Abstract
Pulmonary surfactant contains homeostatic and antimicrobial hydrolases. When Mycobacterium tuberculosis is initially deposited in the terminal bronchioles and alveoli, as well as following release from lysed macrophages, bacilli are in intimate contact with these lung surfactant hydrolases. We identified and measured several hydrolases in human alveolar lining fluid and lung tissue that, at their physiological concentrations, dramatically modified the M. tuberculosis cell envelope. Independent of their action time (15 min to 12 h), the effects of the hydrolases on the M. tuberculosis cell envelope resulted in a significant decrease (60–80%) in M. tuberculosis association with, and intracellular growth of the bacteria within, human macrophages. The cell envelope-modifying effects of the hydrolases also led to altered M. tuberculosis intracellular trafficking and induced a protective proin-flammatory response to infection. These findings add a new concept to our understanding of M. tuberculosis–macrophage inter-actions (i.e., the impact of lung surfactant hydrolases on M. tuberculosis infection).
World Health Organization data indicate that 8 million people are newly infected with Mycobacterium tuberculosis, and 3 million people die of tuberculosis every year (1). M. tuberculosis is an intracellular pathogen highly adapted to its natural host: the human and its major host cell reservoirs, monocytes, and macrophages (2). The outermost components of the M. tuberculosis cell envelope, comprised predominately of lipids and carbohydrates, are the first to contact host cellular and molecular constituents and therefore play a major role in facilitating host cell recognition and modulation of host cell responses against infection (3).
When M. tuberculosis infection occurs by airborne transmission, bacilli are deposited in the alveolar spaces of the lungs. The alveolar spaces are covered by surfactant, secreted by type II alveolar epithelial cells. The primary role of pulmonary surfactant is to prevent alveolar collapse at low lung volumes by reducing the surface tension in the alveolar space. Lipids comprise ∼80% of total surfactant (4, 5). The surfactant protein fraction includes a highly variable amount of serum proteins, a wide variety of specific hydrolases (6), and four surfactant proteins termed SP-A, -B, -C, and -D that contribute to its specific function (7). Surfactant also contains several products secreted by alveolar myeloid and epithelial cells. Some of them, such as the bacteriolytic ly-sozyme, are already defined in host defense (8, 9). However, the wide variety of hydrolases secreted into surfactant resemble but are distinct from lysosomal enzymes (10).
Little attention has been paid to the environmental immune pressure that M. tuberculosis encounters in the alveolar space. Cells of the alveolar space such as macrophages, monocytes, lymphocytes, neutrophils, and type I and II epithelial cells contain an array of hydrolases, including those associated with surfactant, that are released to the alveolar environment (11–14). When M. tuberculosis is initially deposited in the terminal bronchioles and alveoli, as well as following release from lysed macrophages, the bacilli are in intimate contact with these hydrolases. In this scenario, it is likely that the action of pulmonary hydrolases will alter the M. tuberculosis cell envelope surface, influencing the M. tuberculosis–host interface, and play a role in determining the immune response to M. tuberculosis and ultimately the fate of M. tuberculosis within the host.
We identified hydrolases in the lung that alter the M. tuberculosis cell envelope, regulate M. tuberculosis interaction with host cells, and impact M. tuberculosis intracellular survival. Biochemical analyses showed that M. tuberculosis bacilli exposed to human alveolar lining fluid (ALF), and to the most abundant surfactant hydrolases, are significantly altered in their mannose (Man)- and glucose (Glc)-containing cell envelope molecules. This alteration included a reduction in two major virulence factors on the M. tuberculosis surface [the Man-capped lipoarabinomannan (Man-LAM) (3, 15) and trehalose-6,69-dimycolate (TDM) (16, 17)], changing the course of events in the M. tuberculosis–human macrophage interaction. We also demonstrate that these M. tuberculosis cell envelope alterations induced a proinflammatory response. Thus, we provide evidence that the interaction of M. tuberculosis with lung surfactant hydrolases is a previously unrecognized, important determinant of the initial pathway of infection.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with United States Code of Federal Regulations, local regulations, and Good Clinical Practice as approved by the National Institutes of Health (National Institute of Allergy and Infectious Diseases/Division of Microbiology and Infectious Diseases branch). All research involving human participants was reviewed and approved by the Biomedical Sciences Institutional Review Board at The Ohio State University (OSU; no. 2008H0119 and no. 2008H0135). All human subject written informed consents were provided by study participants and/or their legally authorized representatives and documented prior to research involvement.
Chemical reagents and Abs
All chemicals reagents were of the highest grade from Sigma-Aldrich unless otherwise specified. For confocal studies, PE mouse anti-human CD63 and PE mouse IgG1 isotype control (BD Biosciences) were used as previously described (18). CS-35 and CS-40 mAbs against ManLAM were kindly provided by the Tuberculosis-Research-Materials and Vaccine-Testing contract (NOI-AI-75320) and Leprosy-Research-Support contract (NOI-AI-25469). Anti-TDM polyclonal IgG Ab was collected and purified from rabbits immunized with M. tuberculosis TDM as previously described (19).
Growth conditions of M. tuberculosis strains and radiolabeling
GFP-M. tuberculosis Erdman (provided by Dr. Horwitz, University of California, Los Angeles) and M. tuberculosis H37Rv (ATCC 27294; American Type Culture Collection) strains were grown as previously described (20). For radiolabeling, M. tuberculosis bacilli were grown to an OD of 0.7 at 600 nm in salt medium containing 0.05% Tween and 2 mCi/ml [1-3H-]Glc (sp. act. of 40–60 mCi/mmol) and 2 mCi/ml [1,2-14C]sodium acetate (sp. act. of 50–120 mCi/mmol). An aliquot of ∼1 3 106 bacilli was quantified by scintillation counting, giving a total of 8.3 3 109cpm radioactivity incorporation in the final pellet (23.6% incorporation). To determine if M. tuberculosis cell envelope surface components were radiolabeled, harvested cells were killed by delipidation using chloroform/methanol (2:1, v/v) followed by chloroform/methanol/water (10:10:3, v/v/v) for a total of 48 h. Then, delipidated cells were dried (7.83 g dried cells), and an aliquot of 50 mg was quantified by scintillation counting. For the [3H] isotope and [14C] isotope, there was ∼3.7 3 108cpm and 3.1 3 109cpm in the total lipid extract, respectively (together accounting for 41.8% of the total counts present in the radiolabeled bacilli). We then purified specific cell envelope components to evaluate if they were properly radiolabeled. We purified radiolabeled phosphatidyl-myo-inositol (myo-Inos) mannosides (1.47 3 107cpm; [3H]/[14C] ratio = 1:1.8), as well as a mixture of ManLAM/Lipomannan (1.85 3 107cpm; [3H]/[14C] ratio = 1:1.86). For experiments depicted in Fig. 2A and Supplemental Fig. 1A and 1B, we used radiolabeled whole live M. tuberculosis bacilli.
Figure 2.
