Abstract
Oxidative stress may be the unifying factor for the injury caused by hyperglycemia in diabetic peripheral neuropathy. Puerarin is the major isoflavonoid derived from Radix puerariae and has been shown to be effective in increasing superoxide dismutase activity. This study sought to investigate the neuroprotective effect of puerarin on high glucose-induced oxidative stress and Schwann cell apoptosis in vitro. Intracellular reactive oxygen radicals and mitochondrial transmembrane potential were detected by flow cytometry analysis. Apoptosis was confirmed by TUNEL and oxidative stress was monitored using an enzyme-linked immunosorbent assay for the DNA marker 8-hydroxy-2-deoxyguanosine. The expression levels of bax and bcl-2 were analyzed by quantitative real-time reverse transcriptase-PCR, while protein expression of cleaved caspase-3 and -9 were analyzed by means of western blotting. Results suggested that puerarin treatment inhibited high glucose-induced oxidative stress, mitochondrial depolarization and apoptosis in a dose-dependent manner. Furthermore, puerarin treatment downregulated Bax expression, upregulated bcl-2 expression and attenuated the activation of caspase-3 and -9. Overall, our results indicated that puerarin antagonized high glucose-induced oxidative stress and apoptosis in Schwann cells.
Keywords: puerarin, diabetic peripheral neuropathy, hyperglycemia, Schwann cell, apoptosis, caspase, mitochondrial transmembrane potential, oxidative stress, 8-hydroxy-2-deoxyguanosine, reactive oxygen radical
Research Highlights
-
(1)
High glucose-induced oxidative stress and mitochondrial dysfunction-associated Schwann cell apoptosis.
-
(2)
Schwann cell apoptosis occurred via the caspase-dependent pathway.
-
(3)
Puerarin inhibited oxidative stress-induced apoptosis of Schwann cells.
-
(4)
Neurotoxicity induced by high glucose was not associated with osmolarity.
Abbreviations DPN, diabetic peripheral neuropathy; ROS, reactive oxygen species; 8-OHdG, 8-hydroxy-2-deoxyguanosine; ΔΨm, mitochondrial transmembrane potential
INTRODUCTION
Diabetic peripheral neuropathy (DPN) is one of the most common microvascular complications of diabetes. Depending on the methods used to diagnose DPN, it is generally held that at least 50% of all diabetic patients will develop neuropathy[1,2,3]. However, the precise pathogenesis of DPN remains unclear and is known to involve multiple pathways, including the polyol, advanced glycation end-products, protein kinase C and hexosamine pathway. Recent evidence indicates that all of the four pathways result from increased levels of cellular reactive oxygen species (ROS), which impairs nerve function, resulting in apoptosis and subsequent neuronal injury[4,5,6,7,8,9,10]. Apparently, inhibition of ROS may restore metabolic and vascular imbalances and block the progression of neuropathy[6,11].
Radix puerariae (kudzu root) is a well-known traditional Chinese medicinal herb, which has been widely used for treating various diseases including cardiovascular and brain disorders[12]. Puerarin (daidzein-8-C-glucoside) is the major isoflavonoid derived from Radix puerariae and has been proven to be effective in treating heart diseases[13]. Recent evidence indicates that the protective mechanisms of puerarin are at least partly related to its function in increasing superoxide dismutase activity[13,14]. However, whether puerarin can benefit patients with DPN has not been explored.
Schwann cells play a critical role in maintaining peripheral nerve function and are susceptible to hyperglycemic toxicity because they take up glucose through the insulin-independent glucose transporter[15,16]. Schwann cells may be a natural target for the treatment of DPN because defects in Schwann cells are reversible[17,18]. Hyperglycemia has been proposed to be a potent initiator of apoptosis, and Schwann cell apoptosis has been detected in diabetic models[10,19,20,21]. In fact, oxidative stress reduces mitochondrial transmembrane potential (ΔΨm) and triggers apoptosis by activating caspase-9 and -3. The apoptotic process is mainly regulated by BcI-2 family proteins.
Little is known about the effect of puerarin on high glucose-induced oxidative stress and the downstream signaling that leads to apoptosis. This study aimed to examine the impact of high glucose on Schwann cell apoptosis and determine if puerarin treatment could modulate the effect of high glucose on Schwann cell apoptotic cell death in vitro.
RESULTS
Puerarin inhibits high glucose-induced ROS generation in Schwann cells in vitro
The levels of intracellular ROS in different groups of cells were determined by flow cytometry (Figures 1A and B). In comparison with the control, the relative levels of ROS in the high glucose group increased by 2.26 folds (P < 0.01). The relative levels of intracellular ROS in puerarin-treated cells were reduced by 13.9% (P < 0.05), 26.9% (P < 0.01) and 39.5% (P < 0.01), at 1, 10 and 100 μM, respectively. It is quite clear that puerarin inhibited the high glucose-induced overproduction of ROS in Schwann cells in a dose-dependent manner from 1 to 100 μM. Interestingly, no significant difference in the relative levels of intracellular ROS was observed between mannitol-treated and control cells (P > 0.05).
