Abstract
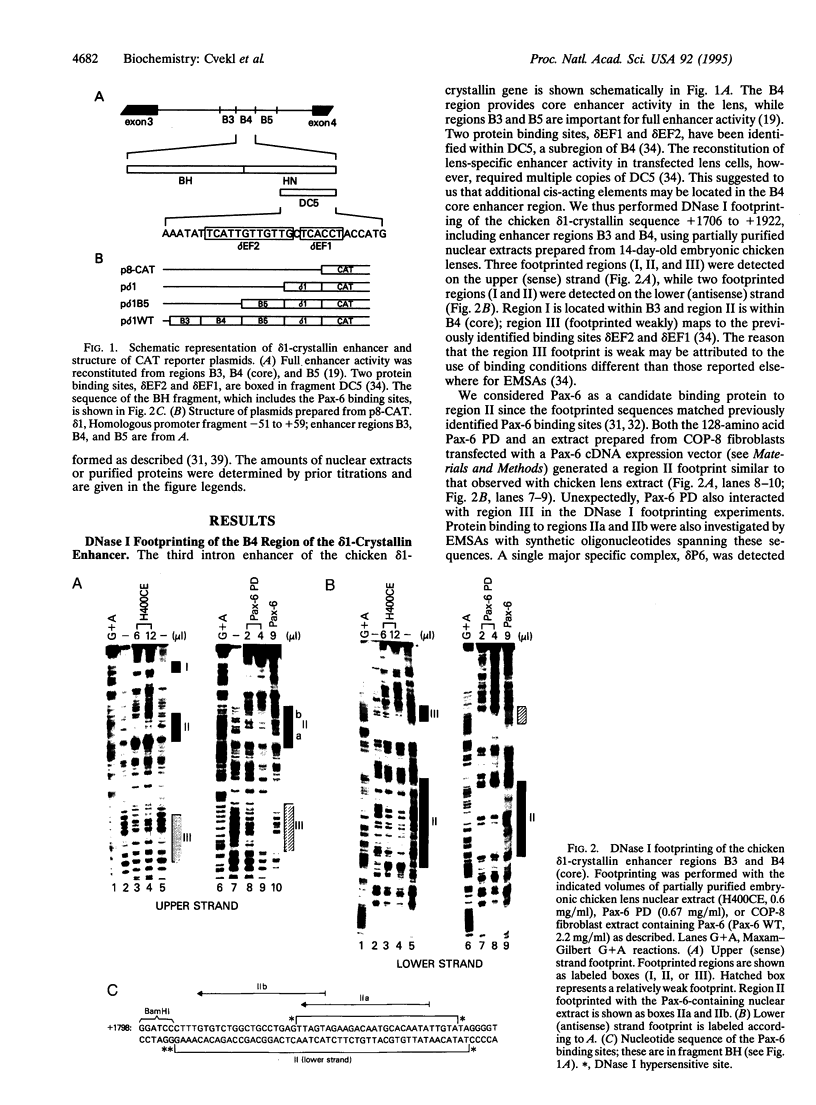
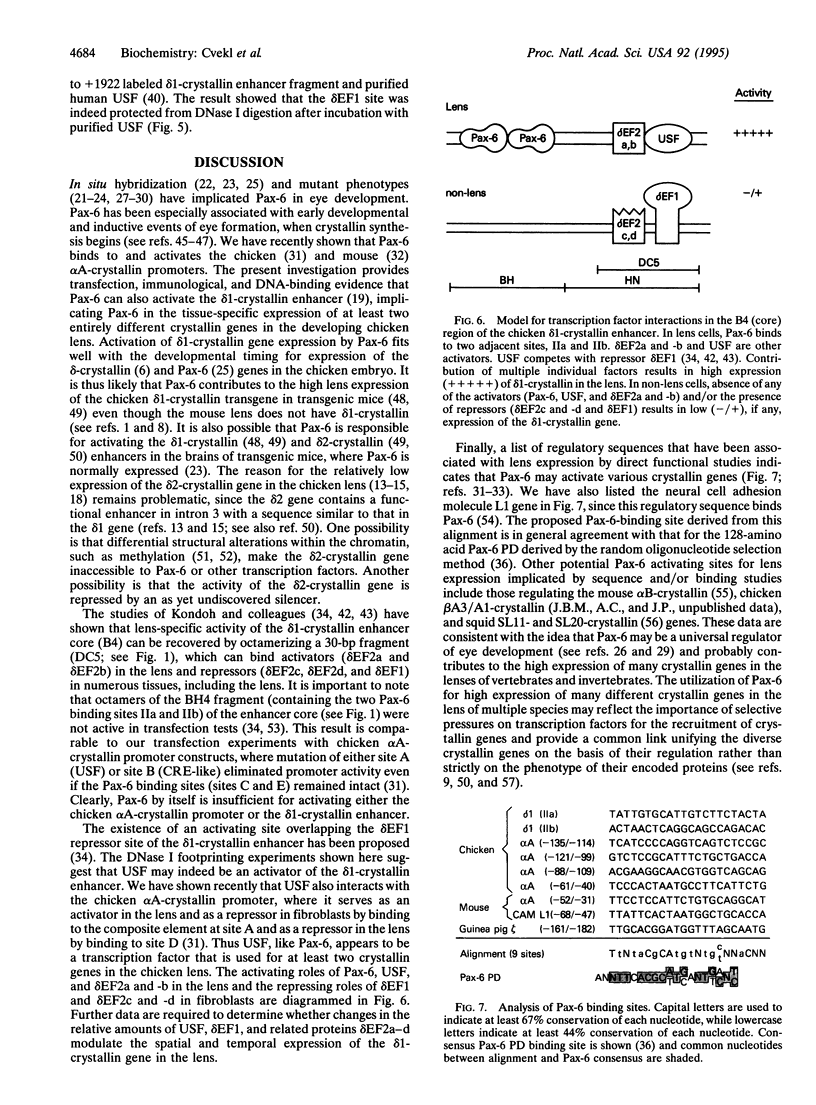
The abundance of delta-crystallin in the chicken eye lens provides an advantageous marker for tissue-specific gene expression during cellular differentiation. The lens-specific expression of the delta 1-crystallin gene is governed by an enhancer in the third intron, which binds a positive (delta EF2) and negative (delta EF1) factor in its core region. Here we show by DNase I footprinting, electrophoretic mobility-shift assays, and cotransfection experiments with the delta 1-promoter/enhancer fused to the chloramphenicol acetyltransferase reporter gene that the delta 1-crystallin enhancer has two adjacent functional Pax-6 binding sites. We also demonstrate by DNase I footprinting that the delta EF1 site can bind the transcription factor USF, raising the possibility that USF may cooperate with Pax-6 in activation of the chicken delta 1- and alpha A-crystallin genes. These data, coupled with our recent demonstration that Pax-6 activates the alpha A-crystallin gene, suggest that Pax-6 may have been used extensively throughout evolution to recruit and express crystallin genes in the lens.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbosa P., Cialkowski M., O'Brien W. E. Analysis of naturally occurring and site-directed mutations in the argininosuccinate lyase gene. J Biol Chem. 1991 Mar 15;266(8):5286–5290. [PubMed] [Google Scholar]
- Bloemendal H., de Jong W. W. Lens proteins and their genes. Prog Nucleic Acid Res Mol Biol. 1991;41:259–281. doi: 10.1016/s0079-6603(08)60012-4. [DOI] [PubMed] [Google Scholar]
- Bresnick E. H., Felsenfeld G. Evidence that the transcription factor USF is a component of the human beta-globin locus control region heteromeric protein complex. J Biol Chem. 1993 Sep 5;268(25):18824–18834. [PubMed] [Google Scholar]
- Carriere C., Plaza S., Martin P., Quatannens B., Bailly M., Stehelin D., Saule S. Characterization of quail Pax-6 (Pax-QNR) proteins expressed in the neuroretina. Mol Cell Biol. 1993 Dec;13(12):7257–7266. doi: 10.1128/mcb.13.12.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalepakis G., Wijnholds J., Giese P., Schachner M., Gruss P. Characterization of Pax-6 and Hoxa-1 binding to the promoter region of the neural cell adhesion molecule L1. DNA Cell Biol. 1994 Sep;13(9):891–900. doi: 10.1089/dna.1994.13.891. [DOI] [PubMed] [Google Scholar]