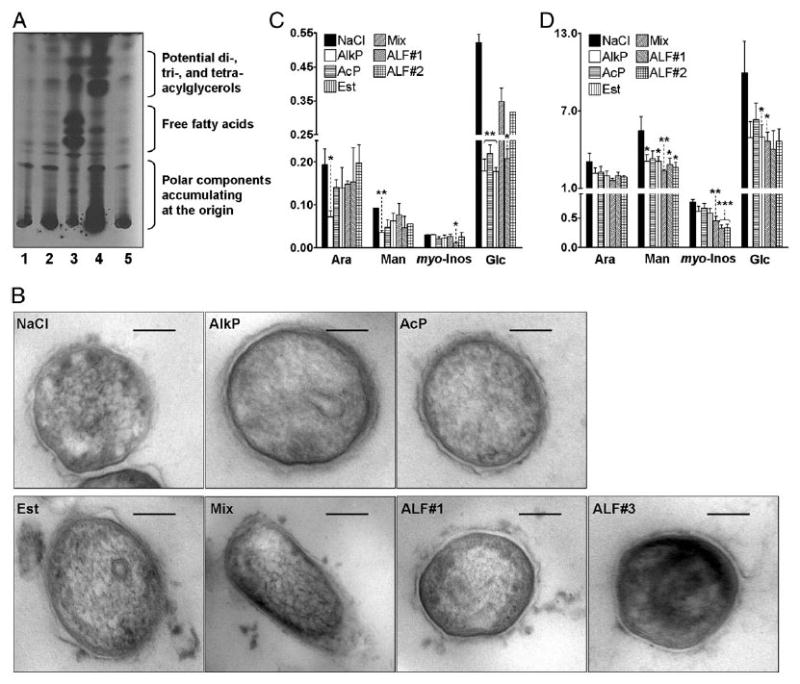
Alveolar hydrolases modify the M. tuberculosis cell envelope. A, TLC analysis of hydrophobic fragments released from live M. tuberculosis upon surfactant hydrolase activity (TLC, chloroform/methanol, 96:4 v/v) is shown (n = 3). Treatments were: 1. 0.9% NaCl; 2. human monocyte lysate; 3. human AM lysate; 4. human ALF; and 5. 0.9% NaCl. B, EM of treated-M. tuberculosis. Morphologies of the bacterial surface were compared among groups in which the bacteria were treated with 0.9% NaCl (control), AlkP, AcP, Est, Mix (AlkP+AcP+Est), or ALF from two independent human donors (ALF#1 and ALF#3) before fixation. Lower-power electron micrographs were obtained at original magnification ×98,000. Scale bars, 100 nm. C, Car-bohydrate composition analysis of hydrolase-treated M. tuberculosis. M. tuberculosis (2 × 107) was treated with 0.9% NaCl (control) or physiological concentrations of purified hydrolases (AlkP, AcP, Est, or Mix) or human ALF from a healthy donor. Alditol acetates obtained from treated-whole bacteria were further analyzed by GC/MS based on bacterial numbers. Shown are cumulative data of n = 3 each performed in duplicate (mean ± SEM). D, Carbohydrate composition analysis of soluble M. tuberculosis cell envelope components. M. tuberculosis-treated lysate fractions were converted to alditol acetates and analyzed by GC/MS based on equal amounts of protein (10 mg/ml). Shown are cumulative data of n = 4, each performed in duplicate (mean ± SEM): Ara, Man, myo-Inos, and Glc. Student t test, *p < 0.05, **p 0.005, ***p < 0.001.
Hydrolase activity content in ALF and human lung tissue
ALF was obtained from bronchoalveolar lavage fluid (BALF) after each donor (n = 8) by BAL in 80 ml sterile 0.9% NaCl (following an approved Institutional Review Board protocol at OSU and National Institutes of Health/National Institute of Allergy and Infectious Diseases/Division of Microbiology and Infectious Diseases protocol), concentrated 20-fold by using a 10 kDa molecular mass cutoff membrane Centricon Plus (Amicon Bioseparations) device at 4°C to achieve the surfactant volume present within the lungs. This removed surfactant lipids (free or in micelles), leaving the functional hydrolases in ALF (.10 kDa molecular mass cut-off). Lipid removal from ALF was confirmed by mass spectrometry (MS) of the fatty acid methyl ester derivatives (data not shown). Protein content (performed by the bicinchoninic acid method following the manufacturer's instructions [Bio-Rad]) and phospholipid (21) content were also determined in crude surfactant and ALF. ALF (defined in this study as BALF .10 kDa fraction) was frozen at 280°C until use. Following an approved Institutional Review Board at OSU, fresh harvested cadaveric human lung tissue was mechanically homogenized in 1 ml 0.9% NaCl, centrifuged at 10,000 3 g, and the supernatant filtered through a 0.22-mm filter. Hydrolase activities present in ALF and lung tissue were measured using a colorimetric method based on the release of p-nitrophenol upon specific substrate cleavage as previously described (22). Briefly, up to 25 ml substrate (2.5 mM) and 25 ml human ALF or 25 ml human lung homogenate (each containing in vivo-relevant alveolar levels of 0.5–1.5 mg/ml phospholipids by phosphate assay) were loaded in a 96-well plate. After 1 h incubation at 37°C with gentle shaking, reactions were stopped by adding 65 ml 1 M sodium carbonate and the OD read at 450 nm. For negative controls, substrates were incubated with 25 ml buffer under the same conditions, and reactions were stopped as described, followed by the addition of 25 ml ALF or lung homogenate. As a positive control, we incubated 0.1 U available a-mannosidase (Prozyme, Hayward, CA) with 10 mM p-nitrophenyl-a-O-mannoside in 0.125 M sodium acetate buffer (pH 5) as substrate (data not shown). Results were normalized to negative controls. Substrates used to determine the hydrolase content in ALF and human lung tissue are indicated in parentheses: 1) Acid phosphatase (p-nitrophenyl-phosphate); 2) a-mannosidase (p-nitrophenyl-a-mannoside); 3) a-galactosidase (p-nitrophenyl-a-galactopyranoside); 4) b-galactosidase (p-nitrophenyl-b-galactopyranoside); 5) a-glucosidase (p-nitrophenyl-a-D-glucoside); 6) b-glucosidase (b-Glc;p-nitrophenyl-b-D-glucoside); 7) a-xylosidase (p-nitrophenyl-a-D-xylopyranoside); 8) a-fucosidase (p-nitrophenyl-a-L-fucoside); 9) arylsulfatase (dipotassium p-nitrocatechol sulfate); 10) fatty acid esterase-I (p-nitrophenylpalmitate ester); 11) nonspecific esterase (p-nitrophenylacetate); 12) alkaline phosphatase (AlkP; p-nitrophenyl-phosphate); 13) alkaline phosphodiesterase (bis[p-nitrophenyl]phosphate); 14) phospholipase C (p-nitrophenylphosphorylcholine); 15) peroxidase (O-phenylenediamine HCl); 16) a-rhamnosidase (p-nitrophenyl-a-L-rhamnopyranoside; and 17) fatty acid esterase-II (p-nitrophenylstearate ester).
M. tuberculosis-radiolabeled experiments
Aliquots of live radiolabeled M. tuberculosis (1 3 1012) were incubated with 200 ml ALF (1 mg phospholipids/ml) or cell lysate (1 mg protein/ml) in 0.9% NaCl at 37°C for 12 h followed by centrifugation to obtain the supernatant containing released fragments by the action of hydrolyses (Supplemental Fig. 1A). To confirm the nature of the hydrophobic fragments (chloroform phase) released from the M. tuberculosis cell envelope upon the action of hydrolases derived from ALF and alveolar myeloid cell lysates, TLC (chloroform/methanol, 96:4, v/v) was performed.
Hydrolase and ALF M. tuberculosis treatment for infections
Single-cell suspensions of M. tuberculosis (2 3 107) were incubated with 0.9% NaCl (control), ALF in 0.9% NaCl (obtained as described above), commercially available human AlkP (1.414 U/ml, 0.9% NaCl), acid phosphatase (AcP; 0.5 U/ml 0.9% NaCl), esterase (Est; 0.52 U/ml, 0.9% NaCl) (as determined in Supplemental Table I), or a hydrolase mixture (AlkP + AcP + Est) for 15 min, 1, 3, 6, 12, and 24 h, 37°C, 5% CO2, gently washed, and resuspended in RPMI 1640 containing 2.5% HSA immediately prior to infection. Infections were performed using bacilli counted in Petroff Hauser chambers after treatment and washing.
Monosaccharide composition of treated M. tuberculosis whole cells and lysates
Samples were hydrolyzed and converted to alditol acetates using scylloinositol as internal standard and analyzed by gas chromatography (GC) as we previously described (23).
Whole-cell ELISA for ManLAM or TDM on the treated M. tuberculosis surface
Surface-exposed ManLAM or TDM on treated M. tuberculosis bacilli was analyzed by ELISA using anti-ManLAM mAb CS-35 (24) and CS-40 (25) or anti-TDM rabbit Ab (19) as we previously described (20).
