Abstract
The endothelins are a family of endothelium-derived peptides that possess a variety of functions, including vasoconstriction. Endothelin-1 (ET-1) is up-regulated during tissue repair and promotes myofibroblast contraction and migration, hence contributing to matrix remodeling during tissue repair. Here, we show that addition of ET-1 to normal lung fibroblasts induces expression of proteins that contribute to a contractile phenotype, including α-smooth muscle actin (α-SMA), ezrin, moesin, and paxillin. We confirm that ET-1 enhances the ability of lung fibroblasts to contract extracellular matrix, a function essential for tissue repair, through induction of de novo protein synthesis. Blockade of the Akt/phosphoinositide 3-kinase (PI3-kinase) pathway with LY294002 and wortmannin prevents the ability of ET-1 to induce α-SMA, ezrin, paxillin, and moesin and to promote matrix contraction. Dominant negative rac and Akt blocked the ability of ET-1 to promote formation of α-SMA stress fibers. Using specific ET-1 receptor inhibitors, we show that ET-1 induces collagen matrix contraction through the ETA, but not the ETB, receptor. Relative to normal pulmonary fibroblasts, fibroblasts cultured from scars of patients with the fibrotic disease systemic sclerosis (scleroderma) show enhanced ET-1 expression and binding. Systemic sclerosis lung fibroblasts show increased ability to contract a collagen matrix and elevated expression of the procontractile proteins α-SMA, ezrin, paxillin, and moesin, which are greatly reduced by antagonizing endogenous ET-1 signaling. Thus, blocking ET-1 or the PI3-kinase/Akt cascades might be beneficial in reducing scar formation in pulmonary fibrosis.
INTRODUCTION
A complex histological and architectural structure is a prerequisite for effective lung function. In the lung, specialized structures, the alveoli, increase the surface area of the lung, allowing for efficient gas exchange. The maintenance of these specialized structures is in turn dependent on the underlying connective tissue, comprised principally of fibroblasts and extracellular matrix (ECM; for review, see Gadek et al., 1984), which is essential for the mechanical and structural integrity of the lung. As a response to environmental insults, or as a consequence of local inflammatory processes, structural damage to the lung can occur, resulting in a wound healing response. This response consists of an integrated series of biochemical, immunological, and structural changes that result in the de novo synthesis of a new epithelium, blood vessels, and connective tissue (Razzaque and Taguchi, 2003). The proper repair of connective tissue requires synthesis of new ECM components, such as collagen and fibronectin (Badylak, 2002). In addition, repair of connective tissue requires the proper reconstitution of its support function; that is, an appropriate tensile strength must be recreated. This tensile strength results from the remodeling of the newly formed ECM through a combination of cell locomotion and translocation of the flexible collagen fibrils that are carried out through the contractile ability of a specialized type of fibroblast, the myofibroblast (Bell et al., 1979; Grinnell, 1994; Tomasek et al., 2002). The myofibroblast executes functional activity in part through the expression of α-smooth muscle actin (Bell et al., 1979; Grinnell, 1994; Tomasek et al., 2002), which promotes contraction and hence contributes to matrix remodeling. Ideally, wound repair replaces ECM appropriately, myofibroblasts disappear, and organ function is restored (Desmoulière, 1995). However, if the wound healing process does not appropriately terminate, myofibroblasts persist at the site of the lesion, resulting in an extensive, exaggerated amount of excessively contracted ECM. α-Smooth muscle actin (α-SMA)-producing myofibroblasts not only promote wound contraction (Tomasek et al., 2002) but also they promote tissue repair and wound healing by synthesizing elevated levels of ECM components, such as collagen (Grinnell, 2003). The persistence of the myofibroblasts within the fibrotic lesion is believed to result in elevated levels of matrix synthesis and contraction, causing scar formation (Schmitt-Graff et al., 1994), and the resultant functional impairment of the affected organ, which can eventually lead to death (Panos et al., 1990). Overall, it has become generally accepted that the modulation of fibroblastic cells toward the myofibroblastic phenotype, with acquisition of specialized contractile features, is essential for connective tissue remodeling during normal and pathological wound healing (Tomasek et al., 2002). Understanding of the origin of the myofibroblast and how this cell type performs its functions will have a profound influence on the future effectiveness not only of tissue engineering but also of regenerative medicine.
A growing body of evidence implicates the vasoconstrictive peptide endothelin-1 (ET-1) as a mediator of organbased fibrosis (Abraham et al., 1997; Teder and Noble, 2000; Shi-Wen et al., 2001). Each of the three known endothelin isoforms (-1, -2, and -3) arise by proteolytic processing of large precursors (∼200 amino acid residues). Intermediates, termed big ET-1, -2, and -3 (38–41 aa) are excised from prepropeptides at sites containing paired basic amino acids. Big endothelins, which have low biological activity (Yanagisawa, 1994), are cleaved at Trp-21-Val/Ile-22 to produce mature 21-residue, biologically active peptides (Anggard et al., 1990; Rubanyi and Botelho, 1991). The enzyme responsible for the specific cleavage at Trp-21 has been termed endothelin-converting enzyme; it is a neutral membrane-bound metalloproteinase with Mr = 120 kDa, belonging to the endo-peptidase-24.11 family found in brain (Ohnaka et al., 1993; Turner and Murphy, 1996). Injury and the wound healing response lead to stabilization of endothelin-converting enzyme-1 mRNA and to the generation of bioactive endothelin (Shao et al., 2003).
ET-1 demonstrates a wide range of biological properties on normal cells (Rubanyi and Botelho, 1991; Levin, 1995), including significant mitogenic activity toward a number of cell types, such as smooth muscle cells and fibroblasts, and the modification of extracellular matrix metabolism (Levin, 1995; Saita et al., 1998; Xu et al., 1998; Shi-Wen et al., 2001). In addition, ET-1 has been reported to promote the contractile ability of normal dermal fibroblasts (Guidry and Hook, 1991; Appleton et al., 1992), which is essential for wound closure and reconstitution of the dermis (Grinnell, 1994), but also contributes to scar formation (Schmitt-Graff et al., 1994). ET-1 also induces expression of α-SMA in lung fibroblasts (Shahar et al., 1999). However, the signaling pathways through which ET-1 promotes ECM contraction are not known.
The myofibroblast seems to be important for the pathogenesis of scarring in lung fibrosis (Bogatkevich et al., 2001). Indeed, histological examination of fibrotic lesions of the lung of patients with the systemic fibrotic disease systemic sclerosis (scleroderma, SSc) revealed the presence of α-SMA–producing fibroblasts (Sappino et al., 1990). Furthermore, fibroblasts cultured from SSc dermal lesions express both elevated levels of ECM (Varga and Bashey, 1995) and express α-smooth muscle actin (Kirk et al., 1995) relative to their normal counterparts. We hypothesized that the SSc lung fibroblast might show an enhanced ability to contract a collagen lattice, relative to normal lung fibroblasts and thus may contribute to scar formation by displaying excessive ECM contraction. In support of this hypothesis, ET-1 has been shown to contribute to perpetuation of the fibrogenic process (Rockey and Chung, 1996) and is overexpressed in plasma from SSc patients, as well as in fibroblasts cultured from SSc skin lesions (Miyauchi et al., 1992; Kawaguchi et al., 1994). ET-1 expression also is induced by myofibroblasts in response to injury (Rockey and Chung, 1996).
We have previously shown, using skin fibroblasts, that ET-1 induces matrix contraction in an endothelin A receptor (ETA) receptor-dependent manner (Shi-Wen et al., 2001). However, it is not clear whether ET-1 promotes matrix contraction through inducing expression of procontractile proteins, or whether ET-1 directly enhances the ability of myofibroblasts to contract matrix. In addition, the potential contribution of elevated levels of ET-1 production by SSc lung fibroblasts to the phenotype of these cells has not been evaluated. In this report, we use three-dimensional collagen matrices and genome-wide arrays to investigate the molecular mechanism through which ET-1 promotes ECM remodeling. We show that ET-1 induces the expression of procontractile genes. We then identify the signaling pathways required for this activity of ET-1. We also demonstrate that SSc pulmonary fibroblasts show markedly enhanced ability to contract a collagen matrix and elevated levels of procontractile proteins that depend on an elevated level of endogenous ET-1 signaling. Thus, the elevated level of ET-1 expression observed in SSc fibroblasts directly contributes to lung fibrosis by causing the enhanced contractile ability of the SSc fibroblast, thereby promoting the formation of scar tissue.
EXPERIMENTAL PROCEDURES
Patients and Cell Culture
Fibroblasts were grown by explant culture from open lung biopsy specimens from SSc patients taken for histological staging of lung fibrosis, and control samples were taken from normal lungs not used for transplant. The group of seven SSc patients fulfilled the criteria of the American College of Rheumatology for the diagnosis of SSc with lung involvement. The sex ratio was 6 femles:1 male. Fibroblasts were used between passages 2 and 5 (Shi-Wen et al., 1997).
Gene Array Analysis
Lung fibroblasts were serum starved for 18 h and treated with 100 nM ET-1 for 4 h. At the end of the treatment period, total RNA was harvested (TRIzol; Invitrogen, Carlsbad, CA) and quantified, and integrity was verified by denaturing gel electrophoresis. Equal amounts of identically treated RNA were pooled and reverse transcribed (Invitrogen) into cDNA that was then in vitro transcribed into biotinylated cRNA. The target cRNA was then fragmented and hybridized to the human U133A array (Affymetrix, Santa Clara, CA), covering 14,500 well characterized human genes, as described by the manufacturer. Hybridization of cRNA to U133A chips (Affymetrix), signal amplification and data collection were performed using a fluidics station and chip reader (Affymetrix), following Affymetrix protocol. Arrays were scaled to an average intensity of 100 per gene and analyzed using the Affymetrix version 5.0 (MAS5) comparison analysis software. Criteria indicated by Affymetrix were used to determine robust changes in gene expression. Briefly, transcripts were defined as up-regulated by ET-1 only when identified as “present” (ET-1–treated chip) by the Affymetrix detection algorithm and as significantly increased as determined by the Affymetrix change algorithm, with a change in p value of <0.001. The fold change between treated and untreated samples had to be at least 1.5-fold to identify a transcript as being altered. This cutoff was chosen as α-SMA, a known target of ET-1 (Shahar et al., 1999), was increased by 1.5-fold in our system.
