Abstract
Background.
The need for preoperative chemoradiation or short-course radiation in all T3 rectal tumors is a controversial issue. A multicenter phase II trial was undertaken to evaluate the efficacy and safety of neoadjuvant capecitabine and oxaliplatin combined with bevacizumab in patients with intermediate-risk rectal adenocarcinoma.
Methods.
We recruited 46 patients with T3 rectal adenocarcinoma selected by magnetic resonance imaging (MRI) who were candidates for (R0) resection located in the middle third with clear mesorectal fascia and who were selected by pelvic MRI. Patients received four cycles of neoadjuvant capecitabine and oxaliplatin combined with bevacizumab (final cycle without bevacizumab) before total mesorectal excision (TME). In case of progression, preoperative chemoradiation was planned. The primary endpoint was overall response rate (ORR).
Results.
On an intent-to-treat analysis, the ORR was 78% (n = 36; 95% confidence interval [CI]: 63%–89%) and no progression was detected. Pathologic complete response was observed in nine patients (20%; 95% CI: 9–33), and T downstaging was observed in 48%. Forty-four patients proceeded to TME, and all had R0 resection. During preoperative therapy, two deaths occurred as a result of pulmonary embolism and diarrhea, respectively, and one patient died after surgery as a result of peritonitis secondary to an anastomotic leak (AL). A 13% rate of AL was higher than expected. The 24-month disease-free survival rate was 75% (95% CI: 60%–85%), and the 2-year local relapse rate was 2% (95% CI: 0%–11%).
Conclusion.
In this selected population, initial chemotherapy results in promising activity, but the observed toxicity does not support further investigation of this specific regimen. Nevertheless, these early results warrant further testing of this strategy in an enriched population and in randomized trials.
Author Summary
Discussion
To the best of our knowledge, this is the first multicenter phase II trial evaluating neoadjuvant systemic therapy without radiation in rectal cancer. The high-quality staging procedure with a central reviewer in this trial ensured a very homogeneous population. Early trials on rectal cancer have no validated early endpoints that could serve as surrogates for long-term outcome [1]. Consequently, we used radiological overall response rate (ORR) as the primary endpoint in this study. Response Evaluation Criteria In Solid Tumors quantification of the response correlated with survival in early clinical trials [2]. Furthermore, due to the particular design of this trial, the radiological restaging allowed us to capture the specific efficacy of neoadjuvant capecitabine and oxaliplatin combined with bevacizumab (CAPOX-B), The incidence of grade 3–4 adverse events was similar to that reported for this regimen in large phase III trials [3,], but the rate of anastomotic leak (AL; 13%) was higher than expected. Although the findings have not been uniform, phase II trials combining preoperative bevacizumab with chemotherapy and/or chemoradiation for locally advanced rectal cancer have revealed an increased rate of wound complications [4]. We also observed a high frequency (6%) of fatal adverse events. Two of the three deaths (i.e., one massive pulmonary thromboembolism in an 84-year-old man and one peritonitis secondary to AL) were attributable, at least in part, to bevacizumab.
The ORR was 78% after preoperative CAPOX-B, and because no disease progression was observed, no patient received preoperative chemoradiation (CRT). The R0 resection rate was 100%, and the pathological complete response (pCR) rate was 20%. Although the rate of local relapse (LR) is just 2%, these data must be interpreted with caution because follow-up is short for this endpoint. Our early efficacy endpoints (Fig. 1) and the low rate of LR were similar to those recently reported by Scrhag et al. [5]; however, the observed mortality in our series was higher. Although both studies have a similar approach, the investigators from Memorial Sloan Kettering Cancer Center used bevacizumab combined with FOLFOX in a single-center trial, and that may, at least in part, explain the differences. In addition, our efficacy results seem very similar to those observed in patients randomly assigned to combined preoperative fluoropyrimidines and radiation in recently published phase III trials. In the German CAO/ARO/AIO-04 [6] trial with a population composed mainly of stage cT3 tumors (84%), pCRs were achieved in 13% of patients who underwent surgery in the 5-fluorouracil/RT group, with ypN0 of 69%.
Figure 1.
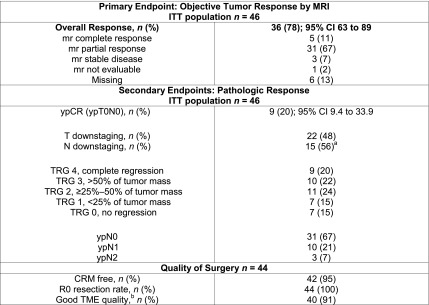
Radiological and pathological response. T downstaging defined as lower pathologic T stage compared with the pretreatment mrT stage. N downstaging defined as N negative pathologic stage compared with mrN positive at baseline. a15 ypN negative of 29 mrN positive at baseline. bDefined as intact mesorectum with only minor irregularities.
Abbreviations: CRM, circumferential resection margin; ITT, intention to treat; MRI, magnetic resonance imaging; mr, magnetic resonance; pCR, pathological complete response; TME, Total mesorectal excision; TRG, tumor regression grade.
A limitation of our trial is patient selection. Increases in thromboembolic events have been described in older patients treated with bevacizumab [7]. Consequently, careful patient selection remains important and is a major concern for this potentially curable population.
In summary, the observed toxicity does not warrant further investigation of this specific regimen, but our findings provide justification for additional investigation of this strategy. The phase II/III Alliance for Clinical Trials in Oncology trial (NCT01515787) is under way and will compare neoadjuvant FOLFOX with selective CRT versus standard preoperative CRT.
Supplementary Material
Acknowledgments
We thank the patients and study investigators; Patricia Romero, Paz Torres, and Dolores Ferrandez (Roche); and José Javier Garcia and his team (Pivotal) for statistical advice and monitoring support.
Footnotes
Access the full results at: FernandezMartos-14-233.theoncologist.com
ClinicalTrials.gov Identifier: NCT00909987
Sponsor(s): Gemcad Group
Principal Investigators: Carlos Fernandez-Martos, Gina Brown
IRB Approved: Yes
Author disclosures and references available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


