Abstract
The incidence of Barrett’s esophageal cancer is one of the most rapidly increasing among all cancers in the West, and it is also expected to increase in Japan. The optimal treatment for early Barrett’s esophageal cancer remains controversial. En bloc esophagectomy with regional lymph node dissection has been considered the standard therapy. Endoscopic therapies are currently being evaluated as alternatives to esophagectomy because they can provide the least postoperative morbidity and the best quality of life. Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) allow for removal of visible lesions and histopathologic review of resected tissue, which help in diagnostic staging of the disease. EMR is limited with respect to resection size, and large lesions must be resected in several fragments. Piecemeal resection of lesions is associated with high local recurrence rates, probably because of minor remnants of neoplastic tissue being left in situ. ESD provides larger specimens than does EMR in patients with early Barrett’s neoplasia. This in turn allows for more precise histological analysis and higher en bloc and curative resection rates, potentially reducing the incidence of recurrence. Detailed endoscopic examination to determine the invasion depth and spread of Barrett’s esophageal cancer is essential before ESD. The initial inspection is usually conducted with white-light imaging followed by narrow-band imaging. The ESD procedure is similar to that for lesions in other parts of the gastrointestinal tract. However, the narrow space of the esophagogastric junction and contraction of the lower esophageal sphincter sometimes disturb the visual field and endoscopic control. Skilled endoscope handling, sometimes including retroflexion, is required during ESD for Barrett’s esophageal cancer. Previous reports have shown that ESD achieves en bloc resection in >80% of lesions. Although promising short-term results are reported, a long-term, large-scale study is required for better understanding of ESD for Barrett’s esophageal cancer.
Keywords: Barrett’s esophageal cancer, Barrett’s esophagus (BE), endoscopic resection, endoscopic submucosal dissection (ESD), endoscopic treatment
Barrett’s esophageal (BE) cancer
BE was first described in 1950 (1). This condition is thought to be a complication of chronic gastroesophageal reflux disease and may be found in both symptomatic and asymptomatic individuals (2). The annual incidence of adenocarcinoma arising from BE is 0.12% to 0.50% (3-7). There is geographic variation in the prevalence of BE, which is much more common in the West than in the East (8). The increase in the incidence of BE has led to a four-fold increase in the incidence of BE cancer in the West (9). Similar data are not available from the East. However, it is suggested that the rate of BE and BE cancer will increase in Asia in the future (10,11) because of the decreasing prevalence of Helicobacter pylori infection and Westernization of the diet.
Barrett’s esophageal cancer in Japan
BE is defined as replacement of the stratified squamous epithelium that normally lines the distal esophagus with columnar epithelium (12). Histological confirmation of intestinal metaplasia is not required for the diagnosis of BE in Japan. In Japan, there are few reports on the prevalence of BE and incidence of BE cancer. BE is usually classified into two categories according to the extent of columnar epithelium above the gastroesophageal junction: (I) long-segment BE, in which the extent of the columnar epithelium is ≥3 cm; and (II) short-segment BE, in which the extent of the columnar epithelium is <3 cm (13). In Japanese patients, because the prevalence of long-segment BE (≥3 cm) is extremely low (11), most esophageal adenocarcinoma in Japanese patients arises from short-segment BE. The risk of cancer in BE appears to vary with the extent of BE; therefore, patients with long-segment disease may have a higher incidence of adenocarcinoma than those with short-segment BE (14). In a Spanish cohort, for example, the annual risk of BE cancer was 0.57% for patients with long-segment BE and only 0.26% for patients with short-segment disease (15).
Treatment for Barrett’s esophageal cancer
BE cancer survival rates correlate with the disease stage. Locally advanced diseases show a 5-year survival rate of approximately 20% (16,17). Because of the poor 5-year survival rates for advanced BE cancer, surveillance and early detection of BE cancer has become a critical issue (18,19). Rigorous surveillance of BE and a systematic biopsy protocol improves detection of dysplasia and early cancer (20).
