Abstract
Numerous evidence demonstrates that dynein is crucial for organization of microtubules (MTs) into radial arrays, but its exact function in this process is unclear. Here, we studied the role of cytoplasmic dynein in MT radial array formation in the absence of the centrosome. We found that dynein is a potent MT nucleator in vitro and that stimulation of dynein activity in cytoplasmic fragments of melanophores induces nucleation-dependent formation of MT radial array in the absence of the centrosome. This new property of dynein, in combination with its known role as an MT motor that is essential for MT array organization in the absence and presence of the centrosome, makes it a unique molecule whose activity is necessary and sufficient for the formation and maintenance of MT radial arrays in cells.
INTRODUCTION
System of cytoplasmic microtubules (MTs) plays a key role in organization and functioning of living cells. MTs mediate intracellular transport, a process that ensures the delivery of organelles to various destinations inside the cell in interphase and coordinates the equal segregation of chromosomes and other cellular components in mitosis. A key property that allows MTs to function efficiently is their ability to form focused radial arrays, with plus ends exploring the peripheral parts of the cytoplasm and minus ends clustered at the center where MT nucleation takes place.
Radial MT arrays are generally formed by the centrosome, which can induce outgrowth of MTs with their plus ends directed outwards. It has been shown that γ-tubulin–dependent MT nucleation at the centrosome is largely responsible for the radial array formation (reviewed in Doxsey, 2001). However, numerous evidence suggests that there is another, centrosome-independent mechanism of radial array organization, driven by minus end-directed MT motors, such as cytoplasmic dynein that contributes to MT organization in centrosome-containing systems and substitutes for it in the absence of the centrosome (reviewed in Merdes and Cleveland, 1997; Compton, 1998; Hyman, 2000; Karsenti and Vernos, 2001).
A good experimental system to study centrosome-independent MT organization is cytoplasmic fragments of fish melanophores. These cells can rapidly redistribute pigment granules throughout the cytoplasm and aggregate them at the cell center through the activity of molecular motors, kinesin and myosin for pigment dispersion, and dynein for pigment aggregation (reviewed in Tuma and Gelfand, 1999). In melanophore fragments, stimulation of dynein activity by application of aggregation stimulus induces clustering of pigment granules at the center of the fragment and the formation of MT radial array (Rodionov and Borisy, 1997). This self-organization depends on dynein activity and occurs through a combination of MT-based granule transport and MT nucleation on the pigment granules (Vorobjev et al., 2001). Although it is known that dynein mediates MT-based granule transport, it has been unclear what factor contributes the MT nucleation activity. Our previous work has shown that the nucleation of MTs on granules is inhibited by dynein antibody, suggesting that dynein itself may play a role in MT nucleation.
Here, we studied directly whether dynein can nucleate MTs. We found that purified dynein has a high MT nucleating capacity that depends on its activity and that inhibition of dynein activity in vivo blocks MT nucleation on pigment granules. Furthermore, we found that this ability is specific for dynein and not other MT motors, such as kinesin and that it most likely accounts for the observed MT nucleation in our centrosome-free system. Therefore, dynein alone can contribute both MT-based transport and MT nucleation on pigment granules and thus possesses all the properties necessary and sufficient for the organization of MT radial arrays in vivo.
MATERIALS AND METHODS
Melanophore Cultures and Microsurgery
Cultures of black tetra melanophores were prepared as described in Rodionov et al. (1994). To prepare melanophore fragments, melanophore with elongated processes were selected, and the processes were dissected with microneedles with a tip diameter of ∼0.1 μm. Dynein-dependent aggregation of pigment granules in the fragments was induced with 0.5 μM adrenalin or 0.8 μM melanin-concentrating hormone. To activate kinesin-dependent movement of pigment granules, fragments were washed to remove adrenalin and treated with 8-bromo-cAMP (1 mM) and 3-isobutyl-1-methylxanthine (IBMX) (5 mM). To disrupt actin filaments, fragments were treated with latrunculin A (5 μM) as described in Rodionov et al. (1998).
Image Acquisition and Analysis
Live imaging of MTs in the fragments was performed as described in Rodionov et al. (2001)). For fluorescence labeling of MTs, melanophores were injected with Cy3-tubulin before the dissection of fragments. Fluorescence microscopy was performed using a Nikon Diaphot 300 inverted microscope equipped with Plan ×100 1.25 numerical aperture objective. Images of microtubules were captured with a slow-scan back-illuminated cooled charge-coupled device camera (CH350; Roper Scientific, Trenton, NJ) driven by MetaMorph imaging software (Universal Imaging, Downingtown, PA).
