Abstract
Ovulation in the nematode Caenorhabditis elegans is coordinated by interactions between the somatic gonad and germ cells. Myoepithelial sheath cells of the proximal ovary are smooth muscle-like cells, but the regulatory mechanism of their contraction is unknown. We show that contraction of the ovarian muscle requires tropomyosin and troponin, which are generally major actin-linked regulators of contraction of striated muscle. RNA interference of tropomyosin or troponin C caused sterility by inhibiting ovarian contraction that is required for expelling mature oocytes into the spermatheca where fertilization takes place, thus causing accumulation of endomitotic oocytes in the ovary. Tropomyosin and troponin C were associated with actin filaments in the myoepithelial sheath, and the association of troponin C with actin was dependent on tropomyosin. A mutation in the actin depolymerizing factor/cofilin gene suppressed the ovulation defects by RNA interference of tropomyosin or troponin C. These results strongly suggest that tropomyosin and troponin are the actin-linked regulators for contraction of ovarian muscle in the C. elegans reproductive system.
INTRODUCTION
In reproductive systems of multicellular organisms, somatic gonads provide the proper environment for germ cells for their development, transport, and fertilization. Ovulation in the nematode Caenorhabditis elegans requires a signal from sperm that induces ovary contraction and oocyte maturation, which are followed by spermathecal dilation and fertilization (Ward and Carrel, 1979; McCarter et al., 1997, 1999; Hubbard and Greenstein, 2000). Myoepithelial sheath cells of the proximal ovary are morphologically smooth muscle-like cells with distinct thick and thin filaments that are organized into a nonstriated manner (Strome, 1986; Ardizzi and Epstein, 1987; Hall et al., 1999) and are required for ovulation of mature oocytes (McCarter et al., 1997). However, the same myosin heavy chain isoforms are expressed in the myoepithelial sheath and the striated body wall muscle (Ardizzi and Epstein, 1987), suggesting that the sheath cells are physiologically similar to striated muscle. Intense contraction of the myoepithelial sheath is induced by sperm in the absence of oocytes (McCarter et al., 1997). Purified major sperm protein induces contraction through an Eph receptor (Miller et al., 2001; Miller et al., 2003), but its downstream regulation of cytoskeletal activity is not understood.
To date, only several cytoskeletal proteins are implicated in sheath contraction. The UNC-54 myosin heavy chain is a component of the thick filaments in the myoepithelial sheath (Ardizzi and Epstein, 1987), but its mutation causes only weak ovulation defects (McCarter et al., 1997). The MYO-3 myosin heavy chain is also a component of the thick filaments in the myoepithelial sheath (Ardizzi and Epstein, 1987) and may have a partially redundant function with UNC-54. However, mutations in the myo-3 gene are homozygous lethal (Waterston, 1989), and its function is not investigated in the sheath cells. Disturbance of the functions of MUP-2 troponin T (Myers et al., 1996), PAT-3 β-integrin (Lee et al., 2001), or talin (Cram et al., 2003) also causes ovulation defects, but their subcellular localization in the sheath cells is not examined in detail. In addition, perturbation of β-integrin or talin causes defects in the gonadal morphogenesis (Lee et al., 2001; Cram et al., 2003), suggesting that the ovulation defects might be partly due to structural defects rather than defects in the regulation of contraction.
Tropomyosin (TM) is a major actin-associated protein among eukaryotes. In striated muscle, TM is coupled with troponin (TN) and transmits the calcium signal to activate actin-myosin interaction (Gordon et al., 2000). Mutations in the human TM gene are associated with nemaline myopathy and familial hypertrophic cardiomyopathy (Michele and Metzger, 2000; Tubridy et al., 2001). In both muscle and nonmuscle cells, TM stabilizes actin filaments by protecting them from disassembly (Cooper, 2002). Null or severe loss-of-function mutations of a TM gene are lethal in yeast (Balasubramanian et al., 1992; Drees et al., 1995), mice (Blanchard et al., 1997; Rethinasamy et al., 1998), and C. elegans (Williams and Waterston, 1994; Anyanful et al., 2001). Thus, TM is an important regulator of actin-dependent processes in a variety of cells. In contrast, TN is expressed only in striated muscle in vertebrates and functions as an actin-linked calcium switch to activate actomyosin interaction (Gordon et al., 2000). However, there are two instances for the presence of TN in smooth muscle in other animals: adult body wall muscle of sea squirt (Endo and Obinata, 1981) and adductor muscle of scallop (Ojima and Nishita, 1986; Nishita et al., 1997). Although these smooth muscle TNs have activity to regulate actin-myosin interaction in a calcium-dependent manner in vitro, their physiological roles in muscle contraction are yet to be established.
In this study, we demonstrate that TM and TN are the components of thin filaments in the sheath cells of the C. elegans ovary and are required for ovarian contraction during ovulation. We also find that the ovulation defects by RNA interference (RNAi) of TM or TN are suppressed by a mutation of actin depolymerizing factor (ADF)/cofilin, which enhances actin filament turnover (reviewed in Bamburg, 1999; Bamburg et al., 1999; Ono, 2003). Thus, our results indicate that these cytoskeletal regulators play crucial roles in the C. elegans reproduction.
MATERIALS AND METHODS
Nematode Strains
Wild-type C. elegans strain N2 was obtained from the Caenorhabditis Genetics Center (Minneapolis, MN). unc-60 (r398) (McKim et al., 1988) was obtained from D. Baillie (Simon Fraser University, Burnaby, BC, Canada). The strain DH1033 expressing YP170::GFP (Grant and Hirsh, 1999) was obtained from B. Grant (Rutgers University, Piscataway, NJ). Nematodes were grown at 20°C as described previously (Brenner, 1974).
RNA Interference Experiments
Nematodes were treated with RNAi for CeTM or pat-10 by feeding as described previously (Ono and Ono, 2002). Phenotypes were analyzed in their F1 generation. Worm motility was quantified as described previously (Epstein and Thomson, 1974; Ono et al., 1999). Two different vectors for RNAi of CeTM were used: TM1 was used to suppress the CeTMI and CeTMII isoforms and TM2 to suppress all four CeTM isoforms (Ono and Ono, 2002). To construct a vector for pat-10 (RNAi), a 1087-base pair genomic DNA fragment of the pat-10 gene (F54C1.7) was amplified from genomic DNA of wild-type C. elegans by polymerase chain reaction by using a forward primer 5′-GATCAGATCTGGCTGAGGATATCGAAGAGATTC and a reverse primer 5′-GATCGCTAGCTTGAAGATTGTAGATCAGCGCTG. The amplified fragments were digested by BglII and NheI at the sites introduced in the polymerase chain reaction primers and cloned into L4440 (provided by A. Fire, Stanford University, Stanford, CA) at the cloning site between two oppositely oriented T7 promoters (Timmons and Fire, 1998).
Time-Lapse Nomarski Microscopy
Control worms were anesthetized in 0.1% tricaine, 0.01% tetramisole in M9 for 30 min and mounted on 2% agarose pads (McCarter et al., 1997). Tricaine/tetramisole paralyzes body wall movement but does not block several rounds of oocyte maturation and ovulation. They were set on a Nikon Eclipse TE2000 inverted microscope and observed with a 40× CFI Plan Fluor objective (numerical aperture 1.4). Images were captured at room temperature by a SPOT RT Monochrome charge-coupled device camera (Diagnostic Instruments, Sterling Heights, MI) and recorded every 15 s for 60-100 min by the IPLab imaging software (Scanalytics, Fairfax, VA).
