Abstract
The cation-dependent mannose 6-phosphate receptor (CD-MPR) mediates the transport of lysosomal enzymes from the trans-Golgi network to endosomes. Evasion of lysosomal degradation of the CD-MPR requires reversible palmitoylation of a cysteine residue in its cytoplasmic tail. Because palmitoylation is reversible and essential for correct trafficking, it presents a potential regulatory mechanism for the sorting signals within the cytoplasmic domain of the CD-MPR. Characterization of the palmitoylation performing an in vitro palmitoylation assay by using purified full-length CD-MPR revealed that palmitoylation of the CD-MPR occurs enzymatically by a membrane-bound palmitoyltransferase. In addition, analysis of the localization revealed that the palmitoyltransferase cycles between endosomes and the plasma membrane. This was identified by testing fractions from HeLa cell homogenate separated on a density gradient in the in vitro palmitoylation assay and further confirmed by in vivo labeling experiments by using different treatments to block specific protein trafficking steps within the cell. We identified a novel palmitoyltransferase activity in the endocytic pathway responsible for palmitoylation of the CD-MPR. The localization of the palmitoyltransferase not only fulfills the requirement of our hypothesis to be a regulator of the intracellular trafficking of the CD-MPR but also may affect the sorting/activity of other receptors cycling through endosomes.
INTRODUCTION
Lysosomes are intracellular organelles containing numerous acid hydrolases and serve as a major degradative compartment in eukaryotic cells (Kornfeld, 1992; Hille-Rehfeld, 1995). Delivery of newly synthesized soluble lysosomal enzymes to lysosomes is dependent on their acquisition of mannose 6-phosphate (M6P) residues in the Golgi and the trans-Golgi network (TGN). This tag acts as a recognition signal for high-affinity binding to the M6P receptors (MPRs) in the TGN. The receptor ligand complexes then leave the TGN in clathrin-coated vesicles, which fuse with acidified endosomes (Le Borgne and Hoflack, 1997). After the pHinduced dissociation of the complexes, the lysosomal enzymes are further delivered to lysosomes, whereas the receptors recycle back to the TGN to repeat this process.
The 46-kDa cation-dependent mannose 6-phosphate receptor (CD-MPR) is a type I integral membrane protein. The intracellular trafficking of the CD-MPR is directed by sorting signals located in its 67-amino acid cytoplasmic tail. Internalization is mediated by three separate internalization sequences through clathrin-coated pits: a pair of phenylalanine residues (Phe13-X-X-X-X-Phe18), a classical tyrosine motif (Tyr45-X-X-Val48), and probably a C-terminal dileucine motif (Leu64-Leu65) (Johnson et al., 1990; Denzer et al., 1997). Overlapping with one of the internalization motifs is a diaromatic motif (Phe18-Trp19) that binds to the MPR-tail interacting protein of 47 kDa (TIP47) and is required for the sorting of the receptor from late endosomes back to the TGN, thereby preventing degradation of the CD-MPR in the lysosomes (Schweizer et al., 1997; Diaz and Pfeffer, 1998; Nair et al., 2003). In addition to the diaromatic motif, palmitoylation of the Cys34 is required to avoid lysosomal degradation (Schweizer et al., 1996). Palmitoylation of Cys34 will anchor this portion of the cytoplasmic tail of CD-MPR to the lipid bilayer, influencing the conformation of the entire cytoplasmic tail and thereby modulating the accessibility of the sorting signals. Palmitoylation of the CD-MPR is reversible with a rapid turnover of palmitate (τ1/2 ≤ 2 h) (Schweizer et al., 1996). These findings led to the suggestion that palmitoylation functions to regulate the presentation of overlapping sorting signals in the cytoplasmic tail of the CD-MPR. Such a tight regulation of the signals would require that palmitoylation of the CD-MPR occur enzymatically.
Enzymatic as well as nonenzymatic palmitoylation has been described previously (O'Brien et al., 1987; Duncan and Gilman, 1996), although the latter might only occur in vitro. The two most promising candidates for such an enzymatic palmitoylation were identified in yeast, Akr1p, and the Erf2p/Erf4p complex that palmitoylate the casein kinase Yck2p and Ras2p, respectively (Lobo et al., 2002; Roth et al., 2002). In addition to these two palmitoyltransferases, many palmitoyltransferase activities have been reported mostly along the exocytic pathway. Some proteins (vesicular stomatitis virus G, influenza hemagglutinin, and CC chemokine receptor 5) are palmitoylated in the endoplasmic reticulum-Golgi intermediate compartment or cis-Golgi (Bonatti et al., 1989; Veit and Schmidt, 1993; Blanpain et al., 2001). Further in the secretory pathway, a palmitoyltransferase activity localized to the Golgi palmitoylates p21N-ras (Gutierrez and Magee, 1991). For many cytosolic and membrane proteins (Fyn, edGα-subunits of G protein, and transferrin receptor), palmitoylation seems to occur at the plasma membrane (Adam et al., 1984; Dunphy et al., 1996; van't Hof and Resh, 1997; Fishburn et al., 1999). Furthermore, there is a palmitoyltransferase activity in mitochondria (Corvi et al., 2001) and in yeast on the vacuole (Veit et al., 2001). The various subcellular sites and the diversity of the substrates for palmitoylation suggest that numerous palmitoyltransferases with different subcellular locations and specificities may exist.
Three thioesterases that remove the palmitate residue from proteins were identified so far in mammals (Camp and Hofmann, 1993; Soyombo and Hofmann, 1997; Duncan and Gilman, 1998). Two of these thioesterases are soluble lysosomal enzymes, palmitoyl-protein thioesterase 1 and 2 (PPT1 and PPT2), required for degradation of palmitoylated substrates (Verkruyse and Hofmann, 1996; Soyombo and Hofmann, 1997). The third thioesterase, acyl protein thioesterase 1, is a cytoplasmic protein and was shown to depalmitoylate substrates, such as G protein α subunits, p21ras and endothelial nitric-oxide synthase (Duncan and Gilman, 1998; Yeh et al., 1999). Whether the CD-MPR gets enzymatically palmitoylated and/or depalmitoylated has not been investigated so far.
To confirm or overthrow our hypothesis that reversible palmitoylation of the CD-MPR is regulating its sorting a detailed characterization of its palmitoylation is required.
MATERIALS AND METHODS
Materials
Enzymes used in molecular cloning were obtained from Roche Diagnostics (Mannheim, Germany), New England Biolabs (Beverly, MA), or Promega (Madison, WI); general chemicals were from Fluka (Buchs, Switzerland); protease inhibitors, CoA, and wortmannin were from Sigma-Aldrich (St. Louis, MO); DMEM, fetal calf serum, G418, and LipofectAMINE Plus were from Invitrogen (Carlsbad, CA); cell culture dishes from Falcon (Franklin Lakes, NJ); nitrocellulose was from Schleicher & Schuell (Dassel, Germany); enhanced chemiluminescence Western blotting reagents from PerkinElmer Life and Analytical Sciences (Boston, MA); protein A-Sepharose beads were from Repligen (Cambridge, MA); Percoll, activated CH Sepharose 4B, and low-molecular-weight protein markers were from Amersham Biosciences (Piscataway, NJ); Centricon Plus-20 was from Millipore (Bedford, MA); [3H]palmitate was from ARC (St. Louis, MO) or from ANAWA Trading SA (Zürich, Switzerland); and acyl-CoA synthetase was from Fluka. Oligonucleotides were synthesized either by the DNA synthesis facility of the Friedrich Miescher Institute (Basel, Switzerland) or Microsynth (Balgach, Switzerland).
Antibodies
Rabbit anti-mouse IgG was purchased from Zymed Laboratories (South San Francisco, CA). Horseradish peroxidase-conjugated antibodies against mouse and rabbit were from Amersham Biosciences. Alexa 488-conjugated goat anti-mouse antibody was from Molecular Probes (Eugene, OR). The monoclonal antibody (mAb) 22D4 specific for the bovine CD-MPR was generously provided by D. Messner (Messner, 1993). This mAb is specific for the bovine CD-MPR and does not cross-react with the endogenous mouse CD-MPR. The monoclonal antibodies for NaKATPase (N1/123/33) and p63 (G1/296/22) were a gift from H.P. Hauri (Marxer et al., 1989; Schweizer et al., 1993). The mAb for Rab5 was generously provided by J. Gruenberg (Gorvel et al., 1991), and the polyclonal antibody for Rab7 (no 14-1) was kindly supplied by P. Chavrier (Chavrier et al., 1990). The mAb for β1-4-galactosyltransferase-1 was a gift from E. Berger (Berger et al., 1986).
