Abstract
Sprague-Dawley neonatal rats within 7 days after birth were used in this study. The left common carotid artery was occluded and rats were housed in an 8% O2 environment for 2 hours to establish a hypoxic-ischemic brain damage model. 17β-estradiol (1 × 10-5 M) was injected into the rat abdominal cavity after the model was successfully established. The left hemisphere was obtained at 12, 24, 48, 72 hours after operation. Results showed that malondialdehyde content in the left brain of neonatal rats gradually increased as modeling time prolonged, while malondialdehyde content of 17β-estrodial-treated rats significantly declined by 24 hours, reached lowest levels at 48 hours, and then peaked at 72 hours after injury. Nicotinamide-adenine dinucleotide phosphate histochemical staining showed the nitric oxide synthase-positive cells and fibers dyed blue/violet and were mainly distributed in the cortex, hippocampus and medial septal nuclei. The number of nitric oxide synthase-positive cells peaked at 48 hours and significantly decreased after 17β-estrodial treatment. Our experimental findings indicate that estrogen plays a protective role following hypoxic-ischemic brain damage by alleviating lipid peroxidation through reducing the expression of nitric oxide synthase and the content of malondialdehyde.
Keywords: hypoxic-ischemic encephalopathy, hypoxic-ischemic brain damage, estrogen, malondialdehyde, free radical, nitric oxide synthase, lipid peroxidation, neonatal rats, neuroprotection, neural regeneration
Research Highlights
(1) Estrogen was observed to have a neuroprotective effect against hypoxic-ischemic brain damage in neonatal rats, which was most likely due to its antioxidant capacity.
(2) The expression of malondialdehyde and nitric oxide synthase in brain tissue of neonatal rats peaked at 48 hours after hypoxic-ischemic brain damage. Sham surgery was able to induce an increase in malondialdehyde expression.
(3) Estrogen inhibited the expression of malondialdehyde and nitric oxide synthase in ischemic brain tissue of neonatal rats following hypoxic-ischemic brain damage.
Abbreviation
TTC, 2,3,5-triphenyltetrazolium chloride
INTRODUCTION
Neonatal hypoxic-ischemic encephalopathy is a common complication after neonatal asphyxia, and can cause neonatal cerebral palsy, mental retardation and other serious sequelae. Currently, there is no specific treatment. When cerebral hypoxia ischemia occurs, ATP degrades and adenosine transforms into hypoxanthine. After cerebral blood reperfusion occurs and blood oxygen levels are restored, hypoxanthine may produce oxygen free radicals under the action of hypoxanthine oxidase, thus causing damage to cell and nuclear membranes, inducing brain cell apoptosis and necrosis, and generating corresponding clinical symptoms. That is to say that oxygen free radicals are an important cause of neonatal hypoxic ischemic encephalopathy[1,2,3,4]. Therefore, reducing the production of oxygen free radicals can effectively protect brain tissue and improve the prognosis of neonatal hypoxic ischemic encephalopathy.
Estrogen can function in the reproductive system and protect the brain from injury in adults[5,6,7,8,9]. The neuroprotective mechanism of estrogen is multiple and its antioxidant capacity is an important factor. However, the role of estrogen in children, particularly in neonates, remains unclear.
This study aimed to observe the malondialdehyde content in brain tissue before and after estrogen intervention and the number of nitric oxide synthase-positive cells in newborn rats with hypoxic ischemic brain damage. Overall, we attempted to investigate the neuroprotective effect and underlying mechanism associated with estrogen against hypoxic-ischemic brain damage.
RESULTS
Quantitative analysis of experimental animals
A total of 123 neonatal Sprague-Dawley rats, within 7 days after birth, were used in this study. Rats (n = 120) were randomly divided into three groups: sham operation group (only the left common carotid artery was ligated, without hypoxia), model group (left common carotid artery ligation + hypoxia for 2 hours, to establish the hypoxic-ischemic brain damage model), and the treatment group (hypoxic-ischemic brain damage model + estrogen treatment). Ten rats in each group were selected for experiments at 12, 24, 48, 72 hours after modeling, five for detection of malondialdehyde content and five for detection of nitric oxide synthase. Another three rats were used for 2,3,5-triphenyltetrazolium chloride (TTC) staining at 24 hours after modeling. In total, 123 rats were involved in the results analysis.