- Cvekl A., Horská K., Vlcek C., Paces V. Protein-binding A + T-rich motifs flank the duck beta A-globin enhancer. Gene. 1991 Jul 22;103(2):253–257. doi: 10.1016/0378-1119(91)90282-g. [DOI] [PubMed] [Google Scholar]
- Cvekl A., Kashanchi F., Sax C. M., Brady J. N., Piatigorsky J. Transcriptional regulation of the mouse alpha A-crystallin gene: activation dependent on a cyclic AMP-responsive element (DE1/CRE) and a Pax-6-binding site. Mol Cell Biol. 1995 Feb;15(2):653–660. doi: 10.1128/mcb.15.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvekl A., Sax C. M., Bresnick E. H., Piatigorsky J. A complex array of positive and negative elements regulates the chicken alpha A-crystallin gene: involvement of Pax-6, USF, CREB and/or CREM, and AP-1 proteins. Mol Cell Biol. 1994 Nov;14(11):7363–7376. doi: 10.1128/mcb.14.11.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerny T., Schaffner G., Busslinger M. DNA sequence recognition by Pax proteins: bipartite structure of the paired domain and its binding site. Genes Dev. 1993 Oct;7(10):2048–2061. doi: 10.1101/gad.7.10.2048. [DOI] [PubMed] [Google Scholar]
- Epstein J., Cai J., Glaser T., Jepeal L., Maas R. Identification of a Pax paired domain recognition sequence and evidence for DNA-dependent conformational changes. J Biol Chem. 1994 Mar 18;269(11):8355–8361. [PubMed] [Google Scholar]
- Funahashi J., Kamachi Y., Goto K., Kondoh H. Identification of nuclear factor delta EF1 and its binding site essential for lens-specific activity of the delta 1-crystallin enhancer. Nucleic Acids Res. 1991 Jul 11;19(13):3543–3547. doi: 10.1093/nar/19.13.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi J., Sekido R., Murai K., Kamachi Y., Kondoh H. Delta-crystallin enhancer binding protein delta EF1 is a zinc finger-homeodomain protein implicated in postgastrulation embryogenesis. Development. 1993 Oct;119(2):433–446. doi: 10.1242/dev.119.2.433. [DOI] [PubMed] [Google Scholar]
- Genis-Galves J. M., Maisel H., Castro J. Changes in chick lens proteins with aging. Exp Eye Res. 1968 Oct;7(4):593–602. doi: 10.1016/s0014-4835(68)80014-4. [DOI] [PubMed] [Google Scholar]
- Glaser T., Jepeal L., Edwards J. G., Young S. R., Favor J., Maas R. L. PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat Genet. 1994 Aug;7(4):463–471. doi: 10.1038/ng0894-463. [DOI] [PubMed] [Google Scholar]
