Abstract
Vitamin D synthesis and signaling affects numerous cellular processes including: proliferation, differentiation and apoptosis. It is now commonly recognized that low levels of vitamin D are associated with a greater risk of tumorigenesis. Cancers of the gastrointestinal tract are most often difficult to diagnose and treat as patients typically present with progressed disease. Basic research, clinical trials and population studies have supported the concept that treatment with Vitamin D may be a therapeutic option when treating GI cancers, however treatments must be individualized and monitored closely as the side effects from Vitamin D treatment can be increasingly harmful. This review will highlight the most recent findings regarding Vitamin D signaling and GI cancers.
Keywords: Vitamin D, cancer, signaling, enzymes, esophagus, stomach, pancreas, liver, colon
Vitamin D synthesis
Vitamin D exists in two major forms: vitamin D2 (ergocalciferol) and D3 (cholecalciferol) and can be obtained from diet or supplements (1-3). Besides from food and supplements, vitamin D is also derived from sunlight. This process involves the conversion of 7-dehydrocholesterol in the skin after exposure to sunlight (UV-B light). Both D2 and D3 forms of vitamin D utilize vitamin D binding proteins (VDBP) in the bloodstream to reach the liver where they are converted to 25(OH)D by the enzyme, 25-hydroxylase (1-3). The biologically active form of vitamin D [1,25(OH)2D3 (referred to as vitamin D3 throughout this review) is synthesized in the kidney by 1a-hydroxylase (CYP27B1) and is metabolized by the enzyme, 24 hydroxylase (CYP24A1)], that limits calcitriol actions via catabolism. Renal CYP27B1 gene expression is regulated and activated by the parathyroid hormone. After its synthesis, vitamin D3 is released into the serum and can act on the intestine, bone and kidney to regulate calcium metabolism (2,3). CYP27B1 and CYP24A1 are found in numerous tissues throughout the body including the skin, colon, pancreas, liver, brain and placenta allowing for vitamin D3 synthesis and degradation. Vitamin D3 is thought to be the metabolite responsible for the cellular anticancer actions of vitamin D (2,3).
Vitamin D receptor signaling
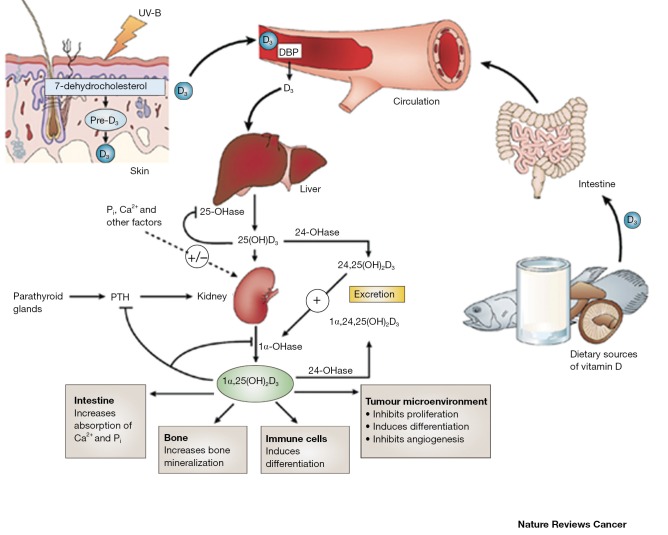
Vitamin D3 binds to the vitamin D receptor (VDR). Vitamin D3 and VDR form a heterodimer with the retinoid X receptor (RXR) and bind to the vitamin D responsive element on the respective responsive gene (1-3). After binding, transcription and translation occur leading to protein formation, for example the formation of the calcium binding protein or osteocalcin. Classically, vitamin D3 enters the cell through membrane proteins. For example, in intestinal cells, vitamin D3 binds to VDR synthesizing the calcium binding protein, which can regulate transport through the cell (2). While the VDR is predominantly a nuclear protein, it has been found in the cytoplasm of vitamin D3 target cells. The interaction between RXR and VDR is essential for VDR transcriptional activity (2,3). Vitamin D-response elements (VDREs) are also utilized to initiate gene transcription. The RXR-VDR complex recruits specific coactivator molecules like steroid receptor coactivators, histone acetyltransferases and the mediator complex subunit 1. The VDR-RXR complex translocates to the nucleus binding to VDREs that allows for promotion or suppression of specific cellular events, including tumorigenesis (1-3). Figure 1 depicts the metabolism/catabolism and signaling pathway of vitamin D3 (reprinted with permission from Nature Reviews Cancer 2007;7:684-700).
Figure 1.
