Abstract
In this study, PC12 Adh cells and Neuro-2a cells were treated with Rho-associated kinase inhibitors (Y27632 and Fasudil), a cyclooxygenase-1 selective inhibitor (SC560), and a cyclooxygenase-2 inhibitor (NS398). We found that these cells became tolerant to Rho-associated kinase inhibitors, as neurite outgrowth induced by these inhibitors diminished following more than 3 days of exposure in either cell line. The proteins cyclooxygenase-2 and cytosolic prostaglandin E synthetase were upregulated at day 3. NS398 decreased the tolerance to neurite outgrowth induction in both cell lines, whereas SC560 had almost no effect. These findings indicate that cells become tolerant to neurite outgrowth induced by Rho-associated kinase inhibitors, this is at least partly associated with upregulation of proteins involved in the cyclooxygenase-2 pathway, and cyclooxygenases-2 inhibition prevents this tolerance.
Keywords: Rho-associated kinase inhibitors, Y27632, fasudil, neurite, cyclooxygenase 2 inhibitors, drug tolerance
Research Highlights
-
(1)
Rho-associated kinase inhibitors (Y27632 and Fasudil) promote neurite outgrowth in PC12 Adh cells. This effect peaks at 2 days and then ceases or diminishes.
-
(2)
Rho-associated kinase inhibitors effectively induce neurite outgrowth, and increase expression of cyclooxygenase-2 and cytosolic prostaglandin E synthetase.
-
(3)
Cyclooxygenases-2 inhibitor ameliorates tolerance to neurite outgrowth induced by Rho-associated kinase inhibitors.
Abbreviations
ROCK, Rho-associated kinase; COX, cyclooxygenase; NGF, nerve growth factor
INTRODUCTION
Rho-associated kinase (ROCK), known as Rho-associated coiled-coil forming protein serine/threonine kinase or Rho-associated kinase, is a central regulator of cytoskeleton control, cell adhesion, and gene expression[1]. There are two isoforms of ROCK, which are encoded by two different genes and have been named ROCK I (ROK β or p160 ROCK) and ROCK II (ROK α). ROCK II is preferentially expressed in the nervous system and plays a central role in neurite outgrowth[2]. By binding to its Rho binding domain, active Rho proteins (mainly Rho A-GTP), which are activated by membrane signals like adrenalin receptor activation and prostaglandin E receptor activation[3,4,5], can activate ROCK II by unmasking its catalytic domain. This leads to substrate phosphorylation (mainly myosin light chain) and trigger cytoskeleton contraction[2]. Eventually, this cytoskeleton contraction will cause neurite collapse[2].
Many reports have confirmed ROCK II expression in PC12 cells, Neuro-2a cells, and other cell lines[6,7,8,9]. ROCK II over-expression and ROCK II activators inhibit neurite outgrowth induced by nerve growth factor (NGF) and other stimuli. In contrast, low expression of ROCK II and ROCK II inhibitors trigger or promote neurite outgrowth[4,10]. In a previous study in PC12 cells, we found that neurite outgrowth induced by NGF was different from that induced by a ROCK inhibitor, since the ROCK inhibitor Y27632 decreased noradrenalin synthesis and release whereas NGF increased these processes[7]. ROCK upregulation and/or activation are observed in many nerve disorders, and ROCK inhibition promotes the recovery of neural functions after nerve damage or brain ischemia/reperfusion injuries in animal experiments[11,12,13]. Since neurite outgrowth is an essential step for neural network reconstruction and is a basic foundation for neural function recovery, the relationship between ROCK upregulation and nerve disorders has been roughly established[2]. Accordingly, ROCK inhibitors are promising agents for treating neural disorders.
However, most neural disorders are chronic and do not recover in 1–2 days, because neural disorders need a relatively long treatment time both for neurite outgrowth and for functional remodeling to occur. It could even take more than 2 days for ROCK inhibitors to treat nerve disorders. Because most experiments in vitro on neurite outgrowth induced by ROCK inhibitors were conducted over 1 or 2 days, the chronic effects of ROCK inhibitors on neurite outgrowth are not fully understood. To determine the mechanisms underlying tolerance to ROCK inhibition, we investigated the cyclooxygenase (COX) pathways. COX-1, COX-2, and prostaglandin E synthetase are inducible enzymes in the COX pathway, and can be upregulated by some cytokines and stressful stimuli[14]. Previous study confirmed that prostaglandin E2 is a product of the COX-2 pathway (yielded via COX-2 and prostaglandin E synthetase)[15], and that it can strongly cause neurite collapse in PC12 cells following treatment with NGF[4].
