Abstract
Dark Agouti rat donor hind limbs were orthotopically transplanted into Lewis rat recipients to verify the effects of bone marrow mesenchymal stem cells on neural regeneration and functional recovery of allotransplanted limbs in the microenvironment of immunotolerance. bone marrow mesenchymal stem cells were intramuscularly (gluteus maximus) injected with FK506 (tacrolimus) daily, and were transplanted to the injured nerves. Results indicated that the allograft group not receiving therapy showed severe rejection, with transplanted limbs detaching at 10 days after transplantation with complete necrosis. The number of myelinated axons and Schwann cells in the FK506 and FK506 + bone marrow mesenchymal stem cells groups were significantly increased. We observed a lesser degree of gastrocnemius muscle degeneration, and increased polymorphic fibers along with other pathological changes in the FK506 + bone marrow mesenchymal stem cells group. The FK506 + bone marrow mesenchymal stem cells group showed significantly better recovery than the autograft and FK506 groups. The results demonstrated that FK506 improved the immune microenvironment. FK506 combined with bone marrow mesenchymal stem cells significantly promoted sciatic nerve regeneration, and improved sensory recovery and motor function in hind limb allotransplant.
Keywords: FK506 (tacrolimus), bone marrow mesenchymal stem cells, allotransplant, hind limb transplant, function recovery, sensory function, motor function, peripheral nerve injury, regeneration, neural regeneration
Research Highlights
-
(1)
FK506 and bone marrow mesenchymal stem cells significantly improved sensory function in rat hind limbs and sciatic function index.
-
(2)
FK506 and bone marrow mesenchymal stem cells increased the number of myelinated axons and Schwann cells, and improved pathological changes in gastrocnemius muscle.
-
(3)
Effects of FK506 and bone marrow mesenchymal stem cells on hindlimb allografts were better than autograft and FK506 use alone.
Abbreviation
BMSCs, bone marrow mesenchymal stem cells
INTRODUCTION
The first human hand transplant in this era of immunosuppression was performed in September 1998 in Lyon, France, by a team led by Jean-Michel Dubernard[1,2]. Some patients have had successful hand allografts, but others have failed[3,4,5]. Until now it is difficult to predict the functional recovery of transplanted hands. Hand transplantation is quite different from visceral organ transplantation. Several years are required to recover grafted hand function. Many animal experiments have been performed, but most of them have focused on the control of acute rejection[6,7,8,9,10]. Finding an effective way to improve hand function after transplantation is very important, but currently only a few studies have focused on limb functional recovery.
FK506 (tacrolimus) is a neutral macrolide with immunosuppressive properties, and is isolated from Streptomyces tsukubaensis[11]. FK506 selectively and rapidly inhibits the accumulation of interleukin-2 mRNA, as well as the accumulation of genes such as interleukin-3 and interleukin-4[12]. FK506 was proven to be 10–100 times more effective as an immunosuppressant than cyclosporine. A previous study showed that use of FK506 alone cannot effectively recover motor function[13].
Bone marrow mesenchymal stem cells (BMSCs) are pluripotent stem cells that can be easily harvested, cultured and used in autologous transplantation. They have the potential to differentiate into muscle, cartilage, bone and adipose tissue. They also act as support cells by producing an array of trophic factors and cytokines[14]. This study is designed to evaluate the effect of FK506 combined with BMSCs on the recovery of sensory and motor function in allograft rats.
RESULTS
Quantitative analysis of experimental animals
A total of 35 hind limb transplantation Lewis rats were divided into the allograft (n = 5), autograft (n = 10), FK506 (n = 10) and FK506 + BMSCs groups (n = 10). Dark Agouti rat donor hind limbs were orthotopically transplanted into Lewis rat recipients in the allograft, FK506 and FK506 + BMSCs groups. Rats from the FK506 and FK506 + BMSCs groups were intramuscularly injected with FK506 daily. Simultaneously, rats in the FK506 + BMSCs group were injected with BMSCs in the same region. Severe rejection appeared in the allograft group, and the allograft limbs showed complete necrosis and finally detached at 10 days after surgery. None of the recipients from the other three groups showed acute rejection. Three rats in the FK506 group and two rats in the FK506 + BMSCs group died from hemorrhage and operative infection, and were supplemented with new rats. Ten rats each from the autograft, FK506 and FK506 + BMSCs groups were included in the final analysis.
FK506 and BMSCs recovered hindlimb sensation in allograft rats
The pinch test revealed that sensory recovery improved gradually in the autograft, FK506 and FK506 + BMSCs groups over 1 year after surgery. The FK506 + BMSCs group showed significantly better recovery than the autograft and FK506 groups (P < 0.05). There were no significant differences between the FK506 and autograft groups (P > 0.05; Figure 1).