Recombinant DNA
All basic DNA procedures were as described previously (Sambrook et al., 1998). The polymerase chain reaction procedure of Ho et al., (1989) was used to generate the MPR-FFWYLL-A and MPR-C30,34A constructs with pSFFV-MPR (Rohrer et al., 1995) serving as a template together with MPR-BglII.down (5′-CCGAGATCTCCCACTTAAGCGTGG-3′) and pSFFVneo.up2 (5′-CTGCCATTCATCCGCTTATTATC-3′) as the down- and upstream primers, respectively. Appropriate partial complementary pairs of oligonucleotides in which the desired amino acid replacement had been incorporated were chosen as internal primers. The final PCR products were subcloned into pSFFVneo as described previously (Rohrer et al., 1995) and confirmed by sequencing.
Cell Culture and Transfection
A mannose-6-P/insulin-like growth factor-II receptor-deficient mouse L cell line designated D9 (LRec-) was transfected as described previously (Nair et al., 2003).
Synthesis of [3H]Palmitoyl-CoA
[3H]Palmitoyl-CoA was prepared from 750 μCi of [3H]palmitate (60 Ci/mmol, 10 mCi/ml) by incubation with 0.05 U of acyl-CoA synthetase in 1 ml of 0.05% Triton X-100, 0.5 mM CoA, 1 mM ATP, 1 mM MgCl2, 40 mM KH2PO4, pH 7.5, for 1 h at 37°C. The sample was dried in a Speed-Vac centrifugal evaporator (Savant Instruments, Hicksville, NY) and resuspended in 75 μl of 50 mM Tris, pH 8.0. The purity was determined by thin-layer chromatography on Merck Silica Gel 60 plate by using propanol/water/5% ammonia (70:10:20) as the developing solvent, resulting in an Rf value of 0.43 for palmitoyl-CoA and 0.61 for palmitate. The analysis showed ≥90% radio-chemical purity.
CD-MPR Purification
Mouse L cells stably expressing bovine CD-MPR wild type (wt) were grown in suspension. Cells (1.5 × 109) were centrifuged for 5 min at 260 × g at 4°C, washed with phosphate-buffered saline (PBS), lysed in 8 ml of buffer-2 containing 1:500 dilution of a protease inhibitor cocktail, and sonicated. After solubilizing for 30 min on ice, the cell lysates were centrifuged for 30 min at 40,000 rpm at 4°C in a Ti 50 rotor (Beckman Coulter, Fullerton, CA). The supernatant was loaded onto a 22D4 antibody column. This column was prepared by coupling 5 mg of purified 22D4 mAb to 5 ml of activated CH-Sepharose 4B according to the manufacturer's protocol. The column was washed with 150 ml of buffer-2 containing protease inhibitors at a flow rate of 1 ml/min. Bound CD-MPR was eluted with elution buffer (0.1 M glycine, pH 3.0, 0.05% Triton X-100) in 1-ml fractions containing 100 μl of 1 M Tris, pH 8.2, to neutralize. Ten microliters of each fraction was subjected to SDS-PAGE and immunoblotting with 22D4 antibody. The fractions containing CD-MPR were pooled and concentrated with Centricon Plus-20 to obtain a concentration of 1 mg/ml. The Triton X-100 content was measured in a spectrophotometer at 277-nm wavelength, and the protein concentration was determined using a Bio-Rad protein assay (Bio-Rad, Hercules, CA). The sample was aliquoted and frozen at -20°C.
In Vitro Palmitoyltransferase Assay
Ten micrograms of purified CD-MPR and 200 μCi of [3H]palmitoyl-CoA were incubated with 150 μg of protein from HeLa cell postnuclear supernatant (PNS) or membrane fraction in a total volume of 500 μl of assay buffer (45 mM Tris, pH 8.0, 40.5 mM glycine, 2 mM ATP, 130 mM KCl, 10 mM NaCl, 1 mM dithiothreitol [DTT], 0.02% Triton X-100). The sample was incubated at 37°C for 30 min. Then, 500 μl of 2× buffer-2 containing protease inhibitor cocktail and phenylmethylsulfonyl fluoride was added to the sample and solubilized on ice for 30 min followed by a centrifugation at 100,000 × g in a Ti50 rotor for 30 min at 4°C. The resulting supernatants were subjected to immunoprecipitation and subsequently analyzed by SDS-PAGE and fluorography as described below.
Percoll Density Gradient
HeLa cells were grown on 15-cm culture dishes. Cells from 12 dishes were washed with PBS and scraped in 5 ml of homogenization buffer (10 mM Tris, pH 7.4, 0.25 M sucrose). The cells were centrifuged at 260 × g for 5 min at 4°C, resuspended in 5 ml of homogenization buffer containing protease inhibitors, and homogenized in a ball-bearing homogenizer (HMG, Heidelberg, Germany) with 12 strokes. The homogenate was centrifuged at 700 × g for 10 min at 4°C, and 5 mg of the resulting PNS was diluted in 2.3 ml of homogenization buffer and mixed with 9.2 ml of 15% Percoll. The resulting 12% Percoll sample was loaded on top of a 0.5-ml 2.5 M sucrose pillow into a thin-walled open-top centrifugation tube and centrifuged at 20,000 rpm (28,000 × g) in a Ti70.1 rotor for 45 min at 4°C. Fractions (1 ml) were collected from the bottom of the gradient, and the membranes of each fraction were pelleted by centrifugation at 80,000 rpm in a TLA 120.2 rotor for 30 min at 4°C and resuspended in equal volumes. The fractions were assayed for β-hexosaminidase activity (lysosomal enzyme) and subjected to SDS-PAGE and immunoblotting as described below with antibodies against NaK-ATPase, galactosyltransferase, p63, Rab5, and Rab7 to determine the expression levels of these marker enzymes.
Internalization Assay
Internalization assay was performed as described previously (Schweizer et al., 2000) by biotinylation of surface proteins with sulfo-NHS-SS-biotin on ice, internalization for different periods of time at 37°C, followed by removal of surface biotin with glutathione. The amount of biotinylated internalized CD-MPR was then compared with a sample that was only incubated on ice (0% biotinylation) and a sample that was not treated with glutathione after biotinylation (100% biotinylation).
Steady-State Surface Distribution of CD-MPR
The steady-state surface distribution was of wild-type and mutant CD-MPR was measured by binding of iodinated antibodies as described previously (Schweizer et al., 1997). The iodination of the antibody was performed by ANAWA Trading SA according to their standard protocol.
Metabolic Labeling with [3H]Palmitate
Cells were grown in six-well plates. For the treatment at 19°C, the cells were washed twice with PBS and pretreated at 19 or 37°C for 30 min in 1 ml of DMEM containing 20 mM HEPES, pH 7.4, and 5% low-lipid calf serum. For the treatment with wortmannin, the cells were washed twice with PBS and preincubated with DMEM containing 20 mM HEPES, pH 7.4, 5% low-lipid calf serum, and 1 μM wortmannin or 0.1% dimethyl sulfoxide (DMSO) for control cells for 45 min at 37°C. After preincubation, all cells were incubated with 150 μCi of [3H]palmitate in 1 ml of preincubation medium for 90 min at 37°C. For the wortmannin treatment, 1 μM wortmannin was added again to the labeling media after 45 min. After labeling, cells were chilled on ice, washed once with ice-cold PBS, scraped in 1 ml of ice-cold PBS, and centrifuged for 5 min at 260 × g at 4°C. The pellets were lysed in 1 ml of buffer-2 (100 mM sodium phosphate, pH 8.0, containing 1% Triton X-100 and a 1:500 dilution of a protease inhibitor cocktail [5 mg/ml benzamidine and 1 mg/ml each of pepstatin A, leupeptin, antipain, and chymostatin in 40% dimethyl sulfoxide/60% ethanol]) and phenylmethylsulfonyl fluoride (40 μg/ml) and passed five times through a 25-gauge needle connected to a 1-ml syringe. After solubilizing for 30 min on ice, the cell lysates were centrifuged for 30 min at 40,000 rpm in a Ti50 rotor (Beckman Coulter). The resulting supernatants were subjected to immunoprecipitation and subsequently analyzed by SDS-PAGE and fluorography as described below.
For the pulse-chase experiment, cells were pretreated at 37°C for 30 min in 1 ml of DMEM containing 20 mM HEPES, pH 7.4, and 5% low-lipid calf serum. Then, the cells were incubated with 150 μCi of [3H]palmitate in 1 ml of preincubation medium for 90 min at 37°C. The cells were washed twice with PBS containing 0.1 mM nonradioactive palmitate, which is 40-fold in excess over the radioactive palmitate. Subsequently, the cells were chased for 2 h in DMEM, containing 10% fetal calf serum and 0.1 mM nonradioactive palmitate at 37°C in the presence or absence of 1 μM wortmannin or at 19°C. For the wortmannin-treated samples, 1 μM wortmannin was repeatedly added after every hour. After the chase, the cells were chilled on ice and the procedure was followed as described above for the labeled cells.