Immunofluorescence Staining
Cells were seeded at 4 × 103 cells/well in DMEM-10% normal calf serum into chamber slides (Labtek, Nunc; Fisher Scientific UK, Loughborough, United Kingdom), grown to subconfluence, made quiescent in serum-free DMEM overnight, and exposed to control media (DMEM) or ET-1 (100 nM) for 24 h. The cell monolayer was washed three times with phosphate-buffered saline (PBS), and fixed in 4% formaldehyde for 5 min. Cells were washed three times in PBS before permeabilization and after each later step. Permeabilization was performed using 0.1% Triton in 50 mM PIPES (pH 7.0), 90 mM HEPES (pH 7.0), 0.5 mM MgCl2, 0.5 mM EGTA, and 75 mM KCl for 30 s, at room temperature. Chamber slides were incubated with mouse monoclonal anti-α-SMA antibody (Sigma-Aldrich, St. Louis, MO) and rhodamine-labeled anti-mouse antibody (Jackson ImmunoResearch Laboratories, West Grove, PA), each for 60 min at room temperature. Nuclei were stained for 1 min in 1 μg/ml 4,6-diamidino-2-phenylindole (Molecular Probes, Eugene, OR). After extensive washing with PBS, a single drop of CitiFluor AF1 (Chem Lab, Canterbury, United Kingdom) was added and cells were then visualized and photographed using Axioskop Z fluorescence microscope (Carl Zeiss, Jena, Germany).
Cell Transfection
Primary lung fibroblasts were transfected (FuGene; Roche Diagnostics, Indianapolis, IN) with expression vector encoding dominant negative Akt (Upstate Biotechnology, Lake Placid, NY) or rac (a generous gift from Prof. A. Hall, University College, London, United Kingdom) and an expression vector (cytomegalovirus, CMV) encoding enhanced green fluorescent protein (CMV-EGFP; BD Biosciences Clontech, Palo Alto, CA) used to identify transfected cells. After an 18-h serum starvation step, cells were treated with and without 100 nM ET-1 for 24 h. Cells were then fixed with paraformaldehyde and stained for α-SMA expression as described above. Transfected cells (that is, cells expressing green fluorescent protein [GFP]) were identified using a fluorescein isothiocyanate filter.
Floating and Fixed Collagen Gel Cultures and Quantitation of Gel Contraction
Experiments were performed essentially as described previously (Grinnell et al., 1999). Briefly, 24-well tissue culture plates were precoated with bovine serum albumin (BSA). Trypsinized normal and lung fibrosis associated with systemic sclerosis lung fibroblasts were suspended in MCDB medium and mixed with collagen solution (1 part of 0.2 M HEPES, pH 8.0, 4 parts collagen [Vitrogen-100, 3 mg/ml], and 5 parts of MCDB X 2), yielding a final concentration of 80,000 cells/ml and 1.2 mg/ml collagen. Collagen/cell suspension (1 ml) was added to each well. After polymerization, gels were detached from wells by adding 1 ml of MCDB medium in the presence or absence of ET-1 (100 nM). Contraction of the gel was quantified by loss of gel weight and decrease in gel diameter over a 24-h period. For fixed gel experiments, gels remained attached to a plastic substrate for 24 h and were then detached using a rubber policeman. ET-1 was then added for 1 h, and contraction was assessed as described above (Grinnell et al., 1999).
For inhibition experiments, cells were preincubated in the presence of endothelin receptor antagonist or protein synthesis inhibitor cycloheximide (1 μg/ml; Sigma-Aldrich) for 30 min before initiation of the assay. The specific receptor antagonists used were as follows: ETA receptor antagonist (ETA-RA), 10 μM PD156707, sodium 2-benzo[1,3]dioxol-5-yl-4-(4-methoxy-phenyl)-4-oxo-3-(3,4,5-trimethoxy-benzyl)-but-2-enoate; ETB receptor antagonist (ETB-RA), 10 μM BQ788, N-cis-2,6-dimethylpiperidimocarbonyl-l-gMeLeuD-Nle-ONa; and the mixed ETA/B receptor antagonist (Bosentan), 10 μM Ro 47-0203, 4-tert-butyl-N-[6-(2-hydroxy-ethoxy)-5-(2-methoxy-phenoxy)-2,2′-bipyrimidin-4-yl]-benzene-sulfonamine (from Dr. M. Clozel, Actelion Pharmaceuticals, Allschwil, Switzerland). Other inhibitors used (Calbiochem-Novabiochem, San Diego, CA) were as follows: the PI3-kinase inhibitors wortmannin (100 nM) and LY294002 (20 μM) and the ras/mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) inhibitors U0126 (10 μM) and PD98059 (50 μM), and the p38 inhibitor SB203580 (30 μM). Comparison of collagen gel contraction between ET-1, ET-1 receptor antagonists, cycloheximide, and medium only-treated fibroblasts was performed by using Student's paired t-test, whereas Student's unpaired t-test was used to compare SSc with normal lung fibroblasts. A p value of <0.05 was considered as statistically significant.
Fibroblast Populated Collagen Lattices (FPCL)
Measurement of tension across a three-dimensional, free-floating fibroblast-populated collagen lattice (Eastwood et al., 1996) was performed as described previously (Tomasek et al., 2002). Briefly, with 1 × 106 cells/ml collagen gel, we measured the force generated across the collagen lattice, by using a tensioning-culture force monitor that is capable of measuring the minute forces exerted by cells within a collagen lattice (Eastwood et al., 1994), >24 h as fibroblasts attach, spread, and migrate. In brief, a rectangular fibroblastseeded collagen gel was cast and floated in medium, tethered to two flotation bars on either end of the short edges, in turn attached to an anchor point at one end and a force transducer at the other. Cell-generated tensional forces in the collagen gel were detected by the force transducer and logged into a personal computer. Graphical readings are produced every 10 min, averaged from 600 readings (1/s), providing a continuous output of force (Dynes) generated (Eastwood et al., 1994).
In Vitro Autoradiography
Membrane binding sites were localized using high-resolution autoradiography. For localization of ET-1 binding sites, normal and SSc lung fibroblasts were grown in three-dimensional gels and fixed with paraformaldehyde [3.0% (wt/vol)]. Gels were sectioned, and cells were incubated for 18 h with 50 μl of buffer containing 150 pM 125I-labeled ET-1 in humidified chambers with nonspecific binding being established on paired slides incubated in the presence of unlabeled ET-1. After incubation, slides were washed in buffer, dipped in distilled water (both at 4°C) and dried in a stream of cold air. Binding sites were identified by dipping postfixed cells (paraformaldehyde vapor, 2 h at 80°C) in molten nuclear emulsion at 50°C and exposing for 4–7 d in light-proof boxes at 4°C. The slides were then processed in undiluted D19 developer (Eastman Kodak, Rochester, NY), followed by Hypam fixative, both for 5 min at 22°C. Cells were then stained with Mayer's hematoxylin and eosin for histology, examined under bright-field illumination on a Vanox microscope (Olympus, Tokyo, Japan), and photographed using Raster plus software. Data were then exported into Adobe Photoshop.
Measurement of ET-1 in Control and SSc Lung Fibroblast Culture Supernatants
Endothelin-1 secretion was measured in supernatants collected from confluent monolayer cultures of normal and SSc lung fibroblasts in serum-free medium, by using an enzyme-linked immunosorbent assay (ELISA) kit according to manufacturer's instructions (Biomedica, Vienna, Austria). This assay uses two antibodies directed against different epitopes of ET-1 and has a sensitivity of <1.0 pg/ml. These data were adjusted in accordance with cell counts at the time of sampling, and values are given as ET-1 per milliliter per 106 cells.
Western Blot Analysis
To examine biochemical or functional differences between control and ET-1-treated lung fibroblasts, a series of Western blot experiments were performed. Fibroblasts were cultured and treated with ET-1 in floating or fixed collagen gels as described above. Alternatively, fibroblasts were grown to confluence in DMEM with 10% fetal calf serum and then serum starved in DMEM with 0.5% bovine serum albumin (BSA) for 24 h. After serum starvation, cells were stimulated with 100 nM ET-1 for 24 h with 0.5% BSA. Cell layer lysates were examined. SDS-PAGE was performed on 12% polyacrylamide gels, and the separated proteins were transferred onto nitrocellulose membranes at 30 V for 90 min. Membranes were blocked by incubation for 1 h with 5% nonfat milk in PBS containing 0.2% Tween 20, and antigens were detected using specific antibodies. Cell layer lysates (10 μg/sample) were probed using antibodies directed against α-SMA (Sigma-Aldrich), Akt, p-Akt, p42/44 MAPK, phospho-p42/44 MAPK, anti-paxillin, anti-ezrin, and anti-moesin (all from Cell Signaling Technology, Beverly, MA), followed by incubation with appropriate horseradish peroxidase-conjugated bound secondary antibody (Jackson ImmunoResearch Laboratories). Signal was detected using the enhanced chemiluminescence protocol (Amersham Biosciences, Piscataway, NJ) as described by the manufacturer.