Figure 1.
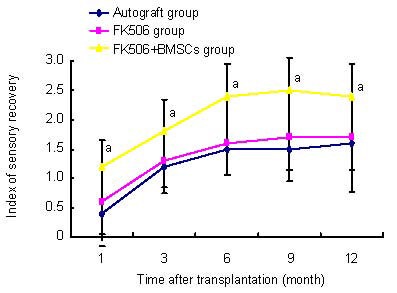
Effects of FK506 and bone marrow mesenchymal stem cells (BMSCs) on sensory function in rat hind limb after allotransplantation.
Results of sensory recovery using cutaneous pain reaction: grade 0: no reaction; grade 1: little reaction; grade 2: strong pain reaction; grade 3: normal reaction. Data were expressed as mean ± SD. In each group, six rats were tested (Student's t-test. aP < 0.05, vs. autograft and FK506 groups).
Recovered sciatic nerve function in limb transplant rats
In all groups, the sciatic function index showed significant improvement at 1–6 months after transplantation, and thereafter the value reached a plateau. At 12 months after transplantation, sciatic function index for all groups had increased to approximately 60 points. No significant difference in sciatic function index was detected between groups (P > 0.05; Figure 2).
Figure 2.
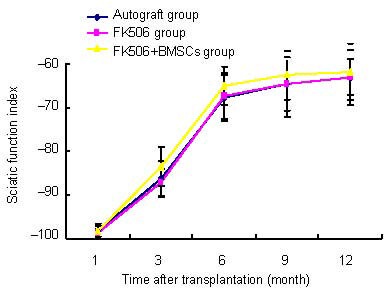
Effects of FK506 and bone marrow mesenchymal stem cells (BMSCs) on sciatic function index in allograft rats.
A score of “0” using these indices indicated normality, whereas a score of “–100” corresponded to total loss of function. In all groups, sciatic function index showed significant improvement between 1 to 3 and 3 to 6 months, and thereafter the value reached a plateau at 1 year after transplantation. Data were expressed as mean ± SD. Six rats were tested in every group (Student's t-test).
FK506 and BMSCs inhibited hind limb muscle atrophy in allograft rats
In the autograft, FK506 and FK506 + BMSCs groups, muscle atrophy was obvious on the experimental side. At 12 months, muscle weight was significantly greater in the FK506 + BMSCs group than the autograft group (P < 0.05). There was no significant difference between the FK506 and FK506 + BMSCs groups (P > 0.05; Figure 3).
Figure 3.
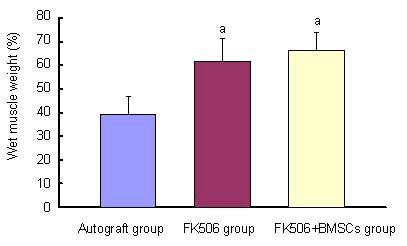
Effects of FK506 and bone marrow mesenchymal stem cells (BMSCs) on wet weight of rat hind limbs.
Wet weight of muscle was expressed as a percentage of that of the normal contralateral muscle. Data were expressed as mean ± SD. 10 rats were used in every group. aP < 0.05, vs. autograft group (Student's t-test).
FK506 and BMSCs increased the number of myelinated axons and Schwann cells in the sciatic nerve of allograft rats
At 12 months after surgery, quantitative analysis of myelinated axons revealed a significant difference between the FK506 and FK506 + BMSCs groups (P < 0.05). There were significant differences (P < 0.05) between the FK506 and FK506 + BMSCs groups in the total number of Schwann cells in nerves, and also between FK506 and the autograft groups (P < 0.05; Figures 4–6).
Figure 4.
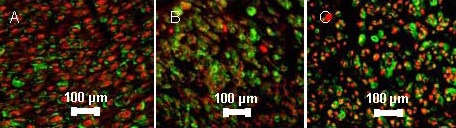
Myelinated axons and Schwann cells in sciatic nerve of rats at 12 months after transplantation (immunofluorescence staining, fluorescence microscope, × 400).
The quantity of myelinated axons and Schwann cells in sciatic nerve of rats from highest to lowest was FK506 + bone marrow mesenchymal stem cells (BMSCs) group (A) > FK506 group (B) > autograft group (C). S-100 is a tumor marker of Schwann cells[15], fluorescein isothiocyanate (FITC) as fluorescence indicator, green; neurofilament 200 as a tumor marker of myelinated axons[16], Cy3 as a fluorescence indicator, red.
Figure 6.
