SUMMARY
Activity-dependent CREB phosphorylation and gene expression are critical for long-term neuronal plasticity. Local signaling at CaV1 channels triggers these events but how information is relayed onward to the nucleus remains unclear. Here we report a novel mechanism that mediates long-distance communication within cells: a shuttle that transports Ca2+/calmodulin from the surface membrane to the nucleus. We show that the shuttle protein is γCaMKII, that its phosphorylation at Thr287 by βCaMKII protects the Ca2+/CaM signal, and that CaN triggers its nuclear translocation. Both βCaMKII and CaN act in close proximity to CaV1 channels, supporting their dominance, while γCaMKII operates as a carrier, not as a kinase. Upon arrival within the nucleus, Ca2+/CaM activates CaMKK and its substrate CaMKIV, the CREB kinase. This mechanism resolves longstanding puzzles about CaM/CaMK-dependent signaling to the nucleus. The significance of the mechanism is emphasized by dysregulation of CaV1, γCaMKII, βCaMKII and CaN in multiple neuropsychiatric disorders.
INTRODUCTION
Enduring neuronal plasticity requires communication between surface electrical events and nuclear gene expression (Deisseroth et al., 2003; Dolmetsch et al., 2001; Lonze and Ginty, 2002). Such “excitation-transcription (E-T) coupling” is exemplified by activity-dependent regulation of the transcription factor CREB (cAMP-response element binding protein), which is functionally important for learning and memory (Bartsch et al., 1998; Impey et al., 1996; Kandel, 2001; Yin et al., 1995). In mammalian neurons, this signaling is potently initiated by Ca2+ influx through surface CaV1 (L-type) channels (Greenberg et al., 1986; Morgan and Curran, 1986; Murphy et al., 1991), but culminates micrometers away with activation of nuclear CREB (Deisseroth et al., 1996; Dolmetsch et al., 2001). Despite much study, basic questions persist about mechanisms that link neuronal activity to nuclear events.
Ca2+ binding to calmodulin (CaM) and consequent activation of Ca2+/CaM-dependent protein kinases (Hudmon and Schulman, 2002; Kennedy, 2000) helps support E-T coupling (Wheeler et al., 2008; Wheeler et al., 2012; Wu et al., 2001). The CaM kinase family is most famous for αCaMKII, which contributes strongly to synaptic potentiation, learning and memory (Giese et al., 1998; Lisman et al., 2002; Malinow et al., 1989; Silva et al., 1992; Wayman et al., 2008). Together with βCaMKII, αCaMKII participates in E-T coupling by gathering in signaling clusters within CaV1 channel nanodomains (Wheeler et al., 2008; Wheeler et al., 2012). Additional CaM kinases, CaMKIV and CaMKK form a CaMK cascade within the nucleus (Means, 2000; Soderling, 1999). Neuronal activity and Ca2+/CaM drive CaMKK to phosphorylate and activate nuclear CaMKIV, which phosphorylates CREB (Bito et al., 1996) and CREB-binding protein (CBP)(Impey et al., 2002), thus triggering gene expression. CaMK actions near the surface and within the nucleus are clearly distinct, but the link between these remains unclear.
This paper features another CaMK family member, γCaMKII, which is enriched in mammalian brain, but also expressed in heart, smooth muscle, liver, and immune cells (Bayer et al., 1999; Gangopadhyay et al., 2003; Tobimatsu and Fujisawa, 1989). Variations in its gene (CAMK2G) are associated with unreliable memory (de Quervain and Papassotiropoulos, 2006) and mental retardation (de Ligt et al., 2012). Here we show that γCaMKII provides a conduit between Ca2+ entry at the neuronal surface and nuclear transcriptional events. γCaMKII operates as a vectorial transporter of sequestered Ca2+/CaM, independent of any catalytic activity. Once delivered to the nucleus by γCaMKII, Ca2+/CaM triggers a highly cooperative activation of the nuclear CaMK cascade, rapid phosphorylation of CREB and transcription of target genes. Our experiments demonstrate that the γCaMKII shuttle is a long-sought mechanism to rapidly link voltage-gated opening of CaV1 Ca2+ channels to activity-dependent transcription.
RESULTS
Changes in spatial distribution of γCaMKII upon stimulation
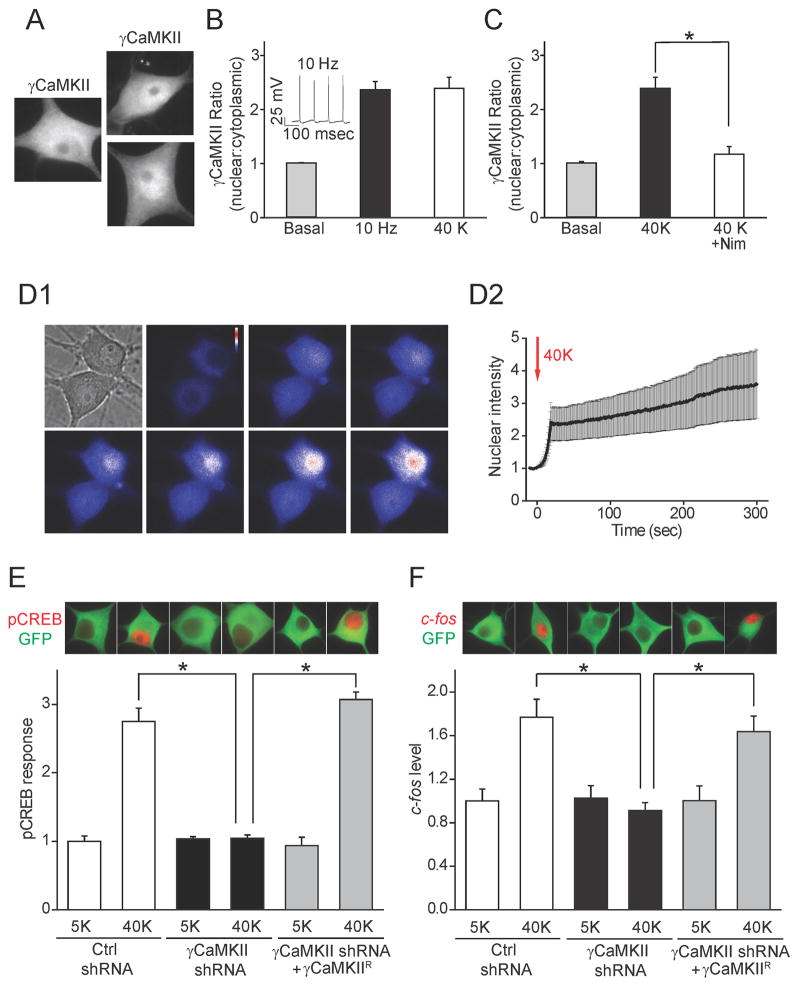
Studies in cultured superior cervical ganglion (SCG) neurons indicated that E-T coupling is initiated in nanodomains just beneath the plasma membrane and involves clustering of CaMKII (α and β isoforms) near CaV1 channels (Wheeler et al., 2008; Wheeler et al., 2012). These findings explained the reliance of E-T coupling on CaV1 channels but left open how a local aggregation of CaMKII at the surface causes signaling to the nucleus. Neither αCaMKII nor βCaMKII showed nuclear translocation following K+-depolarization, as in hippocampal neurons (Deisseroth et al., 1998); this also held for δCaMKII (Supplemental Fig. 1A–C). However, the distribution of the γCaMKII underwent a ~2.5-fold increase in the nuclear: cytoplasmic intensity ratio upon depolarization with 40 mM K+, due to elevated nuclear intensity and diminished cytoplasmic intensity (Fig. 1A, Supplemental Fig. 1D). Similar changes were evoked by trains of spikes (10 Hz field stimulation) (Fig. 1A, B). The depolarization-induced translocation of γCaMKII to the nucleus was abolished by nimodipine (Nim, Fig. 1C), a CaV1-specific channel blocker, indicating that CaV1 channels initiated the movement. To monitor the translocation in real time, we expressed a monomeric GFP-tagged γCaMKII. The GFP-γCaMKII fluorescence intensity consistently rose in the nucleus upon stimulation (Fig. 1D1, D2) and progressively decreased in the distal dendrites (Supplemental Fig. 1E, F), consistent with translocation of tagged γCaMKII.
Figure 1. γCaMKII translocates to the nucleus in an activity-dependent manner and is critical for E-T coupling.
(A) SCG neurons were stimulated at 10 Hz for 60 s or exposed to 40 mM K+ for 300 s and stained with a γCaMKII antibody. Scale bar, 10 μm. (B) Increase in nuclear: cytoplasmic ratio ofγCaMKII intensity resulting from stimulation at 10 Hz for 60 s (solid bar) or from exposure to 40 mM K+ for 300 s (open bar). Inset, recorded action potentials evoked by 10 Hz field stimulation. (C) γCaMKII translocation triggered by 40 mM K+ stimulation was abolished by the CaV1 specific blocker Nim (10 μM). (D1) mGFP-γCaMKII translocation upon 40 mM K+ stimulation. The bright field image indicates the cell shape, and other panels show epifluorescent images (union jack format) captured at indicated time points. (D2) Nuclear mGFP intensity, before and during 40 mM K+ stimulation (onset, t=0 s, n=4). (E) SCG neurons expressing γCaMKII shRNA or non-silencing control shRNA were stimulated with 40 mM K+ for 10 s and stained for pCREB (red). GFP (green) was used to confirm expression of plasmids. The pCREB response was prevented by γCaMKII knockdown and rescued by overexpressing the shRNA-resistant γCaMKII construct γCaMKIIR (see Supplemental Fig. 1H–J for details). (F) c-fos protein levels in SCG neurons transduced as in (E) and stimulated with 40 mM K+ for 300 s, followed by 40 min incubation in normal culture medium to allow time for gene expression. γCaMKII knockdown prevented the expression of c-fos, which was rescued by overexpressed γCaMKIIR (see also Supplemental Fig. 1M). In all Figures, * denotes p<0.001, as determined by Student’s t-test. Data are represented as mean +/− SEM. See also Figure S1.
γCaMKII is critical for rapid CREB phosphorylation and gene transcription
To find out whether γCaMKII is required for E-T coupling, we studied the pCREB response following knockdown of γCaMKII, performed with a lentivirus expressing short hairpin RNA (shRNA) specific to γCaMKII. Stimulation by exposure to 40 mM K+ for 10 s gave a robust but non-saturating pCREB response (Supplemental Fig. 1G). Importantly, this pCREB response was strongly attenuated by γCaMKII knockdown, but completely rescued by cotransfecting γCaMKII cDNA that is resistant to γCaMKII shRNA (indicated as γCaMKIIR, Fig. 1E; Supplemental Fig. 1H–J). Furthermore, the sustained increase of CREB phosphorylation evoked by a 5 min depolarization was similarly abolished by γCaMKII knockdown (Supplemental Fig. 1K), while the Ca2+ rise was not reduced (Supplemental Fig. 1L). Thus, regardless of whether stimuli were brief or prolonged, γ CaMKII contributed to Ca2+-triggered CREB phosphorylation.
To examine effects of γCaMKII on transcription, we tracked the CREB-dependent gene c-fos (Sheng et al., 1990). Depolarizing neurons with 40 K+ for 5 min led to a ~2-fold increase in c-fos levels (protein, Fig. 1F; mRNA, Supplemental Fig. 1M). Strikingly, this increase in gene expression was prevented by γCaMKII knockdown and rescued by γCaMKIIR (Fig. 1F; Supplemental Fig. 1M). Thus γCaMKII plays a critical role in supporting pCaMKII and CaM redistribution to the nucleus and in driving CREB phosphorylation and gene expression.
