Abstract
Mutations affecting skeletal muscle isoforms of the tropomyosin genes may cause nemaline myopathy, cap myopathy, core-rod myopathy, congenital fiber-type disproportion, distal arthrogryposes, and Escobar syndrome. We correlate the clinical picture of these diseases with novel (19) and previously reported (31) mutations of the TPM2 and TPM3 genes. Included are altogether 93 families: 53 with TPM2 mutations and 40 with TPM3 mutations. Thirty distinct pathogenic variants of TPM2 and 20 of TPM3 have been published or listed in the Leiden Open Variant Database (http://www.dmd.nl/). Most are heterozygous changes associated with autosomal-dominant disease. Patients with TPM2 mutations tended to present with milder symptoms than those with TPM3 mutations, DA being present only in the TPM2 group. Previous studies have shown that five of the mutations in TPM2 and one in TPM3 cause increased Ca2+ sensitivity resulting in a hypercontractile molecular phenotype. Patients with hypercontractile phenotype more often had contractures of the limb joints (18/19) and jaw (6/19) than those with nonhypercontractile ones (2/22 and 1/22), whereas patients with the non-hypercontractile molecular phenotype more often (19/22) had axial contractures than the hypercontractile group (7/19). Our in silico predictions show that most mutations affect tropomyosin–actin association or tropomyosin head-to-tail binding.
Keywords: congenital myopathy, genotype–phenotype correlation, TPM2, TPM3, actin, hypercontractile phenotype
Introduction
The congenital myopathies are clinically, histologically, and genetically variable neuromuscular disorders, often caused by mutations in genes encoding sarcomeric proteins [Wallgren-Pettersson et al., 2011]. These myopathies are defined on the basis of static or only slowly progressive muscle weakness and a range of structural abnormalities in the muscle fibers. In recent years, a number of different myopathy entities have been associated with mutations in one of the two tropomyosin genes, TPM2 and TPM3. Nemaline myopathy (NM; MIMs #161800, #256030, #605355, #609273, #609284, #609285, #610687, #615348) is a genetically heterogeneous disorder showing wide clinical variability. It is characterized by muscle weakness and nemaline (rod) bodies on muscle biopsy [Wallgren-Pettersson et al., 2004]. NM was described by two separate groups in 1963 [Conen et al., 1963; Shy et al., 1963]. “Cap myopathy” or “cap disease” (MIMs #609284, #609285) was first characterized by Fidzianska et al. (1981). The name derives from the cap-like structures located under the sarcolemma. The first cases of congenital fiber-type disproportion (CFTD; MIM #255310) was described by Brooke (1973) in patients with hypotrophy of type 1 muscle fibers (Fig. 1). Arthrogryposis is a very heterogeneous disease category in which distal arthrogryposes (DA) is one subgroup of dominantly inherited disorders (Fig. 2). Daentl et al. (1974) described three patients with DA type 1A (MIM #108120). Another form, DA type 2B (MIM #601680) was first identified by Krakowiak et al. (1997)]. Escobar syndrome is a nonlethal type of multiple pterygium syndrome (MIM #265000). It was first characterized by Norum et al. (1969) and associated in one case with NM [Monnier et al., 2009]. Core-rod myopathy and autosomal-dominanttrismus-pseudocamptodactyly syndrome (DA type 7) have also recently been associated with TPM2 mutations [Davidson et al., 2013].
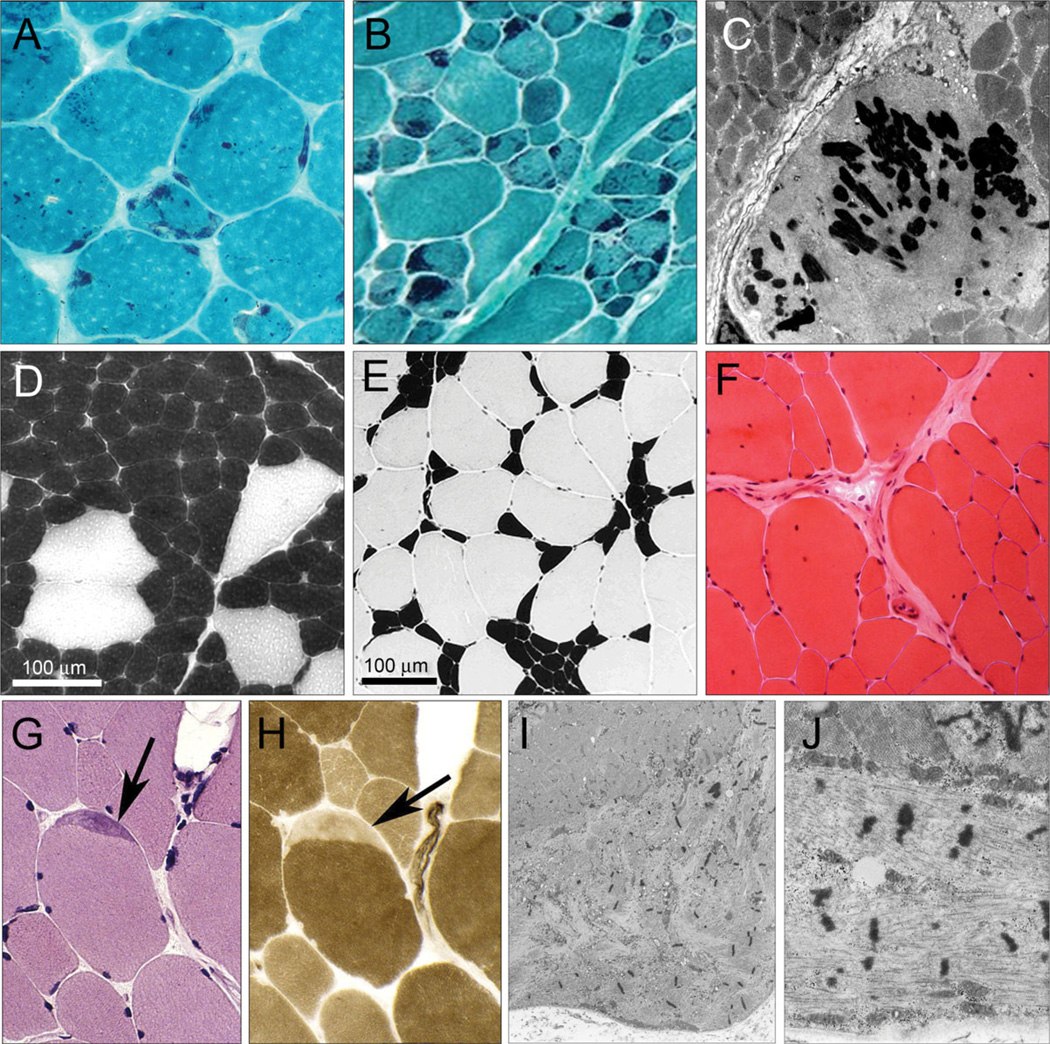
Figure 1.
Histological characteristics of NM, cap myopathy, and CFTD. Histology images showing the range of abnormalities in skeletal muscle that can arise with mutations in tropomyosin. A–C: NM. In TPM2, NM (A: p.K7del mutation) rods are often randomly scattered. In TPM3, NM (B: p.M9R), rods are confined to type 1 fibers that are usually hypotrophic. C: Nemaline bodies on electron microscopy. D–F: CFTD due to mutations in TPM3D and E: ATPase (4.3) showing the consistent difference in size between type 1 fibers (dark) and type 2 fibers (pale). Type 1 fiber predominance is common (D) but a range is seen (E). D: Increased internalized nuclei in large (type 2) fibers in an adult CFTD patient with the TPM3 p.L100M mutation. G–J: Cap myopathy due to the TPM2 p.Glu139del mutation. Caps are most reliably seen on ATPase stains (H) but are sometimes visible on H&E stains (G) or oxidative stains. I and J: Electron microscopy of a typical cap structure.
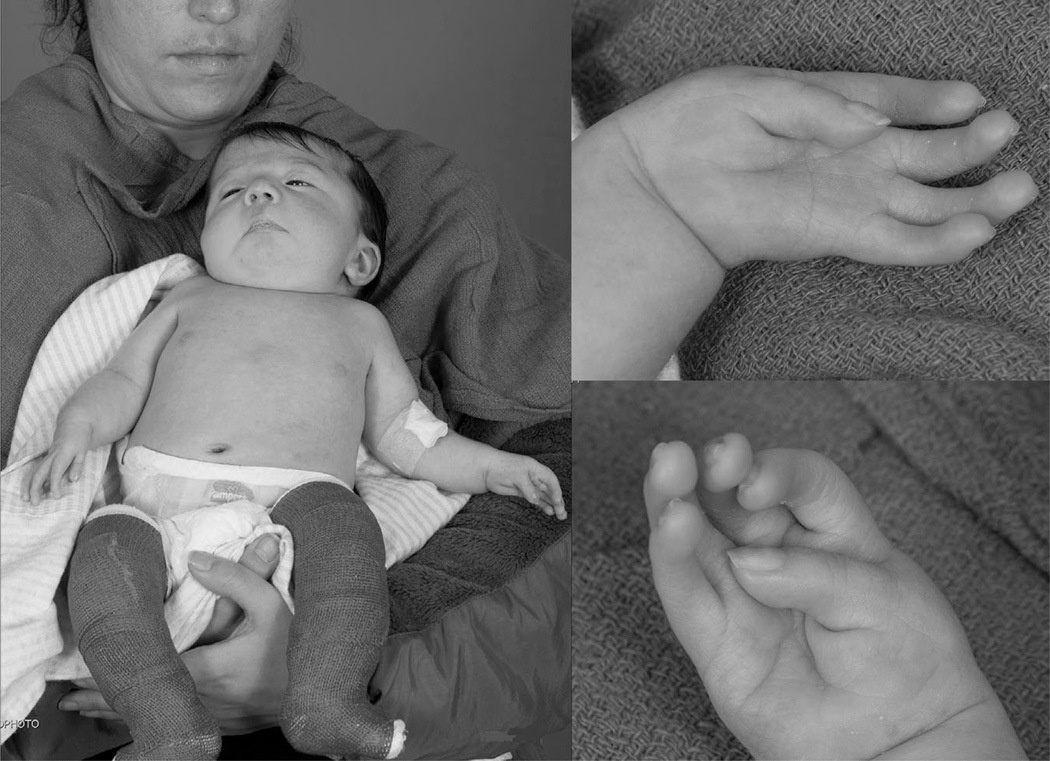
Figure 2.