Protein Purification
Tubulin was isolated from porcine brain by two cycles of disassembly and reassembly (Borisy et al., 1975) followed by phosphocellulose chromatography (Weingarten et al., 1975). Cytoplasmic dynein was purified from bovine brain by using MT affinity purification protocol of Bingham et al. (1998) with minor modifications. Specifically, glycerol was excluded from homogenization buffer and 10 mg/ml glucose and 1 U/ml hexokinase were used instead of 5′-adenylylimidodiphosphonate to enhance binding of dynein to MTs. Kinesin was isolated from bovine brain by using initial batch ion exchange chromatography method as described in Hackney (1991).
Dynein Inactivation
To obtain heat-treated dynein, dynein preparation was incubated for 15 min in a boiling waterbath at 100°C and clarified by centrifugation for 5 min at 13,000 × g to remove the denatured proteins. For UV cleavage experiments, dynein preparation was supplemented with 50 μM Mg-ATP and 50 μM sodium orthovanadate and incubated on ice under UV light (365 nm) for 2 h (King, 1995).
Tubulin Polymerization Assays
Polymerization was carried out in BRB buffer (80 mM K-PIPES, pH 6.8, 1 mM MgCl2, 1 mM EGTA) containing 3.5 M glycerol and monitored turbidimetrically at 350 nm, 37°C, with a 1-cm path thermostated cell in a Shimatzu UV1601PC spectrophotometer. Initial rate of tubulin polymerization was determined from the turbidity curves by linear regression analysis. Normalized initial rate of tubulin polymerization was determined as a ratio of the initial polymerization rates of tubulin mixed with dynein or control proteins to pure tubulin. Critical concentration of tubulin polymerization was determined by MT sedimentation assay as described in Leguy et al. (2000). The size of the MT nucleus in the absence and presence of dynein was determined as described in Flyvbjerg et al. (1996), by using the formula n = 3(k + 2), where n is the size of the nucleus, and (k + 2) is the extrapolated value of the mean slope derived from a family of curves of tubulin polymerization at the increasing concentrations of tubulin. MT capping assay was performed by seeded MT assembly by using ethylene glycol bis[succinimidylsuccinate]-stabilized fluorescent MT seeds as described in Howard and Hyman (1993). The components were mixed in the following order: MT seeds were mixed with dynein preparation or buffer (for control experiments) and incubated at 37°C for 1 min. The mixture was then supplemented with the monomeric tubulin solution containing 10% of Cy3-labeled fluorescent tubulin and 1 mM GTP and incubated 50 min at 37°C. The resulting MTs were fixed with 0.5% glutaraldehyde in phosphate-buffered saline and pelleted onto glass coverslips by centrifugation at 3000 × g for 20 min. The length of MTs assembled on seeds was determined by the length measurement function of MetaMorph software.
Immunoblotting
Immunoblotting was performed by the method of Towbin et al. (1979), by using monoclonal anti-γ-tubulin antibody (Sigma-Aldrich, St. Louis, MO). Immunoreactive bands were detected with SuperSignal West Femto maximum sensitivity substrate (Pierce Chemical, Rockford, IL).
RESULTS
To study the role of dynein in the organization of MT radial arrays, we used the centrosome-free cytoplasmic fragments of fish melanophores. Our previous work showed that MT radial array formation in melanophore fragments involves dynein-dependent nucleation of MTs on the pigment granule aggregate (Vorobjev et al., 2001). This nucleation was found to be specifically associated with dynein activation and not with the clustering of pigment granules per se, suggesting a direct or indirect role for dynein in MT nucleation.
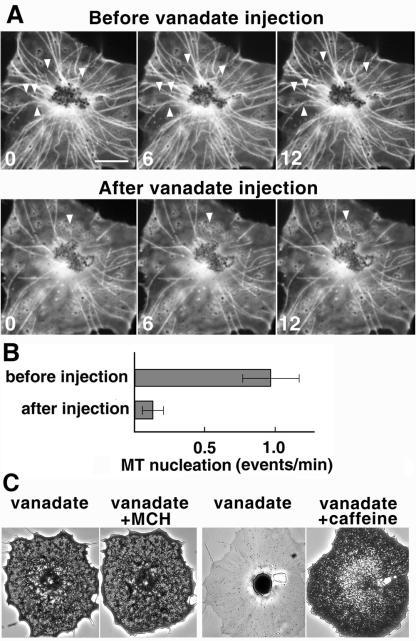
To test whether this nucleation depends on the activity of dynein, we injected melanophore fragments with sodium orthovanadate, which has been shown to inhibit dynein activity in vitro and in vivo (Beckerle and Porter, 1982; Merdes et al., 2000). Injection of vanadate dramatically affected the ability of pigment aggregate to nucleate MTs (Figure 1, A and B), as seen by the reduced number of MTs and the decreased rate of events of MT outgrowth from the pigment mass. Control experiments confirmed that, consistent with previous observations (Beckerle and Porter, 1982), injection of vanadate into black tetra melanophores specifically inhibited dynein-dependent pigment aggregation, but not kinesin- and myosin-dependent pigment dispersion (Figure 1C). Thus, MT nucleation on pigment granules depends on the activity of dynein.