Immunoprecipitation, SDS-PAGE, Fluorography, and Immunoblotting
Immunoprecipitation of CD-MPR with anti-CD-MPR mAb 22D4 was carried out as described previously (Rohrer et al., 1995). The immunocomplexes were released from the beads by boiling for 3 min in nonreducing SDS-PAGE sample buffer (94 mM Tris-HCl, pH 6.8, 3% SDS, 15% glycerol, 0.001% bromphenol blue). The proteins were separated on a 10% SDS-polyacrylamide minigel by using the Laemmli system (Laemmli, 1970). For fluorography, the gel was stained with 0.25 g Coomassie Brilliant Blue R-250 in 100 ml of destaining solution (25% methanol, 7% acetic acid in H2O) for 20 min, destained for 1 h in destaining solution, incubated in H2O for 5 min, treated with 1 M sodium salicylate for 20 min, dried, and exposed to XOmat AR film (Eastman Kodak, Rochester, NY) for 3–10 d. For Western blotting, the gel was transferred onto a nitrocellulose membrane according to the method of Towbin et al. (1979). The membrane was blocked with 3% nonfat dry milk powder (Sano Lait, Coop, Switzerland) in PBS. The blot was subsequently incubated with anti-CD-MPR monoclonal antibody 22D4 or other antibodies as stated (diluted 1:500 in PBS-3% powdered milk), followed by a horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibody (diluted 1:2000 in PBS-3% powdered milk). Immunoreactive proteins were visualized using the enhanced chemiluminescence detection system according to the manufacturer's directions. The autoradiographs were quantitated using a personal densitometer (Amersham Biosciences).
Confocal Immunofluorescence Microscopy
Cells were grown on coverslips, washed with PBS, and fixed in 3% paraformaldehyde, pH 8.3, for 20 min followed by four washes with 20 mM glycine in PBS. The cells were permeabilized in saponin buffer (0.1% saponin, 20 mM glycine in PBS) for 20 min. All following steps were performed in saponin buffer. Cells were incubated with 22D4 antibody (1:500) for 30 min and washed four times, followed by incubation with goat anti-mouse Alexa 488 antibody. The coverslips were washed four times and mounted on glass slides with ProLong Antifade (Molecular Probes) for viewing with a Leica SP2 AOBS UV confocal laser scanning microscope. Serial sections in the z-axis through the entire cells were taken, and the resulting stacks of images were analyzed with the use of the Imaris program (Bitplane AG, Zürich, Switzerland).
Assays and Miscellaneous Methods
β-Hexosaminidase activity was determined as described previously (Rohrer et al., 1995). Protein concentration was determined with the Bio-Rad protein assay kit by using protein standard I according to the manufacturer's protocol.
RESULTS
CD-MPR Is Enzymatically Palmitoylated
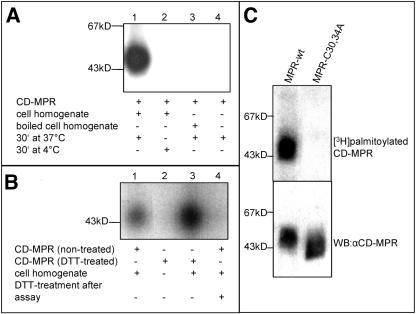
CD-MPR is reversibly palmitoylated at Cys30 and Cys34, wherein palmitoylation of the Cys34 is essential for the trafficking of the CD-MPR from endosomes to the TGN (Schweizer et al., 1996). To explore the nature of this reversible palmitoylation of the CD-MPR, an in vitro palmitoylation assay was developed. Ten micrograms of CD-MPR, purified as a full-length membrane protein, was incubated with 200 μCi of [3H]palmitoyl-CoA for 30 min at 37°C with HeLa cell homogenate. To maintain the CD-MPR in solubilized form, 0.02% Triton X-100 was used. In a first step, the assay was validated by demonstrating that palmitoylation of the CD-MPR occurs enzymatically. Incubation of purified CD-MPR with [3H]palmitoyl-CoA alone did not result in palmitoylated CD-MPR in contrast to the incubation of the receptor with [3H]palmitoyl-CoA and HeLa cell extract (Figure 1A, lane 1 vs. lane 4). Next, a potential palmitoyltransferase was either denatured by boiling the HeLa cell homogenate for 15 min before the incubation at 37°C; or alternatively, the entire reaction was carried out at 4°C (Figure 1A, lanes 2 and 3). In both cases, no palmitoylated CD-MPR was detected. Furthermore, experiments with varying time and temperature of the incubation revealed that the assay is dependent on those parameters (our unpublished data). Together, these findings demonstrate that palmitoylation of the CD-MPR in this assay requires an active enzyme. Moreover, no autocatalytic palmitoylation was obtained when purified CD-MPR was treated with 50 mM DTT for 2 h at 50°C to hydrolyze already attached palmitate moieties before the incubation with [3H]palmitoyl-CoA (Figure 1B, lane 2). However, DTT-treated CD-MPR was readily palmitoylated when HeLa cell homogenate was added to the assay (Figure 1B, lane 3). This showed that the enzymatic requirement was specific for palmitoylation and not the hydrolysis of the palmitate. The palmitic acid was attached via a thioester linkage, which was demonstrated by treating the [3H]palmitoylated CD-MPR after the in vitro palmitoylation assay with 50 mM DTT for 2 h at 50°C. The [3H]palmitate was released by DTT that hydrolyzes thioesters (Figure 1B, lanes 1 and 4), confirming previous results in vivo (Schweizer et al., 1996). To show, that the palmitoyl-transferase in the in vitro assay specifically palmitoylated the cytoplasmic cysteines and not the lumenal ones, we used PNS from mouse L cells stably transfected with wt CD-MPR and with a mutant CD-MPR, with both cytoplasmic cysteines replaced by alanines (C30,34A). In this experiment, the PNS comprised the palmitoyltransferase activity as well as the appropriate substrate, which was immunoprecipitated after incubation with [3H]palmitoyl-CoA. We could show that the lumenal cysteines of the CD-MPR in the C30,34A were not substrate for the palmitoyltransferase, because this mutant did not show incorporation of [3H]palmitate (Figure 1C).
Figure 1.
CD-MPR palmitoylation occurs enzymatically. (A) Ten micrograms of purified CD-MPR was incubated with [3H]palmitoyl-CoA and 150 μg of HeLa cell homogenate for 30 min at 37°C (lane 1) or at 4°C (lane 2). In lane 3, 150 μg of HeLa cell homogenate was boiled for 15 min before incubation with CD-MPR and [3H]palmitoyl-CoA for 30 min at 37°C. Lane 4 shows the sample of an assay without HeLa cell homogenate. CD-MPR was immunoprecipitated and subjected to SDS-PAGE (10% gel). [3H]Palmitate incorporation into CD-MPR was visualized by autoradiography. (B) Ten micrograms purified CD-MPR was incubated with [3H]palmitoyl-CoA and 150 μg of HeLa cell homogenate for 30 min at 37°C (lane 1). Ten micrograms of purified CD-MPR was incubated with 50 mM DTT for 2 h at 50°C before incubation with [3H]palmitoyl-CoA and either without (lane 2) or with 150 μg of HeLa cell homogenate (lane 3) for 30 min at 37°C. CD-MPR was immunoprecipitated. The sample in lane 4 corresponds to the sample in lane 1 that was subsequently incubated with 50 mM DTT for 2 h at 50°C. The samples were separated on SDS-PAGE (10% gel). [3H]palmitate incorporation into CD-MPR was visualized by autoradiography. (C) PNS of mouse L cells stably transfected with CD-MPR wild-type (wt) or CD-MPR C30,34A were incubated with [3H]palmitoyl-CoA for 30 min at 37°C. CD-MPR wild type and mutant were subsequently immunoprecipitated and subjected to SDS-PAGE (10% gel). [3H]Palmitate incorporation into CD-MPR was visualized by autoradiography (top). Expression levels of the CD-MPR wild-type and C30,34A in the PNS were determined by Western blotting (WB) with the anti-CD-MPR mAb 22D4 (bottom).