Distal arthrogryposis patient. Little girl with distal arthrogryposis and suspected myopathy.
The roles of tropomyosins in stabilizing the thin (actin) filament of the sarcomere [Cooper, 2002] and in regulating muscle contraction [Gordon et al., 2000] have been well defined in skeletal muscle. The tropomyosins exist as coiled-coil homo- or hetero-dimers forming head-to-tail polymers, running along the length of the actin filament [Phillips et al., 1979; Matsumura et al., 1983; Holmes et al., 1990; Lin et al., 1997]. They are encoded by four different genes; TPM1 (MIM # 191010), TPM2 (MIM # 190990), TPM3 (MIM # 191030), and TPM4 (MIM # 600317) [Pittenger et al., 1994], generating more than 40 different tropomyosin isoforms due to the use of different promoters or variable intragenic splicing [Pittenger et al., 1994; Dufour et al., 1998; Cooley and Bergtrom, 2001]. The tropomyosin genes TPM1, TPM2, and TPM3 encode the skeletal muscle isoforms Tm1 (α-tropomyosinfast), Tm2 (β-tropomyosin), and Tm3 (α-tropomyosinslow), respectively. TPM1 is mainly expressed in fast muscle fibers and has lower expression in skeletal muscle than in cardiac muscle. TPM2 is expressed in both slow, and, to a lesser extent, in fast muscle fibers. TPM3 is expressed exclusively in slow muscle fibers. When both α- and β-tropomyosins are expressed, α,β-heterodimers are preferentially formed over α-homodimers, and β-homodimers are rare [Perry, 2001].
Here, we compare the nature and location of novel, and to the best of our knowledge, all previously described mutations of the TPM2 and TPM3 genes in patients with NM, CFTD, cap myopathy, core-rod myopathy, DA type 1A, DA type 2B, DA type 7, and NM with Escobar syndrome to explore the relationships between mutation type and position and the phenotypes that arise. Ninety-three families were included in this study: 53 with TPM2 mutations and 40 with TPM3 mutations. Altogether 30 distinct pathogenic variants of TPM2 and 20 of TPM3 have been reported in the literature (including the 14 novel TPM2 and 5 novel TPM3 mutations, a total of 19 mutations reported for the first time in the current paper). We have also studied previously known and novel phosphorylation sites in tropomyosin with known patient mutations, and characterized the phosphorylation patterns in purified proteins containing known patient mutations compared with wild-type (wt) protein.
Families
Ascertainment
Families reported here for the first time were identified by neuromuscular services in different countries: Argentina, Australia, Canada, Finland, France, Germany, Israel, Italy, The Netherlands, UK, and USA. All patients had consented to research or diagnostic genetic testing. Institutional review board approval was obtained to access patient information, and written consent was obtained where appropriate.
TPM2
Fifty-three families had mutations of the TPM2 gene. Patients in 13 of the families had been diagnosed with NM and in five families a diagnosis of cap myopathy was made. CFTD was the diagnosis in seven families. Ten families had a proband with DA. In one family, a member had NM with Escobar syndrome. NM and cap myopathy had both been diagnosed in three families. Affected members of two families with a diagnosis of NM also had significant fiber-type disproportion. Cap myopathy with fiber-type disproportion had been diagnosed in two families. One family had DA with unspecified congenital myopathy (CM) and one with core-rod myopathy. One proband had a diagnosis of CM and affected members of seven families had no specified diagnosis.
TPM3
Forty families had mutations in the TPM3 gene. Patients in nine families had a diagnosis of NM and in 20 families, of CFTD with no specific histological hallmarks. Three probands had been diagnosed with cap myopathy. Two families had members with a diagnosis of NM and cap myopathy. Probands of four families had CM, and in two there was no specified diagnosis.
Mutations
Nomenclature of TPM2 and TPM3 Mutations and Database
For the description of sequence variants, we used reference sequence NM 003289.3 for TPM2 and NM 152263.3 for TPM3. Amino acid coordinates are provided relative to NP 003280.2 and NP_689476.2, respectively. Note that TPM3 mutations are numbered according to the Human Genome Variation Society recommendations [den Dunnen and Antonarakis, 2000], meaning the first two ATG codons in the primary cDNA were both included. TPM2 and TPM3 variants described in the article are listed in the Leiden Open Variant Database (http://www.dmd.nl/) at the Center for Human and Clinical Genetics, Leiden University Medical Center.
Novel Mutations in TPM2 and TPM3
We used Sanger sequencing and dHPLC to identify novel mutations in the TPM2 and TPM3 genes. Including previously published studies, 94 families were included in the study. Of these, 53 had TPM2 mutations and 41 had TPM3 mutations. There were 30 different mutations in TPM2 (14 novel mutations we report and 16 previously described) and 20 different mutations in TPM3 (five novel and 15 previously reported) (Tables 1 and 2; Fig. 3). No clinical details were available for 12 TPM2 families and five TPM3 families. None of these changes were found in the 1000 Genomes Dataset (www.1000genomes.org, accessed November 2013), nor in the Exome Variant Server, NHLBI Exome Sequencing Project (ESP) (Seattle, WA) (URL: http://evs.gs.washington.edu/EVS/, accessed November 2013).
Table 1.
Novel and Recurrent TPM2 Mutations in 28 Patients from 27 Families
| Family | Patient ID, gender |
Mutation(s) in cDNA RefSeq NM_003289.3 |
Altered protein site and predicted effect |
Diagnosis | Phenotype | PolyPhen-2/FATHMM prediction |
Reference |
|---|---|---|---|---|---|---|---|
| TPM2 novel mutations | |||||||
| 1 | 2,693, M | c.8C>G | p.Ala3Gly | NM | Proximal muscle weakness (66 years) | Benign/damaging | Present study (Family 1) |
| 2# | 20–625, F | c.41A>T | p.Asp14Val | NM | progressive course of the disease |
Possibly damaging/damaging |
Present study (Family 12) |
| 3 | 20–561, F | c.124G>A | p.Glu41Lys | NM | Severe neonatal hypotonia, weakness of neck flexors |
Benign/tolerated | Present study (Family 15) |
| 4 | F | c.240+2T>G | Spl | CFTD | Distal arthrogryposis and weakness |
- | Present study (Family 19) |
| 5 | F | c.240+5G>A | Spl | DA | Hypotonia, joint contractures, and bilateral club foot |
- | Present study (Family 20) |
| 6 | 20–601, F | c.279G>C | p.Gln93His | CM | Neonatal hypotonia with respiratory difficulties |
Probably damaging/damaging |
Present study (Family 22) |
| 7# | F | c.349G>A | p.Glu117Lys | DA | Hypotonia at birth, failure to thrive, scoliosis |
Possibly damaging/damaging |
Present study (Family 24) |
| 8# | 20–393, M | c.382A>G | p.Lys128Glu | CM | Hypotonia at birth mild weakness |
Probably damaging/damaging |
Present study (Family 26) |
| 9# | 3,603, F | c.397C>T | p.Arg133Trp | CFTD | Progressive weakness | Possibly damaging/damaging |
Present study (Family 28) |
| 10# | M | c.398G>C | p.Arg133Pro | CFTD | Severe hypotonia and respiratory failure (at birth) |
Probably damaging/damaging |
Present study (Family 29) |
| 11 | 3,143, F | c.428T>C | p.Leu143Pro | NM CFTD | Distal weakness of lower limbs | Possibly damaging/damaging |
Present study (Family 35) |
| 12# | 3,653, F | c.443T>C | p.Leu148Pro | CFTD | Presented with muscle weakness subsequent to infection |
Possibly damaging/damaging |
Present study (Family 37) |
| Recurrent mutations | |||||||
| 13# | Family A, F | c.19 21delAAG | p.Lys7del | NM | Contractures, mild proximal leg weakness |
– |
Mokbel et al. (2013) (Family 2) |
| 14# | Family B, M |
c.19 21delAAG | p.Lys7del | NM | Contractures, marked fatigue | – |
Mokbel et al. (2013) (Family 3) |
| 15# | Family C, F | c.19 21delAAG | p.Lys7del | NM | Contractures, proximal limb weakness |
– |
Mokbel et al. (2013) (Family 4) |
| 16# | Family D, M |
c.19 21delAAG | p.Lys7del | NM | Contractures, mild proximal limb and distal leg weakness |
– |
Mokbel et al. (2013) (Family 5) |
| 17# | Family E, 1,653, F |
c.19 21delAAG | p.Lys7del | NM | Quadriceps weakness, ambulation lost (62 years) |
– |
Mokbel et al. (2013) (Family 6) |
| 18# | 20–747, F | c.19 21delAAG | p.Lys7del | NM | Joint contractures, no weakness |
– | Present study (Family 7) |
| 19# | Family 1 M |
c.19 21delAAG | p.Lys7del | CRM | Contractures, gait unsteadiness |
– | Davidson et al. (2012) (Family 8) |
| 20# | Family 2, F | c.19 21delAAG | p.Lys7del | DA type 7 | Limited ambulation, congenital hip dislocations |
– | Davidson et al. (2012) (Family 9) |
| 21# | Family 3, F | c.19 21delAAG | p.Lys7del | DA type 7 | Contractures, difficulties running, gait difficulties |
– | Davidson et al. (2012) (Family 10) |
| 22# | Family 4, F | c.19 21delAAG | p.Lys7del | DA type 7 | Walked with straightened legs and had “stiff posture” |
– | Davidson et al. (2012) (Family 11) |
| 23# | 291-A, B F, M |
c.415 417delGAG | p.Glu139del | NM Cap | Slowly progressive muscle weakness, rigid spine |
– |
Tasca et al. (2013) (Family 30) |
| 24# | F | c.415 417delGAG | p.Glu139del | CFTD | Able to walk, cervical scoliosis | – | Present study (Family 31) |
| 25# | 3,073, M | c.415 417delGAG | p.Glu139del | Cap CFTD | Generalized muscle weakness, never able to run |
– |
Lehtokari et al. (2007) (Family 32) |
| 26# | F | c.415 417delGAG | p.Glu139del | Cap | Generalized muscle wasting, running in childhood |
– |
Clarke et al. (2009) (Family 33) |
| 27# | F | c.415 417delGAG | p.Glu139del | Cap CFTD | Proximal muscles weaker than distal |
– | Present study (Family 34) |
PolyPhen-2 and FATHMM predictions indicate the possible pathogenicity of the missense mutations.
