Abstract
C/EBP homologous protein, an important transcription factor during endoplasmic reticulum stress, participates in cell apoptosis mediated by endoplasmic reticulum stress. Previous studies have shown that C/EBP homologous protein mediates nerve injury during Alzheimer’s disease, subarachnoid hemorrhage and spinal cord trauma. In this study, we introduced C/EBP homologous protein short hairpin RNA into the brains of ischemia/reperfusion rat models via injection of lentiviral vector through the left lateral ventricle. Silencing C/EBP homologous protein gene expression significantly reduced cerebral infarction volume, decreased water content and tumor necrosis factor-α and interleukin-1β mRNA expression in brain tissues following infarction, diminished the number of TUNEL-positive cells in the infarct region, decreased caspase-3 protein content and increased Bcl-2 protein content. These results suggest that silencing C/EBP homologous protein lessens cell apoptosis and inflammatory reactions, thereby protecting nerves.
Keywords: C/EBP homologous protein, endoplasmic reticulum stress, Alzheimer’s disease, subarachnoid hemorrhage, tumor necrosis factor-α, ischemia/reperfusion, interleukin-1β, cerebral infarction, neural regeneration
Research Highlights
(1) C/EBP homologous protein gene silencing can downregulate tumor necrosis factor-α and interleukin-1β expression in models of ischemia/reperfusion injury.
(2) C/EBP homologous protein not only promotes apoptosis, but also aggravates acute brain injury.
Abbreviations
CHOP, C/EBP homologous protein; shRNA, short hairpin RNA; LV, lentiviral vector; TNF-α, tumor necrosis factor-α; siRNA, short interfering RNA
INTRODUCTION
C/EBP homologous protein (CHOP), an important transcription factor during endoplasmic reticulum stress, is activated following ischemia/hypoxia and oxidative stress, and participates in cell apoptosis[1,2]. A previous study showed that CHOP overexpression in cells led to decreased Bcl-2 levels, increased Bim levels and the occurrence of cell apoptosis[3]. RNAi-mediated CHOP silencing has been shown to protect smooth muscle cells against endoplasmic reticulum stress-mediated apoptosis[4]. CHOP knockout increased Bcl-2 content in myocardial cells and lessened myocardial cell apoptosis[5]. CHOP is probably associated with inflammation. Indeed, CHOP knockout also suppressed caspase-11 activities and inhibited inflammation in a mouse model of lipopolysaccharides-induced pneumonitis[6]. Moreover, inflammatory factor expression was reduced, and inflammatory cell infiltration was lessened in the infarcted myocardium of mice with CHOP knockout following myocardial ischemia/reperfusion[5].
CHOP plays an important role in the occurrence and development of nervous system disease. A previous study confirmed that amyloid precursor protein activated intracellular CHOP expression and that this expression mediated neural cell apoptosis[7]. CHOP silencing reduced β-amyloid deposition, relieved inflammatory reaction and cell apoptosis in an in vitro model of Alzheimer's disease[8]. Following subarachnoid hemorrhage, silencing of CHOP expression lessened cerebral vasospasm-induced acute cerebral injury[9]. A large amount of CHOP is detectable in brain tissue following ischemia/reperfusion injury[10]. However, it remains unknown whether CHOP is associated with inflammatory reactions or cell apoptosis following cerebral ischemia/reperfusion.
In the present study, we established rat models of ischemia/reperfusion injury using the suture occlusion method, introduced CHOP short hairpin RNA (shRNA) into the brain via injection of a lentiviral vector (LV) through the left lateral ventricle, and analyzed the effects of CHOP gene silencing on acute brain injury following ischemia/reperfusion.
RESULTS
Quantitative analysis of experimental animals
A total of 36 rats were randomly and equally assigned to control, vector and LV-shRNA groups. PBS, LV-cytomegalovirus (CMV)-control plasmid and LV-CMV-CHOP shRNA plasmids were respectively injected into the left lateral ventricles of rats from the control, vector and LV-shRNA groups. Forty-eight hours later, rat models of ischemia/reperfusion were established using the suture occlusion method. A total of 36 rats were included in the final analysis, with no drop outs.