The optimal treatment for early BE cancer remains controversial. En bloc esophagectomy with regional lymph node dissection has been considered to be the standard therapy. Esophagectomy definitively eliminates all portions of the esophagus lined by BE and allows for the removal of associated lymph nodes that could harbor metastases. Nevertheless, en bloc esophagectomy is associated with high mortality (4-19%) (21), high postoperative morbidity (20-47%) (22), and low postoperative quality of life (23). The morbidity and mortality associated with surgical esophagectomy and the low rates of metastases associated with early esophageal cancer have led to an interest in newer, less invasive therapies as alternatives to esophagectomy.
New modalities such as endoscopic therapies or less aggressive surgical operations are currently being evaluated in an effort to achieve the least postoperative morbidity and the best quality of life. Although limited data are available on the risk of metastasis related to subdivisions of T1 lesions, studies of esophagectomy specimens indicate that a low risk is present, ranging from 0.0% to 1.3% for T1a carcinomas and 18.0% to 22.0% for T1b tumors (24-26). This low rate of metastasis has provided a rationale for the endoscopic treatment of mucosal (T1a) BE cancer or high-grade dysplasia for curative intent. Endoscopic therapies can be further subdivided into tissue-acquiring and non-tissue-acquiring modalities. Tissue acquisition can be achieved through endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR), while thermal, photochemical, or radiofrequency energy is used to destroy the BE without providing a tissue specimen (27-29). Favorable outcomes have been reported after endoscopic ablative techniques such as photodynamic therapy, radiofrequency ablation, and cryotherapy. Modalities such as argon plasma coagulation, multipolar electrocoagulation, and laser therapy are not current mainstay therapies because of high BE relapse rates and their infrequent usage. In endoscopic eradication treatment, it is recommended that any visible abnormalities be removed by endoscopic resection followed by ablation of all remaining BE according to United States guidelines (30). However, this strategy is not commonly utilized in Japan because of the unknown risk of metachronous lesion development in the residual BE after endoscopic resection in the Japanese population.
ESD and EMR for Barrett’s esophageal cancer
Endoscopic resection in the form of EMR and ESD allows for removal of visible lesions and histopathologic review of resected tissue, facilitating more accurate diagnostic staging of the disease. If submucosal invasion is found, patients can then be referred for surgical resection because these lesions have a substantial risk of metastasis. If the lesion is confined to the mucosa and the resection margins are clear, endoscopic resection can be curative because of the very low risk of lymph node metastases. Notably, most adverse events associated with endoscopic resection are amenable to endoscopic treatment (31-33).
The various modalities of EMR include the use of a transparent cap, two-channel endoscope, and ligation. These modalities are limited with respect to resection size, and large lesions must be resected in several fragments. In addition, the targeted area cannot be precisely controlled by the endoscopist, which might result in unnecessary resection of non-neoplastic mucosa. When lesions are resected in small fragments, histological assessment of cancer invasion depth can be inaccurate. Histological evaluation of several specimens cannot usually identify the outer margins of the neoplastic area, and thus complete resection cannot be confirmed. In addition, piecemeal resection of early neoplasia in BE is associated with a high local recurrence rate, probably because of minor remnants of neoplastic tissue left in situ (34-37). In one trial, the rate of complete resection (R0) was only 30% with a lesion diameter of <20 mm (36). Repeated sessions of EMR are sometimes needed to achieve complete local remission, and recurrent lesions develop in 10% to 30% of cases after EMR without eradication of the residual non-neoplastic BE (34-36,38,39).
In patients with early BE neoplasia, ESD provides larger specimens than does EMR, for more precise histological analysis and higher en bloc and curative resection rates, potentially reducing the incidence of recurrence. Variations of this method have been used increasingly more frequently for early gastrointestinal neoplasia, mainly in Asian countries. Although no large randomized prospective studies of ESD and EMR for neoplastic lesions have been performed, the results of several retrospective studies have been reported (40-42). A recent meta-analysis of nonrandomized studies showed that ESD for early gastrointestinal tumors is superior to EMR in terms of en bloc and curative resection rates, but that it is more time-consuming and is associated with higher rates of bleeding and perforation (43). Because limited data are available on ESD for BE cancer, we herein introduce our view of the Japanese standard practice of ESD for BE cancer.