Ischemic changes in brain tissue of newborn rats after hypoxic-ischemic brain damage
TTC staining revealed that 24 hours after left common carotid artery ligation in neonatal rats, the majority of cerebral cortical and medial septal nuclei on the left hemisphere were damaged due to ischemia and hypoxia. Staining at the damage zone became lighter, while the right cerebral hemisphere was present with normal red dye (Figure 1). This is evidence that common carotid artery ligation can effectively block blood supply to the brain.
Figure 1.
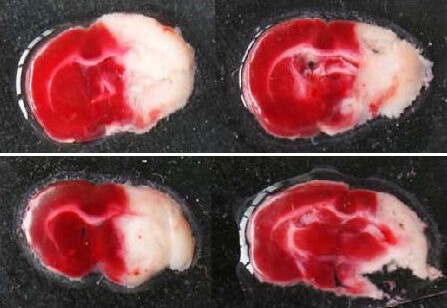
Ischemic condition of brain tissue in neonatal rats after hypoxic ischemic brain damage (2,3,5-triphenyltetrazolium chloride staining).
Normal brain tissue is deep red, and infarct tissue is white.
Estrogen decreased malondialdehyde levels in ischemic brain tissue of neonatal rats after hypoxic-ischemic brain damage
The malondialdehyde content in the sham operation group gradually increased during 12–72 hours after modeling. Malondialdehyde content in the model group was similar to that in the sham operation group, but significantly increased at 72 hours compared with the sham operation group (P < 0.05). In the treatment group, malondialdehyde content began to decrease at 24 hours, peaked at 48 hours and gradually increased at 72 hours, which was still significantly lower than that in the model group (P < 0.05; Figure 2).
Figure 2.
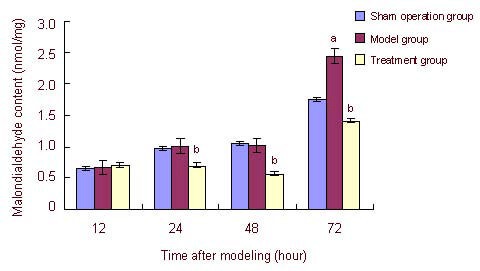
Effects of estrogen on malondialdehyde content in ischemic brain tissue of neonatal rats with hypoxic ischemic brain damage.
Data were expressed as mean ± SD of five rats in each group at each time point. aP < 0.05, vs. sham operation group; bP < 0.05, vs. model group, analysis of variance. Differences between groups were compared using the least significant difference t-test.
Estrogen decreased nitric oxide synthase expression in ischemic brain tissue of neonatal rats after hypoxic-ischemic brain damage
Nicotinamide-adenine dinucleotide phosphate histochemical staining showed that nitric oxide synthase-positive cells dyed blue/violet, with visible cell bodies and a clear boundary in surrounding tissue. Nitric oxide synthase-positive cells and fibers were mainly distributed in the cortex, hippocampus and medial septal nuclei. The number of nitric oxide synthase-positive cells in the model group was significantly higher than that in the treatment group, and nicotinamide-adenine dinucleotide phosphate histochemical staining became darker, especially at 48 hours. Compared with the model group, the number of nitric oxide synthase-positive cells decreased significantly in the treatment group (P < 0.05) and reached a minimum in the sham operation group (Figure 3).
Figure 3.
