Abstract
As the incidence of cystic fibrosis (CF) bone disease is increasing, we analyzed cystic fibrosis transmembrane conductance regulator (CFTR) deficient mice (CF mice) to gain pathogenic insights. In these studies comparing adult (14 wk) CF and C57BL/6J mice, both bone length and total area were decreased in CF mice. Metaphyseal trabecular & cortical density were also decreased, as well as diaphyseal cortical & total density. Trabecular bone volume was diminished in CF mice. Female CF mice revealed decreased trabecular width and number compared to C57BL/6J, whereas males demonstrated no difference in trabecular number. Female CF mice had reduced mineralizing surface and bone formation rates. Conversely, male CF mice had increased mineralizing surface, mineral apposition and bone formation rates compared to C57BL/6J males. Bone formation rate was greater in males compared to female CF mice. Smaller bones with decreased density in CF, despite absent differences in osteoblast and osteoclast surfaces, suggest CFTR influences bone cell activity rather than number. Differences in bone formation rate in CF mice are suggestive of inadequate bone formation in females, but increased bone formation in males. This pro-anabolic observation in male CF mice is consistent with other clinical sex differences in CF.
Keywords: cystic fibrosis, CFTR, osteopenia, sex-related, bone formation
Cystic Fibrosis (CF) is a relatively common single gene disorder characterized by chloride channel dysfunction in multiple organ systems, several of which contribute to a systemic inflammatory state in most patients (1, 2). In Caucasians, it is the leading life-limiting recessive genetic disorder, and originates from a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene (3). The first clinical manifestations may occur during the fetal period, with intestinal obstruction and perforation of the bowel. However, the major manifestations that afflict these individuals include the inability to absorb fats and other nutrients from the intestine, as well as poor lung function secondary to airway inflammation and infection. Other problems associated with CF include diabetes, bone mineral disease, poor growth, liver disease, infertility, and chronic sinusitis. In 1938, the mean life expectancy for children with CF was less than one year (4). Over the past 30 years, the life expectancy of children with CF in the United States has increased from 16 years of age to 37 years (2, 4). This change has been attributed to improvements in airway clearance, antibiotics, and nutritional support.
As life expectancy of CF patients has increased, chronic complications of the disorder have become more prominent. Bone disease is a major impediment to the quality of life and overall health of adolescents and young adults with CF (5). The frequency of bone disease in CF has increased to 16.7% of the nearly 25,000 patients currently enrolled in the CF Foundation Patient Registry (2). Currently, the CF Foundation recommends that all patients above the age of 18 years be screened for osteoporosis (6, 7). Although controversy exists over whether fractures are increased in individuals with CF, case reports of severe outcomes persist (5, 8, 9). In one extreme case, Latzin et al. describe a 16-year-old girl with CF and osteoporosis, who died from respiratory failure following a spontaneous fracture of the mid-sternum (10).
However the full impact of decreased bone mineral density (BMD) and its pathogenesis in CF are not known. Many factors weigh against the development of normal bone health in individuals with CF. Some problems develop during the childhood years of a patient with CF, including poor nutrition, malabsorption of vitamin D, poor growth and delayed pubertal development (7). Malabsorption of fats and proteins secondary to pancreatic insufficiency is reported in 59% of newborns with CF and by one year of life, 90% are affected (4). The resultant inability to absorb fat-soluble vitamins, most importantly vitamin D, but also vitamin K, is linked to abnormalities in bone mineral metabolism (11, 12). Problems with malabsorption inherently result in poor growth, although in the presence of adequate nutrition via gastrostomy feedings, growth may still be impaired (13). Other contributors to bone disease that tend to occur as individuals age include: chronic pulmonary infections, decreased physical activity, hypogonadism and corticosteroid usage (6, 7).
Elkin et al. illustrate complexities of CF bone disease with a comparison of bone formation rates in CF individuals versus controls (14). Although demonstrating a reduction in bone formation rate in CF subjects, the study was confounded by pancreatic insufficiency, ongoing lung disease and chronic corticosteroid therapy. Corticosteroids interfere with bone formation and increase bone resorption (15, 16). Use of animal models such as CFTR-deficient mice allow investigators to separate out variables.
Our hypothesis is that an inherent dysfunction of bone mineral metabolism is directly associated with CFTR dysfunction in CF, and subsequently the loss of normal function will result in decreased bone density in the murine model. Previous studies utilizing non-gut corrected CFTR-deficient mice described decreased bone density without evidence of sex-related differences (17-19). To our knowledge, this is the first and most comprehensive analysis of bone in gut-corrected CF mice, including dynamic evidence of differences in bone formation between sexes.
Methods
Mice
The primary CFTR knockout strain utilized in this study was the Cftr S489X -/- neo insertion in C57BL/6J mice, initially developed at the University of North Carolina and then modified with the transgenic overexpression of gut-specific human CFTR from the fatty acid binding promoter (FABP) in order to prevent intestinal obstruction, allowing for weaning to a normal solid chow diet, and overall improving viability. C57BL6 Cftr(-/-) FABP-hCftr(+/+) have been back crossed against the C57BL/6J mouse for greater than ten generations, providing a knockout comparison to the C57BL/6J mouse. Mice are bred via heterozygote breeding pairs, and housed under Specific Pathogen Free (SPF) conditions at the University of Florida Animal Care Services according to NIH guidelines and allowed food and water ad libitum. All experimental procedures were approved by University of Florida IACUC.
Pre-Necropsy and Necropsy Procedures
All mice were injected subcutaneously with the fluorochrome compounds declomycin and calcein at a dose of 15 mg/kg body weight on the 5th and 2nd days prior to sacrifice, respectively (20). Ten CF mice (5 male & 5 female) and 14 C57BL/6J mice (5 male & 9 female) were sacrificed at 14 weeks of age. Following sacrifice, both femora from each animal were stripped of musculature and placed in 10% phosphate-buffered formalin (pH 7.4) for 24 hours for tissue fixation. These bone specimens were then transferred to 70% ethanol and processed as described below.