Redistribution of phospho-CaMKII upon stimulation
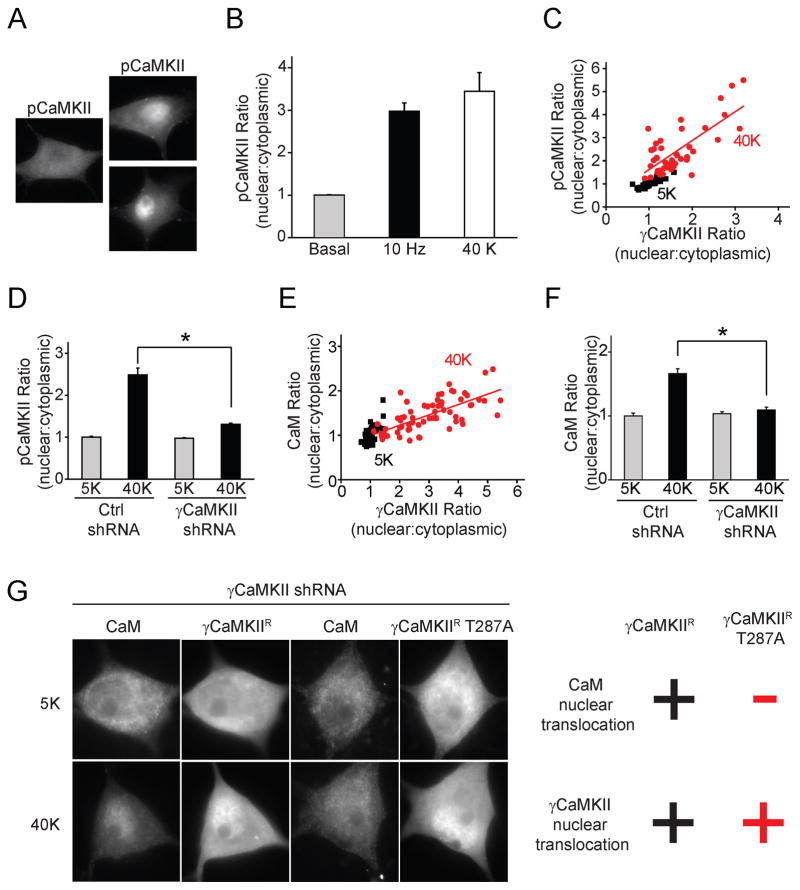
Like other CaMKIIs, γCaMKII is regulated by phosphorylation at a particular threonine (α : Thr286; β, γ and δ : Thr287) (Hudmon and Schulman, 2002; Kennedy, 2000). Finding that γCaMKII is critical for CREB phosphorylation led us to ask whether γCaMKII translocates to the nucleus in a Thr287-phosphorylated state. We used an antibody specific for phospho-Thr286/287 CaMKII (pCaMKII) (not specific to any particular isoform) and compared the distributions of γCaMKII and pCaMKII. Indeed, pCaMKII immunostaining revealed a clear nuclear redistribution in neurons subjected to field stimulation or 40 mM K+ (Fig. 2A, B; Supplemental Fig. S2A, B). Like γCaMKII translocation, redistribution of pCaMKII in response to 40 mM K+ was prevented by Nim (Supplemental Fig. 2C, D).
Figure 2. Activity-dependent nuclear pCaMKII redistribution is driven by γCaMKII/CaM translocation.
(A) SCG neurons were stimulated at 10 Hz for 60 s or with 40 mM K+ for 300 s and stained for phospho-Thr-286/287-CaMKII (pCaMKII). Scale bar, 10 μm. (B) Pooled data for increase in ratio of nuclear:cytoplasmic pCaMKII intensities. (C, E) Single-cell correlation of nuclear:cytoplasmic intensity ratio between pCaMKII and γCaMKII (R=0.8) or CaM and γCaMKII (R=0.7) in response to 40 mM K+, 300 s. (D, F) SCG neurons expressing γCaMKII shRNA or non-silencing control shRNA stimulated as in (C). Increased nuclear:cytoplasmic ratios for pCaMKII and CaM were prevented by γCaMKII knockdown. (G) With endogenous γCaMKII knocked down, shRNA-resistant γCaMKIIR or γCaMKIIR T287A were overexpressed in SCG neurons. Upon stimulation as in (C), both γCaMKIIR and γCaMKIIR T287A translocated to the nucleus; however, only γCaMKIIR, not γCaMKIIR T287A, rescued CaM translocation. “+” and “−“ indicate a significant or insignificant change respectively (see Supplemental Fig. 2 I, J for details). See also Figure S2.
A strong correlation was found between depolarization-driven pCaMKII and γCaMKII redistributions across a population of neurons (Fig. 2C, Supplemental Fig. 2E). A simple interpretation is that γCaMKII translocated into the nucleus in a phospho-Thr287 form. We tested this by knocking down γCaMKII with shRNA, and found that the depolarization-dependent alteration in pCaMKII distribution was largely abolished (Fig. 2D), indicating that pCaMKII redistribution was dominated by nuclear translocation of phosphorylated γCaMKII.
CaM redistribution correlates with the translocation of γCaMKII
One consequence of CaMKII autophosphorylation at Thr-286/287 is a >1000-fold increase in the affinity of CaMKII for Ca2+/CaM (Meyer et al., 1992). Thus, phosphorylated CaMKII might sequester Ca2+/CaM long after cytosolic Ca2+ had subsided (Deisseroth et al., 1998; Ma et al., 2012; Mermelstein et al., 2001). We next asked whether phospho-γCaMKII and CaM might travel to the nucleus together, in line with the proposed role of CaM redistribution in E-T coupling (Deisseroth et al., 1998; Mermelstein et al., 2001). We co-stained neurons for CaM and γCaMKII after 40 K+ stimulation (Supplemental Fig. 2F). The ratios of nuclear: cytoplasmic intensities for CaM and γCaMKII were highly correlated (Fig. 2E). Similarly, correlation was found by directly comparing CaM translocation and pCaMKII redistribution (Supplemental Fig. 2G, H). Knockdown of γCaMKII with shRNA eliminated the stimulation-dependent increase in nuclear: cytoplasmic ratio of CaM staining seen with control shRNA (Fig. 2F). These results suggested that γCaMKII translocated to the nucleus in a T287-phosphorylated form, accompanied by CaM. This would account for the reciprocal changes in nuclear and cytoplasmic levels of pCaMKII and CaM. Finally, the necessity of T287 phosphorylation was probed by an shRNA-resistant construct (γCaMKIIR) encoding γCaMKII with Thr287 mutated to Ala (γCaMKIIR T287A). With endogenous γCaMKII knocked down, “wild-type” γCaMKIIR fully rescued CaM translocation (Fig. 2G left; Supplemental Fig. 2I, J). In contrast, γCaMKIIR T287A was unable to rescue CaM translocation even though it translocated to the nucleus just as well as γCaMKIIR (Fig. 2G right; Supplemental Fig 2I, J). Taken together, these results suggest that γCaMKII carries CaM into the nucleus because Thr287 phosphorylation engages CaM trapping (Hudmon and Schulman, 2002; Meyer et al., 1992).
γCaMKII needs to be dephosphorylated by CaN near the cell surface in order to translocate to the nucleus
How is the nuclear translocation of γCaMKII regulated? Unlike γCaMKII in the other tissues (Gangopadhyay et al., 2003; Takeuchi and Fujisawa, 1998), neuronal specific γCaMKII isoforms (γA, γA′ and γA.B) each possess a nuclear localization signal (NLS) (Takeuchi et al., 2002). If the NLS confers nuclear localization, how might this be regulated? Notably, a serine (Ser-334 in γA′CaMKII) lies just C-terminal to the NLS in γCaMKII (Supplemental Fig. 3A); phosphorylation of a similarly positioned serine inhibits nuclear localization in αBCaMKII and δBCaMKII (Heist et al., 1998).
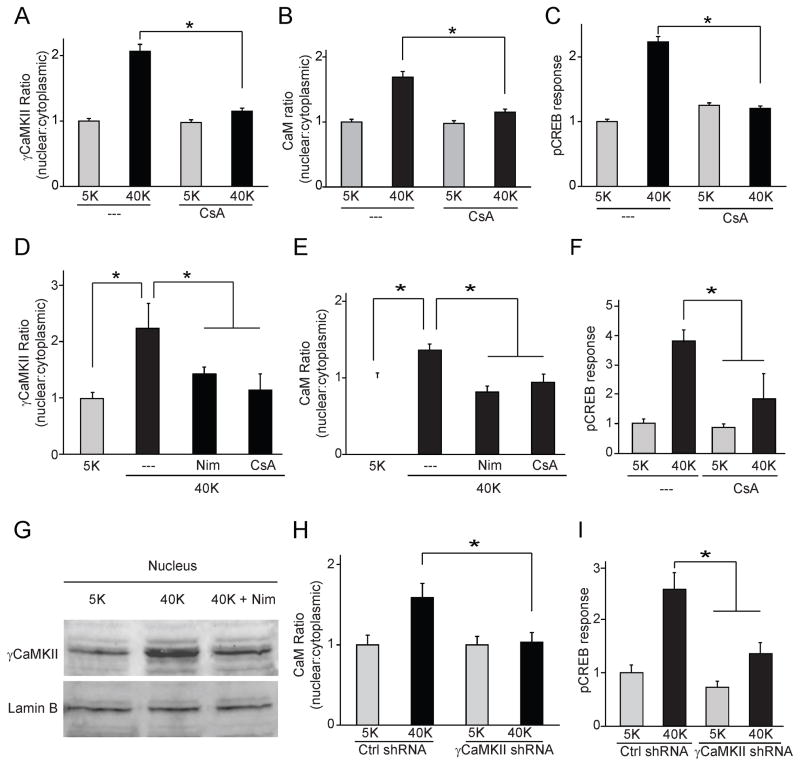
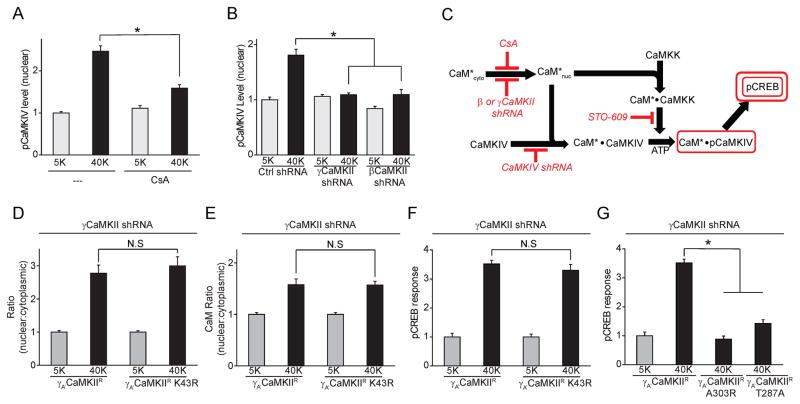
Seeking clues to such regulation, we first applied a broad range phosphatase inhibitor, PhosSTOP (PS), and found that PS inhibited γCaMKII translocation and pCaMKII redistribution, without diminishing Ca2+ influx (Supplemental Fig. 3B–D). These results implied that some kind of phosphatase activity is required for γCaMKII translocation. γCaMKII translocation was unaffected by okadaic acid (OA) at 2 μM, high enough to inhibit both PP1 and PP2A (Supplemental Fig. 3E). In contrast, γCaMKII translocation was abolished by selective inhibition of calcineurin (CaN, also known as PP2B), using cyclosporin A (CsA) (Fig. 3A) or FK506 (Supplemental Fig. 3E). CsA also prevented CaM translocation and the pCREB response (Fig. 3B, C).
Figure 3. CaN is necessary for γCaMKII/CaM translocation and pCREB response in SCG and cortical neurons.