Isolation and preparation of human macrophages
Monocyte-derived macrophage (MDM) monolayers for microscopy and CFUs were prepared from healthy tuberculin-negative human volunteers as we previously described (26).
Assay of treated M. tuberculosis association with macrophages
Association of treated M. tuberculosis (GFP-M. tuberculosis Erdman or M. tuberculosis H37Rv) with MDM monolayers was performed at a multiplicity of infection (MOI) 10:1 and determined by counting bacilli on $300 consecutive MDMs per coverslip using phase-contrast and fluorescence microscopy as we previously described (20, 26).
CFU analyses of treated M. tuberculosis
For CFU experiments, 12-d-old MDM monolayers were washed and infected with treated M. tuberculosis bacilli (MOI 1:1, triplicate wells) and CFUs determined as we previously described (27).
Quantitative phagosome–lysosome fusion assay of M. tuberculosis in macrophages
Experiments to determine the degree of phagosome–lysosome (P–L) fusion observed in MDMs infected with treated M. tuberculosis were performed by staining for the late endosomal/lysosomal marker CD63 (0.5 mg/ml final concentration) or IgG1 isotype control as we previously described (18). The percentage of treated M. tuberculosis that colocalized with CD63 was quantified by counting .350 consecutive phagosomes in each test group in $3 independent experiments using a minimum of three independent donors.
Electron microscopy experiments
Single-cell suspensions of treated M. tuberculosis (for 12 h) were fixed in 2% paraformaldehyde and processed and analyzed by transmission electron microscopy (TEM) as we previously described (18). Briefly, single-cell suspensions of 0.9% NaCl-, hydrolase-, or ALF-treated M. tuberculosis (for 12 h at 37°C, 5% CO2) were fixed with 2% paraformaldehyde, washed, and processed for TEM. Morphologies of the bacterial surface were compared with groups in which the bacteria were either treated with 0.9% NaCl, AlkP, AcP, Est, Mix, or ALF from two independent human donors (ALF#1 and ALF#3) before fixation. Fixed bacteria were rinsed three times in cacodylate buffer and postfixed with 1% osmium tetroxide in 0.1 M cacodylate buffer with 1.5% potassium ferrocyanide for 1.5 h. After rinsing with buffer and distilled water, cells were stained with 2.5% uranyl acetate for 30 min. A stepwise prolonged dehydration procedure was followed that consisted of 50% acetone for 15 min, 70% acetone two times for 20 min each, 95% acetone three times for 20 min each, and absolute acetone four times for 1 h each. A 1:1 mixture of absolute acetone and propylene oxide for 1 h was then followed by two incubations in 100% propylene, the first for 45 min and the second overnight. The samples were embedded in an increasing concentration of Spurr's resin (Polysciences, Warrington, PA) mixed with propylene oxide for 2 h. Two overnight incubations in 100% Spurr's resin were then performed before curing at 60°C for 48 h. Thin sections were cut at 100 nm and viewed by TEM using an FEI Tecnai G2 Spirit Transmission Electron Microscope (Hillsboro, OR). Lower-power electron micrographs were obtained at original magnification 398,000.
Statistical analysis
We used Prism software (GraphPad) to determine the statistical significance of differences in the means of experimental groups with unpaired, two-tailed Student t tests. Overall data were compared by one-way ANOVA followed by Tukey's multiple comparison test of the means. The p values ,0.05 were considered significant (*p , 0.05, **p , 0.005, and ***p , 0.001).
Results
Enzymatic activity of hydrolases present in human ALF and human lung tissue
To address which hydrolase activities are present in the human alveolar space with the potential to alter the cell envelope of M. tuberculosis within this microenvironment, we obtained BALF in 0.9% NaCl from healthy donors and human lung tissue from the OSU Lung Cell Isolation Core for analysis.
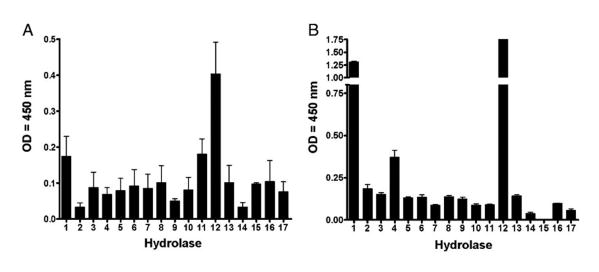
A colorimetric method based on the cleavage of p-nitrophenol from a specific substrate was used to measure the hydrolase activities present in samples that contained in vivo-relevant alveolar levels of 0.5–1.5 mg/ml phospholipids as determined by a phosphate assay (21, 28). Results in Fig. 1 show evidence of hydrolase activities present in ALF (defined as lipid-free BALF .10 kDa fraction; see Materials and Methods) and lung tissue (Fig. 1A, 1B, respectively), where the most abundant hydrolytic activities capable of affecting the M. tuberculosis cell envelope were AlkP, AcP, and nonspecific Est (Fig. 1A). We further calculated their relevant physiological concentrations in vivo using a p-nitrophenol standard curve (Supplemental Table I).
Figure 1.
Basal expression of several hydrolase activities in ALF and human lung tissue. Hydrolase activities present in ALF (n = 8 in triplicate, mean ± SEM) (A) and human lung tissue (a representative experiment [mean ± SD]) of n = 3 in triplicate (B) were monitored using a colorimetric method based on the release of p-nitrophenol upon specific substrate cleavage. Results show the presence of several hydrolases with different levels of activity that have the potential to remodel the cell envelope of M. tuberculosis within the alveolar environment. 1, Acid phosphatase; 2, α-mannosidase; 3, α-galactosidase; 4, β-galactosidase; 5, α-glucosidase; 6, β-glucosidase; 7, α-xylosidase; 8, α-fucosidase; 9, arylsulfatase; 10, fatty acid esterase-I; 11, nonspecific esterase; 12, AlkP; 13. alkaline phosphodiesterase; 14, phospholipase C; 15, peroxidase; 16, α-rhamnosidase; 17, fatty acid esterase-II. See also Supplemental Table I.
Effect of hydrolase activities derived from ALF on live M. tuberculosis
We reasoned that these hydrolase activities at their basal levels have the potential to remodel the cell envelope of M. tuberculosis in the alveoli prior to interaction with host alveolar compartment cells, as well as following release from lysed macrophages. To assess if hydrolases present in ALF were capable of altering the cell envelope of M. tuberculosis at their physiological concentrations in vivo, [3H] and [14C] double-radiolabeled live M. tuberculosis was exposed to ALF (1 mg phospholipid/ml) and to human monocyte and alveolar macrophage (AM) lysates (1 mg protein/ml, characterized for low and high hydrolase content, respectively) (29–31) obtained from cells from the same donors. Reactions were stopped by the addition of chloroform, which served to stop hydrolase activities present in the reactions by denaturation of the enzymes and also to extract cleaved lipidic fragments (i.e., fatty acids, GPI anchors) from the aqueous phase of the reaction. Results in Supplemental Fig. 1A and 1B show a release of radioactive counts derived from radiolabeled M. tuberculosis in both aqueous and organic phases after treatment with monocyte and AM lysates and, importantly, from ALF when compared with control (radiolabeled M. tuberculosis incubated in 0.9% NaCl). The radioactive counts found in the aqueous phase corresponded to hydrophilic components of the cell envelope that were released upon hydrolase activity. The observed low levels of counts in the control condition may be from secreted M. tuberculosis enzymes that had the ability to cleave components of the bacterial cell envelope (32) or from the effect of the isotonic buffer used. Calculating the total released radiolabeled counts ([3H], [14C] H2O phase + [3H], [14C] chloroform phase) (Supplemental Fig. 1B), myeloid cells and ALF-treated bacilli contained up to 2-fold and 4.3-fold higher radioactive counts than control treatment, respectively. To confirm the nature of the hydrophobic fragments (organic phase) released from the M. tuberculosis cell envelope in response to hydrolases, TLC was performed. A TLC autoradiogram showed a large release of hydrophobic fragments from the M. tuberculosis cell envelope upon exposure to ALF and AM lysate (Fig. 2A). Interestingly, fragments released by the action of AM and ALF hydrolases were different (Fig. 2A, lane 3 versus lane 4), indicating their specificity in potentially altering the M. tuberculosis cell envelope. Overall, these results indicate that alveolar hydrolases cleave the cell envelope surface of M. tuberculosis. Importantly, lysate or ALF treatment did not reduce the viability of M. tuberculosis; lysate- or ALF-treated M. tuberculosis grew slightly faster on agar plates than control-treated or untreated M. tuberculosis (data not shown). Consistent with this, electron microscopy of treated bacteria did not show discernable changes in the M. tuberculosis cell envelope (Fig. 2B).