In the present study, we examined the mechanisms underlying tolerance to ROCK inhibition in two cell lines. One is the PC12 Adh cell line (from rat pheochromocytoma), and the other is the Neuro-2a cell line (from mouse neuroblast). Both are sensitive to ROCK inhibitors[16,17], and are cell lines often used in the study of neurite outgrowth. The main aim of the present study was to clarify the chronic effects (more than 3 days) of ROCK inhibitors on neurite outgrowth.
RESULTS
Tolerance to neurite outgrowth induced by ROCK inhibitors
When exposed to ROCK inhibitors (Y27632 and Fasudil) for 1 or 2 days, both PC12 Adh cells (Figures 1A, B) and Neuro-2a cells (Figures 1C, D) grew neurites. At day 3, neurite outgrowth ceased or began diminishing. In PC12 Adh cells (Figures 1A, B), there was no apparent tolerance to neurite outgrowth induced by NGF, which reached a persistent positive cell rate near 100%[6]. Because there are no NGF receptors on Neuro-2a cells[18], NGF did not induce neurite outgrowth in Neuro-2a cells (Figures 1C, D).
Figure 1.

Neurite outgrowth induced by Rho-associated kinase (ROCK) inhibitors in PC12 Adh cells (A, B) and Neuro-2a cells (C, D).
Cells with longer neurite(s) than their soma were defined as neurite positive cells. The neurite positive cell rate (%) was calculated as positive cells/total cells × 100%.
Neurite outgrowth in PC12 Adh cells following continuous exposure to ROCK inhibitors (33 μM Y27632 or 33 μM fasudil) decreased by the third day of treatment as shown in optical microscope images (A) and graphically (B).
Neurite outgrowth in Neuro-2a cells following continuous exposure to ROCK inhibitors (33 μM Y27632 or 33 μM fasudil) persisted beyond the third day of treatment as shown in optical microscope images (C) and graphically (D). NGF (1.0 μg/mL) induced neurite outgrowth in PC12 Adh cells (A and B) but not in Neuro-2a cells (C and D).
Scale bars in A, C: 100 μm. Data in Figures B and D are expressed as mean ± SD. aP < 0.05, vs. 0 day (one-way analysis of variance, n = 3). NGF: Nerve growth factor.
The median inhibitory concentration (IC50) of the ROCK inhibitors fasudil and Y27632 was similar (fasudil 0.158 μM; Y27632 0.162 μM)[19]. However, both ROCK inhibitors induced higher positive cell rate in Neuro-2a cells (Figure 1D) at day 3 and later than that in PC12 Adh cells (Figure 1B), which suggested that PC12 Adh cells have a greater propensity than Neuro-2a cells to become tolerant to ROCK inhibition.
Different reactiveness of PC12 Adh and Neuro-2a cells to ROCK inhibitors
We investigated the relationship between concentration and effect to determine the sensitivities of both cell lines to both ROCK inhibitors. We exposed cells to ROCK inhibitors or NGF at different concentrations for 2 days, and the concentration-effect curves are shown in Figure 2. The median effective concentration (EC50) was calculated based on these curves (Table 1). Based on the results in Table 1, both cell lines were more sensitive to the concentration of Y27632 than to that of fasudil, though both ROCK inhibitors had similar inhibitor effectiveness. As shown in Figure 2, the efficacy of ROCK inhibitors in Neuro-2a cells (near to 90%) was greater than that in PC12 cells (about 65%). This suggests that Neuro-2a cells are more reactive to ROCK inhibitors and more prone to growing neurites.
Figure 2.