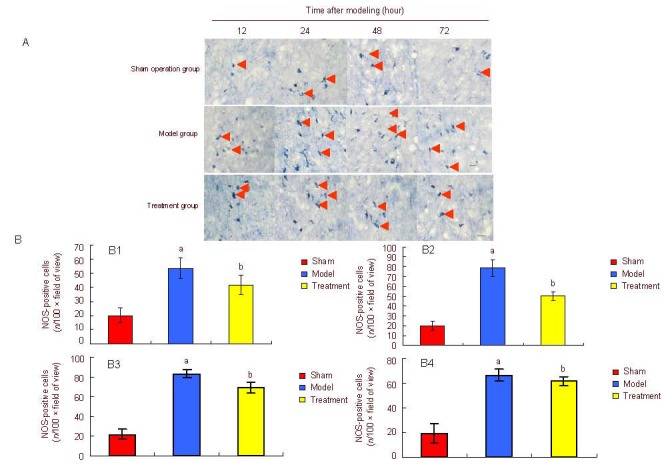
Effect of estrogen on nitric oxide synthase (NOS) expression in the medial septal nuclei of neonatal rats on the damaged side.
(A) Nicotinamide-adenine dinucleotide phosphate histochemical staining (bar: 100 μm) revealed the expression of NOS-positive neurons (arrows). NOS-positive cells in the model group showed small cell bodies and short neurites. The number of NOS-positive neurons in the treatment group was significantly lower than that in the model group.
(B) Quantification of NOS expression in medial septal nuclei of neonatal rats on the damaged side.
Data are expressed as mean ± SD of five rats in each group at each time point. At 12 (B1), 24 (B2), 48 (B3), 72 (B4) hours after modeling, aP < 0.05, vs. sham operation (sham) group; bP < 0.05, vs. model group, analysis of variance. Differences between two groups were compared using the least significant difference t-test.
DISCUSSION
Oxidative stress-induced free radical damage is considered to be one of the main causes of hypoxic-ischemic encephalopathy.
Under normal physiological conditions, free radical generation and removal are in a dynamic balance. Small amounts of free radicals produced by organisms can be removed by free radical scavengers in vivo, such as superoxide dismutase, glutathione peroxidase, vitamin E, vitamin C and catalase[10,11,12]. When the brain undergoes hypoxic ischemic injury, free radical generation sharply rises, thus causing large amounts of oxygen free radicals to accumulate in the inner environment, which is greater than the clearing capacity of the body. Therefore, the balance of oxygen free radicals is disrupted. Oxygen free radicals are extremely active, and easily bind to unsaturated fatty acids, proteins and folic acid on cell membranes, resulting in lipid peroxidation, destruction of membrane surface structure and leading to loss of function. Some scholars believe that the neonatal brain is more susceptible than the adult brain to lipid peroxidation. The reason for this may be that compared with adults, the neonatal brain contains higher levels of unsaturated fatty acids and are accordingly more vulnerable to damage triggered by lipid peroxidation when the production of free radicals and the capacity of clearing free radicals is unbalanced[13].
Secondly, the neonatal brain has not yet fully developed. Immature oligodendrocytes react more severely to free radical-induced injury compared with mature neurons[14]. In addition, activated xanthine oxidase aggregates in the cerebral vascular endothelium during the lipid peroxidation process, and disrupts the blood-brain barrier[15,16], which further causes angiorrhexis and triggers intracranial hemorrhage, thereby aggravating hypoxic-ischemic encephalopathy.
The existing treatment for hypoxic-ischemic encephalopathy includes inhibiting free radical generation, removing excessive free radicals, and stabilizing cell membrane structure and mitochondrial membrane structure. Although allopurinol cannot improve the mortality rates of neonatal children with hypoxic-ischemic encephalopathy in an epidemiological perspective, timely intervention can still effectively reduce S-100B protein content in umbilical cord blood when fetal hypoxia occurs[17,18]. In another study, cerebral ischemic reperfusion rats were treated with the combined therapy of chitosan nanoparticles and superoxide dismutase. The results showed that levels of malondialdehyde, a free radical byproduct, in superoxide dismutase-treated rats was significantly lower than that in the control group[19]. Oxygen intervention after hypoxic-ischemic encephalopathy can increase in vivo superoxide dismutase levels, which contribute to the reduction of lipid peroxidation[20]. However, it has been identified that no drug has this precise effect. Therefore, it is critical to develop a clinically accepted drug that has a clear neuroprotective effect. Estrogen serves as a natural antioxidant and neuroprotective agent[21,22,23,24,25,26]. In this study, we have shown that estrogen acts as a free radical scavenger during hypoxic-ischemic encephalopathy and protects against brain injury. Therefore, our results may provide new insights into therapies that specifically target lipid peroxidation, a major cause of hypoxic ischemic encephalopathy.