Palmitoyltransferase Is a Membrane Protein
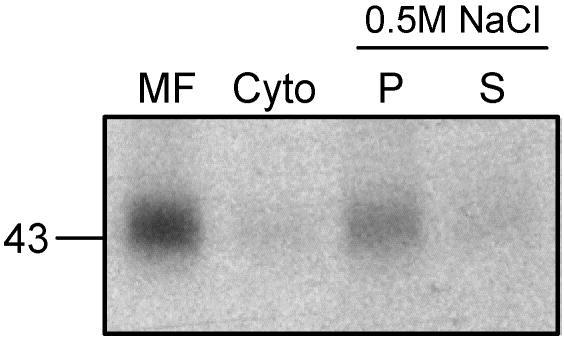
The localization of the palmitoyltransferase was analyzed by fractionating the cell homogenate. The 700 × g supernatant (PNS) was subjected to a 100,000 × g spin. The pellet containing the membranes and the supernatant containing the cytosol were both tested for palmitoyltransferase activity in the in vitro assay. From the total activity of the PNS, 91% was found in the pellet; thus, in the membrane fraction and only 9% was found in the cytosol (Figure 2). To differentiate between peripheral and integral membrane proteins, the pellet of the 100,000 × g spin was incubated with 0.5 M NaCl for 30 min at 4°C and then centrifuged once more at 100,000 × g for 30 min at 4°C. The resulting supernatant contained peripheral membrane proteins that were detached from the membrane, whereas the pellet consisted of integral membrane proteins. The total CD-MPR palmitoyltransferase activity determined in the in vitro assay after the treatment with high salt was reduced to 37% compared with nontreated membranes, which indicates the sensitivity of the palmitoyltransferase to high salt concentration. However, the majority (75%) of the palmitoyltransferase activity of the membranes, treated with high salt, was recovered in the pellet (Figure 2). Furthermore, a carbonate wash was applied to the membrane fraction. This harsher treatment more accurately separates peripheral from integral membrane proteins. The sample was incubated with 0.1 M sodium carbonate, pH 11, for 30 min at 4°C, neutralized with HCl, and subsequently centrifuged at 100,000 × g for 30 min at 4°C. When tested in the in vitro assay, no palmitoylated CD-MPR was detected in the pellet or in the supernatant (our unpublished data), indicating that the exposure to high pH irreversibly destroyed the palmitoyltransferase activity.
Figure 2.
Palmitoyltransferase is membrane bound. The postnuclear supernatant of HeLa cells was centrifuged at 100,000 × g for 30 min at 4°C. The pellet contained the membrane fraction (MF), and the supernatant consisted of the cytosol (Cyto). For the salt wash, the membrane fraction was subsequently incubated with 0.5 M NaCl for 30 min at 4°C and centrifuged again. The resulting pellet (P) and the supernatant (S) as well as the MF and the Cyto were assayed for palmitoyltransferase activity in vitro. Equal amounts of protein (MF, P) and according amount of supernatant (C, S) were used in the palmitoyltransferase assay .The samples were incubated with [3H]palmitoyl-CoA and purified CD-MPR at 37°C for 30 min. CD-MPR was immunoprecipitated and subjected to SDS-PAGE. [3H]Palmitate incorporation into CD-MPR was visualized by autoradiography and quantitated by densitometric scanning.
Cell Fractionation to Localize the Palmitoyltransferase Activity
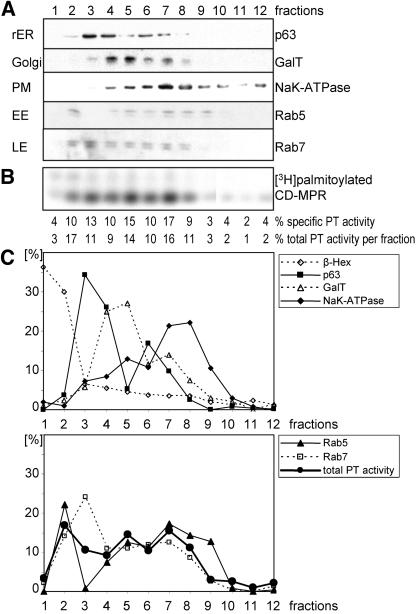
To determine the localization of the palmitoyltransferase, HeLa cell homogenate was separated on a self-forming Percoll density gradient, and 12 individual fractions were harvested from the bottom. The fractions were then centrifuged at 100,000 × g to pellet the membranes and subsequently resuspended in equal volumes. With this step, we isolated the membranes and organelles free from cytosol, and therefore they did not contain the thioesterase acyl protein thioesterase 1, which might act on the CD-MPR and thus influence the experimental data. The same percentage of the fractions of the gradient was tested for expression of marker enzymes of the different organelles. A typical result is shown in Figure 3. The gradient clearly separated peaks of β-hexosaminidase (lysosomes), p63 (rough endoplasmic reticulum), galactosyltransferase (Golgi) and NaK-ATPase (plasma membrane) (Figure 3, A and C). The distribution of marker proteins for the early (Rab5) and late endosomes (Rab7) was not as clearly separated as the other marker proteins. Rab5 had two major peaks, one very dense peak close to the lysosomal β-hexosaminidase (fraction 2), reflecting a heavy, probably coated early endosomal structure and a broad peak partially coinciding with the marker of the plasma membrane, representing a light early endosomal structure. The majority of Rab7 was found on a dense fraction (fraction 3) trailing off into lighter fractions (fractions 4–8), reflecting the heterogeneous nature of late endosomes. For each fraction, 150 μg of protein was tested in the in vitro assay, to determine the specific palmitoyltransferase activity (Figure 3B). The specific activity of the palmitoyltransferase was quantified; subsequently, the total activity per fraction was calculated and plotted in a graph together with the marker proteins for comparison (Figure 3C). The CD-MPR palmitoyltransferase activity showed a distribution with several small peaks that coincided with the distribution of the early endosomes, possibly reflecting an association of the palmitoyltransferase with this organelle. Alternatively, the palmitoyltransferase could be localized to several organelles, including the Golgi, the plasma membrane, and heavy early endosomal structures (Figure 3C).
Figure 3.
HeLa cell fractionation assayed for palmitoyltransferase (PT) activity in vitro. (A) Postnuclear supernatant of HeLa cells was separated on a 15% Percoll density gradient. Fractions were collected from bottom (fraction 1) to top (fraction 12) of the gradient. Membranes of fractions were centrifuged, and pellets were resuspended in equal volumes. Equal volumes of fractions were tested for expression of marker enzymes of the different organelles by immunoblotting with the respective antibodies: galactosyltransferase (GalT) for Golgi, NaK-ATPase for plasma membrane (PM), p63 for rough endoplasmic reticulum (rER), Rab7 for late endosomes (LE), and Rab5 for early endosomes (EE). (B) One hundred fifty micrograms of each fraction was assayed for palmitoyltransferase activity in vitro. The samples were incubated with [3H]palmitoyl-CoA and purified CD-MPR at 37°C for 30 min. CD-MPR was immunoprecipitated and subjected to SDS-PAGE. [3H]Palmitate incorporation into CD-MPR was visualized by autoradiography. The percentage of specific activity in each fraction was quantitated by densitometric scanning. The total activity per fraction was calculated using the protein concentration of each fraction. (C) Two graphs with the percentages of marker enzymes per fraction and the total palmitoyltransferase activity per fraction. Immunoblot shown in A was quantitated by densitometric scanning. Levels of β-hexosaminidase, a lysosomal marker enzyme, were measured with an activity assay with equal volumes of fractions.
