Abstract
The immunomodulatory and anti-oxidative activities of differentiated mesenchymal stem cells contribute to their therapeutic efficacy in cell-replacement therapy. Mesenchymal stem cells were isolated from human umbilical cord and induced to differentiate with basic fibroblast growth factor, nerve growth factor, epidermal growth factor, brain-derived neurotrophic factor and forskolin. The mesenchymal stem cells became rounded with long processes and expressed the neural markers, Tuj1, neurofilament 200, microtubule-associated protein-2 and neuron-specific enolase. Nestin expression was significantly reduced after neural induction. The expression of immunoregulatory and anti-oxidative genes was largely unchanged prior to and after neural induction in mesenchymal stem cells. There was no significant difference in the effects of control and induced mesenchymal stem cells on lymphocyte proliferation in co-culture experiments. However, the expression of human leukocyte antigen-G decreased significantly in induced neuron-like cells. These results suggest that growth factor-based methods enable the differentiation of mesenchymal stem cell toward immature neuronal-like cells, which retain their immunomodulatory and anti-oxidative activities.
Keywords: umbilical cord, mesenchymal stem cell, immunomodulation, oxidative stress, neural induction, neural regeneration
Research Highlights
-
(1)
Umbilical cord-derived mesenchymal stem cells can differentiate into neuron-like cells following induction with basic fibroblast growth factor, nerve growth factor, epidermal growth factor, brain-derived neurotrophic factor and forskolin.
-
(2)
Real-time quantitative reverse transcription-PCR and co-culture of induced mesenchymal stem cells with lymphocytes verified that neural induction with growth factors did not greatly influence the immunomodulatory and anti-oxidative activities of mesenchymal stem cells.
Abbreviation
UC-MSCs, umbilical cord-derived mesenchymal stem cells
INTRODUCTION
Mesenchymal stem cells (MSCs) are a highly promising source of adult stem cells for cell-based therapeutics because of their substantial multilineage differentiation capacity and secretory activities[1,2,3,4]. Allogeneic umbilical cord and autologous bone marrow may be ideal practical sources of MSCs because of their accessibility and lack of ethical concerns. In addition to their differentiation into osteoblasts, chondroblasts and adipocytes[5], MSCs also have the capacity to differentiate into neuron-like cells[6,7,8]. Therapeutic benefit has been shown in cerebral ischemia[9,10] and trauma[11] following administration of MSCs. In vitro neurally induced human MSCs have been shown to influence injured brain tissue repair in vivo[12].
Recent studies have suggested that the immunomodulatory activity of MSCs is of crucial importance to cell therapy using MSCs[13]. There is in vitro evidence that MSCs can directly modulate the function of T-cells. Moreover, they inhibit the maturation and migration of various antigen-presenting cells, suppress B-cell activation, induce suppressor T-cell formation, and alter the expression of several receptors necessary for antigen capture and processing[14,15]. This immunosuppressive activity of MSCs may play an important role in the repair of nervous system injuries. In addition, the anti-oxidative effects of MSCs can improve the survival of injured neuronal cells. Expression of the heme-oxygenase-1 protein within MSCs decreased cytotoxicity and inhibited apoptosis induced by oxidative stresses[16].
Immunomodulatory and anti-oxidative activities are key properties of MSCs. However, whether neurally-differentiated MSCs retain these properties is unclear. In the present study, we isolated MSCs from umbilical cord and analyzed the immunomodulatory and anti-oxidative properties of these umbilical cord-derived MSCs (UC-MSCs) before and after neural induction at the cellular and molecular levels.
RESULTS
Biological characteristics of UC-MSCs
Adherent cells with a fibroblastic morphology were observed as early as 48 hours after establishing explant cultures of umbilical cord tissue[17]. The cells formed a monolayer of homogeneous bipolar spindle-like cells with a whirlpool like morphology within 2 weeks (Figure 1A). Surface antigens expressed by the cultured cells at passage 5 were detected by fluorescence-activated cell sorting. The results showed that the cells expressed CD29, CD44, CD73, CD90, CD105 and CD106, but did not express CD34 and CD45, consistent with the phenotype of MSCs (Figure 1B).
Figure 1.
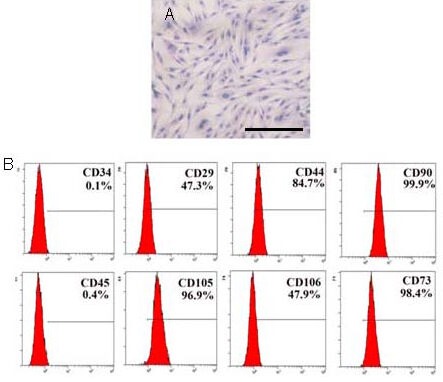
Morphology and immunophenotype of umbilical cord-derived cells at passage 5.
(A) Hematoxylin-eosin staining of umbilical cord-derived cells. The cells exhibited a bipolar spindle-shaped morphology and grew as a monolayer. Scale bar: 200 μm.
(B) Surface markers were detected by flow cytometric analysis. The cells expressed CD29, CD44, CD73, CD90, CD105 and CD106, but not CD34 and CD45.