Malondialdehyde is a stable metabolite of lipid peroxidation. Therefore, malondialdehyde content indirectly reflects the content of free radicals and the degree of free radical-triggered lipid peroxidation, and ultimately detects the degree of cell damage[27,28,29,30,31]. In this study, the content of malondialdehyde in neonatal rat brain tissue of different groups at varying time points was determined.
Results showed that malondialdehyde content in the sham operation group gradually increased over time, which may indicate that the stress response in sham-operated rats increased the production of oxygen free radicals. The malondialdehyde content in the model group was similar to that in the sham operation group, but significantly increased at 72 hours, suggesting that generation of oxygen free radicals increased when hypoxic-ischemic brain damage occurred, and that changes were apparent at 72 hours. No difference was found at 24 and 48 hours when compared with the sham operation group. The inability of oxygen free radical scavenging enzymes to remove reactive oxygen species resulted in an increase in oxygen free radicals at 72 hours. Compared with the model group, malondialdehyde content in the treatment group decreased, indicating that estrogen had a strong antioxidant effect in neonatal rats and could relieve ischemic reperfusion injury. Other experimental findings are consistent with our research[32].
Nitric oxide is a novel neurotransmitter and gas molecule, produced during the oxidation of L-arginine following catalysis by nitric oxide synthase. Nitric oxide itself is also a kind of free radical that contributes to membrane lipid peroxidation. Nitric oxide may generate more toxic superoxide radicals, and in combination this can lead to the production of peroxynitrite ions. Peroxynitrite degrades into hydroxyl free radicals and nitrogen dioxide, these products can damage cellular proteins, nucleic acids and lipid membranes[33,34,35]. In this study, nicotinamide-adenine dinucleotide dehydrogenase histochemical staining found a significant difference in the number of nitric oxide synthase-positive cells between the treatment group and model group, especially at 48 hours. In neonatal rats with hypoxic-ischemic brain damage, nitric oxide content positively correlated with changes in nitric oxide synthase[36,37,38].
Estrogen decreased nitric oxide synthase expression and reduced nitric oxide production, thereby alleviating oxygen free radical production, which was consistent with the significant decrease in malondialdehyde content. Nie et al[38] found that the expression of inducible nitric oxide synthase gradually increased at 12–72 hours after cerebral ischemia reperfusion, while expression of neuronal nitric oxide synthase and endothelial nitric oxide synthase gradually decreased. We speculate that the neuroprotective mechanism of estrogen is associated with down-regulation of inducible nitric oxide synthase expression. In this study, nitric oxide synthase-positive cells were not identified, however, this will be the focus of our future studies. The significant increase in malondialdehyde content in the treatment group at 72 hours may depend on the dose and duration of estrogen. Therefore, further work is required to define the most suitable dose of estrogen. In addition, an estrogen substitute may be a potential research topic due to the various adverse reactions associated with estrogen exposure.
MATERIALS AND METHODS
Design
A randomized, controlled animal experiment.
Time and setting
Experiments were performed from June 2010 to December 2011 at the Department of Human Anatomy, Nantong University School of Medicine, China.
Materials
A total of 123 Sprague-Dawley neonatal rats aged 7 days, of clean grade, males and females, were provided by the Animal Experimental Center of Nantong University, China, with license number SYXK-(Su)-2007-0021. All experimental procedures were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[39].