Figure 1.
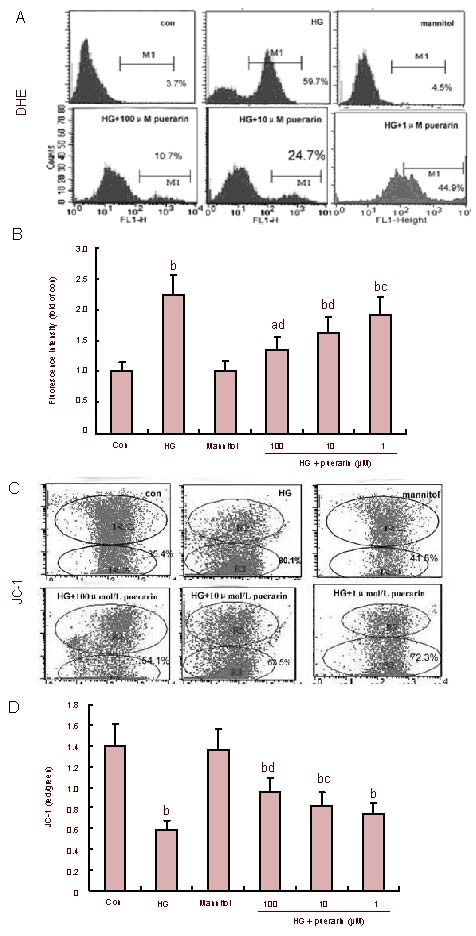
Effect of puerarin on reactive oxygen species (ROS) and mitochondrial transmembrane potential (Ψm) in Schwann cells treated with high glucose (HG).
The levels of intracellular ROS and Ψm in different groups of cells were measured by flow cytometry.
(A) The levels of ROS. Data shown are representative histograms of different groups of cells. (B) Quantitative analysis. Data are expressed as the mean ± SEM of each group of cells from three separate experiments.
(C) Depolarization of Ψm became evident as indicated by an increasing cell population in R3. (D) The changes of Ψ were analyzed using the fluorescent dye JC-1. The results were calculated as the ratio of red/green fluorescence quantitative analysis. Data are expressed as mean ± SEM of each group of cells from three separate experiments. aP < 0.05, bP < 0.01, vs. control (con); cP < 0.05, dP < 0.01, vs. HG.
Puerarin mitigated high glucose-induced mitochondrial depolarization and DNA damage in Schwann cells
As shown in Figures 1C and D, the values of ΔΨm in the high glucose group significantly decreased (P < 0.01). However, values of ΔΨm in puerarin-treated cells were significantly increased in a dose-dependent manner. 8-hydroxy-2-deoxyguanosine (8-OHdG) is a sensitive and specific marker of oxidative DNA damage. The experimental data showed that concentrations of 8-OHdG in the high glucose group significantly increased when compared with the control (P < 0.01; Figure 2). Furthermore, the high glucose groups treated with high and medium concentrations of puerarin decreased 8-OHdG levels (P < 0.05, P < 0.01). There was no significant difference in the values of ΔΨm and concentrations of 8-OHdG between the control and mannitol-treated group (P > 0.05). Therefore, puerarin inhibited the high glucose-related oxidative stress-mediated loss of ΔΨm and DNA damage in Schwann cells.
Figure 2.
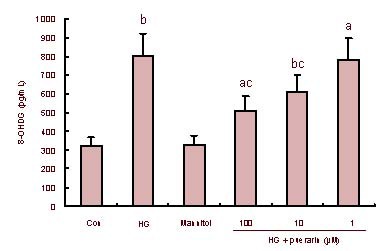
Effect of puerarin on DNA damage in Schwann cells treated with high glucose (HG).
The concentrations of 8-hydroxy-2-deoxyguanosine (8-OHdG), a sensitive and specific marker of oxidative DNA damage, in different groups of Schwann cells were determined using an enzyme-linked immunosorbent assay. Data are expressed as the mean ± SEM of each group of cells from three separate experiments. aP < 0.05, bP < 0.01, vs. control (con); cP < 0.05, vs. HG.
Puerarin inhibits high glucose-induced apoptosis of Schwann cells
Apoptotic cells were identified by a black-brown signal following 3,3’-diaminobenzidine staining (Figure 3A). As shown in Figure 3B, quantitative analysis indicated that the percentage of apoptotic cells in the high glucose group was significantly higher than that in control cells (P < 0.01). While treatment with mannitol did not change the apoptotic rate of Schwann cells, treatment with puerarin did (P < 0.01). Therefore, it can be concluded that puerarin inhibited high glucose-induced apoptosis of Schwann cells in vitro.
Figure 3.