To assess how alveolar hydrolases biochemically alter the cell envelope of M. tuberculosis, we treated M. tuberculosis with: 1) the most abundant hydrolases alone or in combination (as quantified in Supplemental Table I); 2) ALF obtained from two in-dependent donors (ALF#1, donor 1 or ALF#2, donor 2); or 3) 0.9% NaCl as the control for 12 h at 37°C, 5% CO2 and analyzed he bacterial cell envelope carbohydrates (Fig. 2C, 2D) and fatty acid (data not shown) content after hydrolase exposure. Results showed that live M. tuberculosis exposed to hydrolases was reduced in its overall content of arabinose (Ara), Man, myo-Inos, and Glc (Fig. 2C). Ara, Man, and myo-Inos are carbohydrate constituents of cell envelope determinants that are critical in M. tuberculosis recognition by human macrophages and can regulate intracellular survival (e.g., ManLAM) (3, 15). Glc is found in the M. tuberculosis cell envelope as trehalose, constituting the polar groups of major factors described for M. tuberculosis pathogenesis [i.e., TDM (16, 17)]. Furthermore, when we analyzed the carbohydrate content in M. tuberculosis cell lysates (fraction containing soluble M. tuberculosis cell envelope components), we also observed a reduction in the carbohydrate constituents of ManLAM and TDM (Fig. 2D), indicating that important cell envelope components on the surface of M. tuberculosis can be affected by hydrolases present in human surfactant.
To assess if alveolar hydrolases or ALF altered the presence of ManLAM and/or TDM on the surface of M. tuberculosis, we analyzed the overall surface content of ManLAM and TDM on the surface of bacteria following treatment with hydrolases. We incubated M. tuberculosis with the most abundant hydrolases that we found in the ALF or with ALF from two independent healthy donors (ALF#1 or ALF#2) for 15 min at 37°C, 5% CO2. After treatment, we assayed for surface exposed ManLAM or TDM on whole live bacteria by ELISA. As shown in Fig. 3A and 3C, hydrolase- or ALF-treated M. tuberculosis bacilli had a significant reduction in surface-exposed ManLAM and TDM, respectively, when compared with control M. tuberculosis. Fig. 3B and 3D show the percent decrease in surface ManLAM and TDM after treatment. These results provide evidence that alveolar surfactant hydrolases affect the cell envelope of M. tuberculosis.
Figure 3.
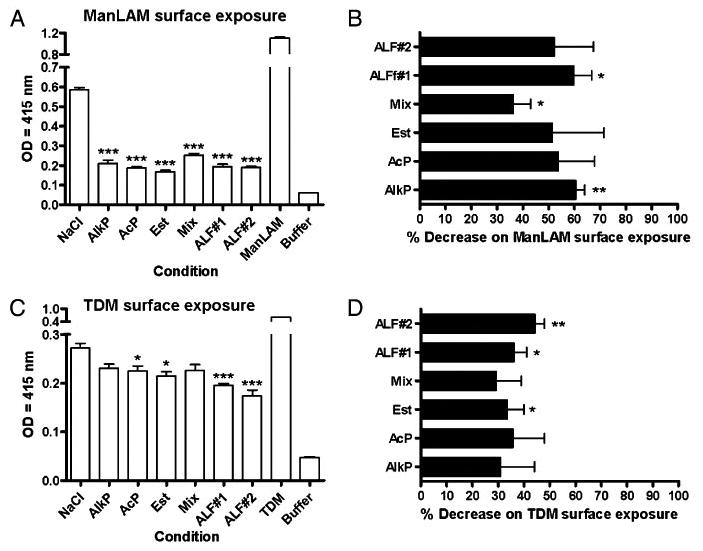
Presence of ManLAM and TDM on the bacterial surface of hydrolase- and/or ALF-treated M. tuberculosis. Whole bacterial ELISA using live treated M. tuberculosis and anti-LAM mAb CS-35 (for Man-LAM) (A, B) or anti-TDM polyclonal Ab (for TDM) (C, D). A and B are representative experiments of n = 3, each performed in triplicate (mean ± SD); Student t test, treatment versus control. C and D are overall data from n = 3, each performed in trip-licate (mean ± SEM). One-way ANOVA followed by post-Tukey's multiple comparison test, *p < 0.05; **p < 0.005; ***p < 0.001, treatment versus control. ManLAM (5 μg) or TDM (5 μg) were used aspositive control. Buffer: 0.9% NaCl. *p < 0.05, **p < 0.005, ***p < 0.001. Mix, AlkP+AcP+Est.
Effect of specific alveolar hydrolases on the association of virulent M. tuberculosis with macrophages and the production of proinflammatory cytokines
Because alveolar hydrolases have the ability to alter the M. tuberculosis cell envelope, we reasoned that such cell envelope modifications would impact interactions between M. tuberculosis and determinants of the innate immune system such as human macrophages. Thus, we tested whether hydrolases derived from ALF, at their relevant concentrations in vivo, altered the recognition of M. tuberculosis by human macrophages. Results in Fig. 4A show that M. tuberculosis treated with human ALF, with AlkP, AcP, or Est, or a mixture of all three significantly decreased bacterial association with human macrophages when compared with control-treated bacilli. Effects were seen with treatment for as little as 15 min, suggesting that minimal contact time in the alveolus is sufficient to impact M. tuberculosis-host cell inter-actions. Fig. 4B shows the percent decrease in association for cumulative data, which ranged from 55–70% depending on the specific treatment. The decrease in association was more prominent when M. tuberculosis was exposed to ALF or hydrolases for 12 h. Similar results were found with hydrolase or ALF treatment for 1, 3, 6, and 24 h. To further assess the specificity of these results, M. tuberculosis was also treated with physiological concentrations of b-Glc, a human surfactant hydrolase that should not affect the M. tuberculosis cell envelope due to the absence of b-Glc in the M. tuberculosis cell envelope. Results in Fig. 4C and 4D show that M. tuberculosis treated with b-Glc did not affect bacterial association with macrophages. Importantly, as shown for ALF-treated M. tuberculosis, purified hydrolase-treated bacilli showed no differences in viability when compared with control or untreated M. tuberculosis (data not shown).
Figure 4.