(A, B) SCG neurons stimulated with 40 mM K+ for 300 s in absence or presence of calcineurin-specific inhibitor cyclosporin A (CsA, 50 nm). Translocation of both γCaMKII (A) and CaM (B) prevented by exposure to CsA. (C) pCREB response upon 40 mM K+ stimulation for 10 s was prevented by CsA. (D, E) Cultured cortical neurons were either mock-stimulated with 5 mM K+ or stimulated with 40 mM K+ for 60 s and stained for γCaMKII or CaM (costaining with αCaMKII antibody to label excitatory neurons). CaV1 inhibitor Nim (10 μm) or CaN inhibitor CsA (50 nm) prevented translocation ofγCaMKII and CaM. (F) Cortical neurons treated as in (C), in additional presence of specific MEK inhibitor PD98059 (50 μm), were stained for pCREB. pCREB response inhibited by CsA. (G) Nuclei isolated from cultured cortical neurons and subjected to Western blot analysis to probe for γCaMKII. 40 mM K+ stimulation of intact cells induced an increase of nuclear γCaMKII that was prevented by Nim (N=7). Lamin B is a nuclear marker. (H) Cortical neurons transfected with either γCaMKII shRNA or non-silencing control shRNA, stimulated as in (D) and stained for CaM. γCaMKII knockdown prevented CaM translocation. (I) Cortical neurons expressing either γCaMKII shRNA or a non-silencing control shRNA. Stimulated pCREB response prevented by γCaMKII knockdown. See also Figure S3.
Cortical neurons also display CaN-regulated translocation of γCaMKII and CaM
We sought next to determine whether CaN-regulated γCaMKII translocation exists in CNS neurons, focusing on pyramidal cells cultured from neocortex where γCaMKII transcripts are prominent. Indeed, K+-depolarization induced nuclear translocation of γCaMKII and CaM in cortical pyramidal neurons, but not with Nim or CsA present (Fig. 3D, E). To test whether CaN participates in CaMK-dependent excitation-pCREB coupling, we examined the pCREB response in cortical pyramidal neurons with a MEK1 inhibitor (PD98059) present to avoid engagement of the MAPK pathway (Dolmetsch et al., 2001; Wu et al., 2001). Importantly, depolarization-triggered CREB phosphorylation was inhibited by CaN inhibition with CsA (Fig. 3F).
Capitalizing on the abundance of cultured cortical neurons, we isolated their nuclei and analyzed nuclear γCaMKII levels by western blot. Consistent with immunostaining data, K+ stimulation markedly elevated nuclear γCaMKII, but not with Nim present (Fig. 3G); cytoplasmic γCaMKII was, if anything, slightly diminished (Supplemental Fig. 3F). Importantly, γCaMKII knockdown also prevented CaM translocation and pCREB response (Fig. 3H, I). Thus, CaM translocation and pCREB response in cortical neurons depends on both CaN and γCaMKII, similar to what we found in SCG neurons.
Dephosphorylation of Ser334 by CaN is critical for γCaMKII translocation
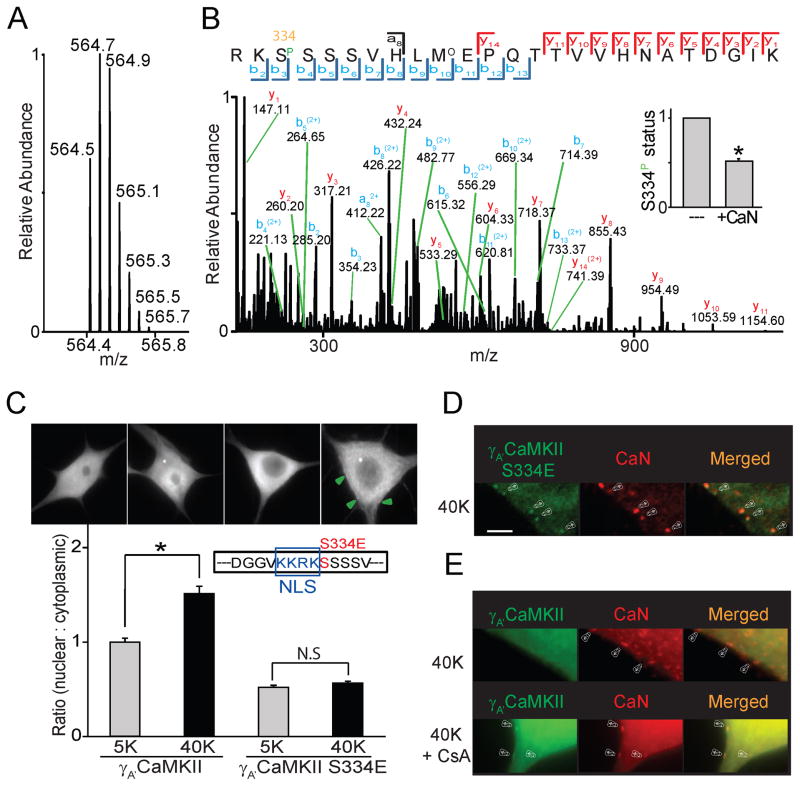
To identify structural determinants of the translocation, we expressed HA-tagged γA′CaMKII protein and probed its phosphorylation state with mass spectrometry (LC-MS/MS) (Fig. 4A, B). The phosphorylation status of Ser-334 and other candidate sites downstream was directly assessed. Ser-334 was phosphorylated under basal conditions in HEK293 cells (Fig. 4A, B), whereas the level of S334 phosphorylation was strongly decreased in vitro by exposure to purified CaN (Fig. 4B, insert). This buttressed pharmacological evidence that endogenous γCaMKII is a target of CaN (Fig. 3A), and supported Ser-334 as a site of dephosphorylation.
Figure 4. γCAMKII is dephosphorylated by CaN near the cell surface to drive nuclear translocation.
(A) Full scan mass spectrum of the quintuply charged ion (m/z 564.4773) for the phosphopeptide RKS(Phospho)SSSVHLM(oxidation)EPQTTVVHNATDGIK from γA′CaMKII. (B) Tandem mass spectrometry (MS/MS) of the phosphopeptide RKS(Phospho)SSSVHLM(Oxidation)EPQTTVVHNATDGIK. MS/MS was performed on the quintuply charged ion (m/z 564.4773) by using higher energy collision induced dissociation. The sequence of the peptide as well as the site of phosphorylation were confidently identified based on the matched b- and y-ion series indicated by the spectrum annotations. Note that all b-ions except the b2 ion correspond to fragments after neutral loss of phosphoric acid. The bar graph (insert) indicates that the phosphorylation status of S334 (marked by the orange square) was attenuated to ~50% of the control group after adding calcineurin and CaM for 30 min. N = 3 experiments, each done in triplicate. (C) SCG neurons expressing γA′CaMKII or γA′CaMKII S334E stimulated with 40 mM K+ for 300 s; γA′CaMKII but not γA′CaMKII S334E translocated to the nucleus upon stimulation. Puncta of γA′CaMKII S334E formed on the cell surface upon stimulation marked by green arrows. Scale bar, 10 μm. Inset, position of S334E point mutation, just carboxyl to the nuclear localization sequence (NLS). (D, E) Representative images of SCG neurons overexpressing γA′CaMKII S334E or γA′CaMKII, stimulated with 40 mM K+ for 60 s. Arrowheads, sites where γA′CaMKII S334E puncta colocalize with CaN (D) or γA′CaMKII puncta colocalize with CaN in presence of CaN inhibitor CsA (lower panel) (E). No γA′CAMKII puncta formed without CaN inhibitor (upper panel) (E). Scale bar, 5 μm. See also Figure S4.
To mimic the negative charge that phosphorylation confers, Ser-334 of HA-tagged γA′CaMKII was mutated to Glu (γA′CaMKII S334E) and modified protein was localized with anti-HA antibody (Fig. 4C). Like endogenous γCaMKII, the HA-tagged “wild-type” γ A′CaMKII protein translocated to the nucleus upon 40 mM K+ stimulation (Fig. 4C, left; Supplemental Fig. 4A), an event prevented by Nim, CsA or FK506 (Supplemental Fig. 4B). In contrast, the phosphomimetic γA′CaMKII S334E protein was mostly cytoplasmic under basal conditions, and failed to undergo translocation upon stimulation (Fig. 4C, right). Thus, dephosphorylation of Ser-334 of γCaMKII appears necessary for its nuclear translocation.
While failing to translocate to the nucleus, γA′CaMKII S334E nonetheless underwent redistribution, forming puncta near the surface membrane upon depolarization (Fig. 4D). Such puncta were not seen with γA′CaMKII itself (Fig. 4E, upper row) unless CaN was inhibited with CsA (Fig. 4E, lower row). Evidently, inhibition of dephosphorylation, by phosphomimetic mutation or CaN inhibition, creates a biochemical bottleneck, causing γA′CaMKII to pile up. The puncta mark sites where CaN-mediated dephosphorylation would normally initiate translocation to the nucleus. Indeed, puncta of γA′CaMKII S334E coincided with puncta of CaN (Fig. 4D, merged image). Similar colocalization was seen in multiple planes of focus (Supplemental Fig. 4C). γA′CaMKII puncta also colocalized with CaN when CaN was inhibited with CsA (Fig. 4E, bottom row). CaN puncta appeared no different when phosphatase activity was not inhibited (Fig. 4E, top row). Without an imposed bottleneck, the lack of γA′CaMKII puncta can be attributed to prompt CaN-triggered translocation to the nucleus.
γCaMKII travels to signaling checkpoint in CaM-bound but not Thr287-phosphorylated form
We next focused on how γCaMKII is recruited to these signaling checkpoints. The puncta formed by γA′CaMKII S334E provided an assay of biochemical steps preceding its arrival at the checkpoint. Consistent with previous reports (Shen and Meyer, 1999), CaM binding to γCaMKII was necessary to enable its recruitment to surface sites (see Supplemental Fig. 4D–I and legends for details). At what subsequent stage is γCaMKII phosphorylated for sake of CaM trapping? A γA′CaMKII construct with a phosphomimetic T287E mutation remained dispersed within the cytosol after stimulation (Supplemental Fig. 4J1, J3). Similarly, a doubly altered γA′CaMKII T287E, S334E construct failed to form puncta upon stimulation (Supplemental Fig. 4J2, J4). Evidently, γCaMKII cannot be mobilized from the cytosol and targeted to the cell surface when Thr287 is fully phosphorylated (see also Hudmon et al., 2005; Shen and Meyer, 1999). Thus, it is CaM-bound, but not fully Thr287-phosphorylated γCaMKII that is recruited to the CaV1 checkpoints (Supplemental Fig. 4M). Such phosphorylation likely occurs before translocation to the nucleus because expression of the nuclear translocation-defective γA′CaMKII S334E strongly increased pCaMKII levels in the cytoplasm and at surface puncta (Supplemental Fig. 4K, L). Thus, Thr287 phosphorylation of γCaMKII likely occurs after its mobilization from the cytosol, but before dephosphorylation of Ser334.