Endoscopic examination before ESD
Detailed endoscopic examination to determine invasion depth and lesion spread is usually performed before ESD. Initial inspection is conducted with white-light imaging (Figure 1). Cancer invasion depth is diagnosed based on the lesion color, elevation, depression, and hardness. Spread of the lesion is determined by the presence of redness, an irregular surface, slight elevation, or slight depression. Non-magnifying white-light imaging observation is usually followed by magnifying narrow-band imaging observation. Lesion spread is determined by the presence of an irregular surface pattern or irregular vessel pattern with narrow-band imaging (Figure 2). Endoscopic diagnosis of the lateral extension of BE cancer is sometimes difficult because the margin can be unclear and the cancer can spread under the squamous epithelium. When these two modalities fail to delineate the lesion, biopsies are taken for further assessment. Screening for synchronous lesions is also performed with white-light imaging and narrow-band imaging. Autofluorescence imaging is commercially available, but the combination of this modality and random biopsy is not commonly used in clinical practice of BE cancer treatment in Japan.
Figure 1.
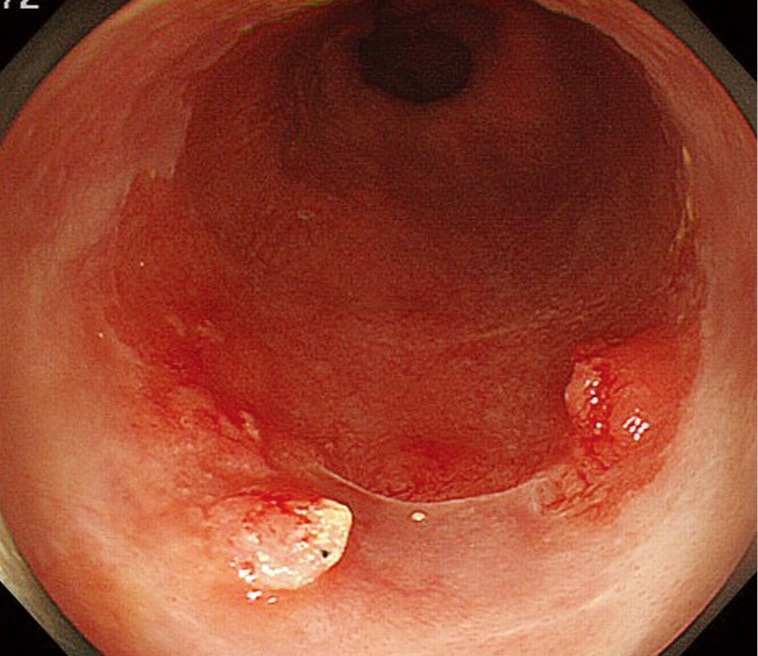
Endoscopic image of Barrett’s esophageal cancer with small elevations and slightly reddish areas.
Figure 2.
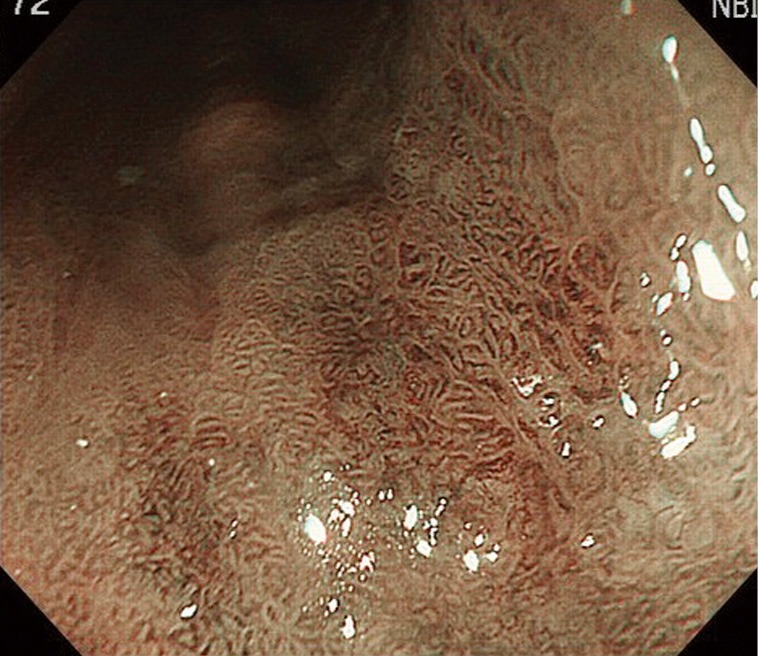
Narrow-band image of a slightly reddish area shows an irregular surface pattern.