Photochemical synthesis of vitamin D3 (cholecalciferol, D3) occurs cutaneously where pro-vitamin D3 (7-dehydrocholesterol) is converted to pre-vitamin D3 (pre-D3) in response to ultraviolet B (sunlight) exposure. Vitamin D3, obtained from the isomerization of pre-vitamin D3 in the epidermal basal layers or intestinal absorption of natural and fortified foods and supplements, binds to vitamin D-binding protein (DBP) in the bloodstream, and is transported to the liver. D3 is hydroxylated by liver 25-hydroxylases (25-OHase). The resultant 25-hydroxycholecalciferol [25(OH)D3] is 1α-hydroxylated in the kidney by 25-hydroxyvitamin D3-1α-hydroxylase (1α-OHase). This yields the active secosteroid 1α,25(OH)2D3 (calcitriol), which has different effects on various target tissues. The synthesis of 1α,25(OH)2D3 from 25(OH)D3 is stimulated by parathyroid hormone (PTH) and suppressed by Ca2+, Pi and 1α,25(OH)2D3 itself. The rate-limiting step in catabolism is the degradation of 25(OH)D3 and 1α,25(OH)2D3 to 24,25(OH)D3 and 1α,24,25(OH)2D3, respectively, which occurs through 24-hydroxylation by 25-hydroxyvitamin D 24-hydroxylase (24-OHase), encoded by the CYP24A1 gene. 24,25(OH)D3 and 1α,24,25(OH)2D3 are consequently excreted [reprinted with permission from Nature Reviews Cancer 2007;7:684-700].
Vitamin D and cancer
The traditional role of vitamin D has been centered on the control of calcium and bone metabolism, however, recent studies have begun to elucidate the role that vitamin D plays in the development and progression of numerous cancers. Because vitamin D is known to participate in cell cycle regulation, cellular proliferation and apoptosis, angiogenesis and molecular cell signaling it stands to reason that this metabolite is involved in tumorigenic activity (1-3). It has been found that low serum vitamin D3 levels are associated with increased cancers of the breast (4), colon (5) and prostate (6) and animals lacking VDR or with severe vitamin D deficiency are prone to increased tumorigenesis (7,8). In our review we will focus on the recently examined (within the last 5-10 years) role of vitamin D3 in the cancers of the GI tract including: esophagus, gastric (stomach), liver, pancreas and colon.
Esophageal cancer
Cancer of the esophagus (esophageal cancer) is a relatively rare form of cancer that is characterized by two types: adenocarcinoma and squamous cell carcinoma. It has been shown that heavy smoking or alcohol use increases the incidence of squamous cell esophageal cancer (9). Typically the diagnosis, treatment and outcome for patients afflicted with esophageal cancers are difficult and grim. The majority of patients present in advanced stages of the disease with dysphagia (difficulty swallowing) being the main cause for seeking medical care. The five-year survival rate is approximately 15%, however, most patients do not survive the first year after diagnosis (10). Treatments depend upon the stage at which the diagnosis is made and may include esophagectomy, radiotherapy, and chemotherapy or laser photodynamic therapy (9). No reliable markers have been discovered for the detection of esophageal cancer, however, some studies suggest that vitamin D and VDR may allow for earlier detection or alternative therapeutic strategies. The majority of studies that examine the role that vitamin D may alter esophageal cancer are primarily done in specific population cohorts (11,12). Findings from these studies have been inconclusive and controversial. For example, in a study by Trowbridge et al. the authors found that in human esophageal adenocarcinomas VDR expression declined with tumor de-differentiation. The Authors also found that VDR translocated out to the cytoplasm during neoplastic transformation (13). While the Authors postulate that VDR expression may serve as a marker for neoadjuvant therapy they also disclose that these findings maybe aberrant and should be confirmed (13). A cohort study from Italy has found that increased dietary vitamin D intake (>3.5 mg/day) reduced the risk of esophageal cancer by ~40% suggesting a protective role for vitamin D in this cancer (14). In contrast, a study in China found that increased vitamin D serum levels predicted an increase in squamous dysplasia, the precursor for esophageal squamous cell carcinoma (15). The primary precursor to esophageal adenocarcinoma is Barrett’s Esophagus and it has been found that VDR expression is upregulated in Barrett’s mucosa when compared to the normal squamous epithelium of the esophagus (16) although no conclusive evidence regarding the definite role of vitamin D could be demonstrated. With regards to the role that might be played by the enzymes regulating vitamin D synthesis, a study found that overexpression of CYP24A1 coupled with low VDR expression is indicative of poor prognosis of esophageal cancer (17). These studies have provided preliminary insight into the potential impact that vitamin D may have on this GI cancer.
Gastric (stomach) cancer
Gastric cancer includes any cancer arising from any part of the stomach and is the fourth most common cancer worldwide (18). This cancer causes approximately 800,000 deaths worldwide and incurs poor prognosis, as most patients will present with advanced disease (18). The majority of gastric cancers arise from Helicobacter pylori infection, however, there are also dietary risks associated with gastric cancer. Smoking has also been found to significantly increase the risk of developing gastric cancer as well as alcohol consumption (19). Surgery is the most common treatment for stomach cancer wherein part or all of the stomach may be removed. Chemotherapy and radiotherapy are also utilized, however, there is no established standard of care (20). Similar to esophageal cancer, there are numerous studies linking vitamin D to gastric cancer, however, there is also confusion on the exact role and mechanism of vitamin D during tumorigenesis. A recent cohort study found that increased serum vitamin D levels were associated with decreased risk of gastric cancer and poor prognosis (21). It is also been shown that paricalcitol (an analog to calcitriol) suppresses the growth of gastric cancer cells by regulating cell cycle, apoptosis and inflammation without inducing the hypercalcemia effects seen by calcitriol (22). Bao et al. found that direct usage of 1,25-dihydroxyvitamin D3 induces cellular apoptosis in gastric cancer cells and also increased the expression of VDR and CYP24A1 (23) further supporting the anti-tumoral role that vitamin D may activate in gastric cancer. Evidence that vitamin D3 may act through the hedgehog signaling pathway has also been demonstrated in gastric cancer cells. In this study, treatment with vitamin D3 decreased cell viability by the inhibition of the expression of numerous hedgehog signaling target genes including patched1 and Gli1 in gastric cancer cells (24). A synergistic effect by vitamin D3 was also seen when it was combined with different chemotherapeutic drugs including paclitaxel (24). Finally, in an early study, treatment with vitamin D3 was found to induce chemopreventative effects in rats with glandular stomach cancer by reducing both cancerous and precancerous lesions in the stomach (25). Taken together, these studies have laid important groundwork to continue the efforts in understanding the role of vitamin D in gastric cancers.