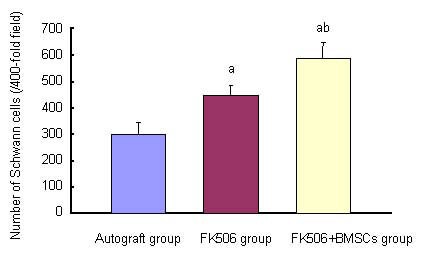
Effects of FK506 and bone marrow mesenchymal stem cells (BMSCs) on number of Schwann cells in sciatic nerve of rats at 12 months after transplantation.
Data were expressed as mean ± SD. 10 rats were used in each group. aP < 0.05, vs. autograft group; bP < 0.05, vs. FK506 group (Student's t-test).
Figure 5.
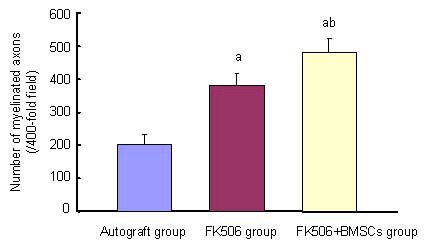
Effects of FK506 and bone marrow mesenchymal stem cells (BMSCs) on number of myelinated axons in sciatic nerve of rats at 12 months after transplantation.
Data were expressed as mean ± SD. 10 rats were used in each group. aP < 0.05, vs. autograft group; bP < 0.05, vs. FK506 group (Student's t-test).
FK506 and BMSCs improved gastrocnemius appearance in allograft rats
At 12 months after surgery, histological studies of gastrocnemius muscle revealed varying degrees of muscle degeneration. Polymorphic fibers and connective tissue increased, instead of normal muscle fibers. A lesser degree of these changes was found in FK506 + BMSCs group compared with FK506 and autograft groups (Figure 7).
Figure 7.
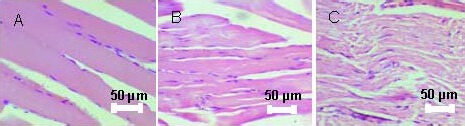
Morphology of gastrocnemius muscles in rats at 12 months after transplantation (hematoxylin and eosin staining, light microscope, × 200).
Varying degree of muscle degeneration, where polymorphic fibers and connective tissue increased, is seen instead of normal muscle fibers. Pathological changes in order of magnitude were as follows: FK506 + bone marrow mesenchymal stem cells group (A) > FK506 group (B) > autograft group (C).
DISCUSSION
This study confirmed that all rats treated with FK506 showed no acute rejection. FK506 accelerates neural regeneration in vivo and increases neurite elongation in vitro[17,18,19,20,21]. In the present study, Schwann cells in nerves from the FK506 group were significantly greater in number than the autograft group. FK506 is inactive on its own and requires binding to an FK506 binding protein-12, or immunophilin, for activation. In this regard, FK506 is analogous to cyclosporin A, which must bind to its immunophilin (cyclophilin A) to display activity. This FK506-FK506 binding protein-12 complex inhibits the activity of the serine/threonine protein phosphatase 2B (calcineurin), which is the basis for the immunosuppressant action of FK506. The discovery that immunophilins are also present in the nervous system introduces a new level of complexity in the regulation of neuronal function[22,23]. Immunophilins are a group of proteins that serve as receptors for the immunosuppressant drugs cyclosporin A and FK506. The level of immunophilin designated FK-506 binding protein-12 is more than 10 times higher in the brain than in immune tissues. Crushing the sciatic nerve markedly augments expression of FK506 binding protein-12 mRNA in lumbar motor neurons and dorsal root ganglia neuronal cells. Increased FK506 binding protein-12 expression appears linked to regeneration[24,25,26,27].
Grand et al[28] indicated that the combination of FK506 treatment with cold preservation of nerve allografts resulted in functional and histomorphometric recovery superior to that with nerve autograft alone. Furthermore, daily administration of low dose FK506 enhances peripheral nerve recovery after transection injury[29]. Additionally, treatment with FK506 improved the rate of functional recovery after nerve resection and autograft repair[21,30,31].
BMSC transplantation was well studied, indicating that the nerve system had some immune tolerance and was a relatively immune privileged site. Excessive immune response is an important factor hindering neural regeneration[32,33,34,35,36,37,38].
In the present study, the FK506 + BMSCs group showed better functional recovery than the FK506 and autograft groups. The FK506 + BMSCs group also showed significantly greater muscle weight than the autograft group. Results implied that FK506 helped improve the microenvironment, creating surroundings favoring neural regeneration in the limb allograft. These findings were consistent with previous studies[39,40]. Many studies confirmed that with immunosuppressant intervention, inflammatory cell infiltration decreased and limb function recovery improved, which may explain the above mechanism. However, further experiments are needed to verify the specific mode of action.