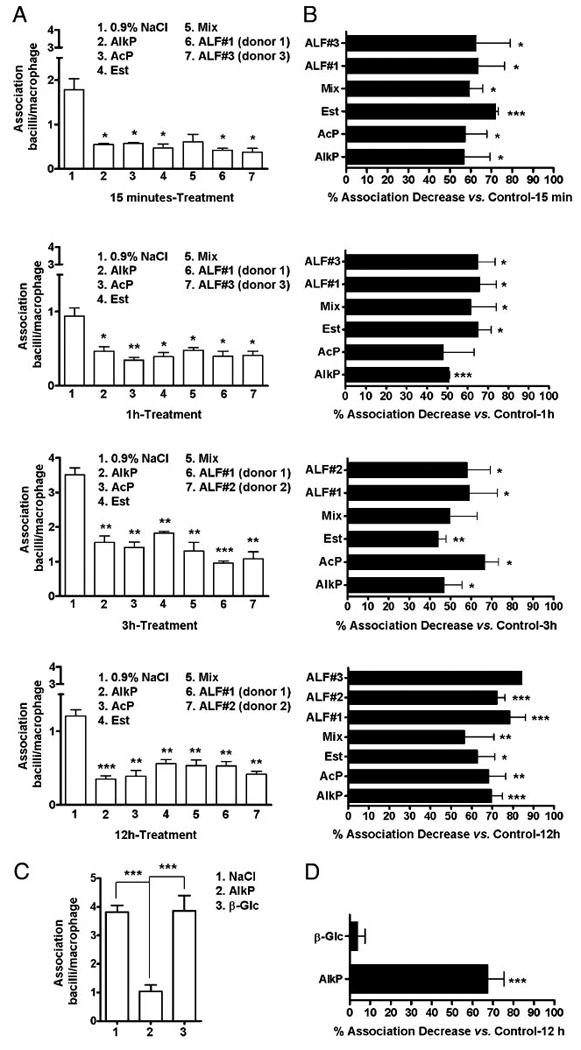
Association of treated-M. tuberculosis with human macrophages. Single-cell suspensions of M. tuberculosis (2 × 107) were incubated with 0.9% NaCl (control), ALF in 0.9% NaCl, commercially available human AlkP (1.414 U/ml 0.9% NaCl) or AcP (0.5 U/ml 0.9% NaCl), or a hydrolase mixture (AlkP + AcP + Est [Est at 0.52 U/ml 0.9% NaCl]) for 15 min, 1, 3, 12, and 24 h (the latter not shown). Infections (using bacilli counted after treatment and washing) and cell-association studies using human macrophage mono-layers on coverslips were performed at an MOI of 10:1. A, A representative experiment for each length of hydrolase exposure performed in triplicate (mean ± SD). B, Overall data showing the percent association decrease for each length of hydrolase exposure studied (mean ± SEM); n = 2 for all the treatments except for 12 h treatment, for which n = 4 for AlkP, AcP, Mix, and ALF#1 treatments, n = 3 for Est and ALF#2 treatments, and n = 1 for ALF#3 treatment. ALF#1, ALF#2, and ALF#3 are ALFs obtained from three independent donors. C and D, Decrease in association with human macrophages is hydrolase treatment dependent. M. tuberculosis bacilli were treated with β-glucosidase, AlkP, or 0.9% NaCl. Data showing the number of M. tuberculosis associated with human macrophages (n = 3 in triplicate) (C) and the overall percent association decrease versus control (n = 3 in triplicate) (D) are depicted (mean ± SEM). Student t test, treatment versus control, *p < 0.05, **p < 0.005, ***p < 0.001.
To further evaluate how ALF and its most abundant hydrolases influence M. tuberculosis activation of the human macrophage, we assessed TNF levels by ELISA from supernatants of macrophage-infected monolayers incubated with hydrolase-treated M. tuberculosis. Results in Fig. 5 showed that incubation with hydrolase-or ALF-treated M. tuberculosis led to a significant increase in TNF production (Fig. 5A, 5C). This increase was observed following macrophage incubation with M. tuberculosis that had been ex-posed to hydrolases for as little as 15 min. Increases were between 1.5- and 10.0-fold except for AcP-treated M. tuberculosis, which induced even higher TNF levels (Fig. 5B). Similar results were observed for M. tuberculosis exposed to hydrolases for 12 h, in which macrophage TNF production was also significantly in-creased (2.0–6.0-fold) (Fig. 5D). These data provide further evidence that hydrolases present within human surfactant play an important role in regulating M. tuberculosis–host interactions by altering and protective proinflammatory responses.
Figure 5.
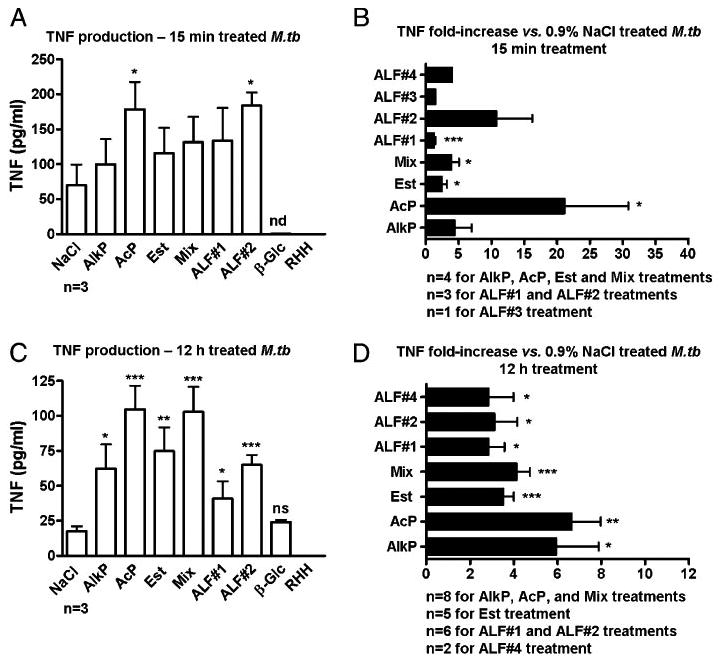
Proinflammatory cytokine response induced by hydrolase- or ALF-treated M. tuberculosis. Human macrophage mono-layers were infected with 15 min- or 12 h-treated M. tuberculosis that was gently washed prior to addition to macrophages. TNF levels were measured from isolated supernatants by ELISA. TNF production induced by 15 min-treated M. tuberculosis (A, B) and TNF production induced by 12 h-treated M. tuberculosis (C, D) (mean ± SEM); n values are depicted in each graph. ALF#1, ALF#2, ALF#3, and ALF#4 are ALFs obtained from four independent donors. Student t test, treatment versus control, *p < 0.05, **p < 0.005, ***p < 0.001. nd, not detected.
Effect of specific alveolar surfactant hydrolases on the intracellular survival of M. tuberculosis in macrophages
To evaluate the effect of surfactant hydrolases on intracellular survival of M. tuberculosis in human macrophages, virulent M. tuberculosis was treated for 12 h with or without ALF, with specific hydrolases in combination or alone at their in vivo concentrations. Macrophages were infected with single-cell suspensions of treated M. tuberculosis and CFUs determined at 24–120 h as we previously described (27). Intact macrophage monolayers were verified by inverted-phase microscopy throughout the infection period. Results in Fig. 6A show that surfactant hydrolases reduced intracellular growth of M. tuberculosis. The results were evident as early as 24 h (Fig. 6B).
Figure 6.