The reference column links families to the corresponding Supp. Tables S1 and S2. The possible pathogenicity of the mutations was assessed with PolyPhen-2, version 2.2.2, HumVar option, and FATHMM version 2.3 with the prediction algorithm weighted for human mutations and disease ontology as the option for phenotypic associations. Nucleotide numbering according to the TPM2 cDNA sequence with +1 corresponding to the A of the ATG translation initiation codon in the reference sequence NM_003289.3. Amino acid coordinates are provided relative to NP_003280.2; the ATG translation initiation codon is codon 1.
NM, nemaline myopathy; Cap, cap myopathy; CFTD, congenital fiber-type disproportion; DA, distal arthrogryposis; CRM, core-rod myopathy; CM, undefined congenital myopathy; spl, splice-site mutation; #, gain-of-function mutation resulting in hypercontractile phenotypes; M, male; F, female.
Table 2.
Novel and Recurrent TPM3 Mutations in 24 Patients from 22 Families
| Family | Patient ID, gender |
Mutation(s) in cDNA RefSeq NM_152263.3 |
Altered protein site and predicted effect |
Diagnosis | Phenotype | PolyPhen-2/FATHMM prediction |
Reference |
|---|---|---|---|---|---|---|---|
| TPM3 novel mutations | |||||||
| 1 | 3,363, M | c.263C>T | (p.Ser87Phe) p.Ser88Phe |
NM Cap | Myalgia, progressive weakness | Probably damaging/damaging |
Present study (Family 3) |
| 2 | 20–799, M | c.271C>T | (p.Arg90Cys) p.Arg91Cys |
CM | Moderate scapular and pelvic weakness |
Probably damaging/damaging |
Present study (Family 5) |
| 3 | F | c.298C>G | (p.Leu99Val) p.Leu100Val |
CFTD | Neonatal hypotonia, head lag, running |
Benign/damaging | Present study (Family 7) |
| 4 | 20–607, F | c.452A>C | (p.Glu150Ala) p.Glu151Ala |
Cap | Weakness of neck flexor | Probably damaging/damaging |
Present study (Family 8) |
| 5 | 20–834, F | c.734G>T | (p.Arg244Ile) p.Arg245Ile |
Cap | Proximal weakness, able to sit but not to stand up |
Probably damaging/damaging |
Present study (Family 29) |
| 6 | 20–872, F | c.758C>A | (p.Thr252Lys) p.Thr253Lys |
CM | Myopathic facies, walking | Possibly damaging/tolerated |
Present study (Family 30) |
| Recurrent mutations | |||||||
| 7 | F | c.502C>T | (p.Arg167Cys) p.Arg168Cys |
NM | Proximal and distal weakness, walking with difficulty |
Probably damaging/damaging |
Present study (Family 10) |
| 8 | 1,963, F | c.502C>T | (p.Arg167Cys) p.Arg168Cys |
CFTD | Moderate weakness, running in childhood, walks, stairs difficult |
Probably damaging/damaging |
Clarke et al. (2008) Family 5 (Family 1) |
| 9 | BOS1109-1, F | c.502C>T | (p.Arg167Cys) p.Arg168Cys |
CFTD | Moderate proximal and distal weakness |
Probably damaging/damaging |
Lawlor et al. (2010) (Family 12) |
| 10 | 20–837, M | c.502C>T | (p.Arg167Cys) p.Arg168Cys |
NM | Walks with support, kyphosis | Probably damaging/damaging |
Present study (Family 13) |
| 11 | M | c.502C>T | (p.Arg167Cys) p.Arg168Cys |
CFTD | Weakness of the feet, ambulant, slow runner |
Probably damaging/damaging |
Present study (Family 14) |
| 12 | F | c.502C>G | (p.Arg167Gly) p.Arg168Gly |
CFTD | Mild facial and neck flexor weakness, slow runner |
Probably damaging/damaging |
Clarke et al. (2008) (Family 3) (Family 15) |
| 13 | F | c.503G>A | (p.Arg167His) p.Arg168His |
CFTD | Neck flexor weakness, achieved walking |
Benign/damaging | Present study (Family 16) |
| 14 | M | c.503G>A | (p.Arg167His) p.Arg168His |
CFTD | Hypotonic, mild weakness in distal muscles, climbs stairs |
Benign/damaging | Present study (Family 17) |
| 15 | F, M | c.503G>A | (p.Arg167His) | NM | Proximal limb girdle weakness, mild facial weakness, runs |
Benign/damaging | Durling et al. (2002) |
| p.Arg168His | CFTD |
Clarke et al. (2008) (Family 6) (Family 18) |
|||||
| 16 | M | c.503G>A | (p.Arg167His) p.Arg168His |
NM | Walks, has never been able to run or jump |
Benign/damaging | Penisson-Besnier et al. (2007) (Family 19) |
| 17 | M | c.503G>A | (p.Arg167His) p.Arg168His |
Cap | Distal weakness of lower limbs, unable to run |
Benign/damaging |
De Paula et al. (2009) (Family 20) |
| 18 | BOS 247-4, F | c.503G>A | (p.Arg167His) p.Arg168His |
CFTD | Axial weakness, able to jump, but not very high |
Benign/damaging |
Lawlor et al. (2010) (Family 21) |
| 19 | BOS 343-1, M | c.503G>A | (p.Arg167His) p.Arg168His |
NM | Difficulty raising arms and holding head, respiration difficulty |
Benign/damaging |
Lawlor et al. (2010) (Family 22) |
| 20 | F | c.503G>A | (p.Arg167His) p.Arg168His (RYR p.Arg3539His) |
CFTD | Never able to walk far, run or jump, ambulant |
Benign/damaging |
Klein et al. (2012) (Family 23) |
| 21 | F | c.503G>A | (p.Arg167His) p.Arg168His |
CFTD | Spinal rigidity and scoliosis, facial weakness |
Benign/damaging |
Munot et al. (2010) (Family 24) |
PolyPhen-2 and FATHMM predictions indicate the possible pathogenicity of the missense mutations. The reference column links families to the corresponding Supp. Tables S3 and S4. The possible pathogenicity of the mutations was assessed with PolyPhen-2, version 2.2.2, HumVar option, and FATHMM version 2.3 with the prediction algorithm weighted for human mutations and disease ontology as the option for phenotypic associations. Nucleotide numbering according to the coding sequence of the TPM3 cDNA reference sequence NM_152263.3. The first two ATG codons in the primary cDNA were both included according to the Human Genome Variation Society recommendations. Amino acid coordinates are provided relative to NP_689476.2; the ATG translation initiation codon is codon 1.
NM, nemaline myopathy; Cap, cap myopathy; CFTD, congenital fiber-type disproportion; CM, undefined congenital myopathy; spl, splice-site mutation; #, gain-of-function mutation resulting in hypercontractile phenotypes; M, male; F, female.