Rat limb allografts treated with FK506 showed no acute rejection and the same functional recovery as autografts. Combining FK506 with BMSCs produced a synergistic effect, potentially paving the way for future research and demonstrating possible implications for clinical use.
MATERIALS AND METHODS
Design
A randomized, controlled animal study.
Time and setting
Experiments were performed at the Affiliated Hospital of Chengde Medical College in China from October 2010 to November 2011.
Materials
Animals
Genetically inbred rats with the major histocompatibility complex were used. Graft donors were adult Dark Agouti rats (genetic expression, RT1a; Seiwa Experimental Animals, Beijing, China; SCXK 2006-0009). Rats were specific pathogen free, male, aged 12 weeks old, and weighed 200–250 g. Recipients were 12 weeks old, male Lewis rats (genetic expression, RT1l; Seiwa Experimental Animals, Beijing, China, SPF level, SCXK 2006-0009), and weighed 200–250 g. Experimental procedures were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[41].
Drugs
FK506 (purity 98.7%, powder, used for animals, Shengtianyu Company, Wuhan, China) was dissolved in saline and kept in a refrigerator at 4°C.
Methods
Culture of BMSCs
After sacrifice with ketamine, BMSCs were obtained from the thigh bone of Lewis rats (n = 5). The marrow suspension was seeded on 100-mm dishes in Dulbecco's modified Eagle's medium-high glucose (Gibco, Grand Island, NY, USA) containing 10% heat-inactivated fetal bovine serum (Sigma, St Louis, MO, USA). Cultures were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2. Medium was replaced every 4 days.
Model establishment of rat hind limb transplantation
The rat hind limb transplantation model described by Muramatsu et al[10] was used. Rats were anesthetized with a ketamine/xylazine cocktail and operated upon aseptically. The donor's right hindlimb, including bone, muscle, femoral vessels, sciatic nerve and skin, was amputated at the midfemoral level. A similar amputation was made in the recipient rat. After the donor's hind limb was orthotopically transplanted into the recipient, osteosynthesis was performed, using an 18-gauge needle as an intramedullary rod. The donor femoral artery and vein were anastomosed end-to-end to the recipient's femoral artery and vein with 10-0 nylon using standard microsurgical techniques, whereas the sciatic nerve was sutured epineurally with 8-0 nylon. Thigh muscles were approximated using 4-0 nylon sutures, and the skin envelope of the recipient rats was used to cover the denuded thigh and lower leg of the donor limb. For autografts, the right hindlimb of Lewis rats was elevated, similarly amputated, and returned to the same site, and the femoral vessels and sciatic nerve were repaired in a similar manner (Figure 8). Recipient rats were inspected daily for one year. They were housed in a plastic cage where they could walk freely.
Figure 8.
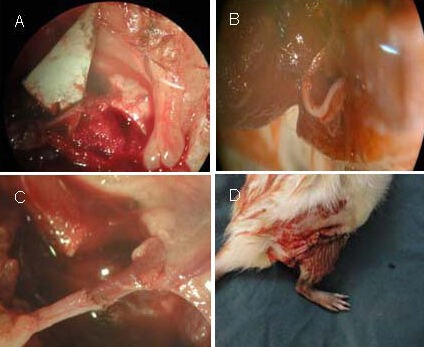
Model establishment of rat limb allotransplant.
(A) Anastomosed femoral artery and vein with 10-0 nylon, using standard microsurgical techniques. (B) Sciatic nerve was sutured epineurally with 10-0 nylon.
(C) Anastomosed artery and vein with 10-0 nylon. (D) The skin envelope of the recipient rat was used to cover the denuded thigh and lower leg of the donor limb.
FK506 therapy and BMSC transplantation
After surgery, the allograft groups were treated with intramuscular (gluteus maximus) injection of FK506 at a dose of 1 mg/kg per day[10,13] to prevent rejection until sacrificed (12 months).
BMSC suspension (1 × 107/μL; 10 μL) was injected into the injured nerve. The distal end of the injured nerve was injected (5 mm distance from the injured nerve point) at four points, the needle was maintained in every point for 1 minute and removed slowly (Figure 9).
Figure 9.
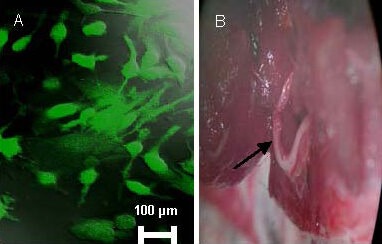
Bone marrow mesenchymal stem cells transplantation.