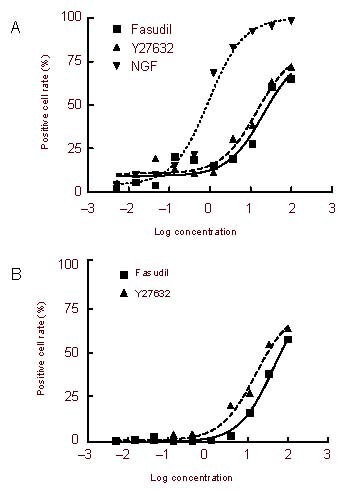
Concentration-response relationship for neurite outgrowth induced in PC12 Adh (A) and Neuro-2a cells (B) by exposure to Rho-associated kinase inhibitors for 2 days.
The curves were fitted with a sigmoidal dose-response curve. The concentration of fasudil and Y27632 are expressed in μM, whereas that of nerve growth factor (NGF) is expressed in ng/mL. Neuro-2a cells did not respond to NGF.
Table 1.
The median effective concentration of Rho-associated kinase inhibitors for the induction of neurite outgrowth in two cell lines

ROCK inhibitors induced upregulation of proteins in the COX-2 pathway in PC12 Adh cells
Results of western blot assays in PC12 Adh cells showed that exposure to Y27632 at different concentrations for 3 days upregulated COX-2 and cytosolic prostaglandin E synthetase, the key proteins in the COX-2 pathway. Meanwhile, COX-1 expression was unaffected (Figure 3).
Figure 3.
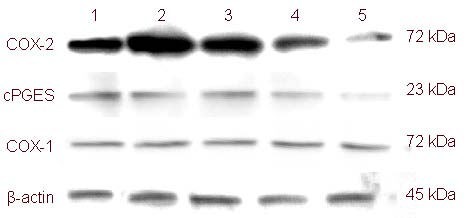
Protein expression of cyclooxygenase (COX)-1, COX-2 and cytosolic prostaglandin E synthetase (cPGES) in PC12 Adh cells exposed to the Rho-associated kinase inhibitor Y-27632 for 3 days as assessed by western blot assay.
(1) 33 μM Y-27632; (2) 3.7 μM Y-27632; (3) 0.41 μM Y-27632; (4) PBS; (5) 100 ng/mL nerve growth factor.
A COX-2 selective inhibitor attenuated tolerance to ROCK inhibition
As shown in Figure 3, downregulation or inhibition of the COX-2 pathway decreased tolerance to ROCK inhibitors. Combination treatments of a COX inhibitor and a ROCK inhibitor were performed in both PC12 Adh and Neuro-2a cells for more than 3 days. Combination treatment with NS398 and a ROCK inhibitor engendered higher positive cell rates than treatment with a ROCK inhibitor alone, both in PC12 Adh cells and Neuro-2a cells, especially at day 3 and later. The differences between combined treatment with SC560 and a ROCK inhibitor and treatment with a ROCK inhibitor alone were not significant in either PC12 Adh cells or Neuro-2a cells. COXs inhibitors by themselves had minimal effects on neurite outgrowth in either PC12 cells or Neuro-2a cells (Figure 4).
Figure 4.
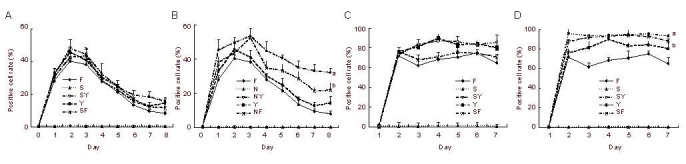
Neurite outgrowth induced by combined treatment with cyclooxygenase (COX) inhibitors and Rho-associated kinase (ROCK) inhibitors in PC12 Adh and Neuro-2a cells.
The neurite positive cell rate (%) = (positive cells/total cells) × 100%.
F: 33.3 μM fasudil; Y: 33.3 μM Y27632; S: 15.2 nM SC560; SF: 15.2 nM SC560 + 33.3 μM fasudil; SY: 15.2 nM SC560 + 33.3 μM Y27632; N: 1.25 μM NS398; NF: 1.25 μM NS398 + 33.3 μM fasudil; NY: 1.25 μM NS398 + 33.3 μM Y27632.