Ex Vivo Bone Structural Analyses
The length of the left femur was measured in each animal with a precision caliper (General Hardware, New York, NY), after which these long bones were scanned by peripheral quantitative computed tomography (pQCT) with a Stratec XCT Research M instrument (Norland Medical Systems, Fort Atkinson, WI). Scans were performed at distances of 2.5 and 7.5 mm proximal to the distal end of the femur for measurements of cancellous and cortical bone structure, respectively. The first site (2.5 mm) was at the level of the secondary spongiosa of the distal femoral metaphysis whereas the second site (7.5 mm) was in the femoral diaphysis composed entirely of cortical bone. The structural variables that were measured include: total mineral content, trabecular content, cortical content, total mineral density, trabecular density, cortical density, total area, trabecular area, cortical area, marrow area, cortical thickness and periosteal circumference.
Quantitative Bone Histomorphometry
The right distal femur from each animal was dehydrated in increasing concentrations of ethanol and embedded undecalcified in modified methyl methacrylate (21). The embedded bones were sectioned longitudinally at 4 and 8 μm thickness with Leica/Jung 2050 or 2165 microtomes. The 4 μm-thick sections were stained according to the Von Kossa method with a tetrachrome counterstain. Structural and cellular variables were measured in these sections with the Osteometrics System (Atlanta, GA) and the Bioquant Elite Bone Morphometry System (R&M Biometrics, Nashville, TN). The sample area within the distal femoral metaphysis began at 1 mm proximal to the growth plate to exclude the primary spongiosa. The following bone variables were measured in the thin sections: cancellous bone volume (%), trabecular number (#/mm), trabecular separation (μm), trabecular width (μm), osteoblast surface (%), and osteoclast surface (%). In addition, the following fluorochrome-based data were collected from unstained, 8 μm-thick bone sections with the same quantitative system: mineralizing surface (%), mineral apposition rate (μm/day), and bone formation rate (surface referent, μm3/μm2/day).
Statistical Analysis
Data were analyzed via an unpaired t test. Results are reported as mean values with standard deviation. All statistical analyses were conducted using GraphPad Prism 5.0 software with a two-tailed p < 0.05 considered significant (GraphPad Software, San Diego, CA).
Results
Cftr-/- Genotype Results in Decreased Femur Size
Femur size of C57BL/6J and CF mice was compared (Table 1) based on femur length (measured by precision calipers) and area (obtained by pQCT). Femur length was shorter in both male and female CF mice compared to gender-matched C57BL/6J controls (males p<0.001 and females p<0.0001). Metaphyseal total area was reduced in both male and female CF mice (p<0.001 for both), as well as total area of diaphysis (p<0.01 and p<0.001 respectively). In addition, cortical bone area was reduced in both male and female CF mice at both the metaphysis and diaphysis (metaphysis: male p<0.001 and female p<0.0001; diaphysis: male p<0.01 and female p<0.0001).
Table 1. Femur Length and Area Comparison.
Femurs from 14-week-old C57BL/6J Cftr-/- mice were compared to C57BL/6J mice for length, total bone area (bone + bone marrow), and cortical area. C57BL/6J Cftr-/- mice (both male and female) had shorter bones, and had decreased bone and cortical area at the metaphysis (M) and diaphysis (D) compared to C57BL/6J control mice.
| Measurement | Female C57BL/6J (n = 9) |
Female C57BL/6J Cftr-/- (n = 5) |
Male C57BL/6J (n = 5) |
Male C57BL/6J Cftr-/- (n = 5) |
|---|---|---|---|---|
| Femur Length (mm) | 15.89±0.3 | 14.13±0.33 ‡ | 16.01±0.13 | 15.16±0.31 † |
| M Total Area (mm2) | 2.81±0.21 | 2.30±0.16 † | 3.084±0.14 | 2.548±0.13 † |
| M Cortical Area (mm2) | 1.15±0.05 | 0.88±0.03 ‡ | 1.16±0.05 | 0.98±0.04 † |
| D Total Area (mm2) | 1.57±0.1 | 1.31±0.11 † | 1.774±0.09 | 1.446±0.14 ** |
| D Cortical Area (mm2) | 0.94±0.03 | 0.78±0.05 ‡ | 1.06±0.06 | 0.85±0.08 ** |
M=Metaphysis, D=Diaphysis, p values for comparison to same-sex C57BL/6J mice:
p<0.05,
p<0.01,
p<0.001, and
p<0.0001.
CF Mice have Decreased Bone Content and Density
Bone content and density were compared by pQCT (Table 2 and Figure 1) at both the metaphysis and diaphysis in male and female CF mice and C57BL/6J controls. At the metaphysis, CF mice have decreased total bone content and density (bone content: males p<0.001 and females p<0.0001; bone density: males p<0.01 and females p<0.001). Trabecular bone content and density were reduced in CF mice (bone content: males p<0.01 and females p<0.001; bone density: males p<0.05 and females p<0.01). In addition, cortical bone content and density were reduced in CF mice compared to C57BL/6J controls (bone content: males p<0.01 and females p<0.0001; bone density: males p<0.05 and females p<0.0001).
Table 2. Peripheral Quantitative Computerized Tomography of Femur.