Figure 1.
MT nucleation on pigment granules in melanophore fragments depends on the activity of dynein. (A) Time series of fluorescence images of MTs nucleating from the pigment aggregate in melanophore fragments stimulated with melanin concentrating hormone to induce pigment aggregation. The same fragment was observed before (top row) and after (bottom row) the injection of 0.5 mM needle concentration of sodium orthovanadate. Arrows indicate new MT ends emerging from the aggregate. Numbers indicate time in seconds. Bar, 10 μm. (B) Quantification of MT nucleation events per minute of observation, seen as the emergence of new MT ends. The rate of nucleation events reduces dramatically upon injection of sodium orthovanadate. (C) Phase contrast images of intact fish melanophores injected with sodium orthovanadate and stimulated for pigment aggregation (pair on the left) or pigment dispersion (pair on the right). Injection of sodium orthovanadate into intact melanophores inhibits pigment aggregation but not pigment dispersion.
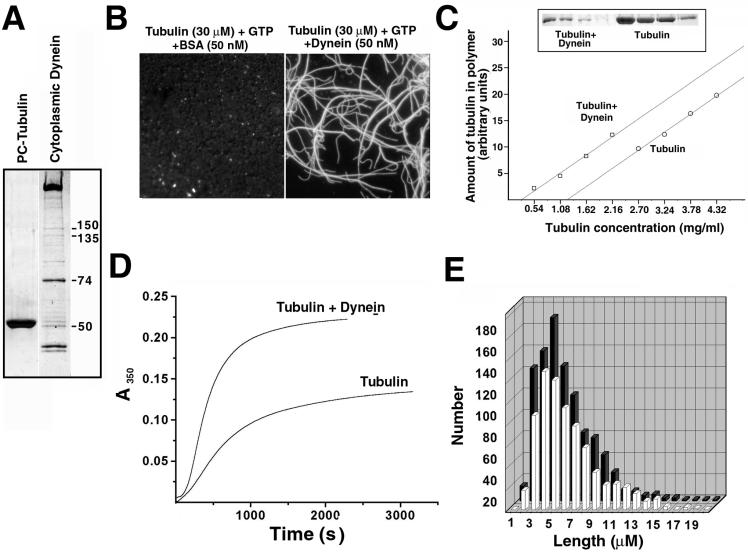
To test whether dynein can induce MT nucleation in vitro, we purified dynein from bovine brain and mixed it with purified tubulin in various nucleation assays (Figure 2). Our results show that dynein can indeed nucleate MTs as seen in various types of assays (Fig. 2, B–E). Addition of 50 nM dynein to a mixture of fluorescent tubulin and GTP induced visible formation of MT polymers not seen in the pure tubulin preparation or a mixture of tubulin with 50 nM bovine serum albumin (BSA) (Figure 2B). Quantification showed that critical concentration for tubulin polymerization reduced from 1.2 mg/ml for pure tubulin to 0.4 mg/ml for tubulin/dynein mixture (Figure 2C). The rate of MT polymerization in the presence of glycerol increased almost threefold upon addition of dynein to the tubulin preparation (Figure 2D). The average length of MTs in the dynein–tubulin mixture decreased from 5.9 to 4.7 μM (Figure 2E). The average size of the nucleus, determined by the kinetic analysis of the light scattering curves at different tubulin concentrations, decreased from 6.5 to 3.5 tubulin dimers in the presence of dynein. These results show that dynein is capable to nucleate MTs in vitro.
Figure 2.
Cytoplasmic dynein induces MT nucleation in vitro. (A) SDS-PAGE of the preparations of tubulin (PC tubulin) and cytoplasmic dynein used for the nucleation experiments. Marked in the dynein preparation are the molecular weights of dynein IC (74) and the components of the dynactin complex. (B) Fluorescence images of Cy3-labeled tubulin in the presence of 50 nM BSA (left) or 50 nM dynein (right). Addition of dynein but not BSA induces rapid tubulin polymerization. (C) Measurements of the critical concentration of tubulin polymerization in the presence and absence of dynein. Top, SDS-PAGE of MT pellets at different tubulin concentrations in the presence (left) and absence (right) of 46 nM dynein. Bottom, plots of the amount of polymeric tubulin versus tubulin concentration in the mixture in the presence (left) and absence (right) of dynein. Addition of dynein reduces the critical concentration of tubulin polymerization from 1.2 to 0.4 mg/ml. (D) Light scattering curves of tubulin (15 μM) polymerization in the absence and presence of 46 nM dynein in the presence of 3.5 M glycerol. Addition of dynein increases the rate of tubulin polymerization almost threefold. (E) Histograms of the length distributions of MTs in the presence (black) and absence (white) of 46 nM dynein. Addition of dynein decreases the average length of MTs from 5.9 to 4.7 μM.