Fluorescence Microscopy
Staining of whole animals with tetramethylrhodamine-phalloidin (Sigma-Aldrich, St. Louis, MO) was performed as described previously (Ono, 2001). 4′6-Diamidino-2-phenylindole, dihydrochloride (DAPI) (Sigma-Aldrich) was included at 0.1 μg/ml in the phalloidin solution to stain DNA. For immunofluorescent staining of the C. elegans gonads, gonads were dissected by cutting adult worms at the level of the pharynx as described previously (Rose et al., 1997), attached on poly-lysine-coated slides by a freeze-crack method (Epstein et al., 1993), and fixed with 4% formaldehyde in cytoskeleton buffer (10 mM MES-KOH, pH 6.1, 138 mM KCl, 3 mM MgCl2, 2 mM EGTA) containing 0.32 M sucrose for 30 min at room temperature. They were permeabilized with methanol for 5 min at -20°C and stained with anti-actin antibody (C4; MP Biomedicals, Irvine, CA), DAPI, and anti-CeTM (Ono and Ono, 2002), or anti-PAT-10 antibody (Terami et al., 1999) (provided by H. Kagawa, Okayama University, Okayama, Japan). Alexa 488-conjugated goat anti-guinea pig IgG or goat anti-rabbit IgG (Molecular Probes, Eugene, OR) and Cy3-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) were used as secondary antibodies. For staining of the gonads only by tetramethylrhodamine-phalloidin, the dissected gonads were fixed with 4% formaldehyde in cytoskeleton buffer containing 0.32 M sucrose for 30 min at room temperature and incubated with 0.2 μg/ml tetramethylrhodaminephalloidin in phosphate-buffered saline containing 0.5% Triton X-100, 30 mM glycine, 1 mM EDTA, and 0.05% sodium azide for 1 h at room temperature. Samples were observed with a Nikon Eclipse TE2000 inverted microscope with a 40× CFI Plan Fluor objective (numerical aperture 1.4). Images were captured at room temperature by a SPOT RT Monochrome charge-coupled device camera (Diagnostic Instruments) and edited by the IPLab imaging software (Scanalytics) and Adobe Photoshop 6.0 with no gamma adjustment.
Western Blot
The worm lysates were prepared and Western blot performed as described previously (Ono and Ono, 2002). Primary antibodies used were anti-PAT-10 (Terami et al., 1999), anti-CeTM (Ono and Ono, 2002), and anti-actin (C4; MP Biomedicals) antibodies.
RESULTS
RNA Interference of Tropomyosin Causes Sterility
We previously showed that suppression of TM by RNAi caused paralysis or reduced motility and disorganization of actin filaments in body wall muscle of C. elegans (Ono and Ono, 2002). In addition, we found that suppression of TM caused strong sterility (Table 1). The TM1 RNAi construct suppresses the CeTMI and CeTMII isoforms of the four TM isoforms present in worms [CeTMI,II (RNAi)] and reduced the total level of TM protein to 50% (see Figure 7 of Ono and Ono, 2002;Figure 3b). Decreased motility of the affected worms to approximately one-half the speed of control worms was also observed as reported previously (Ono and Ono, 2002) (Table 1). In addition, the CeTMI,II (RNAi) worms produced no progeny (Table 1), whereas control worms produced ∼300 progeny per worm (Table 1). Suppression of all four TM isoforms by the TM2 RNAi construct [CeTMI,II,III,IV (RNAi)] reduced the TM protein level to ∼10% (see Figure 7 of Ono and Ono, 2002; Figure 3b) and caused a more severe motility defect than that by TM1 as reported previously (Ono and Ono, 2002) (Table 1). Nonetheless, the sterile phenotypes induced by both CeTMI,II (RNAi) and CeTMI,II,III,IV (RNAi) were equally severe (Table 1). These results strongly suggest that TM is required for the reproductive system of C. elegans and that CeTMI and CeTMII are the TM isoforms that play major roles in this event or that the reproductive system is more sensitive to the level of TM than body wall muscle.
Table 1.
RNAi phenotypes for CeTM and pat-10
| Strain | RNAi | Motility (beats/30 s, n = 10) | Brood size (n = 10) | Emo phenotype (%, n = 200) |
|---|---|---|---|---|
| Wild-type | Control | 105 ± 5.5 | 314 ± 33 | 0 |
| CeTMI,II | 46 ± 6.8 | 0 ± 0 | 100 | |
| CeTMI,II,III,IV | 13 ± 5.9 | 0 ± 0 | 100 | |
| pat-10 | 0.6 ± 1.1 | 0 ± 0 | 100 | |
| unc-60(r398) | Control | 48.9 ± 11 | 251 ± 46 | 0 |
| CeTMI,II | 47 ± 12 | 271 ± 39 | 0 | |
| CeTMI,II,III,IV | 39 ± 5.2 | 72 ± 50 | 59 | |
| pat-10 | 1.7 ± 1.3 | 8.3 ± 11 | 10 |
Figure 7.
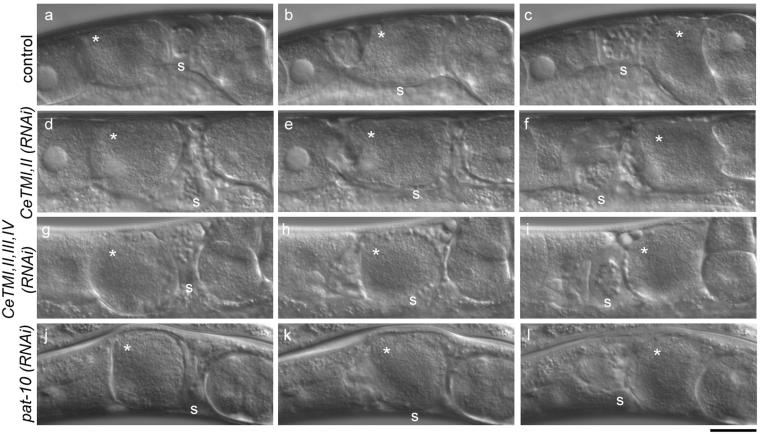
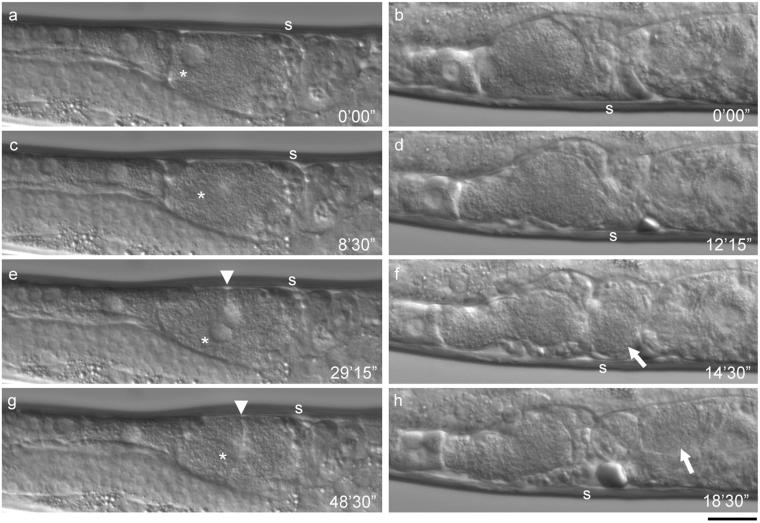
Ovulation processes in the unc-60B mutant after RNAi treatment. Time-lapse observation of ovulation processes in the unc-60 (r398) worms after control treatment (a-c; also see Video 5), CeTMI,II (RNAi) (d-f; also see Video 6), CeTMI,II,III,IV (RNAi) (g-i; also see Video 7), or pat-10 (RNAi) (j-l; also see Video 8). Oocytes (asterisks) are shortly after maturation (a, d, g, and j), transported in the spermatheca (b, e, h, and k), and expelled into the uterus (c, f, i, and l). Positions of the spermatheca are indicated by s. Bar, 20 μm.
Figure 3.
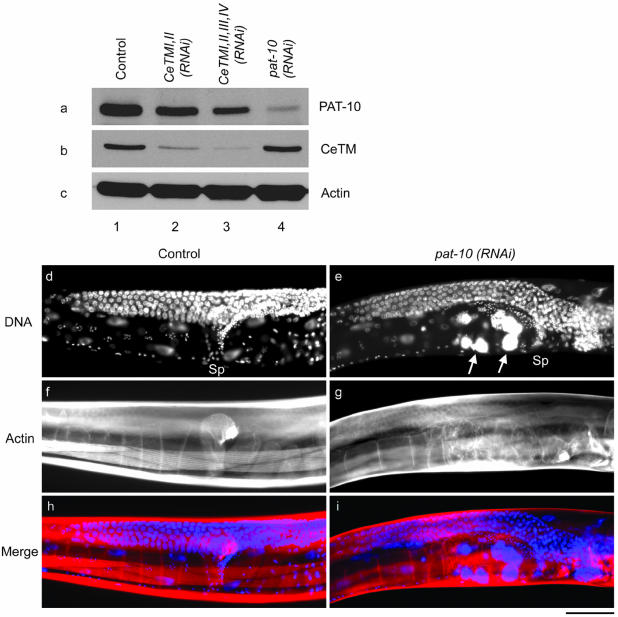
RNAi of pat-10 troponin C results in ovulation defects. (a-c) Western blot analysis of PAT-10 (a), CeTM (b), and actin (c) after control RNAi with a blank vector (lane 1), CeTMI,II (RNAi) (lane 2), CeTMI,II,III,IV (RNAi) (lane 3), or pat-10 (RNAi) (lane 4). PAT-10 was significantly reduced by pat-10 (RNAi) (a, compare lanes 1 and 4) and slightly reduced by CeTMI,II (RNAi) (a, lane 2), CeTMI,II,III,IV (RNAi) (a, lane 3). In contrast, the level of CeTM was not altered by pat-10 (RNAi) (b, compare lanes 1 and 4). (c-i) Control (d, f, and h) or pat-10 (RNAi) (e, g, and i) worms were stained by DAPI (d and e) and rhodamine-phalloidin (f and g). Merged images of DNA (blue) and F-actin (red) are shown in h and i. Positions of the spermatheca (Sp) are indicated in d and e. Large accumulations of DNA in endomitotic oocytes are indicated by arrows in e. Bar, 50 μm.