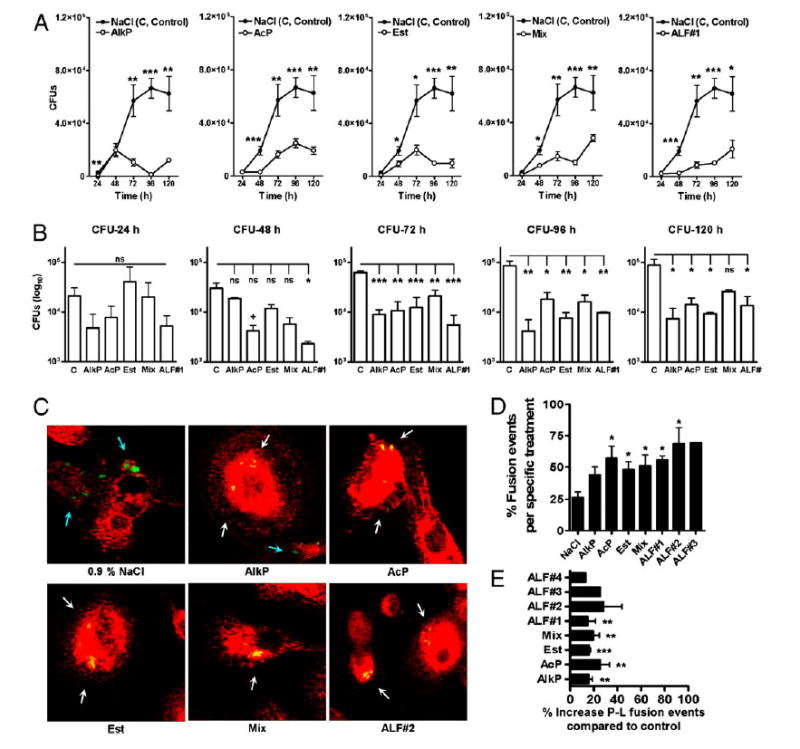
Effects of human ALF and hydrolase activities on M. tuberculosis intracellular survival and trafficking in macrophages. M. tuberculosis bacilli were treated with 0.9% NaCl (control), ALF#1 (donor #1), a mixture of AlkP, AcP, and Est, or AlkP, AcP, and Est alone at relevant in vivo concentrations. Human macrophages were infected at an MOI of 1:1. M. tuberculosis survival was determined at the indicated intervals. A, A representative experiment in triplicate is shown (mean ± SD); Student t test, treatment versus control, *p < 0.05, **p < 0.005, ***p < 0.001. B, Cumulative data from three independent experiments each performed in triplicate (mean ± SEM); one-way ANOVA, Tukey-Post test, *p < 0.05, **p < 0.005, ***p < 0.001. Where no significance was reached by ANOVA analyses, direct pairwise comparisons between control and a specific hydrolase treatment were analyzed at each time point by Student t test, +p < 0.05. C, Fluorescence microscopy images of P–L fusion events in human macrophages in response to hydrolase- or ALF-treated M. tuberculosis. Human macrophages were adhered to glass coverslips and incubated with treated GFP-M. tuberculosis (MOI 10:1) for 2 h using a synchronized phagocytosis assay. Cell monolayers were fixed, permeabilized, and stained with anti-human CD63-PE–conjugated mouse anti-human IgG. Shown are merge images where CD63 positive compartments are red, GFP-treated-M. tuberculosis in unfused phagosomes are green (blue arrows), and those colocalized with CD63 are yellow (white arrows). P–L fusion was examined and enumerated via confocal microscopy, counting >350 P-L events per coverslip. Original magnification ×650. D, A representative experiment from n = 3 performed in triplicate (mean ± SD); Student t test treatment versus control, *p < 0.05. E, Overall percent P–L fusion increase from n = 3 in triplicate (mean ± SEM); Student t test treatment versus control, **p < 0.005, ***p < 0.001. ALF#1, ALF donor 1; C, Control (0.9% NaCl).
Considering that hydrolase-treated bacilli demonstrated reduced phagocytosis and early intracellular growth in macrophages, we next examined whether phagocytosis of treated bacilli resulted in an increase in P–L fusion events in the macrophage (potentially through an altered entry pathway into the cell). We incubated human macrophages with control M. tuberculosis or bacilli ex-posed to ALF or hydrolases and assessed P–L fusion events for up to 2 h. The overall levels of P–L fusion were significantly in-creased when M. tuberculosis was exposed to ALF or with a specific hydrolase (alone or in combination) (Fig. 6C–E).
Discussion
Studies, including those from our own laboratory (reviewed in Refs. 3, 15), have focused on the host–bacillus interaction at different levels (33, 34), but primarily on the molecular mechanism(s) of M. tuberculosis entry and survival within its natural niche, the AM. However, little attention has been paid to how environmental immune pressure influences M. tuberculosis out-side of this niche.
In this context, type II alveolar epithelial cells are located in the corners of the alveolus, where their physiological functions include surfactant production, secretion, and recycling via specific organelles called lamellar bodies (35). During active surfactant secretion into the alveolus and during its recycling process, a variety of hydrolases have been associated with these organelles (10, 36–40), many of which have lysosomal-type degradative functions but are distinct from lysosomal enzymes (10). To what extent M. tuberculosis bacilli directly interact with epithelial cells and their secreted hydrolases remains unknown. However, the location of the alveolar epithelium as well as the relatively large alveolar surface area (estimated at 1022 m2) (41, 42) make it likely that M. tuberculosis will interact with components of the alveolar space prior to and following its residence in the AM. Moreover, the low alveolar surfactant volume relative to the alveolar surface area (0.02 ml/m2) likely increases the local concentration of released hydrolases when compared with other tissue compartments (43, 44). This, in turn, increases the probability that secreted hydrolases will impact M. tuberculosis in the alveoli, altering its cell envelope and possibly its metabolism. The exact period of time that M. tuberculosis remains in the alveoli after deposition remains unknown. In healthy people, it is estimated that only 8–12 AMs are found per alveolar sac (41), with each sac having a surface area of 206.9 mm2 (41). The low number of AMs per sac during health enables optimal gas exchange to occur. In this scenario, we believe that M. tuberculosis will remain submerged within the surfactant monolayer or the surfactant hypophase for some time and thus be in contact with surfactant hydrolases as well as other surfactant components (43). Our results show that even short-term exposure to hydrolases (15 min) influences the nature of the M. tuberculosis cell envelope and interactions of the bacilli with macrophages (and potentially with other cellular and soluble components of the innate immune system).
Previous studies have determined the extracellular hydrolase composition of the lung (22) as well as the hydrolase content in mononuclear and polymorphonuclear exudate cells and pulmonary AMs in different animal models (10, 45). In these studies, AlkP, AcP, Est, succinic dehydrogenase, aminopeptidase, and cytochrome oxidase were the most abundant hydrolases measured. Our results with human ALF and lung tissue indicate that alveolar hydrolases like AlkP, AcP, and Est are also the most abundant ones in healthy individuals. We found that these hydrolases alter the cell envelope of M. tuberculosis by decreasing the Man and Glc content on the M. tuberculosis surface, which correlated with decreased surface ManLAM and TDM. As AlkP and AcP are nonspecific enzymes that hydrolyze many types of phosphate esters, releasing inorganic phosphate (44), a plausible explanation for their effects on M. tuberculosis is that they may target specific molecular anchoring points on the cell envelope surface or affect neighboring molecules, thereby disrupting hydrophobic/electro-static interactions, resulting in the release of ManLAM and TDM to the milieu. In this context, AlkP is capable of hydrolyzing phospholipids due to its phospho-monoesterase activity, which shows high specificity at neutral pH under surfactant-like conditions (46). This esterase activity also has the potential to affect M. tuberculosis surface-exposed lipids, lypoglycans, and lipoproteins by cleaving ester linkages in these molecules. Interestingly, increased esterase activity has been reported in the lesions of cutaneous tuberculosis (47, 48), bacillus Calmette-Guérin infection (49), leprosy, and leishmaniasis (48, 49), pre-dominantly due to infection-driven tissue destruction. Related studies show that high concentrations of natural lipases and esterases are capable of slowing down M. tuberculosis growth in vitro and in vivo (50). Our results show that hydrolase- or ALF-treated M. tuberculosis bacilli also have limited intracellular growth within human macrophages over time. This could be explained by the intracellular killing observed, but could, in addition, be a result of a differential intracellular growth rate.
The effects of lung hydrolases on the M. tuberculosis cell envelope have important implications for host cell interactions. M. tuberculosis ManLAM and higher-order phosphatidyl-myo-inositol mannosides interact with the mannose receptor, a major C-type lectin on human macrophages (3, 15). The mannose receptor directs the intracellular fate of M. tuberculosis within the human host by limiting P–L fusion events (18, 51) and promoting anti-inflammatory responses (52–54). The observed decrease of Man, specifically in the form of ManLAM, on hydrolase-exposed M. tuberculosis may direct the bacillus to other macrophage surface receptors in vivo, triggering an immune response that may initially be detrimental to M. tuberculosis. Our results support this notion in which an increase in proinflammatory mediators along with an increase in P–L fusion events was observed when hydrolase-exposed M. tuberculosis bacilli were incubated with macrophages.