PC12 Adh cells did not grow neurites when exposed to either the COX-1 inhibitor SC560 (A) or the COX-2 inhibitor NS398 (B). Neurite outgrowth was induced by both ROCK inhibitors (33 μM Y27632 and 33 μM fadusil) following one or more days (A and B). However, tolerance developed in the third day (A and B). Combined treatment with NS398 decreased this tolerance (B), but combined treatment with SC560 did not (A).
Neuro-2a cells did not grow neurites when exposed to the either COX-1 inhibitor SC560 (C) or the COX-2 inhibitor NS398 (D). Neurite outgrowth was induced by both ROCK inhibitors (33 μM Y27632 and 33 μM fadusil) following one or more days (C and D). However, tolerance developed in the third day (C and D).
Combined treatment with NS398 decreased this tolerance (B), but combined treatment with SC560 did not (C). The data in B and D are expressed as mean ± SD. aP < 0.05, vs. Y; bP < 0.05, vs. F (two-way analysis of variance, n = 3).
DISCUSSION
ROCK inhibitors are promising agents for neural disorders based on the evidence from cellular and animal models[2]. Although ROCK inhibitors promote neurite outgrowth in vivo, neurite outgrowth induced by ROCK inhibitors in vitro has been measured over periods less than 3 days, and their chronic effects on neurite outgrowth are not well understood. We previously found different effects of the ROCK inhibitor Y27632 and NGF in PC12 cells. Specifically, NGF increased noradrenalin synthesis and release, whereas Y27632 decreased these processes, even when neurite outgrowth was induced to a similar extent[7].
The PC12 cell line was derived from the rat pheochromocytoma, and there are at least two cell lines associated with the name PC12 in the ATCC, which are called the PC12 cell line and the PC12 Adh cell line[6,20]. According to our previous findings, the PC12 Adh cell line is more suitable for neurite outgrowth studies than the PC12 cell line[6]. The Neuro-2a cell line was derived from mouse neuroblastomas. Therefore, we used the PC12 Adh and Neuro-2a cell lines to investigate the chronic effects of ROCK inhibitors on neurite outgrowth. In the present study, we found that PC12 Adh cells become tolerant to ROCK inhibitors, which was verified in Neuro-2a cells. The tolerance developed in the third day of exposure. This suggests that this tolerance is a result of gene expression regulation, and it could be stronger following longer exposure to ROCK inhibitors. However, PC12 Adh cells did not become tolerant to neurite outgrowth induced by NGF, because the longest neurites would extend beyond the microscope field if exposed to NGF (100 ng/mL) for 14 days (data not shown).
Our present study found proteins in the COX-2 pathway were upregulated following exposure to ROCK inhibitors for 3 or more days. It is possible that these changes in the COX-2 pathway mediated tolerance to neurite outgrowth induced by ROCK inhibitors. To answer this question, we treated both cell lines with the COX-1 selective inhibitor SC560 and the COX-2 selective inhibitor NS398, and we found that NS398 decreased tolerance in these cells whereas SC560 was ineffective. Therefore, chronic treatment with ROCK inhibitors may upregulate proteins in the COX-2 pathway and concomitantly increases the amount of prostaglandins, especially prostaglandin E2. Prostaglandin E2 and other prostaglandins may have diffused out of cells and activated their receptors in cell membranes, resulting in cytoskeleton contraction via activation of ROCK or another pathway.
In the ROCK pathway, ROCK is activated by Rho A-GTP. When Rho A-GTP binds to Rho binding domain of ROCK, ROCK's catalytic domain is unmasked. Unmasking of the catalytic domain allows it to bind much more ATP or ROCK inhibitors, which means that the amount of the inhibitor may no longer be sufficient to prevent activation of all of the available enzymes. The activation of ROCK strongly induces neurite collapse and perhaps tolerance to ROCK inhibitors. The relationship between the COX pathway and ROCK is presented in Figure 5.
Figure 5.
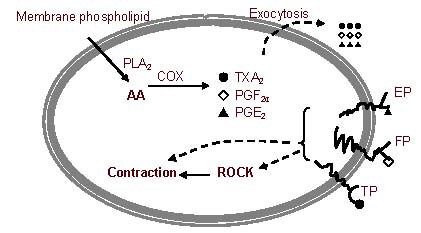
The relationship between the cyclooxygenase (COX) pathway and Rho-associated kinase (ROCK).