Indication for ESD
ESD is indicated when a lesion is diagnosed as high-grade dysplasia or mucosal cancer during the pretreatment evaluation. The depth of cancer invasion is further assessed by histological examination of the resected specimen. When the lesion is identified as high-grade dysplasia or cancer limited to the lamina propria, ESD is regarded as curative. When the lesion invades the muscularis mucosa, a substantial risk of metastasis exists and additional surgical resection is considered based on the patient’s condition. When submucosal invasion is confirmed histologically, additional surgical resection is usually performed. A lesion with a circumferential spread of two-thirds or less is a generally accepted indication for ESD. Lesions with a circumferential spread of more than two-thirds can be treated by ESD; however, surgical resection is sometimes indicated because of the risk of severe stricture after ESD.
Process of ESD
Marking dots are usually made 2 to 3 mm outside the margins of the lesion. However, the margin of BE cancer is sometimes unclear and difficult to delineate. Marking dots are made 5 to 10 mm outside lesions with unclear margins. When the oral side of the lesion is adjacent to the squamous epithelium, marking dots are made at least 10 mm outside the oral margins because the cancer can spread invisibly under the squamous epithelium (Figure 3). A solution such as glycerin solution or hyaluronic acid is injected into the submucosa, and the mucosa is incised outside the marking dots. In the lower part of the esophagus, most of the submucosal vessels run longitudinally. Mucosal incision in the transverse direction readily results in bleeding when longitudinally running vessels are cut. The submucosal layer beneath the lesion is then meticulously dissected until total removal of the lesion has been achieved (Figures 4,5). This part of the procedure is the most challenging and requires expert control and skill. Most BE cancers in Japan arise from short-segment BE, which is usually located near the esophagogastric junction. The narrow space of the esophagogastric junction and contraction of the lower esophageal sphincter sometimes disturb the visual field and control of the endoscope. Detailed handling of the endoscope, sometimes retroflexed handling, is required in the narrow space during ESD for BE cancer.
Figure 3.
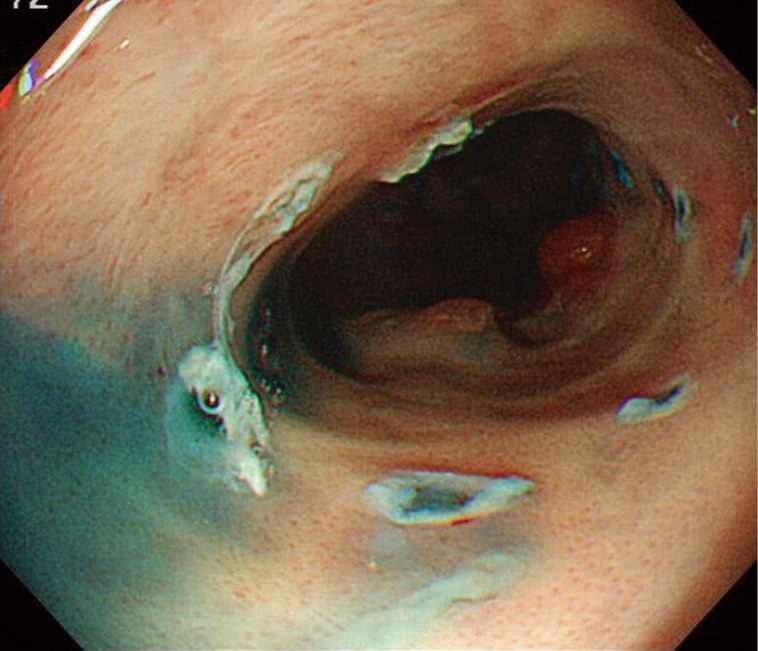
Endoscopic image of Barrett’s esophageal cancer after marking.
Figure 4.