Methods
Establishment of neonatal rat hypoxic-ischemic brain damage models and intervention
Hypoxic-ischemic brain damage models were prepared as previously described[40]. In brief, neonatal rats were anesthetized by ether inhalation and fixed in a supine position; a neck incision was made to free the left common carotid artery. The proximal and distal ends of the wound were ligated using 4-0 sterile suture, and the skin wound was disinfected and sutured. After surgery, neonatal rats were fed by mother rats for 1 hour, and then placed in a homemade organic glass hypoxia cabin covered with soda lime to absorb CO2 and moisture. The bottom of the hypoxia cabin was soaked in electrically heated water (37°C), with nitrogen ventilation. The oxygen concentration in the cabin was determined using a CY-12C type portable digital oxygen measuring instrument (Hangzhou Electrochemical Analytical Instrument Factory, Hangzhou, Zhejiang Province, China). After the oxygen concentration was maintained at 8 ± 0.5%, the hypoxia cabin was ventilated with a mixture of 8% O2 and 92% N2 standard gas at a speed of 1.5–2.5 L/min (Nanjing Special Gas Company Limited, Nanjing, Jiangsu, China). The oxygen concentration was maintained at 8 ± 0.5% to sustain hypoxia for 2 hours[41]. After experimentation, neonatal rats were returned to the mother. In the sham operation group, only the left carotid artery was isolated, but not ligated, followed by wound suture. No hypoxia treatment was given. In the treatment group, rats were intraperitoneally injected with 1 × 10-5 M 17β-estradiol (0.1 mL; Sigma, St. Louis, MO, USA) immediately after modeling. The model group and sham operation group were injected with equal volumes of normal saline.
TTC staining to visualize ischemic damage
Twenty-four hours after hypoxic-ischemic brain damage, three neonatal rats were deeply anesthetized and killed by decapitation. Rat brains were immediately placed in a –20°C refrigerator for 10 minutes. The cerebellum and brain stem were removed, and brain tissue was cut into serial coronal slices at an interval of 2 mm. The brain slices were immersed and incubated in 2% (w/v) TTC solution (Greensynchem, Nanjing, Jiangsu Province, China) at 37°C in the dark for 30 minutes to observe the ischemia in brain tissue of neonatal rats.
Determination of malondialdehyde content in ischemic brain tissue of neonatal rats
Rats were killed at 12, 24, 48, 72 hours after modeling. The left hemisphere of the brain was taken out using sterile operation scissors and tweezers, and then weighed. Each 100 mg of brain tissue was precooled with 1 mL sterile saline and homogenated in a glass homogenizer tube, which was placed in a container filled with a mixture of water and ice and ground for 6–8 minutes. The homogenate was harvested and centrifuged at 2 000 r/min, 4°C for 5 minutes, and the supernatant was extracted and stored on ice. The total protein concentration of samples was determined using the Coomassie Blue Staining Solution protein assay kit (Nanjing Jiancheng Bioengineering Company, Nanjing, Jiangsu Province, China). Malondialdehyde content was detected using the thiobarbituric acid method[42] according to the kit instructions. Malondialdehyde (nmol/mg) = (absorbance of detected tube – absorbance of blank tube)/(absorbance of standard tube – absorbance of standard blank tube) × 10 nmol/mL/protein (mg/mL).
Nicotinamide-adenine dinucleotide phosphate histochemical staining for detection of nitric oxide synthase expression in the neonatal rat brain
Under anesthesia by ether inhalation, the chest of neonatal rats was opened, and a blunt trumpet scalp needle was carefully inserted from the cardiac apex and terminated at the aorta. Rats were fixed with hemostatic forceps and injected with sterile saline using a 50-mL syringe. At the same time, the right atrial appendage was cut until the outflow liquid was clear, and approximately 40 mL of 4% (v/v) neutral formalin was injected. The neonatal rats were decapitated and brain tissue was harvested. Tissue was preserved at 4°C for 24 hours, followed by 20% (w/v) and 30% (w/v) sucrose gradient dehydration. The brain tissue at the damage zone was cut into 20-μm-thick frozen sections, which were incubated with 1 mM nicotinamide-adenine dinucleotide phosphate (Roche Diagnostics (Shanghai) Limited, Shanghai, China) and 0.2 mM nitro blue tetrazolium (Haorui Chemicals, Shanghai, China) droplets in the dark at 37°C for 90 minutes. The reaction was terminated with PBS, followed by routine dehydration, transparency and mounting. Slices were observed under a Leica microscope (10 × magnification; Wetzlar, Germany). Eight sections at the same site from each rat (mainly in the cortex, medial septal nuclei and striatum), five sets, were selected. The number of nitric oxide synthase-positive cells was measured using Jetta 801 series morphological analysis software (Nanjing, China) and the average value was calculated.