Osteogenic and adipogenic differentiation capacities of MSCs
The differentiation capacity of the MSCs was assessed using passage 4 cells derived from umbilical cord. When induced to differentiate under osteogenic conditions, the MSCs increasingly congregated with increasing time of induction and formed a mineralized matrix, as confirmed by alizarin red staining (Figure 2A). Most MSC-like cells became positive for alkaline phosphatase by the end of 14 days (Figure 2B). No mineralized matrix was observed in the cells kept in regular growth medium. The spindle-shaped MSCs flattened and broadened after 1 week of adipogenic induction (Figure 2C). Small oil droplets gradually appeared in the cytoplasm. By the end of the second week, almost all of the cells contained numerous oil red O-positive lipid droplets (Figure 2D). The cells maintained in regular growth medium did not stain with oil red O.
Figure 2.
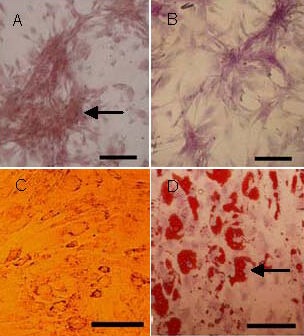
Differentiation capacities of umbilical cord-derived mesenchymal stem cells (scale bars: 100 μm).
By the end of 14 days, osteogenic differentiation was reflected by the formation of a mineralized matrix (arrow) positively stained by alizarin red (A) and alkaline phosphatase expression (B). Adipocytic differentiation was demonstrated by the presence of a broadened morphology (C) and the formation of lipid vacuoles (arrow) by oil-red O staining (D).
Induced differentiation of UC-MSCs into neuron-like cells
When MSCs were exposed to neural induction medium, they rapidly underwent dramatic morphological changes. Within a few hours, the majority of the cells had rounded up and extended long dendritic cellular processes. MSCs in the control group maintained their flattened morphology. The morphology of the induced cells was almost indistinguishable from control cells after 6 days of continuous induction because the cells became confluent (Figure 3A). In addition to the morphological evidence, we therefore compared the expression of neural specific markers in MSCs, i.e., nestin, Tuj1 (neuronal class III beta-tubulin), neurofilament 200, microtubule-associated protein 2, and neuron-specific enolase, by immunocytochemistry prior to and after neural induction. As shown in Figure 3B, MSCs expressed neurofilament 200, neuron-specific enolase, microtubule-associated protein 2 and Tuj1 after 7 days of induction. Cells in the untreated control group did not show any immunoreactivity for neurofilament 200, neuron-specific enolase, microtubule-associated protein 2 and Tuj1. Nestin expression was significantly reduced in cells cultured in neural induction medium. The reduction of nestin expression in the induced cells reflected their differentiation[18]. Overall, the results suggest that UC-MSCs can be induced to differentiate into neuron-like cells in vitro.
Figure 3.
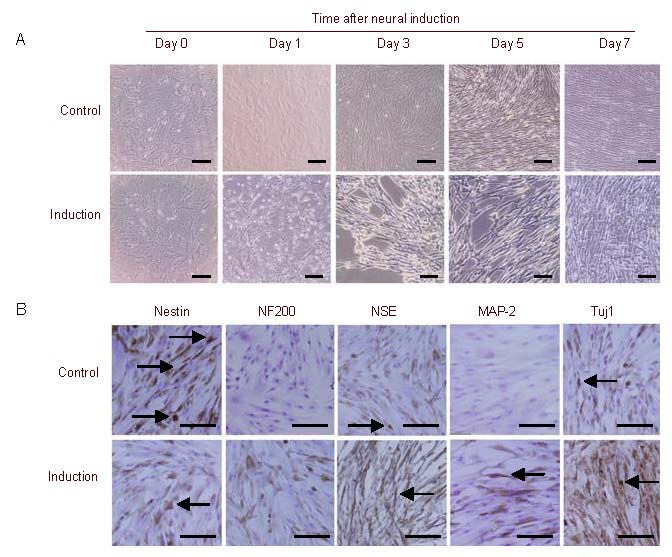
Neural differentiation of umbilical cord-derived mesenchymal stem cells (UC-MSCs; scale bars: 100 μm).
(A) Morphological changes in MSCs following neural differentiation. Cells before induction were recorded as day 0. Cultures of untreated MSCs display a characteristic fibroblastic morphology. Neurally induced MSCs underwent morphological changes and acquired a pseudo-neuronal shape with neurite-like processes, whereas MSCs in the control group maintained their flattened morphology.
(B) Immunocytochemical analysis of neural marker expression by MSCs after neural differentiation. After 7 days of induction, cells expressed neurofilament 200 (NF200), neuron-specific enolase (NSE), microtubule-associated protein 2 (MAP-2) and Tuj1, while the untreated control group did not express these markers. Nestin expression was significantly reduced in the neural induction group. Arrows indicate positive expression.
Neural induction does not change the immunomodulatory activity of MSCs
The immunosuppressive effects of MSCs in response to neural induction were analyzed in vitro using a lymphocyte co-culture assay. Approximately 1.9 × 104 MSCs and 4 × 104 peripheral blood mononuclear cells were seeded into each well of a 96-well plate. Our results showed that MSCs both prior to and after neural induction inhibited phytohemagglutinin-stimulated peripheral blood mononuclear cell proliferation. Although the inhibitory activity of uninduced MSCs was higher than neurally induced MSCs, there was no significant difference (P = 0.209; Figure 4A).