RESULTS
Endothelin-1 Promotes the Ability of Lung Fibroblasts to Contract Floating Collagen Matrices through the ETA Receptor
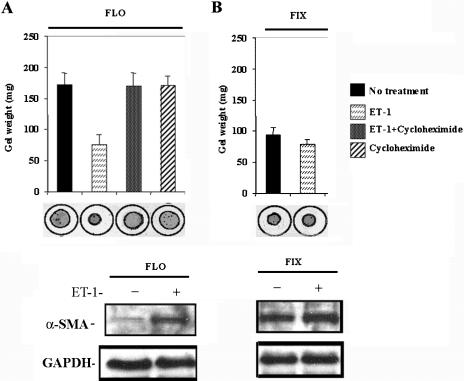
To assess the contribution of ET-1 to the ability of lung fibroblasts to contract ECM, we seeded normal lung fibroblasts within a floating collagen matrix. We then incubated cells for 24 h in the presence or absence of 100 nM ET-1. During this period, we assessed collagen gel contraction by measuring the maximum gel diameter by using an ocular micrometer. We found that normal lung fibroblasts incubated with 100 nM ET-1 contracted their gels significantly more than cells incubated without ET-1 (paired Student's t-test, p < 0.05; Figure 1A). Thus, ET-1 induces normal lung fibroblasts to contract ECM. The ability of ET-1 to promote gel contraction paralleled an ET-1-mediated increase, as visualized by Western blot analysis, in α-SMA protein (Figure 1A). We then assessed whether the ability of ET-1 to induce contraction depended on de novo protein synthesis of procontractile machinery. We found that addition of the protein synthesis inhibitor cycloheximide blocked the ability of ET-1 to promote matrix contraction within floating gels (Figure 1A). To evaluate whether ET-1 induced proteins that constitute the contractile machinery, we found, using Western blot analysis, that little α-SMA was detected in the absence of added ET-1, but that addition of 100 nM ET-1 potently induced expression of additional α-SMA (Figure 1A). Thus, we concluded that the ability of ET-1 to promote ECM contraction in floating gels was dependent on de novo synthesis of proteins, including proteins that comprised the contractile cytoskeletal network.
Figure 1.
Induction of collagen gel contraction by ET-1 in primary normal lung fibroblasts. (A) Floating (FLO) gels. Normal lung fibroblasts were seeded into a collagen gel matrix, and after gel polymerization, the gel was detached from the tissue culture plate and incubated in the presence or absence of ET-1 (100 nM) for 24 h. Cycloheximide was added to the gels 1 h before addition of ET-1. ET-1 promotes contraction of floating, but not fixed, gels. ET-1–mediated contraction of floating gels required protein synthesis, because the ability of ET-1 to promote contraction was blocked by cycloheximide. In addition, at the end of the experiment, cells treated were subjected to Western blot analysis to detect α-SMA protein, which was potently induced by ET-1 treatment. Thus, ET-1 promotes ECM contraction through the protein synthesis. (B) Fixed (FIX) gels. Normal lung fibroblasts were seeded into a collagen gel matrix, and the mixture remained attached to the tissue culture plate, and after 24 h, the gel was then detached, and incubated in the presence or absence of ET-1 (100 nM) for 1 h. ET-1 induces α-SMA protein expression in floating gels. Cells treated as in were subjected to Western blot analysis to detect α-SMA protein. Expression of α-SMA is elevated in fixed, mechanically stressed gels was markedly elevated, even in the absence of added ET-1. Thus, ET-1 does not directly promote mechanocontraction of already formed α-SMA stress fibers.
To assess whether ET-1 directly promotes matrix contraction, we investigated whether ET-1 could induce contraction of fixed collagen gels. In this system, cells already possess a stress fiber network due to the induction of myofibroblasts occurring secondary to mechanical tension (Lin et al., 1997; Grinnell et al., 1999). Agents that directly promote mechano-contraction act within 1 h after release of the tethered gel (Grinnell et al., 1999). We allowed gels containing fibroblasts and collagen matrix to remain attached to a plastic substratum for 24 h. Gels were then released and were allowed to contract in the presence or absence of ET-1 for 1 h. Using Western blot analysis, we confirmed that α-SMA was readily detected in the absence of added ET-1 (Figure 1B). ET-1 was not able to further augment contraction of fixed gels (Figure 1B). Collectively, the results are consistent with the notion that ET-1 does not directly promote ECM contraction, but rather it contributes to this process indirectly via the induction of expression of procontractile proteins.
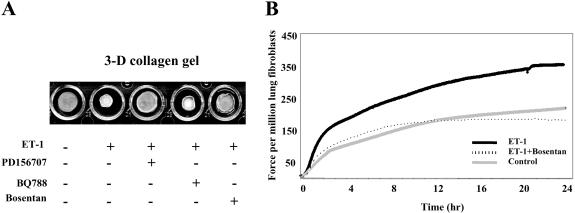
The ability of ET-1 to promote collagen gel contraction was blocked by an endothelin receptor A/endothelin receptor B (ETA/ETB) receptor antagonist, bosentan (Veniant et al., 1994), and an ETA receptor antagonist, PD156707 (Maguire et al., 1997), but not an ETB antagonist, BQ788 (Hamroun et al., 1995; Figure 2A). Addition of inhibitor alone, in the absence of added ET-1 had no effect on the basal ability of the fibroblast to contract collagen (our unpublished data). These results are similar to previous data showing that ET-1 promotes the ability of skin fibroblasts to promote ECM contraction through the ETA receptor (Shi-Wen et al., 2001); however, our studies are the first to suggest that ET-1 promotes contraction of ECM in lung fibroblasts through the ETA receptor.
Figure 2.
Induction of collagen gel contraction by ET-1 in primary normal lung fibroblasts. (A) Floating gel contraction assay. Normal lung fibroblasts were seeded into a collagen gel matrix, and after gel polymerization, the gel was detached from the tissue culture plate and incubated in the presence or absence of ET-1 (100 nM) for 24 h. Normal lung fibroblasts have contracted their gels significantly more in the presence of ET-1. Incubation of fibroblasts with the ET-1 receptor antagonist bosentan and the ETA receptor antagonist PD156707 blocked the ability of ET-1 to induce gel contraction. Conversely, the ETB receptor antagonist BQ788 had no effect. (B) FPCL assay. Normal lung fibroblasts were seeded into a floating collagen gel matrix and incubated in the presence or absence of ET-1 (100 nM) for 24 h. Mechanical force (Dynes) was assessed by attaching the collagen gel to a tensioning-culture force monitor. Representative plot from normal lung fibroblasts treated with ET-1 (100 nM) showing the contraction stimulated force generation >24 h.
We then performed a similar experiment, but this time using the FPCL model, in which force is measured across a floating gel containing collagen and cells tethered at one end (Eastwood et al., 1996). By using this model, contraction was first apparent after 4-h postpopulation and was maximal by about 24 h of culture. As for the gel contraction model, 100 nM ET-1 potently induced the ability of fibroblasts to contract a collagen matrix (Figure 2B).
Endothelin-1 Promotes the Ability of Lung Fibroblasts to Contract a Collagen Matrix by a PI3-Kinase/Akt-dependent Pathway
We then sought to identify the signaling mechanism through which ET-1 promotes the ability of normal fibroblasts to contract a collagen gel matrix. Using Western blot analysis with anti-phospho-ERK, anti-phospho-p38, and anti-phospho-Akt antibodies, we showed that ET-1 activated p42/p44, p38, and PI3-kinase/Akt pathways in lung fibroblasts (Figure 3). The induction of phospho-p42/p44 and phospho-p38 seemed to be short-lived, whereas the induction of phospho-Akt seemed to be sustained. When we used specific inhibitors of these pathways, namely, the p38 inhibitor SB203580 (Cuenda et al., 1995), the p42/p44 inhibitors PD98059 (Dudley et al., 1995) and U0126 (Favata et al., 1998), and the PI3-kinase/Akt inhibitors LY294002 (Vlahos et al., 1994) and wortmannin (Okada et al., 1994), only the PI3kinase/Akt inhibitors LY294002 and wortmannin blocked the ability of ET-1 to promote the ability of lung fibroblasts to contract a collagen gel matrix (Figure 4, A and B). Addition of inhibitor alone, in the absence of added ET-1 had no effect on the basal ability of the fibroblast to contract collagen (our unpublished data).
Figure 3.
ET-1 induces p38 MAPK, p42/44 MAPK, and Akt phosphorylation in normal primary lung fibroblasts. Normal lung fibroblasts were cultured, serum starved for 18 h, and treated with 100 nM ET-1. Whole cell protein extracts were made, and equal amounts of protein were subjected to SDS-PAGE and Western blot analysis with anti-ERK, anti-phospho-ERK, anti-p38, anti-phospho-p38, anti-Akt and anti-phospho-Akt antibodies. Treatment of lung fibroblasts induces all three pathways tested.
Figure 4.
Inhibition of the Akt/PI-3 kinase blocks collagen gel contraction by ET-1. Collagen-cell suspensions were polymerized in the absence and presence of 100 nM ET-1, and the rates of subsequent gel contraction measured >24 h. (A) Gel contraction assay. The extent of ET-1–induced contraction (100 nM) in the presence of each inhibitor was assessed 24 h after lattice seeding as described in MATERIALS AND METHODS. The promotion of fibroblast-mediated gel contraction by ET-1 was found to be specifically blocked by the Akt/PI3 kinase inhibitors wortmannin and LY294002, but not by the p42/p44 inhibitors PD98059 and U0126 or the p38 inhibitor SB203580 (*p < 0.05 compared with ET-1–induced matrix contraction, Student's paired t-test). (B) FPCL assay. As described in the legend for Figure 2, force across a collagen gel was measured in the presence or absence of ET-1 and wortmannin, as indicated.