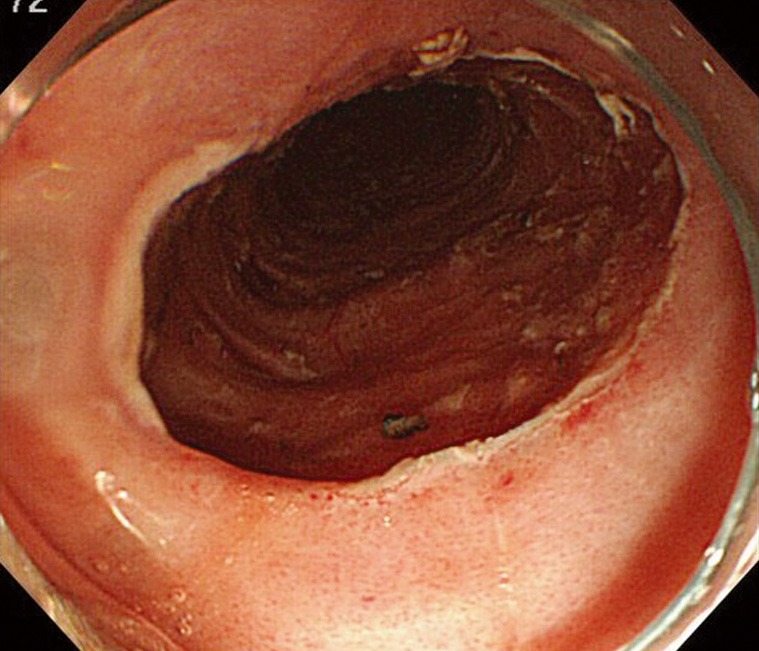
Esophageal ulcer after endoscopic submucosal dissection.
Figure 5.
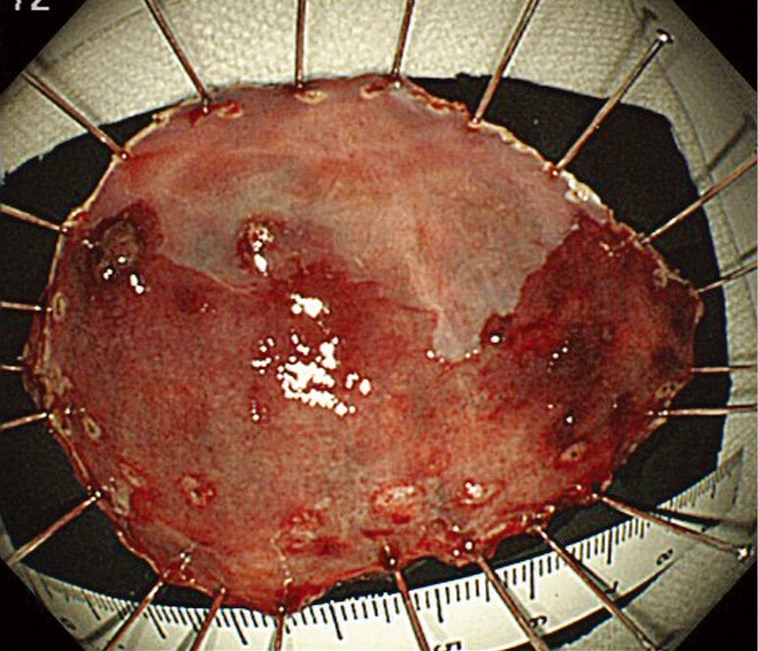
Endoscopic image of resected specimen.
Management of adverse events associated with ESD
The adverse event profile of endoscopic resection includes stricture formation, bleeding, and perforation. Perforation is usually treated by endoscopic clipping, and bleeding is treated by ablation with hemostatic forceps. The risk of stricture rises with the extent of the resection area. When more than three-fourths of the circumference is resected by ESD, the risk of stricture increases (44). Repeated balloon dilatation was previously required to treat stricture after ESD. However, triamcinolone injection (45,46) or oral prednisolone (47) can reportedly reduce the stricture after wide spread endoscopic resection.
Outcome after endoscopic resection for Barrett’s esophageal cancer
Only two English-language case series of ESD for BE cancer (48,49), and four peer-reviewed English articles on ESD for esophagogastric junctional cancer have been published (50-53). BE cancer is probably included within the group of esophagogastric junctional cancers; however, the actual number of cases of BE cancers is not described in these articles. Some non-peer-reviewed Japanese articles involving five to six patients with BE cancer have also been published (54,55). Short-term outcomes were evaluated in these Japanese articles. En bloc resection was achieved in 80% to 83% of lesions, and en bloc resection with cancer-free margins was achieved in 80% to 83% of patients.