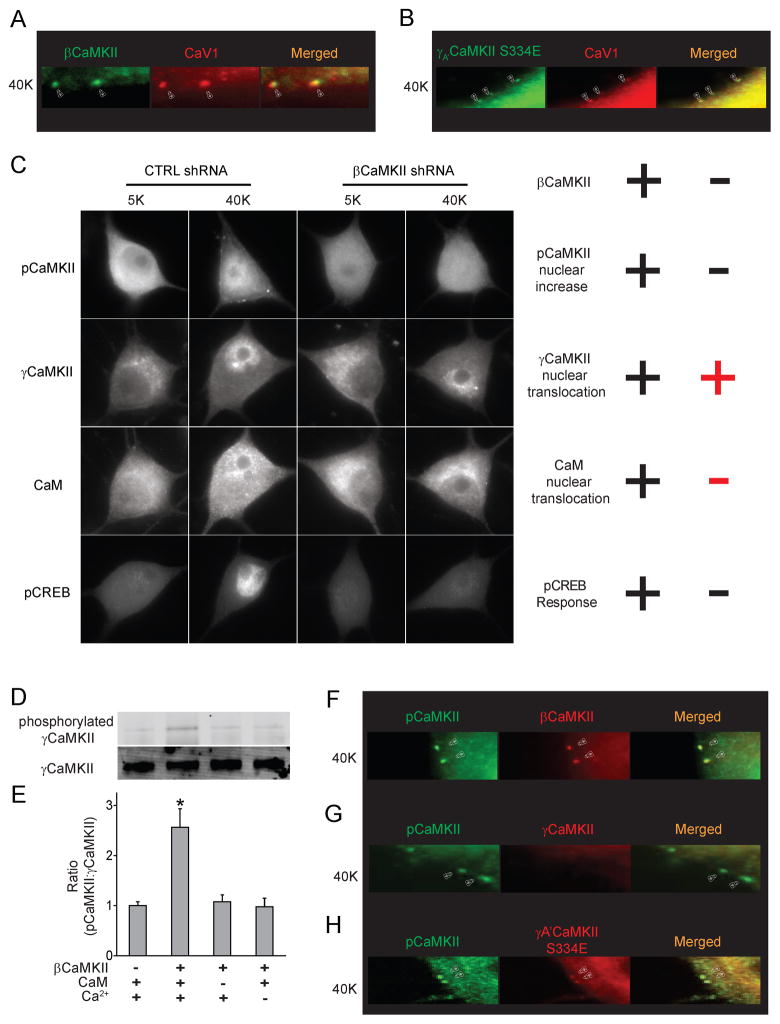
βCaMKII is dispensable for γCaMKII translocation, but required for nuclear accumulation of pCaMKII and CaM
Upon stimulation, CaMKII isoforms other than γCaMKII, principally γCaMKII in cultured SCG neurons, congregate at surface puncta and help initiate signaling to the nucleus (Wheeler et al., 2012). Although βCaMKII does not translocate to the nucleus (Supplemental Fig. 1B), its aggregation suits the role of phosphorylating γCaMKII to enable the CaM trapping in two key respects: specific localization and activity. First, βCaMKII puncta coincided with CaV1 channel puncta (Wheeler et al., 2012) (Fig. 5A); γCaMKII was also mobilized to CaV1 channels, judging by colocalization of γA′CaMKII S334E puncta with CaV1 channel puncta (Fig. 5B). Second, the clusters of γCaMKII appeared to be activated, insofar as they coincided with puncta of pCaMKII (Wheeler et al., 2012), not attributable to γCaMKII (Fig. 4C, left; Fig. 4E, top). Thus, βCaMKII clusters appear well-suited to drive Thr287 phosphorylation of γCaMKII.
Figure 5. Phosphorylation of γCaMKII by βCaMKII: dispensable for γCaMKII translocation but required for CaM translocation and CREB phosphorylation.
(A, B) Arrowheads, sites where endogenous βCaMKII (A) or overexpressed γA′CaMKII S334E (B) colocalize with puncta of CaV1.3 channels upon 40 mM K+ stimulation for 60 s. Scale bar, 1 μm. (C) SCG neurons expressing βCaMKII shRNA or non-silencing control shRNA were stimulated with 40 mM K+ for 300 s and stained for pCaMKII (first row), co-stained for γCaMKII and CaM (second and third row), stained for pCREB (fourth row). Scale bar, 10 μm. βCaMKII knockdown prevented the increase in nuclear pCaMKII levels (−), CaM translocation (−) and pCREB response (−), but did not affect γCaMKII translocation (+). “+” and “−” indicate a significant or insignificant change respectively (see Supplemental Fig. 5A–D for details). (D–E) HA-tagged γA′CaMKII K43R was concentrated and immobilized with agarose beads coated with HA antibody. After a 10-s exposure to 25μM ATP, with or without the presence of 44 μg/ml βCaMKII, 1 mM CaCl2 and 1 μM CaM, the immobilized γA′CaMKII K43R was eluted from the beads and collected for western blot analysis. Phosphorylation state and amount of γA′CaMKII K43R detected by specific antibodies against pCaMKII and γCaMKII (D). A specific antibody to βCaMKII was used to confirm that the pCaMKII was not caused by βCaMKII accumulation on the beads. (E) Ratio of pCaMKII: γCaMKII increased significantly in the presence of βCaMKII (n=7), but only if both CaM and Ca2+ were present (n=4). (F-H) Arrowheads indicate sites where endogenous βCAMKII (F) and overexpressed γA′CaMKII S334E (G), but not γCaMKII (H), colocalize with pCaMKII puncta upon 40 mM K+ stimulation for 60 s. Scale bar, 1 μm. See also Figure S5.
To test this, we knocked down βCaMKII with an shRNA construct that reduced βCaMKII mRNA levels by ~90% while leaving γCaMKII transcripts unchanged (Wheeler et al., 2008). The distributions of pCaMKII, γCaMKII, and CaM were monitored in cells infected with the knockdown construct or a non-silencing control (Fig. 5C). Knockdown of βCaMKII largely abolished the K+-induced increase in nuclear pCaMKII (Fig. 5C, top row), but spared the nuclear γCaMKII rise (Fig. 5C, second row). βCaMKII knockdown also prevented CaM translocation (Fig. 5C, third row), reminiscent of the pattern seen with γCaMKII T287A (Fig. 2G right; Supplemental Fig 2I, J right). Finally, knocking down βCaMKII also prevented CREB phosphorylation (Fig. 5C, bottom row) (Wheeler et al., 2012). These observations were corroborated in pooled data (Supplemental Fig. 5A–D). Evidently, without βCaMKII-dependent phosphorylation at T287, nuclear translocation of the γCaMKII shuttle occurs without cargo and CREB activation fails. Critically, βCaMKII shRNA resistant βCaMKIIR (Supplemental Fig. 5E) rescued the nuclear increase in pCaMKII, CaM translocation and pCREB response (Supplemental Fig. 5F–H), without affecting γCaMKII nuclear translocation (Supplemental Fig. 5I). These observations supported the idea that βCaMKII phosphorylates γCaMKII at Thr287 at the surface checkpoint, stabilizes the γCaMKII-CaM complex, and thus ensures proper co-transport of Ca2+/CaM to the nucleus along with γCaMKII. Nuclear delivery of γCaMKII is not sufficient by itself to trigger a pCREB response; cotransfer of Ca2+/CaM is required.
The canonical mechanism of Thr-286/287 phosphorylation, an intersubunit, intraholoenzyme reaction, was first revealed at low concentrations of CaMKII holoenzyme. However, inter-holoenzyme phosphorylation might occur in the concentrated environment of a CaV1-centered signaling nanodomain. To test for inter-holoenzyme phosphorylation, we set up an enriched environment in vitro. The test substrate, immobilized on beads, was a γCaMKII variant that can bind CaM but is catalytically inactive (K43R). Thr287 phosphorylation of bead-concentrated γCaMKII K43R was significantly increased by purified βCaMKII when activated by Ca2+/CaM (Fig. 5D, E). Thus, inter-molecular phosphorylation can occur if βCaMKII and γCaMKII are colocalized in a high concentration environment.
We also tested another scenario wherein endogenous βCaMKII and γCaMKII basally co-assemble into heteromultimers and travel together to the CaV1 checkpoint and then to the nucleus. In contradiction, formation of near-surface puncta of βCaMKII (Fig. 5F) was not accompanied by detectable puncta of γCaMKII (Fig. 5G); γCaMKII S334E puncta verified our ability to detect puncta of γCaMKII (Fig. 5H). Furthermore, no increase in nuclear βCaMKII was detected, despite clear rises in nuclear γCaMKII (Supplemental Fig. 1B, Fig. 1A). Thus, co-translocation of fixed heteromultimers of βCaMKII and γCaMKII was not supported. We further considered the idea that basal co-assembly of βCaMKII and γCaMKII might give way at the checkpoint to autophosphorylation-dependent subunit exchange (Stratton et al., 2014); βCaMKII could conceivably be stranded at the checkpoint while newly assembled γCaMKII homomultimers traveled onward to the nucleus. Against this scenario, subunit exchange requires autophosphorylation of T305/T306, key residues within the CaM footprint (Stratton et al., 2014); such autophosphorylation is mutually exclusive with Ca2+/CaM binding and thus prohibited during continuous Ca2+ elevation (Fig. 1; Supplemental Fig. 1L). Even if heteromultimers of γCaMKII and βCaMKII form, they are unlikely to dominate γCaMKII mobilization to either the surface membrane or the nucleus.
The γCaMKII-CaM shuttle activates a nuclear CaM kinase cascade
What happens once the γCaMKII-Ca2+/CaM complex arrives in the nucleus? We tested for signaling by a well-characterized nuclear “CaM kinase cascade” (Fig. 6A–C)(Means, 2000; Soderling, 1999). Nuclear-resident CaMKIV is thought to be critical for triggering CREB phosphorylation (Bito et al., 1996; Means, 2000; Soderling, 1999). CaMKIV becomes activated via Thr196 phosphorylation by CaM kinase kinase (CaMKK)(Selbert et al., 1995). The role of this cascade was probed by pharmacological and molecular interventions and by monitoring phosphorylation of CaMKIV and CREB (shown in red in Fig. 6C). CaMKIV was concentrated in the nucleus in SCG neurons and did not redistribute upon depolarization (Supplemental Fig. 6A, C). Its phosphorylation, assessed with an antibody against phospho-Thr196 CaMKIV (pCaMKIV) (Selbert et al., 1995), increased 3-fold upon K+-depolarization (Supplemental Fig. 6A, B). Knockdown of CaMKIV by shRNA (near-complete loss of protein; Supplemental Fig. 6D) abolished the depolarization-dependent increase in pCREB (Supplemental Fig. 6E). The specific CaMKK inhibitor STO-609 prevented the increases in nuclear pCaMKIV (Supplemental Fig. 6F) and pCREB (Supplemental Fig. 6G), confirming the Involvement of CaMKK. These data show that SCG neurons rely on activation of CaMKK and CaMKIV for CREB phosphorylation.
Figure 6. Activation of nuclear CaMK cascade and distinct roles of γCaMKII and CaM in driving CREB phosphorylation.
(A) SCG neurons stimulated with 40 mM K+ for 10 s in the absence or presence of CaN inhibitor CsA (50 nm). Increase in nuclear pCaMKIV levels inhibited by CsA. (B) SCG neurons expressing γCaMKII shRNA, βCaMKII shRNA or nonsilencing control shRNA were stimulated with 40 mM K+ for 10 s and stained for pCaMKIV. Knockdown of γCaMKII or βCaMKII prevented the activation of CaMKIV. (C) Schema showing free Ca2+/CaM (denoted CaM*) delivered by γCaMKII activating the CaMK cascade comprised of CaMKK and CaMKIV. Prevention of critical steps by applying different inhibitors (CsA, STO-609) or shRNA (against βCaMKII, γCaMKII or CaMKIV) indicated in red; rectangles show monitored variables (see Supplemental Fig. 6 for details). (D–F) SCG neurons expressing γCaMKII shRNA with γCaMKII shRNA-resistant γA′CaMKIIR or γA′CaMKIIR K43R were stimulated with 40 mM K+ for 300 s (D, E) or 10 s (F). Both γA′CaMKIIR and γA′CaMKIIR K43R can translocate to the nucleus upon the stimulation (D), and support CaM translocation (E) and pCREB response (F). (G) SCG neurons expressing γCaMKII shRNA with γCaMKII shRNA resistant γA′CaMKIIR A303R or γA′CaMKIIR T287A were stimulated as in (F). Neither γA′CaMKIIR A303R nor γA′CaMKIIR T287A rescued the pCREB response. See also Figure S6.