COX catalyzes arachidonic acid (AA), which comes from membrane phospholipids, to prostaglandins (PGs), including thromboxane A2 (TXA2), PGF2α, and PGE2. These PGs are able to diffuse out of the cell.
Then, the PGs are able to activate their membrane receptors, and these activations can result in cytoskeleton contraction via activation of the ROCK pathway and other pathways. Cytoskeleton contraction activated by ROCK causes neurite collapse.
EP: Prostaglandin E receptor; FP: prostaglandin F receptor; TP: thromboxane receptor; PLA2: phospholipase A2; PGE2: prostaglandin E2. PGF2: prostaglandin E2α.
Experimental and clinical evidence also shows that COX-2 inhibitors can promote recovery from neural injuries[21,22], though COX-2 inhibition itself has no direct effects on neurite outgrowth. Since chronic exposure to ROCK inhibitors produces tolerance to their induction of neurite outgrowth, and COX-2 inhibitors decreased this tolerance, combination treatments with ROCK inhibitors and COX-2 inhibitors may have synergistic therapeutic effects for neural disorders, though this suggestion needs further investigation in animal models.
Taken together, our findings demonstrate that chronic exposure to ROCK inhibitors produces tolerance to their induction of neurite outgrowth, and this is associated with upregulation of proteins in the COX-2 pathway.
MATERIALS AND METHODS
Design
An observational cytology experiment.
Time and setting
This study was performed at the Key Laboratory of Molecular Biology for Sinomedicine, Yunnan University of Traditional Chinese Medicine, China from March 2009 to May 2011.
Materials
Cells
PC12 Adh cells were obtained from the American Type Culture Collection via the Kunming Institute of Zoology, Chinese Academy of Sciences, China. Neuro-2a cells were purchased from the Beijing Dingguo Biotechnology Company Ltd., China. Both cell lines were identified by comparing their morphologies with those described online (http://www.atcc.org/ATCCAdvancedCatalogSearch/ProductDetails/tabid/452/Default.aspx?ATCCNum=CRL-1721.1&Template=cellBiology, http://www.atcc.org/ATCCAdvancedCatalogSearch/ProductDetails/tabid/452/Default.aspx?ATCCNum=CCL-131&Template=cellBiology).
Drugs
The ROCK inhibitors Y-27632 ((R)-(+)-trans-N-(4-pyridyl)-4-(1-aminoethyl)-cyclohexanecarboxamide.2H CI, purity ≥ 98%) and fasudil (5-(1,4-diazepan-1-ylsulfonyl) isoquinoline, purity ≥ 98%) were purchased from Alexis Biochemicals (San Diego, USA). The COX-1 selective inhibitor SC560 (5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-trifluoromethylpyrazole, purity ≥ 98%, IC50 = 9 nM)[23] and the COX-2 selective inhibitor NS398 (N-[2-(cyclohexoxy)-4-nitro-phenyl]-methanesulfonamide, purity ≥ 98%, IC50 = 1.77 μM)[23] were purchased from Cayman Chemical Company (San Diego, USA). NGF-7S (130 kDa) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Methods
Cell cultures
PC12 Adh cells were incubated in Ham's F12K medium (Invitrogen, New York, NY, USA) with 2 mM L-glutamine adjusted to contain 1.5 g/L sodium bicarbonate with 15% horse serum (Invitrogen) and 2.5% fetal bovine serum (Invitrogen) in an atmosphere containing 5% CO2 and 95% air at 37°C. When the PC12 Adh cells occupied 80% of the flask, they were dispersed and seeded in 96-well plates wrapped with 0.01% polylysine for 4 hours. Then, we exposed the PC12 Adh cells to mixtures of ROCK inhibitors, COXs inhibitors, and NGF at different concentrations. We renewed the media every 2 days, and the concentration of the ROCK inhibitors, COXs inhibitors, and NGF in the media was maintained after each renewal[6,24].