Figure 4.
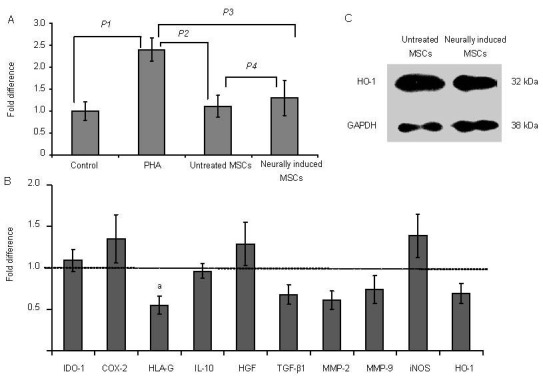
Influence of neural induction on the immunomodulatory and anti-oxidative activities of mesenchymal stem cells (MSCs).
(A) Neural induction does not affect the cells’ capacity to suppress allogeneic lymphocyte proliferation. The proliferation of lymphocytes in the MSC and induced groups was significantly lower than in the phytohemagglutinin (PHA) stimulated group (P values: P1 = 0.003 1; P2 = 0.007 8; P3 = 0.012 1), while there was no significant difference between the MSCs and induced cells (P values: P4 = 0.209). Quantitative data are expressed as mean ± SD.
(B) Real-time PCR quantification of immunoregulatory-related genes. The untreated control group values are set at fold change = 1. Only the expression of human leukocyte antigen-G (HLA-G) was reduced significantly (aP = 0.034, vs. control group by unpaired Student's t-test). Quantitative data are expressed as mean ± SD.
(C) Western blotting showed no significant difference in heme-oxygenase-1 (HO-1) expression before and after neural induction. GAPDH was used as the internal control.
IDO-1: Indoleamine 2,3-dioxygenase-1; COX-2: cyclooxygenase-2; IL-10: interleukin-10; HGF: hepatocyte growth factor; TGF-β1: tumor growth factor-β1; MMP-2: matrix metalloproteinase-2; MMP-9: matrix metalloproteinase-9; iNOS: inducible nitric oxide synthase; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
Expression of immunoregulatory genes by MSCs prior to and after neural induction
Real-time PCR quantification of eleven immunoregulatory-related genes was performed. Upregulation of four genes indoleamine 2,3-dioxygenase-1 (IDO-1), cyclooxygenase-2, hepatocyte growth factor, and inducible nitric oxide synthase) and downregulation of six genes (human leukocyte antigen-G, interleukin-10, tumor growth factor-β1, matrix metalloproteinase-2, matrix metalloproteinase-9, and heme-oxygenase-1) were confirmed in neurally induced MSCs, but no significant difference was observed compared with the control group (P > 0.05; Figure 4B). It is also worth noting that human leukocyte antigen-G was significantly downregulated in induced MSCs (P = 0.034; Figure 4B).
The expression of heme-oxygenase-1 protein was chosen as an indicator of anti-oxidative activity[19]. On western blot analysis, there were no significant differences between the heme-oxygenase-1 expression levels before and after neural induction (Figure 4C). The ratios of heme-oxygenase-1 to GAPDH in the untreated group and the neurally induced group were 1.18 and 1.07, respectively.
DISCUSSION
Cell-based therapy for nerve repair has achieved promising functional recovery. MSCs constitute an interesting cellular source for promoting neural regeneration in patients with neurodegenerative diseases because MSCs have the capacity to differentiate into cells with a neuronal phenotype in vivo[20,21] or in vitro under specific culture conditions[7,22,23]. However, the purity and yield of cells differentiated from stem cells must be sufficient to exert meaningful therapeutic effects. Therefore, pre-induction differentiation in vitro prior to transplantation is essential. The earliest neuronal differentiation protocols were based on the use of a mixture of chemical compounds (beta-mercaptoethanol, dimethyl sulfoxide and butylated hydroxyanisole) that induced MSCs to rapidly acquire a neuronal-like morphology characterized by the expression of specific neuronal markers[8,24,25]. However, many subsequent studies demonstrated that the morphological and molecular changes in MSCs were probably because of a stress response, rather than real differentiation into neuronal cells. Despite the neuronal-like morphology and neural protein expression, induced MSCs did not have basic neuronal functional properties[26].
The latest methods for inducing the differentiation of MSCs into neuron-like cells in vitro include the use of cytokines[27,28,29] and signal pathway inhibitors[30]. These combinations are more moderate but equally effective for inducing differentiation compared with chemical methods. Thus, in the present study, we also attempted to induce the neuronal differentiation of MSCs using a cytokine combination that included nerve growth factor, epidermal growth factor, basic fibroblast growth factor and brain-derived neurotrophic factor together with forskolin. Our observations were in accordance with previously published results[31,32,33]. Morphological changes in MSCs were accompanied by protein expression changes: a decrease in the immature stem cell marker, nestin, together with an increase in the neuronal immature marker, Tuj1, and thereafter of the more mature neuronal markers, microtubule-associated protein 2, neurofilament 200 and neuron-specific enolase. Moreover, a decrease in nestin expression, which is consistent with ongoing maturation, was also observed when MSCs were differentiated according to the protocol of Woodbury et al[8]. Although microtubule-associated protein 2 is not specific to neuronal cells, the increased expression of this gene might be involved in neurite initiation[34]. However, because transfusion therapy does not require terminal differentiation, we did not analyze electrophysiological and other functional indicators.