Cell suspension [A: 1 × 107 cells/μL, (10 μL)] was injected into the injured nerve (B). Arrow indicates the transplant site.
Cutaneous pain reaction testing for the recovery of sensory function in rats
At 1, 3, 6, 9, and 12 months after surgery, modified pain reaction tests[13,42] were performed in a quiet room. The recipient was held in one hand with its hind limbs hanging downward. After the rat became quiet and stopped struggling, the transplanted hind limb was pinched with a towel clip on the middle of the foot. This procedure was repeated five times. The grades of pain reaction were described according to the following: grade 0, no reaction to pinch; grade 1, little reaction to pinch, with a very slow reaction speed without violent struggling; grade 2, strong pain reaction to pinch stimulation with a reaction speed faster than grade 1, and violent flouncing when pinch was performed; and grade 3,with faster and more sensitive pain reaction than grade 2 (normal)[13,42].
Walking track analysis of rat sciatic nerve function
The sciatic nerve functional recovery was assessed using the sciatic function index. The procedure was repeated when an unsatisfactory result was obtained. Bain et al[43] and De Medinaceli et al[44] have described a detailed method, but some steps were modified in the present experiment. Walking tracks were obtained and were 8.2 cm × 42 cm, made of cardboard with walls and darkened at one end. Paper was put at the bottom of the track to act as film. The model rat's hind limb was painted with black ink and allowed to walk down the track. On each piece of paper, several footprints could be seen. Footprint was measured for length (PL), the distance from the first to the fifth toe (TS) and the distance from the second to the fourth toe (IT). These measurements were performed for both the experimental and normal sides and coded with the prefixes E for experimental and N for the normal. The data (NPL, EPL, NSF, ESF, NIT and EIT) were fed into the computer. The following formula was derived by Bain et al[43], who calculated the sciatic function index as follows:
Sciatic function index = –38.3 (EPL – NPL)/NPL + 109.5 (ETS – NTS)/NTS + 13.3 (EIT – NIT)/NIT – 8.8
A score of “0” using these indices indicated normality, whereas a score of “–100” corresponded to total loss of function.
Wet weight of rat gastrocnemius muscle
After an overdose of pentobarbital anesthesia (120 mg/kg), animals were perfused through the aorta with PBS (pH 7.6), followed by 300–500 mL of fixative composed of 4% paraformaldehyde in PBS (pH 7.4). Wet weight of gastrocnemius muscle was recorded for each group at 12 months after surgery. Gastrocnemius muscle was harvested without the tendon and gently blotted with absorbent paper to remove any blood or serum, and promptly weighed. The results were expressed as a percentage of the normal contralateral muscle.
Immunofluorescence staining to determine the number of myelinated axons and Schwann cells in sciatic nerve of allograft rats
After rats were sacrificed with a fatal dose of pentobarbital sodium (2%) injected in the abdominal cavity, rats were fixed in the dorsal position and their sciatic nerves taken. Nerve tissues were postfixed in 4% glutaraldehyde in 0.1 M PBS at pH 7.4 for 12 hours at room temperature. After that, each sample was immersed in graded sucrose solution (from 15% to 30% in 0.1 M PBS) for 12 hours and kept in a refrigerator at 4°C.
After this embedding, a series of 5 μm thick transected serial sections were cut on a cryostat microtome (Frigocut, Germany) at –20°C. The sections were for immunochemistry (dried in room temperature for 24 hours), and were fixed with freezing acetone for 20 minutes, then washed with 0.01 M PBS containing 0.3% Triton X-100 three times, 5 minutes each time. Subsequently, sections were incubated in 10% normal goat serum for 1 hour at room temperature and incubated with rabbit anti-S100 polyclonal (Chemicon) and mouse anti-rat neurofilament 200 monoclonal antibodies (Sigma) at room temperature (1:200) in 0.01 M PBS for over 8 hours in a humidity chamber. Sections were washed three times with PBS and 0.3% Triton X-100, whereupon they were incubated in fluorescein isothiocyanate-conjugated rabbit anti-mouse IgG (1:200; Sigma) and Cy3-conjugated rabbit anti-mouse IgG (1:200; Sigma) in PBS for 1 hour at room temperature. After the same washing procedure, sections were cover slipped. The number of Schwann cells (S-100 positive cells[15]) and myelinated axons (neurofilament 200 positive expression[16]) in sciatic nerve tissue samples from limbs of transplanted rats was observed using fluorescence microscopy (Nikon, Japan, × 400).