Metaphysis (M) and diaphysis (D) regions from 14-week-old C57BL/6J Cftr-/- mice were compared to C57BL/6J mice via peripheral quantitative computerized tomography (pQCT at 2.5 mm and 7.5 mm proximal to the distal end of the femur respectively). Bone mineral content and density were decreased in male and female C57BL/6J Cftr-/- mice at both the metaphysis and diaphysis.
| Measurement | Female C57BL/6J (n = 9) |
Female C57BL/6J Cftr-/- (n = 5) |
Male C57BL/6J (n = 5) |
Male C57BL/6J Cftr-/- (n = 5) |
|---|---|---|---|---|
| M Total Content (mg/mm) | 1.24±0.08 | 0.85±0.06 ‡ | 1.45±0.1 | 1.07±0.11 † |
| M Total Density (mg/cm3) | 443.6±25.2 | 369.9±23.1 † | 471.2±16.8 | 420.5±25.8 ** |
| M Trabecular Content (mg/mm) | 0.05±0.01 | 0.02±0.01 † | 0.13±0.02 | 0.07±0.04 ** |
| M Trabecular Density (mg/cm3) | 60.8±12.5 | 35.5±5.8 ** | 144.8±14.5 | 89.6±45.8 * |
| M Cortical Content (mg/mm) | 1.03±0.06 | 0.71±0.03 ‡ | 1±0.07 | 0.80±0.06 ** |
| M Cortical Density (mg/cm3) | 902.4±274 | 805.5±22.5 ‡ | 857.1±20 | 814.1±34.8 * |
| D Total Content (mg/mm) | 1.01±0.05 | 0.79±0.06 ‡ | 1.17±0.07 | 0.87±0.08 † |
| D Total Density (mg/cm3) | 642.1±17.3 | 604.1±45.9 * | 660.2±19.5 | 599.1±3.2 † |
| D Cortical Content (mg/mm) | 1.01±0.04 | 0.79±0.06 ‡ | 1.17±0.07 | 0.87±0.73 † |
| D Cortical Density (mg/cm3) | 1075±21.4 | 1018±18.1 † | 1097±11 | 1024±134 ‡ |
| D Cortical Thickness (mm) | 0.26±0.006 | 0.24±0.015 † | 0.276±0.01 | 0.243±0.012 ** |
| D Periosteal Circumference (mm) | 443±0.14 | 4.05±0.17 † | 4.72±0.12 | 4.26±0.21 ** |
M=Metaphysis, D=Diaphysis, p values for comparison to same-sex C57BL/6J mice:
p<0.05,
p<0.01,
p<0.001, and
p<0.0001.
Figure 1.
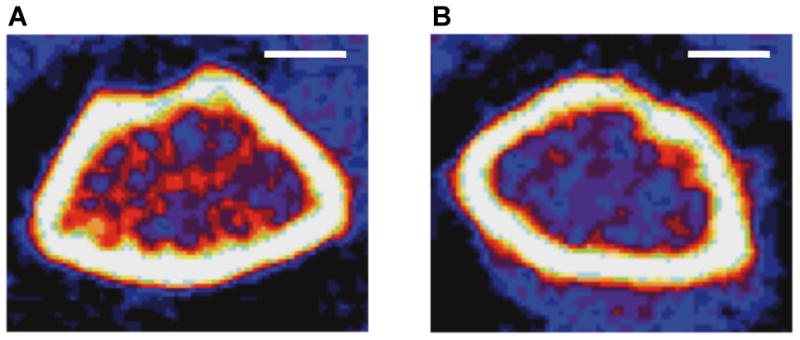
Peripheral quantitative computed tomography (pQCT) compared femoral metaphysis from female adult (14 week) C57BL/6J (1A, n=9) and C57BL/6J Cftr-/- (1B, n=5) mice. Total, cortical and trabecular densities of C57BL/6J Cftr-/- mice were decreased. The reduced red areas indicative of lower trabecular density in C57BL/6J Cftr-/- mice are apparent. Detailed results are included in Table 2. Scale bars= 1 mm
Total bone content and density were reduced in CF mice compared to C57BL/6J mice at the diaphysis (bone content: males p<0.001 and females p<0.0001; bone density: males p<0.001 and females p<0.05). Cortical bone content and density were decreased in CF mice (cortical bone content: males p<0.001 and females p<0.0001; cortical bone density: males p<0.0001 and females p<0.001). In addition, cortical thickness and periosteal circumference were less in male and female CF mice (cortical thickness: males p<0.01 and females p<0.001; periosteal circumference: males p<0.01 and females p<0.001).
Quantitative Bone Histomorphometry
Bone histomorphometry of the distal femur (Table 3) revealed decreased trabecular bone volume in CF mice (males p<0.05 and females p<0.01) as well as decreased trabecular width and number (trabecular width: males p<0.05 and females p<0.01; trabecular number: males p=NS and females p<0.01). In addition, trabecular separation was increased in female CF mice (p<0.01). No difference was detected in the percentage of osteoclast surface in trabecular bone, although there was a trend towards a significant decrease in the percentage of osteoblast surface in CF female mice (p=0.09). See detailed values and SD in table 3. Figure 2 shows representative distal femurs from C57BL/6J and CF mice.
Table 3. Histomorphologic Analysis of Cancellous Bone in Distal Femur.
Histomorphological comparison of distal femurs from 14-week-old C57BL/6J Cftr-/- to C57BL/6J mice. C57BL/6J Cftr-/- animals exhibited decreased indices of cancellous bone mass, which were more pronounced in female mice. No significant differences were observed in % osteoblast and osteoclast surfaces. Dynamic indices of bone formation demonstrate decreased bone formation rate (BFR) in females, but increased BFR in male C57BL/6J Cftr-/- mice. BFR was greater in male compared to female C57BL/6J Cftr-/- mice (P<0.5, see Figure 3).
| Measurement | Female C57BL/6J (n = 9) |
Female C57BL/6J Cftr-/- (n = 5) |
Male C57BL/6J (n = 5) |
Male C57BL/6J Cftr-/- (n = 5) |
|---|---|---|---|---|
| Trabecular Bone Volume (%) | 545±1.09 | 2.74±1.47 ** | 10.05±2.05 | 7.21±1.31 * |
| Trabecular Bone Width (μm) | 23.98±2.64 | 17.93±442 ** | 27.94±3.93 | 22.21±1.57 * |
| Trabecular Number (#/mm) | 2.73±0.44 | 1.73±0.54 ** | 4.29±0.38 | 3.91±0.72 |
| Trabecular Separation (μm) | 355.1±59.04 | 610.1±198.1 ** | 210.9±21.21 | 244.3±46.37 |
| Osteoclast Surface (%) | 1.9±1 | 2.3±0.9 | 1±0.2 | 1.5±0.7 |
| Osteoblast Surface (%) | 38±4.7 | 28.5±144 | 264±4.9 | 24.7±8.1 |
| Mineralizing Surface (%) | 22.77±3.58 | 10.86±3.05 ‡ | 948±1.35 | 17.72±2.24 † |
| Mineral Apposition Rate (μm/day) | 1.98±0.14 | 1.75±0.34 | 143±0.07 | 1.79±0.25 * |
| Bone Formation Rate (10-2μm3/μm2/d) | 45.07±7.33 | 18.89±646 ‡ | 13.57±1.78 | 32.06±8.29 ** |
p values for comparison to same-sex C57BL/6J mice:
p<0.05,
p<0.01,
p<0.001, and
p<0.0001.
