Abstract
Background.
EGFR and Src are frequently activated in non-small cell lung cancer (NSCLC). In preclinical models, combining EGFR and Src inhibition has additive synergistic effects. We conducted a phase I/II trial of the combination of Src inhibitor dasatinib with EGFR inhibitor erlotinib to determine the maximum tolerated dose (MTD), pharmacokinetic drug interactions, biomarkers, and efficacy in NSCLC.
Methods.
The phase I 3+3 dose-escalation study enrolled patients with solid tumors to determine the MTD. The phase II trial enrolled patients with advanced NSCLC who had undergone no previous treatments to determine progression-free survival (PFS) and response. Pharmacokinetic and tissue biomarker analyses were performed.
Results.
MTD was 150 mg of erlotinib and 70 mg of dasatinib daily based on 12 patients treated in the phase I portion. No responses were observed in phase I. The 35 NSCLC patients treated in phase II had an overall disease control rate of 59% at 6 weeks. Five patients (15%) had partial responses; all had activating EGFR mutations. Median PFS was 3.3 months. Epithelial-mesenchymal transition markers did not correlate with outcomes.
Conclusion.
The combination of erlotinib and dasatinib is safe and feasible in NSCLC. The results of this study do not support use of this combination in molecularly unselected NSCLC.
Author Summary
Discussion
One strategy to enhance the efficacy of EGFR inhibitors in NSCLC is to combine them with Src inhibitors. This combination has additive effects in NSCLC cell lines in vitro, with synergistic effects observed in EGFR mutant cells [1–4]. In addition, NSCLC with an epithelial phenotype may be more sensitive to EGFR inhibition, and the addition of Src inhibition may overcome EGFR inhibitor resistance in mesenchymal tumors [5–10]. These findings led to the initiation of this study combining erlotinib with the Src inhibitor dasatinib [11–13] in NSCLC patients to evaluate the clinical activity of this combination and to test predictive biomarkers and pharmacokinetic interactions.
We performed a phase I/II study of this combination. The phase I portion enrolled patients with advanced solid tumors with any number of previous therapies. The phase II portion included stage IV NSCLC. The primary objective of the phase I portion was to assess the safety and tolerability of the combination and to determine the appropriate dose for phase II. The primary objective of the phase II portion was to determine the antitumor activity of the combination in NSCLC patients. Tumor size was assessed by computed tomography at baseline and every 6 weeks. We used existing tissue to assess E-cadherin and vimentin expression by immunohistochemistry [14] and to detect mutations in EGFR and KRAS by polymerase chain reaction, as described previously [14–16]. Erlotinib and dasatinib are both metabolized primarily by CYP3A4 [17–19]. Treatment with a CYP3A4 inhibitor or inducer increases or decreases exposure to both drugs, respectively [17, 18]. Dasatinib and erlotinib plasma concentrations were estimated, as described previously [20–22].
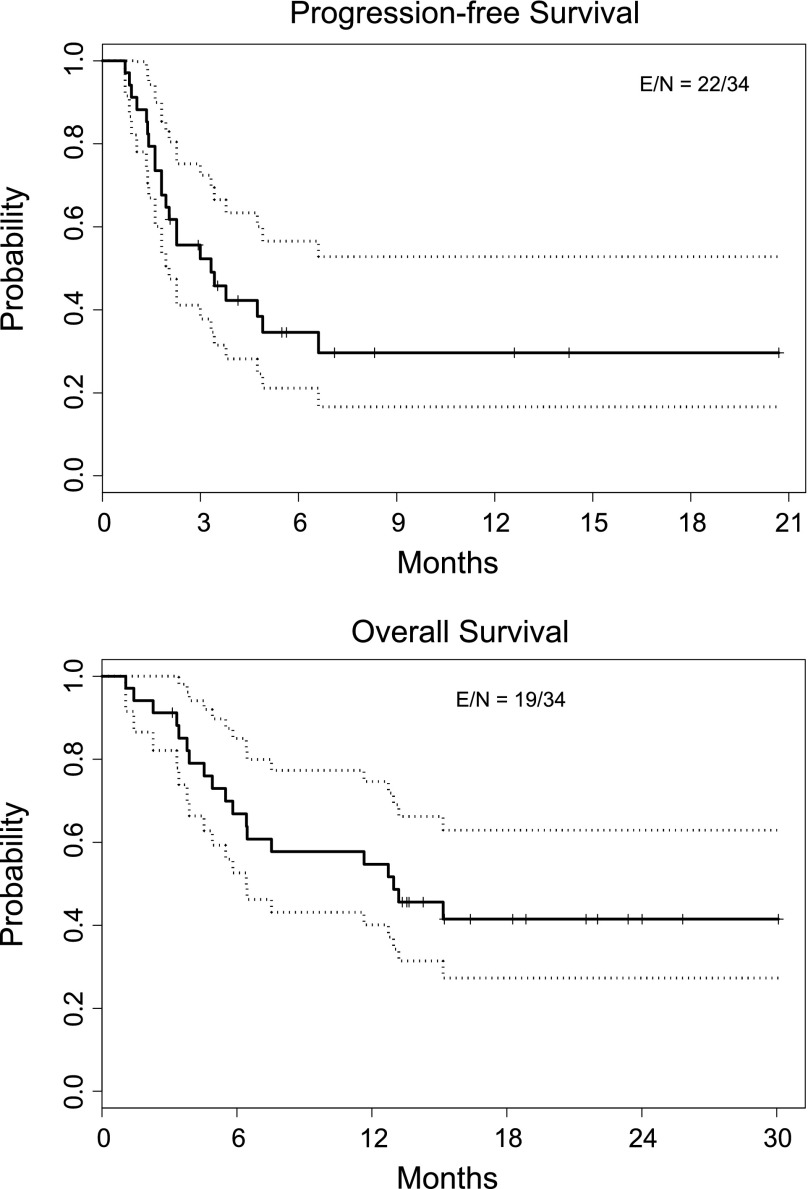