CHOP silencing reduced infarct volume in rats following cerebral ischemia/reperfusion
At 1 day following cerebral ischemia/reperfusion, 2,3,5-triphenyltetrazolium chloride staining revealed a large gray infarct region (276.7 ± 56.4 mm3) in the left cerebral hemisphere, which involved the cortex, hippocampus and basal ganglia in the control group. The infarct region was distributed evenly and infarct volume did not obviously change in the vector group (254.4 ± 74.6 mm3; P > 0.05). Infarct volume in the LV-shRNA group was significantly smaller than that in the control and vector groups (145.2 ± 52.1 mm3; P < 0.01; Figure 1).
Figure 1.
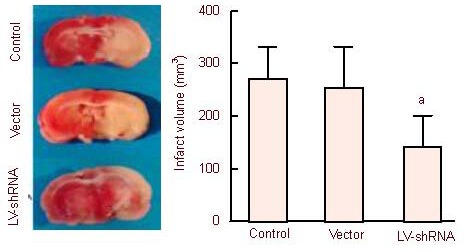
Infarct volume in rats from the control, vector and lentiviral vector (LV)-short hairpin RNA (shRNA) groups (2,3,5-triphenyltetrazolium chloride staining).
There was an obvious, large, gray infarct region, which involved the cortex, hippocampus and basal ganglia in each group following model induction using the suture occlusion method. Volume in the LV-shRNA group: aP < 0.01, vs. control and vector groups. Mean ± SD, n = 3, one-way analysis of variance and Student-Newman-Keuls test.
CHOP silencing reduced tumor necrosis factor-α (TNF-α) mRNA and interleukin-1β mRNA expression in the infarct region
Real-time quantitative PCR was utilized to determine TNF-α mRNA and interleukin-1β mRNA expression levels to study the role of CHOP in inflammatory reactions in the infarct region. Interleukin-1β mRNA expression was significantly lower in the LV-shRNA group compared with the control and vector groups (P < 0.05; Figure 2).
Figure 2.
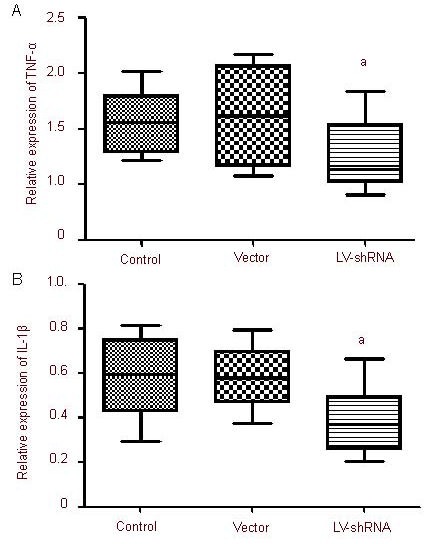
C/EBP homologous protein gene silencing effects tumor necrosis factor-α (TNF-α) mRNA (A) and interleukin-1β (IL-1β) mRNA (B) expression in the infarct region of rats following ischemia/reperfusion.
Results are expressed as the ratio of the absorbance values of TNF-α and IL-1β mRNA to that of the house-keeping gene GAPDH. aP < 0.01, vs. control and vector groups. Mean ± SD, n = 6, one-way analysis of variance and Student-Newman-Keuls test.
CHOP silencing increased Bcl-2 content and decreased caspase-3 content in the infarct region
A western blot assay revealed that CHOP and caspase-3 contents were lower (P < 0.01), while Bcl-2 content was higher (P < 0.05) in the LV-shRNA group compared with the control and vector groups (Figure 3).
Figure 3.
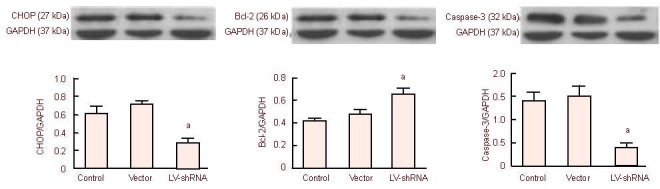
Effects of C/EBP homologous protein (CHOP) gene silencing on Bcl-2 and caspase-3 protein expression in the cerebral infarct regions of rats following ischemia/reperfusion.