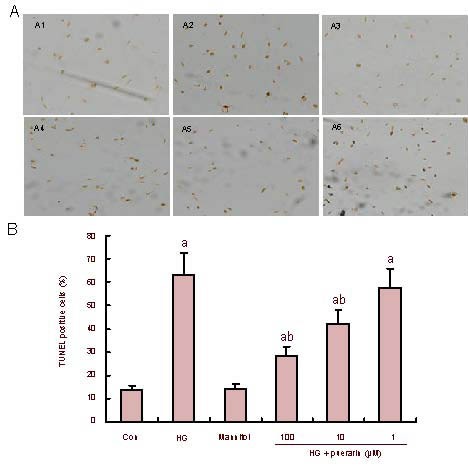
Effect of puerarin on Schwann cell apoptosis following treatment with high glucose (HG).
(A) Apoptosis was confirmed by TUNEL with 3,3’-diaminobenzidine (× 100). Representative photomicrographs of TUNEL in Schwann cells are shown (black-brown signal).
(B) Quantitative analysis. At least 500 cells from each group were examined in a blinded manner. Data are expressed as mean ± SEM of each group of cells from three separate experiments. aP < 0.01, vs. control (con); bP < 0.01, vs. HG. A1: control; A2: HG; A3: mannitol; A4: HG + puerarin 100 μM; A5: HG + puerarin 10 ìM; A6: HG + puerarin 1 μM.
Effects of puerarin on high glucose-related bax and bcl-2 expression
To understand the mechanisms underlying the role of puerarin in high glucose-related Schwann cell apoptosis, the relative levels of anti-apoptotic bcl-2 and proapoptotic bax mRNA transcripts in different groups of cells were determined by quantitative reverse transcription-PCR (Figures 4A and B).
Figure 4.
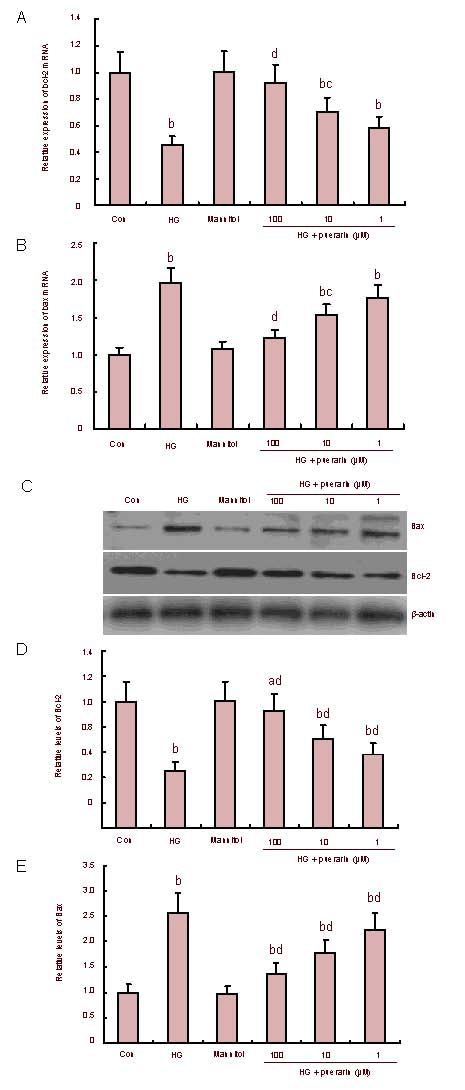
Effect of puerarin on the expression of Bax and Bcl-2 in Schwann cells treated with high glucose (HG).
(A, B) The relative levels of bcl-2 and bax mRNA transcripts. (C) The representative images of Western blots. Whole cell lysates were separated by SDS-PAGE, followed by immunoblotting. β-actin levels are shown as a control for equal loading.
(D, E) Quantitative analysis of Bcl-2 and Bax protein. Data are expressed as mean ± SEM of each group of cells from three separate experiments and the value of the control cells was designated as 1. aP < 0.05, bP < 0.01, vs. control; cP < 0.05, dP < 0.01, vs. HG.
Quantitative analysis indicated that Schwann cells in high glucose conditions significantly enhanced the transcription of the bax gene, but reduced the transcription of the bcl-2 gene when compared with that of the control (P < 0.01). Treatment with mannitol did not change the expression of these genes; however, treatment with puerarin significantly attenuated the effect of high glucose on the expression of these genes, particularly in cells treated with 100 and 10 μM of puerarin (P < 0.05, P < 0.01). As shown in Figures 4C–E, western blot analysis revealed that Schwann cells in high glucose conditions significantly enhanced the expression of bax, but reduced the expression of bcl-2 when compared with the control (P < 0.01). Expression of bax in puerarin-treated cells was significantly reduced while the relative levels of bcl-2 in these groups of cells were significantly elevated when compared with that of the high glucose group (P < 0.01). There was no significant difference in protein expression between the control and osmotic control (P > 0.05).