- Glaser T., Walton D. S., Maas R. L. Genomic structure, evolutionary conservation and aniridia mutations in the human PAX6 gene. Nat Genet. 1992 Nov;2(3):232–239. doi: 10.1038/ng1192-232. [DOI] [PubMed] [Google Scholar]
- Gopal-Srivastava R., Piatigorsky J. Identification of a lens-specific regulatory region (LSR) of the murine alpha B-crystallin gene. Nucleic Acids Res. 1994 Apr 11;22(7):1281–1286. doi: 10.1093/nar/22.7.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger R. M. Embryonic lens induction: shedding light on vertebrate tissue determination. Trends Genet. 1992 Oct;8(10):349–355. doi: 10.1016/0168-9525(92)90280-h. [DOI] [PubMed] [Google Scholar]
- Hanson I. M., Fletcher J. M., Jordan T., Brown A., Taylor D., Adams R. J., Punnett H. H., van Heyningen V. Mutations at the PAX6 locus are found in heterogeneous anterior segment malformations including Peters' anomaly. Nat Genet. 1994 Feb;6(2):168–173. doi: 10.1038/ng0294-168. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Goto K., Okada T. S., Kondoh H. Lens-specific enhancer in the third intron regulates expression of the chicken delta 1-crystallin gene. Genes Dev. 1987 Oct;1(8):818–828. doi: 10.1101/gad.1.8.818. [DOI] [PubMed] [Google Scholar]
- Head M. W., Triplett E. L., Clayton R. M. Independent regulation of two coexpressed delta-crystallin genes in chick lens and nonlens tissues. Exp Cell Res. 1991 Apr;193(2):370–374. doi: 10.1016/0014-4827(91)90109-8. [DOI] [PubMed] [Google Scholar]
- Hejtmancik J. F., Beebe D. C., Ostrer H., Piatigorsky J. delta- and beta-Crystallin mRNA levels in the embryonic and posthatched chicken lens: temporal and spatial changes during development. Dev Biol. 1985 May;109(1):72–81. doi: 10.1016/0012-1606(85)90347-1. [DOI] [PubMed] [Google Scholar]
- Hill R. E., Favor J., Hogan B. L., Ton C. C., Saunders G. F., Hanson I. M., Prosser J., Jordan T., Hastie N. D., van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991 Dec 19;354(6354):522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- Kamachi Y., Kondoh H. Overlapping positive and negative regulatory elements determine lens-specific activity of the delta 1-crystallin enhancer. Mol Cell Biol. 1993 Sep;13(9):5206–5215. doi: 10.1128/mcb.13.9.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
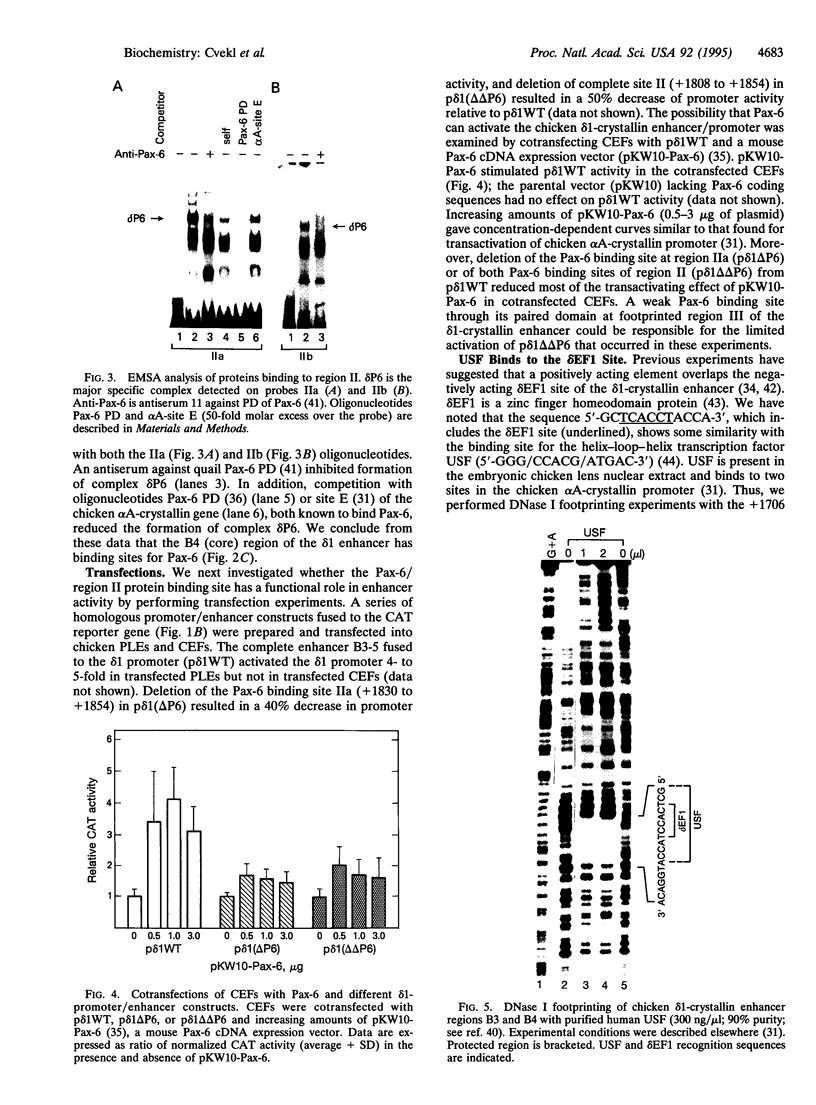