Tropomyosin Is Essential for Ovulation
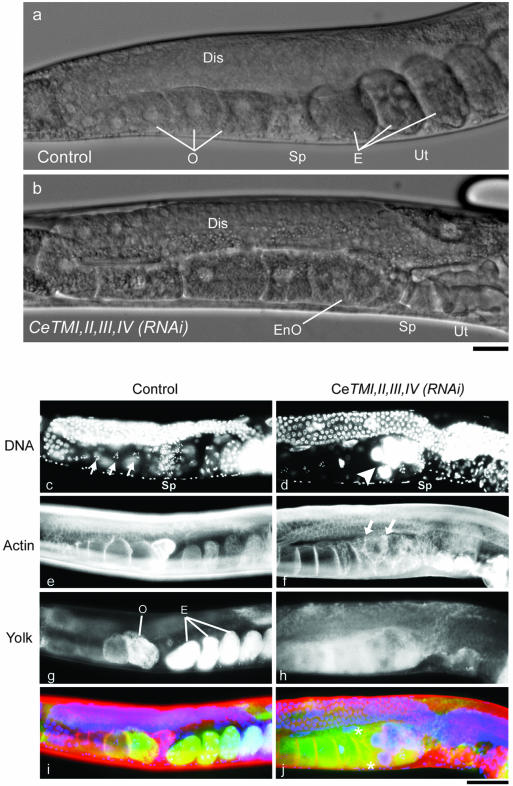
Examination of the adult gonads in CeTM (RNAi) worms revealed that the oocytes at the very proximal ends of the ovary became endomitotic and no embryos resided in the uterus (compare Figure 1, a and b). In control worms, the oocytes were located in the proximal ovary (Figure 1a) and were arrested at meiosis with condensed chromosomes (Figure 1c, arrows). However, in both CeTMI,II (RNAi) and CeTMI,II,III,IV (RNAi) worms, oocytes at the proximal gonads had a large accumulation of DNA (Figure 1d, arrowhead), indicating that there was endomitotic replication of DNA in the oocytes, which became highly polyploid. This phenotype resembles the previously described endomitotic oocytes in gonadal arms phenotype (Emo) that is observed when ovulation is defective (Iwasaki et al., 1996). The Emo phenotype of CeTM (RNAi) worms was very severe, such that oocytes or embryos were not observed in the spermatheca or uterus (Figure 1b and Table 1), suggesting that there was no ovulation. In addition, visualization of the actin filaments revealed that the endomitotic oocytes had irregular cell compartments (Figure 1f, arrows) and that some cells were anuclear (Figure 1j, asterisks), suggesting that these oocytes underwent aberrant cell division in the ovary. The two RNAi treatments, CeTMI,II (RNAi) and CeTMI,II,III,IV (RNAi), caused nearly identical Emo phenotype (Table 1). Therefore, the phenotypes shown in the Figures 1 and 2 are after treatment with only CeTMI,II,III,IV (RNAi).
Figure 1.
RNA interference of tropomyosin causes ovulation defects and endomitotic DNA replication in the oocytes. (a and b) Nomarski images of the adult hermaphroditic gonad of control (a) and CeTMI,II,III,IV (RNAi) (b) worms. Germ cells are proliferated in the distal gonad (Dis) and developed into oocytes (O) in the proximal gonad. A mature oocyte is ovulated and fertilized in the spermatheca (Sp) and initiates embryogenesis in the uterus (Ut). In the CeTMI,II,III,IV (RNAi) worm, the proximal oocytes (EnO) had ambiguous nuclear-cytoplasmic boundary, which is characteristic of endomitotic oocytes, and there was no embryos in the uterus. Bar, 20 μm. (c-j) Visualization of DNA by DAPI staining showed that control oocytes had condensed chromosomes (c, arrows), whereas the proximal oocytes in the CeTMI,II,III,IV (RNAi) worm had large accumulations of DNA (d, arrowhead). Staining of actin filaments with rhodamine-phalloidin demonstrated that oocytes were regularly compartmentalized in the control ovary (e) but that they had irregular cell compartments in the CeTMI,II,III,IV (RNAi) ovary (f, arrows). Transgenically expressed GFP-tagged yolk protein YP170 accumulated in control oocytes and embryos by endocytosis (g). The oocytes in the CeTMI,II,III,IV (RNAi) worm also had the yolk protein (h), suggesting that endocytosis was normal. Merged in i and j are DNA (blue), actin (red), and yolk (green). Asterisks in j indicate anuclear cells. Bar, 50 μm.
Figure 2.
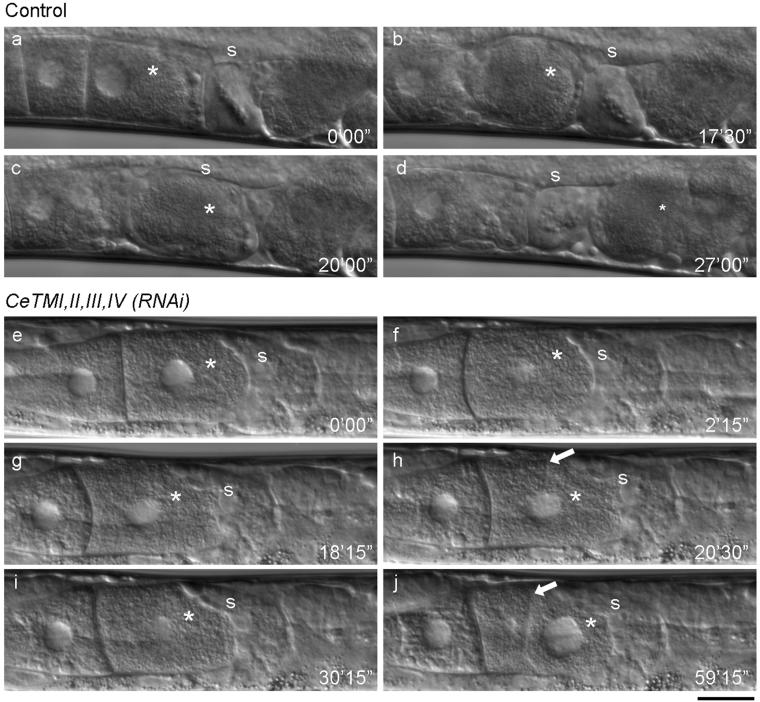
Ovulation, but not oocyte maturation, is defective in the tropomyosin-RNAi worms. Ovulation processes of control (a-d) and CeTMI,II,III,IV (RNAi) (e-j) worms were recorded by time-lapse Nomarski microscopy (also see Videos 1 and 2). In a control worm, the most proximally located oocyte (a, asterisk) became mature and showed morphological change into a round shape and nuclear envelope breakdown (b). It was pushed by intense contraction of the ovary and fertilized in the spermatheca (c). Then, embryogenesis was initiated in the uterus after ovulation (d). In the CeTMI,II,III,IV (RNAi) worm, the most proximal oocyte (e, asterisk) became mature (f), but it was not ovulated due to weak contraction of the ovary (g). In the absence of ovulation, the nuclear envelope reappeared (g, asterisk) and a cleavage furrow was formed (h, arrow). The furrow was dynamic and sometimes regressed (i). However, when the cleavage is complete, the daughter cell on the left became anuclear and the large nucleus is segregated into the other cell (j). Positions of the spermatheca are indicated by “s”. Numbers indicate time (minute seconds) from the first frame. Bar, 20 μm.
