Abstract
Repetitive transcranial magnetic stimulation is a noninvasive treatment technique that can directly alter cortical excitability and improve cerebral functional activity in unconscious patients. To investigate the effects and the electrophysiological changes of repetitive transcranial magnetic stimulation cortical treatment, 10 stroke patients with non-severe brainstem lesions and with disturbance of consciousness were treated with repetitive transcranial magnetic stimulation. A quantitative electroencephalography spectral power analysis was also performed. The absolute power in the alpha band was increased immediately after the first repetitive transcranial magnetic stimulation treatment, and the energy was reduced in the delta band. The alpha band relative power values slightly decreased at 1 day post-treatment, then increased and reached a stable level at 2 weeks post-treatment. Glasgow Coma Score and JFK Coma Recovery Scale-Revised score were improved. Relative power value in the alpha band was positively related to Glasgow Coma Score and JFK Coma Recovery Scale-Revised score. These data suggest that repetitive transcranial magnetic stimulation is a noninvasive, safe, and effective treatment technology for improving brain functional activity and promoting awakening in unconscious stroke patients.
Keywords: repetitive transcranial magnetic stimulation, consciousness disturbance, stroke, quantitative electroencephalography, nervous excitation, consciousness, neuroregenerative disease, regeneration, neural regeneration
Research Highlights
(1) The therapeutic effect of repetitive transcranial magnetic stimulation on the disturbance of consciousness was observed by quantitative electroencephalography spectral power analysis, which can reflect the degree of conscious disturbance through the distribution, power, and amplitude of the spectrum bands. This technique is superior to traditional electroencephalogram in evaluating the effect of magnetic stimulation.
(2) Repetitive transcranial magnetic stimulation can alter the excitability of the cerebral cortex and improve the disturbance of consciousness after stroke.
(3) The absolute power in the alpha band was increased after repetitive transcranial magnetic stimulation treatment.
(4) The relative power value in the alpha band was positively related to Glasgow Coma Score and JFK Coma Recovery Scale-Revised score.
Abbreviations
rTMS, repetitive transcranial magnetic stimulation; GCS, Glasgow Coma Score; CRS-R, JFK Coma Recovery Scale-Revised
INTRODUCTION
Repetitive transcranial magnetic stimulation (rTMS) given at a particular cortical site is a painless, noninvasive, simple, and convenient technique in the field of neurophysiological therapy. Through a rapidly changed pulse current, a magnetic stimulating coil placed on the human scalp can generate a strong magnetic field that can cross through the skull, causing secondary induction current at adjacent nerve tissues[1]. This current acts on the cell membrane of cerebral cortical neurons, resulting in excitatory or inhibitory postsynaptic potentials, which contribute to altered cortical function[2]. rTMS is currently used for the treatment of epilepsy, Parkinson's disease, cognitive dysfunction, psychiatric illness, and depression, and achieves satisfactory curative effects[3,4,5]. Different frequencies of rTMS produce a differing degree of effects. For example, low-frequency rTMS (< 1 Hz) can reduce neuronal excitability and inhibit cortical activity[6], while high-frequency rTMS (5–25 Hz) can improve neuronal excitability and enhance cortical activity[7]. Further, differences in cerebral blood flow, cortical function, and metabolites have been reported with different rTMS frequencies[8,9], with high-frequency stimulation increasing local metabolism levels and cerebral blood flow, while low-frequency stimulation reduces these parameters. In addition, rTMS may alter the transmission of monoamine neurotransmitters and genetic expression of neuronal excitability in the brain[9].
Electroencephalography is a technique used for rapid and real-time detection of cerebral functional activity, and is closely related to cerebral metabolites and behavioral neurological symptoms. The recovery and/or suppression of consciousness is dependent on the function of the cerebral cortex. Electroencephalography signals contain abundant cortical network information, which is associated with consciousness and cognition, and plays an important role in the diagnosis of conscious disorder and prediction of recovery[10]. Quantitative electroencephalography spectral power analysis using fast Fourier transformation can transform changes in electroencephalography amplitude into changes in electroencephalography energy. These spectral analyses can directly display the spatial distribution and energy changes of α, β, θ, and δ electroencephalography frequency bands in the cerebral cortex, and help to examine functional brain activity in patients with consciousness disturbance[11]. For example, Fingelkurts et al[12] demonstrated that static quantitative electroencephalography analysis had potential predictive values for the clinical outcome of patients under vegetative or minimally conscious states, with a poor outcome prognosis in patients predominated by δ wave and/or θ slow wave, but a good prognosis outcome in patients with α wave and/or θ wave.