In this study, we show that hydrolases present in the alveolar space are actively involved in shaping the nature of the dominant M. tuberculosis cell envelope carbohydrates and lipids and thereby play a role in dictating M. tuberculosis–AM interactions and in determining the fate of M. tuberculosis within the host. This finding adds a new concept to our understanding of M. tuberculosis infection. In this context, a significant challenge is how our findings can be verified during M. tuberculosis infection in vivo, given that few studies have successfully determined the changes that occur in the lungs during the earliest bacteria–host inter-actions. Our current studies are addressing the complexities of how this can be accurately measured in vivo using animal models; however, our in vitro assays using human samples provide strong evidence that hydrolases are important determinants in dictating the destiny of M. tuberculosis within the host, and we anticipate that they will play a critical role in M. tuberculosis pathogenesis.
Supplementary Material
Acknowledgments
We thank Dr. Mark Wewers for assistance during bronchoalveolar lavage procedures and Dr. Daren Knoell for providing cadaveric human lung tissues. We also thank the Campus Chemical Instrument Center and the Campus Microscopy and Imaging Facility at OSU for technical support.
This work was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases (AI-073856) and the Parker B. Francis Fellowship (to J.B.T.).
Abbreviations used in this article
- AcP
acid phosphatase
- ALF
alveolar lining fluid
- AlkP
alkaline phosphatase
- AM
alveolar macrophage
- Ara
arabinose
- BALF
bronchoalveolar lavage fluid
- Est
esterase
- GC
gas chromatography
- b-Glc
b-glucosidase
- Glc
glucose
- Man
mannose
- ManLAM
mannose-capped lipoarabinomannan
- MDM
monocyte-derived macrophage
- MOI
multiplicity of infection
- MS
mass spectrometry
- myo-Inos
myo-inositol
- OSU
The Ohio State University
- P–L
phagosome–lysosome
- TDM
trehalose-6,69-dimycolate
- TEM
transmission electron microcopy
Footnotes
The online version of this article contains supplemental material.
Disclosures: The authors have no financial conflicts of interest.
References
- 1.World Health Organization. Multidrug and Extensively Drug-Resistant TB (M/XDR-TB): 2010 Global Report on Surveillance and Response. WHO Press; Geneva, Switzerland: 2010. [Google Scholar]
- 2.Schlesinger LS. The role of mononuclear phagocytes in tuberculosis. In: Lipscomb MF, Russell SW, editors. Lung macrophages and dendritic cells in health and disease. Marcel Dekker, Inc.; New York: 1997. pp. 437–480. [Google Scholar]
- 3.Torrelles JB, Schlesinger LS. Diversity in Mycobacterium tuberculosismannosylated cell wall determinants impacts adaptation to the host. Tuberculosis (Edinb) 2010;90:84–93. doi: 10.1016/j.tube.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King RJ. Pulmonary surfactant. J Appl Physiol. 1982;53:1–8. doi: 10.1152/jappl.1982.53.1.1. [DOI] [PubMed] [Google Scholar]
- 5.vanGolde LM. Synthesis of surfactant lipids in the adult lung. Annu Rev Physiol. 1985;47:765–774. doi: 10.1146/annurev.ph.47.030185.004001. [DOI] [PubMed] [Google Scholar]
- 6.Griese M. Pulmonary surfactant in health and human lung diseases: state of the art. Eur Respir J. 1999;13:1455–1476. doi: 10.1183/09031936.99.13614779. [DOI] [PubMed] [Google Scholar]
- 7.Weaver TE, Whitsett JA. Function and regulation of expression of pulmonary surfactant-associated proteins. Biochem J. 1991;273:249–264. doi: 10.1042/bj2730249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh G, Katyal SL, Brown WE, Collins DL, Mason RJ. Pulmonary lysozyme—a secretory protein of type II pneumocytes in the rat. Am Rev Respir Dis. 1988;138:1261–1267. doi: 10.1164/ajrccm/138.5.1261. [DOI] [PubMed] [Google Scholar]
- 9.Haller EM, Shelley SA, Montgomery MR, Balis JU. Immunocytochemical localization of lysozyme and surfactant protein A in rat type II cells and extracellular surfactant forms. J Histochem Cytochem. 1992;40:1491–1500. doi: 10.1177/40.10.1527372. [DOI] [PubMed] [Google Scholar]
- 10.Hook GE, Gilmore LB. Hydrolases of pulmonary lysosomes and lamellar bodies. J Biol Chem. 1982;257:9211–9220. [PubMed] [Google Scholar]
- 11.Nicod LP. Lung defenses: An overview. Eur Resp Rev. 2005;14:45–50. [Google Scholar]
- 12.Hiemstra PS, Bals R. Series introduction: Innate host defense of the respiratory epithelium. J Leukoc Biol. 2004;75:3–4. doi: 10.1189/jlb.0903410. [DOI] [PubMed] [Google Scholar]
- 13.Williams MC. Alveolar type I cells: molecular phenotype and development. Annu Rev Physiol. 2003;65:669–695. doi: 10.1146/annurev.physiol.65.092101.142446. [DOI] [PubMed] [Google Scholar]
- 14.Mason RJ. Biology of alveolar type II cells. Respirology. 2006;11(Suppl):S12–S15. doi: 10.1111/j.1440-1843.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- 15.Schlesinger LS, Azad AK, Torrelles JB, Roberts E, Vergne I, Deretic V. Determinants of phagocytosis, phagosome biogenesis and autophagy for Mycobacterium tuberculosis. In: Kaufmann SHE, Britton WJ, editors. Handbook of Tuberculosis. Immunology and Cell Biology. Wiley-VCH Verlag; Weinheim, Germany: 2008. pp. 1–22. [Google Scholar]
- 16.Ryll R, Kumazawa Y, Yano I. Immunological properties of trehalose dimycolate (cord factor) and other mycolic acid-containing glycolipids— a review. Microbiol Immunol. 2001;45:801–811. doi: 10.1111/j.1348-0421.2001.tb01319.x. [DOI] [PubMed] [Google Scholar]
- 17.Hunter RL, Olsen MR, Jagannath C, Actor JK. Multiple roles of cord factor in the pathogenesis of primary, secondary, and cavitary tuberculosis, including a revised description of the pathology of secondary disease. Ann Clin Lab Sci. 2006;36:371–386. [PubMed] [Google Scholar]
- 18.Kang PB, Azad AK, Torrelles JB, Kaufman TM, Beharka A, Tibesar E, DesJardin LE, Schlesinger LS. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med. 2005;202:987–999. doi: 10.1084/jem.20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujiwara N, Pan J, Enomoto K, Terano Y, Honda T, Yano I. Production and partial characterization of anti-cord factor (trehalose-6,69-dimycolate) IgG antibody in rabbits recognizing mycolic acid subclasses of Mycobacterium tuberculosis or Mycobacterium avium. FEMS Immunol Med Microbiol. 1999;24:141–149. doi: 10.1111/j.1574-695X.1999.tb01275.x. [DOI] [PubMed] [Google Scholar]
- 20.Torrelles JB, Knaup R, Kolareth A, Slepushkina T, Kaufman TM, Kang PB, Hill PJ, Brennan PJ, Chatterjee D, Belisle JT, et al. Identification of Mycobacterium tuberculosis clinical isolates with altered phagocytosis by human macrophages due to a truncated lipoarabinomannan. J Biol Chem. 2008;283:31417–31428. doi: 10.1074/jbc.M806350200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ames BN. Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol. 1966;8:115–118. [Google Scholar]
- 22.Hook GE. Extracellular hydrolases of the lung. Biochemistry. 1978;17:520–528. doi: 10.1021/bi00596a023. [DOI] [PubMed] [Google Scholar]