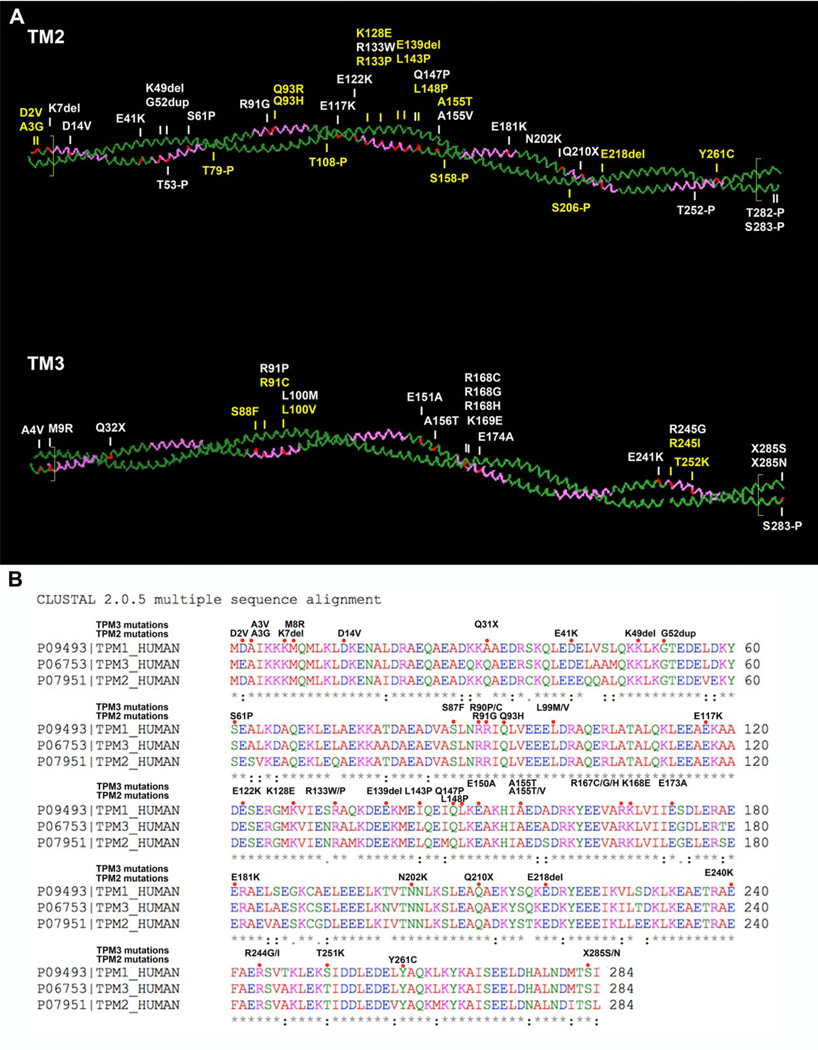
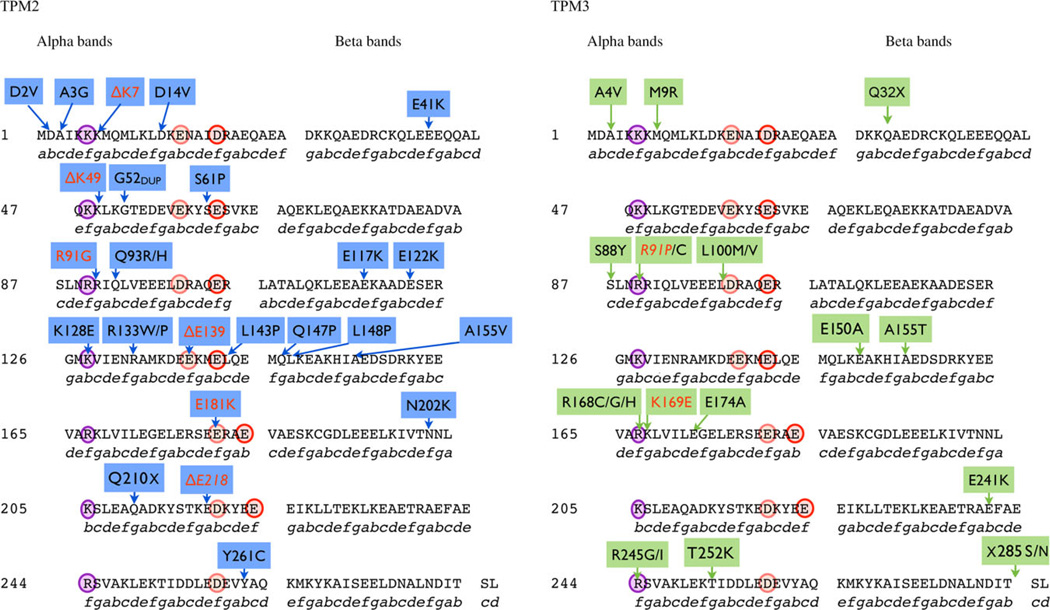
Figure 3.
Sequence comparison of Tm1, Tm2, and Tm3 and Tm2–Tm3 dimer with disease-causing mutations and phosphorylation sites. A: Schematic presentation of Tm2 and Tm3 dimers with disease-causing mutations (shown in red and above the molecules), α-zones (purple), and overlapping regions (separated by green lines in N- and C-terminal ends of the molecules). Phosphorylation sites are marked underneath the molecules. Novel mutations/phosphorylation sites are shown in yellow. The figure was created using the PyMol software (http://www.pymol.org) and the Protein Databank structure 1C1GA. B: Sequence comparison of Tm1, Tm2, and Tm3. Mutations are indicated in the sequence by red dots. In the Tm3 protein amino acid sequence (P06753), the initiation codons have been processed, thus the nomenclature is different compared with that in other figures. Stars underneath the sequences indicate conserved amino acids, and dots show the sites where sequences diverge.
Prediction of Pathogenicity of Mutations
PolyPhen-2, version 2.2.2, HumVar option (http://genetics.bwh.harvard.edu/pph2/index.shtml) [Adzhubei et al., 2010], and FATHMM (functional Analysis through hidden Markov models), version 2.3 with the prediction algorithm weighted for human mutations and disease ontology as the option for phenotypic associations (http://fathmm.biocompute.org.uk) [Shihab et al., 2013] were used to predict the functional effect of the missense mutations identified in TPM2 and TPM3. Molecular modeling is described in detail by Memo and Marston (2013).
In TPM2, 17 of the 22 missense mutations were predicted to be possibly or probably damaging by PolyPhen-2, and FATHMM. However, there were slight discrepancies in the predictions by the two programs. PolyPhen-2 predicted three mutations (p.Asp2Val, p.Ala3Gly, and p.Arg91Gly) to be benign, whereas FATHMM predicted the same three mutations to be damaging. On the other hand, FATHMM predicted the mutation p.Gln147Pro to be tolerated, whereas PolyPhen-2 predicted the same mutation to be probably damaging. Both programs predicted two mutations (p.Glu41Lys and p.Asn202Lys) to be benign or tolerated (Table 1 and Supp. Tables S1 and S2). p.Glu41Lys has, however, been shown to affect muscle contractility due to decreased calcium sensitivity [Marttila et al., 2012], and p.Asn202Lys has been hypothesized to affect Tm– Troponin T (TnT) interactions [Ohlsson et al., 2008].
In TPM3, 16 of the 18 missense mutations were predicted to be possibly or probably damaging by PolyPhen-2, and 17 were predicted to be damaging by FATHMM. PolyPhen-2 predicted two mutations (p.Leu100Val and p.Arg168His) to be benign, whereas FATHMM predicted the same mutations to be damaging. On the other hand, FATHMM predicted the mutation p.Thr253Lys to be tolerated, whereas PolyPhen-2 predicted the same mutation to be possibly damaging (Table 2 and Supp. Tables S3, S4, and S5). Our analysis shows that both PolyPhen-2 and FATHMM were able to correctly predict more than 80% of the pathogenic Tm mutations (82.5% for PolyPhen-2 and 85% for FATHMM).
Recurrent Mutations in TPM2 and TPM3
Two recurrent mutations were found in TPM2. The first is p.Lys7del, which was found in 10 families [Davidson et al., 2013; Mokbel et al., 2013; present study, see Table 1]. The second one is p.Glu139del, which has been reported in five families [Lehtokari et al., 2007; Clarke et al., 2009; Tasca et al., 2013; present study, see Table 1].
One mutational hotspot was identified in TPM3. The p.Arg168 residue was mutated in 21 families. The recurrent mutation p.Arg168His was present in 12 families [Durling et al., 2002; Penisson-Besnier et al., 2007; Clarke et al., 2008; De Paula et al., 2009; Lawlor et al., 2010; present study, see Table 2 and Supp. Table S6]. Eight families had the p.Arg168Cys mutation [Clarke et al., 2008; present study, see Table 2 and Supp. Table S6] and one family had the p.Arg168Gly mutation [Clarke et al., 2008; see Table 2.].
Clinical Relevance
Tropomyosins are actin-binding, coiled-coil proteins expressed in all eukaryotic cells. Their major function in skeletal muscle is to stabilize actin and to regulate actin–myosin interactions by limiting access to myosin-binding sites along the major groove of the actin filament [Gunning et al., 2005]. The tropomyosin α-helical coiled coil consists of heptapeptide repeats (abcdefg) where the a and d residues are generally nonpolar and form the interhelical interface (or core) of the double-stranded structure [Perry, 2001].
CM phenotypes can be classified into two types: those that are characterized by congenitally weak muscles and those with congenitally normal or hypercontractile muscles (e.g., DA). Contractility is the major factor that is altered by mutations in contractile proteins such as tropomyosin. The classification of these disorders is a challenge because a single mutation shared between patients can cause a range of phenotypes. This was seen in patients in two recently published papers on the Lys7del mutation [Davidson et al., 2013; Mokbel et al., 2013]. Within the 10 families described, the variability in phenotype is quite remarkable, complicating the recognition of any definite clinical and histological phenotype–genotype correlation.
NM, cap myopathy, and CFTD are closely related entities with largely overlapping clinical features, whereas DA and Escobar syndrome may be seen as more distinct clinical entities with less clinical overlap with other disorders within the group. Distal contractures are, however, seen in all these disease groups. The age at presentation did not appear to differ between the TPM2 and TPM3 groups. In both, the majority presented perinatally or in infancy, a smaller number in childhood, with delayed motor milestones, and few in adulthood.
Nine of the patients with TPM2 mutations presented with distal arthrogryposis, whereas none of the patients with TPM3 mutations presented with this feature. This may be due to Tm2 being expressed earlier during development than Tm3 [Muthuchamy et al., 1993]. Patients with the recurrent deletion mutation Lys7del in the 5′-end of the gene presented with jaw contractures, camptodactyly (2/10) or core-rod myopathy (1/10), contractures (4/10), and difficulty in opening the mouth (4/10) [Davidson et al., 2013; Mokbel et al., 2013]. Nemaline bodies were also present in patients with a diagnosis of DA type 7 (3/3), exemplifying the overlap between phenotypic characteristics of these disease entities (Supp. Tables S1 and S2).