Before the oocytes became endomitotic, there were no morphological abnormalities in the germ cells in CeTM (RNAi) worms. Sperm was properly stored in the spermatheca (Figure 1d) and sperm that was supplied by mating with control males did not rescue the RNAi phenotype (our unpublished data). Developing oocytes were morphologically normal before they became endomitotic (our unpublished data). In addition, the endocytic process of the yolk proteins in oocytes seemed to be normal (Figure 2, g and h), although the actin cytoskeleton plays important roles in endocytosis (Schafer, 2002; Engqvist-Goldstein and Drubin, 2003). We followed the dynamics of a green fluorescent protein (GFP)-tagged yolk protein (YP170) in control, CeTMI,II (RNAi), or CeTMI,II,III,IV (RNAi) worms. In control worms, the yolk protein accumulated in maturing oocytes and early embryos (Figure 1g). CeTM (RNAi) worms also had the yolk protein in the cytoplasm of the oocytes (Figure 1h) and did not show a typical endocytosis-defective phenotype (Grant and Hirsh, 1999).
To observe the processes of the ovulation defect, we recorded the live activity of the gonads of CeTM (RNAi) worms by time-lapse Nomarski microscopy (Figure 2; Videos 1 and 2). In control worms, only the most proximal oocyte became mature, which was characterized by nuclear envelope breakdown and rounding up of the oocyte (McCarter et al., 1999) (Figure 2, a and b, asterisks; Video 1). This was accompanied by frequent and intense myoepithelial sheath contraction and spermathecal dilation that allowed the oocyte to enter the spermatheca where it was fertilized (McCarter et al., 1999) (Figure 2c; Video 1). The fertilized egg was subsequently expelled into the uterus and initiated embryogenesis (Figure 2d; Video 1). In the CeTMI,II,III,IV (RNAi) worms, the proximal oocyte became mature normally (Figure 2, e and f, asterisks; Video 2), but neither intense contraction of the myoepithelial sheath nor dilation of the spermatheca took place. As a result, the oocyte remained in the gonad, rearranged its cortex into a square shape, reformed the nuclear envelope (Figure 2g; Video 2), and, surprisingly, initiated cytokinesis (Figure 2h, arrow; Video 2). The cleavage furrow was often dynamic and sometimes regressed (Figure 2i; Video 2). However, the nucleus was not divided. Thus, when the cleavage was complete, the nucleus was restricted to only one of the two daughter cells, leaving the other cell anuclear (Figure 2j, asterisk; Video 2). In the absence of ovulation, maturation of the second or third proximal oocyte was often observed (our unpublished data), indicating that a signal to induce oocyte maturation can reach distally located oocytes as demonstrated previously (Iwasaki et al., 1996). Therefore, suppression of TM specifically caused a defect in the contractile activity of the ovary, but not in the process of oocyte maturation. The CeTMI,II (RNAi) (n = 5) and CeTMI,II,III,IV (RNAi) (n = 10) worms showed a nearly identical ovulation defect by time-lapse microscopy (Table 3).
Table 3.
Dissection of the ovulation process by time-lapse recording
| Strain | RNAi | Sheath contraction | Spermathecal dilation | Oocyte maturationa | Aberrant cytokinesis | Ovulation | n |
|---|---|---|---|---|---|---|---|
| Wild-type | Control | 100b | 89 | 100 | 0 | 78 | 9 |
| CeTMI,II | 0 | 0 | 100 | 100 | 0 | 5 | |
| CeTMI,II,III,IV | 0 | 0 | 100 | 100 | 0 | 10 | |
| pat-10 | 0 | 43 | 100 | 71 | 43c | 7 | |
| unc-60(r398) | Control | 83 | 83 | 83 | 0 | 83 | 6 |
| CeTMI,II | 60 | 100 | 100 | 0 | 80 | 5 | |
| CeTMI,II,III,IV | 0 | 80 | 100 | 20 | 60 | 5 | |
| pat-10 | 0 | 50 | 83 | 17 | 50 | 6 |
Maturation was characterized by nuclear envelope breakdown
All values except “n” indicate percentages
This includes two cases (29%) of abnormal ovulation, which cleaves the oocyte before completion
PAT-10 Troponin C Is Required for Ovulation
A previous report by Myers et al. (1996) demonstrated that a mutation in the mup-2 troponin T gene impaired contraction of the myoepithelial sheath and caused a very similar sterile phenotype to CeTM (RNAi). This observation strongly suggests that troponin is also a regulator of sheath contraction. We extracted available functional data on all genes for the C. elegans troponin components (two troponin Cs [TNCs], four troponin Is, and four troponin Ts) (Table 2) and found that the pat-10/tnc-1 TNC gene was the only troponin component that caused sterility by RNAi (Kamath et al., 2003). These data led us to hypothesize that PAT-10 TNC might be a regulatory component of contraction of the myoepithelial sheath cells. PAT-10 TNC was previously reported as a component of thin filaments in body wall muscle (Terami et al., 1999), but its expression and localization in the gonad are not known. Mutations in the pat-10 gene cause embryonic arrest (Williams and Waterston, 1994) and do not allow us to investigate its role in the gonad that develops during larval stages (Hubbard and Greenstein, 2000).
Table 2.
Tropomyosin and troponin in C. elegans
| Protein | Gene | Length (aa) | Mutanta | RNAia |
|---|---|---|---|---|
| Tropomyosin | lev-11/tmy-1 (Y105E8B.1 I) | 256-301 | Levb, Patc | Embd, Unce, Stef |
| Troponin C | pat-10/tnc-1 (F54C1.7 I) | 161 | Patg | Stef,h |
| tnc-2 (ZK673.7 II) | 160 | ND | Lvah | |
| Troponin I | tni-1 (F42E11.4 X) | 250 | ND | WTh |
| unc-27/tni-2 (ZK721.2 X) | 242 | Unci | WTh | |
| tni-3 (T20B3.2 V) | 260 | ND | WTh | |
| tni-4 (W03F8.1 IV) | 197 | ND | Groh | |
| Troponin T | mup-2/tnt-1 (T22E5.5 X) | 405 | Mup, Stej | WTh |
| tnt-2 (F53A9.10 X) | 428 | ND | WTh | |
| tnt-3 (C14F5.3 X) | 328-1216 | ND | WTh | |
| tnt-4 (T08B1.2 V) | 347-375 | ND | WTh |
Designations for the phenotypes: Lev, levamisole-resistant; Pat, paralysed arrest at twofold; Unc, uncoordinated; Ste, sterile; Lva, larval arrest; Gro, slow growth; Mup, muscle positioning; WT, wild type; ND, not determined
This study
We performed RNAi of pat-10 by feeding and found that it allowed the treated worms to grow into adults and caused defective worm motility and sterility, which were very similar to the CeTM (RNAi) phenotypes (Figure 3). RNAi of pat-10 significantly reduced the PAT-10 protein (Figure 3a, compare lanes 1 and 4) without affecting the level of CeTM (Figure 3b, compare lanes 1 and 4). The level of PAT-10 was slightly reduced by RNAi of CeTM (Figure 3a, lanes 2 and 3). The pat-10 (RNAi) worms were nearly paralyzed and produced no progeny (Table 1). Examination of the pat-10 (RNAi) gonads showed that endomitotic oocytes with large accumulations of DNA were present in the proximal gonad (100%, n = 200) (Figure 3e and Table 1). Time-lapse recording of the live pat-10 (RNAi) gonads showed that contraction of the proximal gonad was absent or very weak even after the proximal oocyte became mature and the oocyte remained in the gonad (57%, n = 7) (Figure 4, a, c, e, and g; Video 3). Such oocytes underwent multiple rounds of nuclear envelope breakdown and reappearance (Figure 4, c and e, asterisks) and aberrant cytokinesis (71%, n = 7) (Figure 4, e and g, arrowheads). In some pat-10 (RNAi) worms (43%, n = 7), the spermatheca was able to dilate and allowed ovulation of the proximal oocyte in the absence of intense sheath contraction (Figure 4, b, d, f, and h; Video 4). However, in two of three such cases, the spermatheca contracted before ovulation was completed, which resulted in cleavage of the oocyte and ovulation of only a portion of the oocyte (Figure 4, f and h, arrows; Video 4), indicating that the spermatheca can dilate but its dilation and contraction are uncoordinated during ovulation. These results indicate that sterility by pat-10 (RNAi) is due to a strong defect in contraction of the myoepithelial sheath as observed for RNAi of CeTM.