Is co-translocation of γCaMKII/CaM necessary for activation of the CaMKK-CaMKIV cascade? Activation of CaMKIV was indeed prevented (a) by exposure to CsA to prevent γCaMKII and CaM translocation (Fig. 6A), (b) by shRNA knockdown of γCaMKII (Fig. 6B), and (c) by βCaMKII knockdown (Fig. 6B). These findings account for the inhibition of CREB phosphorylation by the same maneuvers. Activation of the nuclear CaMK cascade is the critical intermediary between γCaMKII-CaM cotransport to the nucleus and activation of CREB.
γCaMKII catalytic activity is not necessary for CREB phosphorylation
Does γCaMKII operates as a protein kinase or merely as a transporter of Ca2+/CaM? To answer this, we knocked down endogenous γCaMKII with γCaMKII shRNA and compared the effects of shRNA-resistant rescue constructs, γA′CaMKIIR and γA′CaMKIIR K43R (kinase dead). The catalytically inactive protein still translocated to the nucleus (Fig. 6D), rescued CaM translocation (Fig. 6E) and supported the pCREB response (Fig. 6F), each to the same extent as kinase active γA′CaMKIIR. In sharp contrast, attempted rescue by expression of γA′CaMKIIR A303R or γA′CaMKIIR T287A failed to support pCREB response (Fig. 6G), consistent with the inability of these variants to support respectively CaM binding or sequestration, and hence CaM translocation (Supplemental Fig. 2I; 4F). Indeed, inhibiting nuclear CaM untrapping from γCaMKII also prevented the pCREB response, as tested by blocking PP2A dephosphorylation of phospho-γCaMKII (see Supplemental Fig. 6H, I and legend for details). Taken together, our results indicated that nuclear Ca2+/CaM, not the catalytic activity of γCaMKII, is necessary for triggering CREB phosphorylation.
Sufficiency of nuclear CaM delivery in the absence of γCaMKII translocation
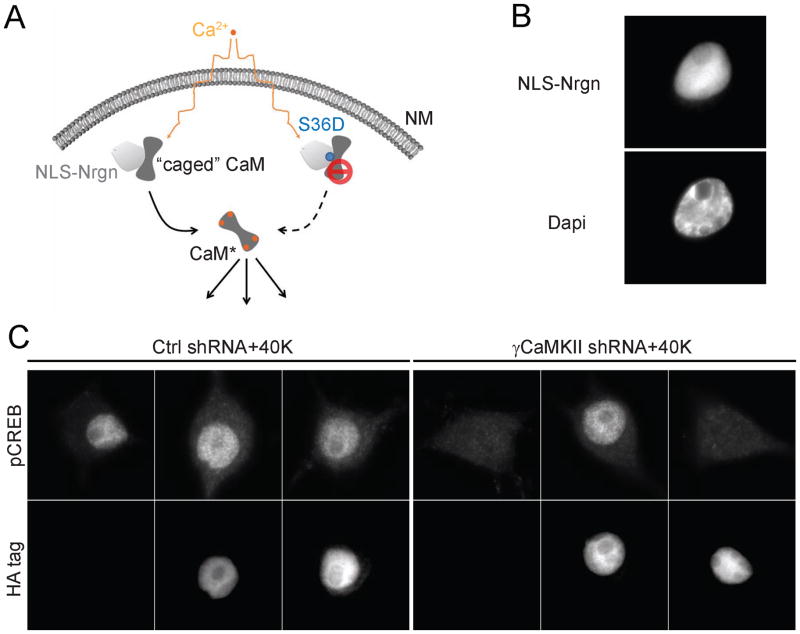
Given the necessity of nuclear CaM delivery, over and above elevation of γCaMKII, we asked whether delivery of CaMnuc is also sufficient, even without γCaMKII. Nuclear liberation of CaM was accomplished by exploiting neurogranin (Nrgn), an endogenous CaM buffer that sequesters apoCaM but releases CaM once it becomes Ca2+ bound (Baudier et al., 1991). By fusing Nrgn with an N-terminal NLS, we targeted NLS-Nrgn (and thus “caged CaM”) to the nucleus of quiescent SCG neurons (Fig. 7 A, B). Expression of this construct had no effect on CREB phosphorylation without stimulation (Supplemental Fig. 7A, B), and slightly increased the pCREB response (p<0.03) to depolarization (Fig. 7C; Supplemental Fig. 7B, left bars). In the test of sufficiency, we examined the effect of NLS-Nrgn following γCaMKII knockdown to eliminate normal cytoplasm-to-nucleus shuttling of CaM (Fig. 2F). Blockade of this route of CaM delivery verifiably prevented CREB phosphorylation in 40 K+. In sharp contrast, the pCREB response was restored to normal levels (P=0.8) by the NLS-Nrgn construct (Fig. 7C; Supplemental Fig. 7B, right bars). To establish that nuclear Nrgn acts to liberate Ca2+/CaM, we tested an S36D variant of NLS-Nrgn, which lacks apoCaM binding; NLS-Nrgn S36D failed to rescue CREB phosphorylation (Fig. 7C, Supplemental Fig. 7B, right bars). Finally, the CaMKK inhibitor STO-609 abolished the NLS-Nrgn-rescued pCREB response (Supplemental Fig. 7C), confirming that the uncaged Ca2+/CaM operates via the nuclear CaMK cascade. This set of NLS-Nrgn experiments demonstrated that direct delivery of nuclear Ca2+/CaM is sufficient to drive nuclear CREB phosphorylation if the normal shuttling of CaM is disabled. Thus, nuclear Ca2+/CaM is both necessary and sufficient to support this form of E-T coupling.
Figure 7. Nuclear delivery of Ca2+/CaM is sufficient to drive CREB phosphorylation.
(A) Testing the sufficiency of Ca2+/CaM with a nuclear-localized, caged CaM. (B) HA-tagged NLS-Nrgn or NLS-Nrgn S36D successfully targeted to the nucleus. Scale bar, 10 μm. (C) SCG neurons expressing γCaMKII shRNA or non-silencing control shRNA, stimulated with 40 mM K+ for 10 s. NLS-Nrgn but not NLS-Nrgn S36D was able to restore the pCREB response, consistent respectively with the ability or inability to harbor CaM for release upon nuclear Ca2+ elevation. Under basal conditions, overexpressing NLS-Nrgn or NLS-Nrgn S36D did not affect pCREB levels (see Supplemental Fig. 7A, B for details). See also Figure S7.
DISCUSSION
Our experiments demonstrate a novel mechanism for transmitting information within cells: shuttling Ca2+/CaM from one location to another. γCaMKII, largely overlooked in neuroscience since its discovery 25 years ago (Tobimatsu et al., 1988), is identified here as the surface-to-nucleus shuttle in sympathetic and neocortical pyramidal neurons. The shuttle works as follows. Neuronal membrane depolarization opens CaV1 channels that deliver Ca2+ to the cytoplasm and promote binding of Ca2+/CaM to γCaMKII. The Ca2+/CaM-γCaMKII complex is recruited to CaV1 channels where another isoform of CaMKII (βCaMKII in sympathetic neurons, α, βCaMKII in cortical neurons) also clusters. Two regulatory events ensue: the phosphorylation of γCaMKII at T287 by βCaMKII, trapping the Ca2+/CaM cargo, and the CaN-mediated dephosphorylation of γCaMKII at S334, enabling an adjacent NLS to direct the loaded shuttle to the nucleus. Nuclear delivery of Ca2+/CaM activates the nuclear CaMK cascade, thus driving CREB phosphorylation, and transcription of CRE-regulated genes (Schematic Summary).
Clarifying how Ca2+/CaM translocation serves as a long-range communication mechanism
CaM movements from cytoplasm to nucleus upon elevation of [Ca2+]cyto have previously been reported in neurons (Deisseroth et al., 1998), mammalian cell lines (Teruel et al., 2000; Thorogate and Torok, 2004) and heart cells (Wu and Bers, 2007). Skepticism about the functional relevance of these observations has persisted (Hagenston and Bading, 2011; Hardingham et al., 2001). The abundance of nuclear CaM may seem to obviate any need for CaM translocation. However, nuclear free CaM is in short supply (Teruel et al., 2000) because of the abundance of nuclear CaM-binding proteins (Bachs et al., 1994), leaving opportunity for freshly imported Ca2+/CaM to drive further signaling (Teruel et al., 2000). Another concern is the putatively rapid dissociation of Ca2+ from CaM once [Ca2+]free begins to fall. It has been unclear how CaM, lacking an NLS, might travel to the nucleus other than by passive diffusion (Thorogate and Torok, 2004). We proposed that Ca2+/CaM stabilization and nuclear targeting could both arise from binding to a CaMK, but were unable to find such a partner (Deisseroth et al., 1998; Mermelstein et al., 2001). These issues are now resolved by the identification of γCaMKII as the long-sought molecular shuttle.
Functional Rationale of CaM-driven signaling
The shuttle mechanism helps clarify the functional logic of excitation-pCREB coupling. Clusters of CaV1 channels provide a nucleation point for participants in surface-to-nucleus communication, including CaN and βCaMKII (Wheeler et al., 2008; Wheeler et al., 2012), thus illuminating the long-observed reliance of E-T coupling on CaV1 channels (Deisseroth et al., 1996; Greenberg et al., 1986; Murphy et al., 1991; Wheeler et al., 2012). The joint involvement of βCaMKII and calcineurin accounts for previously puzzling findings that CaM kinase and CaN work synergistically rather than in opposition to promote CREB phosphorylation (Hahm et al., 2003) and CRE-mediated gene expression (Kingsbury et al., 2007; Schwaninger et al., 1995). The localization of multiple signaling events within the nanodomain of CaV1 channels accounts for differential effects of fast and slow on-rate Ca2+ buffers (BAPTA, EGTA) in preventing CaMKII relocation (Wheeler et al., 2012) and CREB phosphorylation (Deisseroth et al., 1996). Launching the signaling at a CaV1-centered Ca2+ nanodomain frees up separate clusters of CaV2 channels for other functions such as signaling to ER and mitochondria (Wheeler et al., 2012). By temporarily trapping Ca2+/CaM, a shuttle mechanism can go on signaling even after rises in cytosolic Ca2+ have subsided, thus reducing the duration of Ca2+ elevation needed to trigger a response. Whether similar ‘private line’ communication also holds for the wide array of Ca2+ entry and release pathways within cells (Berridge, 2012; Clapham, 2007) requires further investigation.
Our experiments indicate that γCaMKII serves to sequester and convey Ca2+/CaM, and that its kinase activity is dispensible for driving CREB phosphorylation. Progressive accumulation of nuclear Ca2+/CaM provides a means for integrating the effects of multiple weak stimuli (Mermelstein et al., 2001). Because CaMKK and CaMKIV both require Ca2+/CaM binding to drive CaMKIV activation (Means, 2000; Wayman et al., 2008), CREB phosphorylation should increase in a steep power law relationship with Δ [Ca2+/CaM]nuc, consistent with our data. Activation of nuclear CaMKIV has multiple regulatory effects beyond CREB phosphorylation. CaMKIV-mediated phosphorylation activates CREB binding protein (CPB)(Impey et al., 2002), an important cofactor of CREB, CREST and other transcription factors. CBP also regulates the nuclear localization of certain histone deacetylases (McKinsey et al., 2000). Furthermore, CaMKIV helps control alternative splicing of pre-mRNA (Xie and Black, 2001). Thus, by clarifying how nuclear CaMKIV becomes activated, our findings help explain the dominant role of CaV1 channels in phenomena ranging from activation of CBP to histone deacetylation and exon selection.