Although rTMS has been widely used in the field of neural science and technology, including studies of mechanisms of cortical plasticity[13], there are few reports on the use of rTMS in consciousness disorder in stroke patients. Disturbance of consciousness includes disorders of consciousness and wakefulness, and the major clinical manifestations include coma, vegetative state, and minimal consciousness state[14]. The neuroanatomical structures that maintain consciousness and control conscious activity include the bilateral hemisphere functional cortex and the ascending reticular activating system[15]. The use of rTMS for effective stimulation of the brain functional cortex and the ascending reticular activating system is being widely examined.
As a noninvasive, safe, and effective treatment, rTMS can improve cerebral functional activity, regulate cortical and nervous excitation, and promote the recovery of patients with disturbance of consciousness. Piccione et al[16] reported that a single treatment of 20 Hz rTMS was effective for 6 hours in patients under a minimally conscious state, with an improvement in neural behavior and changes in electroencephalography. Louise-Bender Pape et al[17] demonstrated that after 5 Hz rTMS treatment, neurobehavioral functions of patients in a vegetative state were improved after 15 stimulations, and this improvement lasted for 25 stimulations, after which a fatigue state appeared. Nevertheless, existing studies on the use of rTMS for treatment of consciousness disorder are mostly case reports with small numbers of samples. Thus, in the present study, we investigated the effect of rTMS treatment on neural electrophysiology in 10 stroke patients with impaired consciousness using quantitative electroencephalography spectral power analysis, in a broader attempt to understand the onset time and efficacy duration of rTMS treatment, and to analyze the correlation between electroencephalography changes and improvement of neurobehavioral functions.
RESULTS
Clinical characteristics of stroke patients with disturbance of consciousness
All 10 patients were right-handed. Their clinical characteristics including age, sex, duration, Glasgow Coma Score (GCS), and JFK Coma Recovery Scale-Revised (CRS-R) score are shown in Table 1.
Table 1.
Clinical characteristics of stroke patients with disturbance of consciousness
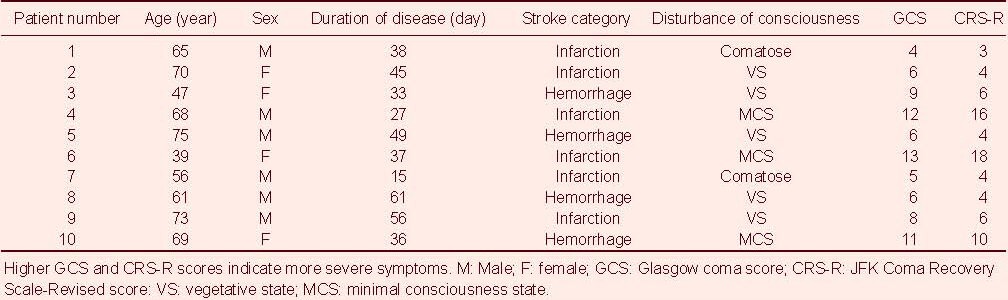
Absolute power values at α, β, θ, and δ bands in all brain regions of patients with disturbance of consciousness immediately after rTMS treatment
The absolute power values at α, β, θ, and δ bands in the electroencephalography recording sites (Fp1, Fp2, F3, F4, Fz, C3, C4, T3, T4, P3, P4, O1, O2) were compared by a paired t-test (Figure 1).
Figure 1.
Absolute power values at α, β, θ, and δ bands in all brain regions of disturbance of consciousness patients immediately after repetitive transcranial magnetic stimulation (rTMS) treatment. Data are presented as mean ± SD. aP < 0.05, vs. before treatment (paired samples t-test).