To ensure that the observed effect is the property of dynein itself and not a contaminating MT nucleating factor in the dynein preparation, we tested for the presence of microtubule-associated proteins (reviewed in Olmsted, 1986) and γ-tubulin (reviewed in Doxsey, 2001) that have been shown to induce MT nucleation. Heat treatment of the dynein preparation by incubation at 100°C for 15 min, the conditions that have been shown to preserve the heat-stable brain microtubule-associated proteins and retain their capacity to nucleate MTs while inactivating most of the other brain proteins, abolished the MT nucleating capacity of the dynein preparation (Figure 3A). Immunoblotting with a monoclonal antibody against γ-tubulin has revealed no γ-tubulin immunoreactivity in the dynein preparation (Figure 3B). Thus, the observed MT nucleation is a specific property of dynein.
Figure 3.
Dynein MT nucleation depends on dynein and not the contaminating MT nucleation factors. (A) Addition of intact, but not heat-treated dynein, increases the normalized initial rates of tubulin polymerization more than twofold. (B) Immunoblotting of bovine brain extract (right) and a preparation of bovine brain dynein (left) with a monoclonal anti-γ-tubulin antibody. Unlike brain extract, the preparation of dynein shows no γ-tubulin immunoreactivity.
Next, we tested the effect of dynein activity on the rate of dynein-induced MT polymerization in vitro (Figure 4). Because dynein has a MT-dependent ATPase activity, we first checked how its MT nucleation capability is affected by the addition of ATP. We found that in the presence of 1 mM ATP, similar to its physiological concentration, dynein nucleating capacity is slightly reduced as seen by the decreased rate of MT polymerization (Figure 4A). This decrease may be explained by the ATP-induced release of dynein from the forming MTs. Dynein MT nucleation in the absence or presence of ATP is significantly higher than in the presence of ADP and sodium orthovanadate, which completely inhibit dynein activity. Therefore, MT nucleation by dynein in vitro, like in vivo, depends on dynein activity.
Figure 4.
Dynein dependent MT nucleation in vitro depends on the activity of dynein. (A) Changes in the light scattering curves of tubulin (17 μM) polymerization upon addition of 46 nM dynein in the presence of 1 mM Mg-ATP, or 1 mM ADP and 10 μM sodium orthovanadate. Addition of ATP somewhat lowers dynein nucleating activity, whereas addition of ADP and vanadate abolishes it, indicating that dynein MT nucleation requires dynein activity. (B) Normalized initial rates of MT polymerization quantified from the curves in A. Gel inset shows the corresponding MT pellets; gel densitometry results are similar to the normalized initial rates and show more than twofold stimulation of MT assembly by dynein and dynein in the presence of ATP, but not ADP and vanadate.
Because our dynein preparation also contained the dynactin complex, which interacts with tubulin independently of dynein (Waterman-Storer et al., 1995), we next tested whether the dynactin component contributes to the dynein nucleation ability. Dynein MT nucleation rate did not change upon addition of dynactin antibody that blocks the tubulin-binding site of dynactin (King and Schroer, 2000) (Figure 5A). To test which of the two components of the dynein/dynactin complex possesses MT nucleation capability, we separated dynein and dynactin by MonoQ chromatography and tested their independent abilities to nucleate MTs (Figure 5B). We found that although dynein MT-nucleating activity was slightly reduced in the absence of dynactin, similar concentration of dynactin alone had no significant MT nucleation capacity. Therefore, dynein and not dynactin contributes the major MT nucleation activity.
Figure 5.
Dynein nucleating capacity is independent of dynactin. (A) Normalized initial rates of tubulin (11 μM) polymerization show similar increase upon addition of dynein or a mixture of dynein and an antibody to the p150 subunit of dynactin that inhibits the interaction of dynactin with MTs. (B) Dynein but not dynactin induces MT nucleation. Left, SDS-PAGE of the biochemically separated preparations of dynactin and dynein complexes. Right, normalized initial rates of tubulin polymerization does not change upon addition of dynactin, but increases upon addition of dynein.