Figure 2.
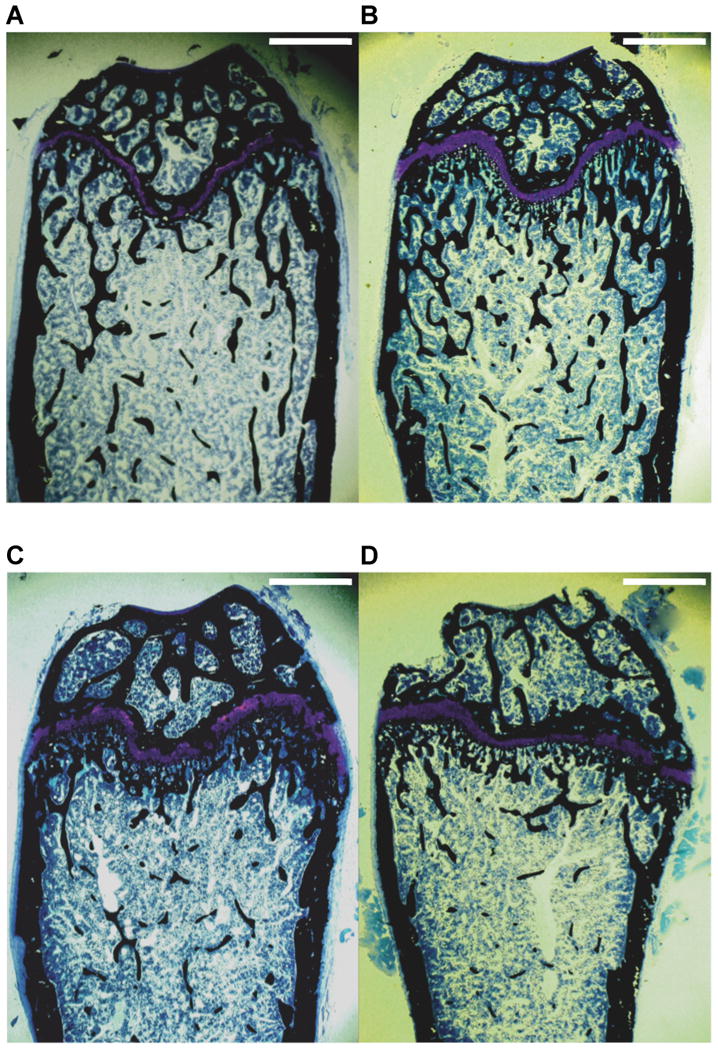
Histomorphological sections obtained from distal femurs of 14-week-old male C57BL/6J (2A, n=5), male C57BL/6J Cftr-/- (2B, n=5), female C57BL/6J (2C, n=9) and female C57BL/6J Cftr-/- (2D, n=5) mice at 20X. C57BL/6J Cftr-/- mice have decreased trabecular bone volume as well as decreased trabecular width. The reduced number of black-stained cancellous bone spicules is especially apparent in the female C57BL/6J Cftr-/- mouse. Magnification=20x, Scale bars= 1 mm
Table 3 details the mean values and SD of the dynamic bone measurements. Percentage of mineralizing surface was reduced in female CF mice (p<0.0001), but increased in male CF mice (p<0.001). In addition, mineral apposition rate was increased in male CF mice (p<0.05) and approached a significant decrease in female CF mice (p=0.09). The calculated bone formation rate was decreased in CF female mice compared to controls (p<0.0001) and increased in CF male mice compared to male controls (p<0.01) and female CF mice (p<0.05)(Figure 3).
Figure 3.
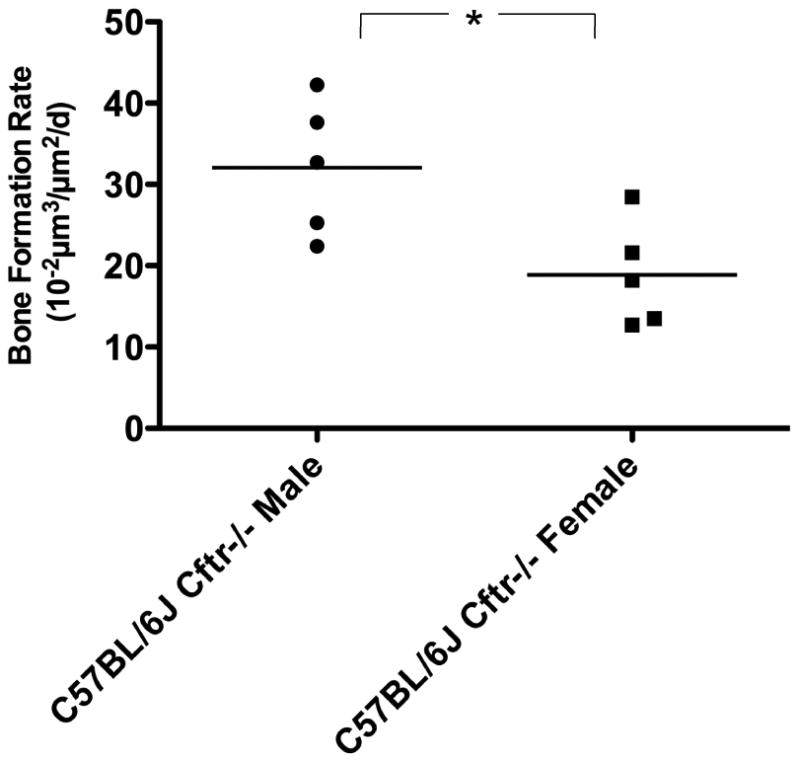
Cancellous bone formation rates are greater (*p<0.05) in male C57BL/6J Cftr-/- mice (n=5) than C57BL/6J Cftr-/- female mice (n=5).