(A) Compared with before treatment, the α band absolute power at Fp1, Fp2, and O2 electrodes was significantly increased immediately after the first rTMS treatment. (B, D) Compared with before treatment, the β band and θ band absolute power values were not increased at each electrode recording sites immediately after the first rTMS treatment. (C) Compared with before treatment, the δ band activity was significantly reduced in the F3 and C3 electrodes immediately after the first rTMS treatment.
Immediately after the first rTMS treatment, the α band absolute power at Fp1, Fp2, and O2 electrodes was significantly increased (t = –2.231, P = 0.039; t = –2.125, P = 0.048; t = –2.372, P = 0.029). The δ band activity in F3 and C3 electrodes was significantly reduced (t = 2.314, P = 0.033; t = 3.169, P = 0.005). There was no significant difference in the absolute power values at β and θ bands at each electrode recording site after treatment (Figure 1).
Relative power values at α, β, θ, and δ bands at different time points after rTMS treatment of disturbance of consciousness patients
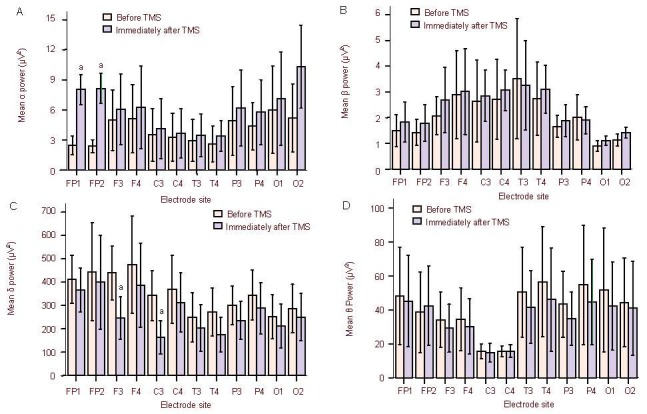
The relative power values at α, β, θ, and δ bands in 10 patients were compared before treatment, immediately after treatment, 1 day after treatment, and weekly after treatment. Results showed that the relative power value at the α band was increased (P < 0.01), while the value at the δ band was decreased immediately after the rTMS treatment (P < 0.01). At 1 day post-treatment, the α band relative power value was reduced, but remained higher than before treatment (P < 0.01). At 2 weeks post-treatment, the α band relative power remained stable (P < 0.01). There were no significant differences in the relative power values at the β and θ bands before and after treatment (Figure 2).
Figure 2.
Comparison of relative power values at α, β, θ, and δ bands at different time points after treatment.
Data are presented as mean ± SD. aP < 0.05, vs. before treatment (paired samples t-test).
Correlation between electroencephalography and clinical behavioral evaluation post-treatment
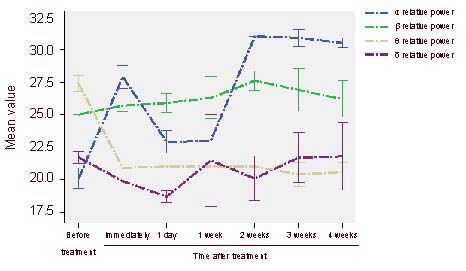
Ten patients were assessed with GCS and CRS-R before and after rTMS treatment. As shown in Figure 3, the GCS and CRS-R scores were not apparently changed after the first treatment, although the relative power value at the α band was increased. At 2 weeks post-treatment, the GCS and CRS-R scores were significantly increased. Further, Pearson correlation showed that the differences between 2 weeks post-treatment and before treatment of the relative power value at the α band was positively correlated with that of the GCS score and the CRS-R score (r = 0.900, P < 0.001; r = 0.892, P < 0.01).
Figure 3.