- Kessel M., Gruss P. Murine developmental control genes. Science. 1990 Jul 27;249(4967):374–379. doi: 10.1126/science.1974085. [DOI] [PubMed] [Google Scholar]
- Klement J. F., Wawrousek E. F., Piatigorsky J. Tissue-specific expression of the chicken alpha A-crystallin gene in cultured lens epithelia and transgenic mice. J Biol Chem. 1989 Nov 25;264(33):19837–19844. [PubMed] [Google Scholar]
- Kondoh H., Araki I., Yasuda K., Matsubasa T., Mori M. Expression of the chicken 'delta 2-crystallin' gene in mouse cells: evidence for encoding of argininosuccinate lyase. Gene. 1991 Mar 15;99(2):267–271. doi: 10.1016/0378-1119(91)90137-z. [DOI] [PubMed] [Google Scholar]
- Kondoh H., Katoh K., Takahashi Y., Fujisawa H., Yokoyama M., Kimura S., Katsuki M., Saito M., Nomura T., Hiramoto Y. Specific expression of the chicken delta-crystallin gene in the lens and the pyramidal neurons of the piriform cortex in transgenic mice. Dev Biol. 1987 Mar;120(1):177–185. doi: 10.1016/0012-1606(87)90116-3. [DOI] [PubMed] [Google Scholar]
- Li H. S., Yang J. M., Jacobson R. D., Pasko D., Sundin O. Pax-6 is first expressed in a region of ectoderm anterior to the early neural plate: implications for stepwise determination of the lens. Dev Biol. 1994 Mar;162(1):181–194. doi: 10.1006/dbio.1994.1077. [DOI] [PubMed] [Google Scholar]
- Li X., Zelenka P. S., Piatigorsky J. Differential expression of the two delta-crystallin genes in lens and non-lens tissues: shift favoring delta 2 expression from embryonic to adult chickens. Dev Dyn. 1993 Feb;196(2):114–123. doi: 10.1002/aja.1001960205. [DOI] [PubMed] [Google Scholar]
- Martha A., Ferrell R. E., Mintz-Hittner H., Lyons L. A., Saunders G. F. Paired box mutations in familial and sporadic aniridia predicts truncated aniridia proteins. Am J Hum Genet. 1994 May;54(5):801–811. [PMC free article] [PubMed] [Google Scholar]
- Nickerson J. M., Wawrousek E. F., Borras T., Hawkins J. W., Norman B. L., Filpula D. R., Nagle J. W., Ally A. H., Piatigorsky J. Sequence of the chicken delta 2 crystallin gene and its intergenic spacer. Extreme homology with the delta 1 crystallin gene. J Biol Chem. 1986 Jan 15;261(2):552–557. [PubMed] [Google Scholar]
- Nickerson J. M., Wawrousek E. F., Hawkins J. W., Wakil A. S., Wistow G. J., Thomas G., Norman B. L., Piatigorsky J. The complete sequence of the chicken delta 1 crystallin gene and its 5' flanking region. J Biol Chem. 1985 Aug 5;260(16):9100–9105. [PubMed] [Google Scholar]