In Vivo Labeling with [3H]Palmitate to Localize Palmitoyltransferase Activity
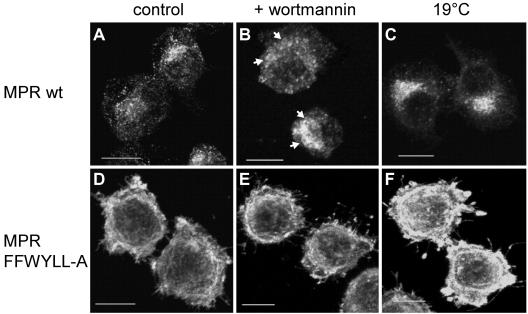
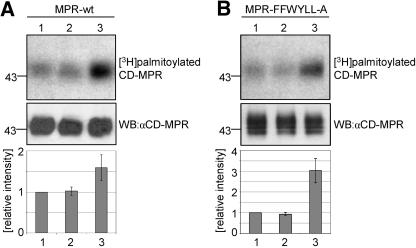
Further investigation to localize the palmitoyltransferase was performed with in vivo labeling experiments by adding [3H]palmitate to the cells, which was incorporated by the CD-MPR. These experiments were carried out with two CD-MPR constructs, the wt and a mutant construct (FFW-YLL-A; Figure 4A), stably transfected into mouse L cells. In the mutant CD-MPR, the important amino acids of all three internalization signals (Denzer et al., 1997) were mutated to alanines. Therefore, the mutant CD-MPR was not internalized, whereas the wt CD-MPR was rapidly internalized (Figure 4B). This led to an accumulation of the mutant CD-MPR at the plasma membrane of 88%, whereas wt CD-MPR was expressed at the plasma membrane only to 19% (Figure 4C). The cells were subjected to two treatments to block certain transport steps within the cell, to accumulate the proteins in a specific organelle. The levels of palmitoylated CD-MPR in control cells and treated cells were compared with determine whether the palmitoyltransferase and CD-MPR accumulated in the same organelle. One block was evoked by wortmannin, a fungal metabolite that inhibits phosphatidylinositol 3-OH kinase. It has been shown that the addition of wortmannin to mammalian cells at micromolar concentration for 1 h causes an accumulation of the CI-MPR and furin in enlarged endosomes (Brown et al., 1995; Kundra and Kornfeld, 1998; Mallet and Maxfield, 1999). In mouse L cells, 1 μM wortmannin causes maximal inhibition of the exit of the CI-MPR from endosomes, but it does not affect internalization (Kundra and Kornfeld, 1998). To accumulate proteins in endosomes, cells were pretreated with 1 μM wortmannin or as a control with 0.1% DMSO for 45 min and labeled with [3H]palmitate for 90 min at 37°C in the presence of 0.1% DMSO or wortmannin, which was repeatedly added every 45 min due to the short half-life of wortmannin. On treatment with wortmannin, wt CD-MPR accumulated in enlarged endosomes (Figure 5, A and B, arrows), whereas the mutant CD-MPR at the plasma membrane had no altered distribution (Figure 5, D vs. E). wt CD-MPR had a twofold increased level of palmitoylation upon addition of wortmannin compared with control cells, whereas the mutant CD-MPR was 33% less palmitoylated than in control cells (Figure 6). This result demonstrated that the palmitoyltransferase is not only present at the plasma membrane (high level of palmitoylation of the mutant CD-MPR) but also in the endosomes. On treatment with wortmannin, the palmitoyltransferase accumulated in the endosomes, where also wt CD-MPR accumulated and hence the level of palmitoylation of wt CD-MPR was twofold elevated. Furthermore, palmitoylation of the mutant CD-MPR decreased by 33% upon treatment with wortmannin, indicating that the palmitoyltransferase was depleted from the plasma membrane. Together, these data show that the palmitoyltransferase cycles between the plasma membrane and endosomes and that the addition of wortmannin trapped the enzyme in endosomes. To further investigate the internal trafficking pathway of the palmitoyltransferase, a different block of intracellular transport was applied. Incubation of cells at 19°C blocks the exit out of the TGN, thereby accumulating proteins that cycle through the TGN (Matlin and Simons, 1983; Griffiths et al., 1985). Cells were preincubated either at 19 or 37°C for 30 min and subsequently labeled with [3H]palmitate for 90 min at 19 or 37°C. As expected, the temperature block accumulated wt CD-MPR in the TGN, whereas the localization of the mutant CD-MPR at the plasma membrane was not affected (Figure 5, C and F). The level of palmitoylation of the mutant CD-MPR was not changed by the temperature block, whereas the extent of palmitoylation of the wt CD-MPR was reduced at 19°C by 50% (Figure 7). These data indicate that the palmitoyltransferase does not cycle through the Golgi or the TGN, and therefore its trafficking is not affected by this temperature block.
Figure 4.
Increased surface levels and decreased internalization rate of MPR-FFWYLL-A. (A) Schematic illustration of the cytoplasmic tails of the CD-MPR constructs. The amino acids are shown in single-letter code. The internalization signals that are mutated to alanines in the mutant construct and the palmitoylated cysteines are indicated by bold letters. (B) Internalization rates. Cell surface proteins of mouse L cells stably expressing MPR wt (▪), MPR-FFWYLL-A (•), and MPR-CC-A (▴) were derivatized at 4°C by using sulfo-NHS-SS-biotin. The cells were then incubated at 37°C for the indicated time and subsequently chilled on ice. The biotin groups remaining at the cell surface were removed by incubation in a reducing glutathione solution. The cells were lysed, and the wild-type and mutant forms of MPR were immunoprecipitated. Immunoprecipitates were resolved by SDS-PAGE and subjected to immunoblotting by using a streptavidin-horseradish peroxidase conjugate. The immunoblots were quantitated for each construct, and the values were expressed as their percentage of the sample that was kept at 4°C and not treated with glutathione. The values are averages of three individual experiments. (C) Surface levels. Mouse L cells stably expressing MPR wt and MPR-FFWYLL-A were incubated with iodinated antibodies against CD-MPR for 2 h on ice either without saponin for the surface levels or with 0.1% saponin to determine the total CD-MPR levels. The cells were lysed and the cell-associated radioactivity was determined with a gamma-counter. The bars represent the percentage of wt and mutant CD-MPR that were present at the cell surface at steady state. The values are expressed as mean ± SEM from four separate experiments.
Figure 5.
Effect of wortmannin and 19°C temperature block on the localization of CD-MPR wt and FFWYLL-A. Mouse L cells stably expressing MPR wt (A–C) and MPR-FFW-YLL-A (D–F) were fixed, permeabilized, and incubated with the mAb 22D4 against CD-MPR followed by goat anti-mouse Alexa 488. Before fixation, B and E were treated with 1 μM wortmannin for 90 min, whereas C and F were incubated at 19°C for 90 min. Bars, 10 μm. Arrows in B indicate enlarged endosomes.
Figure 6.
CD-MPR palmitoylation upon wortmannin treatment in vivo. (A) Mouse L cells stably expressing MPR wt and MPR-FFW-YLL-A were pretreated with 1 μM wortmannin or 0.1% DMSO for 45 min and then labeled with [3H]palmitate for 90 min in the presence of 1 μM wortmannin (repeatedly added after every 45 min) or 0.1% DMSO. The cells were then chilled on ice and lysed. CD-MPR was immunoprecipitated and subjected to SDS-PAGE. [3H]Palmitate incorporation into CD-MPR was visualized by autoradiography. (B) Level of CD-MPR expression. Ten percent of the immunoprecipitated sample was subjected to SDS-PAGE and Western blotting (WB) with 22D4 mAb against CD-MPR. (C) Quantitation of [3H]palmitate incorporation into CD-MPR wt and FFW-YLL-A. The fluorography and the Western blot shown in A and B, respectively, and those from additional experiments were quantitated by densitometric scanning. In each experiment, the values obtained for the [3H]palmitate incorporation were corrected for the CD-MPR expression levels. The value obtained with the DMSO-treated CD-MPR wt was set to 1. The values are expressed as mean ± S.D. from three separate experiments.
Figure 7.
CD-MPR palmitoylation upon 19°C temperature block in vivo. (A) Mouse L cells stably expressing MPR wt and MPR-FFW-YLL-A were preincubated at 19 or 37°C for 30 min and then labeled with [3H]palmitate for 90 min at 19 or 37°C, respectively. The cells were then chilled on ice and lysed. CD-MPR was immunoprecipitated and subjected to SDS-PAGE. [3H]palmitate incorporation into CD-MPR was visualized by autoradiography. (B) Level of CD-MPR expression. Ten percent of the immunoprecipitated sample was subjected to SDS-PAGE and Western blotting (WB) with 22D4 mAb against CD-MPR. (C) Quantitation of [3H]palmitate incorporation into CD-MPR wt and FFWYLL-A. The fluorography and the Western blot shown in A and B, respectively, and those from additional experiments were quantitated by densitometric scanning. In each experiment, the values obtained for the [3H]palmitate incorporation were corrected for the CD-MPR expression levels. The value obtained with the CD-MPR wt incubated at 37°C was set to 1. The values are expressed as mean ± S.D. from three separate experiments.
To exclude, that the in vivo labeling results are due to an accumulation of a putative thioesterase, a control experiment was performed to test the effect of the different treatments (wortmannin and 19°C) on the depalmitoylation of the CD-MPR. Mouse L cells containing wild-type CD-MPR or mutant CD-MPR were labeled with [3H]palmitate for 90 min at 37°C and then chased for 2 h in the presence of 0.1 mM nonradioactive palmitate at 37°C in the presence or absence of 1 μM wortmannin or at 19°C. Thus, the radioactively palmitoylated wild-type CD-MPR was either distributed normally (untreated cells incubated at 37°C), or accumulated in endosomes (cells incubated with wortmannin) or in the TGN (cells incubated at 19°C), whereas the radioactively labeled mutant CD-MPR was always localized to the plasma membrane. The levels of radioactively palmitoylated CD-MPR after the chase were then compared. There was no difference in the levels of depalmitoylation between the nontreated and the wortmannin-treated CD-MPR, for both the wild-type and the mutant CD-MPR, indicating that wortmannin had no effect on the thioesterase activity (Figure 8). The levels of palmitoylation of the wild-type and mutant CD-MPR after the chase at 19°C were 1.5-fold and 3-fold increased, indicating that there was less thioesterase activity under these conditions (Figure 8). However this reduction in depalmitoylation was not specific to the TGN localization, because we also observed less thioesterase activity for the mutant CD-MPR at the plasma membrane, and thus it is a general effect of the lower temperature. Therefore, none of the effects on the level of palmitoylation by the different treatments that were observed in the in vivo labeling experiments (Figures 6 and 7) were due to an influence on a thioesterase by the treatment.