Results are expressed as the ratio of the absorbance values of target protein to the house-keeping protein GAPDH. Expression levels in the lentiviral vector (LV)-short hairpin RNA (shRNA) group: aP < 0.05, vs. control and vector groups. Mean ± SD, n = 6, one-way analysis of variance and Student-Newman-Keuls test.
CHOP silencing lessend cell apoptosis in the infarct region
A large number of TUNEL-positive cells in the rat cortex, hippocampus and basal ganglia in each group. There were large numbers of round or elliptic apoptotic cells showing pyknosis and karyorrhexis. The number of apoptotic cells in the LV-shRNA group was significantly less than that in the control and vector groups (P < 0.05; Figure 4).
Figure 4.
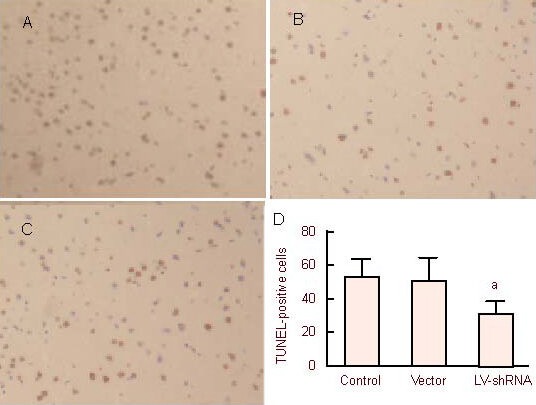
Effects of C/EBP homologous protein gene silencing on cell apoptosis in the rat cerebral infarct region following ischemia/reperfusion.
Cell apopotsis in control group, vector group and lentiviral vector (LV)-short hairpin RNA (shRNA) group, respectively (A–C; TUNEL staining, × 200) there were round or elliptic apoptotic cells showing pyknosis and karyorrhexis. (D) Quantification of TUNEL-positive cells. aP < 0.05, vs. control and vector groups. Mean ± SD, n = 3, one-way analysis of variance and Student-Newman-Keuls test.
DISCUSSION
shRNAs are RNA sequences with a tight hairpin turn which can be used in RNA interference. shRNAs are introduced into cells using a vector, and the U6 promoter in the vector ensures shRNA expression. The shRNA vector can be transmitted into daughter cells, and gene silencing can be inherited. The hairpin structure of shRNAs can be cut into short interfering RNAs (siRNAs), which then become conjugated to an RNA-induced silencing complex. This complex can conjugate to and degrade corresponding mRNAs. RNA interference is an important method for studying pathogenesis and performing gene therapy and has been extensively applied in studies of nervous system diseases[11,12,13]. RNA interference has recently emerged as a specific and efficient method with which to silence gene expression either by transfection with siRNAs or, more recently, by transcription of shRNAs[14,15]. Of all shRNA vectors, lentiviral vectors have been characterized to show transfection efficiency, stable expression, low immunogenicity, and an ability to effectively infect and integrate unseparated cells such as neuronal cells, and they are widely used in studies of the nervous system[16,17].
This study analyzed the effect of CHOP gene silencing in neural cells, especially neurons, on brain injury following ischemia/reperfusion. Thus, lentiviruses carrying CHOP shRNAs were suitable for our study. A western blot assay confirmed that CHOP expression was dramatically lower in the LV-shRNA group than that in the vector and control groups, thereby validating the approach used.
When histiocytes experience hypoxia/ischemia, inflammation and oxidative stress, intracellular endoplasmic reticulum stress are observed[18,19]. Although homeostasis can be maintained during endoplasmic reticulum stress, excessive endoplasmic reticulum stress leads to disruption of homeostasis because of the continuous presence of the insult, finally resulting in cell apoptosis[20]. Numerous previous studies have revealed that endoplasmic reticulum stress exerts a crucial role in the occurrence and development of many nervous system diseases such as cerebral infarction, Parkinson's disease, Alzheimer's disease and amyotrophic lateral scherosis[21,22,23,24]. CHOP is an important transcription factor in endoplasmic reticulum stress. It is not expressed in normal neural cells, but it is expressed in cells following stimulation by pathological factors, finally resulting in apoptosis. In this study, a high level of CHOP expression was seen following cerebral ischemia/reperfusion, consistent with the findings of a previous study[10]. By using RNA interference to silence CHOP, the number of TUNEL-positive cells was remarkably decreased and infarct volume was reduced in the injured region, suggesting that CHOP mediates cell apoptosis and aggravates brain injury following cerebral ischemia/reperfusion. The mechanisms underlying this CHOP-mediated cell injury remain unclear, but they are associated with inhibition of Bcl-2 expression and induction of oxidative stress[25]. In the present study, CHOP silencing led to an increase in Bcl-2 expression and a decrease in caspase-3 expression. These findings suggest that CHOP decreases Bcl-2 expression, activates caspase-3, and mediates apoptosis. Therefore, silencing CHOP could protect the nervous system.