Effect of puerarin on caspase activation (Figure 5)
Figure 5.
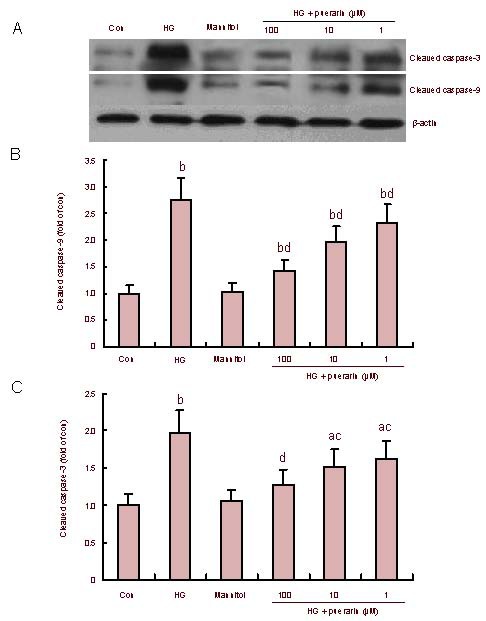
Effect of puerarin on caspase-3 and caspase-9 activation in Schwann cells treated with high glucose (HG; western blotting).
(A) Representative images.
(B, C) Quantitative analysis of cleaved caspase-9 and cleaved caspase-3. Data are representative images or expressed as mean ± SEM of each group of cells from three separate experiments and the value of the control cells was designated as 1. aP < 0.05, bP < 0.01, vs. control; cP < 0.05, dP < 0.01, vs. HG.
To further characterize hyperglycemia-related apoptosis, relative levels of cleaved caspase-9 and -3 were determined by western blot analysis (Figure 5A). The quantitative analysis revealed that relative levels of cleaved caspase-9 and-3 in the high glucose group of cells were significantly higher than those of the control (P < 0.01; Figures 5B and C). Furthermore, there was no significant difference in the levels of these proteins between the mannitol-treated and untreated controls (P > 0.05). However, treatment with puerarin significantly inhibited caspase-9 and-3 activation and their inhibitory effect appeared to be dose-dependent in the high glucose group. These data clearly demonstrated that puerarin inhibited high glucose-related caspase activation in Schwann cells.
DISCUSSION
Hyperglycemia, which occurs under diabetic conditions, is widely recognized as a major etiological factor causing diabetic complications[22]. For nervous cells, previous studies have shown that hyperglycemic injury can occur when glucose levels are higher than 35 mM. However, lower concentrations of glucose have been shown to induce neuronal death in culture[8]. Moreover, 30 to 150 mM glucose has been used in individual experiments to produce hyperglycemic injury in Schwann cells, which is higher than human serum glucose concentrations[21,23,24,25,26]. However, the reason why these hyperglycemic concentrations lead to high levels of Schwann cell injury (in vitro) remains unclear. In this study, we used 50 mM glucose as a high dose according to the previous studies[27,28].
Increasing evidence indicates that mitochondria are both the source and target of ROS[29]. As hyperglycemic cells metabolize more glucose, increased turnover of mitochondrial energy-generating complexes occur, increasing the production of free radicals[30]. Hyperglycemia can induce oxidative stress in mitochondria, which in turn activates four known pathways: the polyol, hexosamine, protein kinase C and advanced glycation end-products pathways[5]. Schwann cells are susceptible to oxidative stress because of their large population of mitochondria. ROS, generated by high glucose, leads to nervous system damage and Schwann cells are specific targets of oxidative injury[4,21]. Hyperglycemia-related stress results in apoptotic cell death and ROS, which is also a well-known initiator of apoptosis in many cell types[31,32,33]. This study examined the effect of constant high glucose on ROS production and apoptosis of Schwann cells in vitro. The present study found that high glucose produced higher levels of ROS and DNA-associated 8-OHdG and promoted more significant changes in ΔΨm than the control. In agreement with previous studies[34,35,36], this study confirmed that stable high glucose led to an increase in apoptosis as a result of oxidative stress generation. This suggests a cause-effect relationship between mitochondrial oxidative stress and Schwann cell apoptosis.
According to traditional Chinese medicine, Radix puerariae is a popular herb, which has been widely and successfully used for many years. Puerarin (daidzein-8-C-glucoside) is a major isoflavonoid derived from Radix puerariae. It was reported to possess many biological activities[13,14]. Nevertheless, there is insufficient information on its protective properties in DPN. The present study revealed that puerarin significantly inhibited high glucose-stimulated ROS production, 8-OHdG formation, loss of ΔΨm and apoptosis in Schwann cells in a dose-dependent manner. These data indicated that treatment with puerarin can reduce oxidative stress-related apoptosis and may be beneficial for patients with DPN.
