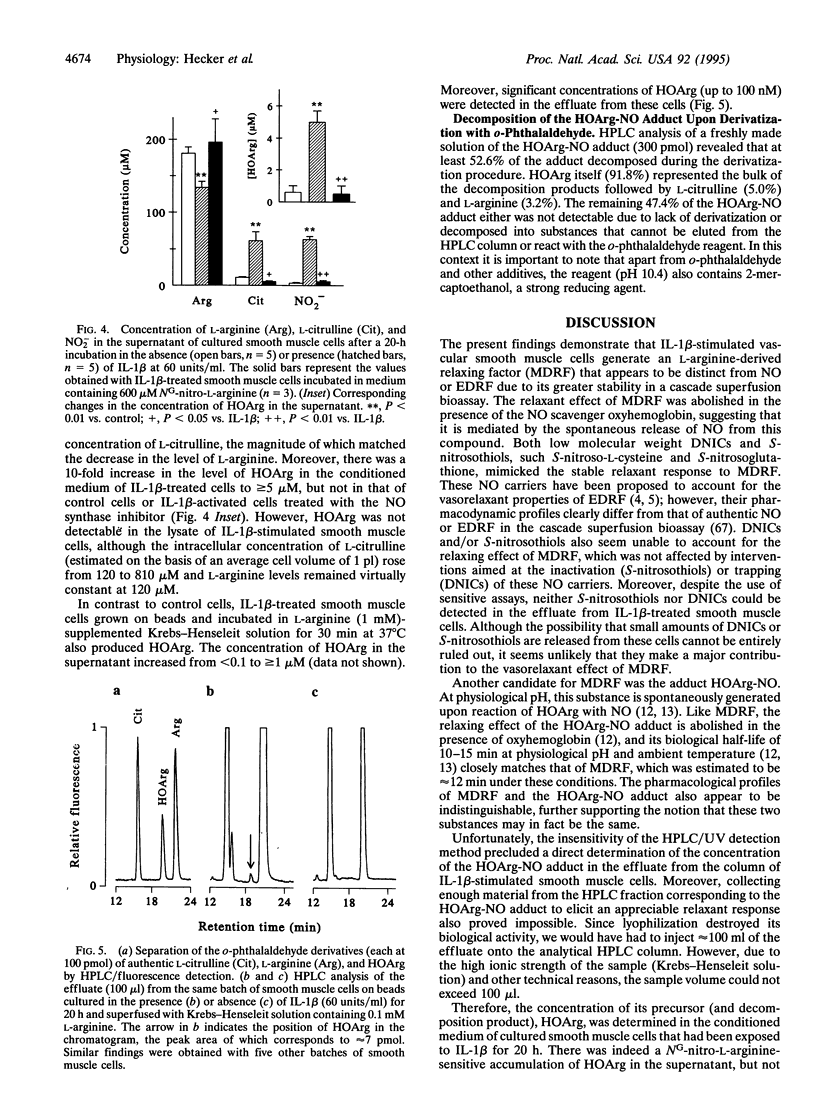
The nature of an L-arginine-derived relaxing factor released from vascular smooth muscle cells cultured on microcarrier beads and stimulated for 20 h with interleukin 1 beta was investigated. Unlike the unstable relaxation elicited by authentic nitric oxide (NO) in a cascade superfusion bioassay system, the effluate from vascular smooth muscle cells induced a stable relaxation that was susceptible to inhibition by oxyhemoglobin. Three putative endogenous NO carriers mimicked this stable relaxing effect: S-nitroso-L-cysteine, low molecular weight dinitrosyl-iron complexes (DNICs), and the adduct of NG-hydroxy-L-arginine (HOArg) with NO. Inactivation of S-nitroso-L-cysteine by Hg2+ ions or trapping of DNICs with agarose-bound bovine serum albumin abolished their relaxing effects, whereas that of the vascular smooth muscle cell effluate remained unaffected. In addition, neither S-nitrosothiols nor DNICs were detectable in the effluate from these cells, as judged by UV and electron spin resonance (ESR) spectroscopy. The HOArg-NO adduct was instantaneously generated upon reaction of HOArg with authentic NO under bioassay conditions. Its pharmacological profile was indistinguishable from that of the vascular smooth muscle cell effluate, as judged by comparative bioassay with different vascular and nonvascular smooth muscle preparations. Moreover, up to 100 nM HOArg was detected in the effluate from interleukin 1 beta-stimulated vascular smooth muscle cells, suggesting that sufficient amounts of HOArg are released from these cells to spontaneously generate the HOArg-NO adduct. This intercellular NO carrier probably accounts for the stable L-arginine-derived relaxing factor released from cytokine-stimulated vascular smooth muscle cells and also from other NO-producing cells, such as macrophages and neutrophils.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chénais B., Yapo A., Lepoivre M., Tenu J. P. N omega-hydroxy-L-arginine, a reactional intermediate in nitric oxide biosynthesis, induces cytostasis in human and murine tumor cells. Biochem Biophys Res Commun. 1993 Nov 15;196(3):1558–1565. doi: 10.1006/bbrc.1993.2429. [DOI] [PubMed] [Google Scholar]
- Feelisch M., te Poel M., Zamora R., Deussen A., Moncada S. Understanding the controversy over the identity of EDRF. Nature. 1994 Mar 3;368(6466):62–65. doi: 10.1038/368062a0. [DOI] [PubMed] [Google Scholar]
- Gordon D., Mohai L. G., Schwartz S. M. Induction of polyploidy in cultures of neonatal rat aortic smooth muscle cells. Circ Res. 1986 Dec;59(6):633–644. doi: 10.1161/01.res.59.6.633. [DOI] [PubMed] [Google Scholar]
- Hecker M., Nematollahi H., Hey C., Busse R., Racké K. Inhibition of arginase by NG-hydroxy-L-arginine in alveolar macrophages: implications for the utilization of L-arginine for nitric oxide synthesis. FEBS Lett. 1995 Feb 13;359(2-3):251–254. doi: 10.1016/0014-5793(95)00039-c. [DOI] [PubMed] [Google Scholar]