Comparison of long-term survival after surgical resection and endoscopic resection would provide helpful information with regard to the most optimal standard treatment. Although the ideal design would be a randomized controlled trial to compare outcomes between these two treatment modalities, this would be difficult to achieve given the small number of cases of mucosal BE cancer and the difficulty in randomizing patients to these two radically different treatment approaches. The available literature suggests that the long-term outcomes of endoscopic therapy for early esophageal cancer, including the median cancer-free survival period, are similar to those of surgical therapy but with fewer adverse events (37,56-58). ESD allows for detailed histologic examination and a reduced risk of recurrence. Improved outcomes are expected with the use of an ESD-based treatment strategy for BE cancer. Although previous reports show promising short-term results (48-55), a long-term, large-scale study is required for better understanding of ESD for BE cancer.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Barrett NR. Chronic peptic ulcer of the oesophagus and ‘oesophagitis’. Br J Surg 1950;38:175-82. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman DA, Oehlke M, Helfand M. Risk factors for Barrett’s esophagus in community-based practice. GORGE consortium. Gastroenterology Outcomes Research Group in Endoscopy. Am J Gastroenterol 1997;92:1293-7. [PubMed] [Google Scholar]
- 3.Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med 2011;365:1375-83. [DOI] [PubMed] [Google Scholar]
- 4.Wani S, Falk G, Hall M, et al. Patients with nondysplastic Barrett’s esophagus have low risks for developing dysplasia or esophageal adenocarcinoma. Clin Gastroenterol Hepatol 2011;9:220-7; quiz e26. [DOI] [PubMed]
- 5.Drewitz DJ, Sampliner RE, Garewal HS. The incidence of adenocarcinoma in Barrett’s esophagus: a prospective study of 170 patients followed 4.8 years. Am J Gastroenterol 1997;92:212-5. [PubMed] [Google Scholar]
- 6.Shaheen NJ, Crosby MA, Bozymski EM, et al. Is there publication bias in the reporting of cancer risk in Barrett’s esophagus? Gastroenterology 2000;119:333-8. [DOI] [PubMed] [Google Scholar]
- 7.Hameeteman W, Tytgat GN, Houthoff HJ, et al. Barrett’s esophagus: development of dysplasia and adenocarcinoma. Gastroenterology 1989;96:1249-56. [DOI] [PubMed] [Google Scholar]
- 8.Shaheen NJ, Richter JE. Barrett’s oesophagus. Lancet 2009;373:850-61. [DOI] [PubMed] [Google Scholar]
- 9.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst 2008;100:1184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu JC. Gastroesophageal reflux disease: an Asian perspective. J Gastroenterol Hepatol 2008;23:1785-93. [DOI] [PubMed] [Google Scholar]
- 11.Hongo M, Nagasaki Y, Shoji T.Epidemiology of esophageal cancer: Orient to Occident. Effects of chronology, geography and ethnicity. J Gastroenterol Hepatol 2009;24:729-35. [DOI] [PubMed] [Google Scholar]
- 12.American Gastroenterological Association , Spechler SJ, Sharma P, et al. American gastroenterological association medical position statement on the management of Barrett’s esophagus. Gastroenterology 2011;140:1084-91. [DOI] [PubMed] [Google Scholar]
- 13.Sharma P, Morales TG, Sampliner RE. Short segment Barrett’s esophagus--the need for standardization of the definition and of endoscopic criteria. Am J Gastroenterol 1998;93:1033-6. [DOI] [PubMed] [Google Scholar]
- 14.Thomas T, Abrams KR, De Caestecker JS, et al. Meta analysis: cancer risk in Barrett’s oesophagus. Aliment Pharmacol Ther 2007;26:1465-77. [DOI] [PubMed] [Google Scholar]
- 15.Alcedo J, Ferrández A, Arenas J, et al. Trends in Barrett’s esophagus diagnosis in southern Europe: implications for surveillance. Dis Esophagus 2009;22:239-48. [DOI] [PubMed] [Google Scholar]
- 16.Gillison EW, Powell J, Mcconkey CC, et al. Surgical workload and outcome after resection for carcinoma of the oesophagus and cardia. Br J Surg 2002;89:344-8. [DOI] [PubMed] [Google Scholar]