Because other known MT nucleating factors, such as γ-tubulin, are able to cap MT minus ends (Leguy et al., 2000; Wiese and Zheng, 2000), we next tested whether dynein also has the minus end-capping ability, by observing MT polymerization in the seeded assembly assays. In the absence of dynein, MT growth rate plotted against the monomeric tubulin concentration was significantly higher than in the presence of dynein (Figure 6A), consistent with MT growth from one end only in the presence of dynein. To confirm this result, we assembled fluorescently labeled MTs on ethylene glycol bis[succinimidylsuccinate]-stabilized fluorescent seeds and determined the amount of MTs elongated on one end only in the absence and presence of dynein. We found that in dynein-free mixture, ∼40% of MTs elongated on one end only, and in the presence of dynein this amount increased to >80% (Figure 6B), indicating that dynein indeed prevents polymerization on one of the MT ends. To confirm that this effect represents capping of the minus end, we quantified the length distribution for plus and minus ends of the MTs grown on fluorescently labeled seeds in the dynein-containing mixture and compared it with the length of MT ends grown on one-ended seeds in the same mixture. Average length of the shorter (minus) ends on the control seeds was 36.1 ± 9.28 μm and the longer (plus) ends were on average 99.8 ± 26.7 μm in length. The average length of the MT assembled on one end of the seeds only was 85.7 ± 28.8 μm, similar to the length of the plus ends, indicating that the minus ends of such seeds were capped and could not serve as the assembly sites. Therefore, dynein, like other MT nucleating factors, has the ability to cap MT minus ends.
Figure 6.
Dynein caps MT minus ends. (A) Seeded rate of MT growth is significantly higher in the absence of dynein, consistent with MT growth on both ends in the absence of dynein and on only one end in the presence of dynein. (B) Top, images of the representative MTs assembled on fluorescent seeds in the absence (left) and presence (right) of dynein show that in the dynein-containing preparation MTs assemble predominantly on one end of the seed, whereas in the absence of dynein the majority of MTs assemble on both ends of the seed. Bottom, growth of MTs on fluorescent seeds indicates a significant increase in the percentage of seeds elongated on one end only in the presence of dynein compared with control. Average length of MT ends on such seeds was similar to the length of the longer (plus) ends in “uncapped” seeds, indicating that such seeds were capped on the minus ends.
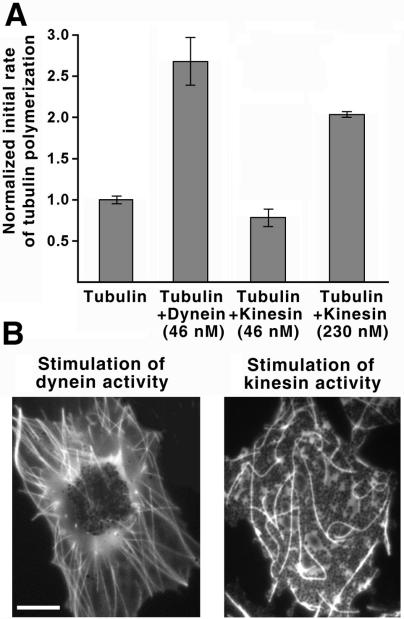
Next, we asked the question whether other MT motors, such as kinesin, are also capable of MT nucleation. We found that addition of 46 nM of purified bovine brain kinesin (the concentration similar to that in which dynein increases MT nucleation 2.5-fold compared with pure tubulin) did not cause any increase of MT nucleation (Figure 7A). Some increase of MT nucleation (2-fold) was observed in the presence of kinesin at 5 times higher concentration (230 nM) (Figure 7A), indicating that kinesin is an extremely weak MT nucleator compared with dynein. We conclude that the ability to induce MT nucleation is a specific property of dynein.
Figure 7.
Kinesin, unlike dynein, has weak MT nucleating activity and is unable to induce MT radial array formation. (A) Normalized initial rate of tubulin (11 μM) polymerization increases threefold in the presence of 46 nM dynein and does not change in the presence of 46 nM kinesin. Addition of 230 nM kinesin induces a weaker (2-fold) increase in the rate of tubulin polymerization, indicating its poor ability to nucleate MTs. (B) Images of fluorescent MTs in melanophore fragments treated with latrunculin A for actin filament disassembly and stimulated with adrenalin (left) to induce dynein-dependent pigment aggregation or 8-bromo-cAMP/IBMX (right) to stimulate kinesin-dependent pigment dispersion. Dynein stimulation induces the formation of a MT radial array, whereas kinesin stimulation does not. Bar, 10 μm.
It has been shown that in vitro both kinesin and dynein can organize taxol-stabilized MTs into focused radial arrays of opposite polarity, plus ends outward in the case of dynein, and minus ends outward in the case of kinesin (Nedelec et al., 1997; Surrey et al., 2001). In vivo, however, the situation is more complex, because MTs are dynamic and MT nucleation itself can play a role in radial array formation. To test whether the nucleating capacity of a motor is important for the radial array formation, we compared the ability of kinesin and dynein to organize MTs into radial arrays in the cytoplasmic fragments of melanophores, by treating them with pigment aggregation or dispersion stimuli that activate dynein or kinesin MT-based transport, respectively. To exclude the interference of AF-dependent transport, which is active during pigment dispersion, we treated the melanophore fragments with actin-disrupting drug latrunculin A (Rodionov et al., 1998). Stimulation of pigment aggregation and dynein activity by adrenalin induced concentration of pigment granules at the center of the melanophore fragment and the formation of MT radial array that extended from the pigment aggregate to the periphery of the fragment (Figure 7B, left). Stimulation of pigment dispersion by a combined treatment with 8-bromo-cAMP and IBMX, which induces constitutive activation of kinesin transport and pigment hyperdispersion in intact cells (Rodionov et al., 2003), failed to result in the formation of orderly MT array (Figure 7B, right). Therefore kinesin, unlike dynein, is not capable to induce radial array formation, possibly due to its inability to induce MT nucleation.