Statistical analysis
Measurement data were expressed as mean ± SD and statistical processing was performed using SPSS 16.0 software (SPSS, Chicago, IL, USA) for analysis of variances. Differences between two groups were compared with the least significant difference t-test. A P value < 0.05 indicated a statistically significant difference.
Footnotes
Funding: This study was supported by the Project of Nantong Application Plan, No. BK2011055 and the Project of Nantong University, No. 09Z032.
Conflicts of interest: None declared.
Ethical approval: All procedures involving animals were approved by the Institutional Animal Care and Use Committee of Nantong University in China.
(Edited by Liu YL, Guo JS/Yang Y/Song LP)
REFERENCES
- [1].Shao XM. 4th ed. Beijing: People's Medical Publishing House; 2011. Practical of Neonatology. [Google Scholar]
- [2].Weis SN, Schunck RV, Pettenuzzo LF, et al. Early biochemical effects after unilateral hypoxia-ischemia in the immature rat brain. Int J Dev Neurosci. 2011;29(2):115–120. doi: 10.1016/j.ijdevneu.2010.12.005. [DOI] [PubMed] [Google Scholar]
- [3].Greggio S, Rosa RM, Dolganov A, et al. NAP prevents hippocampal oxidative damage in neonatal rats subjected to hypoxia-induced seizures. Neurobiol Dis. 2009;36(3):435–444. doi: 10.1016/j.nbd.2009.08.008. [DOI] [PubMed] [Google Scholar]
- [4].Alkan T, Gören B, Vatansever E, et al. Effects of hypoxic preconditioning in antioxidant enzyme activities in hypoxic-ischemic brain damage in immature rats. Turk Neurosurg. 2008;18(2):165–171. [PubMed] [Google Scholar]
- [5].Wu L, Chen WJ. Neuro-protection of estrogen. Shenjing Jiepou Xue Zazhi. 2005;21(1):95–97. [Google Scholar]
- [6].Ooishi Y, Mukai H, Hojo Y, et al. Estradiol rapidly rescues synaptic transmission from corticosterone-induced suppression via synaptic/extranuclear steroid receptors in the hippocampus. Cereb Cortex. 2012;22(4):926–936. doi: 10.1093/cercor/bhr164. [DOI] [PubMed] [Google Scholar]
- [7].Kipp M, Berger K, Clarner T, et al. Sex steroids control neuroinflammatory processes in the brain: relevance for acute ischemia and degenerative demyelination. J Neuroendocrinol. 2012;24(1):62–70. doi: 10.1111/j.1365-2826.2011.02163.x. [DOI] [PubMed] [Google Scholar]
- [8].Gillies GE, McArthur S. Independent influences of sex steroids of systemic and central origin in a rat model of Parkinson's disease: a contribution to sex-specific neuroprotection by estrogens. Horm Behav. 2010;57(1):23–34. doi: 10.1016/j.yhbeh.2009.06.002. [DOI] [PubMed] [Google Scholar]
- [9].Thompson CK, Brenowitz EA. Caspase inhibitor infusion protects an avian song control circuit from seasonal-like neurodegeneration. J Neurosci. 2008;28(28):7130–7136. doi: 10.1523/JNEUROSCI.0663-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jin HM. 5th ed. Beijing: People's Medical Publishing House; 2000. Pathophysiology. [Google Scholar]
- [11].Harris C, Hansen JM. Oxidative stress, thiols, and redox profiles. Methods Mol Biol. 2012;889:325–346. doi: 10.1007/978-1-61779-867-2_21. [DOI] [PubMed] [Google Scholar]
- [12].Bozok S, Ilhan G, Yilmaz Y, et al. Protective effects of hyperbaric oxygen and iloprost on ischemia/reperfusion- induced lung injury in a rabbit model. Eur J Med Res. 2012;17(1):14. doi: 10.1186/2047-783X-17-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ferriero DM. Oxidant mechanisms in neonatal hypoxia-ischemia. Dev Neurosci. 2001;23(3):198–202. doi: 10.1159/000046143. [DOI] [PubMed] [Google Scholar]