SSc Lung Fibroblasts Show Elevated Levels of ET-1 Expression and Cell Surface Binding
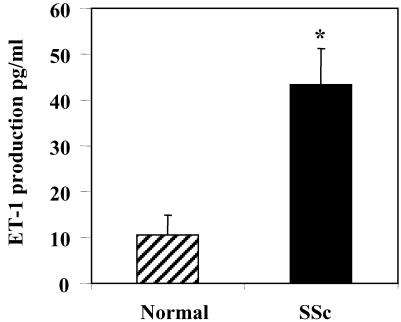
One aspect of the excessive scarring associated with fibrotic disease is markedly enhanced contraction of ECM. We thus decided to probe the functional relevance of the ability of ET-1 to activate Akt/PI3-kinase to the fibrotic phenotype of lung fibroblasts isolated from SSc patients. To assess whether SSc lung fibroblasts secrete elevated levels of ET-1, we assessed levels of ET-1 in supernatants from normal and SSc lung fibroblasts. We plated equal numbers of SSc and normal fibroblasts, and the next day measured equal amounts of media for levels of secreted ET-1 by an ET-1 ELISA. We found that SSc lung fibroblasts secreted slightly >4 times (43.48 ± 7.29 ng/ml; average ± SD, p < 0.02) the amount of ET-1 relative to normal lung fibroblasts [10.72 ± 4.83 ng/ml; average ± SD, p < 0.02; Figure 5).
Figure 5.
Secretion of ET-1 by normal and SSc lung fibroblasts. The concentration of ET-1 in conditioned media was measured by an ELISA that detects ET-1 levels. Medium conditioned for 24 h by normal and SSc lung fibroblasts (SScLF) was examined. Data shown are means (+SEM) based on five replicate wells for three independent experiments by using different fibroblast strains (*p < 0.05, Student's unpaired t-test).
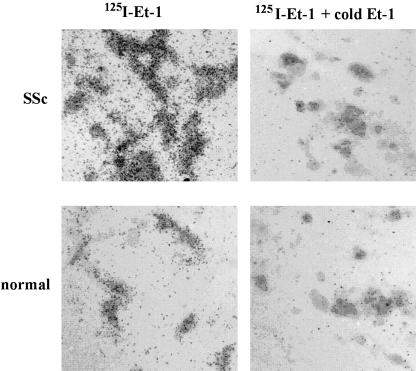
To assess whether SSc lung fibroblasts also showed elevated levels of ET-1 receptors, we incubated normal and SSc lung fibroblasts with 125I-labeled ET-1 for 18 h. After the unbound 125I-ET-1 was removed by extensive washing, total cell surface ET-1 binding was assessed by autoradiography. Adding to our previous data showing elevated ET-1 expression by lesional SSc lung fibroblasts, we found that, relative to normal lung fibroblasts, SSc lung fibroblasts possessed a markedly increased ability to bind ET-1 (Figure 6). Together, these results suggest that SSc fibroblasts would be expected to show elevated, constitutive levels of ET-1 signaling.
Figure 6.
Lesional SSc lung fibroblasts display elevated levels of ET-1 receptor binding. Normal and SSc fibroblasts were grown in collagen gels. Cells were assessed for binding of exogenously added 150 pM 125I-ET-1, in the presence or absence of 100-fold molar excess cold ET-1, as described in Materials and Methods. Brightfield illumination autoradiographs of normal and SSc fibroblasts are shown. Accumulation of grains indicate increased binding to the cell surface.
SSc Lung Fibroblasts Show an Elevated Ability to Contract a Collagen Gel Matrix, Which Depends on Endogenous ET-1 and the ET-A Receptor
Previously, it was shown that dermal fibroblasts cultured from lesions of SSc patients expressed α-SMA (Sappino et al., 1990) and thus might be expected to demonstrate an enhanced ability, relative to normal fibroblasts, to contract a collagen matrix. Therefore, we decided to investigate whether lung fibroblasts cultured from SSc fibrotic lung disease also displayed a similar phenotype and whether these cells showed an enhanced contractile phenotype relative to normal lung fibroblasts. We further sought to determine whether this elevated contractile activity might be due to an elevated level of ET-1 expressed by SSc fibroblasts. To begin to perform this analysis, we compared the ability of SSc and normal lung fibroblasts to contract a collagen gel matrix over a 24-h period. We found that, consistent with their endogenous expression of α-SMA, SSc lung fibroblasts showed a greatly increased ability, relative to normal lung fibroblasts, to contract a collagen matrix (Figure 7, A and B). To assess whether this ability of SSc lung fibroblasts could be due to elevated, constitutive ET-1 signaling, we tested the ability of the ETA/ETB receptor antagonist bosentan (Veniant et al., 1994), the ETA receptor antagonist PD156707 (Maguire et al., 1997), or the ETB receptor antagonist BQ788 (Hamroun et al., 1995) to reduce the contractile phenotype of SSc lung fibroblasts. Because we had earlier determined that wortmannin and LY2494002 blocked the ability of exogenously added ET-1 to promote the ability of normal fibroblasts to contract ECM, we also evaluated the ability of wortmannin and LY294002 to suppress the contractile phenotype of SSc fibroblasts. We found that addition of bosentan, PD156707, LY294002, or wortmannin potently reduced the contractile ability of SSc lung fibroblasts (Figure 7, A and B). Conversely, addition of the ETB receptor antagonist BQ788 did not affect the ability of the SSc lung fibroblast to contract a collagen gel matrix (Figure 7). In summary, antagonizing endogenous ET signaling through the ETA receptor reduced the contractile ability of SSc lung fibroblasts (Figure 7).
Figure 7.
SSc lung fibroblasts show an enhanced ability to contract a collagen gel matrix that is dependent on endogenous ET signaling via the ET-A receptor. Normal and SSc lung fibroblasts were subjected to a gel contraction assay (A) or force generation assay (B) in the presence or absence of 100 nM ET-1, the PI3-kinase inhibitor wortmannin, the Akt inhibitor LY294002, the MEK inhibitors PD98059 and U0126, the p38 inhibitor SB203580, the ETA/ETB receptor antagonist bosentan, the ETA receptor antagonist PD156707, or the ETB receptor antagonist BQ788. Wortmannin and bosentan potently reduced the contractile ability of the SSc fibroblast in the absence of exogenously added ET-1 (p < 0.05, paired Student's t-test).
ET-1 Induces Expression of Procontractile Proteins in Lung Fibroblasts
To further investigate the role of ET-1 in fibroblast biology, we serum starved normal lung fibroblasts for 18 h and then treated cells for an additional 4 h with or without 100 nM ET-1. RNA was extracted from lung fibroblasts and subjected to genome-wide expression analysis by using U133A arrays (Affymetrix). ET-1 induced several transcripts that encoded genes expected to contribute to the contractile phenotype of myofibroblasts (Table 1), including mRNAs encoding smooth muscle alpha and γ-actins, contraction promoting proteins, and smooth muscle actin-associated cytoskeletal proteins such as caldesmon, gelsolin, tensin, and filamin (Table 1; Janson et al., 1991; Morgan and Gangopadhyay, 2001). In addition, transcripts encoding ezrin, radixin, and moesin, proteins that cross-link actin to the plasma membrane (Louvet-Vallee, 2000), were found to be induced by ET-1 (Table 1).
Table 1.
Transcripts elevated (≥1.5-fold) in normal lung fibroblasts by a 4-h treatment with 100 nM ET-1
| Affymetrix ID | GenBank ID | Transcript name | Fold inductiona |
|---|---|---|---|
| Muscle actin | |||
| 200974_at | NM_001613.1 | Actin, alpha 2, smooth muscle, aorta | 1.5 |
| 202274_at | NM_001615.2 | Actin, gamma 2, smooth muscle, enteric | 1.9 |
| 201950_x_at | NM_004930.1 | Capping protein (actin filament) beta | 1.6 |
| 200720_s_at | NM_005736 | Actin-related protein 1, centractin alpha | 1.7 |
| 200729_s_at | NM_005722 | Actin-related protein 2 homolog | 2.6 |
| 200996_at | NM_005721 | Actin-related protein 3 homolog | 1.7 |
| 211672_s_at | NM_005718 | Actin-related protein 2/3 complex, subunit 4 | 2.0 |
| Proteins promoting actin-mediated contraction | |||
| 208613_s_at | AV712733 | Filamin B, beta (actin-binding protein-278) | 2.1 |
| 207876_s_at | NM_001458.1 | Filamin C, gamma (actin-binding protein-280) | 1.5 |
| 214040_s_at | BE675337 | Gelsolin | 2.5 |
| 218864_at | AF116610.1 | Tensin | 3.7 |
| 210978_s_at | BC002616.1 | Transgelin 2 | 2.5 |
| 214726_x_at | AL556041 | Adducin 1 (alpha) | 1.6 |
| 201616_s_at | M64110.1 | Caldesmon 1 | 3.7 |
| 206116_s_at | NM_000366.1 | Tropomyosin 1 (alpha) | 3.5 |
| 212481_s_at | AI214061 | Tropomyosin 4 | 3.7 |
| 212365_at | BF215996 | Myosin IB | 2.0 |
| Actin/cell membrane cross-linking proteins | |||
| 217234_s_at | AA670344 | Villin 2 (ezrin) | 4.0 |
| 200600_at | NM_002444.1 | Moesin (MSN) | 1.5 |
| 212398_at | AI057093 | Radixin | 4.0 |
Transcripts were identified as changed by the Affymetrix change algorythm (p < 0.001)
To confirm the array results, we used Western blot analysis to verify that 100 nM ET-1 induced expression of α-SMA, ezrin, and moesin proteins. To further confirm the notion that ET-1 might contribute to the contractile phenotype of fibroblasts by inducing expression of procontractile proteins, we assessed whether ET-1 could induce expression of paxillin, a protein found in focal adhesions (Schaller, 2001), whose recruitment to the cellular membrane has recently been found to be necessary for the development of tension during smooth muscle contraction (Opazo Saez et al., e pub 2003). We found that a 24-h treatment of lung fibroblasts with 100 nM ET-1 induced α-SMA, ezrin, moesin, and paxillin proteins (Figure 8). This ability was blocked by bosentan and wortmannin (Figure 8).
Figure 8.