DISCUSSION
Our results demonstrate that dynein, besides its MT motor activity, is capable to nucleate MTs and that this ability depends on its properties as the MT-activated ATPase. We have previously shown that the combination of minus end-directed MT transport and MT nucleation on pigment granules in melanophore fragments is sufficient to organize MTs into a radial array (Vorobjev et al., 2001). Here, we show that besides its known role in minus-end directed transport, dynein also possesses MT nucleation capacity and thus can contribute both activities necessary and sufficient to organize MT arrays in vivo.
It has been previously shown that dynein can bind to tubulin dimers when mixed at a high molar ratio with tubulin in solution (Haimo and Fenton, 1988). This capacity of dynein to induce interaction between tubulin dimers and stimulate MT assembly is in good agreement with our discovery of cytoplasmic dynein MT nucleating ability. If the two tubulin binding sites of a dynein molecule were both occupied with tubulin monomers, this could reduce the critical size of the microtubule nucleus and thus support polymerization as depicted in Figure 8.
Figure 8.
Dynein activity is necessary and sufficient to organize MTs into radial arrays in vivo. Dynein molecules (black) are bound to organelles or particles and transport them to the MT minus ends. In addition, dynein molecules can induce MT nucleation by capturing two tubulin dimers that can serve as sites of new MT assembly.
The newly discovered property of cytoplasmic dynein in MT nucleation makes it a unique molecule that possesses all the necessary and sufficient properties for organizing MT radial arrays in vivo. In melanophore fragments in the absence of centrosome, dynein transports pigment granules to the MT minus ends and remains there, providing nucleation sites for new MTs that outgrow from the forming pigment aggregate (Figure 8). Thus, it likely contributes both components of the MT radial array organization mechanism, MT-based pigment granule transport and MT nucleation on the granules. We believe that dynein acts by similar mechanisms in other centrosome-free systems, combining MT minus end-directed transport of the nucleation sites and MT nucleation at the resulting organizing centers. Indeed, it has been shown that the formation of centrosome-free MT arrays in meiotic spindles and Xenopus egg extracts depends on the activity of MT minus end motors (Heald et al., 1996; Matthies et al., 1996). It has been suggested that the role of the MT minus end motors in this process is to tether the MT minus ends into focused spindle poles. Our data suggest that the role of dynein in this process may be more extensive and that it not only tethers the MTs together but also provides the new nucleation sites at the forming spindle poles. Remarkably, MT minus end motor ncd, which is involved in the organization of the meiotic centrosome-free spindle poles in Drosophila oocytes, also possesses MT nucleating activity (Karabay and Walker, 1999; Highsmith et al., 2001). Furthermore, such spindle poles contain no detectable γ-tubulin (Matthies et al., 1996). Therefore, it is possible that ncd contributes MT nucleation as well as the tethering activity and that in other centrosome-free systems cytoplasmic dynein plays the same role.
Our data suggest that besides contributing to the formation of the noncentrosomal MT arrays, dynein may play an additional role in the formation and maintenance of the centrosomal MT arrays. Numerous studies have demonstrated that the role of dynein in the maintenance of the integrity of the centrosome, spindle poles, and radial MT arrays is critically important. Mutations affecting dynein function or dissociation of dynein adapter dynactin disrupt MT arrays in interphase cells (Koonce et al., 1999; Ma et al., 1999; Quintyne et al., 1999; Quintyne and Schroer, 2002). In mitosis, disruption of dynein/dynactin induces dissociation of the otherwise active centrosome from the mitotic spindle (Echeverri et al., 1996; Gaglio et al., 1997). It has been shown that dynein delivers to the MT minus ends MT nucleation sites containing pericentrin and γ-tubulin in interphase cells (Young et al., 2000), or dynactin/NuMa complexes involved in spindle pole formation during mitosis (Merdes et al., 2000). Dynein-dependent transport of MTs to the mitotic spindle poles has been observed in vivo (Rusan et al., 2002). Together, these studies strongly suggest that dynein-dependent delivery of centrosomal components, MT nucleation sites, and MTs themselves is essential for the formation and maintenance of the centrosomal MT arrays in interphase and mitosis. Our data suggest a new role for dynein in the formation and maintenance of the centrosomal arrays by serving as a MT nucleation factor. Indeed, dynein accumulates at the centrosome when cells approach mitosis (G2/M transition), which requires massive MT nucleation at the centrosome (Quintyne and Schroer, 2002).