The consequences of physical injuries and neurodegenerative diseases in the central nervous system are severe because of acute inflammation/immune response, trophic factor withdrawal, oxidative stress, excitotoxicity, hypoxia or anoikis. To exploit the immunosuppressive and neuroprotective activities of MSCs, we further hope that MSC-derived neural cells may retain the secretory and anti-oxidative activities of MSCs. Our neural induction protocol led to changes in the expression of immune-related genes such as IDO-1, cyclooxygenase-2, hepatocyte growth factor, inducible nitric oxide synthase, interleukin-10, tumor growth factor-β1, matrix metalloproteinase-2, matrix metalloproteinase-9 and heme-oxygenase-1, but without statistical significance. IDO-1 and cyclooxygenase-2 participate in the synthesis of prostaglandin E2 in MSCs. Prostaglandin E2 is the major soluble factor responsible for the in vitro inhibition of the allogeneic lymphocyte reaction[35]. IDO-1 is an enzyme that catabolizes tryptophan, an essential amino acid, and plays a critical role in immunosuppression mediated by MSCs of various tissue origins[36]. In accordance with the role of this secretory protein, we showed similar results for other well-known immunosuppressive factors, i.e., hepatocyte growth factor, interleukin-10 and tumor growth factor-β1[37,38,39]. Matrix metalloproteinase-2 and matrix metalloproteinase-9 secreted by MSCs modulate the immune system by reducing the surface expression of CD25 on responding T-cells[40]. More importantly, we showed that inducible nitric oxide synthase and heme-oxygenase-1, which play important roles in both immunoregulation and anti-oxidative processes[41,42], did not change significantly after neural induction. Altogether, these phenomena are in accordance with the results of the lymphocyte proliferation assays. There was no significant difference between the activities of MSCs and induced cells in lymphocyte co-cultures.
Although the immunosuppressive activity of neurally induced MSCs was retained, human leukocyte antigen-G was significantly downregulated in MSCs and the cells lost their non-immunogenic properties. It is widely regarded that human leukocyte antigen-G expressed on placenta and umbilical cord inhibits immune responses to foreign antigens via these actions on immune cells[43].
In conclusion, neural induction does not change the immunosuppressive and anti-oxidative activities of MSCs. The suppression of host innate inflammatory responses may be a key factor for improving MSC survival and engraftment[29]. Neuronal-primed human UC-MSCs may be clinically useful for treating Parkinson's disease, spinal cord injuries and optic nerve injuries. However, their immune privilege may gradually diminish upon differentiation in vivo. Hence, the question of how to maintain the immunoprivileged status of the cells has attracted particular attention.
In summary, growth factor-based methods allowed MSC differentiation toward immature neuronal-like cells. The induced neuron-like cells retained their immunomodulatory properties in vitro, but their immunoprivileged status was gradually lost following neural differentiation.
MATERIALS AND METHODS
Design
A comparative observation at the cellular and molecular levels.
Time and setting
The experiments were performed at the Cryomedicine Laboratory of Qilu Hospital, Shandong University, China from October 2011 to May 2012.
Materials
Umbilical cords (n = 10; clinically normal pregnancies) were obtained from Qilu Hospital of Shandong University after normal full-term deliveries. Informed consent was obtained from the mothers. Tissue collection for research was approved by the Ethics Committee of Qilu Hospital.
Methods
Isolation and culture of MSCs
Umbilical cords were excised and washed in 0.1 M PBS (pH 7.4) to remove excess blood. The cords were dissected and blood vessels removed. The remaining tissue was cut into small pieces (1–2 cm2) and placed in plates containing low-glucose Dulbecco's modified Eagle's medium (Gibco-BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco-BRL), 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco-BRL). Cultures were maintained at 37°C in a humidified atmosphere with 5% CO2. The medium was changed every 3–4 days. After 7 to 12 days in culture, adherent cells were observed growing out from the individual tissue explants. The adherent fibroblast-like cells became confluent after 2–3 weeks in culture. They were then trypsinized using 0.25% trypsin (Gibco-BRL) and passaged at 1 × 104 cells/cm2 in the medium described above. After three or more passages, the cells were analyzed[44].
Cell surface antigen phenotyping by flow cytometry
Passages 15–17 cells were collected and treated with 0.25% trypsin. The cells were individually stained with fluorescein isothiocyanate or phycoerythrin-conjugated anti-marker monoclonal antibodies in 100 μL PBS for 15 minutes at room temperature or for 30 minutes at 4°C, as recommended by the manufacturer. The antibodies used were specific for the human antigens, CD29, CD34, CD44, CD45, CD73, CD90, CD105 and CD106 (10 μL for 1 × 106 cells; SeroTec, Raleigh, NC, USA). Cells were analyzed on a flow cytometry system (Cytometer 1.0, Cytomics™FC500, Beckman Coulter, Brea, CA, USA). Positive cells were counted and the signals for the corresponding immunoglobulin isotypes were compared[45].