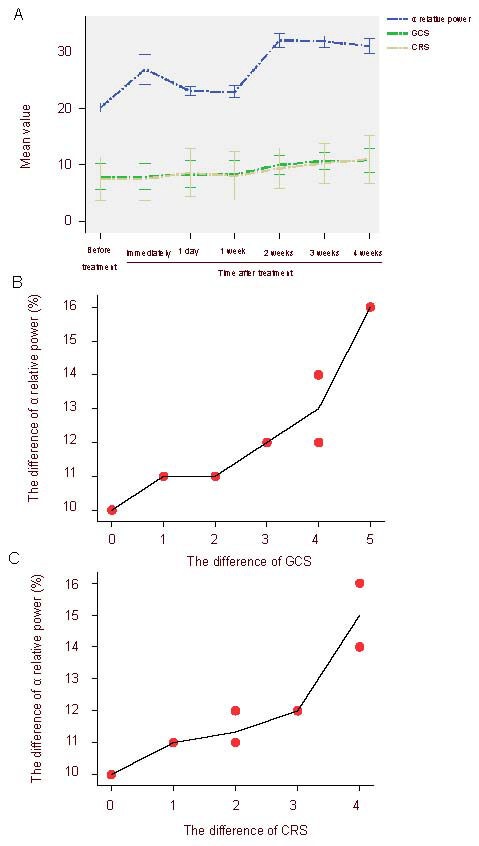
Correlation between the relative power values in the α band and the clinical neurobehaviors after repetitive transcranial magnetic stimulation treatment in patients with consciousness disturbance.
Glasgow Coma Score (GCS) and Coma Recovery Scale-Revised (CRS-R) score showed statistically significant differences at 2 weeks post-treatment compared with before treatment (P < 0.05). Pearson correlation analysis indicated a positive correlation between relative power value at α band and GCS score and CRS-R score.
(A) Changes of α relative power values and GCS and CRS-R scores at different time points after treatment. (B) The correlation between the difference of α relative power values and the difference of GCS score (the differences between 2 weeks post-treatment and before treatment) (r = 0.900, P < 0.001). (C) Correlation between the difference of α relative power values and the difference of CRS-R score (the differences between 2 weeks post-treatment and before treatment) (r = 0.892, P < 0.01).
DISCUSSION
In the present study, we examined the electroencephalography and neurobehavioral changes in stroke patients with disturbance of consciousness after rTMS treatment. Analysis of quantitative electroencephalography spectral power parameters showed that single rTMS treatment significantly increased the absolute power values at the α band in the Fp1, Fp2, and O2 electrode recording sites, and significantly decreased the δ band activity in the F3 and C3 electrodes. Further, the neurobiological effects of electroencephalography lasted until the following day, while neural behavior improvement was visible at 2 weeks.
Stimulation frequency
The therapeutic effect of rTMS mainly depends on the stimulation parameters, including stimulation frequency, stimulation intensity, stimulation site, coil direction, stimulation pattern, stimulation interval and duration, stimulation frequency ranges of 1–25 Hz, and a stimulation intensity that is 80–110% of the motor threshold. At differing parameters, TMS can activate, suppress, or interfere with the activities of neural cells at different cortical layers, thus resulting in different neurophysiological effects[18]. In the present study, electroencephalography in patients with a disorder of consciousness appeared to change after high-frequency (5 Hz) stimulation treatment. This is consistent with a previous report that high-frequency rTMS (5–25 Hz) can improve neuronal excitability and enhance cortical activity[7].
Stimulation locus
The spatial resolution of rTMS is controlled within 1 cm, with a penetration depth of approximately 3 cm[19]. Thus, rTMS largely acts on cerebral cortex cells, while the brain areas located deep below the scalp (including the hippocampus, thalamus, and amygdala) remain impractical to target at present[18]. The neural anatomical structure involved in the maintenance of consciousness and performing conscious activity include the wide cerebral cortex and ascending reticular activating system. As such, it is important to select the cortical locus for rTMS for promoting consciousness recovery. In this study, the right dorsolateral prefrontal cortex was defined as the rTMS stimulation site for the following reasons:
(1) The dorsolateral prefrontal cortex is closely linked with the brain network structure, with the ascending reticular activating system connecting to the dorsolateral prefrontal cortex, and even the entire cortex, via the thalamic relay nucleus and raphe nucleus[17]. (2) The dorsolateral prefrontal cortex is believed to enhance learning, memory, and attention, while attention, memory, and consciousness have the closest correlation with awareness[20,21]. (3) The dorsolateral prefrontal cortex in the right cerebral hemisphere is primarily responsible for the regulation of attention networks and mediates attention, which interacts with the human sensory system[17].