- 23.Torrelles JB, Khoo KH, Sieling PA, Modlin RL, Zhang N, Marques AM, Treumann A, Rithner CD, Brennan PJ, Chatterjee D. Truncated structural variants of lipoarabinomannan in Mycobacterium leprae and an ethambutol-resistant strain of Mycobacterium tuberculosis. J Biol Chem. 2004;279:41227–41239. doi: 10.1074/jbc.M405180200. [DOI] [PubMed] [Google Scholar]
- 24.Kaur D, Lowary TL, Vissa VD, Crick DC, Brennan PJ. Characterization of the epitope of anti-lipoarabinomannan antibodies as the terminal hexaarabinofuranosyl motif of mycobacterial arabinans. Microbiology. 2002;148:3049–3057. doi: 10.1099/00221287-148-10-3049. [DOI] [PubMed] [Google Scholar]
- 25.Chatterjee D, Lowell K, Rivoire B, McNeil MR, Brennan PJ. Lipoarabinomannan of Mycobacterium tuberculosis. Capping with mannosyl residues in some strains. J Biol Chem. 1992;267:6234–6239. [PubMed] [Google Scholar]
- 26.Schlesinger LS. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–2930. [PubMed] [Google Scholar]
- 27.Olakanmi O, Britigan BE, Schlesinger LS. Gallium disrupts iron metabolism of mycobacteria residing within human macrophages. Infect Immun. 2000;68:5619–5627. doi: 10.1128/iai.68.10.5619-5627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Notter RH. Lung Surfactants: Basic Science and Clinical Applications. Marcel Dekker; New York: 2000. pp. 1–444. [Google Scholar]
- 29.Cohn ZA, Wiener E. The particulate hydrolases of macrophages. I. Comparative enzymology, isolation, and properties. J Exp Med. 1963;118:991–1008. doi: 10.1084/jem.118.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorber WA, Leake ES, Myrvik QN. Comparative densities of hydrolase-containing granules from normal and BCG-induced alveolar macro-phages. Infect Immun. 1973;7:86–92. doi: 10.1128/iai.7.1.86-92.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Furth R. Phagocytic cells: Development and distribution of mono-nuclear phagocytes in normal steady state and inflammation. In: Gallin JI, Goldstein IM, Snyderman R, editors. Inflammation: Basic Principles and Clinical Correlates. Raven; New York: 1988. pp. 281–295. [Google Scholar]
- 32.Dong X, Bhamidi S, Scherman M, Xin Y, McNeil MR. Development of a quantitative assay for mycobacterial endogenous arabinase and ensuing studies of arabinase levels and arabinan metabolism in Mycobacterium smegmatis. Appl Environ Microbiol. 2006;72:2601–2605. doi: 10.1128/AEM.72.4.2601-2605.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 34.Fenton MJ, Riley LW, Schlesinger LS. Receptor-mediated recognition of Mycobacterium tuberculosis by host cells. In: Cole ST, Eisenach KD, McMurray DN, Jacobs WR Jr, editors. Tuberculosis and the Tubercle Bacillus. ASM Press; New York: 2005. pp. 405–426. [Google Scholar]
- 35.Fehrenbach H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res. 2001;2:33–46. doi: 10.1186/rr36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DiAugustine RP. Lung concentric laminar organelle. Hydrolase activity and compositional analysis. J Biol Chem. 1974;249:584–593. [PubMed] [Google Scholar]
- 37.Gilder H, Haschemeyer RH, Fairclough GF, Jr, Mynarcik DC. Isolation and characterization of lamellar body material from rat lung homogenates by continuous linear sucrose gradients. J Lipid Res. 1981;22:1277–1285. [PubMed] [Google Scholar]
- 38.Edelson JD, Shannon JM, Mason RJ. Alkaline phosphatase: a marker of alveolar type II cell differentiation. Am Rev Respir Dis. 1988;138:1268–1275. doi: 10.1164/ajrccm/138.5.1268. [DOI] [PubMed] [Google Scholar]
- 39.de Vries AC, Schram AW, Tager JM, Batenburg JJ, van Golde LM. A specific acid alpha-glucosidase in lamellar bodies of the human lung. Biochim Biophys Acta. 1985;837:230–238. doi: 10.1016/0005-2760(85)90046-3. [DOI] [PubMed] [Google Scholar]
- 40.Young SL, Fram EK, Larson E, Wright JR. Recycling of surfactant lipid and apoprotein-A studied by electron microscopic autoradiography. Am J Physiol. 1993;265:L19–L26. doi: 10.1152/ajplung.1993.265.1.L19. [DOI] [PubMed] [Google Scholar]
- 41.Stone KC, Mercer RR, Gehr P, Stockstill B, Crapo JD. Allometric relationships of cell numbers and size in the mammalian lung. Am J Respir Cell Mol Biol. 1992;6:235–243. doi: 10.1165/ajrcmb/6.2.235. [DOI] [PubMed] [Google Scholar]
- 42.Stone KC, Mercer RR, Freeman BA, Chang LY, Crapo JD. Distribution of lung cell numbers and volumes between alveolar and nonalveolar tissue. Am Rev Respir Dis. 1992;146:454–456. doi: 10.1164/ajrccm/146.2.454. [DOI] [PubMed] [Google Scholar]
- 43.Chroneos ZC, Midde K, Sever-Chroneos Z, Jagannath C. Pul-monary surfactant and tuberculosis. Tuberculosis (Edinb) 2009;89(Suppl 1):S10–S14. doi: 10.1016/S1472-9792(09)70005-8. [DOI] [PubMed] [Google Scholar]
- 44.Patton JS. Mechanisms of macromolecule absorption by the lungs. Adv Drug Deliv Rev. 1996;19:3–36. [Google Scholar]
- 45.Dannenberg AM, Jr, Burstone MS, Walter PC, Kinsley JW. A histochemical study of phagocytic and enzymatic functions of rabbit mono-nuclear and polymorphonuclear exudate cells and alveolar macrophages. I. Survey and quantitation of enzymes, and states of cellular activation. J Cell Biol. 1963;17:465–486. doi: 10.1083/jcb.17.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ehle H, Müller E, Horn A. Alkaline phosphatase of the calf intestine hydrolyzes phospholipids. FEBS Lett. 1985;183:413–416. doi: 10.1016/0014-5793(85)80822-x. [DOI] [PubMed] [Google Scholar]
- 47.Findlay GH. The simple esterases of human skin. Br J Dermatol. 1955;67:83–91. doi: 10.1111/j.1365-2133.1955.tb12696.x. [DOI] [PubMed] [Google Scholar]
- 48.Wells GC. Esterases in cutaneous granulomata. Br J Dermatol. 1957;69:415–427. doi: 10.1111/j.1365-2133.1957.tb13216.x. [DOI] [PubMed] [Google Scholar]
- 49.Steigleder GK, Schultis K. Histochemistry of skin esterases. Arch Klin Exp Dermatol. 1957;205:196–211. [PubMed] [Google Scholar]
- 50.Annenkov GA, Klepikov NN, Martynova LP, Puzanov VA. Wide range of the use of natural lipases and esterases to inhibit Mycobacterium tuberculosis. Probl Tuberk Bolezn Legk. 2004;6:52–56. [PubMed] [Google Scholar]
- 51.Torrelles JB, Azad AK, Schlesinger LS. Fine discrimination in the recognition of individual species of phosphatidyl-myo-inositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J Immunol. 2006;177:1805–1816. doi: 10.4049/jimmunol.177.3.1805. [DOI] [PubMed] [Google Scholar]
- 52.Astarie-Dequeker C, N'Diaye EN, Le Cabec V, Rittig MG, Prandi J, Maridonneau-Parini I. The mannose receptor mediates uptake of pathogenic and nonpathogenic mycobacteria and bypasses bactericidal responses in human macrophages. Infect Immun. 1999;67:469–477. doi: 10.1128/iai.67.2.469-477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nigou J, Zelle-Rieser C, Gilleron M, Thurnher M, Puzo G. Mannosylatedlipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J Immunol. 2001;166:7477–7485. doi: 10.4049/jimmunol.166.12.7477. [DOI] [PubMed] [Google Scholar]
- 54.Chieppa M, Bianchi G, Doni A, Del Prete A, Sironi M, Laskarin G, Monti P, Piemonti L, Biondi A, Mantovani A, et al. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol. 2003;171:4552–4560. doi: 10.4049/jimmunol.171.9.4552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.