Discussion
The incidence of CF bone disease continues to rise as the mean life expectancy of CF patients continues to increase (2, 5, 6). The origin of osteoporosis is postulated to be multifactoral in healthy individuals. In CF, factors associated with bone disease appear to be intensified. The contribution of the CF genetic background has only recently arrived on the scene of mechanisms of disease. Our novel findings demonstrate decreased bone density in adult CF mice, but also report sex-related differences in bone metabolism. Our model is based on CFTR deficient mice with a transgenic human gut correction. The advantage of this model is that it improves viability and life expectancy of the mice, and eliminates concerns for gut absorption and nutritional deficiency.
For many years, increased corticosteroid usage was blamed for decreased bone density in CF. Corticosteroids interfere with calcium absorption from the gut, decrease renal tubular reabsorption of calcium, suppress osteoblastic formation of new bone, as well as decreasing gonadal hormone production (15). Regarding bone resorption, corticosteroids increase osteoclastogenesis via increased production of receptor activator of nuclear factor-kappa B ligand (RANKL) (16). Stimulation of the receptor activator of nuclear factor-kappa B results in osteoclastogenesis. Aris et al. described increased markers of bone turnover in CF, reporting elevated urinary N-telopeptides and serum bone-specific alkaline phosphatase in CF subjects compared to healthy age-matched controls (22).
New insights into the development of osteoporosis implicate the impact of chronic systemic inflammation on the loss of bone mass (23). Inflammatory influences may originate in the bone marrow, peripheral blood, or directly at the bone. Reports suggest that proinflammatory cytokines and other biochemical signals are driving osteoclastogenesis in CF. The most common CFTR mutation (ΔF508) is a missense mutation which produces a misfolded version of CFTR that has been shown to trigger an endoplasmic reticulum overload response within cells, (24) resulting in widespread changes in gene expression (25-28). Among the most prominent of these changes is an upregulation of pro-inflammatory cytokines associated with innate immunity (26). This may correlate with the observation that CF patients (29) and CF mice (30) show an exaggerated pro-inflammatory cytokine response to bacterial challenge, as compared with normal individuals or control mouse strains. This same upregulation of proinflammatory cytokines may be driving the delineation of osteoclast formation and subsequent bone loss. A study on the impact of inflammation on bone mineral content in CF by Haworth et al, reported increased levels of urinary markers for osteoclast activity related to serum levels of IL-6 (31). Shead et al detected increased levels of osteoclast precursors in CF patients during infective exacerbations, specifically CD14/33/45-positive CD 34-negative cell populations (23). In an earlier study, Haworth et al demonstrated decreased osteoblastic and increased osteoclastic activity via bone histomorphology in adult CF patients (32). Whereas the reduced osteoblastic activity was the result of both decreased osteoblast numbers and diminished action via osteoid production, larger osteoclast numbers and evidence of increased absorption determined greater osteoclastic activity. In our study, we observed no difference in osteoclast surfaces in CF mice of both genders. Our findings suggest that an increase in bone resorption in these animals is due to an increase in osteoclast activity rather than an increase in osteoclast numbers. Although this differs slightly from the findings previously described in humans, further investigations to induce inflammation in this mouse model will help delineate the relationship.
Despite strong evidence of systemic modulators, we must not overlook the potential impact of CFTR itself on bone formation. Originally described in 2004 by Dif et al., three-week old CFTR-deficient mice demonstrated reduced bone density and bone formation compared to heterozygote (Cftr -/+) and wildtype (Cftr+/+) controls (17). The CFTR-deficient mouse model utilized by Dif and colleagues typically succumbs to intestinal obstruction when weaned to a solid chow diet. Thus, mice were compared prior to weaning and utilizing only female mice controlled for gender differences. Evidence further surfaced on the significance of CFTR in bone metabolism when Shead and colleagues demonstrated expression of CFTR in human osteoblasts, osteocytes and osteoclasts (33). However, as with other tissues in the body, CFTR expression does not necessarily translate to function.
Two recent studies have described decreased bone density persisting into adulthood in CFTR-deficient mice (18, 19). Both studies utilized CFTR-deficient mice without gut correction, and required supplemented feeds. Haston et al described reduced bone density in both 12 and 28-week CFTR-deficient mice, but found no difference in male or female mice (19). Our model for bone disease in CF requires no additional nutritional supplementation to prevent intestinal obstruction, eliminating influences of medical intervention, and improving the overall nutritional patency of the animal.
In addition to the direct contribution of CFTR on bone metabolism, which is suggested by protein expression, indirectly the production of anabolic hormones involved in bone remodeling must be considered. Although normal growth hormone values are reported in patients with CF, decreased levels of the downstream product IGF-I are described (34). Both are involved with bone metabolism and growth. IGF-I, a nutritionally dependent, growth hormone driven regulator of bone growth, is decreased in children with CF, even with gastrostomy nutritional supplementation (13). Furthermore, Rosenberg et al detailed decreased concentrations of IGF-I and liver IGF-I mRNA in both male and female CFTR-deficient mice (35).
Gut correction of CF mice used in our study, although allowing a normal diet, does not improve growth. Rosenberg et al demonstrated growth impairment (weight and length) in CFTR-deficient mice (35). To control for size differences, we utilized pQCT to analyze bone sections. Size artificially increases measured BMD in a two-dimensional analysis by dual-energy X-ray absorptiometry (DEXA, which measures areal BMD in g/cm2). pQCT eliminates these artifacts and measures a volumetric BMD (g/cm3). Bones of different sizes, from individuals of variable weight, can be compared (36).