The onset time and duration of effect
The optimal rTMS treatment course, onset time, and duration remain unclear. The present study showed that a dynamic electroencephalography change was visible after single rTMS treatment. This result is consistent with the findings of Piccione et al[16], who reported that single rTMS treatment improved neural behavior in patients with disorder of consciousness, although these effects only lasted for 6 hours. In the present study, electroencephalography changes were still observed at 1 day post-treatment, with a decrease in the absolute power values at the α band compared with immediately after treatment. We also found that the neurobehavioral improvements occurred at 2 weeks post-treatment. Although there are few studies reporting the long-term efficacy of electroencephalography, Louise-Bender pape et al[17] reported that the neurobiological effects of rTMS in patients with disturbance of consciousness remained at 6 weeks after cessation of treatment. The present study is a small-sample short-term observation, and further studies are required to investigate the efficacy duration.
Neurobiological effect of rTMS treatment
The results of our study showed that rTMS treatment could significantly increase the power values at the α band in stroke patients with impaired consciousness, and that the relative power value showed a linear positive correlation with GCS and CRS-R scores. These data suggest that the improved neurobehavioral functions in patients with consciousness disorder following rTMS may be attributed to cortical neural electrophysiological changes. Although the rTMS stimulation site is localized in the right dorsolateral prefrontal cortex, the neural electrophysiological effects are widely observed, including other electroencephalography recording sites. Electroencephalography α band waves are closely linked to human behaviors, and the electroencephalography energy of cortical α rhythm can predict consciousness recovery of patients in a vegetative state[22]; the higher the energy, the better the recovery. After rTMS treatment, the enhanced α band energy can also improve cognitive ability[23], and electrophysiological activity at the α band may be enhanced because of the improvement of the cortex-thalamus interactions[24]. The energy increase at the α band is related to improved brain function[16], and the increase of absolute power at the α band and the decrease of δ band activity both represent altered metabolic activity in the central nervous system. Our study showed similar electroencephalography changes, and this electrophysiological improvement is closely attributed to neural behaviors such as consciousness recovery, thus demonstrating a positive role of rTMS in improving brain functions and promoting consciousness recovery in patients with disturbance of consciousness.
Safety analysis
The safety of rTMS has been widely recognized, and there have been no reports of obvious abnormal reaction in animals and clinical studies. There are a few side effects of rTMS reported in humans, including epilepsy, headache, head discomfort, tinnitus, hearing impairment, and local skin burns. In our study, all patients tolerated rTMS treatment well, with no seizures or other adverse reactions during the treatment process. However, clinical practice and experience in performing rTMS remains insufficient. Thus, further studies are required to examine the most effective treatment parameters, and clinicians should remain cautious in the selection of cases, treatment parameters, and complications when performing rTMS.
Limitations
Stroke patients with disorders of consciousness can be grouped into cortical injury and brainstem injury, which may exhibit different prognoses and rTMS effects. The present study was a short-term study with a small sample size. Thus, we did not examine patients suffering from disturbance of consciousness as a result of severe brainstem injury. Future studies are required to focus on the comparison of the two types of stroke patients with disorders of consciousness after rTMS treatment. Further, the small sample size in our study did not allow comparisons of patients at different states (e.g., coma, vegetative state, or minimally conscious state).
Problems and prospects
A number of problems remain in the use of rTMS for treatment of consciousness disorders, as follows. (1) rTMS location: the precise positioning of rTMS stimulator is the greatest problem encountered in clinical application. Application of computer-aided frameless stereotactic navigation rTMS and combination of rTMS with functional magnetic resonance imaging have greatly improved the accuracy of rTMS location. (2) The optimal stimulation parameters: the rTMS treatment effect depends on stimulus parameters. However, there is no uniform definition for specific parameters. (3) The optimal treatment course, duration, and evaluation remain unclear. (4) There is little known of the clinical efficacy and underlying neurobiological mechanism of rTMS for treatment of consciousness disorders awakening therapy. (5) The safety of long-term rTMS application remains unknown.