Based on 12 patients in phase I and in a similar study [23], we chose 150 mg of erlotinib and 70 mg of dasatinib daily for the phase II study. Toxic effects were consistent with those observed in previous studies with these agents. Five (15%) of 33 evaluable patients enrolled in the phase II study had partial responses, and no patients had complete responses. Fifteen patients (44%) had stable disease (Fig. 1). All responders had activating EGFR mutations; EGFR mutation status was associated with overall response rate (62% in mutant EGFR and 0% in wild-type EGFR, p = .005). Our pharmacokinetic results corroborate previous studies showing erlotinib-mediated inhibition of dasatinib clearance [24]. E-cadherin and vimentin expression did not correlate with efficacy. Our results do not support continued study of the combination of erlotinib and dasatinib in molecularly unselected NSCLC.
Figure 1.
Kaplan Meier Curves showing progression-free and overall survival durations for 34 evaluable patients in the phase II study.
Abbreviations: E, events (i.e., progression or death); N, total number of patients.
Supplementary Material
Footnotes
Access the full results at: Johnson-14-228.theoncologist.com
ClinicalTrials.gov Identifier: NCT00826449
Sponsor(s): Bristol-Myers Squibb and Astellas Pharmaceuticals
Principal Investigator: Faye M. Johnson
IRB Approved: Yes
For Further Reading:Brett Hughes, Linda Mileshkin, Peter Townley et al. Pertuzumab and Erlotinib in Patients With Relapsed Non-Small Cell Lung Cancer: A Phase II Study Using 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Imaging. The Oncologist 2014;19:175–176.
Abstract:
Background. Combination blockade of human epidermal growth factor receptor (HER) family signaling may confer enhanced antitumor activity than single-agent blockade. We performed a single-arm study of pertuzumab, a monoclonal antibody that inhibits HER2 dimerization, and erlotinib in relapsed non-small cell lung cancer (NSCLC).
Methods. Patients received pertuzumab (840-mg loading dose and 420-mg maintenance intravenously every 3 weeks) and erlotinib (150-mg or 100-mg dose orally, daily). The primary endpoint was response rate (RR) by 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) at day 56 in all patients and those with EGFR wild-type tumors.
Results. Of 41 patients, 28 (68.3%) experienced treatment-related grade ≥3 adverse events, including pneumatosis intestinalis (3 patients), resulting in early cessation of enrollment. Tissue samples from 32 patients showed mutated EGFR status in 9 of 41 (22%) and wild-type EGFR in 23 of 41 (56%). The FDG-PET RR for patients with assessments at day 56 was 19.5% in all patients (n = 41) and 8.7% in patients with wild-type EGFR NSCLC (n = 23). Investigator-assessed computed tomography RR at day 56 was 12.2%.
Conclusion. FDG-PET suggests that pertuzumab plus erlotinib is an active combination, but combination therapy was poorly tolerated, which limits its clinical applicability. More research is warranted to identify drug combinations that disrupt HER receptor signaling but that exhibit improved tolerability profiles.
Author disclosures and references available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.