However, pQCT does not remove differences in pubertal status. Previous studies in CFTR-deficient mice examined animals at three weeks (prepubertal), and also attempted to evaluate mice at the pubertal age of eight weeks (17, 19). Comparison of eight-week-old CFTR-deficient mice is problematic, due to differences in pubertal progression. Puberty (determined by vaginal opening) is delayed up to four weeks in CF mice (37). In a study by Jin et al, puberty occurred at 31.3±1.4 days for wild type female mice versus 48.9±9.3 days for CFTR-deficient females. To further eliminate variables in our study, BMD was only compared in mice of the same sex. Comparisons between male and female mice were made by bone formation rates, calculated from histomorphometry.
Interestingly, we found increased bone formation in male CF mice compared to females. This reflects general clinical differences in disease phenotype of men and women with CF. The concept of sex-related differences in CF are well established. These differences have included a more rapid decline in pulmonary function (38), alterations in voltage across the nasal epithelium (as measured by nasal potential difference) depending on menstrual cycles (39), colonization with Pseudomonas aeruginosa (40), and even emotional impact of disease (41). One of the most prominent differences in males and females with CF occurs with CF related diabetes. Milla et al reported a decreased survival in females with CF related diabetes by 16.3 years, whereas only 2.1 years for males (42). The etiology of these differences remains unclear.
Hodges et al detailed decreased fertility in female CFTR deficient mice (43). They found reduced uterine and ovarian size, decreased ovulation rates and abnormalities in estrous cycles. Gonadotropins, follicle stimulating hormone (FSH) and luteinizing hormone (LH), were within normal range reported for mice. Furthermore, exogenous human chorionic gonadotropin (hCG) corrected differences in organ size and ovulation of CFTR deficient mice. This suggests hypothalamic hypogonadism, a central dysfunction of sex hormone production. Similar findings occur in women with CF, including a delay in puberty and abnormalities in hormone production suggestive of hypothalamic origins (44-47). Amenorrhea, anovulation, irregular cycles, smaller uteri and decreased estrogen levels are associated with CF women (45, 48). Moreover, estrogen is a major contributor to bone health, growth and remodeling. The effects are primarily pro-osteoblastic and anti-osteoclastic through both direct and indirect signaling (49).
The increased rate of bone formation in male CF mice would lead one to expect a greater bone density in male CF mice compared to controls. However, bone density is determined by the equilibrium (or lack of equilibrium) between bone formation and bone resorption (6, 22, 49). Increased bone formation, coupled with a reduced bone density, suggests bone loss through increased bone resorption. Additional studies of osteoclast dynamics, as well as markers of bone breakdown, are needed to fully detail bone resorption in these animals. It is this inequality of reduced bone formation and increased bone resorption that we propose leads to the resultant state of osteopenia in CF.
In summary, CF related bone disease is the quintessential multifactoral disease. Data support a genetic susceptibility that combines with an environment rich in modifiers to increase bone resorption and poorly promote bone formation. Gender differences in disease represent magnifications of these modifiers. Improving our understanding of CF related bone disease has the potential to decrease the incidence, develop better therapies, and advance the overall understanding of mechanisms in metabolic bone disease.
Acknowledgments
Financial support: No additional financial assistance was received for this study.
Abbreviations
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- BMD
bone mineral density
Contributor Information
Troy D. Pashuck, Department of Pathology, Immunology and Laboratory Medicine, University of Florida, College of Medicine, Gainesville, FL 32610
Sarah E. Franz, Department of Physiological Sciences, University of Florida, College of Veterinary Medicine, Gainesville, FL 32610
Molly K. Altman, Department of Physiological Sciences, University of Florida, College of Veterinary Medicine, Gainesville, FL 32610
Clive H. Wasserfall, Department of Pathology, Immunology and Laboratory Medicine, University of Florida, College of Medicine, Gainesville, FL 32610
Mark A. Atkinson, Department of Pathology, Immunology and Laboratory Medicine, University of Florida, College of Medicine, Gainesville, FL 32610
Thomas J. Wronski, Department of Physiological Sciences, University of Florida, College of Veterinary Medicine, Gainesville, FL 32610
Terence R. Flotte, Department of Pediatrics, University of Massachusetts Medical School, Worcester, MA 01655
Michael S. Stalvey, Department of Pediatrics, University of Massachusetts Medical School, Worcester, MA 01655; Department of Pediatrics, University of Florida, College of Medicine, Gainesville, FL 32610
References
- 1.Boat TF, Welsh MJ, Beaudet AL. Cystic Fibrosis. In: Scriver C, Beaudet A, Sly W, Valle D, editors. The Metabolic Basis of Inherited Disease. McGraw-Hill; New York: 1989. pp. 2649–2680. [Google Scholar]
- 2.Patient Registry 2006 Annual Report. Cystic Fibrosis Foundation; Bethesda, Maryland: 2008. [Google Scholar]