Endothelin induces α-SMA, paxillin, moesin, and ezrin expression; SSc fibroblasts overexpress α-SMA, paxillin, moesin, and ezrin in a manner that is dependent on endogenous ET-1 signaling. Normal and SSc fibroblasts were treated with and without 100 nM ET-1 (24 h) as indicated in the presence or absence of bosentan or wortmannin. Cell layers were harvested, and equal amounts of protein were subjected to SDS-PAGE and Western blot analysis with anti-α-SMA, anti-paxillin, anti-moesin, and anti-ezrin antibodies, as indicated. As a loading control, Western analysis with an anti-GAPDH antibody also was performed.
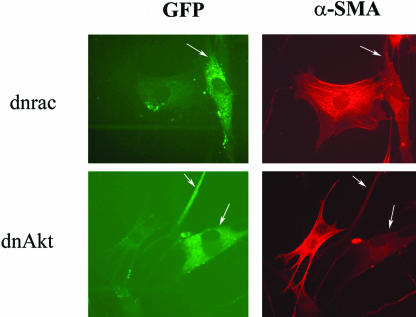
To extend these data showing that ET-1 promoted induction of procontractile proteins via Akt/PI3-kinase, we transfected cells with expression vectors encoding dominant negative Akt or rac, the latter which is upstream of PI3-kinase/Akt. Cells were cotransfected with expression vector encoding GFP, which was used to identify transfected cells. After a serum starvation step, cells were treated with ET-1 for 24 h, fixed, and α-SMA expression and incorporation into stress fibers was assessed using an anti-α-SMA antibody. Compared with cells receiving empty expression vector, cells transfected with dominant negative Akt or rac showed no induction of α-SMA stress fibers upon ET-1 treatment (Figure 9). These results confirm our impression that the rac/PI3 kinase/Akt cascade is necessary for the ET-1 mediated induction of α-SMA stress fibers and the resultant ability of ET-1–treated cells to contract ECM. Collectively, our results suggest that ET-1 treatment promotes normal lung fibroblasts to exhibit a contractile phenotype and show features of myofibroblasts by directly inducing expression of procontractile genes via PI3 kinase.
Figure 9.
Dominant negative rac or Akt block ET-1–dependent induction of the α-SMA stress fiber network. Lung fibroblasts were transfected with vector encoding dominant negative rac (dnrac) or dominant negative Akt (dnAkt). Cells were also transfected with an expression vector encoding mammalian enhanced-GFP (CMV-EGFP), which was used to identify transfected cells (denoted by white arrow). Eighteen hours after transfection, cells were treated with 100 nM ET-1 for 24 h. Cells were fixed and stained with anti-α-SMA antibody that was detected with a Texas Red-conjugated secondary antibody. Cells transfected with dominant negative versions of signal transduction mediators were identified by cells expressing GFP protein from the cotransfected CMV-EGFP construct. Cells transfected with either dnrac or dnAkt (white arrows), relative to untransfected cells, showed marked reduction in α-SMA stress fiber formation.
Endogenous ET-1 Produced by Lung SSc Fibroblasts Promotes the Expression of Procontractile Proteins
We then further tested the relevance of endogenous ET-1 and the PI3-kinase pathway to the ability of ET-1 to induce matrix contraction in SSc fibroblasts by assessing whether endogenous ET-1 resulted in the overexpression of α-SMA, ezrin, moesin, and paxillin protein expression in SSc fibroblasts. We found, using Western blot analysis, that SSc fibroblasts constitutively produced elevated levels α-SMA, ezrin, moesin, and paxillin protein expression (Figure 8). We further found that the overexpression of these proteins by lung SSc fibroblasts was markedly reduced by bosentan and wortmannin (Figure 8). Similarly, by immunofluorescence analysis, we showed that SSc fibroblasts possessed elevated levels of α-SMA stress fibers that were reduced with bosentan treatment (Figure 10). Thus, ET-1 directly contributed to the enhanced contractile ability of fibrotic lung fibroblasts by not only enhancing matrix contraction but also by inducing expression of proteins involved with formation of actin stress fibers. Collectively, our results strongly suggest that endogenous ET-1 produced by fibrotic fibroblasts directly contributes to the fibrogenic phenotype.
Figure 10.
SSc lung fibroblasts show elevated formation of α-SMA stress fibers that is dependent on endogenous ET signaling. Normal and SSc lung fibroblasts were treated with or without bosentan for 24 h and subjected to immunofluorescence analysis with a mouse anti-α-SMA antibody and a fluorescein isothiocyanate-conjugated anti-mouse antibody. Nuclei were detected by 4,6-diamidino-2-phenylindole staining. Constitutive expression and assembly of α-SMA stress fibers by SSc lung fibroblast was potently reduced by the ET-1 dual receptor antagonist bosentan.
DISCUSSION
In this study, we have used primary human lung fibroblasts as an in vitro model with which to examine the role of ET-1 in ECM remodeling and fibrosis. In vitro and in vivo studies have consistently shown that SSc fibroblasts directly contribute to the excessive scarring observed in fibrosis by showing enhanced production of ECM components (Harrison et al., 1991; Ivarsson et al., 1993; Jimenez et al., 1996; Shi-Wen et al., 1997). An additional characteristic of scar tissue is the presence of α-SMA–enhanced contraction of the extracellular matrix by lesional fibroblasts (Grinnell, 1994; Tomasek et al., 2002; Hinz et al., 2002). In this report, for the first time we show that SSc lung fibroblasts cultured from fibrotic lesions contribute to scar formation by possessing an enhanced ability, relative to normal fibroblasts, to contract a collagen matrix.
Previously, we and others showed that elevated levels of circulating ET-1 occurred in patients with skin and lung fibrosis, which correlated with the severity of the fibrotic phenotype (Miyauchi et al., 1992; Kawaguchi et al., 1994; Abraham et al., 1997). This increase in circulating ET-1 was paralleled by an increase in ET-1 synthesis in vivo (Miyauchi et al., 1992; Kawaguchi et al., 1994; Abraham et al., 1997). To begin to probe the role of ET-1 in the fibrotic phenotype, we first determined that ET-1 promoted the ability of normal lung fibroblasts to contract a collagen gel matrix in vitro, through the Akt/PI3-kinase–dependent pathway.
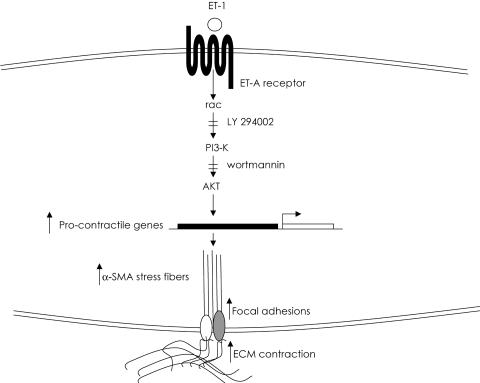
To assess whether ET-1 could be involved in enhanced ECM contraction by lesional SSc pulmonary fibroblasts, we showed that, relative to normal lung fibroblasts, SSc fibroblasts expressed elevated levels of ET-1 protein and showed substantially higher levels of ET-1 cell surface binding. We then found that endogenous ET-1 activity in lung SSc fibroblasts directly contributed to the contractile phenotype of the SSc fibroblasts, because blocking ET-1 signaling by the specific ET-1 dual receptor antagonist bosentan, the ET-A antagonist PD156707, and the PI3-kinase inhibitor wortmannin greatly reduced the ability of SSc fibroblasts to contract a collagen gel matrix. In addition, SSc fibroblasts produced elevated levels of α-SMA, ezrin, moesin, and paxillin, which depended on endogenous ET-1 signaling and PI3-kinase. Therefore, the enhanced contractile ability of the SSc fibroblast seemed to depend on the elevated levels of endogenous ET-1 expression and cell binding demonstrated by lung SSc fibroblasts (Figure 11). Our results are consistent with the notion that the elevated levels of ET-1 produced by the SSc fibroblast directly contribute to scar formation by being responsible for the enhanced ability of the SSc fibroblast to contract ECM via a PI3-kinase/Akt-dependent mechanism.
Figure 11.
Schematic diagram showing the contribution of ET-1 to ECM contraction in SSc lung fibroblasts. ET-1 promotes ECM contraction by normal and SSc lung fibroblasts through Akt/PI3 kinase. Endogenous ET-1 activity contributes to the enhanced ability of lesional SSc fibroblasts to contract ECM.
In addition to ET-1, other factors present in serum, namely, epidermal growth factor, platelet-derived growth factor (PDGF)-BB, transforming growth factor-β (TGFβ), and connective tissue growth factor (CTGF), act on fibroblasts to promote matrix contraction (Grinnell, 1994; Shi-Wen et al., 2000; Allen et al., 2002). Thus, in vivo, elevation of these factors in bronchial lavage fluid or serum of patients with fibrotic disease (Ludwicka et al., 1995; Sato et al., 2000; Leask et al., 2004) might collectively contribute to the enhanced contractile phenotype of fibroblasts in these individuals. Consistent with the notion that several factors cooperate in vivo to generate a fibrotic phenotype, in this report we showed, in low serum, that antagonizing ET-1 signaling did not revert the contractile ability of SSc fibroblasts entirely to that of normal fibroblasts. Similarly, if we performed experiments in full serum, bosentan did not significantly affect the contractile ability of SSc fibroblasts (our unpublished data).
TGFβ has been shown to be a potent inducer of myofibroblast formation (Desmoulière, 1995). Factors such as ET-1 and CTGF may be downstream effectors of the ability of TGFβ to promote ECM contraction because TGFβ induces expression of ET-1 and CTGF (Holmes et al., 2001; Leask et al., 2003; Rodriguez-Pascual et al., 2003). Giving further support to this idea, recent experiments using antisense oligonucleotides to reduce CTGF expression showed that TGFβ-induced matrix contraction in free-floating gels was at least partially mediated by CTGF via the induction of matrix metalloprotenases (Daniels et al., 2003). Furthermore, the ability of TGFβ to induce matrix contraction in free-floating gels is greatly impaired in Smad3 -/-fibroblasts (Liu et al., 2003). Given that Smad3 is generally considered to be a mediator of TGFβ-induced gene transcription (Attisano and Wrana, 2002), these results suggest that the ability of TGFβ to induce matrix contraction may be mediated by proteins such as CTGF and ET-1, induced in a Smad3-dependent manner. Conversely, the ability of PDGF-BB to promote matrix contraction did not depend on Smad3, suggesting that PDGF-BB and TGFβ independently induce fibroblasts to contract ECM (Liu et al., 2003).