Figure 8.
Effect of different treatments on the depalmitoylation of the CD-MPR in vivo. Mouse L cells stably expressing MPR wt (A) and MPR-FFWYLL-A (B) labeled with [3H]palmitate for 90 min at 37°C and chased for 2 h in medium containing nonradioactive palmitate in excess. Cells were chased at 37°C nontreated (A and B, lanes 1) or treated with 1 μM wortmannin (A and B, lanes 2) or incubated at 19°C (A and B, lanes 3). The cells were then chilled on ice and lysed. CD-MPR was immunoprecipitated and subjected to SDS-PAGE. [3H]Palmitate incorporation into CD-MPR was visualized by autoradiography (top). Ten percent of the immunoprecipitated sample was subjected to SDS-PAGE and Western blotting (WB) with 22D4 mAb against CD-MPR (middle). The fluorography and the Western blots were quantitated by densitometric scanning. In each experiment, the values obtained for the [3H]palmitate incorporation were corrected for the CD-MPR expression levels. The value obtained with the CD-MPR chased nontreated at 37°C was set to 1. The values are expressed as mean ± S.D. from three individual experiments.
Palmitoylation Is Not Required for Internalization of the CD-MPR
The in vivo experiments showed that the palmitoyltransferase is active at the plasma membrane (palmitoylation of the mutant CD-MPR at the plasma membrane) and in endosomes (increased palmitoylation of the wild-type CD-MPR upon treatment with wortmannin). To analyze the importance of the palmitoylation for the internalization of the CD-MPR, we analyzed the internalization rate of the mutant CD-MPR lacking the only two cytosolic cysteines, MPR-CC-A, and compared it with the rate of the wild-type CD-MPR. Both wild-type CD-MPR and MPR-CC-A internalized with equal kinetics (Figure 4B), indicating that the palmitoylation is not required for the internalization of the CD-MPR.
DISCUSSION
The results presented in this study demonstrate that a membrane-bound enzyme is responsible for palmitoylation of the CD-MPR. Further characterization revealed that the palmitoyltransferase cycles between the plasma membrane and endosomes.
Palmitoylation of a cysteine residue 34 amino acids distal from the transmembrane domain is rare, and the resulting membrane anchoring by the palmitate implies a drastic change in conformation of the entire cytoplasmic tail. The altered three-dimensional structure might have an effect on the exposure and accessibility of the sorting signals within the cytoplasmic tail of the receptor. To confirm the hypothesis that reversible palmitoylation of the CD-MPR regulates the sorting signals of the receptor, one prerequisite was to prove that an enzyme is involved in palmitoylation. Therefore, we established an in vitro palmitoylation assay with purified CD-MPR and [3H]palmitoyl-CoA as substrates and HeLa cell homogenate, containing the palmitoyltransferase activity. The in vitro assay uses the final substrates also required in vivo for palmitoylation. Hence, the assay is very specific for the palmitoyltransferase activity and not dependent on additional enzymes such as the acyl-CoA synthase to synthesize [3H]palmitoyl-CoA from [3H]palmitate and CoA. The CD-MPR, purified as a full-length membrane protein from tissue culture cells is used in the assay, therefore containing the intact three-dimensional structure that might be essential for recognition by the palmitoyltransferase. The in vitro palmitoylation is abolished by boiling the HeLa cell homogenate to denature the palmitoyltransferase (Figure 1A, lane 3) or by performing the assay on ice, thereby inactivating the palmitoyltransferase (Figure 1A, lane 2). Furthermore, CD-MPR is not autocatalytically palmitoylated when incubated with [3H]palmitoyl-CoA, thus demonstrating the requirement of an enzyme for palmitoylation (Figure 1A, lane 4 and 1B, lane 2). Palmitoylation is time and temperature dependent, which are characteristics of an enzymatic reaction. All these results demonstrate that palmitoylation occurs enzymatically and further characterization revealed that the palmitoyltransferase is membrane bound. The specificity of the palmitoyltransferase activity in the in vitro assay was further confirmed due to the fact that the lumenal cysteine residues were not palmitoylated in a mutant CD-MPR lacking the cytoplasmic cysteine residues (MPR-C30,34A; Figure 1C).
To identify the intracellular localization of the palmitoyltransferase, the cell homogenate was separated on a Percoll density gradient. A good separation of intracellular organelles was achieved for lysosomes, rough endoplasmic reticulum, the Golgi, and the plasma membrane (Figure 3). The early and late endosomal compartments were dispersed over several fractions of the gradient, most likely due to the heterogeneous nature of these organelles. The heavier fractions of the endosomes might represent a coated/heavy subpopulation of an otherwise light organelle. The fractions were tested in the in vitro palmitoylation assay, revealing that most of the total palmitoyltransferase activity was recovered in two peaks, one peak in a dense fraction and a second broad peak in light fractions. The same distribution was found for early endosomes. This could imply that the palmitoyltransferase is localized in early endosomes and therefore cofractionates with this organelle. Alternatively, it is also possible that the palmitoyltransferase is localized in more than one organelle and part of it fractionates with the corresponding organelle like early endosomes and the plasma membrane. To verify the localization of the palmitoyltransferase by additional methods, CD-MPR in vivo labeling experiments with [3H]palmitate were performed, applying blocks to intracellular traffic to accumulate the receptor and possibly the palmitoyltransferase in a particular compartment. These experiments showed that the palmitoyltransferase cycles between the plasma membrane and endosomes. Wortmannin caused an accumulation of wt CD-MPR in the endosomes (Figure 5, A and B) (Brown et al., 1995; Kundra and Kornfeld, 1998). Because it is not clear whether wortmannin only affects early endosomes or in addition also late endosomes, we refer to endosomes in general. The increased level of palmitoylation of the wt CD-MPR upon addition of wortmannin demonstrated that the palmitoyltransferase is accumulated in endosomes as well. This accumulation was confirmed by the decreased extent of palmitoylation of the mutant CD-MPR at the plasma membrane upon treatment with wortmannin, showing that the palmitoyltransferase is depleted from the plasma membrane under this condition. Wortmannin does not affect the palmitoyltransferase reaction in general, which is demonstrated by both effects, a decrease and an increase, in the same experiment with different mutants. In addition, wortmannin does not affect the activity of a putative thioesterase. Thus, wortmannin influences palmitoylation by changing the localization of the enzyme and the substrate, indicating that the palmitoyltransferase cycles between the plasma membrane and endosomes. To determine whether the palmitoyltransferase cycles through the TGN as well, an incubation at low temperature (19°C) was used to specifically block vesicular transport out of the TGN (Matlin and Simons, 1983; Griffiths et al., 1985). Thus, proteins such as the wild-type CD-MPR, which cycle through the TGN as part of their normal trafficking route, are still delivered to the TGN, but fail to leave this organelle and therefore accumulate in the TGN upon reducing the temperature to 19°C. The mutant receptor is not affected by the temperature block because it is stuck at the plasma membrane and does not cycle at all (Figure 5, C and F). The decrease in palmitoylation of the wild-type CD-MPR upon the temperature block demonstrated that the palmitoyltransferase was not accumulated in the TGN together with wild-type receptor. In contrast to the wild-type receptor, both the mutant CD-MPR and the palmitoyltransferase were not affected by the temperature block and hence the level of palmitoylation of the mutant CD-MPR stayed unchanged. Furthermore, the level of palmitoylation of the mutant CD-MPR at 19°C was evidence to show that the incubation time and the kinetics of palmitoylation were sufficient to yield maximal extent of palmitoylation despite the lower temperature. The elevated level of palmitoylation of mutant receptors compared with wild-type receptors in nontreated cells reflects the different localizations of the receptors in relation to the palmitoyltransferase. At steady state, wild-type receptors are mainly localized to the TGN, an organelle lacking the palmitoyltransferase activity, in contrast to mutant receptors that remain at the plasma membrane, which contains the palmitoyltransferase activity.
Together, we suggest a model where the palmitoyltransferase cycles between the plasma membrane and endosomes without passing through the TGN. This confirms the data from the Percoll gradient, where the palmitoyltransferase activity mostly colocalized with endosomes and the plasma membrane.