In most families in the present series, the disease-causing mutations were heterozygous, and the mode of inheritance dominant in 89 families. Of the dominant cases, 43 were due to de novo mutations. Few TPM3 mutations were homozygous and these were all found in the beginning or at the end of the gene. Most recessive mutations were nonsense mutations changing an amino acid to a stop codon, yielding a truncated protein or changing the stop codon to give rise to a longer protein. The dominant ones tended to be missense mutations or in-frame deletions. Among patients with TPM2 mutations, only one, a patient with NM andpterygia, was homozygous for a recessive mutation. There was one case of mosaicism associated with a heterozygous TPM2 mutation. In the TPM3 group, four families showed the recessive mode of inheritance, and there was one case of probable mosaicism. Thus, mosaicism needs to be taken into account in genetic counseling for disorders caused by mutations in either gene.
Type 1 fiber predominance and hypotrophy were common features for all disease entities except for the case of Escobar syndrome. Fiber size disproportion appeared to be consistent among all patients with TPM3 mutations. Type 1 hypotrophy is to be expected in patients with TPM3 mutations because of the exclusive expression of Tm3 in type 1 fibers [Perry, 2001], but small type 1 fibers were common also in the group with TPM2 mutations, despite the expression of Tm2 in both fiber types.
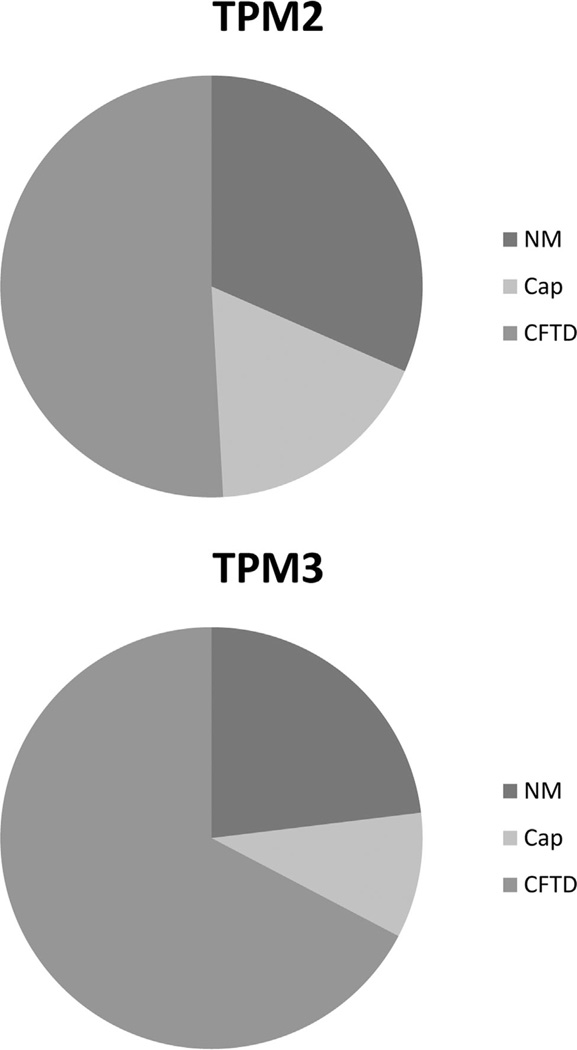
Cap structures and nemaline bodies appeared to be more frequent in biopsies taken at older ages (in three families). This may be explained by the disease process leading to accumulation of sarcomeric protein inclusions over time. Most of the biopsies in CFTD cases had been taken at very young ages (5 months, 6 months, 8 months, 1 year, 14 months, 16 months, 2 years, 11 years, and 29 years). This raises the question of whether the diagnosis of these patients would change if additional biopsies were taken later in life. Such situations have been encountered in a number of CM cases, for example, in one patient with CFTD and cap myopathy caused by a mutation in TPM2. In the biopsy taken at the age of 11 years, only fiber type disproportion was noted, whereas in the later biopsy taken at the age of 33 years, cap structures were obvious [Lehtokari et al., 2007]. Caps were more commonly observed in patients with TPM2 mutations than in patients with TPM3 mutations (10/41 vs. 5/35; Fig. 4), but the small sample size and the possibility of sampling bias at muscle biopsy hinder any firm conclusion that this should be a significant difference. It has been suggested that cap disease might be considered an early stage or variant of NM [Tajsharghi et al., 2007]. The variable observations of caps and/or nemaline bodies in families and even in individual patients over time [Lehtokari et al., 2007; Tajsharghi et al., 2007] support the concept of cap myopathy being a subcategory of NM.
Figure 4.
A pie chart showing histopathological characteristics in biopsies from patients with mutations in the TPM2 and TPM3 genes. Abbreviations: NM, nemaline bodies; Cap, Cap structures; and CFTD, fiber size disproportion.
Patients with hypercontractile molecular phenotypes based on structural analysis of the actin–tropomyosin interface [Marston et al.,2013] (marked by # in the Tables 1 and 2, Supp. Tables S1–S4) more often have contractures of the limb joints (18/19) and jaw (6/19) than those who do not have this type of mutation (2/22 and 1/22). Patients with the nonhypercontractile molecular phenotypes much more often (19/22) have axial contractures (usually scoliosis, but also rigid spine) than the hypercontractile group (7/19). Presentation was early in both groups, so molecular hypercontractility does not, as has been indicated for patients with the Lys7del mutation in previous studies [Davidson et al., 2013; Memo and Marston, 2013; Mokbel et al., 2013] always cause delayed onset of muscle weakness (Supp. Table S1). Only one hypercontractile molecular phenotype was found in TPM3 in this study, p.Lys169Glu [Memo and Marston, 2013]. Three are known altogether (unpublished data).
Our results indicate that NM caused by mutations in TPM2 usually has a milder presentation than NM caused by mutations in TPM3 (TPM2: 10 NM cases, one adult onset, TPM3: 10 NM cases, four severe) (Table 3). Recessive disease caused by mutations in these genes appears mostly to be severe. There is no other clear correlation between the type of mutation and the clinical phenotype. For TPM2, this was also stated by Tajsharghi et al. (2012) in a recent review.
Table 3.
Summary of Clinical Findings in TPM2 and TPM3
| Clinical observation | TPM2 | TPM3 |
|---|---|---|
| Age of onset | Majority perinatally or in infancy, small number in childhood and few in adulthood |
Majority perinatally or in infancy, small number in childhood and few in adulthood |
| Distal arthrogrypos is | Nine patients | None |
| Fiber size disproportion | 29 families | 35 families |
| NM | Milder presentation (10 NM cases, one adult onset) |
More severe presentation (10 NM cases, four severe) |
| Patients with hypercontractile molecular phenotypes have more often contractures than others |
Hypercontractile: contractures of limb joints (18/19) and jaw (6/19) |
Hypercontractile: contractures of limb joints (18/19) and jaw (6/19) |
| Others: contractures of limb joints (2/22) and jaw (1/22) |
Others: contractures of limb joints (2/22) and jaw (1/22) |
Summary of clinical findings in TPM2 and TPM3.
NM, nemaline myopathy.
Biological Relevance
Causative Mutations and Actin Association
In the current models [Perry, 2001; Brown et al., 2005; Li et al., 2011; Memo and Marston, 2013], it is proposed that tropomyosin has a sevenfold repeated amino acid sequence motif that corresponds to the seven actin monomers covered by one tropomyosin. Each motif is subdivided into α- and β-zones. In the relaxed “Off” state, tropomyosin forms contacts with actin through positively charged basic residues in the N-terminal part of an α-zone and acidic residues on the C-terminal side of an α-zone. Ca2+ regulation is imposed on actin–tropomyosin by the troponin complex that switches actin–tropomyosin between the “On” and “Off” conformational states. Thus, mutations in tropomyosin potentially can cause muscle dysfunction leading to myopathy through a variety of mechanisms, including effects on formation of dimers, end-to-end interactions, actin binding, and the regulatory interaction with troponin.
Molecular Modeling
Molecular modeling is described in detail by Memo and Marston (2013).
Tropomyosin interaction sites with actin in the “Off” state were precisely mapped by Li et al. (2011). Actin p.Asp25 is predicted to interact with all seven tropomyosin actin-binding repeats. A TPM2 mutation at one of these tropomyosin interaction sites is p.Lys128Glu and similar TPM3 mutations are p.Arg91Pro/Cys, the hotspot p.Arg168Cys/Gly/His, and p.Arg245Gly/Ile (Fig. 5). Interestingly, gain-of-function mutations that increase contractility are located in the amino acid next to the Tm actin-binding site: p.Lys7del, p.Lys49del, and p.Arg91Gly in TPM2 and p.Lys169Glu in TPM3 [Memo and Marston, 2013]. Actin amino acids 147, 326, and 328 interact with two acidic amino acids in each actin-binding repeat of tropomyosin separated by two to three amino acids at the end of an α-zone. Disease-causing mutations are found affecting only the first acidic amino acid and they are both gain-of-function mutations: TPM2 p.Glu139del and p.Glu181Lys [Li et al., 2011; Ochala et al., 2012; Memo and Marston, 2013].
Figure 5.