Nevertheless, as a noninvasive, easily operated, safe, and effective treatment, rTMS can improve the activity of brain cells, regulate neuronal excitability, and promote functional recovery of injured brains. rTMS treatment can also improve consciousness impairment in post-stroke patients. However, clinical research remains in the exploratory stage, and future large-sample randomized controlled studies are required.
Summary
We found that single rTMS treatment significantly increased the absolute power at the α band and significantly decreased the energy at the δ band in stroke patients. The GCS score and CRS-R score were significantly improved at 2 weeks post-treatment, indicating the improvement of neural behaviors. Further, the relative power value at α band was positively correlated with GCS and CRS-R scores. These data suggest that rTMS therapy can improve brain electrical activities in stroke patients with consciousness disorders, and may promote the recovery of these patients.
SUBJECTS AND METHODS
Design
A self-controlled, clinical study.
Time and setting
This study was performed in the Department of Rehabilitation, Teaching Hospital of Capital Medical University, Beijing Electric Power Hospital, China from September 2010 to October 2011.
Subjects
Ten patients with non-severe brainstem lesions and with disturbance of consciousness who presented at the Department of Rehabilitation, Beijing Electric Power Hospital were involved in this study, including six males and four females, aged 25–65 years old. All patients were assessed by two experienced attending physicians with GCS[25] and CRS-R[16] for the severity of conscious disturbance. Analgesics or anesthetics were forbidden on the day of testing. The Hospital Ethics Committee approved the study. Informed written consent was obtained from the guardians or family members of the involved subjects.
Inclusive criteria: (1) stroke was a factor leading to consciousness disturbance; (2) no severe brainstem lesions were found; (3) cases exhibited stable disease condition and vital signs, with no hydrocephalus or severe cerebral atrophy; (4) all hemorrhage lesions had been absorbed in cerebral hemorrhage patients; (5) disease occurred within the last 3 months.
All patients received a GCS score, CRS-R score, and electroencephalography examination before the first treatment, immediately after the first treatment, 1 day after the first treatment, and every week after treatment (total 4 weeks).
Methods
rTMS treatment
In addition to conventional drug therapy, all patients were given rTMS treatment. Patients in decubitus position were given rTMS treatment on the right dorsolateral prefrontal cortex using a magnetic stimulator (Magstim Rapid-2 type, MagStim Company Ltd., Dyfed, UK), with the coil handle facing upward. The stimulation parameters were as follows: peak stimulation intensity of 2.0T, maximal stimulation intensity of 90% motor threshold, and stimulation frequency of 10 Hz, each for 20 minutes, once per day, 5 times per week, for a total of 20 times within 4 weeks (Figure 4).
Figure 4.
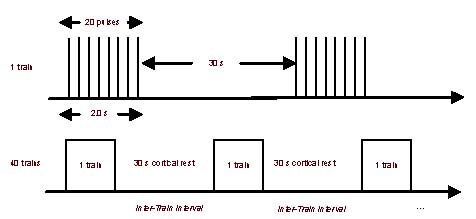
Illustration of repetitive transcranial magnetic stimulation parameters.
Each patient received 40 trains per session over a total of 20 sessions. s: Second.
Electroencephalography recording
According to international standard lead 10/20 system[26] 21 lead electrodes were placed (FP1, FP2, F3, F4, C3, C4, P3, P4, O1, O2, F7, F8, T3, T4, T5, T6, FZ, PZ, CZ) using a digital electroencephalography instrument (Micromed S.r.l, Mogliano Veneto, Italy) and Ag/AgCl electrodes. Double earlobe A1 and A2 were used as reference electrodes. The electroencephalography recording electrode montage and nomenclature are depicted in Figure 5.
Figure 5.
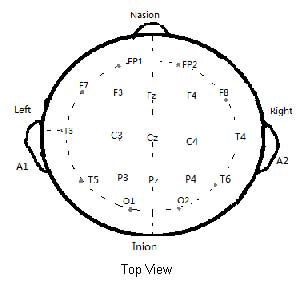
Schematic diagram of the electroencephalography recording electrode montage.