Figure 4.
Time-lapse observation of ovulation in the pat-10 (RNAi) worm. Ovulation process of two representative pat-10 (RNAi) worms. In a, c, e, and g, ovulation was completely unsuccessful (also see Video 3). Nuclear envelope breakdown of the most proximal oocyte (c, asterisk) indicated oocyte maturation. However, due to lack of sheath contraction, the oocyte was not transported and nuclear envelope reappeared (e, asterisk). Aberrant cytokinesis of the oocyte was also observed (e and g, arrowheads). In b, d, f, and h, ovulation was partially successful (also see Video 4). After oocyte maturation (b), the spermatheca dilated (d), and the oocyte entered the spermatheca (f, arrow). However, only a portion of the oocyte was transported into the uterus (h, arrow). Numbers indicate time (minutes seconds) from the first frame. Bar, 20 μm.
Tropomyosin and Troponin C Are the Components of the Nonstriated Thin Filaments in the Myoepithelial Sheath
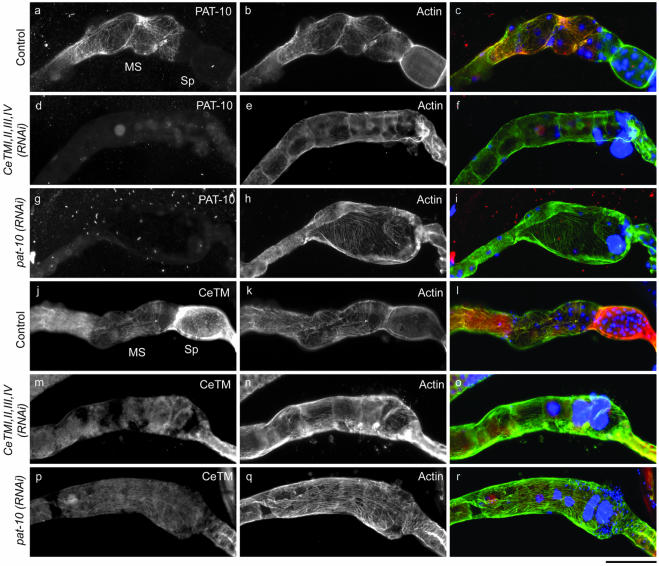
By immunofluorescence microscopy, we found that CeTM and PAT-10 TNC colocalized with actin filaments in the myoepithelial sheath of the ovary (Figure 5). PAT-10 was expressed only in the myoepithelial sheath cells but not in the spermatheca and colocalized with the actin filaments in a nonstriated manner (Figure 5, a-c). In contrast, CeTM was expressed in both myoepithelial sheath and spermatheca and localized to the actin filaments in a nonstriated pattern (Figure 5, j-l). The CeTMI,II,III,IV (RNAi) treatment eliminated filamentous staining by anti-CeTM antibody but not diffuse staining of the gonad (Figure 5, m-o), suggesting that residual CeTM protein or nonspecifically recognized proteins are visualized. In addition, RNAi of CeTM reduced filamentous staining of PAT-10 (Figure 5, d-f), indicating that association of PAT-10 with actin is dependent on CeTM. In contrast, pat-10 (RNAi) nearly completely eliminated PAT-10 in the gonad (Figure 5g) but did not affect the filamentous pattern of CeTM (Figure 5p). Thus, CeTM localizes to actin filaments independently of PAT-10. The organization of actin filaments in the myoepithelial sheath and spermatheca was not significantly altered by RNAi of CeTM (Figure 5, e and n) or pat-10 (Figure 5, h and q) except for minor disarrays of the filaments (also see Figure 6B), which might be due to excessive accumulation of endomitotic oocytes. Together, these observations strongly suggest that both CeTM and PAT-10 TNC are the components of nonstriated thin filaments in the myoepithelial sheath cells and regulate contraction.
Figure 5.
Localization of tropomyosin and PAT-10 troponin C to the thin filament network in the myoepithelial sheath. Hermaphroditic gonads were dissected from control (a-c and j-l), CeTMI,II,III,IV (RNAi) (d-f and m-o), or pat-10 (RNAi) (g-i and p-r) worms and stained with anti-PAT-10 (a, d, and g), or anti-CeTM (j, m, and p), and anti-actin antibody (b, e, h, k, n, and q). Merged images of PAT-10 or CeTM (green), actin (red), and DNA (blue) are shown in c, f, i, l, o, and r. PAT-10 was expressed in the myoepithelial sheath (MS) but not in the spermatheca (Sp) and colocalized with F-actin (a). CeTM was expressed in both myoepithelial sheath and spermatheca and colocalized with F-actin (j). Bar, 50 μm.
Figure 6.
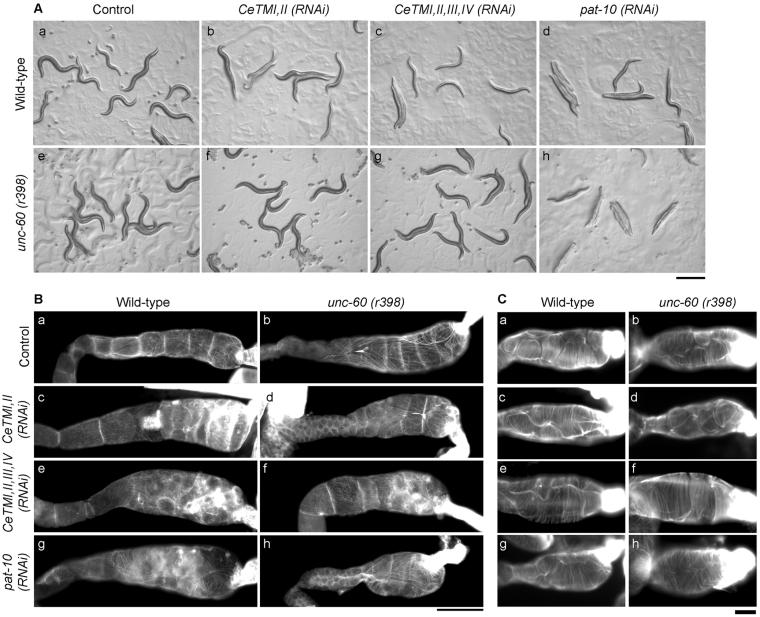
Suppression of ovulation defects by a mutation of unc-60B ADF/cofilin. (A) Micrographs of wild-type (a-d) or unc-60 (r398) (e-h) on agar plates after control (a and e), CeTMI,II (RNAi) (b and f) CeTMI,II,III,IV (RNAi) (c and g), or pat-10 (RNAi) (d and h) treatment. Bar, 0.5 mm. (B and C) The myoepithelial sheath (B) or the spermatheca (C) of dissected gonads from wild-type (a, c, e, and g) or unc-60 (r398) (b, d, f, and h) worms after control treatment (a and b), CeTMI,II (RNAi) (c and d), CeTMI,II,III,IV (RNAi) (e and f), or pat-10 (RNAi) (g and h) were stained by tetramethylrhodamine-phalloidin to visualize F-actin in the myoepithelial sheath. Note that intensely stained aggregates in c, e, f, and g were present in the oocytes not in the sheath cells due to aberrant cell division. Bars, 50 μm (B) or 10 μm (C).
ADF/Cofilin Is Antagonistic to Tropomyosin and Troponin in Ovulation
We previously reported that a mutation in unc-60B, encoding a muscle-specific ADF/cofilin isoform, suppresses the CeTM (RNAi) phenotype in the body wall muscle (Ono and Ono, 2002). We found that the unc-60 (r398) mutation, which inhibits the filament severing activity of UNC-60B (Ono et al., 1999, 2001), also suppressed the sterile phenotype by CeTM (RNAi) (Figure 6A and Table 1). The unc-60 (r398) homozygotes with control RNAi treatment showed reduced motility and brood size compared with wild-type, but no ovulation defects (Emo phenotype) were detected (Figure 7, a-c, and Table 1; Video 5). The CeTMI,II (RNAi) treatment of unc-60 (r398) had no effects on motility, brood size, and ovulation (compare Figure 6A, b and f, and Table 1), and CeTMI,II,III,IV (RNAi) only weakly affected motility and impaired brood size and ovulation to much lesser extents than wild-type (compare Figure 6A, c and g, and Table 1). Nonetheless, the actin filament network in the myoepithelial sheath (Figure 6B) or the spermatheca (Figure 6C) was not significantly different between wild-type (Figure 6, Ba and Ca) and unc-60 (r398) (Figure 6Bb and Cb) and alteration of the actin filaments after the CeTM (RNAi) treatments were relatively minor (Figure 6B, c-f, for the myoepithelial sheath and C, c-f, for the spermatheca). Filamentous localizations of CeTM and PAT-10 TNC in the myoepithelial sheath were significantly reduced by CeTM (RNAi) in unc-60 (r398) as observed in wild-type (our unpublished data). Time-lapse observation of ovulation showed that the defect in contraction of the proximal gonads was partially suppressed in the RNAi-treated unc-60 (r398) worms (Figure 7, d-I, and Table 3; Videos 6 and 7). In addition, spermathecal dilation was not inhibited in the unc-60 (r398) worms (Figure 7, d-I, and Table 3; Videos 6 and 7), whereas it was strongly inhibited in wild-type (Figure 2 and Table 3). Thus, successful ovulation was observed at higher rates in the RNAi-treated unc-60 mutant than wild type (Table 3). These results suggest that contractile activity of the gonad rather than assembly of the contractile structure was altered by the unc-60 mutation and CeTM (RNAi).