- 17.Liu JF, Wang QZ, Hou J.Surgical treatment for cancer of the oesophagus and gastric cardia in Hebei, China. Br J Surg 2004;91:90-8. [DOI] [PubMed] [Google Scholar]
- 18.Inadomi JM, Sampliner R, Lagergren J, et al. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med 2003;138:176-86. [DOI] [PubMed] [Google Scholar]
- 19.Sharma P, Sidorenko EI. Are screening and surveillance for Barrett’s oesophagus really worthwhile? Gut 2005;54Suppl 1:i27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abela JE, Going JJ, Mackenzie JF, et al. Systematic four-quadrant biopsy detects Barrett’s dysplasia in more patients than nonsystematic biopsy. Am J Gastroenterol 2008;103:850-5. [DOI] [PubMed] [Google Scholar]
- 21.Swisher SG, Deford L, Merriman KW, et al. Effect of operative volume on morbidity, mortality, and hospital use after esophagectomy for cancer. J Thorac Cardiovasc Surg 2000;119:1126-32. [DOI] [PubMed] [Google Scholar]
- 22.Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA 1998;280:1747-51. [DOI] [PubMed] [Google Scholar]
- 23.Barr H.High-grade dysplasia in Barrett’s oesophagus. The case against oesophageal resection. Ann R Coll Surg Engl 2007;89:586-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein HJ, Feith M, Bruecher BL, et al. Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg 2005;242:566-73; discussion 573-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leers JM, Demeester SR, Oezcelik A, et al. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann Surg 2011;253:271-8. [DOI] [PubMed] [Google Scholar]
- 26.Barbour AP, Jones M, Brown I, et al. Risk stratification for early esophageal adenocarcinoma: analysis of lymphatic spread and prognostic factors. Ann Surg Oncol 2010;17:2494-502. [DOI] [PubMed] [Google Scholar]
- 27.Sharma VK, Jae Kim H, Das A, et al. Circumferential and focal ablation of Barrett’s esophagus containing dysplasia. Am J Gastroenterol 2009;104:310-7. [DOI] [PubMed] [Google Scholar]
- 28.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med 2009;360:2277-88. [DOI] [PubMed] [Google Scholar]
- 29.Pech O, Gossner L, May A, et al. Long-term results of photodynamic therapy with 5-aminolevulinic acid for superficial Barrett’s cancer and high-grade intraepithelial neoplasia. Gastrointest Endosc 2005;62:24-30. [DOI] [PubMed] [Google Scholar]
- 30.Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology 2011;140:e18-52; quiz e13. [DOI] [PMC free article] [PubMed]
- 31.Seewald S, Akaraviputh T, Seitz U, et al. Circumferential EMR and complete removal of Barrett’s epithelium: a new approach to management of Barrett’s esophagus containing high-grade intraepithelial neoplasia and intramucosal carcinoma. Gastrointest Endosc 2003;57:854-9. [DOI] [PubMed] [Google Scholar]
- 32.Peters FP, Kara MA, Rosmolen WD, et al. Stepwise radical endoscopic resection is effective for complete removal of Barrett’s esophagus with early neoplasia: a prospective study. Am J Gastroenterol 2006;101:1449-57. [DOI] [PubMed] [Google Scholar]
- 33.Chennat J, Konda VJ, Ross AS, et al. Complete Barrett’s eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma--an American single-center experience. Am J Gastroenterol 2009;104:2684-92. [DOI] [PubMed] [Google Scholar]
- 34.Ell C, May A, Gossner L, et al. Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett’s esophagus. Gastroenterology 2000;118:670-7. [DOI] [PubMed] [Google Scholar]
- 35.Peters FP, Kara MA, Rosmolen WD, et al. Endoscopic treatment of high-grade dysplasia and early stage cancer in Barrett’s esophagus. Gastrointest Endosc 2005;61:506-14. [DOI] [PubMed] [Google Scholar]
- 36.Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer). Gastrointest Endosc 2007;65:3-10. [DOI] [PubMed] [Google Scholar]
- 37.Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut 2008;57:1200-6. [DOI] [PubMed] [Google Scholar]