Neuro-2a cells were incubated in Eagle's Minimum Essential Medium (Invitrogen) with 10% fetal bovine serum in an atmosphere containing 5% CO2 and 95% air at 37°C. When Neuro-2a cells occupied 80% of the flask, they were dispersed with a 0.25% trypsin and 0.53 mM ethylene diamine tetraacetic acid solution and seeded in 96-well plates for 4 hours. Then, we exposed the Neuro-2a cells to mixtures of ROCK inhibitors and COX inhibitors at different concentrations. We renewed each medium every 2 days, and the concentrations of the ROCK inhibitors and COX inhibitors in each medium were maintained after each renewal[24].
The baseline period in the PC12 Adh cells and Neuro-2a cells before exposure to ROCK inhibitors, COX inhibitors, or NGF served as the negative control. NGF was used as the positive control for PC12 Adh cells.
Observations of neurite outgrowth
PC12 Adh cells[25] and Neuro-2a cells grew neurites when exposed to ROCK inhibitors. The morphology of the treated PC12 Adh and Neuro-2a cells was categorized into neurite positive cells, round cells, and other cells was observed with a microscope (IX71-A21PH; Olympus, Tokyo, Japan). Cells with neurite(s) longer than their soma were defined as neurite positive cells. Other cells had various features including micro-spikes, ruffles, and a flattened appearance[26,27] (supplementary Figures 1, 2 online).
The number of cells observed in each well was at least 100. If there were fewer than 100 cells in a well, all cells in that well were observed. The neurite positive cell rate was calculated based on the following formula: Positive cell rate (%) = (positive cells/total cells) × 100%. The EC50 for each ROCK inhibitor and NGF was calculated with a sigmoidal dose-response equation[26].
Western blot assays
PC12 Adh cells were exposed to Y-27632 or NGF for 3 days, and the cells were lysed with lyse buffer containing 20 mM Tris-HCI (pH 7.5), 0.1% Triton X-100, 1 mM ethylene diamine tetraacetic acid, 0.2 mM dithiothreitol, and 2 mM NaF. The lysate was spun (6 000 × g) for 10 minutes and the supernatant was collected as a protein sample for western blot assay. Based on previously reported methods[28] with some modifications, protein (20 μg) from different samples was applied for sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The protein in the electrophoresis was transferred to a nitrocellulose membrane by an electrical current of 10 V for 60 minutes in a semi-dry transfer system (Bio-Rad, Hercules, CA, USA). The nitrocellulose membrane was blocked with 3% bovine serum albumin at an ambient temperature (20°C) for 2 hours. It was then bathed in rabbit anti-COXs polyclonal antibodies (1:400; Boster, Wuhan, China), a rabbit anti-cytosolic prostaglandin E2 synthase polyclonal antibody (1:500; Cell Signaling Technology, Beverly, MA, USA), and a rabbit anti-β-actin polyclonal antibody (1:600; Biovision, Milpitas, CA, USA) at 4°C overnight. The membrane was rinsed with TST solution that contained 20 mM Tris HCI (pH 7.5) and 0.05% Tween-20 for 10 minutes three times, and bathed in a solution of goat anti-rabbit antibody linked with horseradish peroxidase (1:1 000; Boster) for another 2 hours at the ambient temperature. The membrane was rinsed with the TST solution for 10 minutes three times and developed through chemiluminescence, and the sodium dodecyl sulfate-polyacrylamide gel electrophoresis bands in nitrocellulose membrane were recorded on X-ray film.
Statistical analysis
The data are expressed as mean ± SD. One-way or two-way analysis of variance was performed using SPSS for Windows 13.0 (SPSS, Chicago, IL, USA) to compare means with the negative control groups. Post-hoc multiple comparisons analyses were conducted with the Student-Neuman-Keuls method for equal variance or Tamhane's T2 method. Statistically significant differences were accepted at P < 0.05.
Footnotes
Funding: This study was financially supported by Yunnan Provincial Science and Technology Department, No. 2009CD079 and by the National Natural Science Foundation of China, No. 81060109.
Conflicts of interest: None declared.
Supplementary information: Supplementary data associated with this article can be found in the online version by visiting www.nrronline.org.