It is interesting to note that ET-1 was able to induce myofibroblast formation, as visualized by α-SMA expression, in floating gels. In this regard, ET-1 is similar to thrombin, which was recently shown to be able to induce myofibroblast formation in lung fibroblasts cultured in floating gels (Bogatkevich et al., 2001, 2003). We interpret these data to mean that the ability of thrombin and ET-1 to promote myofibroblast formation does not depend on mechanical loading. However, studies examining the ability of TGFβ to promote contraction of floating gels have shown that TGFβ is not able to induce myofibroblast formation in this system (Hinz and Gabbiani, 2003). However, to induce α-SMA expression, TGFβ requires the ED-A form of fibronectin (Serini et al., 1998). In floating gels, the fibronectin network cannot properly be maintained or organized (Halliday and Tomasek, 1995). Therefore, the inability of TGFβ to promote myofibroblast formation within floating gels (that is, in the absence of mechanical loading) has been interpreted as arising due to the inability of ED-A fibronectin to be properly organized within floating gels and thus to properly promote TGFβ-induced myofibroblast formation in floating gels (Grinnell and Ho, 2002). However, this hypothesis remains to be thoroughly evaluated. Collectively, these data are consistent with the notion that several agents are capable of inducing myofibroblast formation through different mechanisms.
Different mechanisms seem to be operating in contraction of stressed and floating collagen matrices (Grinnell et al., 1999). In the absence of complications, the process of wound contraction leads to wound closure with little scarring or loss of function; however, in large wounds or in fibrotic disease the consequences of contraction, due to the persistence of myofibroblasts, can result in loss of joint motion or major body deformations referred to as contractures (Desmoulière and Gabbiani, 1988). Contraction of floating collagen matrices is considered to resemble more closely the initial, inductive phase of wound contraction (Ehrlich and Rajaratnam, 1990; Gross et al.,1995; Grinnell et al., 1999), whereas the myofibroblast-like cells in mechanically stressed matrices is considered to be more typical of the late phase of excessive scarring observed in contractures (Grinnell, 1994; Desmoulinère and Gabbiani, 1996.). More accurately, the former model measures cell migration and can measure the induction of myofibroblast formation by growth factors, whereas the latter model measures the direct ability of proteins to enhance contraction, through mechanical means, of an already formed α-SMA network (Grinnell et al., 1999). In this report, we found that the ability of ET-1 to promote contraction in floating gels depended on de novo protein synthesis. Conversely, ET-1 was not able to promote contraction of fixed collagen gels. The divergence between these results presumably reflects the fact that ET-1 promotes matrix contraction via inducing myofibroblast formation, but it does not directly promote stress fiber-mediated contraction by mechanical means.
In this report, we show that ET-1 induction of procontractile proteins is dependent on Akt. Akt (protein kinase B, PKB) is a serine/threonine kinase, which in mammals comprises three highly homologous members known as PKBα (Akt1), PKBβ (Akt2), and PKBγ (Akt3). PKB/Akt is activated in cells exposed to diverse stimuli such as hormones, growth factors, and extracellular matrix components (Franke et al., 2003; Persad and Dedhar, 2003). The activation mechanism remains to be fully characterized but occurs in response to activation of PI3-kinase. PI3-kinases have been shown to play an important role in mitogenic signaling and cell survival, cytoskeletal remodeling, metabolic control, and vesicular trafficking (Coffer et al., 1998; CorveraandCzech,1998).PI3-kinasegeneratesphosphatidylinositol-3,4,5-trisphosphate, a lipid second messenger essential for the translocation of PKB/Akt to the plasma membrane where it is phosphorylated and activated by phosphoinositide-dependent kinase-1 and possibly other kinases (Coffer et al., 1998; Corvera and Czech, 1998). PKB/Akt phosphorylates and regulates the function of many cellular proteins involved in processes that include metabolism, apoptosis, and proliferation (Coffer et al., 1998; Corvera and Czech, 1998). The recent identification of a number of substrates for the serine/threonine kinase Akt suggests that it blocks cell death by both impinging on the cytoplasmic cell death machinery and by regulating the expression of genes involved in cell death and survival (Brunet et al., 2001). Recent evidence indicates that PKB/Akt is frequently constitutively active in many types of human cancer. Constitutive PKB/Akt activation can occur due to amplification of PKB/Akt genes or as a result of mutations in components of the signaling pathway that activates PKB/Akt. Although the mechanisms have not yet been fully characterized, constitutive PKB/Akt signaling is believed to promote proliferation and increased cell survival and thereby contribute to cancer progression (Nicholson and Anderson, 2002).
It has been shown that Akt regulates myocardial contraction (Condorelli et al., 2002) and is activated during contraction of skeletal and smooth muscle (Komalavilas et al., 2001; Sakamoto et al., 2003). Thus, activation of the Akt pathway may be generally required for actin-generated contractile forces. However, relatively little work has been undertaken regarding the role of PI3-kinase/Akt in fibroblast biology, especially in terms of a wound healing response. Recently it was shown that fibroblasts that contract a collagen gel undergo apoptosis (Tian et al., 2002). This result is consistent with the notion that, in a normal wound healing response, myofibroblasts disappear after formation of a repaired dermis. However, apoptosis induced during matrix contraction was attenuated by the ligation of beta1 integrin with an anti-beta1 integrin antibody, through a PI3-kinase/Akt-dependent mechanism (Tian et al., 2002). Thus, it is conceivable that the overexpression of ET-1 in the SSc lesion not only results in the induction of the PI3-kinase/Akt pathway and to the direct enhancement of matrix contraction but also contributes to the persistence of the myofibroblast in the fibrotic lesion by blocking contraction-induced apoptosis. This hypothesis is currently under investigation. We expect that the persistent activation of the Akt pathway after ET-1 treatment reflects the notion that this pathway is required for a sustained activation of a mechanoregulatory response, namely, matrix contraction, which takes place over several hours. Conversely, the transient activation of p38 and ERK implies that these pathways may not be involved with sustained cellular activity but rather may reflect their potential involvement in transient cellular processes. These events might include the activation of gene transcription; indeed, we have found that ET-1 can induce gene expression via ERK (Shi-Wen, Renzoni, Bou-Gharios, du Bois, Black, Leask, and Abraham, unpublished data).
Several genes have been shown to be overexpressed in scleroderma fibroblasts and contribute to the fibrotic phenotype of this disorder (Shi-Wen et al., 2000; Leask et al., 2002); and recently, we showed that the ras/MEK/ERK signaling cascade was involved with the induction of profibrotic genes in fibroblasts (Stratton et al., 2002; Leask et al., 2003). However, this is the first report to causally link a protein overexpressed in SSc to a specific functional role in mediating the fibrotic phenotype of the SSc fibroblast. Specifically, our results suggest that the excessive scarring found in fibrotic lungs of SSc patients may result from the ability of endogenous ET-1 signaling to induce SSc lung fibroblasts to contract ECM. These results suggest there may be potential therapeutic advantage in using PI3-kinase inhibitors or endothelin antagonists to ameliorate the pathological scarring observed in pulmonary fibrosis.
Acknowledgments
This work was supported by the Arthritis Research Campaign, the Raynaud's and Scleroderma Association Trust, The Wellcome Trust, the Welton Foundation, the Golden Charitable Trust and the Nightingale Trust. We also thank the MRC Microarray Centre (London), particularly Helen Causton, Laurence Game, and Helen Banks.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-12-0902. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-12-0902.
References
- Abraham, D.J., Vancheeswaran, R., Dashwood, M.R., Pantelides, P., Shi-Wen, X., du Bois, R.M., and Black, C.M. (1997). Increased levels of endothelin-1 and differential endothelin type A and B receptor expression in scleroderma-associated fibrotic lung disease. Am. J. Pathol. 151, 831-841. [PMC free article] [PubMed] [Google Scholar]
- Allen, F.D., Asnes, C.F., Chang, P., Elson, E.L., Lauffenburger, D.A., and Wells, A. (2002). Epidermal growth factor induces acute matrix contraction and subsequent calpain-modulated relaxation. Wound Repair Regen. 10, 67-76. [DOI] [PubMed] [Google Scholar]
- Anggard, E.E., Botting, R.M., and Vane, J.R. (1990). Endothelins Blood Vessels 27, 269-281. [PubMed] [Google Scholar]
- Appleton, I., Tomlinson, A., Chander, C.L., and Willoughby, D.A. (1992). Effect of endothelin-1 on croton oil-induced granulation tissue in the rat. A pharmacologic and immunohistochemical study. Lab. Investig. 67, 703-710. [PubMed] [Google Scholar]
- Attisano, L., and Wrana, J.L. (2002). Signal transduction by the TGF-beta superfamily. Science 296, 1646-1647. [DOI] [PubMed] [Google Scholar]
- Badylak, S.F. (2002). The extracellular matrix as a scaffold for tissue reconstruction. Semin. Cell Dev. Biol. 13, 377-383. [DOI] [PubMed] [Google Scholar]
- Bell, E., Ivarsson, B., and Merrill, C. (1979). Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc. Natl. Acad. Sci. USA 76, 1274-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black, C.M., and Denton, C.P. (1998). Systemic sclerosis and related disorders in adults and children. In: Oxford Textbook of Rheumatology, ed. P.G. Maddison, D.A. Isenberg, P. Woo, and D.N. Glass, New York: Oxford University Press, 771-789.