Pancreatic cancer
Cancer of the pancreas is a malignant neoplasm that arises from transformed cells of the pancreas. Adenocarcinoma arising within the exocrine component of the pancreas is the most common type of pancreatic cancer. It is the fourth most common cause of cancer-related deaths in the United States and eighth worldwide. The prognosis is very grim for patients that incur this disease. The 1-5-year survival rates are 25% and 6%, respectively and the median survival rate for advanced or metastatic pancreatic cancer is approximately 6-10 months. There are numerous risk factors related to pancreatic cancer including smoking, obesity, chronic pancreatitis and H. pylori infection. Similar to gastric cancer, surgery, radiation and chemotherapy treatments may all be utilized to offer care, however, treatments are dependent upon stage of cancer. Much work has been performed regarding vitamin D and pancreatic tumor progression. It has been demonstrated that the enzyme that catalyzes the conversion of 25(OH)D to 1,25-dihydroxy vitamin D is expressed in ductal cells in the pancreas in both normal and adenocarcinoma tissues and treatment with vitamin D3 decreases the growth of pancreatic cells in vitro (26). The vitamin D receptor, VDR, has been detected in numerous pancreatic cell lines and its expression is greatly decreased when compared to normal pancreatic cells (27). Further, treatment with a vitamin D analog, EB1089, induced a significant decrease in pancreatic cancer cell lines (27). Yu et al. demonstrated that calcitriol, in combination with the chemotherapy drug, gemcitabine increases caspase-dependent apoptosis of human pancreatic cancer cells both in vitro and in vivo (28). In a large cohort study it was found that increased plasma 25-hydroxyvitamin D levels are associated with a decreased risk for pancreatic cancer (29). This study was performed in 451 cases of pancreatic cancer along with over 1,000 controls and provides strong human data supporting the concept that lower vitamin D levels may increase susceptibility to developing pancreatic cancer especially when coupled with any of the known risk factors. In addition, there have been multiple cohort studies demonstrating that increasing serum vitamin D levels are coupled with decreased pancreatic risk, both in men and women (30-33). Interestingly, the link between obesity and vitamin D may offer some insight into pancreatic tumor development and progression. Vitamin D is involved in the regulation of insulin synthesis, binding and response, making insulin regulation directly relevant to pancreatic carcinogenesis since diabetes is also a known risk factor for this cancer (34,35). Evidence shows that low levels of vitamin D may increase the risk of cancer (including pancreatic) in people with diabetes (36). Similar to gastric cancer, vitamin D may act through the hedgehog-signaling pathway to inhibit pancreatic cancer cell growth by inactivating the receptor Smoothened, in vitro, however this effect was not recapitulated when in vivo models of vitamin D3 treatment were used (37). There has been a causal link demonstrated to link vitamin D deficiency and pancreatic disease, including cancer. Kalpdor et al. found that in patients with pancreatic disease, serum vitamin D levels were significantly lower than those of controls, whereas both vitamin A and E levels were normal in all samples (38). Supplementation with vitamin D (dosed dependent upon disease stage) increased circulating vitamin D levels. Most studies stress the importance of monitoring serum vitamin D levels closely when used for treatments, as there can be other unwanted side effects from an overload of vitamin D (39-43). Because the sun is the main source for vitamin D production, treatment with sun exposure to increase serum vitamin D levels may appear to be a natural therapy, but it may not be without risk. A study from Sweden demonstrated that there is an increased risk of developing basal cell carcinoma from prolonged and repetitive sunlight exposure that has no weighted benefit in preventing internal cancers, including pancreatic (44). Clinical studies have examined the potential added benefit of calcitriol to chemotherapy treatments. Blanke et al. demonstrated that out of 25 patients, three had a partial response and seven reached a stable disease state when given a combination of calcitriol and docetaxel (45). The median overall survival was 24 weeks and side effects were likely due to the chemotherapy drug and not calcitriol. While this is a modest effect, it warrants further studies to determine the benefits of vitamin D on pancreatic tumors. An early study using the vitamin D3 analog, EB1089 suggested that, in patients with minimal stage disease, there was an increase in survival time and the duration of stable disease increased for a small number of patients, however advanced pancreatic tumors were not sensitized to this treatment (46). In summary, the investigation of the beneficial effects of vitamin D in pancreatic cancer remains unclear. Most studies support the concept that low levels of vitamin D can result in an increased risk of developing pancreatic cancer especially when coupled with a known risk like diabetes or obesity. Treatment with vitamin D (or one of its many analogs) must be monitored and specific to the patient and stage of disease. However, there may be promising therapies for patients suffering from pancreatic tumors within our reach.