Like apoptosis, inflammatory reactions are an important injury factor following cerebral ischemia/reperfusion. Recent studies have suggested that CHOP is associated with inflammation. CHOP induces caspase-1 and caspase-11 expression, upregulates interleukin-1β, and mediates inflammatory reactions[26]. At present, there is no report concerning the relationship between CHOP and inflammatory reactions following cerebral ischemia/reperfusion.
In our models of cerebral ischemia/reperfusion, CHOP silencing by RNA interference downregulated TNF-α and interleukin-1β expression. Thus, we believe that CHOP not only contributes to apoptosis, but also aggravates acute brain injury.
MATERIALS AND METHODS
Design
A molecular biology randomized controlled study.
Time and setting
Experiments were performed at the Laboratory of Neurology, Translational Medicine Center, Bethune First Hospital, Jilin University, China from October 2011 to March 2012.
Materials
Animals
A total of 36 clean, healthy, male, Sprague-Dawley rats, aged 8–10 weeks, weighing 280–340 g, were supplied by the Animal Experimental Center, Jilin University in China (animal license No. SCXK (Ji) 2003-0002). The rats were housed in individual cages at 21 ± 2°C, with a relative humidity of 30–35%, in a 12-hour light/dark cycle, with free access to food (a full-nutrient diet) and water. The protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[27].
Plasmid
Lentiviral-CMV-CHOP shRNA plasmid and lentiviral-CMV-controlplasmid were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
Methods
Intraventricular injection of CHOP shRNA plasmid
The rats were intraperitoneally anesthetized with 10% chloral hydrate (0.32 mL/100 g), and fixed on a stereotaxic apparatus (Shenzhen Reward Life Science Co., Ltd.). In accordance with the method of Jin et al[28], after sterilization with 75% ethanol, a 2.5-cm-long sagittal incision was made along the median line to expose the cranial bones. A hole was drilled at 0.3 mm anterior to the bregma, 3.6 mm lateral to the ventro, 1.1 mm left of the cranial bones. The cerebral dura mater was punctured with a small needle. A casing was inserted slowly through the hole. PBS, LV-CMV-control plasmid (10 μL, 1 × 108 TU/mL) and LV-CMV-CHOP shRNA plasmid (10 μL, 1 × 108 TU/mL) were respectively injected into the left cerebral ventricle in the control, vector and LV-shRNA groups using a mini-syringe pump. The rats were removed from the stereotaxic apparatus, and placed in an incubator with controllable humidity and temperature at 37°C for 60 minutes. Subsequently, the rats were housed in individual cages at 22°C and allowed to free access to food and water.
Establishment of rat models of cerebral ischemia/reperfusion injury
At 48 hours following injection of lentiviral plasmids into the cerebral ventricle of rats, rat models of cerebral ischemia/reperfusion injury were induced using the suture occlusion method[29]. The rats were subcutaneously anesthetized with ketamine (100 mg/mL, 100 mg/kg), xylazine (20 mg/mL, 2.5 mg/kg) and acepromazine (2.5 mg/mL, 2.5 mg/kg) (Jinan Wanxingda Chemical Co., Ltd., Jinan, Shandong Province, China). The rats were fixed on a surgical splint in the supine position. A median incision was made after sterilizing the neck skin; then, the left common carotid artery, external carotid artery and internal carotid artery were dissociated in order. A small cut proximal to the common carotid artery was made using eye scissors, and a 0.26-mm diameter blunt fishing line was inserted into the original part of the middle cerebral artery through the internal carotid artery. The blood flow of the left middle cerebral artery was blocked for 2 hours, and then the fishing line was pulled out. The external carotid artery was ligated and the incision was sutured. Following regaining of consciousness, the rats were housed at 20–25°C with free access to food and water. Neurological functions were graded[29]. Rats scoring 3 or 4 were considered to show successful model induction.