- Bogatkevich, G.S., Tourkina, E., Silver, R.M., and Ludwicka-Bradley, A. (2001). Thrombin differentiates normal lung fibroblasts to a myofibroblast phenotype via the proteolytically activated receptor-1 and a protein kinase C-dependent pathway. J. Biol. Chem. 276, 45184-45192. [DOI] [PubMed] [Google Scholar]
- Bogatkevich, G.S., Tourkina, E., Abrams, C.S., Harley, R.A., Silver, R.M., and Ludwicka-Bradley, A. (2003). Contractile activity and smooth muscle alphaactin organization in thrombin-induced human lung myofibroblasts. Am. J. Physiol. 285, L334-L343. [DOI] [PubMed] [Google Scholar]
- Brunet, A., Datta, S.R., and Greenberg, M.E. (2001). Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr. Opin. Neurobiol. 11, 297-305. [DOI] [PubMed] [Google Scholar]
- Coffer, P.J., Jin, J., and Woodgett, J.R. (1998). Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem. J. 335, 1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condorelli, G., et al. (2002). Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc. Natl. Acad. Sci. USA 99, 12333-12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvera, S., and Czech, M.P. (1998). Direct targets of phosphoinositide 3-kinase products in membrane traffic and signal transduction. Trends Cell Biol. 8, 442-446. [DOI] [PubMed] [Google Scholar]
- Cuenda, A., Rouse, J., Doza, Y.N., Meier, R., Cohen, P., Gallagher, T.F., Young, P.R., and Lee, J.C. (1995). SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 364, 229-233. [DOI] [PubMed] [Google Scholar]
- Daniels, J.T., Schultz, G.S., Blalock, T.D., Garrett, Q., Grotendorst, G.R., Dean, N.M., and Khaw, P.T. (2003). Mediation of transforming growth factor-beta(1)-stimulated matrix contraction by fibroblasts: a role for connective tissue growth factor in contractile scarring. Am. J. Pathol. 163, 2043-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmoulière, A. (1995). Factors influencing myofibroblast differentiation during wound healing and fibrosis. Cell Biol. Int. 19, 471-476. [DOI] [PubMed] [Google Scholar]
- Desmoulière, A., and Gabbiani, G. (1996). The role of the myofibroblast in wound healing and fibrocontractive diseases. In: The Molecular and Cellular Basis of Wound Repair, 2 ed., ed. R.A.F. Clark, New York: Plenum Press, 391-423.
- Dudley, D.T., Pang, L., Decker, S.J., Bridges, A.J., and Saltiel, A.R. (1995). A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. USA 92, 7686-7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood, M., McGrouther, D.A., and Brown, R.A. (1994). A culture force monitor for measurement of contraction forces generated in human dermal fibroblast cultures: evidence for cell-matrix mechanical signaling. Biochim. Biophys. Acta 1201, 186-192. [DOI] [PubMed] [Google Scholar]
- Eastwood, M., Porter, R., Khan, U., McGrouther, G., and Brown, R. (1996). Quantitative analysis of collagen gel contractile forces generated by dermal fibroblasts and the relationship to cell morphology. J. Cell Physiol. 166, 33-42. [DOI] [PubMed] [Google Scholar]
- Ehrlich, H.P., and Rajaratnam, J.B. (1990). Tissue Cell 22, 407-417. [DOI] [PubMed] [Google Scholar]
- Favata, M.F., et al. (1998). Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273, 18623-18632. [DOI] [PubMed] [Google Scholar]
- Franke, T.F., Hornik, C.P., Segev, L., Shostak, G.A., and Sugimoto, C. (2003). PI3K/Akt and apoptosis: size matters. Oncogene 22, 8983-8998. [DOI] [PubMed] [Google Scholar]
- Gadek, J.E., Fells, G.A., Zimmerman, R.L., and Crystal, R.G. (1984). Role of connective tissue proteases in the pathogenesis of chronic inflammatory lung disease. Environ. Health Perspect. 55, 297-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell, F. (1994). Fibroblasts, myofibroblasts, and wound contraction. J. Cell Biol. 124, 401-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell, F. (2003). Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 13, 264-269. [DOI] [PubMed] [Google Scholar]
- Grinnell, F., and Ho, C.H. (2002). Transforming growth factor beta stimulates fibroblast-collagen matrix contraction by different mechanisms in mechanically loaded and unloaded matrices. Exp. Cell Res. 273, 248-255. [DOI] [PubMed] [Google Scholar]
- Grinnell, F., Ho, C.H., Lin, Y.C., and Skuta, G. (1999). Differences in the regulation of fibroblast contraction of floating versus stressed collagen matrices. J. Biol. Chem. 274, 918-923. [DOI] [PubMed] [Google Scholar]
- Gross, J., Farinelli, W., Sadow, P., Anderson, R., and Bruns, R. (1995). Proc. Natl. Acad. Sci. USA 92, 5982-5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidry, C., and Hook, M. (1991). Endothelins produced by endothelial cells promote collagen gel contraction by fibroblasts. J. Cell Biol. 115, 873-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday, N.L., and Tomasek, J.J. (1995). Mechanical properties of the extracellular matrix influence fibronectin fibril assembly in vitro. Exp. Cell Res. 217, 109-117. [DOI] [PubMed] [Google Scholar]
- Hamroun, D., Mathieu, M.N., Clain, E., Germani, E., Laliberte, M.F., Laliberte, F., and Chevillard, C. (1995). Expression of endothelin and ETB receptors in the megakaryoblastic MEG-01 cell line. J. Cardiovasc. Pharmacol. 26 (suppl 3), S156-S158. [PubMed] [Google Scholar]
- Harrison, N.K., Argent, A.C., McAnulty, R.J., Black, C.M., Corrin, B., and Laurent, G.J. (1991).Collagen synthesis and degradation by systemic sclerosis lung fibroblasts. Responses to transforming growth factor-beta. Chest 99 (suppl 3), 71S-72S [DOI] [PubMed] [Google Scholar]
- Hinz, B., Gabbiani, G., and Chaponnier, C. (2002). The NH2-terminal peptide of alpha-smooth muscle actin inhibits force generation by the myofibroblast in vitro and in vivo. J. Cell Biol. 157, 657-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz, B., and Gabbiani G. (2003). Mechanisms of force generation and transmission by myofibroblasts. Curr. Opin. Biotechnol. 14, 538-546. [DOI] [PubMed] [Google Scholar]
- Holmes, A., Abraham, D.J., Sa, S., Shiwen, X., Black, C.M., and Leask, A. (2001). CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J. Biol. Chem. 276, 10594-10601. [DOI] [PubMed] [Google Scholar]
- Ivarsson, M., McWhirter, A., Black, C.M., and Rubin, K. (1993). Impaired regulation of collagen pro-alpha 1(I) mRNA and change in pattern of collagen-binding integrins on scleroderma fibroblasts. J. Investig. Dermatol. 101, 216-221. [DOI] [PubMed] [Google Scholar]
- Janson, L.W., Kolega, J., and Taylor, D.L. (1991). Modulation of contraction by gelation/solation in a reconstituted motile model. J. Cell Biol. 114, 1005-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez, S.A., Hitraya, E., and Varga, J. (1996). Pathogenesis of scleroderma. Collagen Rheum. Dis. Clin. N. Am. 22, 647-674. [DOI] [PubMed] [Google Scholar]
- Kawaguchi, Y., Suzuki, K., Hara, M., Hidaka, T., Ishizuka, T., Kawagoe, M., and Nakamura, H. (1994). Increased endothelin-1 production in fibroblasts derived from patients with systemic sclerosis. Ann. Rheum. Dis. 53, 506-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk, T.Z., Mark, M.E., Chua, C.C., Chua, B.H., and Mayes, M.D. (1995). Myofibroblasts from scleroderma skin synthesize elevated levels of collagen and tissue inhibitor of metalloproteinase (TIMP-1) with two forms of TIMP-1. J. Biol. Chem. 270, 3423-3428. [DOI] [PubMed] [Google Scholar]
- Komalavilas, P., Mehta, S., Wingard, C.J., Dransfield, D.T., Bhalla, J., Woodrum, J.E., Molinaro, J.R., and Brophy, C.M. (2001). PI3-kinase/Akt modulates vascular smooth muscle tone via cAMP signaling pathways. J. Appl. Physiol. 91, 1819-1827. [DOI] [PubMed] [Google Scholar]
- Leask, A., et al. (2002). Dysregulation of transforming growth factor beta signaling in scleroderma: overexpression of endoglin in cutaneous scleroderma fibroblasts. Arthritis Rheum. 46, 1857-1856. [DOI] [PubMed] [Google Scholar]
- Leask, A., Holmes, A., Black, C.M., and Abraham, D.J. (2003). Connective tissue growth factor gene regulation. Requirements for its induction by transforming growth factor-beta 2 in fibroblasts. J. Biol. Chem. 278, 13008-13015. [DOI] [PubMed] [Google Scholar]
- Leask, A., Denton, C.P., and Abraham, D.J. (2004). Insights into the molecular mechanism of sustained fibrosis: the role of connective tissue growth factor in scleroderma. J. Investig. Dermatol. 122, 1-6. [DOI] [PubMed] [Google Scholar]
- Levin, E.R. (1995). Endothelins. N. Engl. J. Med. 333, 356-363. [DOI] [PubMed] [Google Scholar]