Higher-order Significance and Distinctions from Other Pathways
The importance of nuclear CaM for brain function has been shown with a nucleus-localized trap that curbs rises in nuclear Ca2+/CaM while sparing cytosolic CaM signaling. This inhibited CREB phosphorylation, curbed L-LTP, and abrogated spatial memory (Limback-Stokin et al., 2004); thus functional consequences of thwarting the Ca2+/CaM shuttle were elegantly revealed. This CaM/CaMK-based system adds to known mechanisms for signaling between neuronal (e.g. postsynaptic) membrane and nucleus (Ch’ng and Martin, 2011; Hagenston and Bading, 2011; Jordan and Kreutz, 2009; Saha and Dudek, 2008). The present mechanism likely tracks neuronal depolarization rather than synaptic activity per se, as is likely for the NMDAR-driven transcription factors NFκB (Meffert et al., 2003), Jacob (Karpova et al., 2013) and CRTC1/TORC1 (Ch’ng et al., 2012). These systems may link specific inputs to a restricted set of transcriptional events. In contrast, translocation of Ca2+/CaM and activation of CaMKIV may achieve a broader impact because of the multiplicity of CaMKIV actions. Of known pathways, the CaM kinase-based mechanism is quickest to activate and most sensitive to brief, weak stimuli; even 50 spikes will trigger a 50% activation of CREB over basal levels (Wheeler et al., 2012). Thus, the shuttle mechanism may be tuned for efficacy, speed and sensitivity.
Implications for Schizophrenia, Autism and other neuropsychiatric disorders
Several proteins in the shuttle pathway (subunits of CaV1 channels, γCaMKII, βCaMKII, and CaN) are encoded by genes associated with major neuropsychiatric disorders (autism spectrum disorder (ASD), schizophrenia (SCZ), major depressive disorder (MDD) and bipolar disorder (BPD) (Table 1, Supplemental Materials). Further study is needed to determine which genetic modifications are causal. Already, the extensive representation of the signaling components described here supports the notion that altered activity-dependent regulation of nuclear events may be a general hallmark of mental disorders. For example, CAMK2G, the gene for γCaMKII, crops up repeatedly in neuropsychiatric studies. A polymorphism in CAMK2G is strongly related to variability in human memory performance (de Quervain and Papassotiropoulos, 2006). Protein levels of γCaMKII (and of βCaMKII) appear significantly elevated in the lateral habenulae of rat models of MDD (Li et al., 2013). Likewise, the abundance of γCaMKII protein is increased in NMDAR-related mouse models of schizophrenia (Table 1)(Kocerha et al., 2009). Finally, CAMK2G is a member of a cluster of genes (M12) that has been implicated in autism by transcriptome analysis of autistic brains. Alternative splicing of CAMK2G is sharply altered in ASD samples (Voineagu et al., 2011); this occurs in association with dysregulation of A2BP1, a splicing factor regulated by CaMKIV (Xie and Black, 2001). Thus, our findings could be relevant to brain disorders in multiple ways: by identification of signaling molecules that support CaMKIV-driven alternative splicing as well as activity-dependent transcription.
EXPERIMENTAL PROCEDURES
Additional information regarding primary cultures, plasmids, buffers, antibodies, primer sequences, sample preparation, mass spectrometry analysis and detailed procedures is provided in Extended Experimental Procedures.
Primary neuronal cultures
SCG neurons or cortical neurons were cultured as previously described (Wheeler et al., 2008; Wheeler et al., 2012), with minor modifications (see Extended Experimental Procedures).
Drug treatments and stimulation
We stimulated SCG or cortical neurons with the indicated K solution at 37°C for 10–300 s before fixation (see Extended Experimental Procedures).
Lentiviral transduction of SCG neurons
Lentiviral constructs were made with psPAX2 and pMD2.g. Lentivirus was added to SCG cultures one day after plating, using GFP expression to monitor infection (see Extended Experimental Procedures).
Image analysis
Images for pCREB quantification and puncta weight were analyzed as previously described (Wheeler et al., 2012).
Immunoprecipitation
Pierce Classic IP Kit (Thermo Fisher) was used to concentrate γA′CaMKII K43R on agarose beads, the degree of phosphorylation and the total amount of γA′CaMKII K43R was determined by western blot.
Statistical Analysis
All the data were normalized to corresponding basal conditions. Without further indication, quantification was based on ≥24 fields under a 63× objective from 3–6 platings per condition.
Supplementary Material
Table 1. Various players in the γ CaMKII shuttle mechanism and their association with major neuropsychiatric disorders (autism spectrum disorder (ASD), schizophrenia
(A–C SCG neurons were either mock-stimulated in 5 mM K+ Tyrode solution (containing 2 mM Ca2+) or stimulated with 40 mM K+ Tyrode solution for 300 s. After stimulation, cells were immediately fixed in 4% paraformaldehyde and stained for αCaMKII (A), βCaMKII (B), or δCaMKII (C). Scale bar, 10 μm. There is no significant change in nuclear: cytoplasmic ratio for αCaMKII, βCaMKII, or δCaMKII following stimulation. (D) Representative confocal images of the γCaMKII in SCG neurons after 40 mM K+ stimulation for 300 s compared with 5 mM K+ mock-stimulation. Scale bar, 10 μm. The nuclear: cytoplasmic ratio of γCaMKII intensity increase significantly following stimulation. Quantification was based on ≥16 fields (n≥20 cells) under a 40× objective from 2 platings per condition. (E) The intensity of mGFP-γCaMKII in the distal dendrite was measured upon 40 mM K+ stimulation. The ROI used for the measurement is marked in a red box in the schematic SCG neuron (upper row). The other panels show epifluorescence images captured at time points 0 s, 60 s, and 240 s after onset of stimulation. The GFP signal is presented in the union jack format, with color changing from dark blue to white to red as intensity increases. Scale bar, 2 μm. (F) Time-lapse imaging revealed that γCaMKII in distal dendrites decreased progressively following 40 mM K+ stimulation (n=7). (G) SCG neurons were stimulated with 40 mM K+ for different durations, immediately fixed, and pCREB response was measured. The pCREB response rose rapidly and reached maximum after 40 mM K+ stimulation for 30 s. (H) γCaMKII shRNA was packaged into lentivirus and overexpressed in SCG neurons. Compared to non-silencing control shRNA, γCaMKII shRNA reduced γCaMKII mRNA levels by ~80% in SCG neurons. GAPDH, Ppp1ca and β-actin were used as internal references for RT-PCR. The shRNA against γCaMKII had no detectable effect on mRNA levels of the other isoforms of CaMKII (data not shown). (I, J) Western blot to examine γCaMKII protein levels in SCG neurons following shRNA knockdown. In SCG neurons transduced with γCaMKII shRNA, γCaMKII protein levels were only ~20% of that found in non-silencing control shRNA-transduced neurons; right lane and bar demonstrate rescue by overexpressed γCaMKII shRNA resistant γCaMKIIR. Quantification based on 7 platings per condition. (K) Lentiviral-transduced SCG neurons expressing γCaMKII shRNA or nonsilencing control shRNA were stimulated with 20 mM K+ for 300 s (using 20 mM K+ instead of 40 mM K+ to avoid saturating pCREB response) in the presence of PD98059 (50 μm), a specific inhibitor for Ras/mitogen-activated protein kinase kinase (MEK). This was a precaution against the possibility that γCaMKII knockdown might exert indirect effects on MAPK signaling to CREB phosphorylation, via CaMKIV (Enslen et al., 1996; Wu et al., 2001). γCaMKII knockdown prevented the pCREB response. Quantification was based on ≥24 fields (n≥30 cells) under a 63× objective from 3–6 platings per condition. (L) SCG neurons transduced as in (K) were stimulated with 40 mM K+ for 300 s. The calcium transient upon stimulation was assessed by monitoring the Fura-2 emission ratio (340/380). No difference in amplitude or time course of the rise in cytoplasmic Ca2+ (n≥20). (M) c-fos mRNA levels in SCG neurons transduced as in (K) and stimulated with 40 mM K+ for 300 s, followed by 40 min incubation in normal culture medium to allow time for novel transcription. γCaMKII knockdown prevented transcription of c-fos. Data are normalized against three housekeeping genes and normalized to c-fos levels in the control stimulation condition with non-silencing control shRNA. For RT-PCR, N = 3–4 experiments done in triplicate. *, p<0.001 as determined by Student’s t-test. Data are represented as mean +/− SEM.
(A, B) SCG neurons were mock-stimulated with 5 mM K+ or stimulated with 40 mM K+ and stained for pCaMKII (Thr-286/287). Representative confocal images (A) show a significant change of pCaMKII distribution (B). Quantification was based on ≥16 fields (n≥20 cells) under a 40x objective from 2 platings per condition. (C) pCaMKII redistribution triggered by 40 mM K+ stimulation was abolished by applying the CaV1 specific blocker Nim (10 μM). (D) With calcium influx from CaV1 and CaV2 channels distinguished by the CaV1 specific blocker Nim (10 μm), pCaMKII distribution ratio was plotted against the depolarized membrane potential level (membrane potential data from Wheeler et al., 2012). Compared to CaV2 signaling (gray line), calcium influx through CaV1 channels (black line) was more potent in changing the pCaMKII distribution. (E) SCG neurons treated as in (A) were co-stained with antibodies against pCaMKII and γCaMKII. Representative images show nuclear translocation of γCaMKII is accompanied by pCaMKII redistribution. (F) SCG neurons treated as in (A) were costained with antibodies against γCaMKII and CaM. Representative images show nuclear translocation of γCaMKII is accompanied by CaM translocation. (G) SCG neurons treated as in (A) were co-stained with antibodies against pCaMKII and CaM. Representative images show nuclear translocation of pCaMKII is accompanied by CaM redistribution. (H) Single-cell correlation of nuclear: cytoplasmic intensity ratio between CaM and pCaMKII, in response to 40 mM K+ stimulation for 300 s. The red solid line is a linear fit of the data (R=0.8). (I, J) Lentiviral transduced SCG neurons expressing γCaMKII shRNA with γCaMKII shRNA resistant γCaMKIIR or γCaMKIIR 287A were stimulated with 40 mM K+ for 300 s and co-stained for γCaMKII and CaM. CaM translocation was rescued by shRNA resistant γCaMKIIR but not by γCaMKIIR 287A (I), even though both γCaMKIIR and γCaMKIIR 287A can translocate to the nucleus upon the stimulation (J). Scale bar, 10 μm. *, p<0.001 as determined by Student’s t-test. Data are represented as mean +/− SEM.
(A) The organization of functional domains of the CaMKII protein. γA′CaMKII has a nuclear localization signal (KKRK) in the variable domain, followed by four serines. (B, C) SCG neurons were stimulated with 40 mM K+ for 300 s in the absence or presence of the broad spectrum phosphatase inhibitor PhosStop (PS, 1 tablet in 10 ml Tyrode solution). The γCaMKII translocation (B) and nuclear pCaMKII increase (C) were both inhibited by applying PS. (D) Calcium transients (Fura-2, 340/380 ratio) were measured when SCG neurons were stimulated with 40 mM K+ for 10 s in the absence or presence of the broad phosphatase inhibitor PhosStop (PS, 1 tablet in 10 ml Tyrode solution). After stimulation, SCG neurons were rinsed with 5 K+ Tyrode solution. (E) SCG neurons were stimulated as in (B), in the absence or presence of OA (2 μM, sufficient to block both PP2A and PP1) (Cohen et al., 1990; Nagao et al., 1995) or CaN inhibitor FK506 (1 μM). After stimulation, neurons were stained for γCaMKII. FK506 but not OA prevented γCaMKII translocation. (F) Cytoplasm was isolated from cultured cortical neurons (Thermo Scientific) and Western blot analysis was performed to probe for γCaMKII. 40 mM K+ stimulation of intact cells induced what appeared to be a ~10% decrease in cytosolic levels of γCaMKII (p=0.23), which seemed to be prevented by the CaV1 blocker Nim (p=0.56) (n=4). Lamin B is a nuclear marker to assess the purity of the isolated cytoplasm and GAPDH is a loading control. *p<0.001, as determined by student’s t-test. Data are represented as mean +/− SEM.