A more complex model involving also thioesterases that are potentially affected by the incubation at 19°C and/or the addition of wortmannin could be excluded. The depalmitoylation of the receptor in pulse-chase experiments was not affected by the treatments in a way to account for the results obtained in the in vivo labeling experiments. Wortmannin had no influence on the depalmitoylation of both wild-type and mutant CD-MPR compared with nontreated cells (Figure 8). The reduced depalmitoylation at 19°C (Figure 8) emphasizes that the reduced levels of the palmitoylation of the wild-type CD-MPR under these conditions (Figure 7) result from the lack of a palmitoyltransferase activity in the TGN rather than an increased thioesterase activity. With the depalmitoylation experiment (Figure 8), we can exclude the possibility that the effects we observed in the in vivo labeling experiments (Figures 6 and 7) were due to a thioesterase.
Palmitoylation of the CD-MPR occurred at both the plasma membrane (palmitoylation of the mutant CD-MPR at the plasma membrane) as well as in endosomes (palmitoylation of the wild-type CD-MPR upon wortmannin). Thus, the palmitoyltransferase is active in both organelles. Palmitoylation of the CD-MPR is required to avoid the delivery of the receptor to lysosomes (Schweizer et al., 1996), but it is not required for its internalization, because the CD-MPR mutant lacking the two cytosolic cysteines (MPR-CC-A) is rapidly internalized, with the same kinetics than the wild-type CD-MPR (Figure 4B).
Palmitoyltransferase activities were reported in the early secretory pathway (Veit and Schmidt, 1993), in the Golgi (Gutierrez and Magee, 1991), and at the plasma membrane (Dunphy et al., 1996). Our findings expand the knowledge about the localization of palmitoyltransferase activities with a transferase that cycles between endosomes and the plasma membrane. The variety of palmitoylated substrates and the lack of a clear consensus sequence suggest that several palmitoyltransferases with different specificities might exist. In mammals, no candidate is known that could be the putative palmitoyltransferase of the CD-MPR. Two DHHC-CRD containing palmitoyltransferases identified in yeast, Akr1p and the Erf2p/Erf4p complex (Lobo et al., 2002; Roth et al., 2002), have different intracellular localizations and exhibit high substrate specificities, thereby validating the assumption of various palmitoyltransferases. Database searches revealed that there are >10 DHHC-CRD–containing proteins in Homo sapiens, but none of them have been cloned so far. Further investigations will be required to determine whether there is a cycling palmitoyltransferase among them, which cycles between the plasma membrane and endosomes with a specificity for the CD-MPR.
Furthermore, a palmitoyltransferase that cycles between the plasma membrane and endosomes could play a role in signal transduction. The G protein-coupled receptor β2-adrenergic receptor (β2AR), for example, is palmitoylated, and this may prevent phosphorylation of a nearby phosphorylation site. On activation by an agonist, the β2AR is depalmitoylated, followed by phosphorylation, desensitization, and internalization. In endosomes, β2AR either gets targeted for degradation or dephosphorylated and subsequently recycled back to the plasma membrane with restoration of its native state by palmitoylation, possibly in the endosomes or at the plasma membrane (Pippig et al., 1995; Loisel et al., 1996; Moffett et al., 1996).
Moreover, endosomes are very dynamic compartments displaying a highly complex and pleiomorphic organization, as well as a complex scheme of incoming and outgoing transport routes (Gruenberg, 2001). No membrane protein has been reported so far that is restricted to endosomes, suggesting that a protein that is active in endosomes has to recycle, possibly via the plasma membrane.
Because the half-life of the palmitoylation of the CD-MPR (<2 h) is much less than the half-life of the CD-MPR itself (>40 h) (Schweizer et al., 1996), the receptor gets repeatedly palmitoylated during its lifetime. As a consequence, the palmitoyltransferase should be localized in a compartment that is part of the trafficking itinerary of the CD-MPR. The intracellular distribution of the CD-MPR comprises the TGN, the plasma membrane, and early and late endosomes (Klumperman et al., 1993) and hence includes the organelles containing the palmitoyltransferase, thereby enabling repeated palmitoylation of the CD-MPR. Moreover, palmitoylation is essential for the CD-MPR in the late endosomes to avoid trafficking to the lysosomes (Schweizer et al., 1996). The localization of the palmitoyltransferase at the plasma membrane and endosomes is ideal to ensure that the CD-MPR is palmitoylated in the late endosomes, thereby enabling its proper trafficking. The localization of the palmitoyltransferase to the site of its required function not only validates our results but also supports the hypothesis that palmitoylation might regulate the sorting of the CD-MPR by modulating the presentation of the sorting signals in the cytosolic tail.
Acknowledgments
We thank Drs. H.P. Hauri, J. Gruenberg, P. Chavrier, and E. Berger for generously providing the antibodies. The hybridoma cell line producing mAb 22D4 against CD-MPR was a kind gift of Dr. D. Messner. P. Nair and B. Schaub are acknowledged for critical reading of the manuscript. We acknowledge Dr. E. Berger for continuous support and critical reading the manuscript. This work was supported in part by a Prof. Dr. Max Cloetta Fellowship to J.R. and a Swiss National Foundation grant 31-67274 and a Roche Research Foundation Fellowship to J.S.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-11-0808. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-11-0808.
Abbreviations used: CD-MPR, cation-dependent mannose 6-phosphate receptor; M6P, mannose 6-phosphate; MPR, mannose 6-phosphate receptor; PNS, postnuclear supernatant; TGN, trans-Golgi network.
References
- Adam, M., Rodriguez, A., Turbide, C., Larrick, J., Meighen, E., and Johnstone, R.M. (1984). In vitro acylation of the transferrin receptor. J. Biol. Chem. 259, 15460-15463. [PubMed] [Google Scholar]
- Berger, E.G., Aegerter, E., Mandel, T., and Hauri, H.P. (1986). Monoclonal antibodies to soluble, human milk galactosyltransferase (lactose synthase A protein). Carbohydr. Res. 149, 23-33. [DOI] [PubMed] [Google Scholar]
- Blanpain, C., et al. (2001). Palmitoylation of CCR5 is critical for receptor trafficking and efficient activation of intracellular signaling pathways. J. Biol. Chem. 276, 23795-23804. [DOI] [PubMed] [Google Scholar]
- Bonatti, S., Migliaccio, G., and Simons, K. (1989). Palmitoylation of viral membrane glycoproteins takes place after exit from the endoplasmic reticulum. J. Biol. Chem. 264, 12590-12595. [PubMed] [Google Scholar]
- Brown, W.J., DeWald, D.B., Emr, S.D., Plutner, H., and Balch, W.E. (1995). Role for phosphatidylinositol 3-kinase in the sorting and transport of newly synthesized lysosomal enzymes in mammalian cells. J. Cell Biol. 130, 781-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp, L.A., and Hofmann, S.L. (1993). Purification and properties of a palmitoyl-protein thioesterase that cleaves palmitate from H-Ras. J. Biol. Chem. 268, 22566-22574. [PubMed] [Google Scholar]
- Chavrier, P., Parton, R.G., Hauri, H.P., Simons, K., and Zerial, M. (1990). Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell 62, 317-329. [DOI] [PubMed] [Google Scholar]
- Corvi, M.M., Soltys, C.L., and Berthiaume, L.G. (2001). Regulation of mitochondrial carbamoyl-phosphate synthetase 1 activity by active site fatty acylation. J. Biol. Chem. 276, 45704-45712. [DOI] [PubMed] [Google Scholar]
- Denzer, K., Weber, B., Hille-Rehfeld, A., von Figura, K., and Pohlmann, R. (1997). Identification of three internalization sequences in the cytoplasmic tail of the 46 kDa mannose 6-phosphate receptor. Biochem. J. 326, 497-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz, E., and Pfeffer, S.R. (1998). TIP 47, A cargo selection device for mannose 6-phosphate receptor trafficking. Cell 93, 433-443. [DOI] [PubMed] [Google Scholar]
- Duncan, J.A., and Gilman, A.G. (1996). Autoacylation of G protein alpha subunits. J. Biol. Chem. 271, 23594-23600. [DOI] [PubMed] [Google Scholar]