(Edited by Wang JT, Shi TS/Yang Y/Song LP)
REFERENCES
- [1].Leung T, Chen XQ, Manser E, et al. The p160 RhoAbinding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16(10):5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov. 2005;4(5):387–398. doi: 10.1038/nrd1719. [DOI] [PubMed] [Google Scholar]
- [3].Broggini T, Nitsch R, Savaskan NE. Plasticity-related gene 5 (PRG5) induces filopodia and neurite growth and impedes lysophosphatidic acid- and nogo-A-mediated axonal retraction. Mol Biol Cell. 2010;21(4):521–537. doi: 10.1091/mbc.E09-06-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Katoh H, Aoki J, Ichikawa A, et al. p160 RhoA-binding kinase ROKalpha induces neurite retraction. J Biol Chem. 1998;273(5):2489–2492. doi: 10.1074/jbc.273.5.2489. [DOI] [PubMed] [Google Scholar]
- [5].Sakurada S, Takuwa N, Sugimoto N, et al. Ca2+-dependent activation of Rho and Rho kinase in membrane depolarization-induced and receptor stimulation-induced vascular smooth muscle contraction. Circ Res. 2003;93(6):548–556. doi: 10.1161/01.RES.0000090998.08629.60. [DOI] [PubMed] [Google Scholar]
- [6].Que L, Duan W, Zhang L, et al. 3. Vol. 33. Ziran Kexue Ban: Yunnan Daxue Xuebao; 2011. Differences of neurite outgrowth induced by rho kinase inhibitors between in PC12 cell line and PC12 Adh cell line; pp. 370–372. [Google Scholar]
- [7].Duan WG, Shang J, Jiang ZZ, et al. Rho kinase inhibitor Y-27632 down-regulates norepinephrine synthesis and release in PC12 cells. Basic Clin Pharmacol Toxicol. 2009;104(6):434–440. doi: 10.1111/j.1742-7843.2008.00314.x. [DOI] [PubMed] [Google Scholar]
- [8].Wylie SR, Chantler PD. Myosin IIA drives neurite retraction. Mol Biol Cell. 2003;14(11):4654–4666. doi: 10.1091/mbc.E03-03-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yamazaki M, Miyazaki H, Watanabe H, et al. Phosphatidylinositol 4-phosphate 5-kinase is essential for ROCK-mediated neurite remodeling. J Biol Chem. 2002;277(19):17226–17230. doi: 10.1074/jbc.M109795200. [DOI] [PubMed] [Google Scholar]
- [10].Chan CC, Roberts CR, Steeves JD, et al. Aggrecan components differentially modulate nerve growth factorresponsive and neurotrophin-3-responsive dorsal root ganglion neurite growth. J Neurosci Res. 2008;86(3):581–592. doi: 10.1002/jnr.21522. [DOI] [PubMed] [Google Scholar]
- [11].Shiotani S, Shimada M, Suehiro T, et al. Involvement of Rho-kinase in cold ischemia-reperfusion injury after liver transplantation in rats. Transplantation. 2004;78(3):375–382. doi: 10.1097/01.tp.0000128618.41619.e7. [DOI] [PubMed] [Google Scholar]
- [12].Hiraga A, Kuwabara S, Doya H, et al. Rho-kinase inhibition enhances axonal regeneration after peripheral nerve injury. J Peripher Nerv Syst. 2006;11(3):217–224. doi: 10.1111/j.1529-8027.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- [13].Madura T, Kubo T, Tanag M, et al. The Rho-associated kinase inhibitor fasudil hydrochloride enhances neural regeneration after axotomy in the peripheral nervous system. Plast Reconstr Surg. 2007;119(2):526–535. doi: 10.1097/01.prs.0000246380.40596.29. [DOI] [PubMed] [Google Scholar]