(A) Lentiviral-transduced SCG neurons expressing HA-tagged γA′CaMKII were stimulated with 40 mM K+ for different durations. The maximum translocation of γA′CaMKII was observed with a stimulation for 300 s. (B) Lentiviral transduced SCG neurons expressing HA-tagged γA′CaMKII were stimulated with 40 mM K+ for 300 s in the presence or absence of OA (2 μM), FK506 (1 μM), CsA (50 nM) or Nim (10 μM). As in the case of endogenous γCaMKII, the translocation of overexpressed γA′CaMKII was prevented by 52 the CaN inhibitors FK506 or CsA, the CaV1 blocker Nim, but not by OA. (C) Lentiviral transduced SCG neurons expressing HA-tagged γA′CaMKII S334E were stimulated with 40 mM K+ for 60 s and stained with antibodies against the HA tag on γA′CaMKII S334E and against CaN. Arrows indicate sites at which γA′CaMKII S334E puncta colocalize with CaN. In the upper row, the focal plane was shifted upwards along the z-axis by ~0.37 μm, away from the coverslip, yet colocalized puncta could nevertheless be observed. Scale bar, 5 μm. (D) To test whether γA′CaMKII must bind CaM before it is mobilized to the surface checkpoint, we overexpressed γA′CaMKII S334E and stimulated SCG neurons with 40 mM K+ in the presence of KN-93 (4 μM), an agent that interferes with CaMKIICaM interactions (Sumi et al., 1991), or KN-92 its inactive congener. Representative images; Scale bar, 1 μm. (E) γA′CAMKII S334E puncta weight was calculated as described in Methods. KN-93 prevented γA′CaMKII S334E puncta formation at the cell surface whereas KN-92 did not. (F, G) Representative images of SCG neurons overexpressing γA′CaMKII A303R (deficient in CaM binding) or γA′CaMKII A303R, S334E upon 40 mM K+ stimulation. Scale bar is 10 μm for (F) and 5 μm for (G). γA′CaMKII A303R does not translocate to the nucleus upon 40 mM K+ stimulation (F). Unlike γA′CaMKII, γA′CaMKII A303R, S334E does not form puncta on the cell surface upon the stimulation (G). (H, I) With endogenous γCaMKII knocked down, γA′CaMKIIR A303R failed to translocate to the nucleus (H) or to rescue CaM translocation (I). (J1–J4) Representative images of SCG neurons overexpressing γA′CaMKII T287E (mimicking the phosphorylation at Thr-287) or γA′CaMKII T287E, S334E upon 40 mM K+ stimulation. Scale bar is 10 μm for (J1) and 5 μm for (J2). γA′CaMKII T287E does not translocate to the nucleus upon 40 mM K+ stimulation (J3). Unlike γA′CaMKII S334E, γA′CaMKII T287E S334E does not form puncta on the cell surface upon the stimulation (J4). (K, L) pCaMKII levels in the cytoplasm after stimulation. Following γA′CaMKII S334E overexpression, cytosolic pCaMKII levels increased significantly upon stimulation. (M) Schematic figure indicating that, in order to translocate to the nucleus, γ A′CaMKII must bind CaM in a dephosphorylated (Thr-287) state to be recruited to a checkpoint where CaN is localized. *, p<0.001 as determined by Student’s t-test. Data are represented as mean +/− SEM.
Lentiviral-transduced SCG neurons expressing βCaMKII shRNA or non-silencing control shRNA were stimulated with 40 mM K+ for 300 s and stained for pCaMKII, co-stained for γCaMKII and CaM, or stained for pCREB. βCaMKII knock-down prevented the increase in nuclear pCaMKII levels (A), CaM translocation (B) and pCREB response (C), but did not affect γCaMKII translocation (D). (E) mGFP-tagged βCaMKII or βCaMKII shRNA-resistant mGFP-βCaMKIIR was overexpressed in HEK 293 cells with or without the presence of βCaMKII shRNA. In the presence of βCaMKII shRNA, only ~3% of basal βCaMKII was expressed (left two lanes, p<0.001, n=4), while βCaMKII shRNA spared the expression of βCaMKIIR (right two lanes, p>0.5, n=4). GAPDH was used as a loading control. (F–I) Lentiviral-transduced SCG neurons expressing βCaMKII shRNA with βCaMKII shRNA resistant βCaMKIIR were stimulated with 40 mM K+ for 300 s (F, G, H) or 10 s (I) and stained for pCaMKII, CaM, pCREB or γCaMKII. Nuclear increase of pCaMKII (F), CaM translocation (G), and pCREB response (H) were effectively rescued by shRNA-resistant βCaMKIIR, while γCaMKII translocation was not affected (I). *, p<0.001 as determined by Student’s t-test. All the intensity ratios were normalized to the ratio with 5 mM K+ mockstimulation in control neurons. Quantification was based on ≥24 fields (n≥30 cells) under a 63x objective from 3–6 platings per condition. Data are represented as mean +/− SEM.
(A) Representative images for SCG neurons stimulated with 40 mM K+ for 300 s and stained for pCaMKIV (phospho-Thr-196) and CaMKIV. Scale bar, 10 μm. (B–C) SCG neurons were treated as in (A), and the nuclear levels of pCaMKIV (B) and CaMKIV (C) were measured. While nuclear pCaMKIV levels increased significantly upon stimulation (B), no significant change in the level of nuclear CaMKIV was observed (C). (D) Lentiviral transduced SCG neurons expressing CaMKIV shRNA or non-silencing control shRNA were stained with anti-CaMKIV antibody. After CaMKIV knockdown, less than 1% CaMKIV immunoreactivity remained in the nucleus. (E) Lentiviral transduced SCG neurons expressing CaMKIV shRNA or non-silencing control shRNA were stimulated with 40 mM K+ for 10 s and stained for pCREB. CaMKIV knockdown prevented the pCREB response in the nucleus. (F, G) SCG neurons were stimulated with 40 mM K+ for 10 s in the absence and presence of 3 μm STO-609, a specific inhibitor for CaMKK. Increases in nuclear pCaMKIV (F) and pCREB (G) response were abolished in the presence of the CaMKK inhibitor. (H) Testing for involvement of PP2A in dephosphorylation of nuclear pCaMKII. SCG neurons were stimulated with 40 mM K+ in the absence or presence of 20 nM okadaic acid (OA) to specifically inhibit PP2A. Nuclear pCaMKII level after stimulation was plotted against the nuclear γCaMKII level. Linear regression lines were compared between the groups with (red) and without OA (black). The slope for the OA group is more than two-fold steeper than for the group without OA, indicating that more γCaMKII was in a Thr-287 phosphorylated form after nuclear import with PP2A inhibited by OA. (I) Comparison of nuclear pCaMKII and γCaMKII values from (H). OA increased significantly the nuclear pCaMKII level after stimulation, without changing the γCaMKII and CaM level; notably, nuclear CREB phosphorylation was significantly diminished. These data are consistent with a scenario in which PP2A serves to dephosphorylate T287-phosphorylated γCaMKII, thus liberating trapped CaM and driving the nuclear CaMK cascade; OA would oppose such an action. *p<0.001, as determined by Student’s t-test. Data are represented as mean +/− SEM.
(A–B) Lentiviral transduced SCG neurons expressing γCaMKII shRNA or non-silencing control shRNA were stimulated with 40 mM K+ for 10 s. NLS-Nrgn (but not NLS-Nrgn S36D) was able to restore the pCREB response, consistent, respectively, with its ability 55 (or inability) to release free CaM upon the rise in nuclear Ca2+. Under basal conditions, overexpressing NLS-Nrgn or NLS-Nrgn S36D does not affect pCREB levels. Scale bar, 10 μm. N.S. connotes p>0.05 as determined by Student’s t-test. Quantification was based on ≥24 fields (n≥30 cells) under a 63× objective from 3 platings per condition. Error bars represent SEM. (C) Lentiviral transduced SCG neurons expressing γCaMKII shRNA were stimulated with 40 mM K+ for 10 s. NLS-Nrgn restored the pCREB response and the restoration was prevented by specific CaMKK inhibitor STO-609, consistent with the idea that CaM delivery operates upstream of the activation of nuclear CaMKK-CaMKIV-pCREB cascade.
Acknowledgments
We thank Gord Fishell, Andy Hudmon, Paul De Koninck, Niels Ringstad, and Michael Tadross for insightful comments on an earlier manuscript and Steven Burden for valuable suggestions. We thank Prof. Kathleen Morgan and Cynthia Gallant for their support at initial stages. We also thank Tsien laboratory members for discussions throughout the execution of this project. This work was supported by research grants to R.W.T. from the National Institute of General Medical Sciences, the National Institute of Neurological Disorders and Stroke and the Simons, Mathers and Burnett Family Foundations. S.C. is supported by a Medical Scientist Training Program Fellowship.