- Lin, Y.C., Ho, C.H., and Grinnell, F. (1997). Fibroblasts contracting collagen matrices form transient plasma membrane passages through which the cells take up fluorescein isothiocyanate-dextran and Ca2+. Mol. Biol. Cell 8, 59-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X., Wen, F.Q., Kobayashi, T., Abe, S., Fang, Q., Piek, E., Bottinger, E.P., Roberts, A.B., and Rennard, S.I. (2003). Smad3 mediates the TGF-beta-induced contraction of type I collagen gels by mouse embryo fibroblasts. Cell Motil. Cytoskeleton 54, 248-253. [DOI] [PubMed] [Google Scholar]
- Louvet-Vallee, S. (2000). ERM proteins: from cellular architecture to cell signaling. Biol. Cell 92, 305-316. [DOI] [PubMed] [Google Scholar]
- Ludwicka, A., Ohba, T., Trojanowska, M., Yamakage, A., Strange, C., Smith, E.A., Leroy, E.C., Sutherland, S., and Silver, R.M. (1995). Elevated levels of platelet derived growth factor and transforming growth factor-beta 1 in bronchoalveolar lavage fluid from patients with scleroderma. J. Rheumatol. 22, 1876-1883. [PubMed] [Google Scholar]
- Maguire, J.J., Kuc, R.E., and Davenport, A.P. (1997). Affinity and selectivity of PD156707, a novel nonpeptide endothelin antagonist, for human ET(A) and ET(B) receptors. J. Pharmacol. Exp. Ther. 280, 1102-1108. [PubMed] [Google Scholar]
- Miyauchi, T., Suzuki, N., Yuhara, T., Akama, T., Suzuki, H., and Kashiwagi, H. (1992). Significance of plasma endothelin-1 levels in patients with systemic sclerosis. J. Rheumatol. 19, 1566-1571. [PubMed] [Google Scholar]
- Morgan, K.G., and Gangopadhyay, S.S. (2001). Cross-bridge regulation by thin filament-associated proteins. J. Appl. Physiol. 91, 953-962. [DOI] [PubMed] [Google Scholar]
- Nicholson, K.M., and Anderson, N.G. (2002). The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 14, 381-395. [DOI] [PubMed] [Google Scholar]
- Ohnaka, K., Takayanagi, R., Nishikawa, M., Haji, M., and Nawata, H. (1993). Purification and characterization of a phosphoramidon-sensitive endothelin-converting enzyme in porcine aortic endothelium. J. Biol. Chem. 268, 26759-26766. [PubMed] [Google Scholar]
- Okada, T., Sakuma, L., Fukui, Y., Hazeki, O., and Ui, M. (1994). Blockage of chemotactic peptide-induced stimulation of neutrophils by wortmannin as a result of selective inhibition of phosphatidylinositol 3-kinase. J. Biol. Chem. 269, 3563-3567. [PubMed] [Google Scholar]
- Opazo Saez, A.M., Zhang, W., Wu, Y., Turner, C.E., Tang, D.D., and Gunst, S.J. (2003) Tension development during contractile stimulation of smooth muscle requires recruitment of paxillin and vinculin to the membrane. Am. J. Physiol. 2003 Oct 22 [Epub ahead of print]. [DOI] [PubMed]
- Panos, R.J., Mortenson, R.L., Niccoli, S.A., and King, T.E., Jr. (1990). Clinical deterioration in patients with idiopathic pulmonary fibrosis: causes and assessment. Am. J. Med. 88, 396-404. [DOI] [PubMed] [Google Scholar]
- Persad, S., and Dedhar, S. (2003). The role of integrin-linked kinase (ILK) in cancer progression. Cancer Metastasis Rev. 22, 375-384. [DOI] [PubMed] [Google Scholar]
- Razzaque, M.S., and Taguchi, T. (2003). Pulmonary fibrosis: cellular and molecular events. Pathol. Int. 53, 133-145. [DOI] [PubMed] [Google Scholar]
- Rockey, D.C., and Chung, J.J. (1996). Endothelin antagonism in experimental hepatic fibrosis. Implications for endothelin in the pathogenesis of wound healing. J. Clin. Investig. 98, 1381-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pascual, F., Redondo-Horcajo, M., and Lamas, S. (2003). Functional cooperation between Smad proteins and activator protein-1 regulates transforming growth factor-beta-mediated induction of endothelin-1 expression. Circ Res. 92, 1288-1295. [DOI] [PubMed] [Google Scholar]
- Rubanyi, G.M., and Botelho, L.H. (1991). Endothelins. FASEB J. 5, 2713-2720. [DOI] [PubMed] [Google Scholar]
- Saita, Y., Yazawa, H., Koizumi, T., Morita, T., Tamura, T., Takenaka, T., and Honda, K. (1998). Mitogenic activity of endothelin on human cultured prostatic smooth muscle cells. Eur. J. Pharmacol. 349, 123-128. [DOI] [PubMed] [Google Scholar]
- Sakamoto, K., Aschenbach, W.G., Hirshman, M.F., and Goodyear, L.J. (2003). Akt signaling in skeletal muscle: regulation by exercise and passive stretch. Am. J. Physiol. 285, E1081-E1088. [DOI] [PubMed] [Google Scholar]
- Sappino, A.P., Masouye, I., Saurat, J.H., and Gabbiani, G. (1990). Smooth muscle differentiation in scleroderma fibroblastic cells. Am. J. Pathol. 137, 585-591. [PMC free article] [PubMed] [Google Scholar]
- Sato, S., Nagaoka, T., Hasegawa, M., Tamatani, T., Nakanishi, T., Takigawa, M., and Takehara, K. (2000). Serum levels of connective tissue growth factor are elevated in patients with systemic sclerosis: association with extent of skin sclerosis and severity of pulmonary fibrosis. J. Rheumatol. 27, 149-154. [PubMed] [Google Scholar]
- Schaller, M.D. (2001). Paxillin: a focal adhesion-associated adaptor protein. Oncogene 20, 6459-6472. [DOI] [PubMed] [Google Scholar]
- Schmitt-Graff, A., Desmoulière, A., and Gabbiani, G. (1994). Heterogeneity of myofibroblast phenotypic features: an example of fibroblastic cell plasticity. Virchows Arch. 425, 3-24. [DOI] [PubMed] [Google Scholar]
- Serini, G., Bochaton-Piallat, M.L., Ropraz, P., Geinoz, A., Borsi, L., Zardi, L., and Gabbiani, G. (1998). The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J. Cell Biol. 142, 873-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahar, I., Fireman, E., Topilsky, M., Grief, J., Schwarz, Y., Kivity, S., Ben-Efraim, S., and Spirer, Z. (1999). Effect of endothelin-1 on alpha-smooth muscle actin expression and on alveolar fibroblasts proliferation in interstitial lung diseases. Int. J. Immunopharmacol. 21, 759-775. [DOI] [PubMed] [Google Scholar]
- Shao, R., Shi, Z., Gotwals, P.J., Koteliansky, V.E., George, J., and Rockey, D.C. (2003). Cell and molecular regulation of endothelin-1 production during hepatic wound healing. Mol. Biol. Cell 14, 2327-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi-Wen, X., Denton, C.P., McWhirter, A., Bou-Gharious, G., Abraham, D.J., duBois, R.M., and Black, C.M. (1997). Scleroderma lung fibroblasts exhibit elevated and dysregulated collagen type I biosynthesis. Arthritis Rheum. 40, 1237-1244. [DOI] [PubMed] [Google Scholar]
- Shi-Wen, X., et al. (2000). Autocrine overexpression of CTGF maintains fibrosis: RDA analysis of fibrosis genes in systemic sclerosis. Exp. Cell Res. 259, 213-224. [DOI] [PubMed] [Google Scholar]
- Shi-Wen, X., Denton, C.P., Dashwood, M.R., Holmes, A.M., Bou-Gharios, G., Pearson, J.D., Black, C.M., and Abraham, D.J. (2001). Fibroblast matrix gene expression and connective tissue remodeling: role of endothelin-1. J. Investig. Dermatol. 116, 417-425. [DOI] [PubMed] [Google Scholar]
- Stratton, R., Rajkumar, V., Ponticos, M., Nichols, B., Shiwen, X., Black, C.M., Abraham, D.J., and Leask, A. (2002). Prostacyclin derivatives prevent the fibrotic response to TGF-beta by inhibiting the Ras/MEK/ERK pathway. FASEB J. 16, 1949-1951. [DOI] [PubMed] [Google Scholar]
- Teder, P., and Noble, P.W. (2000). A cytokine reborn? Endothelin-1 in pulmonary inflammation and fibrosis. Am. J. Respir. Cell Mol. Biol. 23, 7-10. [DOI] [PubMed] [Google Scholar]
- Tian, B., Lessan, K., Kahm, J., Kleidon, J., and Henke, C. (2002). beta 1 integrin regulates fibroblast viability during collagen matrix contraction through a phosphatidylinositol 3-kinase/Akt/protein kinase B signaling pathway. J. Biol. Chem. 277, 24667-24675. [DOI] [PubMed] [Google Scholar]
- Tomasek, J.J., Gabbiani, G., Hinz, B., Chaponnier, C., and Brown, R.A. (2002). Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell. Biol. 3, 349-363. [DOI] [PubMed] [Google Scholar]
- Turner, A.J., and Murphy, L.J. (1996). Molecular pharmacology of endothelin converting enzymes. Biochem. Pharmacol. 51, 91-102. [DOI] [PubMed] [Google Scholar]
- Varga, J., and Bashey, R.I. (1995). Regulation of connective tissue synthesis in systemic sclerosis. Int. Rev. Immunol. 12, 187-199. [DOI] [PubMed] [Google Scholar]
- Veniant, M., Clozel, J.P., Hess, P., and Clozel, M. (1994). Endothelin plays a role in the maintenance of blood pressure in normotensive guinea pigs. Life Sci. 55, 445-454. [DOI] [PubMed] [Google Scholar]
- Vlahos, C.J., Matter, W.F., Hui, K.Y., and Brown, R.F. (1994). A specific inhibitor of phosphatidyl-inositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J. Biol. Chem. 269, 5241-5248. [PubMed] [Google Scholar]
- Xu S, Denton CP, Holmes A, Dashwood MR, Abraham DJ, Black CM (1998) Endothelins: effect on matrix biosynthesis and proliferation in normal and scleroderma fibroblasts. J. Cardiovasc. Pharmacol. 31 (suppl 1), S360-S363. [DOI] [PubMed] [Google Scholar]
- Yanagisawa, M. (1994). The endothelin system. A new target for therapeutic intervention. Circulation 89, 1320-1322. [DOI] [PubMed] [Google Scholar]