In contrast, the unc-60 (r398) mutation partially suppressed the effects of pat-10 (RNAi). The paralyzed phenotype by pat-10 (RNAi) was not suppressed by the unc-60 (r398) mutation (Figure 6A, h and Table 1), suggesting that the RNAi effects on body wall muscle was not affected by the unc-60 mutation. Brood size was greatly reduced (8.3 ± 11) but significantly more than wild type (0 ± 0). However, the ovulation defect was greatly suppressed in the unc-60 mutants (Table 1). There were only minor disarrays of actin filaments in the myoepithelial sheath by pat-10 (RNAi) in both wild-type (Figure 6Bg) and unc-60 (r398) (Figure 6Bh). The actin filaments in the spermatheca were not significantly altered by pat-10 (RNAi) in wild-type (Figure 6Cg) and unc-60 (r398) (Figure 6Ch). Immunostaining of PAT-10 TNC in the gonads showed that the pat-10 (RNAi) treatment reduced the PAT-10 protein in unc-60 (r398) as well as in wild-type (our unpublished data). Time-lapse recording of the ovulation process in the pat-10 (RNAi)-treated unc-60 (r398) worms demonstrated that contraction of the proximal gonad was still weak, but the spermatheca was able to dilate and allowed the oocyte to be expelled into the uterus (Figure 7, j-l; Video 8). In the unc-60 (r398) worms, early spermathecal contraction and cleavage of an ovulating oocyte were not observed. Thus, ovulation was successful in 50% of the observed worms (n = 6) (Table 3). Nonetheless, the low brood size of the pat-10 (RNAi) unc-60 (r398) worms suggests that fertilization was often unsuccessful or embryonic lethality occurred.
DISCUSSION
In this study, we identified TM and TNC as essential regulators of contraction of the myoepithelial sheath during ovulation in the C. elegans reproductive system. RNAi of CeTM or pat-10 TNC strongly inhibited sheath contraction, which is essential for expelling a mature oocyte into the spermatheca for fertilization. Spermathecal dilation was strongly inhibited by CeTM (RNAi) but only weakly by pat-10 (RNAi). This observation was supported by immunolocalization, demonstrating that CeTM localized to the actin filament network in the myoepithelial sheath and spermatheca, but PAT-10 TNC was expressed only in the myoepithelial sheath. A mutation in the unc-60B ADF/cofilin gene suppressed ovulation defects by RNAi of CeTM or pat-10, suggesting that ADF/cofilin is antagonistic to TM and TN in ovulation.
Our results show that the nematode myoepithelial sheath is physiologically similar to striated muscle whose contraction is regulated by a TM-TN complex. Smooth muscles of vertebrates and most invertebrates lack troponin, and their contraction is generally regulated by phosphorylation of myosin light chain and/or Ca2+-calmodulin regulation of caldesmon, an actin-associated protein (Marston, 1995; Wang, 2001). However, no caldesmon homolog is found in the C. elegans genome sequence, and, currently, no candidates for myosin-linked regulators of sheath contraction, such as myosin light chain, calmodulin, and myosin light chain kinase, have been characterized. Rho-kinase and myosin phosphatase also regulate smooth muscle contraction in vertebrates by modulating myosin activity (Kureishi et al., 1997; Uehata et al., 1997; Hartshorne et al., 1998) and their C. elegans orthologues LET-502 and MEL-11 are involved in ovulation (Wissmann et al., 1999). However, they are expressed in the spermatheca but not in the myoepithelial sheath and implicated in spermathecal contraction and dilation (Wissmann et al., 1999). Major sperm protein (Miller et al., 2001) and an Eph receptor (Miller et al., 2003) are identified as the upstream signals for sheath contraction. Therefore, based on the established function of troponin as a thin filament-linked calcium-switch for muscle contraction (Gordon et al., 2000), it is likely that intracellular calcium is elevated by major sperm protein and Eph, which is then detected by troponin to activate actomyosin interaction.
The primary effect of RNAi of CeTM or pat-10 was on contraction of the myoepithelial sheath. In addition, spermathecal dilation was severely impaired by CeTM (RNAi) and partially by pat-10 (RNAi). However, CeTM was expressed in the spermatheca, but PAT-10 was not. Therefore, the spermathecal defect in pat-10 (RNAi) worms might be a secondary effect of defective sheath contraction. It is proposed that the myoepithelial sheath pulls dilating spermatheca and facilitates dilation (McCarter et al., 1997). Genetic studies show that spermathecal dilation is mediated by an inositol triphosphate pathway (Clandinin et al., 1998; Bui and Sternberg, 2002), suggesting strongly that calcium is the second messenger. Nonetheless, absence of troponin in the spermatheca suggests that spermathecal dilation is regulated by a different mechanism from sheath contraction. As briefly mentioned above, Rho-kinase and myosin phosphatase regulate spermathecal activity (Wissmann et al., 1999), but the functional relationship between calcium and the Rho-kinase-myosin phosphatase pathway is not understood.
The myoepithelial sheath after RNAi of CeTM or pat-10 did not seem to constrict the enclosed endomitotic oocytes, suggesting that the sheath cells are relaxed. However, if the function of the TM-TN complex is to inhibit the actomyosin interaction when the Ca2+ concentrations are low, perturbation of TM or TN is expected to cause hypercontraction. Therefore, it is possible that the activity of myosin is regulated by a separate mechanism, which may prevent hypercontraction when the TM-TN system is inhibited. Indeed, biochemical studies on isolated C. elegans myosin and actin demonstrated that C. elegans has both actin- and myosin-linked regulatory systems for the actomyosin activity (Harris et al., 1977). Regulatory myosin light chains often play critical roles in the regulation of myosin activity, although the regulatory mechanisms are various. In addition to the phosphoregulation of myosin light chain in vertebrate smooth muscle, molluscan regulatory myosin light chains directly binds to Ca2+ and activate the myosin motor activity (reviewed in Szent-Györgyi, 1996). In C. elegans, two regulatory light chain genes, mlc-1 and mlc-2, are expressed in body wall muscle (Rushforth et al., 1998), but their biochemical properties and expression in the myoepithelial sheath are not understood. Alternatively, the possibility that TNC directly regulates myosin activity is not excluded because TNC is homologous to myosin light chains and calmodulin that are known to regulate myosin activity.
A mutation in the unc-60B ADF/cofilin gene suppressed the ovulation defects by RNAi of CeTM or pat-10. Our phenotypic analysis suggests that this suppression is due to alteration in the spermathecal activity in the unc-60B mutant. This is consistent with our previous immunolocalization of the UNC-60B protein in the spermatheca but not in the myoepithelial sheath (Ono et al., 2003). We previously demonstrated that tropomyosin is antagonistic to UNC-60B-dependent actin filament dynamics in body wall muscle (Ono and Ono, 2002). Similar antagonistic mechanism in the spermatheca may explain why the ovulation defects of CeTM (RNAi) worms are suppressed by the unc-60B mutation. However, our observation that sheath contraction of CeTM (RNAi) worms was partially restored in the unc-60B mutant suggests that UNC-60B is expressed in the myoepithelial sheath at low levels and functions as a regulator of actin organization. In contrast, partial suppression of the ovulation defect of pat-10 (RNAi) worms by the unc-60B mutation is probably due to enhanced spermathecal dilation in the unc-60B mutants. PAT-10 and UNC-60B are not enriched in the same cells in the gonad, and contraction of the myoepithelial sheath was not restored in the unc-60B mutants. Also, the paralyzed phenotype of pat-10 (RNAi) worms was not suppressed by the unc-60B mutation (Figure 6h), suggesting distinct roles of PAT-10 and UNC-60B in muscle contraction and actin filament dynamics, respectively.