- Duncan, J.A., and Gilman, A.G. (1998). A cytoplasmic acyl-protein thioesterase that removes palmitate from G protein alpha subunits and p21(RAS). J. Biol. Chem. 273, 15830-15837. [DOI] [PubMed] [Google Scholar]
- Dunphy, J.T., Greentree, W.K., Manahan, C.L., and Linder, M.E. (1996). Gprotein palmitoyltransferase activity is enriched in plasma membranes. J. Biol. Chem. 271, 7154-7159. [DOI] [PubMed] [Google Scholar]
- Fishburn, C.S., Herzmark, P., Morales, J., and Bourne, H.R. (1999). Gbetagamma and palmitate target newly synthesized Galphaz to the plasma membrane. J. Biol. Chem. 274, 18793-18800. [DOI] [PubMed] [Google Scholar]
- Gorvel, J.P., Chavrier, P., Zerial, M., and Gruenberg, J. (1991). rab5 controls early endosome fusion in vitro. Cell 64, 915-925. [DOI] [PubMed] [Google Scholar]
- Griffiths, G., Pfeiffer, S., Simons, K., and Matlin, K. (1985). Exit of newly synthesized membrane proteins from the trans cisterna of the Golgi complex to the plasma membrane. J. Cell Biol. 101, 949-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg, J. (2001). The endocytic pathway: a mosaic of domains. Nat. Rev. Mol. Cell. Biol. 2, 721-730. [DOI] [PubMed] [Google Scholar]
- Gutierrez, L., and Magee, A.I. (1991). Characterization of an acyltransferase acting on p21N-ras protein in a cell-free system. Biochim. Biophys. Acta 1078, 147-154. [DOI] [PubMed] [Google Scholar]
- Hille-Rehfeld, A. (1995). Mannose 6-phosphate receptors in sorting and transport of lysosomal enzymes. Biochim. Biophys. Acta 1241, 177-194. [DOI] [PubMed] [Google Scholar]
- Ho, S.N., Hunt, H.D., Horton, R.M., Pullen, J.K., and Pease, L.R. (1989). Site-directed mutagenesis by overlap extension using the polymerase chain reaction [see comments]. Gene 77, 51-59. [DOI] [PubMed] [Google Scholar]
- Johnson, K.F., Chan, W., and Kornfeld, S. (1990). Cation-dependent mannose 6-phosphate receptor contains two internalization signals in its cytoplasmic domain. Proc. Natl. Acad. Sci. USA 87, 10010-10014. (erratum published in Proc. Natl. Acad. Sci. USA 1991; 88, 1591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumperman, J., Hille, A., Veenendaal, T., Oorschot, V., Stoorvogel, W., von Figura, K., and Geuze, H.J. (1993). Differences in the endosomal distributions of the two mannose 6-phosphate receptors. J. Cell Biol. 121, 997-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld, S. (1992). Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu. Rev. Biochem. 61, 307-330. [DOI] [PubMed] [Google Scholar]
- Kundra, A., and Kornfeld, S. (1998). Wortmannin retards the movement of the mannose 6-phosphate/insulin-like growth factor II receptor and its ligand out of endosomes. J. Biol. Chem. 273, 3848-3853. [DOI] [PubMed] [Google Scholar]
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680-685. [DOI] [PubMed] [Google Scholar]
- Le Borgne, R., and Hoflack, B. (1997). Mannose 6-phosphate receptors regulate the formation of clathrin-coated vesicles in the TGN. J. Cell Biol. 137, 335-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo, S., Greentree, W.K., Linder, M.E., and Deschenes, R.J. (2002). Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J. Biol. Chem. 277, 41268-41273. [DOI] [PubMed] [Google Scholar]
- Loisel, T.P., Adam, L., Hebert, T.E., and Bouvier, M. (1996). Agonist stimulation increases the turnover rate of beta 2AR-bound palmitate and promotes receptor depalmitoylation. Biochemistry 35, 15923-15932. [DOI] [PubMed] [Google Scholar]
- Mallet, W.G., and Maxfield, F.R. (1999). Chimeric forms of furin and TGN38 are transported from the plasma membrane to the trans-Golgi network via distinct endosomal pathways. J. Cell Biol. 146, 345-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marxer, A., Stieger, B., Quaroni, A., Kashgarian, M., and Hauri, H.P. (1989). (Na+ + K+)-ATPase and plasma membrane polarity of intestinal epithelial cells: presence of a brush border antigen in the distal large intestine that is immunologically related to beta subunit. J. Cell Biol. 109, 1057-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlin, K.S., and Simons, K. (1983). Reduced temperature prevents transfer of a membrane glycoprotein to the cell surface but does not prevent terminal glycosylation. Cell 34, 233-243. [DOI] [PubMed] [Google Scholar]
- Messner, D.J. (1993). The mannose receptor and the cation-dependent form of mannose 6-phosphate receptor have overlapping cellular and subcellular distributions in liver. Arch. Biochem. Biophys. 306, 391-401. [DOI] [PubMed] [Google Scholar]
- Moffett, S., Adam, L., Bonin, H., Loisel, T.P., Bouvier, M., and Mouillac, B. (1996). Palmitoylated cysteine 341 modulates phosphorylation of the beta2-adrenergic receptor by the cAMP-dependent protein kinase. J. Biol. Chem. 271, 21490-21497. [DOI] [PubMed] [Google Scholar]
- Nair, P., Schaub, B.E., and Rohrer, J. (2003). Characterization of the endosomal sorting signal of the cation-dependent mannose 6-phosphate receptor. J. Biol. Chem. 278, 24753-24758. [DOI] [PubMed] [Google Scholar]
- O'Brien, P.J., St Jules, R.S., Reddy, T.S., Bazan, N.G., and Zatz, M. (1987). Acylation of disc membrane rhodopsin may be nonenzymatic. J. Biol. Chem. 262, 5210-5215. [PubMed] [Google Scholar]
- Pippig, S., Andexinger, S., and Lohse, M.J. (1995). Sequestration and recycling of beta 2-adrenergic receptors permit receptor resensitization. Mol. Pharmacol. 47, 666-676. [PubMed] [Google Scholar]
- Rohrer, J., Schweizer, A., Johnson, K.F., and Kornfeld, S. (1995). A determinant in the cytoplasmic tail of the cation-dependent mannose 6-phosphate receptor prevents trafficking to lysosomes. J. Cell Biol. 130, 1297-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth, A.F., Feng, Y., Chen, L., and Davis, N.G. (2002). The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J. Cell Biol. 159, 23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1998). Molecular Cloning: A Laboratory Manual, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Schweizer, A., Ericsson, M., Bachi, T., Griffiths, G., and Hauri, H.P. (1993). Characterization of a novel 63 kDa membrane protein. Implications for the organization of the ER-to-Golgi pathway. J. Cell Sci. 104, 671-683. [DOI] [PubMed] [Google Scholar]
- Schweizer, A., Kornfeld, S., and Rohrer, J. (1996). Cysteine34 of the cytoplasmic tail of the cation-dependent mannose 6-phosphate receptor is reversibly palmitoylated and required for normal trafficking and lysosomal enzyme sorting. J. Cell Biol. 132, 577-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer, A., Kornfeld, S., and Rohrer, J. (1997). Proper sorting of the cation-dependent mannose 6-phosphate receptor in endosomes depends on a pair of aromatic amino acids in its cytoplasmic tail. Proc. Natl. Acad. Sci. USA 94, 14471-14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer, A., Stahl, P.D., and Rohrer, J. (2000). A di-aromatic motif in the cytosolic tail of the mannose receptor mediates endosomal sorting. J. Biol. Chem. 275, 29694-29700. [DOI] [PubMed] [Google Scholar]
- Soyombo, A.A., and Hofmann, S.L. (1997). Molecular cloning and expression of palmitoyl-protein thioesterase 2 (PPT2), a homolog of lysosomal palmitoyl-protein thioesterase with a distinct substrate specificity. J. Biol. Chem. 272, 27456-27463. [DOI] [PubMed] [Google Scholar]
- Towbin, H., Staehelin, T., and Gordon, J. (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76, 4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van't Hof, W., and Resh, M.D. (1997). Rapid plasma membrane anchoring of newly synthesized p59fyn: selective requirement for NH2-terminal myristoylation and palmitoylation at cysteine-3. J. Cell Biol. 136, 1023-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit, M., Laage, R., Dietrich, L., Wang, L., and Ungermann, C. (2001). Vac8p release from the SNARE complex and its palmitoylation are coupled and essential for vacuole fusion. EMBO J. 20, 3145-3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit, M., and Schmidt, M.F. (1993). Timing of palmitoylation of influenza virus hemagglutinin. FEBS Lett. 336, 243-247. [DOI] [PubMed] [Google Scholar]
- Verkruyse, L.A., and Hofmann, S.L. (1996). Lysosomal targeting of palmitoyl-protein thioesterase. J. Biol. Chem. 271, 15831-15836. [DOI] [PubMed] [Google Scholar]
- Yeh, D.C., Duncan, J.A., Yamashita, S., and Michel, T. (1999). Depalmitoylation of endothelial nitric-oxide synthase by acyl-protein thioesterase 1 is potentiated by Ca2+-calmodulin. J. Biol. Chem. 274, 33148-33154. [DOI] [PubMed] [Google Scholar]