- [14].Baud O, Greene AE, Li J, et al. Glutathione peroxidase-catalase cooperativity is required for resistance to hydrogen peroxide by mature rat oligodendrocytes. J Neurosci. 2004;24(7):1531–1540. doi: 10.1523/JNEUROSCI.3989-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Buonocore G, Perrone S, Bracci R. Free radicals and brain damage in the newborn. Biol Neonate. 2001;79(3-4):180–186. doi: 10.1159/000047088. [DOI] [PubMed] [Google Scholar]
- [16].Wong CH, Crack PJ. Modulation of neuro-inflammation and vascular response by oxidative stress following cerebral ischemia-reperfusion injury. Curr Med Chem. 2008;15(1):1–14. doi: 10.2174/092986708783330665. [DOI] [PubMed] [Google Scholar]
- [17].Chaudhari T, McGuire W. Allopurinol for preventing mortality and morbidity in newborn infants with suspected hypoxic-ischaemic encephalopathy. Cochrane Database Syst Rev. 2008;(2):CD006817. doi: 10.1002/14651858.CD006817.pub2. [DOI] [PubMed] [Google Scholar]
- [18].Torrance HL, Benders MJ, Derks JB, et al. Maternal allopurinol during fetal hypoxia lowers cord blood levels of the brain injury marker S-100B. Pediatrics. 2009;124(1):350–357. doi: 10.1542/peds.2008-2228. [DOI] [PubMed] [Google Scholar]
- [19].Jia D, Gao GD, Li YL. Effects of chitosan nanoparticles loaded the copper-superoxide dismutase plasmid on brain tissue after ischemia-reperfusion. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2008;12(45):8827–8830. [Google Scholar]
- [20].Liu L, Yang YJ, Liu LX, et al. Effects of hyperbaric oxygen therapy on neuron apoptosis and brain SOD and MDA production in hypoxic-ischemic brain injury of neonatal rats. Zhongguo Xinsheng Erke Zazhi. 2007;22(2):89–92. [Google Scholar]
- [21].Wang HB, Wang GJ, Sun LH, et al. Effects of estrogen on SOD activity and MDA contents in cerebral ischemia reperfusion. Zhongguo Kangfu. 2003;18(2):72–74. [Google Scholar]
- [22].Shahrokhi N, Haddad MK, Joukar S, et al. Neuroprotective antioxidant effect of sex steroid hormones in traumatic brain injury. Pak J Pharm Sci. 2012;25(1):219–225. [PubMed] [Google Scholar]
- [23].Mohamd EM, Ahmed HH, Estefan SF, et al. Windows into estradiol effects in Alzheimer's disease therapy. Eur Rev Med Pharmacol Sci. 2011;15(10):1131–1140. [PubMed] [Google Scholar]
- [24].Abbas AM, Elsamanoudy AZ. Effects of 17β-estradiol and antioxidant administration on oxidative stress and insulin resistance in ovariectomized rats. Can J Physiol Pharmacol. 2011;89(7):497–504. doi: 10.1139/y11-053. [DOI] [PubMed] [Google Scholar]
- [25].Ozacmak VH, Sayan H. The effects of 17beta estradiol, 17alpha estradiol and progesterone on oxidative stress biomarkers in ovariectomized female rat brain subjected to global cerebral ischemia. Physiol Res. 2009;58(6):909–912. doi: 10.33549/physiolres.931647. [DOI] [PubMed] [Google Scholar]
- [26].Ulas M, Cay M. The effects of 17beta-estradiol and vitamin E treatments on oxidative stress and antioxidant levels in brain cortex of diabetic ovariectomized rats. Acta Physiol Hung. 2010;97(2):208–215. doi: 10.1556/APhysiol.97.2010.2.7. [DOI] [PubMed] [Google Scholar]
- [27].Branghler JM, Hall ED. Central nervous system trauma and stroke Biochemical consideration. Free Radical Biolmed. 1989;6(3):289–301. doi: 10.1016/0891-5849(89)90056-7. [DOI] [PubMed] [Google Scholar]