- [14].Limongelli V, Bonomi M, Marinelli L, et al. Molecular basis of cyclooxygenase enzymes (COXs) selective inhibition. Proc Natl Acad Sci U S A. 2010;107(12):5411–5416. doi: 10.1073/pnas.0913377107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Menter DG, Schilsky RL, DuBois RN. Cyclooxygenase-2 and cancer treatment: understanding the risk should be worth the reward. Clin Cancer Res. 2010;16(5):1384–1390. doi: 10.1158/1078-0432.CCR-09-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dehmelt L, Nalbant P, Steffen W, et al. A microtubulebased, dynein-dependent force induces local cell protrusions: Implications for neurite initiation. Brain Cell Biol. 2006;35(1):39–56. doi: 10.1007/s11068-006-9001-0. [DOI] [PubMed] [Google Scholar]
- [17].Gopalakrishnan SM, Teusch N, Imhof C, et al. Role of Rho kinase pathway in chondroitin sulfate proteoglycanmediated inhibition of neurite outgrowth in PC12 cells. J Neurosci Res. 2008;86(10):2214–2226. doi: 10.1002/jnr.21671. [DOI] [PubMed] [Google Scholar]
- [18].Evangelopoulos ME, Weis J, Kruttgen A. Neurotrophin effects on neuroblastoma cells: correlation with trk and p75NTR expression and influence of Trk receptor bodies. J Neurooncol. 2004;66(1-2):101–110. doi: 10.1023/b:neon.0000013492.37426.0c. [DOI] [PubMed] [Google Scholar]
- [19].Tamura M, Nakao H, Yoshizaki H, et al. Development of specific Rho-kinase inhibitors and their clinical application. Biochim Biophys Acta. 2005;1754(1-2):245–252. doi: 10.1016/j.bbapap.2005.06.015. [DOI] [PubMed] [Google Scholar]
- [20].Wu J, Zhang L, Sun ZW, et al. Mutated recombinant human glucagon-like peptide-1 induces differentiation of PC12 cells. Neural Regen Res. 2011;6(6):457–461. [Google Scholar]
- [21].Rojo Al, Innamorato NG, Martin-Moreno AM, et al. Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson's disease. Glia. 2010;58(5):588–598. doi: 10.1002/glia.20947. [DOI] [PubMed] [Google Scholar]
- [22].Stolp HB, Ek CJ, Johansson PA, et al. Factors involved in inflammation-induced developmental white matter damage. Neurosci Lett. 2009;451(3):232–236. doi: 10.1016/j.neulet.2009.01.021. [DOI] [PubMed] [Google Scholar]
- [23].Duan W, Zhang L. Cyclooxygenase inhibitors not inhibit resting lung cancer A549 cell proliferation. Prostaglandins Leukot Essent Fatty Acids. 2006;74(5):317–321. doi: 10.1016/j.plefa.2006.02.006. [DOI] [PubMed] [Google Scholar]
- [24].Acquatella-Tran Van Ba I, Marchal S, François F, et al. Regenerating islet-derived 1α (Reg-1α) protein is new neuronal secreted factor that stimulates neurite outgrowth via exostosin Tumor-like 3 (EXTL3) receptor. J Biol Chem. 2012;287(7):4726–4739. doi: 10.1074/jbc.M111.260349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Qin Z, Sun Z, Huang J, et al. Mutated recombinant human glucagon-like peptide-1 protects SH-SY5Y cells from apoptosis induced by amyloid-beta peptide (1-42) Neurosci Lett. 2008;444(3):217–221. doi: 10.1016/j.neulet.2008.08.047. [DOI] [PubMed] [Google Scholar]
- [26].Wang TC, Chiu H, Chang YJ, et al. The adaptor protein SH2B3 (Lnk) negatively regulates neurite outgrowth of PC12 cells and cortical neurons. PLoS One. 2011;6(10):e26433. doi: 10.1371/journal.pone.0026433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yamazaki M, Chiba K, Mohri T, et al. Cyclic GMP-dependent neurite outgrowth by genipin and nerve growth factor in PC12h cells. Eur J Pharmacol. 2004;488(1-3):35–43. doi: 10.1016/j.ejphar.2004.02.009. [DOI] [PubMed] [Google Scholar]
- [28].Fukuda T, Takekoshi K, Nanmoku T, et al. Inhibition of the RhoA/Rho kinase system attenuates catecholamine biosynthesis in PC 12 rat pheochromocytoma cells. Biochim Biophys Acta. 2005;1726(1):28–33. doi: 10.1016/j.bbagen.2005.08.008. [DOI] [PubMed] [Google Scholar]