It has been shown that dynein pulling activity at the cell cortex is responsible for the centrosome positioning in interphase cells (Burakov et al., 2003). Added to the ability of dynein to serve as a MT organizing center and nucleation site, it makes dynein a universal factor that alone can organize and maintain the entire MT array in a living cell. Future studies will show the exact contribution of each of these dynein properties to the functioning of MT radial arrays in vivo.
Acknowledgments
This work was supported by the National Institutes of Health grant GM-62290, to V.I.R.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-10-0770. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-10-0770.
References
- Beckerle, M.C., and Porter, K.R. (1982). Inhibitors of dynein activity block intracellular transport in erythrophores. Nature 295, 701-703. [DOI] [PubMed] [Google Scholar]
- Bingham, J.B., King, S.J., and Schroer, T.A. (1998). Purification of dynactin and dynein from brain tissue. Methods Enzymol. 298, 171-184. [DOI] [PubMed] [Google Scholar]
- Borisy, G.G., Marcum, J.M., Olmsted, J.B., Murphy, D.B., and Jonson, K.A. (1975). Purification of tubulin and associated high molecular weight proteins from porcine brain and characterization of microtubule assembly in vitro. Ann. N.Y. Acad. Sci. 253, 107-132. [DOI] [PubMed] [Google Scholar]
- Burakov, A., Nadezhdina, E., Slepchenko, B., and Rodionov, V. (2003). Centrosome positioning in interphase cells. J. Cell Biol. 162, 963-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton, D. (1998). Focusing on spindle poles. J. Cell Sci. 111, 1477-1481. [DOI] [PubMed] [Google Scholar]
- Doxsey, S. (2001). Re-evaluating centrosome function. Nat. Rev. Mol. Cell. Biol. 2, 688-698. [DOI] [PubMed] [Google Scholar]
- Echeverri, C.J., Paschal, B.M., Vaughan, K.T., and Vallee, R.B. (1996). Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 132, 617-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flyvbjerg, H., Jobs, E., and Leibler, S. (1996). Kinetics of self-assembling microtubules: an “inverse problem” in biochemistry. Proc. Natl. Acad. Sci. USA 93, 5975-5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio, T., Dionne, M.A., and Compton, D.A. (1997). Mitotic spindle poles are organized by structural and motor proteins in addition to centrosomes. J. Cell Biol. 138, 1055-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney, D.D. (1991). Isolation of kinesin using initial batch ion exchange. Methods Enzymol. 196, 175-181. [DOI] [PubMed] [Google Scholar]
- Haimo, L.T., and Fenton, R.D. (1988). Interaction of Chlamydomonas dynein with tubulin. Cell Motil. Cytoskeleton 9, 129-139. [DOI] [PubMed] [Google Scholar]
- Heald, R., Tournebize, R., Blank, T., Sandaltzopoulos, R., Becker, P., Hyman, A., and Karsenti, E. (1996). Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature 382, 420-425. [DOI] [PubMed] [Google Scholar]
- Highsmith, S., Thoene, M., Sablin, E., and Polosukhina, K. (2001). NCD activation of tubulin polymerization. Biophys. Chem. 92, 127-139. [DOI] [PubMed] [Google Scholar]
- Howard, J., and Hyman, A.A. (1993). Preparation of marked microtubules for the assay of the polarity of microtubule-based motors by fluorescence microscopy. Methods Cell Biol. 39, 105-110. [DOI] [PubMed] [Google Scholar]
- Hyman, A.A. (2000). Centrosomes: sic transit gloria centri. Curr. Biol. 10, R276-R278. [DOI] [PubMed] [Google Scholar]
- Karabay, A., and Walker, R.A. (1999). The ncd tail domain promotes microtubule assembly and stability. Biochem. Biophys. Res. Commun. 258, 39-43. [DOI] [PubMed] [Google Scholar]
- Karsenti, E., and Vernos, I. (2001). The mitotic spindle: a self-made machine. Science 294, 543-547. [DOI] [PubMed] [Google Scholar]
- King, S.M. (1995). Vanadate-mediated photolysis of dynein heavy chains. Methods Cell Biol. 47, 503-506. [DOI] [PubMed] [Google Scholar]
- King, S.J., and Schroer, T.A. (2000). Dynactin increases the processivity of thecytoplasmic dynein motor. Nat. Cell Biol. 2, 20-24. [DOI] [PubMed] [Google Scholar]
- Koonce, M.P., Kohler, J., Neujahr, R., Schwartz, J.M., Tikhonenko, I., and Gerish, G. (1999). Dynein motor stabilizes interphase microtubule arrays and determines centrosome position. EMBO J. 18, 6786-6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leguy, R., Melki, R., Pantaloni, D., and Carlier, M.-F. (2000). Monomeric γ-tubulin nucleates microtubules. J. Biol. Chem. 275, 21975-21980. [DOI] [PubMed] [Google Scholar]