- Parker D. S., Wawrousek E. F., Piatigorsky J. Expression of the delta-crystallin genes in the embryonic chicken lens. Dev Biol. 1988 Apr;126(2):375–381. doi: 10.1016/0012-1606(88)90147-9. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens crystallins. Innovation associated with changes in gene regulation. J Biol Chem. 1992 Mar 5;267(7):4277–4280. [PubMed] [Google Scholar]
- Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19(3):134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., O'Brien W. E., Norman B. L., Kalumuck K., Wistow G. J., Borras T., Nickerson J. M., Wawrousek E. F. Gene sharing by delta-crystallin and argininosuccinate lyase. Proc Natl Acad Sci U S A. 1988 May;85(10):3479–3483. doi: 10.1073/pnas.85.10.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatigorsky J. Puzzle of crystallin diversity in eye lenses. Dev Dyn. 1993 Apr;196(4):267–272. doi: 10.1002/aja.1001960408. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., Wistow G. The recruitment of crystallins: new functions precede gene duplication. Science. 1991 May 24;252(5009):1078–1079. doi: 10.1126/science.252.5009.1078. [DOI] [PubMed] [Google Scholar]
- Quiring R., Walldorf U., Kloter U., Gehring W. J. Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science. 1994 Aug 5;265(5173):785–789. doi: 10.1126/science.7914031. [DOI] [PubMed] [Google Scholar]
- Richardson J., Cvekl A., Wistow G. Pax-6 is essential for lens-specific expression of zeta-crystallin. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4676–4680. doi: 10.1073/pnas.92.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha M. S., Servetnick M., Grainger R. M. Vertebrate eye development. Curr Opin Genet Dev. 1992 Aug;2(4):582–588. doi: 10.1016/s0959-437x(05)80176-5. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985 Nov;43(1):165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Sekido R., Murai K., Funahashi J., Kamachi Y., Fujisawa-Sehara A., Nabeshima Y., Kondoh H. The delta-crystallin enhancer-binding protein delta EF1 is a repressor of E2-box-mediated gene activation. Mol Cell Biol. 1994 Sep;14(9):5692–5700. doi: 10.1128/mcb.14.9.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D. J., Sharp P. A., Wahli W. W., Keller M. J. A high-efficiency HeLa cell nuclear transcription extract. DNA. 1988 Jan-Feb;7(1):47–55. doi: 10.1089/dna.1988.7.47. [DOI] [PubMed] [Google Scholar]