Electrode skin impedance was always less than 5 kΩ. Unipolar lead plethysmography was performed for more than 30 minutes. Data were stored on a hard drive for further analysis. The electroencephalography epochs were acquired and the epochs of choosing the best quality were used. After eye movements, blinking, ECG, and EMG artifacts were excluded, electroencephalography data were randomly sampled for 30 seconds, and then divided into four frequency bands: δ (1–4 Hz), θ (4–8 Hz), α (8–12 Hz), and β (12–25 Hz). Electroencephalography at each frequency band was analyzed using fast Fourier transformation for the power spectral analysis. The absolute and relative power values at α, β, θ, and δ bands were calculated.
Statistical analysis
Data were statistically analyzed with SPSS 17.0 software (SPSS, Chicago, IL, USA). The changes of absolute power values in each band were compared by paired samples t-test between before the rTMS treatment and immediately after treatment. The electroencephalography spectrum indicators were analyzed with random analysis of variance method before treatment, immediately after treatment, 1 day post-treatment, and weekly post-treatment. Pearson linear analysis was performed for analyzing the correlation between electroencephalography spectrum changes and GCS score, CRS-R score. A P value less than 0.05 was considered a statistically significant difference.
Acknowledgments:
We thank Professor Zhaoran Chen from Higher Brain Function Center, Capital Medical University, China for giving guidance and Technician Lixin Li from Electroencephalography Room of Beijing Electric Power Hospital, China for providing support.
Footnotes
Funding: This study was founded by Committee of Science and Technology, Fengtai District of Beijing City in 2010, No. xm101223.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Ethics Committee, Electric Power Teaching Hospital of Capital Medical University, China.
(Edited by Liu CY, Li DF/Yang Y/Song LP)
REFERENCES
- [1].Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- [2].Di Lazzaro V, Oliviero A, Pilato F, et al. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004;115(2):255–266. doi: 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- [3].Sun W, Fu W, Mao W, et al. Low-frequency repetitive transcranial magnetic stimulation for the treatment of refractory partial epilepsy. Clin EEG Neurosci. 2011;42(1):40–44. doi: 10.1177/155005941104200109. [DOI] [PubMed] [Google Scholar]
- [4].Kodama M, Kasahara T, Hyodo M, et al. Effect of low-frequency repetitive transcranial magnetic stimulation combined with physical therapy on L-dopa-induced painful off-period dystonia in Parkinson's disease. Am J Phys Med Rehabil. 2011;90(2):150–155. doi: 10.1097/PHM.0b013e3181fc7ccd. [DOI] [PubMed] [Google Scholar]
- [5].Schutter DJ. Transcranial magnetic stimulation as a treatment for depression. Tijdschr Psychiatr. 2011;53(6):343–353. [PubMed] [Google Scholar]
- [6].Romero JR, Anschel D, Sparing R, et al. Subthreshold low frequency repetitive transcranial magnetic stimulation selectively decreases facilitation in the motor cortex. Clin Neurophysiol. 2002;113(1):101–107. doi: 10.1016/s1388-2457(01)00693-9. [DOI] [PubMed] [Google Scholar]
- [7].Berardelli A, Inghilleri M, Rothwell JC, et al. Facilitation of muscle evoked responses after repetitive cortical stimulation in man. Exp Brain Res. 1998;122(1):79–84. doi: 10.1007/s002210050493. [DOI] [PubMed] [Google Scholar]
- [8].Hosono Y, Urushihara R, Harada M, et al. Comparison of monophasic versus biphasic stimulation in rTMS over premotor cortex: SEP and SPECT studies. Clin Neurophysiol. 2008;119(11):2538–2545. doi: 10.1016/j.clinph.2008.07.279. [DOI] [PubMed] [Google Scholar]
- [9].Wassermann EM, Lisanby SH. Therapeutic application of repetitive transcranial magnetic stimulation: a review. Clin Neurophysiol. 2001;112(8):1367–1377. doi: 10.1016/s1388-2457(01)00585-5. [DOI] [PubMed] [Google Scholar]