Apoptosis involves a cascade of intracellular events, the process of which could not be ceased once activated[37,38]. We characterized the molecular pathways through which high glucose triggered Schwann cell apoptosis. We found that high glucose significantly upregulated bax expression, but downregulated bcl-2 expression in Schwann cells. Meanwhile, high glucose promoted the activation of caspase-9 and -3. More importantly, puerarin inhibited high glucose-upregulated bax expression, but antagonized high glucose- downregulated bcl-2 expression in Schwann cells. In addition, puerarin inhibited caspase-9 and -3 cleavage in a dose-dependent manner. These changes were accompanied by decreasing Schwann cell apoptosis. Given that puerarin can reduce Schwann cell apoptosis, the protective effect of puerarin on apoptotic pathway- related events were mediated by inhibiting oxidative injury in Schwann cells. Therefore, it is possible that puerarin may be valuable for the treatment of DPN.
In this study, to exclude the role of osmolarity on apoptosis, we used mannitol as an osmotic control. Data showed that the neurotoxicity of glucose was not related to osmolarity. Instead, it is a direct effect of glucose on the cell. In summary, high glucose induced the overproduction of ROS and mitochondrial dysfunction, which triggered a high rate of Schwann cell apoptosis. Furthermore, puerarin inhibited high glucose-induced oxidative stress and mitochondrial dysfunction-associated Schwann cell apoptosis. Although further work is still needed, our findings provide remarkable evidence that puerarin exhibits a protective effect on Schwann cells exposed to high glucose.
MATERIALS AND METHODS
Design
This is a cytological, comparative, observational study.
Time and setting
Experiments were performed at the Institute of Stomatology, Chinese PLA General Hospital, Beijing, China from June 2009 to December 2010.
Materials
New-born Sprague-Dawley rats aged 3 days old were obtained from Wei-tong Lihua Experimental Animal Center (Beijing, China; license No. SCXK 2009-0013). All protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[39].
Methods
Preparation of puerarin
Puerarin was purchased from Phytomarker Ltd (Tianjin, China) and the purity was determined to be 98% by high-performance liquid chromatography. A stock solution of puerarin (1 mM) was dissolved in deionized water and stored at −20 °C.
Cell culture and treatment
Schwann cells were obtained from the sciatic nerves of new-born Sprague-Dawley rats under aseptic conditions, as previously described[27]. Briefly, sciatic nerves were dissected out, placed into D-Hanks medium and minced into roughly 1 mm × 1 mm explants. The nerve explants were cultured in Dulbecco's modified Eagle's medium (Gibco, Grand Island, NY, USA) supplemented with 20% (v/v) heat-inactivated fetal bovine serum (Hyclone, Logan, UT, USA), 100 U/mL penicillin (Gibco), 100 μg/mL streptomycin (Gibco), 2 mM L-glutamine, 1 mM sodium pyruvate and 12.5 mM N-(2-hydroxylethyl)-piperazine- N-2-ethane sulfonic acid (Sigma, St Louis, MO, USA) at 37°C in a humidified 5% CO2 incubator. The cultured Schwann cells were identified by their morphology and immunohistochemistry using anti-S-100 protein (1:100 dilution; Boster, Wuhan, China). Only the second and third passages of Schwann cells with a purity of 95% were used in this study to avoid age-dependent cellular modifications (Figure 6). Schwann cells at 2 × 105/mL were cultured on poly-lysine-coated coverslips in 6-well plates. When they reached 70% confluency, cells were starved overnight and treated in duplicate with different conditions.
Figure 6.

Cultured Schwann cells at passages 2–3 (inverted microscope, × 40).
Cultured Schwann cells were treated in duplicate consistently with 5.6 mM of glucose as a control, consistently with 50 mM of glucose as high glucose, with high glucose in the presence of 1, 10 and 100 μM of puerarin for 48 hours. In addition, the cells were treated with 44.4 mM of mannitol (Sigma, St Louis, MO, USA) plus 5.6 mM glucose for osmotic controls.
Measurement of ROS
Intracellular ROS generation was detected using the Becton Dickinson FACS system and dihydroethidine (Jiamay Biotech, Beijing, China)[28]. Dihydroethidine is mainly oxidized by superoxide anions and is the practice probe used to detect cellular ROS levels. In brief, cells at 2 × 106/mL in different culture conditions were harvested and treated with 10 μM of dihydroethidine at 37°C for 60 minutes in the dark. Results of fluorescent intensity from 10 000 events were analyzed in each sample and corrected for auto-fluorescence from unlabeled cells. ROS values were calculated as relative levels of the experiment to unlabeled control cells.
