Abstract
Background.
The efficacy of ventriculolumbar perfusion (VLP) chemotherapy with methotrexate (MTX) was evaluated for treatment of leptomeningeal carcinomatosis (LMC).
Methods.
The primary outcome was the response rate of increased intracranial pressure (ICP), which was available for comparison from historical data on conventional intraventricular chemotherapy. Secondary endpoints were response rates of other LMC symptoms and overall survival of patients. Artificial cerebrospinal fluid (CSF) premixed with MTX was continuously perfused intraventricularly through a preinstalled intraventricular reservoir and drained via lumbar catheter for 72 hours. The VLP was repeated twice at 3-day intervals for each cycle.
Results.
Forty-five of 65 patients had increased ICP, and 32 patients (71%) showed response after VLP chemotherapy, including 31 patients with normalization of ICP. Altered mentation improved in 7 of 21 patients (33%). Cauda equina symptoms responded in 5 of 27 patients (19%), including 4 patients who became ambulatory from a bedridden state. Median overall survival was 187 days, and the 1-year survival rate was 27%. All side effects, including nausea, vomiting, confusion, and sleep disturbance, were tolerable and transient except for two cases of CSF infection.
Conclusion.
VLP chemotherapy with MTX provided better control of increased ICP, improved symptom response, and prolonged survival at a cost of acceptable toxicity in patients with LMC.
Author Summary
Discussion
The effectiveness of intracerebrospinal fluid chemotherapy for patients with leptomeningeal carcinomatosis (LMC) is doubted, considering marginal survival benefit and poor symptom improvement [1–4]. Cerebrospinal fluid (CSF) flow disturbance, which occurs in more than half of patients with LMC, makes CSF chemotherapy ineffective by hindering even distribution of the injected drug and results in hydrocephalus with increased intracranial pressure (ICP) [5–7].
The potential benefits of ventriculolumbar perfusion (VLP) chemotherapy are uniform drug distribution throughout the CSF space, even under conditions of disturbed CSF flow, and increased cancer-cell killing by enforced drug perfusion [8, 9].
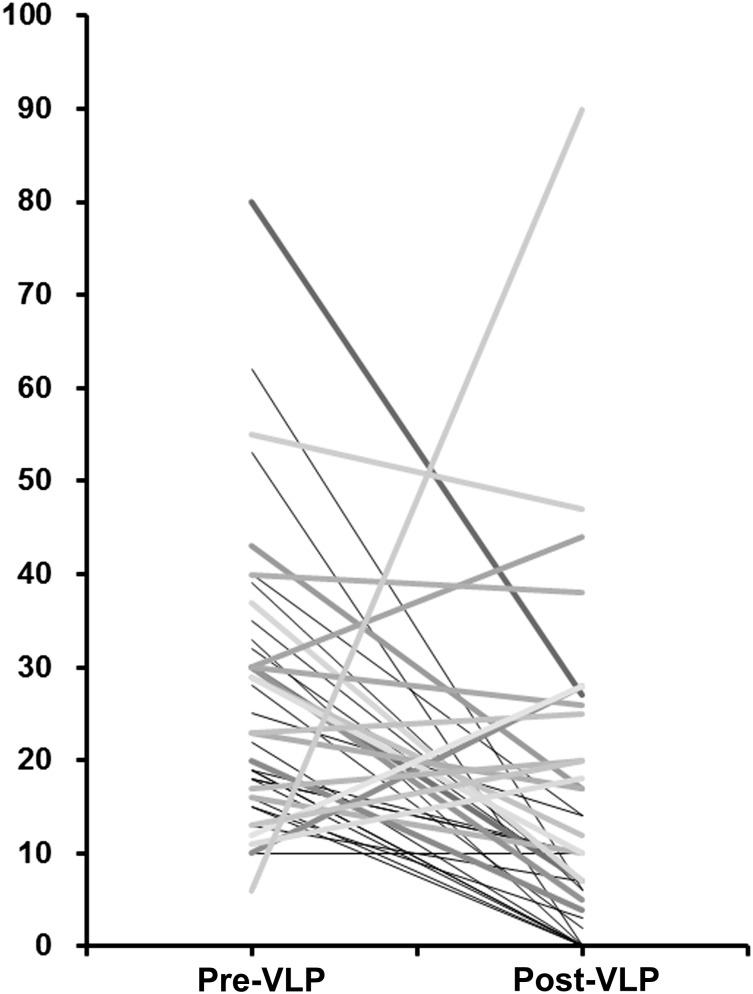
In this phase II study, the primary endpoint was a controlled rate of increased ICP, which was an objective measurement for comparison. VLP chemotherapy with methotrexate (MTX) showed remarkable improvement of increased ICP (71%), altered mentation (33%), and cauda equina symptoms (19%) at a cost of a few, usually transient complications. Wasserstrom et al. reported improvement of increased ICP in 15 of 64 LMC patients (23%) after radiation plus intraventricular chemotherapy [10]. In our previous study of patients with LMC from non-small cell lung cancer (NSCLC) treated by intraventricular chemotherapy, 20 of 69 patients (29%) with increased ICP achieved normal ICP [11]. In this phase II trial, 31 of 41 patients (76%) with increased ICP at the start of VLP were normalized (Fig. 1), and the superiority of VLP in terms of ICP control was statistically significant (chi-square test, p < .001).