On-state actin−tropomyosin contacts and disease mutations. The Tm2 (β-Tm) sequence and the Tm3 (γ-Tm) sequence divided into seven quasi-repeats and α- and β-bands as defined by Mclachlan and Stewart (1976). The purple circles highlight residues interacting with actin Asp25; the orange circles highlight the residues interacting with actin R147, K326, and K328 as defined by Li et al. (2010). The Tm2 mutations are indicated in blue boxes; the Tm3 mutations are indicated in green boxes. The mutations increasing Ca2+-sensitivity are written in red, whereas those decreasing it are in black.
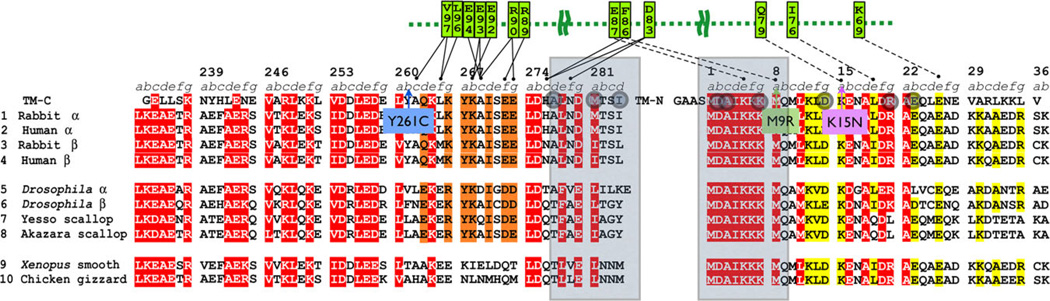
In the “On” state, tropomyosin shifts in position relative to the actin filament and myosin contacts are formed [Behrmann et al., 2012]. The “On” state tropomyosin–actin contacts are less well defined and tropomyosin may simply be pushed into its position by strong myosin–actin binding. Overall, fewer disease-causing mutations are adjacent to the proposed “On” state tropomyosin– actin contacts: the TPM2 mutations p.Asp14Val, p.Glu139del, p.Glu218del, and the TPM3 mutations p.Leu100Met/Val (Tables 1 and 2; Fig. 5; Supp. Table S5). The other main protein that interacts with tropomyosin is TnT. Of the known TPM2 mutations, p.Tyr261Cys, and in TPM3, p.Met9Arg are involved in the interaction with TnT according to Murakami’s structure of TPM2 [Murakami et al., 2008] (Fig. 6). Head-to-tail polymerization of tropomyosin is required for actin binding, and has a role is in actin filament assembly, and for the regulation of actin–myosin contraction [Murakami et al., 2008]. The mutations p.Ala3Gly, p.Lys7del in TPM2 and p.Ala4Val, p.Met9Arg, and p.Stop285Ser/Asn in TPM3 are found at the overlapping region involved in head-to-tail polymerization (Fig. 3).
Figure 6.
Tropomyosin end–end overlap and interactions of tropomyosin with TnT. Tropomyosin end–end overlap (one to nine and 277–284, gray boxes) and interactions of tropomyosin with TnT based on the model of Murakami et al. (2008). Mutations in overlap zone are shown as gray circles; pink outline TPM1 (cardiomyopathy), blue outline TPM2. Only a few mutations are involved in contacts with TnT (green) in this region of tropomyosin. TPM2 Y261C is next to A262, M9R is supposed to bind to F86 in TnT, and K15N (TPM1) is supposed to bind with TnT Q79.
We hypothesize that the charge changes inherent in most hypercontractile mutations would destabilize the “Off” state and favor the equilibrium toward the “On” state, thus accounting for the higher Ca2+ sensitivity as was demonstrated for the ACTA1 p.Lys326Asn mutation [Orzechowski et al., 2013]. This would be the case with the TPM2 mutations p.Lys7del, p.Lys49del, p.Arg91Gly, p.Glu139del, and p.Glu181Lys, and the TPM3 mutation p.Lys169Glu. This seems to correlate with hypercontractile or DA phenotypes that are p.Lys7del, p.Arg91Gly, p.Arg133Trp, and p.Glu181Lys. Congenital muscle weakness correlates with loss-of-function at the molecular level. These mutations are not at the interface of the “Off” state but have an opposite charge change to the gain-of-function mutations and are in a location that could stabilize the “Off” state relative to the “On” state, which may account for the loss-of-function. On the other hand, alteration of the tropomyosin–troponin interface could also have this effect. Mutations shown to be associated with decreased Ca2+ sensitivity include TPM2-Glu41Lys, TPM2-Glu117Lys, TPM3-Arg168His, and TPM3-Arg245Gly.
In this study, we discuss altogether 30 mutations in TPM2 and 20 mutations in TPM3. More than half of them (18/30 for TPM2 and 14/20 for TPM3) are located in α-zones, which contain residues interacting with actin in the “Off” state. In addition, three (p.Asp2Val, p.Ala3Gly, and p.Lys7del) of the TPM2 and four (p.Ala4Val, p.Met8Arg, p.Stop285Ser, and p.Stop285Asn) of the TPM3 mutations are located in the tropomyosin overlapping region (Fig. 3). These regions are essential for tropomyosin function such as actin binding and polymerization. The TPM3-Arg168His and TPM2-null mutations were found to severely reduce the proportion of strong myosin cross-bridges bound to actin filaments at submaximal Ca2+ concentrations and thus to depress force production. The TPM2-Glu181Lys mutant was found to have enhanced Ca2+ sensitivity and force production [Ochala et al., 2012]. Most mutations are in conserved areas of TPM2 and TPM3. Only five mutations (TPM2-Asp2Val, TPM3-Gln31Stop, TPM2-Glu41Lys, TPM2-Leu143Pro, and TPM3-Thr252Lys) are in sites where sequence differs between the genes. Four of the amino acids varying between tropomyosins (TPM2-Asp2Val, TPM2-Glu41Lys, TPM2-Leu143Pro, and TPM3-Thr252Lys) are substituted by similar amino acids, for example, acidic by another acidic one.
Identification of Novel Phosphorylation Sites in Tm2
Phosphorylation has been shown to modify tropomyosin function: it enhances head-to-tail interaction of neighboring tropomyosin dimers, increases binding to TnT and slows relaxation of Ca2+-activated force [Heeley et al., 1989; Hayley et al., 2008; Nixon et al., 2013]. To elucidate a potential role of tropomyosin phosphorylation on pathogenesis, mass spectrometry analyses were performed on purified recombinant tropomyosin, both Tm2 and proteins containing known patient mutations (p.Glu41Lys, p.Lys49del, p.Glu117Lys, p.Glu139del, and p.Gln147Pro). We found four known phosphorylation sites (p.Thr53, p.Thr252, p.Thr282, and p.Ser283) that were present in both the wt and the mutated proteins [Dai et al., 2007; Huttlin et al., 2010]. In addition, we identified four novel phosphorylation sites (p.Thr79, p.Thr108, p.Ser158, and p.Ser206) (Supp. Table S7). The previously unknown phosphorylation sites are situated in the mid region of Tm2 around the mutational hotspot in or near the fourth α-zone (Fig. 3). In all cases examined, the phosphorylation patterns were identical between the wt and the mutated proteins, indicating that changes in phosphorylation patterns are not the main cause of disease for the mutations studied. Also, no patient mutations have been found in the phosphorylated residues in Tm2 and Tm3. Interestingly, in Tm3, only the previously known p.Ser283 was found to be phosphorylated, which corresponds to p.Ser283 in Tm2.
Conclusions
In this report, we have compiled, to the best of our knowledge, all previously reported and novel mutations of TPM2 and TPM3. Altogether we analyzed 27 mutations causing an amino acid change and 3 mutations predicted to affect splicing inTPM2 and 20 mutations in TPM3. Patients with TPM2 mutations tended to present with milder symptoms than those with TPM3 mutations, DA being present only in the TPM2 group. Recessive disease was usually more severe than the dominantly inherited forms. Fiber-type disproportion was consistent in the TPM3 group and was also common with mutations of TPM2. Patients with hypercontractile molecular phenotypes more often had contractures of the limb joints and jaw than those with nonhypercontractile molecular phenotypes, whereas those with no hypercontractility more commonly had spinal deformities or rigidity. No difference was found in the age of onset.
We show that most mutations affect actin association (18/30 in TPM2 and 13/20 in TPM3), resulting in the clinical and histological pictures of NM, cap myopathy, core-rod myopathy, CFTD, CM, DA, and Escobar syndrome with myopathy. Six mutations cause increased Ca2+ sensitivity resulting in hypercontractile phenotypes. Among the remaining mutations, all five so far tested caused decreased Ca2+ sensitivity. Three of the TPM2 and four of the TPM3 mutations were located in the tropomyosin overlapping region affecting head-to-tail binding. We report four novel phosphorylation sites in β-tropomyosin. Although phosphorylation is known to be linked to tropomyosin–actin association, we found no mutations in phosphorylated residues, nor altered phosphorylation patterns in purified tropomyosin proteins containing known patient mutations.
Supplementary Material
Acknowledgements
The authors thank Dr Homa Tajsharghi for helpful discussion of tropomyosin expression during development and Elizabeth DeChene, MS, CGC, for helpful discussions and assistance with clinical diagnostic testing. We would like to thank Leigh B. Waddell, PhD, and we also thank Fabiana Fattori, PhD, for screening for mutations in TPM2 and TPM3 patients in Italy and Adele D’Amico, MD, PhD, for clinical and genetic characterization of congenital myopathies and coordinating the international network of neuromuscular disorders in Italy. F.P. holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund.