2,3,5-triphenyltetrazolium chloride staining of brain tissues of rats with cerebral ischemia/reperfusion injury
At 1 day following model induction, all rats were anesthetized and decapitated. The brains were placed at –20°C for 20 minutes, and then cut into 2 mm-thick sections. The first cut was made on the midpoint of the anterior pole–optic chiasma line. The second cut was made on the optic chiasma. The third cut was made on the infundibular stalk. The fourth cut was made between the infundibular stalk and the caudal end of the posterior lobe. The sections were stained in 2% 2,3,5-triphenyltetrazolium chloride at 37°C for 15–30 minutes. The white region of the sections was considered to be the infarct region.
Real-time quantitative PCR for TNF-α and interleukin-1β mRNA expression in rat models of cerebral ischemia/reperfusion injury
In accordance with the method of Finch et al[30], total RNA was extracted from brain tissues of the left infarct region using Trizol (Sigma, St. Louis, MO, USA). The content of extracted RNA was determined using a UV-240 spectrophotometer (Kaiao, Beijing, China). The A260nm/A280nm ratio of total RNA was between 1.80 and 2.00. A total of 2 μg total RNA was obtained. In strict accordance with the instructions of the Superscript III Reverse Transcriptase kit (Invitrogen, Carlsbad, CA, USA), cDNA was synthesized, followed by real-time quantitative PCR according to instructions of the SYBR Green PCR kit (Beijing TransGen Biotech Co., Ltd., Beijing, China). Then, 1 μL of specific primer (including 0.5 μL upstream primer and 0.5 μL downstream primer), 12.5 μL of 2 × SYBR Green QPCR Master Mix, 2.5 μL of diluted cDNA and 9 μL of nuclease-free PCR-grade water were mixed for denaturation at 95°C for 5 minutes, followed by 40 cycles of 95°C for 30 seconds, 60°C for 1 minute, and 72°C for 30 seconds. PCR products with SYBR green fluorescence were detectable. Product concentration was quantified using GAPDH as an internal reference.
Reference sequences for TNF-α and interleukin-1β were obtained from GeneBank and mRNA-specific primers were designed using Prime primer 5.0 software and synthesized by Shanghai Bioengineering Institute, Shanghai, China. The precise sequences are as follows:

CHOP, Bcl-2 and caspase-3 expression in brain tissues of rats with cerebral ischemia/reperfusion, as detected by western blot assay
Infarcted brain tissues were lysed for 30 minutes on ice, homogenized using a homogenizer for 30 seconds, and then centrifuged at 12 000 r/min for 20 minutes. The supernatant was obtained and diluted using lysis buffer. Total protein concentration was determined by bicinchoninic acid assay. Then, 15 μg of total protein from each sample was added to 4 μL of 6 × Loading Buffer, followed by denaturation in boiling water for 5 minutes and 10% inconsecutive SDS-PAGE at 80 V for 40 minutes, at 110 V for 90 minutes. Proteins were electro-transferred to the membrane by the wet method at 200 mA for 60–90 minutes. The membrane was blocked in bovine serum albumin (Boster, Wuhan, Hubei Province, China) and skim milk for 2 hours, followed by six washes in Tris-buffered saline containing Tween-20, each for 10 minutes (Boster). The specimens were incubated in rabbit anti-CHOP, Bcl-2 and caspase-3 polyclonal antibody IgG (Santa Cruz Biotechnology) at 1: 2 000 at 4°C overnight, eluted six times in Tris-buffered saline containing Tween-20, each for 10 minutes, incubated in horseradish peroxidase-labeled goat anti-rabbit polyclonal antibody IgM (Santa Cruz Biotechnology) at room temperature for 2 hours, followed by enhanced chemiluminescence detection.