- Ma, S., Trivinos-Lagos, L., Graf, R., and Chisholm, R.L. (1999). Dynein intermediate chain mediated dynein-dynactin interaction is required for interphase microtubule organization and centrosome replication and separation in Dictyostelium. J. Cell Biol. 147, 1261-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies, H.J., McDonald, H.B., Goldstein, L.S., and Theurkauf, W.E. (1996). Anastral meiotic spindle morphogenesis: role of the non-claret disjunctional kinesin-like protein. J. Cell Biol. 134, 455-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes, A., and Cleveland, D.W. (1997). Pathways of spindle pole formation: different mechanisms; conserved components. J. Cell Biol. 138, 953-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes, A., Heald, R., Samejima, K., Earnshaw, W.C., and Cleveland, D.W. (2000). Formation of spindle poles by dynein/dynactin-dependent transport of NuMa. J. Cell Biol. 149, 851-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelec, F.J., Surrey, T., Maggs, A.C., and Leibler, S. (1997). Self-organization of microtubules and motors. Nature 389, 305-308. [DOI] [PubMed] [Google Scholar]
- Olmsted, J.B. (1986). Microtubule-associated proteins. Annu. Rev. Cell Biol. 2, 421-457. [DOI] [PubMed] [Google Scholar]
- Quintyne, N.J., Gill, S.R., Eckley, D.M., Crego, C.L., Compton, D.A., and Schroer, T.A. (1999). Dynactin is required for microtubule anchoring at the centrosomes. J. Cell Biol. 147, 321-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintyne, N.J., and Schroer, T.A. (2002). Distinct cell cycle-dependent roles for dynactin and dynein at centrosomes. J. Cell Biol. 159, 245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusan, N.M., Tulu, U.S., Fagerstrom, C., and Wadsworth, P. (2002). Reorganization of the microtubule array in prophase/prometaphase requires cytoplasmic dynein-dependent microtubule transport. J. Cell Biol. 158, 997-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov, V.I., and Borisy, G.G. (1997) Self-centering activity of cytoplasm. Nature 386, 170-173. [DOI] [PubMed] [Google Scholar]
- Rodionov, V.I., Hope, A., Svitkina, T., and Borisy, G.G. (1998). Functional coordination of microtubule-based and actin-based motility in melanophores. Curr. Biol. 8, 165-168. [DOI] [PubMed] [Google Scholar]
- Rodionov, V.I., Lim, S.-S., Gelfand, V.I., and Borisy, G.G. (1994). Microtubule dynamics in fish melanophores. J. Cell Biol. 126, 1455-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov, V.I., Nadezhdina, E., Peloquin, J., and Borisy, G. (2001). Digital fluorescence microscopy of cell cytoplasts with and without the centrosome. Methods Cell Biol. 67, 43-51. [DOI] [PubMed] [Google Scholar]
- Rodionov, V., Yu, J., Kashina, A., Oladipo, A., and Gross, S.P. (2003). Switching between microtubule- and actin-based transport systems in melanophores is controlled by cAMP levels. Curr. Biol. 13, 1-20. [DOI] [PubMed] [Google Scholar]
- Surrey, T., Nedelec, F. Leibler, S., and Karsenti, E. (2001). Physical properties determining self-organization of motors and microtubules. Science 292, 1167-1171. [DOI] [PubMed] [Google Scholar]
- Towbin, H., Staehelin, T., and Gordon, J. (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76, 4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma, M.C., and Gelfand, V.I. (1999). Molecular mechanisms of pigment transport in melanophores. Pigment Cell Res. 12, 283-294. [DOI] [PubMed] [Google Scholar]
- Vorobjev, I., Malikov, V., and Rodionov, V. (2001). Self-organization of a radial microtubule array by dynein-dependent nucleation of microtubules. Proc. Natl. Acad. Sci. USA 98, 10160-10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer, C.M., Karki, S., and Holzbaur, E.L.F. (1995). The p150Glued component of dynactin complex binds to both microtubules and the actin-related protein centractin. Proc. Nat. Acad. Sci. USA 92, 1634-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten, M.D., Lockwood, A.H., Hwo, S.Y., and Kirschner, M.W. (1975). A protein factor essential for microtubule assembly. Proc. Natl. Acad. Sci. USA 72, 1858-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese, C., and Zheng, Y. (2000). A new function for the gamma-tubulin ring complex as a microtubule minus-end cap. Nat. Cell Biol. 2, 358-364. [DOI] [PubMed] [Google Scholar]
- Young, A., Dictenberg, J., Purohit, A., Tuft, R., and Doxsey, S. (2000). Cytoplasmic dynein-mediated assembly of pericentrin and γ-tubulin onto centrosomes. Mol. Biol. Cell 11, 2047-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]