Hematoxylin-eosin staining of changes in rat gastrocnemius muscle appearance
The gastrocnemius muscle tissues of rats were taken and fixed in 10% buffered formalin. Transected serial sections (5 μm) were cut and stained with hematoxylin and eosin for light microscopy (Nikon, Japan).
Statistical analysis
Data were expressed as mean ± SD, and analyzed using SPSS 16.0 software (SPSS, Chicago, IL, USA). Possible differences between the groups were evaluated using one-way analysis of variance. Individual treatments were subjected to multiple comparisons (Student's t-test) to identify specific difference between two groups. A value of P < 0.05 was considered statistically significant.
Acknowledgments:
We thank Professor Chunhui Li from Chengde Medical College in China for providing technical support with pathological section preparation in this study.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, No. 30801171; the Natural Science Foundation of Hebei Province, No. C2009001013 and No. H2012406015.
Conflicts of interest: None declared.
Ethical approval: The study was approved by the Animal Care and Use Ethical Committee of Chengde Medical College, China.
(Edited by Tu QY, Bai H/Qiu Y/Song LP)
REFERENCES
- [1].Jones JW, Gruber SA, Barker JH, et al. Successful hand transplantation. One-year follow-up. Louisville Hand Transplant Team. N Engl J Med. 2000;343(7):468–473. doi: 10.1056/NEJM200008173430704. [DOI] [PubMed] [Google Scholar]
- [2].Jones NF. Concerns about human hand transplantation in the 21st century. J Hand Surg Am. 2002;27(5):771–787. doi: 10.1053/jhsu.2002.34373. [DOI] [PubMed] [Google Scholar]
- [3].Dubernard JM, Owen E, Herzberg G, et al. Human hand allograft: report on first 6 months. Lancet. 1999;353(9161):1315–1320. doi: 10.1016/S0140-6736(99)02062-0. [DOI] [PubMed] [Google Scholar]
- [4].Vögelin E. Hand transplantation-fiction or reality? Ther Umsch. 2011;68(12):730–734. doi: 10.1024/0040-5930/a000237. [DOI] [PubMed] [Google Scholar]
- [5].Kaufman CL, Ouseph R, Blair B, et al. Graft vasculopathy in clinical hand transplantation. Am J Transplant. 2012;12(4):1004–1016. doi: 10.1111/j.1600-6143.2011.03915.x. [DOI] [PubMed] [Google Scholar]
- [6].Arai K, Hotokebuchi T, Miyahara H, et al. Limb allografts in rats immunosuppressed with FK506. I. Reversal of rejection and indefinite survival. Transplantation. 1989;48(5):782–786. doi: 10.1097/00007890-198911000-00011. [DOI] [PubMed] [Google Scholar]
- [7].Ishikane S, Ohnishi S, Yamahara K, et al. Allogeneic injection of fetal membrane-derived mesenchymal stem cells induces therapeutic angiogenesis in a rat model of hind limb ischemia. Stem Cells. 2008;26(10):2625–2633. doi: 10.1634/stemcells.2008-0236. [DOI] [PubMed] [Google Scholar]
- [8].Huang WC, Lin JY, Wallace CG, et al. Vascularized bone grafts within composite tissue allotransplants can autocreate tolerance through mixed chimerism with partial myeloablative conditioning: an experimental study in rats. Plast Reconstr Surg. 2010;125(4):1095–1103. doi: 10.1097/PRS.0b013e3181d0ab80. [DOI] [PubMed] [Google Scholar]
- [9].Muramatsu K, Kuriyama R, Kato H, et al. Prolonged survival of experimental extremity allografts: a new protocol with total body irradiation, granulocyte-colony stimulation factor, and FK506. J Orthop Res. 2010;28(4):457–461. doi: 10.1002/jor.21011. [DOI] [PubMed] [Google Scholar]
- [10].Muramatsu K, Moriya A, Hashimoto T, et al. Immunomodulatory effects of pre-irradiated extremity allograft in the rodent model. J Plast Reconstr Aesthet Surg. 2012;65(7):950–955. doi: 10.1016/j.bjps.2011.12.015. [DOI] [PubMed] [Google Scholar]
- [11].Goto T, Kino T, Hatanaka H, et al. Discovery of FK-506, a novel immunosuppressant isolated from Streptomyces tsukubaensis. Transplant Proc. 1987;19(5 Suppl 6):4–8. [PubMed] [Google Scholar]