- Hecker M., Sessa W. C., Harris H. J., Anggård E. E., Vane J. R. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: cultured endothelial cells recycle L-citrulline to L-arginine. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8612–8616. doi: 10.1073/pnas.87.21.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota K., Yui Y., Hattori R., Uchizumi H., Kawai C. A new relaxing factor in supernatant of incubated rat peritoneal neutrophils. Am J Physiol. 1991 Mar;260(3 Pt 2):H967–H972. doi: 10.1152/ajpheart.1991.260.3.H967. [DOI] [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Mordvintcev P. I., Vanin A. F., Busse R. Formation and release of dinitrosyl iron complexes by endothelial cells. Biochem Biophys Res Commun. 1993 Nov 15;196(3):1303–1308. doi: 10.1006/bbrc.1993.2394. [DOI] [PubMed] [Google Scholar]
- Schini V. B., Junquero D. C., Scott-Burden T., Vanhoutte P. M. Interleukin-1 beta induces the production of an L-arginine-derived relaxing factor from cultured smooth muscle cells from rat aorta. Biochem Biophys Res Commun. 1991 Apr 15;176(1):114–121. doi: 10.1016/0006-291x(91)90897-g. [DOI] [PubMed] [Google Scholar]
- Schott C. A., Bogen C. M., Vetrovsky P., Berton C. C., Stoclet J. C. Exogenous NG-hydroxyl-L-arginine causes nitrite production in vascular smooth muscle cells in the absence of nitric oxide synthase activity. FEBS Lett. 1994 Mar 21;341(2-3):203–207. doi: 10.1016/0014-5793(94)80457-5. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Simon D. I., Osborne J. A., Mullins M. E., Jaraki O., Michel T., Singel D. J., Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Kwon N. S., Nathan C. F., Griffith O. W., Feldman P. L., Wiseman J. N omega-hydroxy-L-arginine is an intermediate in the biosynthesis of nitric oxide from L-arginine. J Biol Chem. 1991 Apr 5;266(10):6259–6263. [PubMed] [Google Scholar]
- Uchizumi H., Hattori R., Sase K., Cai W. J., Kadota K., Sasayama S., Kawai C., Yui Y. A stable L-arginine-dependent relaxing factor released from cytotoxic-activated macrophages. Am J Physiol. 1993 May;264(5 Pt 2):H1472–H1477. doi: 10.1152/ajpheart.1993.264.5.H1472. [DOI] [PubMed] [Google Scholar]
- Vedernikov Y. P., Mordvintcev P. I., Malenkova I. V., Vanin A. F. Similarity between the vasorelaxing activity of dinitrosyl iron cysteine complexes and endothelium-derived relaxing factor. Eur J Pharmacol. 1992 Feb 18;211(3):313–317. doi: 10.1016/0014-2999(92)90386-i. [DOI] [PubMed] [Google Scholar]
- Zembowicz A., Hecker M., Macarthur H., Sessa W. C., Vane J. R. Nitric oxide and another potent vasodilator are formed from NG-hydroxy-L-arginine by cultured endothelial cells. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11172–11176. doi: 10.1073/pnas.88.24.11172. [DOI] [PMC free article] [PubMed] [Google Scholar]