- 3.Cheung JC, Deber CM. Misfolding of the cystic fibrosis transmembrane conductance regulator and disease. Biochemistry. 2008;47:1465–1473. doi: 10.1021/bi702209s. [DOI] [PubMed] [Google Scholar]
- 4.Orenstein DM, Winnie GB, Altman H. Cystic fibrosis: a 2002 update. J Pediatr. 2002;140:156–164. doi: 10.1067/mpd.2002.120269. [DOI] [PubMed] [Google Scholar]
- 5.Aris RM, Renner JB, Winders AD, Buell HE, Riggs DB, Lester GE, Ontjes DA. Increased rate of fractures and severe kyphosis: sequelae of living into adulthood with cystic fibrosis. Ann Intern Med. 1998;128:186–193. doi: 10.7326/0003-4819-128-3-199802010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Aris RM, Merkel PA, Bachrach LK, Borowitz DS, Boyle MP, Elkin SL, Guise TA, Hardin DS, Haworth CS, Holick MF, Joseph PM, O'Brien K, Tullis E, Watts NB, White TB. Guide to bone health and disease in cystic fibrosis. J Clin Endocrinol Metab. 2005;90:1888–1896. doi: 10.1210/jc.2004-1629. [DOI] [PubMed] [Google Scholar]
- 7.Boyle MP. Update on maintaining bone health in cystic fibrosis. Curr Opin Pulm Med. 2006;12:453–458. doi: 10.1097/01.mcp.0000245708.59138.a4. [DOI] [PubMed] [Google Scholar]
- 8.Rossini M, Del Marco A, Dal Santo F, Gatti D, Braggion C, James G, Adami S. Prevalence and correlates of vertebral fractures in adults with cystic fibrosis. Bone. 2004;35:771–776. doi: 10.1016/j.bone.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Rovner AJ, Zemel BS, Leonard MB, Schall JI, Stallings VA. Mild to moderate cystic fibrosis is not associated with increased fracture risk in children and adolescents. J Pediatr. 2005;147:327–331. doi: 10.1016/j.jpeds.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Latzin P, Griese M, Hermanns V, Kammer B. Sternal fracture with fatal outcome in cystic fibrosis. Thorax. 2005;60:616. doi: 10.1136/thx.2005.041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolfenden LL, Judd SE, Shah R, Sanyal R, Ziegler TR, Tangpricha V. Vitamin D and bone health in adults with cystic fibrosis. Clin Endocrinol (Oxf) 2008 doi: 10.1111/j.1365-2265.2008.03216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hecker TM, Aris RM. Management of osteoporosis in adults with cystic fibrosis. Drugs. 2004;64:133–147. doi: 10.2165/00003495-200464020-00002. [DOI] [PubMed] [Google Scholar]
- 13.Hardin DS, Rice J, Ahn C, Ferkol T, Howenstine M, Spears S, Prestidge C, Seilheimer DK, Shepherd R. Growth hormone treatment enhances nutrition and growth in children with cystic fibrosis receiving enteral nutrition. J Pediatr. 2005;146:324–328. doi: 10.1016/j.jpeds.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 14.Elkin SL, Vedi S, Bord S, Garrahan NJ, Hodson ME, Compston JE. Histomorphometric analysis of bone biopsies from the iliac crest of adults with cystic fibrosis. Am J Respir Crit Care Med. 2002;166:1470–1474. doi: 10.1164/rccm.200206-578OC. [DOI] [PubMed] [Google Scholar]
- 15.Goodman SB, Jiranek W, Petrow E, Yasko AW. The effects of medications on bone. J Am Acad Orthop Surg. 2007;15:450–460. doi: 10.5435/00124635-200708000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Hofbauer LC, Gori F, Riggs BL, Lacey DL, Dunstan CR, Spelsberg TC, Khosla S. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology. 1999;140:4382–4389. doi: 10.1210/endo.140.10.7034. [DOI] [PubMed] [Google Scholar]
- 17.Dif F, Marty C, Baudoin C, de Vernejoul MC, Levi G. Severe osteopenia in CFTR-null mice. Bone. 2004;35:595–603. doi: 10.1016/j.bone.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Saeed Z, Guilbault C, De Sanctis JB, Henri J, Marion D, St-Arnaud R, Radzioch D. Fenretinide prevents the development of osteoporosis in Cftr-KO mice. J Cyst Fibros. 2008;7:222–230. doi: 10.1016/j.jcf.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Haston CK, Li W, Li A, Lafleur M, Henderson JE. Persistent osteopenia in adult cystic fibrosis transmembrane conductance regulator-deficient mice. Am J Respir Crit Care Med. 2008;177:309–315. doi: 10.1164/rccm.200705-659OC. [DOI] [PubMed] [Google Scholar]
- 20.Frost H. Bone Histomorphometry: Choice of Marking Agent and Labeling Schedule. In: Recker R, editor. Bone Histomorphometry: Techniques and Interpretation. CRC Press; Boca Raton, FL: 1983. pp. 37–52. [Google Scholar]
- 21.Baron R, Vignery A, Neff L, Silvergate A, Santa Maria A. Processing of Undecalcified Bone Specimens for Bone Histomorphometry. In: Recker R, editor. Bone Histomorphometry: Techniques and Interpretation. CRC Press; Boca Raton, FL: 1983. pp. 13–35. [Google Scholar]
- 22.Aris RM, Ontjes DA, Buell HE, Blackwood AD, Lark RK, Caminiti M, Brown SA, Renner JB, Chalermskulrat W, Lester GE. Abnormal bone turnover in cystic fibrosis adults. Osteoporos Int. 2002;13:151–157. doi: 10.1007/s001980200007. [DOI] [PubMed] [Google Scholar]
- 23.Shead EF, Haworth CS, Gunn E, Bilton D, Scott MA, Compston JE. Osteoclastogenesis during infective exacerbations in patients with cystic fibrosis. Am J Respir Crit Care Med. 2006;174:306–311. doi: 10.1164/rccm.200512-1943OC. [DOI] [PubMed] [Google Scholar]
- 24.Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Y, Clark JC, Aronow BJ, Dey CR, Liu C, Wooldridge JL, Whitsett JA. Transcriptional adaptation to cystic fibrosis transmembrane conductance regulator deficiency. J Biol Chem. 2003;278:7674–7682. doi: 10.1074/jbc.M210277200. [DOI] [PubMed] [Google Scholar]
- 26.Virella-Lowell I, Herlihy JD, Liu B, Lopez C, Cruz P, Muller C, Baker HV, Flotte TR. Effects of CFTR, interleukin-10, and Pseudomonas aeruginosa on gene expression profiles in a CF bronchial epithelial cell Line. Mol Ther. 2004;10:562–573. doi: 10.1016/j.ymthe.2004.06.215. [DOI] [PubMed] [Google Scholar]