- [12].Tocci MJ, Matkovich DA, Collier KA, et al. The immunosuppressant FK506 selectively inhibits expression of early T cell activation genes. J Immunol. 1989;143(2):718–726. [PubMed] [Google Scholar]
- [13].Song YX, Muramatsu K, Kurokawa Y, et al. Functional recovery of rat hind-limb allografts. J Reconstr Microsurg. 2005;21(7):471–476. doi: 10.1055/s-2005-918902. [DOI] [PubMed] [Google Scholar]
- [14].Ide C, Nakai Y, Nakano N, et al. Bone marrow stromal cell transplantation for treatment of sub-acute spinal cord injury in the rat. Brain Res. 2010;1332:32–47. doi: 10.1016/j.brainres.2010.03.043. [DOI] [PubMed] [Google Scholar]
- [15].Mata M, Alessi D, Fink DJ. S100 is preferentially distributed in myelin-forming Schwann cells. J Neurocytol. 1990;19(3):432–442. doi: 10.1007/BF01188409. [DOI] [PubMed] [Google Scholar]
- [16].Paik SK, Lee DS, Kim JY, et al. Quantitative ultrastructural analysis of the neurofilament 200-positive axons in the rat dental pulp. J Endod. 2010;36(10):1638–1642. doi: 10.1016/j.joen.2010.05.005. [DOI] [PubMed] [Google Scholar]
- [17].Doolabh VB, Mackinnon SE. FK506 accelerates functional recovery following nerve grafting in a rat model. Plast Reconstr Surg. 1999;103(7):1928–1936. doi: 10.1097/00006534-199906000-00018. [DOI] [PubMed] [Google Scholar]
- [18].Jensen JN, Brenner MJ, Tung TH, et al. Effect of FK506 on peripheral nerve regeneration through long grafts in inbred swine. Ann Plast Surg. 2005;54(4):420–427. doi: 10.1097/01.sap.0000151461.60911.c0. [DOI] [PubMed] [Google Scholar]
- [19].Rustemeyer J, van de Wal R, Keipert C, et al. Administration of low-dose FK 506 accelerates histomorphometric regeneration and functional outcomes after allograft nerve repair in a rat model. J Craniomaxillofac Surg. 2010;38(2):134–140. doi: 10.1016/j.jcms.2009.03.008. [DOI] [PubMed] [Google Scholar]
- [20].Toll EC, Seifalian AM, Birchall MA. The role of immunophilin ligands in nerve regeneration. Regen Med. 2011;6(5):635–652. doi: 10.2217/rme.11.43. [DOI] [PubMed] [Google Scholar]
- [21].Yan Y, Sun HH, Mackinnon SE, et al. Evaluation of peripheral nerve regeneration via in vivo serial transcutaneous imaging using transgenic Thy1-YFP mice. Exp Neurol. 2011;232(1):7–14. doi: 10.1016/j.expneurol.2011.06.013. [DOI] [PubMed] [Google Scholar]
- [22].Gold BG. FK506 and the role of immunophilins in nerve regeneration. Mol Neurobiol. 1997;15(3):285–306. doi: 10.1007/BF02740664. [DOI] [PubMed] [Google Scholar]
- [23].Yang X, Ewald ER, Huo Y, et al. Glucocorticoid-induced loss of DNA methylation in non-neuronal cells and potential involvement of DNMT1 in epigenetic regulation of Fkbp5. Biochem Biophys Res Commun. 2012;420(3):570–575. doi: 10.1016/j.bbrc.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lyons WE, Steiner JP, Snyder SH, et al. Neuronal regeneration enhances the expression of the immunophilin FKBP-12. J Neurosci. 1995;15(4):2985–2994. doi: 10.1523/JNEUROSCI.15-04-02985.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pereira U, Boulais N, Lebonvallet N, et al. Mechanisms of the sensory effects of tacrolimus on the skin. Br J Dermatol. 2010;163(1):70–77. doi: 10.1111/j.1365-2133.2010.09757.x. [DOI] [PubMed] [Google Scholar]
- [26].Szydlowska K, Gozdz A, Dabrowski M, et al. Prolonged activation of ERK triggers glutamate-induced apoptosis of astrocytes: neuroprotective effect of FK506. J Neurochem. 2010;113(4):904–918. doi: 10.1111/j.1471-4159.2010.06656.x. [DOI] [PubMed] [Google Scholar]
- [27].Shin HJ, Jeon BT, Kim J, et al. Effect of the calcineurin inhibitor FK506 on K+-Cl- cotransporter 2 expression in the mouse hippocampus after kainic acid-induced status epilepticus. J Neural Transm. 2012;119(6):669–677. doi: 10.1007/s00702-011-0746-y. [DOI] [PubMed] [Google Scholar]