Liver cancer
Most cancers found in the liver are metastatic tumors derived from other organs including breast, colon, lung, and kidney. The two primary cancers that arise from cells within the liver are hepatocellular carcinoma (arising from hepatocytes, HCC) and cholangiocarcinoma (CCH, derived from cholangiocytes, cells that line the bile ducts) (47-49). Hepatocellular carcinoma is a primary tumor of the liver that typically results from viral hepatitis infections or cirrhosis (47). Cholangiocarcinoma typically is the result of bile duct damage from diseases like primary sclerosing cholangitis (48,49). Both HCC and CCH are relatively rare; however liver cancer is the third leading cause of cancer death. Hepatocellular and cholangiocarcinomas are difficult to diagnose and typically are well advanced when found. The standard of care includes liver transplantation, partial hepatectomy (to resect tumors when possible), chemotherapy and radiotherapy (47-49). Like most GI cancers, the response to these treatments is minimal and dependent upon the stage of the disease. Numerous studies have been performed and are underway to determine the most beneficial treatment strategies for these cancers and this also involves the investigation of vitamin D on both CCH and HCC. Several studies have shown that both HCC and CCH express high levels of CYP24A1 (50,51). An increase in CYP24A1 can lead to lower levels of vitamin D thereby allowing for tumor growth. In these studies, treatment with vitamin D3 decreased the proliferative rate in numerous HCC and CCH cell lines (50,51). VDR is expressed in cholangiocarcinoma, increasing in expression during the development of CCH, whereas the expression of VDR in normal tissue is negligible (51,52). Figure 2 depicts the expression of VDR, CYP24A1 and CYP27B1 in non-malignant and malignant tumor biopsies from human patient biopsies (reprinted with permission from Elsevier, Digestive and Liver Disease. Kennedy et al. Dysregulation of vitamin D3 synthesis leads to enhanced cholangiocarcinoma growth. 2013;45:316-22). The expression of both VDR and the degrading enzyme are significantly increased compared to non-malignant tissues (Figure 2). In the study by Baek et al. they found that there was a synergistic effect when vitamin D3 was combined with anti-cancer drugs decreasing cholangiocarcinoma cell proliferation (24). Besides using vitamin D3 to reduce cholangiocarcinoma growth, the use of analogs has also proven to be effective. Stimulation with 22-0xa-D3 induced cell cycle arrest of cholangiocarcinoma cell lines and also significantly inhibited tumor growth in CCH-inoculated mice without inducing hypercalcemia (53). Cellular apoptosis was also found in tissue samples from patients with CCH. These studies, though few, demonstrate the importance of examining the role of vitamin D in these malignancies. There are several numerous serum markers to aid in the detection of HCC. A recent study demonstrates that adding vitamin D levels to this list may be of help for physicians when diagnosing hepatocellular carcinoma as there was a significant decrease in vitamin D levels associated with progressive HCC (54). Because vitamin D can exert antiproliferative, pro-differentiation and pro-apoptosis effects on cancer cells that express VDR, the concept that vitamin D can aid in therapeutic development for HCC is also plausible. Numerous studies by Pourgholami et al., have demonstrated that treatment with vitamin D decreases HCC proliferation in vitro and in vivo (55,56). They further looked at the role of vitamin D analogs including EB1089 and CB1093 and found a decrease of growth in hepatoblastoma cell lines and using in vivo models of HCC (55,57,58). Because of the toxicity and hypercalcemic effects of treatment with vitamin D, using analogs appears to be a much better approach to treat these cancers. Finally, a group reports that combining vitamin D with fish oil increases the antiproliferative on human hepatoblastoma cells suggesting that delivery of vitamin D may also have beneficial effects on HCC (59).
Figure 2.