Measurement of ΔΨm
Schwann cells in different culture conditions were harvested and Ψm of those cells were characterized using the lipophilic cationic probe 5,5’,6,6’-tetrachloro-1,1’,3,3’-tetraethylbenzimidazol-carbocyanine iodine assay (Jiamay Biotech). 5,5’,6,6’-tetrachloro-1,1’,3,3’-tetraethylbenzimidazol-carbocyanine iodine is a lipophilic cationic fluorescent dye that exists as an aggregate at very negative membrane potentials (ΔΨm < −100 mV, red fluorescence). As a ΔΨm > −100 mV, 5,5’,6,6’- tetrachloro-1,1’,3,3’-tetraethylbenzimidazol- carbocyanine iodine exists as a monomer (green fluorescence)[40]. In brief, harvested cells at 2 × 106/mL were stained with 3 μM of 5,5’,6,6’-tetrachloro-1,1’,3,3’-tetraethylbenzimidazol-carbocyanine iodine at 37°C for 60 minutes in the dark. A minimum of 10 000 cells per sample were analyzed. The green fluorescence from the 5,5’,6,6’-tetrachloro-1,1’,3,3’-tetraethylbenzimidazol-carbocyanine iodine monomer with a 530-nm excitation and the red fluorescence from the aggregated form of 5,5’,6,6’-tetrachloro-1,1’,3,3’-tetraethylbenzimidazol-carbocyanine iodine with a 590-nm emission were visualized simultaneously. The results were calculated as the ratio of red/green fluorescence.
Detection of 8-OHdG
Schwann cells in different culture conditions were harvested and their DNA was isolated using a genomic DNA extraction kit (Generay Biotechnology, Shanghai, China). The amount of 8-OHdG was determined using the 8-OHdG enzyme-linked immunosorbent assay kit, a competitive enzyme-linked immunosorbent assay (StressMarq Biosciences, Victoria, Canada) according to the manufacturer's instructions[40]. The levels of 8-OHdG in each sample were calculated according to the standard curve.
Determination of apoptotic cells
Apoptotic cells were detected by TUNEL using the in situ cell death detection kit (Roche, Mannheim, Germany) according to the manufacturer's instructions[40]. Apoptotic cells were examined under a light microscope (Olympus, in Shinjuku, Tokyo, Japan) and representative photomicrographs of TUNEL staining in Schwann cells were obtained. At least 500 cells from each group were examined in a blinded manner.
Quantitative real-time reverse transcription-PCR
Total RNA was extracted from Schwann cells using Trizol (Invitrogen, Grand Island, NY, USA). The RNA (1 μg) was reversely transcribed into cDNA using the cDNA synthesis kit (Invitrogen) according to the manufacturer's protocol. The relative levels of each gene mRNA transcripts to control GAPDH were determined using the Eva Green (Biotium, Hayward, CA, USA) dye and specific primers. The sequences of the primers are shown as follows:

The PCR reactions were performed in triplicate at 95°C for 2 minutes and subjected to 40 cycles of 95°C for 20 seconds, 58°C for 20 seconds, and 72°C for 25 seconds. The relative levels of each gene mRNA transcript to GAPDH were analyzed by 2−ΔΔCt method[40].
Western blot analysis
The relative levels of target molecule expression were determined by western blot analysis[40]. After determining the concentration of total protein, cell lysates (50 g/lane) were separated by SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). Subsequently, the membranes were blocked with 5% (w/v) fat-free milk and incubated with either a 1:200 dilution of rabbit-anti-bcl-2, rabbit-anti-bax, rabbit-anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA), or a 1:800 dilution of rabbit-anti-cleaved-caspase-3 and rabbit-anti-cleaved-caspase-9 (Cell Signaling Technology, Beverly, CA, USA). The bound antibodies were detected with a 1:5 000 dilution of goat-anti-rabbit IgG (Santa Cruz Biotechnology) and visualized by enhanced chemiluminescence detection reagents (Applygen Technologies, Beijing, China). The relative levels of each protein to β-actin were determined by densitometry analysis using Image Tool software (Image Tool for Windows, USA).
Statistical analysis
All data were expressed as the mean ± SEM. The difference among groups was analyzed by one-way analysis of variance using SPSS 13.0 software (SPSS, Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
Footnotes
Funding: This project was supported by the National Natural Science Foundation of China, No. 30973354.
Conflicts of interest: None.
Ethical approval: This study was approved by the Animal Ethics Committee of Chinese PLA General Hospital.