TUNEL for cell apoptosis in the rat cerebral infarct region
The sections were washed twice in xylene, each for 5 minutes, washed twice in dehydrated alcohol, each for 3 minutes, separately washed in 95% and 75% ethanol, each 3 minutes, immersed in PBS for 5 minutes and hydrolyzed by proteinase K (20 ug/mL) for 15 minutes at room temperature, followed by four washes in distilled water, each for 2 minutes. PBS containing 2% hydrogen peroxide was added at room temperature for 5 minutes. Redundant solution on the slide was absorbed by filter paper. Two drops of TdT enzyme buffer were added at room temperature for 5 minutes at 37°C for 1 hour, and then stop buffer was added at 37°C for 30 minutes. Following three washes in PBS, the specimens were incubated in peroxidase-labeled anti-digoxin antibody (Boster) for 30 minutes, developed in 0.05% 3, 3’-diaminobenzidine at room temperature for 5 minutes, counterstained in methyl green for 10 minutes, mounted, and dried. The numbers of TUNEL-positive cells in five 100-fold fields surrounding the ischemic region were determined under a light microscope (Olympus, Tokyo, Japan).
Statistical analysis
Measurement data were expressed as mean ± SD, and were analyzed utilizing SPSS 17.0 software (SPSS, Chicago, IL, USA). Intergroup comparisons were conducted by one-way analysis of variance and Student-Newman-Keuls test (α = 0.05).
Footnotes
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Animal Ethics Committee, Jilin University, China.
(Edited by Chen JF, Huang YJ/Qiu Y/Song LP)
REFERENCES
- [1].Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11(4):381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- [2].Oyadomari S, Araki E, Mori M. Endoplasmic reticulum stress-mediated apoptosis in pancreatic beta-cells. Apoptosis. 2002;7(4):335–345. doi: 10.1023/a:1016175429877. [DOI] [PubMed] [Google Scholar]
- [3].McCullough KD, Martindale JL, Klotz LO, et al. Gadd153 sensitizes cells to endoplasmic reticulum stressby down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21(4):1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhang YH, Zhao CQ, Jiang LS, et al. Lentiviral shRNA silencing of CHOP inhibits apoptosis induced by cyclic stretch in rat annular cells and attenuates disc degeneration in the rats. Apoptosis. 2011;16(6):594–605. doi: 10.1007/s10495-011-0596-y. [DOI] [PubMed] [Google Scholar]
- [5].Miyazaki Y, Kaikita K, Endo M, et al. C/EBP homologous protein deficiency attenuates myocardial reperfusion injury by inhibiting myocardial apoptosis and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):1124–1132. doi: 10.1161/ATVBAHA.111.224519. [DOI] [PubMed] [Google Scholar]
- [6].Endo M, Mori M, Akira S, et al. C/EBP homologous protein (CHOP) is crucial for the induction of caspase-11 and the pathogenesis of lipopolysaccharide induced inflammation. J Immunol. 2006;176(10):6245–6253. doi: 10.4049/jimmunol.176.10.6245. [DOI] [PubMed] [Google Scholar]
- [7].Takahashi K, Niidome T, Akaike A, et al. Amyloid precursor protein promotes endoplasmic reticulum stress-induced cell death via C/EBP homologous protein-mediated pathway. J Neurochem. 2009;109(5):1324–1337. doi: 10.1111/j.1471-4159.2009.06067.x. [DOI] [PubMed] [Google Scholar]
- [8].Prasanthi JR, Larson T, Schommer J, et al. Silencing GADD153/CHOP gene expression protects against Alzheimer's disease-like pathology induced by 27-hydroxycholesterol in rabbit hippocampus. PLoS One. 2011;6(10):e26420. doi: 10.1371/journal.pone.0026420. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [9].He Z, Ostrowski RP, Sun X, et al. CHOP silencing reduces acute brain injury in the rat model of subarachnoid hemorrhage. Stroke. 2012;43(2):484–490. doi: 10.1161/STROKEAHA.111.626432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yuan Y, Guo Q, Ye Z, et al. Ischemic postconditioning protects brain from ischemia/reperfusion injury by attenuating endoplasmic reticulum stress-induced apoptosis through PI3K-Akt pathway. Brain Res. 2011;1367:85–93. doi: 10.1016/j.brainres.2010.10.017. [DOI] [PubMed] [Google Scholar]