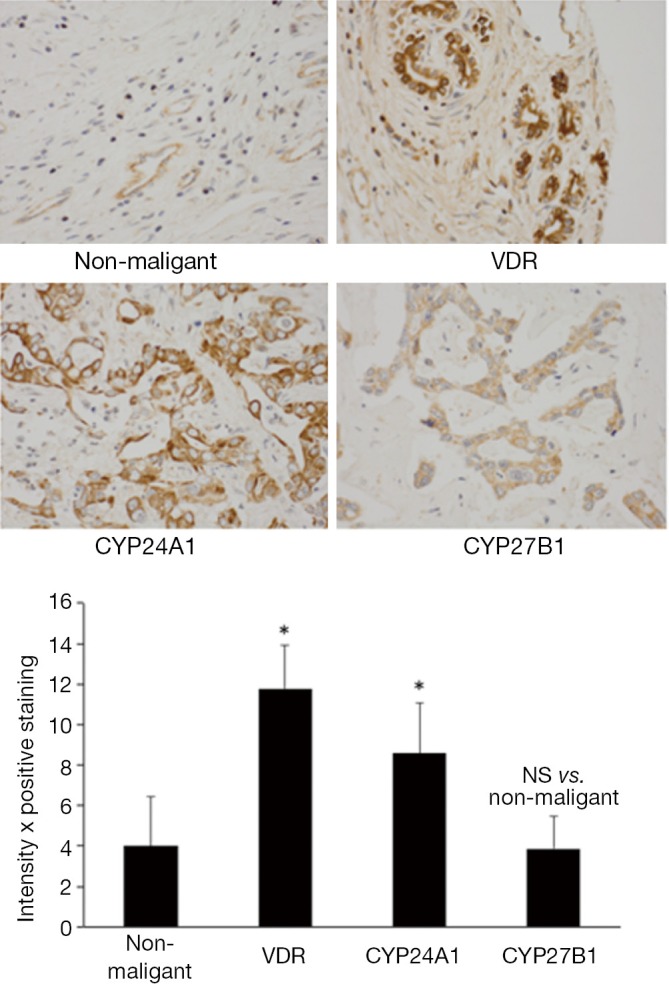
Immunohistochemistry was performed in human cholangiocarcinoma biopsy samples and compared to non-malignant controls. The protein expression of VDR and CYP24A1 are significantly increased in malignant biopsies compared to non-malignant, whereas CYP27B1 expression is decreased (*P<0.05 vs. non-malignant). Data are mean ± SE of 10-blinded evaluations of 10 randomly selected fields of three slides. Original magnification ×40 (reprinted with permission from Elsevier, Digestive and Liver Disease. Kennedy et al. Dysregulation of vitamin D3 synthesis leads to enhanced cholangiocarcinoma growth 2013;45:316-22).
Colon cancer
Finally, we examine the role of vitamin D in colon cancer. Colorectal cancer (CRC) is defined as cancer that arises from the color or rectum (parts of the large intestine) or in the appendix. CRC results in about 0.5 million deaths per year and is the second most common cause of cancer in women and third in men. Unlike a number of the other GI cancers, CRC is more common in developed countries rather than underdeveloped countries. Symptoms of CRC include increased worsening constipation, bloody stool, weight loss and loss of appetite. These symptoms increase with increasing age. Treatment strategies for CRC include surgery, chemotherapy, radiation and palliative care. Preventative care can significantly reduce the likelihood of developing CRC. Numerous studies involving vitamin D and CRC have been reported demonstrating a link between the two. It has been found that during CRC tumor progression, the regulating enzymes that metabolize and catabolize vitamin D are under epigenetic regulation and vitamin D (via VDR) regulates proliferation, differentiation and apoptosis in an autocrine fashion within colonic epithelium (60). An interesting study from Kaler et al. found that vitamin D3 can enhance crosstalk between tumor epithelial cells and the microenvironment suggesting there is also a paracrine regulation by vitamin D (61). More recent work from this group has also found that treatment with vitamin D increases the sensitivity of tumor cells to TRAIL-induced cellular apoptosis (62). CYP24A1 expression was highly increased in adenocarcinomas from human patient samples when compared to normal colon mucosa and the increase in CYP24A1 was correlated with an increase in the proliferative marker, Ki-67 suggesting that degradation of vitamin D allows for tumor progression (63). The authors demonstrate that vitamin D disrupts the activation of STAT1 signaling and the production of Il-1beta in macrophages rendering them unable to activate the Wnt signaling pathway in colon carcinoma cells (61). VDR overexpression has also been found in CRC and was associated with PI3K-AKT pathway and KRAS mutations (64). Besides this finding, another study found that vitamin D actions are mediated via the TLR4 pathway in models of inflammatory bowel disease-induced CRC (65). To add more confusion, a recent study has shown that VDR activation may be dependent upon phosphatidylinositol 5-phosphate r-kinase type II beta (PIPKIIbeta) (66). In vivo, loss of VDR (VDR-/-) in a model of intestinal tumor development (adenomatous polyposis coli (APC) knockout mice), increased tumor burden and the size of tumors compared to APC-/- alone providing strong proof that loss of VDR activation increases CRC tumorigenesis (67). Using a different model of CRC, Aberrant crypt Foci (ACF-/-) crossed with VDR-/-, it was demonstrated that loss of VDR again increased tumor size and enhanced the Wnt/b-catenin pathway (68). Regardless of the pathway, it’s clear that VDR is critical to regulate vitamin D effects on CRC. Like other GI cancers, treatment with vitamin D for CRC should be monitored closely and perhaps even personalized to the individual’s genetic make-up. A recent study found that certain polymorphisms in CYP24A1 and CYP27B1 can regulate and alter vitamin D metabolism in colon cancer (69) and supports the concept of further studies to determine the exact mechanism of vitamin D effects in CRC.
Conclusions
In summary, the work regarding the role of vitamin D in GI cancers is growing and there is potential for therapeutic treatments to be established. However, there must be caution taken when treating with vitamin D and possible personalized treatments may be options for patients suffering from GI malignancies. Serum levels of vitamin D may be a useful biomarker for physicians, but this should also be taken into account with patient diet and sunlight exposure, as both of these are key regulators of vitamin D production. With a growing population of obese patients, vitamin D metabolism is at risk and therefore, the patients risk for developing GI cancers is also increased.