(Edited by Song GB, Yang L/Qiu Y/Wang L)
REFERENCES
- [1].Martin CL, Albers J, Herman WH, et al. Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care. 2006;29:340–344. doi: 10.2337/diacare.29.02.06.dc05-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Little AA, Edwards JL, Feldman EL. Diabetic neuropathies. Pract Neurol. 2007;7:82–92. [PubMed] [Google Scholar]
- [3].Figueroa-Romero C, Sadidi M, Feldman EL. Mechanisms of disease: The oxidative stress theory of diabetic neuropathy. Rev Endocr Metab Disord. 2008;9:301–314. doi: 10.1007/s11154-008-9104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- [5].Brownlee M. The pathobiology of diabetic complications A unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- [6].Cameron NE, Eaton SE, Cotter MA, et al. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia. 2001;44:1973–1988. doi: 10.1007/s001250100001. [DOI] [PubMed] [Google Scholar]
- [7].Russell JW, Golovoy D, Vincent AM, et al. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J. 2002;16:1738–1748. doi: 10.1096/fj.01-1027com. [DOI] [PubMed] [Google Scholar]
- [8].Russell JW, Sullivan KA, Windebank AJ, et al. Neurons undergo apoptosis in animal and cell culture models of diabetes. Neurobiol Dis. 1999;6:347–363. doi: 10.1006/nbdi.1999.0254. [DOI] [PubMed] [Google Scholar]
- [9].Guo C, Quobatari A, Shangguan Y, et al. Diabetic autonomic neuropathy: evidence for apoptosis in situ in the rat. Neurogastroenterol Motil. 2004;16:335–345. doi: 10.1111/j.1365-2982.2004.00524.x. [DOI] [PubMed] [Google Scholar]
- [10].Padilla A, Descorbeth M, Almeyda AL, et al. Hyperglycemia magnifies Schwann cell dysfunction and cell death triggered by PA-induced lipotoxicity. Brain Res. 2011;25:64–79. doi: 10.1016/j.brainres.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Vincent AM, McLean LL, Backus C, et al. Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. FASEB J. 2005;19:638–640. doi: 10.1096/fj.04-2513fje. [DOI] [PubMed] [Google Scholar]
- [12].Chang Y, Hsieh CY, Peng ZA, et al. Neuroprotective mechanisms of puerarin in middle cerebral artery occlusion-induced brain infarction in rats. Biomed Sci. 2009;16:9. doi: 10.1186/1423-0127-16-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gao Q, Yang B, Ye ZG, et al. Opening the calcium- activated potassium channel participates in the cardioprotective effect of puerarin. Eur J Pharmacol. 2007;574:179–184. doi: 10.1016/j.ejphar.2007.07.018. [DOI] [PubMed] [Google Scholar]
- [14].Chen J, Xu J, Li J. Effect of puerarin on fibrinolytic activity and lipid peroxide in patients with coronary heart disease. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1999;19:649–650. [PubMed] [Google Scholar]
- [15].Magnani P, Thomas TP, Tennekoon G, et al. Regulation of glucose transport in cultured Schwann cells. J Peripher Nerv Syst. 1998;3:28–36. [PubMed] [Google Scholar]
- [16].Muona P, Sollberg S, Peltonen J, et al. Glucose transporters of rat peripheral nerve. Differential expression of GLUT1 gene by Schwann cells and perineural cells in vivo and in vitro. Diabetes. 1992;41:1587–1596. doi: 10.2337/diab.41.12.1587. [DOI] [PubMed] [Google Scholar]
- [17].Baumgartner-Parzer SM, Wagner L, Pettermann M, et al. High-glucose--triggered apoptosis in cultured endothelial cells. Diabetes. 1995;44:1323–1327. doi: 10.2337/diab.44.11.1323. [DOI] [PubMed] [Google Scholar]
- [18].Fukunaga M, Miyata S, Higo S, et al. Methylglyoxal induces apoptosis through oxidative stress-mediated activation of p38 mitogen-activated protein kinase in rat Schwann cells. Ann NY Acad Sci. 2005;1043:151–157. doi: 10.1196/annals.1333.019. [DOI] [PubMed] [Google Scholar]
- [19].Delaney CL, Russell JW, Cheng HL, et al. Insulin-like growth factor-I and over-expression of Bcl-xL prevent glucose-mediated apoptosis in Schwann Cells. J Neuropathol Exp Neurol. 2001;60:147–160. doi: 10.1093/jnen/60.2.147. [DOI] [PubMed] [Google Scholar]
- [20].Eckersley L. Role of the Schwann cell in diabetic neuropathy. Int Rev Neurobiol. 2002;50:293–321. doi: 10.1016/s0074-7742(02)50081-7. [DOI] [PubMed] [Google Scholar]
- [21].Eckersley L, Ansselin AD, Tomlinson DR. Effects of experimental diabetes on axonal and Schwann cell changes in sciatic nerve isografts. Brain Res Mol Brain Res. 2001;92:128–137. doi: 10.1016/s0169-328x(01)00163-2. [DOI] [PubMed] [Google Scholar]