- 27.Venkatakrishnan A, Stecenko AA, King G, Blackwell TR, Brigham KL, Christman JW, Blackwell TS. Exaggerated activation of nuclear factor-kappaB and altered IkappaB-beta processing in cystic fibrosis bronchial epithelial cells. Am J Respir Cell Mol Biol. 2000;23:396–403. doi: 10.1165/ajrcmb.23.3.3949. [DOI] [PubMed] [Google Scholar]
- 28.Lory S, Ichikawa JK. Pseudomonas-epithelial cell interactions dissected with DNA microarrays. Chest. 2002;121:36S–39S. doi: 10.1378/chest.121.3_suppl.36s. [DOI] [PubMed] [Google Scholar]
- 29.Kirchner KK, Wagener JS, Khan TZ, Copenhaver SC, Accurso FJ. Increased DNA levels in bronchoalveolar lavage fluid obtained from infants with cystic fibrosis. Am J Respir Crit Care Med. 1996;154:1426–1429. doi: 10.1164/ajrccm.154.5.8912759. [DOI] [PubMed] [Google Scholar]
- 30.Heeckeren A, Walenga R, Konstan MW, Bonfield T, Davis PB, Ferkol T. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J Clin Invest. 1997;100:2810–2815. doi: 10.1172/JCI119828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haworth CS, Selby PL, Webb AK, Martin L, Elborn JS, Sharples LD, Adams JE. Inflammatory related changes in bone mineral content in adults with cystic fibrosis. Thorax. 2004;59:613–617. doi: 10.1136/thx.2003.012047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haworth CS, Webb AK, Egan JJ, Selby PL, Hasleton PS, Bishop PW, Freemont TJ. Bone histomorphometry in adult patients with cystic fibrosis. Chest. 2000;118:434–439. doi: 10.1378/chest.118.2.434. [DOI] [PubMed] [Google Scholar]
- 33.Shead EF, Haworth CS, Condliffe AM, McKeon DJ, Scott MA, Compston JE. Cystic fibrosis transmembrane conductance regulator (CFTR) is expressed in human bone. Thorax. 2007;62:650–651. doi: 10.1136/thx.2006.075887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laursen EM, Lanng S, Rasmussen MH, Koch C, Skakkebaek NE, Muller J. Normal spontaneous and stimulated GH levels despite decreased IGF-I concentrations in cystic fibrosis patients. Eur J Endocrinol. 1999;140:315–321. doi: 10.1530/eje.0.1400315. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg LA, Schluchter MD, Parlow AF, Drumm ML. Mouse as a model of growth retardation in cystic fibrosis. Pediatr Res. 2006;59:191–195. doi: 10.1203/01.pdr.0000196720.25938.be. [DOI] [PubMed] [Google Scholar]
- 36.Sawyer A, Bachrach LK, Fung E, editors. Bone Densitometry in Growing Patients. Humana Press; Totowa, New Jersey: 2007. pp. 15–57. [Google Scholar]
- 37.Jin R, Hodges CA, Drumm ML, Palmert MR. The cystic fibrosis transmembrane conductance regulator (Cftr) modulates the timing of puberty in mice. J Med Genet. 2006;43:e29. doi: 10.1136/jmg.2005.032839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corey M, Edwards L, Levison H, Knowles M. Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. J Pediatr. 1997;131:809–814. doi: 10.1016/s0022-3476(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 39.Sweezey NB, Smith D, Corey M, Ellis L, Carpenter S, Tullis DE, Durie P, O'Brodovich HM. Amiloride-insensitive nasal potential difference varies with the menstrual cycle in cystic fibrosis. Pediatr Pulmonol. 2007;42:519–524. doi: 10.1002/ppul.20624. [DOI] [PubMed] [Google Scholar]
- 40.Levy H, Kalish LA, Cannon CL, Garcia KC, Gerard C, Goldmann D, Pier GB, Weiss ST, Colin AA. Predictors of mucoid Pseudomonas colonization in cystic fibrosis patients. Pediatr Pulmonol. 2008;43:463–471. doi: 10.1002/ppul.20794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patterson JM, Wall M, Berge J, Milla C. Gender differences in treatment adherence among youth with cystic fibrosis: Development of a new questionnaire. J Cyst Fibros. 2008;7:154–164. doi: 10.1016/j.jcf.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Milla CE, Billings J, Moran A. Diabetes is associated with dramatically decreased survival in female but not male subjects with cystic fibrosis. Diabetes Care. 2005;28:2141–2144. doi: 10.2337/diacare.28.9.2141. [DOI] [PubMed] [Google Scholar]
- 43.Hodges CA, Palmert MR, Drumm ML. Infertility in females with cystic fibrosis is multifactorial: evidence from mouse models. Endocrinology. 2008;149:2790–2797. doi: 10.1210/en.2007-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johannesson M, Gottlieb C, Hjelte L. Delayed puberty in girls with cystic fibrosis despite good clinical status. Pediatrics. 1997;99:29–34. doi: 10.1542/peds.99.1.29. [DOI] [PubMed] [Google Scholar]
- 45.Johannesson M, Landgren BM, Csemiczky G, Hjelte L, Gottlieb C. Female patients with cystic fibrosis suffer from reproductive endocrinological disorders despite good clinical status. Hum Reprod. 1998;13:2092–2097. doi: 10.1093/humrep/13.8.2092. [DOI] [PubMed] [Google Scholar]
- 46.Reiter EO, Stern RC, Root AW. The reproductive endocrine system in cystic fibrosis. I. Basal gonadotropin and sex steroid levels. Am J Dis Child. 1981;135:422–426. doi: 10.1001/archpedi.1981.02130290020009. [DOI] [PubMed] [Google Scholar]
- 47.Reiter EO, Stern RC, Root AW. The reproductive endocrine system in cystic fibrosis: 2. Changes in gonadotrophins and sex steroids following LHRH. Clin Endocrinol (Oxf) 1982;16:127–137. doi: 10.1111/j.1365-2265.1982.tb03156.x. [DOI] [PubMed] [Google Scholar]
- 48.Stead RJ, Hodson ME, Batten JC, Adams J, Jacobs HS. Amenorrhoea in cystic fibrosis. Clin Endocrinol (Oxf) 1987;26:187–195. doi: 10.1111/j.1365-2265.1987.tb00776.x. [DOI] [PubMed] [Google Scholar]
- 49.Krum SA, Brown M. Unraveling estrogen action in osteoporosis. Cell Cycle. 2008;7:1348–1352. doi: 10.4161/cc.7.10.5892. [DOI] [PubMed] [Google Scholar]