Acknowledgements
This work was partly supported by internal funds from Scott & White Healthcare.
Disclosure: The authors declare no conflict of interest.
References
- 1.Fleet JC, DeSmet M, Johnson R, et al. Vitamin D and cancer: a review of molecular mechanisms. Biochem J 2012;441:61-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lips P.Vitamin D physiology. Prog Biophys Mol Biol 2006;92:4-8. [DOI] [PubMed] [Google Scholar]
- 3.Picotto G, Liaudat AC, Bohl L, et al. Molecular aspects of vitamin D anticancer activity. Cancer Invest 2012;30:604-14. [DOI] [PubMed] [Google Scholar]
- 4.Engel P, Fagherazzi G, Boutten A, et al. Serum 25(OH) vitamin D and risk of breast cancer: a nested case-control study from the French E3N cohort. Cancer Epidemiol Biomarkers Prev 2010;19:2341-50. [DOI] [PubMed] [Google Scholar]
- 5.Garland CF, Comstock GW, Garland FC, et al. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet 1989;2:1176-8. [DOI] [PubMed] [Google Scholar]
- 6.Ahonen MH, Tenkanen L, Teppo L, et al. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland). Cancer Causes Control 2000;11:847-52. [DOI] [PubMed] [Google Scholar]
- 7.Llor X, Jacoby RF, Teng BB, et al. K-ras mutations in 1,2-dimethylhydrazine-induced colonic tumors: effects of supplemental dietary calcium and vitamin D deficiency. Cancer Res 1991;51:4305-9. [PubMed] [Google Scholar]
- 8.Sitrin MD, Halline AG, Abrahams C, et al. Dietary calcium and vitamin D modulate 1,2-dimethylhydrazine-induced colonic carcinogenesis in the rat. Cancer Res 1991;51:5608-13. [PubMed] [Google Scholar]
- 9.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [DOI] [PubMed] [Google Scholar]
- 10.Polednak AP. Trends in survival for both histologic types of esophageal cancer in US surveillance, epidemiology and end results areas. Int J Cancer 2003;105:98-100. [DOI] [PubMed] [Google Scholar]
- 11.Chang CK, Mulholland HG, Cantwell MM, et al. Vitamin d receptor gene variants and esophageal adenocarcinoma risk: a population-based case-control study. J Gastrointest Cancer 2012;43:512-7. [DOI] [PubMed] [Google Scholar]
- 12.Mulholland HG, Murray LJ, Anderson LA, et al. Vitamin D, calcium and dairy intake, and risk of oesophageal adenocarcinoma and its precursor conditions. Br J Nutr 2011;106:732-41. [DOI] [PubMed] [Google Scholar]
- 13.Trowbridge R, Sharma P, Hunter WJ, et al. Vitamin D receptor expression and neoadjuvant therapy in esophageal adenocarcinoma. Exp Mol Pathol 2012;93:147-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipworth L, Rossi M, McLaughlin JK, et al. Dietary vitamin D and cancers of the oral cavity and esophagus. Ann Oncol 2009;20:1576-81. [DOI] [PubMed] [Google Scholar]
- 15.Abnet CC, Chen W, Dawsey SM, et al. Serum 25(OH)-vitamin D concentration and risk of esophageal squamous dysplasia. Cancer Epidemiol Biomarkers Prev 2007;16:1889-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trowbridge R, Mittal SK, Sharma P, et al. Vitamin D receptor expression in the mucosal tissue at the gastroesophageal junction. Exp Mol Pathol 2012;93:246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mimori K, Tanaka Y, Yoshinaga K, et al. Clinical significance of the overexpression of the candidate oncogene CYP24 in esophageal cancer. Ann Oncol 2004;15:236-41. [DOI] [PubMed] [Google Scholar]
- 18.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [DOI] [PubMed] [Google Scholar]
- 19.Nomura A, Grove JS, Stemmermann GN, et al. Cigarette smoking and stomach cancer. Cancer Res 1990;50:7084. [PubMed] [Google Scholar]
- 20.Scartozzi M, Galizia E, Verdecchia L, et al. Chemotherapy for advanced gastric cancer: across the years for a standard of care. Expert Opin Pharmacother 2007;8:797-808. [DOI] [PubMed] [Google Scholar]
- 21.Ren C, Qiu MZ, Wang DS, et al. Prognostic effects of 25-hydroxyvitamin D levels in gastric cancer. J Transl Med 2012;10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park MR, Lee JH, Park MS, et al. Suppressive effect of 19-nor-1α-25-dihydroxyvitamin D2 on gastric cancer cells and peritoneal metastasis model. J Korean Med Sci 2012;27:1037-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bao A, Li Y, Tong Y, et al. Tumor-suppressive effects of 1,25-dihydroxyvitamin D3 in gastric cancer cells. Hepatogastroenterology 2013;60:943-8. [DOI] [PubMed] [Google Scholar]
- 24.Baek S, Lee YS, Shim HE, et al. Vitamin D3 regulates cell viability in gastric cancer and cholangiocarcinoma. Anat Cell Biol 2011;44:204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikezaki S, Nishikawa A, Furukawa F, et al. Chemopreventive effects of 24R,25-dihydroxyvitamin D3, a vitamin D3 derivative, on glandular stomach carcinogenesis induced in rats by N-methyl-N'-nitro-N-nitrosoguanidine and sodium chloride. Cancer Res 1996;56:2767-70. [PubMed] [Google Scholar]