Contract grant sponsors: National Institute of Arthritis and Musculoskeletal and Skin Diseases of the NIH (grant R01 AR044345); Muscular Dystrophy Association (MDA201302); Australian National Health, Medical Research Council (NHMRC) Project (grant APP1022707); NHMRC Centre of Research Excellence (grant APP1031893); NHMRC of Australia (APP1035828, APP1026933); Telethon GUP08005; European Union Seventh Framework Programme (FP7/2007–2013) (grant agreement no. 305444 (RD-Connect) and 305121 (Neuromics); Association Française contre les Myopathies; Sigrid Jusélius Foundation; Academy of Finland; Finska Läkaresällskapet; Medicinska un-derstödsföreningen Liv och Hälsa r.f.; DHFMR; Foundation for Building Strength for Nemaline Myopathy; Ricerca Finalizzata Ministry of Health; British Heart Foundation.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Disclosure statement: The authors declare that there is no conflict of interest.
References
- Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkari PA, Song Y, Hitchcock-DeGregori S, Blechynden L, Laing N. Expression and biological activity of baculovirus generated wild-type human slow alpha tropomyosin and the Met9Arg mutant responsible for a dominant form of nemaline myopathy. Biochem Biophys Res Commun. 2002;296:300–304. doi: 10.1016/s0006-291x(02)00852-5. [DOI] [PubMed] [Google Scholar]
- Barua B, Pamula MC, Hitchcock-DeGregori SE. Evolutionarily conserved surface residues constitute actin binding sites of tropomyosin. Proc Natl Acad Sci USA. 2011;108:10150–10155. doi: 10.1073/pnas.1101221108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann E, Muller M, Penczek PA, Mannherz HG, Manstein DJ, Raunser S. Structure of the rigor actin–tropomyosin–myosin complex. Cell. 2012;150:327–338. doi: 10.1016/j.cell.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke MH. Clinical studies in myology. Amsterdam: Excerpta Medica; 1973. p. 147. [Google Scholar]
- Brown JH, Zhou Z, Reshetnikova L, Robinson H, Yammani RD, Tobacman LS, Cohen C. Structure of the mid-region of tropomyosin: bending and binding sites for actin. Proc Natl Acad Sci USA. 2005;102:18878–18883. doi: 10.1073/pnas.0509269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke NF, Domazetovska A, Waddell L, Kornberg A, McLean C, North KN. Cap disease due to mutation of the beta-tropomyosin gene (TPM2) Neuromuscul Disord. 2009;19:348–351. doi: 10.1016/j.nmd.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Clarke NF, Kolski H, Dye DE, Lim E, Smith RL, Patel R, Fahey MC, Bellance R, Romero NB, Johnson ES, Labarre-Vila A, Monnier N, Laing NG, North KN. Mutations in TPM3 are a common cause of congenital fiber type disproportion. Ann Neurol. 2008;63:329–337. doi: 10.1002/ana.21308. [DOI] [PubMed] [Google Scholar]
- Conen PE, Murphy EG, Donohue WL. Light and electron microscopic studies of “myogranules” in a child with hypotonia and muscle weakness. Can Med Assoc J. 1963;89:983–986. [PMC free article] [PubMed] [Google Scholar]
- Cooley BC, Bergtrom G. Multiple combinations of alternatively spliced exons in rat tropomyosin-alpha gene mRNA: evidence for 20 new isoforms in adult tissues and cultured cells. Arch Biochem Biophys. 2001;390:71–77. doi: 10.1006/abbi.2001.2347. [DOI] [PubMed] [Google Scholar]
- Cooper JA. Actin dynamics: tropomyosin provides stability. Curr Biol. 2002;12:R523–R525. doi: 10.1016/s0960-9822(02)01028-x. [DOI] [PubMed] [Google Scholar]
- Daentl DL, Berg BO, Layzer RB, Epstein CJ. A new familial arthrogryposis without weakness. Neurology. 1974;24:55–60. doi: 10.1212/wnl.24.1.55. [DOI] [PubMed] [Google Scholar]
- Dai J, Jin WH, Sheng QH, Shieh CH, Wu JR, Zeng R. Protein phosphorylation and expression profiling by yin-yang multidimensional liquid chromatography (yin-yang MDLC) mass spectrometry. J Proteome Res. 2007;6:250–262. doi: 10.1021/pr0604155. [DOI] [PubMed] [Google Scholar]
- Davidson AE, Siddiqui FM, Lopez MA, Lunt P, Carlson HA, Moore BE, Love S, Born DE, Roper H, Majumdar A, Jayadev S, Underhill HR, et al. Novel deletion of lysine 7 expands the clinical, histopathological and genetic spectrum of TPM2-related myopathies. Brain. 2013;136:508–521. doi: 10.1093/brain/aws344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paula AM, Franques J, Fernandez C, Monnier N, Lunardi J, Pellissier JF, Figarella-Branger D, Pouget J. A TPM3 mutation causing cap myopathy. Neuromuscul Disord. 2009;19:685–688. doi: 10.1016/j.nmd.2009.06.365. [DOI] [PubMed] [Google Scholar]
- den Dunnen JT, Antonarakis SE. Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat. 2000;15:7–12. doi: 10.1002/(SICI)1098-1004(200001)15:1<7::AID-HUMU4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Dufour C, Weinberger RP, Schevzov G, Jeffrey PL, Gunning P. Splicing of two internal and four carboxyl-terminal alternative exons in nonmuscle tropomyosin 5 pre-mRNA is independently regulated during development. J Biol Chem. 1998;273:18547–18555. doi: 10.1074/jbc.273.29.18547. [DOI] [PubMed] [Google Scholar]
- Durling HJ, Reilich P, Muller-Hocker J, Mendel B, Pongratz D, Wallgren-Pettersson C, Gunning P, Lochmuller H, Laing NG. De novo missense mutation in a constitutively expressed exon of the slow alpha-tropomyosin gene TPM3 associated with an atypical, sporadic case of nemaline myopathy. Neuromuscul Disord. 2002;12:947–951. doi: 10.1016/s0960-8966(02)00182-7. [DOI] [PubMed] [Google Scholar]
- Fidzianska A, Badurska B, Ryniewicz B, Dembek I. “Cap disease”: new congenital myopathy. Neurology. 1981;31:1113–1120. doi: 10.1212/wnl.31.9.1113. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- Gunning PW, Schevzov G, Kee AJ, Hardeman EC. Tropomyosin isoforms: divining rods for actin cytoskeleton function. Trends Cell Biol. 2005;15:333–341. doi: 10.1016/j.tcb.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Hayley M, Chevaldina T, Mudalige WA, Jackman DM, Dobbin AD, Heeley DH. Shark skeletal muscle tropomyosin is a phosphoprotein. J Muscle Res Cell Motil. 2008;29:101–107. doi: 10.1007/s10974-008-9143-z. [DOI] [PubMed] [Google Scholar]
- Heeley DH, Watson MH, Mak AS, Dubord P, Smillie LB. Effect of phosphorylation on the interaction and functional properties of rabbit striated muscle alpha alpha-tropomyosin. J Biol Chem. 1989;264:2424–2430. [PubMed] [Google Scholar]
- Hitchcock-DeGregori SE, Song Y, Moraczewska J. Importance of internal regions and the overall length of tropomyosin for actin binding and regulatory function. Biochemistry. 2001;40:2104–2112. doi: 10.1021/bi002421z. [DOI] [PubMed] [Google Scholar]
- Holmes KC, Popp D, Gebhard W, Kabsch W. Atomic model of the actin filament. Nature. 1990;347:44–49. doi: 10.1038/347044a0. [DOI] [PubMed] [Google Scholar]
- Huttlin EL, Jedrychowski MP, Elias JE, Goswami T, Rad R, Beausoleil SA, Villen J, Haas W, Sowa ME, Gygi SP. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell. 2010;143:1174–1189. doi: 10.1016/j.cell.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A1, Lillis S, Munteanu I, Scoto M, Zhou H, Quinlivan R, Straub V, Manzur AY, Roper H, Jeannet PY, Rakowicz W, Jones DH, Jensen UB, Wraige E, Trump N, Schara U, Lochmuller H, Sarkozy A, Kingston H, Norwood F, Damian M, Kirschner J, Longman C, Roberts M, Auer-Grumbach M, Hughes I, Bushby K, Sewry C, Robb S, Abbs S, Jungbluth H, Muntoni F. Clinical and genetic findings in a large cohort of patients with ryanodine receptor 1 gene-associated myopathies. Hum Mutat. 2012;33:981–988. doi: 10.1002/humu.22056. [DOI] [PubMed] [Google Scholar]
- Krakowiak PA, O’Quinn JR, Bohnsack JF, Watkins WS, Carey JC, Jorde LB, Bamshad M. A variant of Freeman-Sheldon syndrome maps to 11p15.5-pter. Am J Hum Genet. 1997;60:426–432. [PMC free article] [PubMed] [Google Scholar]
- Lawlor MW, Dechene ET, Roumm E, Geggel AS, Moghadaszadeh B, Beggs AH. Mutations of tropomyosin 3 (TPM3) are common and associated with type 1 myofiber hypotrophy in congenital fiber type disproportion. Hum Mutat. 2010;31:176. doi: 10.1002/humu.21157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtokari VL, Ceuterick-de Groote C, de Jonghe P, Marttila M, Laing NG, Pelin K, Wallgren-Pettersson C. Cap disease caused by heterozygous deletion of the beta-tropomyosin gene TPM2. Neuromuscul Disord. 2007;17:433–442. doi: 10.1016/j.nmd.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Li XE, Tobacman LS, Mun JY, Craig R, Fischer S, Lehman W. Tropomyosin position on F-actin revealed by EM reconstruction and computational chemistry. Biophys J. 2011;100:1005–1013. doi: 10.1016/j.bpj.2010.12.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JJ, Warren KS, Wamboldt DD, Wang T, Lin JL. Tropomyosin isoforms in nonmuscle cells. Int Rev Cytol. 1997;170:1–38. doi: 10.1016/s0074-7696(08)61619-8. [DOI] [PubMed] [Google Scholar]