- [28].Lin H, Chang CP, Lin HJ. Attenuating brain edema, hippocampal oxidative stress, and cognitive dysfunction in rats using hyperbaric oxygen preconditioning during simulated high-altitude exposure. J Trauma Acute Care Surg. 2012;72(5):1220–1227. doi: 10.1097/TA.0b013e318246ee70. [DOI] [PubMed] [Google Scholar]
- [29].Chakrabarty M, Bhat P, Kumari S. Cortico-hippocampal salvage in chronic aluminium induced neurodegeneration by Celastrus paniculatus seed oil: Neurobehavioural, biochemical, histological study. J Pharmacol Pharmacother. 2012;3(2):161–171. doi: 10.4103/0976-500X.95520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yao N, Wang DF, Song X, et al. Neuroprotective effects of combined pretreatment with edaravone and propofol on neonatal rat cerebral cortical neurons with ischemia/reperfusion injury in vitro. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2012;24(5):286–289. [PubMed] [Google Scholar]
- [31].Xia L, Jiang ZL, Wang GH. Treatment with ginseng total saponins reduces the secondary brain injury in rat after cortical impact. J Neurosci Res. 2012;90(7):1424–1436. doi: 10.1002/jnr.22811. [DOI] [PubMed] [Google Scholar]
- [32].Xu CH, Jin ZY, Li HZ. Effect of estrodiol on neonatal rats after hypoxic-ischemia brain injury. Linchuang Erke Zazhi. 2008;26(11):970–972. [Google Scholar]
- [33].Liu Y, Zou LP, Du JB. Nitric oxide-mediated neuronal functional recovery in hypoxic-ischemic brain damaged rats subjected to electrical stimulation. Brain Res. 2011;1383:324–328. doi: 10.1016/j.brainres.2011.01.088. [DOI] [PubMed] [Google Scholar]
- [34].Lin HY, Huang CC, Chang KF. Lipopolysaccharide preconditioning reduces neuroinflammation against hypoxic ischemia and provides long-term outcome of neuroprotection in neonatal rat. Pediatr Res. 2009;66(3):254–259. doi: 10.1203/PDR.0b013e3181b0d336. [DOI] [PubMed] [Google Scholar]
- [35].Lin XY. Change of nitric oxide and nitric oxide synthase in serum and brain tissue after hypoxic-ischemic brain damage in neonatal rats. Fujian Daxue Xuebao. 2004;38(4):381–383. [Google Scholar]
- [36].Kaur C, Ling EA. Antioxidants and neuroprotection in the adult and developing central nervous system. Curr Med Chem. 2008;15(29):3068–3680. doi: 10.2174/092986708786848640. [DOI] [PubMed] [Google Scholar]
- [37].Liu MN, Zhuang SQ, Zhang HY, et al. Long-term effects of early hyperbaric oxygen therapy on neonatal rats with hypoxic-ischemic brain damage. Zhongguo Dang Dai Er Ke Za Zhi. 2006;8(3):216–220. [PubMed] [Google Scholar]
- [38].Nie YX, Guo M, Yu HM. Changes of expression of nNOS, eNOS and iNOS after focal cerebral ischemic reperfusion injury in rats. Zhongguo Xueye Liubianxue Zazhi. 2006;16(3):332–333. [Google Scholar]
- [39].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [40].Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9(2):131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- [41].Chang X, Yin HQ, Li H, et al. Effect of activin A on nestin expression in neonatal rats with hypoxic-ischemic brain damage. Zhongxiyi Jiehe Xinnao Xueguan Bing Zazhi. 2010;8(5):589–591. [Google Scholar]
- [42].Kontos HA, Wei EP. Superoxide production in experimental brain injury. J Neurosurg. 1986;64(5):803–807. doi: 10.3171/jns.1986.64.5.0803. [DOI] [PubMed] [Google Scholar]