- [11].Mathupala SP. Delivery of small-interfering RNA (siRNA) to the brain. Expert Opin Ther Pat. 2009;19(2):137–140. doi: 10.1517/13543770802680195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Boudreau RL, Davidson BL. RNAi therapeutics for CNS disorders. Brain Res. 2010;1338:112–121. doi: 10.1016/j.brainres.2010.03.038. [DOI] [PubMed] [Google Scholar]
- [13].Boudreau RL, Rodríguez-Lebrón E, Davidson BL. RNAi medicine for the brain: progresses and challenges. Hum Mol Genet. 2011;20(R1):R21–27. doi: 10.1093/hmg/ddr137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kelly A, Hurlstone AF. The use of RNAi technologies for gene knockdown in zebrafish. Brief Funct Genomics. 2011;10(4):189–196. doi: 10.1093/bfgp/elr014. [DOI] [PubMed] [Google Scholar]
- [15].Shegokar R, Al Shaal L, Mishra PR. SiRNA delivery: challenges and role of carrier systems. Pharmazie. 2011;66(5):313–318. [PubMed] [Google Scholar]
- [16].Staunstrup NH, Mikkelsen JG. Integrase-defective lentiviral vectors--a stage for nonviral integration machineries. Curr Gene Ther. 2011;11(5):350–362. doi: 10.2174/156652311797415881. [DOI] [PubMed] [Google Scholar]
- [17].Dreyer JL. Lentiviral vector mediated gene transfer and RNA silencing technology in neuronal dysfunctions. Methods Mol Biol. 2010;614:3–35. doi: 10.1007/978-1-60761-533-0_1. [DOI] [PubMed] [Google Scholar]
- [18].Kolattukudy PE, Niu J. Inflammation, endoplasmic reticulum stress, autophagy, and the monocyte chemoattractant protein-1/CCR2 pathway. Circ Res. 2012;110(1):174–189. doi: 10.1161/CIRCRESAHA.111.243212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Parmar VM, Schröder M. Sensing endoplasmic reticulum stress. Adv Exp Med Biol. 2012;738:153–168. doi: 10.1007/978-1-4614-1680-7_10. [DOI] [PubMed] [Google Scholar]
- [20].Jing G, Wang JJ, Zhang SX. ER stress and apoptosis: a new mechanism for retinal cell death. Exp Diabetes Res. 2012;2012:589589. doi: 10.1155/2012/589589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13(3):385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- [22].Nakka VP, Gusain A, Raghubir R. Endoplasmic reticulum stress plays critical role in brain damage after cerebral ischemia/reperfusion in rats. Neurotox Res. 2010;17(2):189–202. doi: 10.1007/s12640-009-9110-5. [DOI] [PubMed] [Google Scholar]
- [23].Holtz WA, O’Malley KL. Parkinsonian mimetics induce aspects of unfolded protein response in death of dopaminergic neurons. J Biol Chem. 2003;278(21):19367–19377. doi: 10.1074/jbc.M211821200. [DOI] [PubMed] [Google Scholar]
- [24].Sokka AL, Putkonen N. Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci. 2007;27(4):901–908. doi: 10.1523/JNEUROSCI.4289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13(3):184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Suyama K, Ohmuraya M, Hirota M, et al. C/EBP homologous protein is crucial for the acceleration of experimental pancreatitis. Biochem Biophys Res Commun. 2008;367(1):176–182. doi: 10.1016/j.bbrc.2007.12.132. [DOI] [PubMed] [Google Scholar]
- [27].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [28].Jin J, Bao X, Wang H, et al. RNAi-induced downregulation of Mecp2 expression in the rat brain. Int J Dev Neurosci. 2008;26(5):457–465. doi: 10.1016/j.ijdevneu.2008.02.009. [DOI] [PubMed] [Google Scholar]
- [29].Hayashi T, Abe K, Itoyama Y. Reduction of ischemic damage by application of vascular endothelial growth factor in rat brain after transient ischemia. J Cereb Blood Flow Metab. 1998;18(8):887–895. doi: 10.1097/00004647-199808000-00009. [DOI] [PubMed] [Google Scholar]
- [30].Finch CE, Marchalonis JJ. Evolutionary perspectives on amyloid and inflammatory features of Alzheimer disease. Neurobiol Aging. 1996;17(5):809–815. doi: 10.1016/0197-4580(96)00119-4. [DOI] [PubMed] [Google Scholar]