Determination of the differentiation capacities of the cultured cells
To investigate the differentiation potential of the fibroblast-like cells, passage 4 cells were cultured under conditions appropriate for inducing their differentiation into each lineage. The cell density was 2 × 104 cells per cm2, and the medium was replaced every 3–4 days for the differentiation assays. The osteogenic differentiation medium consisted of low-glucose Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 0.1 μM dexamethasone, 50 mM β-glycerol phosphate and 0.2 mM ascorbic acid (Sigma, St. Louis, MO, USA). The adipogenic differentiation medium consisted of high-glucose Dulbecco's modified Eagle's medium supplemented with 0.25 mM 3-isobutyl-1-methylxanthine, 0.1 μM dexamethasone, 0.1 mM indomethacin (Sigma), 6.25 μg/mL insulin (Peprotech EC Ltd., London, UK) and 10% fetal bovine serum (Gibco-BRL). Cells kept in the regular growth medium served as controls. For evaluation of mineralized matrix formation, cells were fixed with 4% formaldehyde and stained with 1% alizarin red S (Sigma) solution in water for 10 minutes. Positive staining was indicated by brown staining of the cells. For alkaline phosphatase staining, cells were incubated with 300–400 μL (enough to coat the well) nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate chromogen solution (Jiancheng Bioengineering, Nanjing, China) for 8–10 minutes at room temperature. Positive staining was indicated by distinct purple staining of the cell surface. For oil red O staining, cells were fixed with 4% formaldehyde, stained with oil red O (Sigma) for 10 minutes, and then counterstained with Mayer hematoxylin (Sigma) for 1 minute. The formation of neutral lipid vacuoles was visualized by red staining[45].
Neuronal differentiation of MSCs
To initiate neural differentiation, subconfluent MSCs at passages 4–7 were treated with neural induction medium consisting of Dulbecco's modified Eagle's medium containing 5% fetal bovine serum, 2% B27 (Invitrogen, Carlsbad, CA, USA), 5 μM forskolin (Sigma), 125 μM 3-isobutyl-1-methylxanthine (Sigma), 10 μM β-mercaptoethanol and the following cytokines: 10 ng/mL basic fibroblast growth factor, 50 ng/mL nerve growth factor, 10 ng/mL epidermal growth factor, 10 ng/mL brain-derived neurotrophic factor, and 10 ng/mL fibroblast growth factor-2 (all from Peprotec, Rocky Hill, NJ, USA). The cells were incubated for 7 days before fixation or harvesting[46,47,48].
Immunocytochemical analysis of the expression of neuron-specific markers after neural induction
Immunocytochemical staining was performed in cells grown on glass coverslips. After washing in PBS, cells were fixed with 4% paraformaldehyde (pH 7.4) for 10 minutes and rinsed again with PBS. The cells were permeabilized with 0.1% Tween-PBS for 5 minutes and treated with 3% bovine serum albumin/0.1% Tween/PBS for 30 minutes. Thereafter, they were incubated overnight at 4°C with a primary monoclonal antibody (mouse anti-human nestin, Abcam, 1:250; rabbit anti-human Tuj1, Abcam, 1:200; mouse anti-human neurofilament 200, Sigma, 1:50; rabbit anti-human microtubule-associated protein 2, Sigma, 1:250; rabbit anti-human neuron-specific enolase, Abcam, 1:500) in 1% bovine serum albumin/PBS. Cells were then incubated with goat anti-mouse or mouse anti-rabbit secondary antibodies (1:2 000; Zhongshan Golden Bridge, Beijing, China) at room temperature for 1.5 hours. The 3,3’-diaminobenzidine peroxidase substrate system (Sigma) was used. Images were captured with an Olympus MagnaFire S99800 digital camera mounted on an IX71 Olympus inverted microscope (Olympus, Tokyo, Japan).
Peripheral blood mononuclear cell proliferation assay after neural induction
Neurally induced MSCs and the untreated control MSCs must adapt to co-culture medium (RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin) by graded reduction of the proportion of Dulbecco's modified Eagle's medium. Subsequently, MSCs were plated in triplicate in 96-well plates in 100 μL of co-culture medium at 100% confluence. Allogeneic peripheral blood mononuclear cells were isolated from peripheral blood by Ficoll/Hypaque gradient centrifugation, resuspended at 4 × 105/mL, and added to wells (4 × 104 cells/well in 100 μL of medium) containing or lacking MSCs in the presence of 10 μg/mL phytohemagglutinin (Sigma). After 48 hours, 100 μL of cells from each well were transferred to new 96-well plates containing 10 μL of Cell Counting Kit-8 reagent (Dojindo, Kumamoto, Japan). The absorbance at 450 nm was measured with a model 450 microplate reader (Bio-Rad Labs, Richmond, CA, USA). All experiments were performed in triplicate and were repeated at least twice[49].