- 26.Schwartz GG, Eads D, Rao A, et al. Pancreatic cancer cells express 25-hydroxyvitamin D-1 alpha-hydroxylase and their proliferation is inhibited by the prohormone 25-hydroxyvitamin D3. Carcinogenesis 2004;25:1015-26. [DOI] [PubMed] [Google Scholar]
- 27.Albrechtsson E, Jonsson T, Möller S, et al. Vitamin D receptor is expressed in pancreatic cancer cells and a vitamin D3 analogue decreases cell number. Pancreatology 2003;3:41-6. [DOI] [PubMed] [Google Scholar]
- 28.Yu WD, Ma Y, Flynn G, et al. Calcitriol enhances gemcitabine anti-tumor activity in vitro and in vivo by promoting apoptosis in a human pancreatic carcinoma model system. Cell Cycle 2010;9:3022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolpin BM, Ng K, Bao Y, et al. Plasma 25-hydroxyvitamin D and risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev 2012;21:82-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bao Y, Ng K, Wolpin BM, et al. Predicted vitamin D status and pancreatic cancer risk in two prospective cohort studies. Br J Cancer 2010;102:1422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zablotska LB, Gong Z, Wang F, et al. Vitamin D, calcium, and retinol intake, and pancreatic cancer in a population-based case-control study in the San Francisco Bay area. Cancer Causes Control 2011;22:91-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallicchio L, Helzlsouer KJ, Chow WH, et al. Circulating 25-hydroxyvitamin D and the risk of rarer cancers: Design and methods of the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol 2010;172:10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skinner HG, Michaud DS, Giovannucci E, et al. Vitamin D intake and the risk for pancreatic cancer in two cohort studies. Cancer Epidemiol Biomarkers Prev 2006;15:1688-95. [DOI] [PubMed] [Google Scholar]
- 34.Maestro B, Dávila N, Carranza MC, et al. Identification of a Vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol 2003;84:223-30. [DOI] [PubMed] [Google Scholar]
- 35.Stolzenberg-Solomon RZ, Graubard BI, Chari S, et al. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA 2005;294:2872-8. [DOI] [PubMed] [Google Scholar]
- 36.Ahmad S, Chowdhury TA, Boucher BJ. Diabetes and cancer: Could vitamin D provide the link? J Diabetes Complications 2013;27:184-90. [DOI] [PubMed] [Google Scholar]
- 37.Brüggemann LW, Queiroz KC, Zamani K, et al. Assessing the efficacy of the hedgehog pathway inhibitor vitamin D3 in a murine xenograft model for pancreatic cancer. Cancer Biol Ther 2010;10:79-88. [DOI] [PubMed] [Google Scholar]
- 38.Klapdor S, Richter E, Klapdor R.Vitamin D status and per-oral vitamin D supplementation in patients suffering from chronic pancreatitis and pancreatic cancer disease. Anticancer Res 2012;32:1991-8. [PubMed] [Google Scholar]
- 39.Melamed ML, Manson JE. Vitamin D and cardiovascular disease and cancer: not too much and not too little? The need for clinical trials. Womens Health (Lond Engl) 2011;7:419-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vashi PG, Trukova K, Lammersfeld CA, et al. Impact of oral vitamin D supplementation on serum 25-hydroxyvitamin D levels in oncology. Nutr J 2010;9:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tuohimaa P, Lou YR. Optimal serum calcidiol concentration for cancer prevention. Anticancer Res 2012;32:373-81. [PubMed] [Google Scholar]
- 42.Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA, et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol 2010;172:81-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vieth R.How to optimize vitamin D supplementation to prevent cancer, based on cellular adaptation and hydroxylase enzymology. Anticancer Res 2009;29:3675-84. [PubMed] [Google Scholar]
- 44.Lindelöf B, Krynitz B, Ayoubi S, et al. Previous extensive sun exposure and subsequent vitamin D production in patients with basal cell carcinoma of the skin, has no protective effect on internal cancers. Eur J Cancer 2012;48:1154-8. [DOI] [PubMed] [Google Scholar]
- 45.Blanke CD, Beer TM, Todd K, et al. Phase II study of calcitriol-enhanced docetaxel in patients with previously untreated metastatic or locally advanced pancreatic cancer. Invest New Drugs 2009;27:374-8. [DOI] [PubMed] [Google Scholar]
- 46.Evans TR, Colston KW, Lofts FJ, et al. A phase II trial of the vitamin D analogue Seocalcitol (EB1089) in patients with inoperable pancreatic cancer. Br J Cancer 2002;86:680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907-17. [DOI] [PubMed] [Google Scholar]