It was somewhat surprising that RNAi of CeTM caused aberrant cytokinesis in the endomitotic oocytes, because TM is essential for cytokinesis in fission yeast (Balasubramanian et al., 1992). Our RNAi treatment did not completely eliminate the CeTM protein. Therefore, residual CeTM might be sufficient to support cytokinesis. Alternatively, an uncharacterized CeTM isoform(s) might be expressed in the oocytes. The CeTM gene, tmy-1/lev-11, undergoes alternative splicing to produce multiple isoforms (Kagawa et al., 1995; Anyanful et al., 2001). However, a nonmuscle isoform of CeTM has not been identified. Our anti-CeTM antibody weakly stains early embryos, but CeTM (RNAi) did not affect embryonic cytokinesis (our unpublished data). In vertebrates, >40 TM isoforms are produced and play distinct roles in different cellular events (Gunning et al., 1998). Therefore, an uncharacterized CeTM isoform(s) might not be suppressed by our RNAi constructs and support cytokinesis in the endomitotic oocytes.
Although the mechanisms of ovulation-fertilization are various among different metazoan species, C. elegans is an attractive model to study communication between somatic gonad and germ cells. In this study, we identified TM and TN as essential regulators of cytoskeletal activity in the somatic gonad. Further genetic and cell biological studies on this system should reveal how sperm and oocytes influence activity of the TM-TN-actin system of the somatic gonad and how the gonadal activity affects oocyte maturation and fertilization.
Supplementary Material
Acknowledgments
We thank W. Kelly and S. L'Hernault for critical reading of the manuscript, D. Baillie and B. Grant for the worm strains, A. Fire for the plasmid vector, H. Kagawa for anti-PAT-10 antibody, and T. Allen for the latest information on the C. elegans troponin components. Wild-type C. elegans strain was provided by the Caenorhabditis Genetics Center, which is funded by the National Institute of Health National Center for Research Resources. This work was supported by a grant from the National Institute of Health (R01 AR48615) to S.O.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-03-0179. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-03-0179.
Online version of this article contains supporting material. Online version is available at www.molbiolcell.org.
References
- Anyanful, A., Sakube, Y., Takuwa, K., and Kagawa, H. (2001). The third and fourth tropomyosin isoforms of Caenorhabditis elegans are expressed in the pharynx and intestines and are essential for development and morphology. J. Mol. Biol. 313, 525-537. [DOI] [PubMed] [Google Scholar]
- Ardizzi, J.P., and Epstein, H.F. (1987). Immunochemical localization of myosin heavy chain isoforms and paramyosin in developmentally and structurally diverse muscle cell types of the nematode Caenorhabditis elegans. J. Cell Biol. 105, 2763-2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian, M.K., Helfman, D.M., and Hemmingsen, S.M. (1992). A new tropomyosin essential for cytokinesis in the fission yeast S. pombe. Nature 360, 84-87. [DOI] [PubMed] [Google Scholar]
- Bamburg, J.R. (1999). Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu. Rev. Cell Dev. Biol. 15, 185-230. [DOI] [PubMed] [Google Scholar]
- Bamburg, J.R., McGough, A., and Ono, S. (1999). Putting a new twist on actin: ADF/cofilins modulate actin dynamics. Trends Cell Biol. 9, 364-370. [DOI] [PubMed] [Google Scholar]
- Blanchard, E.M., Iizuka, K., Christe, M., Conner, D.A., Geisterfer-Lowrance, A., Schoen, F.J., Maughan, D.W., Seidman, C.E., and Seidman, J.G. (1997). Targeted ablation of the murine alpha-tropomyosin gene. Circ. Res. 81, 1005-1010. [DOI] [PubMed] [Google Scholar]
- Brenner, S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui, Y.K., and Sternberg, P.W. (2002). Caenorhabditis elegans inositol 5-phosphatase homolog negatively regulates inositol 1,4,5-triphosphate signaling in ovulation. Mol. Biol. Cell 13, 1641-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkeen, A.K., Maday, S.L., Rybicka, K.K., Sulcove, J.A., Ward, J., Huang, M.M., Barstead, R., Franzini-Armstrong, C., and Allen, T.S. (2004). Disruption of Caenorhabditis elegans muscle structure and function caused by mutation of troponin I. Biophys. J. 86, 991-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clandinin, T.R., DeModena, J.A., and Sternberg, P.W. (1998). Inositol trisphosphate mediates a RAS-independent response to LET-23 receptor tyrosine kinase activation in C. elegans. Cell 92, 523-533. [DOI] [PubMed] [Google Scholar]
- Cooper, J. (2002). Actin dynamics: tropomyosin provides stability. Curr. Biol. 12, R523-R525. [DOI] [PubMed] [Google Scholar]
- Cram, E.J., Clark, S.G., and Schwarzbauer, J.E. (2003). Talin loss-of-function uncovers roles in cell contractility and migration in C. elegans. J. Cell Sci. 116, 3871-3878. [DOI] [PubMed] [Google Scholar]
- Drees, B., Brown, C., Barrell, B.G., and Bretscher, A. (1995). Tropomyosin is essential in yeast, yet the TPM1 and TPM2 products perform distinct functions. J. Cell Biol. 128, 383-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo, T., and Obinata, T. (1981). Troponin and its components from ascidian smooth muscle. J. Biochem. 89, 1599-1608. [DOI] [PubMed] [Google Scholar]
- Engqvist-Goldstein, A.E., and Drubin, D.G. (2003). Actin assembly and endocytosis: from yeast to mammals. Annu. Rev. Cell Dev. Biol. 19, 287-332. [DOI] [PubMed] [Google Scholar]
- Epstein, H.F., Casey, D.L., and Ortiz, I. (1993). Myosin and paramyosin of Caenorhabditis elegans embryos assemble into nascent structures distinct from thick filaments and multi-filament assemblages. J. Cell Biol. 122, 845-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein, H.F., and Thomson, J.N. (1974). Temperature-sensitive mutation affecting myofilament assembly in Caenorhabditis elegans. Nature 250, 579-580. [DOI] [PubMed] [Google Scholar]
- Gordon, A.M., Homsher, E., and Regnier, M. (2000). Regulation of contraction in striated muscle. Physiol. Rev. 80, 853-924. [DOI] [PubMed] [Google Scholar]
- Grant, B., and Hirsh, D. (1999). Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol. Biol. Cell 10, 4311-4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning, P., Weinberger, R., Jeffrey, P., and Hardeman, E. (1998). Isoform sorting and the creation of intracellular compartments. Annu. Rev. Cell Dev. Biol. 14, 339-372. [DOI] [PubMed] [Google Scholar]
- Hall, D.H., Winfrey, V.P., Blaeuer, G., Hoffman, L.H., Furuta, T., Rose, K.L., Hobert, O., and Greenstein, D. (1999). Ultrastructural features of the adult hermaphrodite gonad of Caenorhabditis elegans: relations between the germ line and soma. Dev. Biol. 212, 101-123. [DOI] [PubMed] [Google Scholar]
- Harris, H.E., Tso, M-Y.W., and Epstein, H.F. (1977). Actin and myosin-linked calcium regulation in the nematode Caenorhabditis elegans. Biochemical and structural properties of native filaments and purified proteins. Biochemistry 16, 859-865. [DOI] [PubMed] [Google Scholar]
- Hartshorne, D.J., Ito, M., and Erdodi, F. (1998). Myosin light chain phosphatase: subunit composition, interactions and regulation. J. Muscle Res. Cell Motil. 19, 325-341. [DOI] [PubMed] [Google Scholar]
- Hubbard, E.J., and Greenstein, D. (2000). The Caenorhabditis elegans gonad: a test tube for cell and developmental biology. Dev. Dyn. 218, 2-22. [DOI] [PubMed] [Google Scholar]
- Iwasaki, K., McCarter, J., Francis, R., and Schedl, T. (1996). emo-1, a Caenorhabditis elegans Sec61p gamma homologue, is required for oocyte development and ovulation. J. Cell Biol. 134, 699-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa, H., Sugimoto, K., Matsumoto, H., Inoue, T., Imadzu, H., Takuwa, K., and Sakube, Y. (1995). Genome structure, mapping and expression of the tropomyosin gene tmy-1 of Caenorhabditis elegans. J. Mol. Biol. 251, 603-613. [DOI] [PubMed] [Google Scholar]