Real-time quantitative reverse transcription-PCR analysis of immunomodulatory gene mRNA expression after neural induction
Total RNA was purified from each sample of UC-MSCs using Trizol (Invitrogen) as per the manufacturer's protocol. Measures were taken to guarantee that equal amounts of RNA from each subject were used. The expression levels of immunomodulatory genes were analyzed by quantitative reverse transcription-PCR. Total RNA was reverse transcribed to cDNA with the Omniscript cDNA Synthesis Kit (Qiagen, Hamburg, Germany) according to the manufacturer's instructions. Briefly, approximately 0.2 μg of total RNA was reverse transcribed in a 20 μL reaction mixture containing the following components: 1 × RT buffer, deoxynucleotide triphosphate mix (5 mM each), RNase inhibitor (10 U/μL RNase Out, Invitrogen), and 4 U Omniscript RT. The samples were incubated at 37°C for 60 minutes. The resulting cDNA was stored at –80°C prior to real-time PCR.
All reagents and primers were obtained from Bioasi Co., Ltd., Shanghai, China. Beta-actin was validated as an internal control. The expression of each gene relative to β-actin was determined using the 2-ΔCT method, where ΔCT = (CTtarget gene – CTβ-actin). Real-time PCR conditions were as follows: 95°C for 4 minutes, 94°C for 15 seconds, and 60°C for 1 minute, for a total of 40 cycles. Quantitative RT-PCR was performed using an ABI 7500 PCR system (Applied Biosystems, Foster City, CA, USA) and the SYBR green I dye (Toyobo, Osaka, Japan). Successful amplification was defined by the presence of a single dissociation peak on the thermal melting curve. Data were analyzed with Sequence Detection Software 1.4 (Applied Biosystems). Results are given as the fold change relative to untreated control group cDNA, while the control group values are set at fold change = 1.
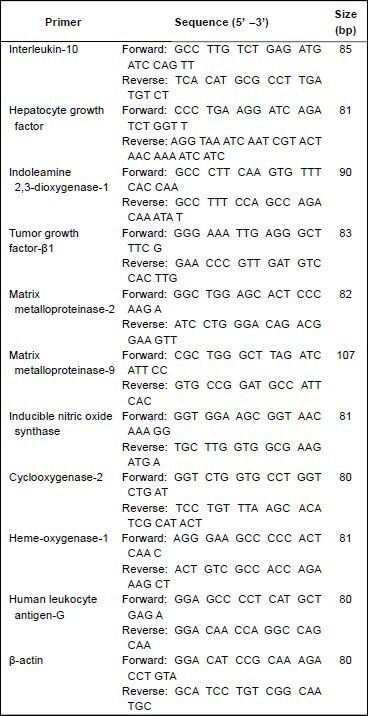
Reported data are representative of at least three independent experiments. The primers used are listed below.
Western blot analysis of heme-oxygenase-1 expression in MSCs after neural induction
For heme-oxygenase-1 western blot analysis, after separation by 7.5% SDS-PAGE, proteins (70 μg) were transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The membranes were blocked for 1 hour in 5% non-fat milk in Tris-buffered saline containing 0.05% Tween 20 and incubated with the mouse anti-heme-oxygenase-1 antibody (7.25 μg/mL; Abcam, Cambridge, UK) and mouse anti-GAPDH antibody (1:1 000; Abcam) overnight at 4°C. After washing in Tris-buffered saline containing 0.05% Tween 20, membranes were incubated for 1 hour at room temperature with peroxidase-labeled goat anti-mouse secondary antibodies (1:2 000; Zhongshan Golden Bridge). The immunoreactive bands were visualized using a SuperEnhanced chemiluminescence detection kit (Applygen Technologies Inc., Beijing, China) and subsequent exposure of the blots to Biomax L film (Kodak). Image analysis software, GIS 1D Ver 4.0 (Tanon Science & Technology Co., Ltd., Shanghai, China), was used to determine band intensities. GAPDH was used as the internal control.
Statistical analysis
Data were analyzed using SPSS software version 14.0 (SPSS Inc, Chicago, IL, USA). Quantitative data were expressed as mean ± SD. Statistical analysis was performed using unpaired Student's t-test for comparisons between two groups, and by one-way analysis of variance followed by Bonferroni test for comparisons among at least three groups. A value of P < 0.05 was considered statistically significant.
Footnotes
Funding: This work was supported by grants from the Shandong Province Science and Technology Program, Grant No. 2011GSF11801; and the Innovation Fund Project of Shandong University, Grant No. 2012ZD023; the Major State Basic Research Development Program, Grant No. 2012CB966504.
Conflicts of interest: None declared.
Ethical approval: All experimental protocols were approved by the Ethics Committee of Qilu Hospital, Shandong University, China.