Figure 1.
Distribution of intracranial pressure. The distribution of individual patients’ intracranial pressure (cm H2O) is shown before (left) and after (right) VLP chemotherapy with methotrexate. Gray scales differentiate individual patients.
Abbreviation: VLP, ventriculolumbar perfusion.
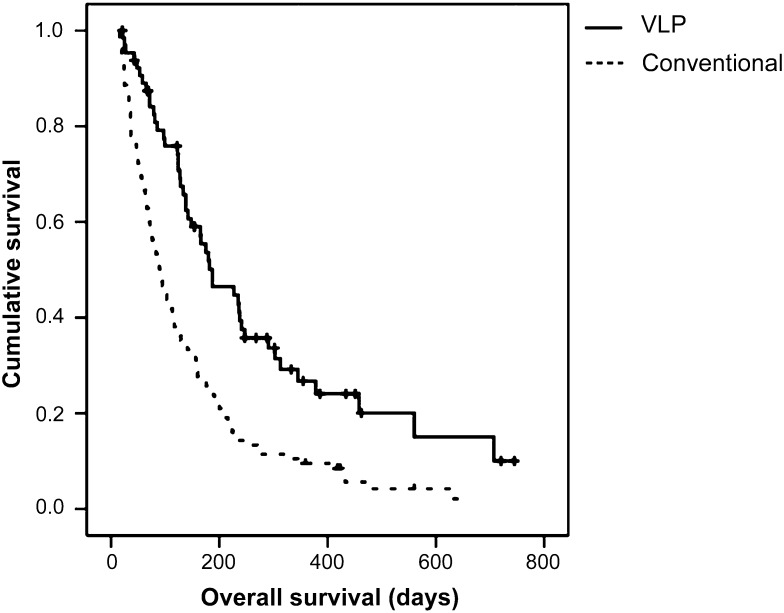
The survival of patients with LMC is affected greatly by the primary cancer diagnosis [10–15]. We were able to retrieve institutional data of overall survival for LMC from NSCLC patients treated with a median of five rounds of conventional intraventricular chemotherapy [11]. In comparison, VLP treatment significantly prolonged patient survival from a median of 89 days with conventional intraventricular chemotherapy to a median of 187 days for NSCLC patients with VLP (Fig. 2).
Figure 2.
Comparison of overall survival time of VLP-treated non-small cell lung cancer patients (n = 51) versus conventional intraventricular chemotherapy-treated patients (n = 105). Data published in [11].
Abbreviation: VLP, ventriculolumbar perfusion.
The technical complexities of VLP and the high incidence of side effects limit its widespread use. Our technical advances included use of a noncollapsible Chemoport, instead of an Ommaya reservoir, to ensure stable needle position with the designated hooked needle [16], and a reduced perfusion rate of 20 mL/hour resulted in more tolerable constitutional side effects than the previously used perfusion rate of 40 mL/hour [17].
Supplementary Material
Footnotes
Access the full results at: Lee-14-199.theoncologist.com
ClinicalTrials.gov Identifier: KCT0000082
Sponsor(s): None
Principal Investigator: Ho-Shin Gwak
IRB Approved: Yes
Author disclosures and references available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.