- Shinohara T., Piatigorsky J. Quantitation of delta-crystallin messenger RNA during lens induction in chick embryos. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2808–2812. doi: 10.1073/pnas.73.8.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan C. H., Norman J. T., Borrás T., Grainger R. M. Developmental regulation of hypomethylation of delta-crystallin genes in chicken embryo lens cells. Mol Cell Biol. 1989 Jul;9(7):3132–3135. doi: 10.1128/mcb.9.7.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan C. H., O'Farrell S., Grainger R. M. Delta-crystallin gene expression and patterns of hypomethylation demonstrate two levels of regulation for the delta-crystallin genes in embryonic chick tissues. Dev Biol. 1991 May;145(1):40–50. doi: 10.1016/0012-1606(91)90211-k. [DOI] [PubMed] [Google Scholar]
- Thomas G., Zelenka P. S., Cuthbertson R. A., Norman B. L., Piatigorsky J. Differential expression of the two delta-crystallin/argininosuccinate lyase genes in lens, heart, and brain of chicken embryos. New Biol. 1990 Oct;2(10):903–914. [PubMed] [Google Scholar]
- Tomarev S. I., Duncan M. K., Roth H. J., Cvekl A., Piatigorsky J. Convergent evolution of crystallin gene regulation in squid and chicken: the AP-1/ARE connection. J Mol Evol. 1994 Aug;39(2):134–143. doi: 10.1007/BF00163802. [DOI] [PubMed] [Google Scholar]
- Ton C. C., Hirvonen H., Miwa H., Weil M. M., Monaghan P., Jordan T., van Heyningen V., Hastie N. D., Meijers-Heijboer H., Drechsler M. Positional cloning and characterization of a paired box- and homeobox-containing gene from the aniridia region. Cell. 1991 Dec 20;67(6):1059–1074. doi: 10.1016/0092-8674(91)90284-6. [DOI] [PubMed] [Google Scholar]
- Walther C., Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991 Dec;113(4):1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- Wistow G. J., Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- Zuker C. S. On the evolution of eyes: would you like it simple or compound? Science. 1994 Aug 5;265(5173):742–743. doi: 10.1126/science.8047881. [DOI] [PubMed] [Google Scholar]
- Zwaan J., Ikeda A. Macromolecular events during differentiation of the chicken lens. Exp Eye Res. 1968 Apr;7(2):301–311. doi: 10.1016/s0014-4835(68)80081-8. [DOI] [PubMed] [Google Scholar]