- 48.Patel T.Worldwide trends in mortality from biliary tract malignancies. BMC Cancer 2002;2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology 2005;41:5-15. [DOI] [PubMed] [Google Scholar]
- 50.Horvath E, Lakatos P, Balla B, et al. Marked increase of CYP24A1 mRNA level in hepatocellular carcinoma cell lines following vitamin D administration. Anticancer Res 2012;32:4791-6. [PubMed] [Google Scholar]
- 51.Kennedy L, Baker K, Hodges K, et al. Dysregulation of vitamin D3 synthesis leads to enhanced cholangiocarcinoma growth. Dig Liver Dis 2013;45:316-22. [DOI] [PubMed] [Google Scholar]
- 52.Seubwai W, Wongkham C, Puapairoj A, et al. Overexpression of vitamin D receptor indicates a good prognosis for cholangiocarcinoma: implications for therapeutics. Cancer 2007;109:2497-505. [DOI] [PubMed] [Google Scholar]
- 53.Seubwai W, Wongkham C, Puapairoj A, et al. 22-oxa-1,25-dihydroxyvitamin D3 efficiently inhibits tumor growth in inoculated mice and primary histoculture of cholangiocarcinoma. Cancer 2010;116:5535-43. [DOI] [PubMed] [Google Scholar]
- 54.Hammad LN, Abdelraouf SM, Hassanein FS, et al. Circulating IL-6, IL-17 and vitamin D in hepatocellular carcinoma: potential biomarkers for a more favorable prognosis? J Immunotoxicol 2013;10:380-6. [DOI] [PubMed] [Google Scholar]
- 55.Ghous Z, Akhter J, Pourgholami MH, et al. Inhibition of hepatocellular cancer by EB1089: in vitro and in vivo study. Anticancer Res 2008;28:3757-61. [PubMed] [Google Scholar]
- 56.Pourgholami MH, Akhter J, Lu Y, et al. In vitro and in vivo inhibition of liver cancer cells by 1,25-dihydroxyvitamin D3. Cancer Lett 2000;151:97-102. [DOI] [PubMed] [Google Scholar]
- 57.Akhter J, Lu Y, Finlay I, et al. 1alpha,25-Dihydroxyvitamin D3 and its analogues, EB1089 and CB1093, profoundly inhibit the in vitro proliferation of the human hepatoblastoma cell line HepG2. ANZ J Surg 2001;71:414-7. [DOI] [PubMed] [Google Scholar]
- 58.Pourgholami MH, Morris DL. 1,25-Dihydroxyvitamin D3 in lipiodol for the treatment of hepatocellular carcinoma: cellular, animal and clinical studies. J Steroid Biochem Mol Biol 2004;89-90:513-8. [DOI] [PubMed] [Google Scholar]
- 59.Chiang KC, Persons KS, Istfan NW, et al. Fish oil enhances the antiproliferative effect of 1alpha,25-dihydroxyvitamin D3 on liver cancer cells. Anticancer Res 2009;29:3591-6. [PubMed] [Google Scholar]
- 60.Cross HS, Nittke T, Peterlik M. Modulation of vitamin D synthesis and catabolism in colorectal mucosa: a new target for cancer prevention. Anticancer Res 2009;29:3705-12. [PubMed] [Google Scholar]
- 61.Kaler P, Augenlicht L, Klampfer L.Macrophage-derived IL-1beta stimulates Wnt signaling and growth of colon cancer cells: a crosstalk interrupted by vitamin D3. Oncogene 2009;28:3892-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaler P, Galea V, Augenlicht L, et al. Tumor associated macrophages protect colon cancer cells from TRAIL-induced apoptosis through IL-1beta-dependent stabilization of Snail in tumor cells. PLoS One 2010;5:e11700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Horváth HC, Lakatos P, Kósa JP, et al. The candidate oncogene CYP24A1: A potential biomarker for colorectal tumorigenesis. J Histochem Cytochem 2010;58:277-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kure S, Nosho K, Baba Y, et al. Vitamin D receptor expression is associated with PIK3CA and KRAS mutations in colorectal cancer. Cancer Epidemiol Biomarkers Prev 2009;18:2765-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murillo G, Nagpal V, Tiwari N, et al. Actions of vitamin D are mediated by the TLR4 pathway in inflammation-induced colon cancer. J Steroid Biochem Mol Biol 2010;121:403-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kouchi Z, Fujiwara Y, Yamaguchi H, et al. Phosphatidylinositol 5-phosphate 4-kinase type II beta is required for vitamin D receptor-dependent E-cadherin expression in SW480 cells. Biochem Biophys Res Commun 2011;408:523-9. [DOI] [PubMed] [Google Scholar]
- 67.Zheng W, Wong KE, Zhang Z, et al. Inactivation of the vitamin D receptor in APC(min/+) mice reveals a critical role for the vitamin D receptor in intestinal tumor growth. Int J Cancer 2012;130:10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Larriba MJ, Ordóñez-Morán P, Chicote I, et al. Vitamin D receptor deficiency enhances Wnt/β-catenin signaling and tumor burden in colon cancer. PLoS One 2011;6:e23524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jacobs ET, Van Pelt C, Forster RE, et al. CYP24A1 and CYP27B1 Polymorphisms Modulate Vitamin D Metabolism in Colon Cancer Cells. Cancer Res 2013;73:2563-73. [DOI] [PMC free article] [PubMed] [Google Scholar]