- Mak A, Smillie LB, Barany M. Specific phosphorylation at serine-283 of alpha tropomyosin from frog skeletal and rabbit skeletal and cardiac muscle. Proc Natl Acad Sci USA. 1978;75:3588–3592. doi: 10.1073/pnas.75.8.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S, Memo M, Messer A, Papadaki M, Nowak K, McNamara E, Ong R, El-Mezgueldi , Li X, Lehman W. Mutations in repeating structural motifs of tropomyosin cause gain of function in skeletal muscle myopathy patients. Hum Mol Genet. 2013;22:4978–4987. doi: 10.1093/hmg/ddt345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marttila M, Lemola E, Wallefeld W, Memo M, Donner K, Laing NG, Marston S, Gronholm M, Wallgren-Pettersson C. Abnormal actin binding of aberrant beta-tropomyosins is a molecular cause of muscle weakness in TPM2-related nemaline and cap myopathy. Biochem J. 2012;442:231–239. doi: 10.1042/BJ20111030. [DOI] [PubMed] [Google Scholar]
- Matsumura F, Yamashiro-Matsumura S, Lin JJ. Isolation and characterization of tropomyosin-containing microfilaments from cultured cells. J Biol Chem. 1983;258:6636–6644. [PubMed] [Google Scholar]
- McLachlan AD, Stewart M. The 14-fold periodicity in alpha-tropomyosin and the interaction with actin. J Mol Biol. 1976;103:271–298. doi: 10.1016/0022-2836(76)90313-2. [DOI] [PubMed] [Google Scholar]
- Memo M, Marston S. New structures of the actin-tropomyosin interface can explain the functional consequences of mutations in skeletal muscle tropomyosin that cause congenital myopathies. J Muscle Res Cell Motil. 2013;34:165–169. doi: 10.1007/s10974-013-9344-y. [DOI] [PubMed] [Google Scholar]
- Mokbel N, Ilkovski B, Kreissl M, Memo M, Jeffries CM, Marttila M, Lehtokari VL, Lemola E, Gronholm M, Yang N, Menard D, Marcorelles P, et al. K7del is a common TPM2 gene mutation associated with nemaline myopathy and raised myofibre calcium sensitivity. Brain. 2013;136:494–507. doi: 10.1093/brain/aws348. [DOI] [PubMed] [Google Scholar]
- Monnier N, Lunardi J, Marty I, Mezin P, Labarre-Vila A, Dieterich K, Jouk PS. Absence of beta-tropomyosin is a new cause of Escobar syndrome associated with nemaline myopathy. Neuromuscul Disord. 2009;19:118–123. doi: 10.1016/j.nmd.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Munot P, Lashley D, Jungbluth H, Feng L, Pitt M, Robb SA, Palace J, Jayawant S, Kennet R, Beeson D, Cullup T, Abbs S, Laing N, Sewry C, Muntoni F. Congenital fibre type disproportion associated with mutations in the tropomyosin 3 (TPM3) gene mimicking congenital myasthenia. 2010;20:796–800. doi: 10.1016/j.nmd.2010.07.274. [DOI] [PubMed] [Google Scholar]
- Murakami K, Stewart M, Nozawa K, Tomii K, Kudou N, Igarashi N, Shirakihara Y, Wakatsuki S, Yasunaga T, Wakabayashi T. Structural basis for tropomyosin overlap in thin (actin) filaments and the generation of a molecular swivel by troponin-T. Proc Natl Acad Sci USA. 2008;105:7200–7205. doi: 10.1073/pnas.0801950105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuchamy M, Pajak L, Howles P, Doetschman T, Wieczorek DF. Developmental analysis of tropomyosin gene expression in embryonic stem cells and mouse embryos. Mol Cell Biol. 1993;13:3311–3323. doi: 10.1128/mcb.13.6.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon BR, Liu B, Scellini B, Tesi C, Piroddi N, Ogut O, John Solaro R, Ziolo MT, Janssen PM, Davis JP, Poggesi C, Biesiadecki BJ. Tropomyosin ser-283 pseudo-phosphorylation slows myofibril relaxation. Arch Biochem Biophys. 2013;535:30–38. doi: 10.1016/j.abb.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norum RA, James VL, Mabry CC. Pterygium syndrome in three children in a recessive pedigree pattern. Birth Defects Orig Art Ser. 1969;2:233–235. [Google Scholar]
- Ochala J, Gokhin DS, Penisson-Besnier I, Quijano-Roy S, Monnier N, Lunardi J, Romero NB, Fowler VM. Congenital myopathy-causing tropomyosin mutations induce thin filament dysfunction via distinct physiological mechanisms. Hum Mol Genet. 2012;21:4473–4485. doi: 10.1093/hmg/dds289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell KL, Stults JT. Identification of mouse liver proteins on two-dimensional electrophoresis gels by matrix-assisted laser desorption/ionization mass spectrometry of in situ enzymatic digests. Electrophoresis. 1997;18:349–359. doi: 10.1002/elps.1150180309. [DOI] [PubMed] [Google Scholar]
- Ohlsson M, Quijano-Roy S, Darin N, Brochier G, Lacene E, Avila-Smirnow D, Fardeau M, Oldfors A, Tajsharghi H. New morphologic and genetic findings in cap disease associated with beta-tropomyosin (TPM2) mutations. Neurology. 2008;71:1896–1901. doi: 10.1212/01.wnl.0000336654.44814.b8. [DOI] [PubMed] [Google Scholar]
- Ohman T, Lietzén N, Välimaki E, Meljchorsen J, Matikainen S, Nyman TA. Cytosolic RNA recognition pathway activates 14-3-3 protein mediated signaling and caspase-dependent disruption of cytoskeleton network in human keratinocytes. J Proteome Res. 2010;9:1549–1564. doi: 10.1021/pr901040u. [DOI] [PubMed] [Google Scholar]
- Orzechowski M, Fischer S, Lehman W. Influence of actin mutation on the energy landscape of actin-tropomyosin filaments. Biophys J Biophys Soc. 2013;104(S1):480a. [Google Scholar]
- Penisson-Besnier I, Monnier N, Toutain A, Dubas F, Laing N. A second pedigree with autosomal dominant nemaline myopathy caused by TPM3 mutation: a clinical and pathological study. Neuromuscul Disord. 2007;17:330–337. doi: 10.1016/j.nmd.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Perry SV. Vertebrate tropomyosin: distribution, properties and function. J Muscle Res Cell Motil. 2001;22:5–49. doi: 10.1023/a:1010303732441. [DOI] [PubMed] [Google Scholar]
- Phillips GN, Jr, Lattman EE, Cummins P, Lee KY, Cohen C. Crystal structure and molecular interactions of tropomyosin. Nature. 1979;278:413–417. doi: 10.1038/278413a0. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Kazzaz JA, Helfman DM. Functional properties of non-muscle tropomyosin isoforms. Curr Opin Cell Biol. 1994;6:96–104. doi: 10.1016/0955-0674(94)90122-8. [DOI] [PubMed] [Google Scholar]
- Ribolow H, Barany M. Phosphorylation of tropomyosin in live frog muscle. Arch Biochem Biophys. 1977;179:718–720. doi: 10.1016/0003-9861(77)90162-x. [DOI] [PubMed] [Google Scholar]
- Shihab HA, Gough J, Cooper DN, Stenson PD, Barker GL, Edwards KJ, Day IN, Gaunt TR. Predicting the functional, molecular, and phenotypic consequences of amino acid substitutions using hidden Markov models. Hum Mutat. 2013;34:57–65. doi: 10.1002/humu.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shy GM, Engel WK, Somers JE, Wanko T. Nemaline myopathy: a new congenital myopathy. Brain. 1963;86:793–810. doi: 10.1093/brain/86.4.793. [DOI] [PubMed] [Google Scholar]
- Tajsharghi H, Ohlsson M, Lindberg C, Oldfors A. Congenital myopathy with nemaline rods and cap structures caused by a mutation in the beta-tropomyosin gene (TPM2) Arch Neurol. 2007;64:1334–1338. doi: 10.1001/archneur.64.9.1334. [DOI] [PubMed] [Google Scholar]
- Tajsharghi H, Ohlsson M, Palm L, Oldfors A. Myopathies associated with beta-tropomyosin mutations. Neuromuscul Disord. 2012;22:923–933. doi: 10.1016/j.nmd.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Tasca G, Fattori F, Ricci E, Monforte M, Rizzo V, Mercuri E, Bertini E, Silvestri G. Somatic mosaicism in TPM2-related myopathy with nemaline rods and cap structures. Acta Neuropathol. 2013;125:169–171. doi: 10.1007/s00401-012-1049-6. [DOI] [PubMed] [Google Scholar]
- Wallgren-Pettersson C, Pelin K, Nowak KJ, Muntoni F, Romero NB, Goebel HH, North KN, Beggs AH, Laing NG ENMCInternational Consortium On Nemaline My-opathy. Genotype-phenotype correlations in nemaline myopathy caused by mutations in the genes for nebulin and skeletal muscle alpha-actin. Neuromuscul Disord. 2004;14:461–470. doi: 10.1016/j.nmd.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Wallgren-Pettersson C, Sewry CA, Nowak KJ, Laing NG. Nemaline myopathies. Semin Pediatr Neurol. 2011;18:230–238. doi: 10.1016/j.spen.2011.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.