- [22].Sharifi AM, Eslami H, Larijani B, et al. Involvement of caspase-8, -9, and -3 in high glucose-induced apoptosis in PC12 cells. Neurosci Lett. 2009;459:47–51. doi: 10.1016/j.neulet.2009.03.100. [DOI] [PubMed] [Google Scholar]
- [23].Sango K, Suzuki T, Yanagisawa H, et al. High glucose-induced activation of the polyol pathway and changes of gene expression profiles in immortalized adlut mouse Schwann cell IMS32. J Neurochem. 2006;98:446–458. doi: 10.1111/j.1471-4159.2006.03885.x. [DOI] [PubMed] [Google Scholar]
- [24].Gumy LF, Bampton ET, Tolkovsky AM. Hyperglycaemia inhibits Schwann cell proliferation and migration and restricts regeneration of axons and Schwann cells from adult murine DRG. Mol Cell Neurosci. 2008;37:298–311. doi: 10.1016/j.mcn.2007.10.004. [DOI] [PubMed] [Google Scholar]
- [25].Suzuki Ti, Sekido H, Kato Ni, et al. Neurotrophin-3- induced production of nerve growth factor is suppressed in Schwann cells exposed to high glucose: involvement of the polyol pathway. J Neurochem. 2004;91:1430–1438. doi: 10.1111/j.1471-4159.2004.02824.x. [DOI] [PubMed] [Google Scholar]
- [26].Askwith T, Zeng W, Eggo MC, et al. Oxidative stress and dysregulation of the taurine transporter in high-glucose- exposed human Schwann cells: implications for pathogenesis of diabetic neuropathy. Am J Physiol Endocrinol Metab. 2009;297:E620–628. doi: 10.1152/ajpendo.00287.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Qu L, Liang XC, Zhang H, et al. Effect of Jinmaitong serum on the proliferation of rat Schwann cells cultured in high glucose medium. Chin J Integr Med. 2008;14:293–297. doi: 10.1007/s11655-008-0293-z. [DOI] [PubMed] [Google Scholar]
- [28].Sun LQ, Zhao J, Zhang TT, et al. Protective effects of Salvianolic acid B on Schwann cells apoptosis induced by high glucose. Neurochem Res. 2012;37:996–1010. doi: 10.1007/s11064-011-0695-8. [DOI] [PubMed] [Google Scholar]
- [29].Lee HC, Wei YH. Oxidative stress, mitochondrial DNA mutation, and apoptosis in aging. Exp Biol Med (Maywood) 2007;232:592–606. [PubMed] [Google Scholar]
- [30].Leinninger GM, Edwards JL, Lipshaw MJ, et al. Mechanisms of disease: mitochondria as new therapeutic targets in diabetic neuropathy. Nat Clin Pract Neurol. 2006;2:620–628. doi: 10.1038/ncpneuro0320. [DOI] [PubMed] [Google Scholar]
- [31].Le Bras M, Clément MV, Pervaiz S, et al. Reactive oxygen species and the mitochondrial signaling pathway of cell death. Histol Histopathol. 2005;20:205–219. doi: 10.14670/HH-20.205. [DOI] [PubMed] [Google Scholar]
- [32].Allen DA, Harwood S, Varagunam M, et al. High glucose-induced oxidative stress causes apoptosis in proximal tubular epithelial cells and is mediated by multiple caspases. FASEB J. 2003;17:908–910. doi: 10.1096/fj.02-0130fje. [DOI] [PubMed] [Google Scholar]
- [33].Ho FM, Liu SH, Liau CS, et al. High glucose-induced apoptosis in human endothelial cells is mediated by sequential activations of c-Jun NH (2)-terminal kinase and caspase-3. Circulation. 2000;101:2618–2624. doi: 10.1161/01.cir.101.22.2618. [DOI] [PubMed] [Google Scholar]
- [34].anaka Y, Gleason CE, Tran PO, et al. Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc Natl Acad Sci U S A. 1999;96:10857–10862. doi: 10.1073/pnas.96.19.10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Von Harsdorf R, Li PF, Dietz R. Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation. 1999;99:2934–2941. doi: 10.1161/01.cir.99.22.2934. [DOI] [PubMed] [Google Scholar]
- [36].Sun LQ, Xue B, Li XJ, et al. Inhibitory effects of salvianolic acid B on apoptosis of Schwann cells and its mechanism induced by intermittent high glucose. Life Sci. 2012;90:99–108. doi: 10.1016/j.lfs.2011.10.001. [DOI] [PubMed] [Google Scholar]
- [37].Gill C, Dowling C, O’Neill AJ, et al. Effects of cIAP-1, cIAP-2 and XIAP triple knockdown on prostate cancer cell susceptibility to apoptosis, cell survival and proliferation. Mol Cancer. 2009;8:39. doi: 10.1186/1476-4598-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gao N, Budhraja A, Cheng S, et al. Phenethyl isothiocyanate exhibits antileukemic activity in vitro and in vivo by inactivation of Akt and activation of JNK pathways. Cell Death Dis. 2011;2:e140. doi: 10.1038/cddis.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [40].Yan X, Zhou T, Tao Y, et al. Salvianolic acid B attenuates hepatocyte apoptosis by regulating mediators in death receptor and mitochondrial pathways. Exp Biol Med. 2010;235:623–632. doi: 10.1258/ebm.2009.009293. [DOI] [PubMed] [Google Scholar]