- 38.May A, Gossner L, Pech O, et al. Local endoscopic therapy for intraepithelial high-grade neoplasia and early adenocarcinoma in Barrett’s oesophagus: acute-phase and intermediate results of a new treatment approach. Eur J Gastroenterol Hepatol 2002;14:1085-91. [DOI] [PubMed] [Google Scholar]
- 39.Behrens A, May A, Gossner L, et al. Curative treatment for high-grade intraepithelial neoplasia in Barrett’s esophagus. Endoscopy 2005;37:999-1005. [DOI] [PubMed] [Google Scholar]
- 40.Ishihara R, Iishi H, Uedo N, et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc 2008;68:1066-72. [DOI] [PubMed] [Google Scholar]
- 41.Oka S, Tanaka S, Kaneko I, et al. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc 2006;64:877-83. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi H, Arimura Y, Masao H, et al. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video) Gastrointest Endosc 2010;72:255-64, 264.e1. [DOI] [PubMed]
- 43.Cao Y, Liao C, Tan A, et al. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy 2009;41:751-7. [DOI] [PubMed] [Google Scholar]
- 44.Ono S, Fujishiro M, Niimi K, et al. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy 2009;41:661-5. [DOI] [PubMed] [Google Scholar]
- 45.Hashimoto S, Kobayashi M, Takeuchi M, et al. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest Endosc 2011;74:1389-93. [DOI] [PubMed] [Google Scholar]
- 46.Hanaoka N, Ishihara R, Takeuchi Y, et al. Intralesional steroid injection to prevent stricture after endoscopic submucosal dissection for esophageal cancer: a controlled prospective study. Endoscopy 2012;44:1007-11. [DOI] [PubMed] [Google Scholar]
- 47.Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc 2011;73:1115-21. [DOI] [PubMed] [Google Scholar]
- 48.Hoteya S, Matsui A, Iizuka T, et al. Comparison of the clinicopathological characteristics and results of endoscopic submucosal dissection for esophagogastric junction and non-junctional cancers. Digestion 2013;87:29-33. [DOI] [PubMed] [Google Scholar]
- 49.Ikeda K, Isomoto H, Oda H, et al. Endoscopic submucosal dissection of a minute intramucosal adenocarcinoma in Barrett’s esophagus. Dig Endosc 2009;21:34-6. [DOI] [PubMed] [Google Scholar]
- 50.Yoshinaga S, Gotoda T, Kusano C, et al. Clinical impact of endoscopic submucosal dissection for superficial adenocarcinoma located at the esophagogastric junction. Gastrointest Endosc 2008;67:202-9. [DOI] [PubMed] [Google Scholar]
- 51.Hirasawa K, Kokawa A, Oka H, et al. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc 2010;72:960-6. [DOI] [PubMed] [Google Scholar]
- 52.Kakushima N, Yahagi N, Fujishiro M, et al. Efficacy and safety of endoscopic submucosal dissection for tumors of the esophagogastric junction. Endoscopy 2006;38:170-4. [DOI] [PubMed] [Google Scholar]
- 53.Omae M, Fujisaki J, Horiuchi Y, et al. Safety, efficacy, and long-term outcomes for endoscopic submucosal dissection of early esophagogastric junction cancer. Gastric Cancer 2013;16:147-54. [DOI] [PubMed] [Google Scholar]
- 54.Hayashi S, Aoki H, Yano M, et al. Case series of Barrett’s esophageal cancer treated by endoscopic submucosal dissection. Journal of Tokushima prefectural central hospital 2013;34:53-8.
- 55.Fumizono U, Fujisaki J, Imai M, et al. Five cases of Barrett’s esophageal cancer treated by endoscopic submucosal dissection. Progress of Digestive Endoscopy 2010;77:31-4 [Google Scholar]
- 56.Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett’s esophagus. Gastroenterology 2009;137:815-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pech O, Bollschweiler E, Manner H, et al. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett’s esophagus at two high-volume centers. Ann Surg 2011;254:67-72. [DOI] [PubMed] [Google Scholar]
- 58.Das A, Singh V, Fleischer DE, et al. A comparison of endoscopic treatment and surgery in early esophageal cancer: an analysis of surveillance epidemiology and end results data. Am J Gastroenterol 2008;103:1340-5. [DOI] [PubMed] [Google Scholar]