- [10].Boccagni C, Bagnato S, Sant Angelo A, et al. Usefulness of standard EEG in predicting the outcome of patients with disorders of consciousness after anoxic coma. J Clin Neurophysiol. 2011;28(5):489–492. doi: 10.1097/WNP.0b013e318231c8c8. [DOI] [PubMed] [Google Scholar]
- [11].John ER, Halper JP, Lowe RS, et al. Source imaging of QEEG as a method to detect awareness in a person in vegetative state. Brain Inj. 2011;25(4):426–432. doi: 10.3109/02699052.2011.558045. [DOI] [PubMed] [Google Scholar]
- [12].Fingelkurts AA, Fingelkurts AA, Bagnato S, et al. Life or death: prognostic value of a resting EEG with regards to survival in patients in vegetative and minimally conscious States. PLoS One. 2011;6(10):e25967. doi: 10.1371/journal.pone.0025967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen R, Udupa K. Measurement and modulation of plasticity of the motor system in humans using transcranial magnetic stimulation. Motor Control. 2009;13(4):442–453. doi: 10.1123/mcj.13.4.442. [DOI] [PubMed] [Google Scholar]
- [14].Wu DY, Cai G, Yuan Y, et al. Application of nonlinear dynamics analysis in assessing unconsciousness: a preliminary study. Clin Neurophysiol. 2011;122(3):490–498. doi: 10.1016/j.clinph.2010.05.036. [DOI] [PubMed] [Google Scholar]
- [15].Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state, and related disorders. Lancet Neurol. 2004;3(9):537–546. doi: 10.1016/S1474-4422(04)00852-X. [DOI] [PubMed] [Google Scholar]
- [16].Piccione F, Cavinato M, Manganotti P, et al. Behavioral and neurophysiological effects of repetitive transcranial magnetic stimulation on the minimally conscious state: a case study. Neurorehabil Neural Repair. 2011;25(1):98–102. doi: 10.1177/1545968310369802. [DOI] [PubMed] [Google Scholar]
- [17].Louise-Bender Pape T, Rosenow J, Lewis G, et al. Repetitive transcranial magnetic stimulation-associated neurobehavioral gains during coma recovery. Brain Stimul. 2009;2(1):22–35. doi: 10.1016/j.brs.2008.09.004. [DOI] [PubMed] [Google Scholar]
- [18].Lapitska N, Gosseries O, Delvaux V, et al. Transcranial magnetic stimulation in disorders of consciousness. Rev Neurosci. 2009;20(3-4):235–250. doi: 10.1515/revneuro.2009.20.3-4.235. [DOI] [PubMed] [Google Scholar]
- [19].Terao Y, Ugawa Y. Basic mechanisms of TMS. J Clin Neurophysiol. 2002;19(4):322–343. doi: 10.1097/00004691-200208000-00006. [DOI] [PubMed] [Google Scholar]
- [20].Müri RM, Vermersch AI, Rivaud S, et al. Effects of single-pulse transcranial magnetic stimulation over the prefrontal and posterior parietal cortices during memory-guided saccades in humans. J Neurophysiol. 1996;76(3):2102–2106. doi: 10.1152/jn.1996.76.3.2102. [DOI] [PubMed] [Google Scholar]
- [21].Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28(5):597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- [22].Babiloni C, Sarà M, Vecchio F, et al. Cortical sources of resting-state alpha rhythms are abnormal in persistent vegetative state patients. Clin Neurophysiol. 2009;120(4):719–729. doi: 10.1016/j.clinph.2009.02.157. [DOI] [PubMed] [Google Scholar]
- [23].Klimesch W, Sauseng P, Gerloff C. Enhancing cognitive performance with repetitive transcranial magnetic stimulation at human individual alpha frequency. Eur J Neurosci. 2003;17(5):1129–1133. doi: 10.1046/j.1460-9568.2003.02517.x. [DOI] [PubMed] [Google Scholar]
- [24].Laureys S. The neural correlate of (un)awareness: lessons from the vegetative state. Trends Cogn Sci. 2005;9(12):556–559. doi: 10.1016/j.tics.2005.10.010. [DOI] [PubMed] [Google Scholar]
- [25].Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- [26].American Clinical Neurophysiology Society. Guideline 6: A proposal for standard montages to be used in clinical EEG. J Clin Neurophysiol. 2006;23(2):111–117. doi: 10.1097/00004691-200604000-00007. [DOI] [PubMed] [Google Scholar]