- [28].Grand AG, Myckatyn TM, Mackinnon SE, et al. Axonal regeneration after cold preservation of nerve allografts and immunosuppression with tacrolimus in mice. J Neurosurg. 2002;96(5):924–932. doi: 10.3171/jns.2002.96.5.0924. [DOI] [PubMed] [Google Scholar]
- [29].Konofaos P, Burns J, Terzis JK. Effect of low-dose FK506 after contralateral C7 transfer to the musculocutaneous nerve: a study in rats. J Reconstr Microsurg. 2010;26(4):225–233. doi: 10.1055/s-0030-1248230. [DOI] [PubMed] [Google Scholar]
- [30].Navarro X, Udina E, Ceballos D, et al. Effects of FK506 on nerve regeneration and reinnervation after graft or tube repair of long nerve gaps. Muscle Nerve. 2001;24(7):905–915. doi: 10.1002/mus.1088. [DOI] [PubMed] [Google Scholar]
- [31].Yeh C, Bowers D, Hadlock TA. Effect of FK506 on functional recovery after facial nerve injury in the rat. Arch Facial Plast Surg. 2007;9(5):333–339. doi: 10.1001/archfaci.9.5.333. [DOI] [PubMed] [Google Scholar]
- [32].Deng W, Obrocka M, Fischer I, et al. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun. 2001;282(1):148–152. doi: 10.1006/bbrc.2001.4570. [DOI] [PubMed] [Google Scholar]
- [33].Akiyama Y, Radtke C, Kocsis JD. Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells. J Neurosci. 2002;22(15):6623–6630. doi: 10.1523/JNEUROSCI.22-15-06623.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Koopmans G, Hasse B, Sinis N. Chapter 19: The role of collagen in peripheral nerve repair. Int Rev Neurobiol. 2009;87:363–379. doi: 10.1016/S0074-7742(09)87019-0. [DOI] [PubMed] [Google Scholar]
- [35].Yang Y, Yuan X, Ding F, et al. Repair of rat sciatic nerve gap by a silk fibroin-based scaffold added with bone marrow mesenchymal stem cells. Tissue Eng Part A. 2011;17(17-18):2231–2244. doi: 10.1089/ten.TEA.2010.0633. [DOI] [PubMed] [Google Scholar]
- [36].Seth R, Revenaugh PC, Kaltenbach JA, et al. Facial nerve neurorrhaphy and the effects of glucocorticoids in a rat model. Otolaryngol Head Neck Surg. 2012;147(5):832–840. doi: 10.1177/0194599812451551. [DOI] [PubMed] [Google Scholar]
- [37].Shichinohe H, Kuroda S, Maruichi K, et al. Bone marrow stromal cells and bone marrow-derived mononuclear cells: which are suitable as cell source of transplantation for mice infarct brain? Neuropathology. 2010;30(2):113–122. doi: 10.1111/j.1440-1789.2009.01050.x. [DOI] [PubMed] [Google Scholar]
- [38].Thompson DM, Meloche M, Ao Z, et al. Reduced progression of diabetic microvascular complications with islet cell transplantation compared with intensive medical therapy. Transplantation. 2011;91(3):373–378. doi: 10.1097/TP.0b013e31820437f3. [DOI] [PubMed] [Google Scholar]
- [39].Rustemeyer J, Dicke U. Allografting combined with systemic FK506 produces greater functional recovery than conduit implantation in a rat model of sciatic nerve injury. J Reconstr Microsurg. 2010;26(2):123–129. doi: 10.1055/s-0029-1243297. [DOI] [PubMed] [Google Scholar]
- [40].Azizi S, Mohammadi R, Amini K, et al. Effects of topically administered FK506 on sciatic nerve regeneration and reinnervation after vein graft repair of short nerve gaps. Neurosurg Focus. 2012;32(5):E5. doi: 10.3171/2012.1.FOCUS11320. [DOI] [PubMed] [Google Scholar]
- [41].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [42].Chen B, Song Y, Liu Z. Promotion of nerve regeneration in peripheral nerve by short-course FK506 after end-to-side neurorrhaphy. J Surg Res. 2009;152(2):303–310. doi: 10.1016/j.jss.2008.03.032. [DOI] [PubMed] [Google Scholar]
- [43].Bain JR, Mackinnon SE, Hudson AR, et al. The peripheral nerve allograft: a dose-response curve in the rat immunosuppressed with cyclosporin A. Plast Reconstr Surg. 1988;82(3):447–457. doi: 10.1097/00006534-198809000-00013. [DOI] [PubMed] [Google Scholar]
- [44].de Medinaceli L, Freed WJ, Wyatt RJ. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol. 1982;77(3):634–643. doi: 10.1016/0014-4886(82)90234-5. [DOI] [PubMed] [Google Scholar]