- Kamath, R.S., et al. (2003). Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421, 231-237. [DOI] [PubMed] [Google Scholar]
- Kureishi, Y., Kobayashi, S., Amano, M., Kimura, K., Kanaide, H., Nakano, T., Kaibuchi, K., and Ito, M. (1997). Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J. Biol. Chem. 272, 12257-12260. [DOI] [PubMed] [Google Scholar]
- Lee, M., Cram, E.J., Shen, B., and Schwarzbauer, J.E. (2001). Roles for βpat-3 integrins in development and function of Caenorhabditis elegans muscles and gonads. J. Biol. Chem. 276, 36404-36410. [DOI] [PubMed] [Google Scholar]
- Lewis, J.A., Wu, C.H., Berg, H., and Levine, J.H. (1980). The genetics of levamisole resistance in the nematode Caenorhabditis elegans. Genetics 95, 905-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston, S. (1995). Ca2+-dependent protein switches in actomyosin based contractile systems. Int. J. Biochem. Cell Biol. 27, 97-108. [DOI] [PubMed] [Google Scholar]
- McCarter, J., Bartlett, B., Dang, T., and Schedl, T. (1997). Soma-germ cell interactions in Caenorhabditis elegans: multiple events of hermaphrodite germline development require the somatic sheath and spermathecal lineages. Dev. Biol. 181, 121-143. [DOI] [PubMed] [Google Scholar]
- McCarter, J., Bartlett, B., Dang, T., and Schedl, T. (1999). On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev. Biol. 205, 111-128. [DOI] [PubMed] [Google Scholar]
- McKim, K.S., Heschl, M.F., Rosenbluth, R.E., and Baillie, D.L. (1988). Genetic organization of the unc-60 region in Caenorhabditis elegans. Genetics 118, 49-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michele, D.E., and Metzger, J.M. (2000). Physiological consequences of tropomyosin mutations associated with cardiac and skeletal myopathies. J. Mol. Med. 78, 543-553. [DOI] [PubMed] [Google Scholar]
- Miller, M.A., Nguyen, V.Q., Lee, M.H., Kosinski, M., Schedl, T., Caprioli, R.M., and Greenstein, D. (2001). A sperm cytoskeletal protein that signals oocyte meiotic maturation and ovulation. Science 291, 2144-2147. [DOI] [PubMed] [Google Scholar]
- Miller, M.A., Ruest, P.J., Kosinski, M., Hanks, S.K., and Greenstein, D. (2003). An Eph receptor sperm-sensing control mechanism for oocyte meiotic maturation in Caenorhabditis elegans. Genes Dev. 17, 187-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, C.D., Goh, P.Y., Allen, T.S., Bucher, E.A., and Bogaert, T. (1996). Developmental genetic analysis of troponin T mutations in striated and nonstriated muscle cells of Caenorhabditis elegans. J. Cell Biol. 132, 1061-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishita, K., Ojima, T., Takahashi, A., and Inoue, A. (1997). Troponin from smooth adductor muscle of Ezo-giant scallop. J. Biochem. 121, 419-424. [DOI] [PubMed] [Google Scholar]
- Ojima, T., and Nishita, K. (1986). Isolation of troponins from striated and smooth adductor muscles of Akazara scallop. J. Biochem. 100, 821-824. [DOI] [PubMed] [Google Scholar]
- Ono, K., Parast, M., Alberico, C., Benian, G.M., and Ono, S. (2003). Specific requirement for two ADF/cofilin isoforms in distinct actin-dependent processes in Caenorhabditis elegans. J. Cell Sci. 116, 2073-2085. [DOI] [PubMed] [Google Scholar]
- Ono, S. (2001). The Caenorhabditis elegans unc-78 gene encodes a homologue of actin-interacting protein 1 required for organized assembly of muscle actin filaments. J. Cell Biol. 152, 1313-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono, S. (2003). Regulation of actin filament dynamics by actin depolymerizing factor/cofilin and actin-interacting protein 1, new blades for twisted filaments. Biochemistry 42, 13363-13370. [DOI] [PubMed] [Google Scholar]
- Ono, S., Baillie, D.L., and Benian, G.M. (1999). UNC-60B, an ADF/cofilin family protein, is required for proper assembly of actin into myofibrils in Caenorhabditis elegans body wall muscle. J. Cell Biol. 145, 491-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono, S., McGough, A., Pope, B.J., Tolbert, V.T., Bui, A., Pohl, J., Benian, G.M., Gernert, K.M., and Weeds, A.G. (2001). The C-terminal tail of UNC-60B (ADF/cofilin) is critical for maintaining its stable association with F-actin and is implicated in the second actin-binding site. J. Biol. Chem. 276, 5952-5958. [DOI] [PubMed] [Google Scholar]
- Ono, S., and Ono, K. (2002). Tropomyosin inhibits ADF/cofilin-dependent actin filament dynamics. J. Cell Biol. 156, 1065-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rethinasamy, P., Muthuchamy, M., Hewett, T., Boivin, G., Wolska, B.M., Evans, C., Solaro, R.J., and Wieczorek, D.F. (1998). Molecular and physiological effects of alpha-tropomyosin ablation in the mouse. Circ. Res. 82, 116-123. [DOI] [PubMed] [Google Scholar]
- Rose, K.L., Winfrey, V.P., Hoffman, L.H., Hall, D.H., Furuta, T., and Greenstein, D. (1997). The POU gene ceh-18 promotes gonadal sheath cell differentiation and function required for meiotic maturation and ovulation in Caenorhabditis elegans. Dev. Biol. 192, 59-77. [DOI] [PubMed] [Google Scholar]
- Rushforth, A.M., White, C.C., and Anderson, P. (1998). Functions of the Caenorhabditis elegans regulatory myosin light chain genes mlc-1 and. mlc-2. Genetics 150, 1067-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, D.A. (2002). Coupling actin dynamics and membrane dynamics during endocytosis. Curr. Opin. Cell Biol. 14, 76-81. [DOI] [PubMed] [Google Scholar]
- Strome, S. (1986). Fluorescence visualization of the distribution of microfilaments in gonads and early embryos of the nematode Caenorhabditis elegans. J. Cell Biol. 103, 2241-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szent-Györgyi, A.G. (1996). Regulation of contraction by calcium binding myosins. Biophys. Chem. 59, 357-363. [DOI] [PubMed] [Google Scholar]
- Terami, H., Williams, B.D., Kitamura, S., Sakube, Y., Matsumoto, S., Doi, S., Obinata, T., and Kagawa, H. (1999). Genomic organization, expression, and analysis of the troponin C gene pat-10 of Caenorhabditis elegans. J. Cell Biol. 146, 193-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons, L., and Fire, A. (1998). Specific interference by ingested dsRNA. Nature 395, 854. [DOI] [PubMed] [Google Scholar]
- Tubridy, N., Fontaine, B., and Eymard, B. (2001). Congenital myopathies and congenital muscular dystrophies. Curr. Opin. Neurol. 14, 575-582. [DOI] [PubMed] [Google Scholar]
- Uehata, M., et al. (1997). Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389, 990-994. [DOI] [PubMed] [Google Scholar]
- Wang, C.L. (2001). Caldesmon and smooth-muscle regulation. Cell Biochem. Biophys. 35, 275-288. [DOI] [PubMed] [Google Scholar]
- Ward, S., and Carrel, J.S. (1979). Fertilization and sperm competition in the nematode Caenorhabditis elegans. Dev. Biol. 73, 304-321. [DOI] [PubMed] [Google Scholar]
- Waterston, R.H. (1989). The minor myosin heavy chain, mhcA, of Caenorhabditis elegans is necessary for the initiation of thick filament assembly. EMBO J. 8, 3429-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, B.D., and Waterston, R.H. (1994). Genes critical for muscle development and function in Caenorhabditis elegans identified through lethal mutations. J. Cell Biol. 124, 475-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissmann, A., Ingles, J., and Mains, P.E. (1999). The Caenorhabditis elegans mel-11 myosin phosphatase regulatory subunit affects tissue contraction in the somatic gonad and the embryonic epidermis and genetically interacts with the Rac signaling pathway. Dev. Biol. 209, 111-127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.