(Edited by Ma CG, Sun DJ/Qiu Y/Song LP)
REFERENCES
- [1].Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109(8):923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Musumeci G, Lo Furno D, Loreto C, et al. Mesenchymal stem cells from adipose tissue which have been differentiated into chondrocytes in three-dimensional culture express lubricin. Exp Biol Med (Maywood) 2011;236(11):1333–1341. doi: 10.1258/ebm.2011.011183. [DOI] [PubMed] [Google Scholar]
- [3].Matsushita K, Morello F, Wu Y, et al. Mesenchymal stem cells differentiate into renin-producing juxtaglomerular (JG)-like cells under the control of liver X receptor-alpha. J Biol Chem. 2010;285(16):11974–11982. doi: 10.1074/jbc.M109.099671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Johnson TV, Bull ND, Hunt DP, et al. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010;51(4):2051–2059. doi: 10.1167/iovs.09-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- [6].Sanchez-Ramos JR. Neural cells derived from adult bone marrow and umbilical cord blood. J Neurosci Res. 2002;69(6):880–893. doi: 10.1002/jnr.10337. [DOI] [PubMed] [Google Scholar]
- [7].Sanchez-Ramos J, Song S, Cardozo-Pelaez F, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164(2):247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- [8].Woodbury D, Schwarz EJ, Prockop DJ, et al. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61(4):364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- [9].Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- [10].Kurozumi K, Nakamura K, Tamiya T, et al. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther. 2005;11(1):96–104. doi: 10.1016/j.ymthe.2004.09.020. [DOI] [PubMed] [Google Scholar]
- [11].Mahmood A, Lu D, Wang L, et al. Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery. 2001;49(5):1196–1204. [PubMed] [Google Scholar]
- [12].Alexanian AR, Fehlings MG, Zhang Z, et al. Transplanted neurally modified bone marrow-derived mesenchymal stem cells promote tissue protection and locomotor recovery in spinal cord injured rats. Neurorehabil Neural Repair. 2011;25(9):873–880. doi: 10.1177/1545968311416823. [DOI] [PubMed] [Google Scholar]
- [13].Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110(10):3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- [14].Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- [15].Atoui R, Shum-Tim D, Chiu RC. Myocardial regenerative therapy: immunologic basis for the potential “universal donor cells”. Ann Thorac Surg. 2008;86(1):327–334. doi: 10.1016/j.athoracsur.2008.03.038. [DOI] [PubMed] [Google Scholar]
- [16].Chen YT, Sun CK, Lin YC, et al. Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med. 2011;9:51. doi: 10.1186/1479-5876-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sun DC, Li DH, Ji HC, et al. In vitro culture and characterization of alveolar bone osteoblasts isolated from type 2 diabetics. Braz J Med Biol Res. 2012;45(6):502–509. doi: 10.1590/S0100-879X2012007500054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Park HE, Kim D, Koh HS, et al. Real-time monitoring of neural differentiation of human mesenchymal stem cells by electric cell-substrate impedance sensing. J Biomed Biotechnol. 2011;2011:485173. doi: 10.1155/2011/485173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kubulus D, Roesken F, Amon M, et al. Mechanism of the delay phenomenon: tissue protection is mediated by heme oxygenase-1. Am J Physiol Heart Circ Physiol. 2004;287(5):H2332–2340. doi: 10.1152/ajpheart.01109.2003. [DOI] [PubMed] [Google Scholar]
- [20].Brazelton TR, Rossi FM, Keshet GI, et al. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290(5497):1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- [21].Nakano K, Migita M, Mochizuki H, et al. Differentiation of transplanted bone marrow cells in the adult mouse brain. Transplantation. 2001;71(12):1735–1740. doi: 10.1097/00007890-200106270-00006. [DOI] [PubMed] [Google Scholar]
- [22].Kohyama J, Abe H, Shimazaki T, et al. Brain from bone: efficient “meta-differentiation” of marrow stroma-derived mature osteoblasts to neurons with Noggin or a demethylating agent. Differentiation. 2001;68(4-5):235–244. doi: 10.1046/j.1432-0436.2001.680411.x. [DOI] [PubMed] [Google Scholar]
- [23].Alexanian AR, Maiman DJ, Kurpad SN, et al. In vitro and in vivo characterization of neurally modified mesenchymal stem cells induced by epigenetic modifiers and neural stem cell environment. Stem Cells Dev. 2008;17(6):1123–1130. doi: 10.1089/scd.2007.0212. [DOI] [PubMed] [Google Scholar]
- [24].Muñoz-Elías G, Woodbury D, Black IB. Marrow stromal cells, mitosis, and neuronal differentiation: stem cell and precursor functions. Stem Cells. 2003;21(4):437–448. doi: 10.1634/stemcells.21-4-437. [DOI] [PubMed] [Google Scholar]
- [25].Woodbury D, Reynolds K, Black IB. Adult bone marrow stromal stem cells express germline, ectodermal, endodermal, and mesodermal genes prior to neurogenesis. J Neurosci Res. 2002;69(6):908–917. doi: 10.1002/jnr.10365. [DOI] [PubMed] [Google Scholar]
- [26].Barnabé GF, Schwindt TT, Calcagnotto ME, et al. Chemically-induced RAT mesenchymal stem cells adopt molecular properties of neuronal-like cells but do not have basic neuronal functional properties. PLoS One. 2009;4(4):e5222. doi: 10.1371/journal.pone.0005222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Peng J, Wang Y, Zhang L, et al. Human umbilical cord Wharton's jelly-derived mesenchymal stem cells differentiate into a Schwann-cell phenotype and promote neurite outgrowth in vitro. Brain Res Bull. 2011;84(3):235–243. doi: 10.1016/j.brainresbull.2010.12.013. [DOI] [PubMed] [Google Scholar]
- [28].Ladak A, Olson J, Tredget EE, et al. Differentiation of mesenchymal stem cells to support peripheral nerve regeneration in a rat model. Exp Neurol. 2011;228(2):242–252. doi: 10.1016/j.expneurol.2011.01.013. [DOI] [PubMed] [Google Scholar]
- [29].Khoo ML, Tao H, Meedeniya AC, et al. Transplantation of neuronal-primed human bone marrow mesenchymal stem cells in hemiparkinsonian rodents. PLoS One. 2011;6(5):e19025. doi: 10.1371/journal.pone.0019025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pacary E, Legros H, Valable S, et al. Synergistic effects of CoCl(2) and ROCK inhibition on mesenchymal stem cell differentiation into neuron-like cells. J Cell Sci. 2006;119(Pt 13):2667–2678. doi: 10.1242/jcs.03004. [DOI] [PubMed] [Google Scholar]
- [31].Padovan CS, Jahn K, Birnbaum T, et al. Expression of neuronal markers in differentiated marrow stromal cells and CD133+ stem-like cells. Cell Transplant. 2003;12(8):839–848. doi: 10.3727/000000003771000183. [DOI] [PubMed] [Google Scholar]
- [32].Tondreau T, Lagneaux L, Dejeneffe M, et al. Bone marrow-derived mesenchymal stem cells already express specific neural proteins before any differentiation. Differentiation. 2004;72(7):319–326. doi: 10.1111/j.1432-0436.2004.07207003.x. [DOI] [PubMed] [Google Scholar]
- [33].Salim A, Nacamuli RP, Morgan EF, et al. Transient changes in oxygen tension inhibit osteogenic differentiation and Runx2 expression in osteoblasts. J Biol Chem. 2004;279(38):40007–40016. doi: 10.1074/jbc.M403715200. [DOI] [PubMed] [Google Scholar]
- [34].Dehmelt L, Smart FM, Ozer RS, et al. The role of microtubule-associated protein 2c in the reorganization of microtubules and lamellipodia during neurite initiation. J Neurosci. 2003;23(29):9479–9490. doi: 10.1523/JNEUROSCI.23-29-09479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bunnell BA, Betancourt AM, Sullivan DE. New concepts on the immune modulation mediated by mesenchymal stem cells. Stem Cell Res Ther. 2010;1(5):34. doi: 10.1186/scrt34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhang Q, Shi S, Liu Y, et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183(12):7787–7798. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Spaggiari GM, Capobianco A, Abdelrazik H, et al. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111(3):1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- [38].DelaRosa O, Lombardo E, Beraza A, et al. Requirement of IFN-gamma-mediated indoleamine 2, 3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Eng Part A. 2009;15(10):2795–2806. doi: 10.1089/ten.TEA.2008.0630. [DOI] [PubMed] [Google Scholar]
- [39].Wang M, Yang Y, Yang D, et al. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology. 2009;126(2):220–232. doi: 10.1111/j.1365-2567.2008.02891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ding Y, Xu D, Feng G, et al. Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9. Diabetes. 2009;58(8):1797–1806. doi: 10.2337/db09-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chabannes D, Hill M, Merieau E, et al. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110(10):3691–3694. doi: 10.1182/blood-2007-02-075481. [DOI] [PubMed] [Google Scholar]
- [42].Schäfer S, Calas AG, Vergouts M, et al. Immunomodulatory influence of bone marrow-derived mesenchymal stem cells on neuroinflammation in astrocyte cultures. J Neuroimmunol. 2012;249(1-2):40–48. doi: 10.1016/j.jneuroim.2012.04.018. [DOI] [PubMed] [Google Scholar]
- [43].Hunt JS, Petroff MG, McIntire RH, et al. HLA-G and immune tolerance in pregnancy. FASEB J. 2005;19(7):681–693. doi: 10.1096/fj.04-2078rev. [DOI] [PubMed] [Google Scholar]
- [44].Lu LL, Liu YJ, Yang SG, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91(8):1017–1026. [PubMed] [Google Scholar]
- [45].Lee OK, Kuo TK, Chen WM, et al. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103(5):1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- [46].Elkabetz Y, Panagiotakos G, Al Shamy G, et al. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 2008;22(2):152–165. doi: 10.1101/gad.1616208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Barber RD, Jaworsky DE, Yau KW, et al. Isolation and in vitro differentiation of conditionally immortalized murine olfactory receptor neurons. J Neurosci. 2000;20(10):3695–3704. doi: 10.1523/JNEUROSCI.20-10-03695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ohori Y, Yamamoto S, Nagao M, et al. Growth factor treatment and genetic manipulation stimulate neurogenesis and oligodendrogenesis by endogenous neural progenitors in the injured adult spinal cord. J Neurosci. 2006;26(46):11948–11960. doi: 10.1523/JNEUROSCI.3127-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kuçi S, Kuçi Z, Kreyenberg H, et al. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. Haematologica. 2010;95(4):651–659. doi: 10.3324/haematol.2009.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]