Footnotes
Author Contributions
H.M. and R.W.T. conceived the project. R.D.G., J.F.E. and H.M. designed the constructs. R.D.G. designed the shRNA and did the RT-PCR. B.-X.L. prepared samples for mass spectrometry and G.-A.Z., E.T. and T.A.N. performed mass spectrometry analysis. S.M.C. and B.-X.L. did the experiments in cortical neuorns. H.M performed all the other experiments. H.M. and R.W.T. wrote the manuscript with comments from all of the other authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachs O, Agell N, Carafoli E. Calmodulin and calmodulin-binding proteins in the nucleus. Cell calcium. 1994;16:289–296. doi: 10.1016/0143-4160(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Bartsch D, Casadio A, Karl KA, Serodio P, Kandel ER. CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell. 1998;95:211–223. doi: 10.1016/s0092-8674(00)81752-3. [DOI] [PubMed] [Google Scholar]
- Baudier J, Deloulme JC, Van Dorsselaer A, Black D, Matthes HW. Purification and characterization of a brain-specific protein kinase C substrate, neurogranin (p17) J Biol Chem. 1991;266:229–237. [PubMed] [Google Scholar]
- Bayer KU, Lohler J, Schulman H, Harbers K. Developmental expression of the CaM kinase II isoforms: ubiquitous gamma- and delta-CaM kinase II are the early isoforms and most abundant in the developing nervous system. Brain Res Mol Brain Res. 1999;70:147–154. doi: 10.1016/s0169-328x(99)00131-x. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Calcium signalling remodelling and disease. Biochem Soc Trans. 2012;40:297–309. doi: 10.1042/BST20110766. [DOI] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- Ch’ng TH, Martin KC. Synapse-to-nucleus signaling. Curr Opin Neurobiol. 2011;21:345–352. doi: 10.1016/j.conb.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch’ng TH, Uzgil B, Lin P, Avliyakulov NK, O’Dell TJ, Martin KC. Activity-dependent transport of the transcriptional coactivator CRTC1 from synapse to nucleus. Cell. 2012;150:207–221. doi: 10.1016/j.cell.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- de Ligt J, Willemsen MH, van Bon BW, Kleefstra T, Yntema HG, Kroes T, Vulto-van Silfhout AT, Koolen DA, de Vries P, Gilissen C, et al. Diagnostic exome sequencing in persons with severe intellectual disability. N Engl J Med. 2012;367:1921–1929. doi: 10.1056/NEJMoa1206524. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Papassotiropoulos A. Identification of a genetic cluster influencing memory performance and hippocampal activity in humans. Proc Natl Acad Sci U S A. 2006;103:4270–4274. doi: 10.1073/pnas.0510212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Bito H, Tsien RW. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Heist EK, Tsien RW. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature. 1998;392:198–202. doi: 10.1038/32448. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Mermelstein PG, Xia H, Tsien RW. Signaling from synapse to nucleus: the logic behind the mechanisms. Curr Opin Neurobiol. 2003;13:354–365. doi: 10.1016/s0959-4388(03)00076-x. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- Gangopadhyay SS, Barber AL, Gallant C, Grabarek Z, Smith JL, Morgan KG. Differential functional properties of calmodulin-dependent protein kinase IIgamma variants isolated from smooth muscle. Biochem J. 2003;372:347–357. doi: 10.1042/BJ20030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- Greenberg ME, Ziff EB, Greene LA. Stimulation of neuronal acetylcholine receptors induces rapid gene transcription. Science. 1986;234:80–83. doi: 10.1126/science.3749894. [DOI] [PubMed] [Google Scholar]
- Hagenston AM, Bading H. Calcium signaling in synapse-to-nucleus communication. Cold Spring Harb Perspect Biol. 2011;3:a004564. doi: 10.1101/cshperspect.a004564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm SH, Chen Y, Vinson C, Eiden LE. A calcium-initiated signaling pathway propagated through calcineurin and cAMP response element-binding protein activates proenkephalin gene transcription after depolarization. Mol Pharmacol. 2003;64:1503–1511. doi: 10.1124/mol.64.6.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJ, Bading H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat Neurosci. 2001;4:261–267. doi: 10.1038/85109. [DOI] [PubMed] [Google Scholar]
- Heist EK, Srinivasan M, Schulman H. Phosphorylation at the nuclear localization signal of Ca2+/calmodulin-dependent protein kinase II blocks its nuclear targeting. J Biol Chem. 1998;273:19763–19771. doi: 10.1074/jbc.273.31.19763. [DOI] [PubMed] [Google Scholar]
- Hudmon A, Lebel E, Roy H, Sik A, Schulman H, Waxham MN, De Koninck P. A mechanism for Ca2+/calmodulin-dependent protein kinase II clustering at synaptic and nonsynaptic sites based on self-association. J Neurosci. 2005;25:6971–6983. doi: 10.1523/JNEUROSCI.4698-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudmon A, Schulman H. Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem. 2002;71:473–510. doi: 10.1146/annurev.biochem.71.110601.135410. [DOI] [PubMed] [Google Scholar]
- Impey S, Fong AL, Wang Y, Cardinaux JR, Fass DM, Obrietan K, Wayman GA, Storm DR, Soderling TR, Goodman RH. Phosphorylation of CBP mediates transcriptional activation by neural activity and CaM kinase IV. Neuron. 2002;34:235–244. doi: 10.1016/s0896-6273(02)00654-2. [DOI] [PubMed] [Google Scholar]
- Impey S, Mark M, Villacres EC, Poser S, Chavkin C, Storm DR. Induction of CRE-mediated gene expression by stimuli that generate long-lasting LTP in area CA1 of the hippocampus. Neuron. 1996;16:973–982. doi: 10.1016/s0896-6273(00)80120-8. [DOI] [PubMed] [Google Scholar]
- Jordan BA, Kreutz MR. Nucleocytoplasmic protein shuttling: the direct route in synapse-to-nucleus signaling. Trends Neurosci. 2009;32:392–401. doi: 10.1016/j.tins.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Karpova A, Mikhaylova M, Bera S, Bar J, Reddy PP, Behnisch T, Rankovic V, Spilker C, Bethge P, Sahin J, et al. Encoding and transducing the synaptic or extrasynaptic origin of NMDA receptor signals to the nucleus. Cell. 2013;152:1119–1133. doi: 10.1016/j.cell.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Kennedy MB. Signal-processing machines at the postsynaptic density. Science. 2000;290:750–754. doi: 10.1126/science.290.5492.750. [DOI] [PubMed] [Google Scholar]
- Kingsbury TJ, Bambrick LL, Roby CD, Krueger BK. Calcineurin activity is required for depolarization-induced, CREB-dependent gene transcription in cortical neurons. J Neurochem. 2007;103:761–770. doi: 10.1111/j.1471-4159.2007.04801.x. [DOI] [PubMed] [Google Scholar]
- Kocerha J, Faghihi MA, Lopez-Toledano MA, Huang J, Ramsey AJ, Caron MG, Sales N, Willoughby D, Elmen J, Hansen HF, et al. MicroRNA-219 modulates NMDA receptor-mediated neurobehavioral dysfunction. Proc Natl Acad Sci U S A. 2009;106:3507–3512. doi: 10.1073/pnas.0805854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Zhou T, Liao L, Yang Z, Wong C, Henn F, Malinow R, Yates JR, 3rd, Hu H. betaCaMKII in lateral habenula mediates core symptoms of depression. Science. 2013;341:1016–1020. doi: 10.1126/science.1240729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limback-Stokin K, Korzus E, Nagaoka-Yasuda R, Mayford M. Nuclear calcium/calmodulin regulates memory consolidation. J Neurosci. 2004;24:10858–10867. doi: 10.1523/JNEUROSCI.1022-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Ma H, Cohen S, Li B, Tsien RW. Exploring the dominant role of Cav1 channels in signalling to the nucleus. Biosci Rep. 2012;33 doi: 10.1042/BSR20120099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Schulman H, Tsien RW. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989;245:862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- McKinsey TA, Zhang CL, Lu J, Olson EN. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature. 2000;408:106–111. doi: 10.1038/35040593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means AR. Regulatory cascades involving calmodulin-dependent protein kinases. Mol Endocrinol. 2000;14:4–13. doi: 10.1210/mend.14.1.0414. [DOI] [PubMed] [Google Scholar]
- Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-kappa B functions in synaptic signaling and behavior. Nat Neurosci. 2003;6:1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Deisseroth K, Dasgupta N, Isaksen AL, Tsien RW. Calmodulin priming: nuclear translocation of a calmodulin complex and the memory of prior neuronal activity. Proc Natl Acad Sci U S A. 2001;98:15342–15347. doi: 10.1073/pnas.211563998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Hanson PI, Stryer L, Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992;256:1199–1202. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Role of ion flux in the control of c-fos expression. Nature. 1986;322:552–555. doi: 10.1038/322552a0. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Worley PF, Baraban JM. L-type voltage-sensitive calcium channels mediate synaptic activation of immediate early genes. Neuron. 1991;7:625–635. doi: 10.1016/0896-6273(91)90375-a. [DOI] [PubMed] [Google Scholar]
- Saha RN, Dudek SM. Action potentials: to the nucleus and beyond. Exp Biol Med (Maywood) 2008;233:385–393. doi: 10.3181/0709-MR-241. [DOI] [PubMed] [Google Scholar]
- Schwaninger M, Blume R, Kruger M, Lux G, Oetjen E, Knepel W. Involvement of the Ca(2+)-dependent phosphatase calcineurin in gene transcription that is stimulated by cAMP through cAMP response elements. J Biol Chem. 1995;270:8860–8866. doi: 10.1074/jbc.270.15.8860. [DOI] [PubMed] [Google Scholar]
- Selbert MA, Anderson KA, Huang QH, Goldstein EG, Means AR, Edelman AM. Phosphorylation and activation of Ca(2+)-calmodulin-dependent protein kinase IV by Ca(2+)-calmodulin-dependent protein kinase Ia kinase. Phosphorylation of threonine 196 is essential for activation. J Biol Chem. 1995;270:17616–17621. doi: 10.1074/jbc.270.29.17616. [DOI] [PubMed] [Google Scholar]
- Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284:162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- Sheng M, McFadden G, Greenberg ME. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron. 1990;4:571–582. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Soderling TR. The Ca-calmodulin-dependent protein kinase cascade. Trends Biochem Sci. 1999;24:232–236. doi: 10.1016/s0968-0004(99)01383-3. [DOI] [PubMed] [Google Scholar]
- Stratton M, Lee IH, Bhattacharyya M, Christensen SM, Chao LH, Schulman H, Groves JT, Kuriyan J. Activation-triggered subunit exchange between CaMKII holoenzymes facilitates the spread of kinase activity. Elife. 2014;3:e01610. doi: 10.7554/eLife.01610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M, Fujisawa H. New alternatively spliced variants of calmodulin-dependent protein kinase II from rabbit liver. Gene. 1998;221:107–115. doi: 10.1016/s0378-1119(98)00422-3. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Fukunaga K, Miyamoto E. Activation of nuclear Ca(2+)/calmodulin-dependent protein kinase II and brain-derived neurotrophic factor gene expression by stimulation of dopamine D2 receptor in transfected NG108-15 cells. J Neurochem. 2002;82:316–328. doi: 10.1046/j.1471-4159.2002.00967.x. [DOI] [PubMed] [Google Scholar]
- Teruel MN, Chen W, Persechini A, Meyer T. Differential codes for free Ca(2+)-calmodulin signals in nucleus and cytosol. Curr Biol. 2000;10:86–94. doi: 10.1016/s0960-9822(00)00295-5. [DOI] [PubMed] [Google Scholar]
- Thorogate R, Torok K. Ca2+-dependent and -independent mechanisms of calmodulin nuclear translocation. Journal of cell science. 2004;117:5923–5936. doi: 10.1242/jcs.01510. [DOI] [PubMed] [Google Scholar]
- Tobimatsu T, Fujisawa H. Tissue-specific expression of four types of rat calmodulin-dependent protein kinase II mRNAs. J Biol Chem. 1989;264:17907–17912. [PubMed] [Google Scholar]
- Tobimatsu T, Kameshita I, Fujisawa H. Molecular cloning of the cDNA encoding the third polypeptide (gamma) of brain calmodulin-dependent protein kinase II. J Biol Chem. 1988;263:16082–16086. [PubMed] [Google Scholar]
- Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, Mill J, Cantor RM, Blencowe BJ, Geschwind DH. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Lee YS, Tokumitsu H, Silva AJ, Soderling TR. Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron. 2008;59:914–931. doi: 10.1016/j.neuron.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DG, Barrett CF, Groth RD, Safa P, Tsien RW. CaMKII locally encodes L-type channel activity to signal to nuclear CREB in excitation-transcription coupling. J Cell Biol. 2008;183:849–863. doi: 10.1083/jcb.200805048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DG, Groth RD, Ma H, Barrett CF, Owen SF, Safa P, Tsien RW. Ca(V)1 and Ca(V)2 channels engage distinct modes of Ca(2+) signaling to control CREB-dependent gene expression. Cell. 2012;149:1112–1124. doi: 10.1016/j.cell.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GY, Deisseroth K, Tsien RW. Activity-dependent CREB phosphorylation: convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. Proc Natl Acad Sci U S A. 2001;98:2808–2813. doi: 10.1073/pnas.051634198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Bers DM. Free and bound intracellular calmodulin measurements in cardiac myocytes. Cell calcium. 2007;41:353–364. doi: 10.1016/j.ceca.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Black DL. A CaMK IV responsive RNA element mediates depolarization-induced alternative splicing of ion channels. Nature. 2001;410:936–939. doi: 10.1038/35073593. [DOI] [PubMed] [Google Scholar]
- Yin JC, Del Vecchio M, Zhou H, Tully T. CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 1995;81:107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1. Various players in the γ CaMKII shuttle mechanism and their association with major neuropsychiatric disorders (autism spectrum disorder (ASD), schizophrenia
(A–C SCG neurons were either mock-stimulated in 5 mM K+ Tyrode solution (containing 2 mM Ca2+) or stimulated with 40 mM K+ Tyrode solution for 300 s. After stimulation, cells were immediately fixed in 4% paraformaldehyde and stained for αCaMKII (A), βCaMKII (B), or δCaMKII (C). Scale bar, 10 μm. There is no significant change in nuclear: cytoplasmic ratio for αCaMKII, βCaMKII, or δCaMKII following stimulation. (D) Representative confocal images of the γCaMKII in SCG neurons after 40 mM K+ stimulation for 300 s compared with 5 mM K+ mock-stimulation. Scale bar, 10 μm. The nuclear: cytoplasmic ratio of γCaMKII intensity increase significantly following stimulation. Quantification was based on ≥16 fields (n≥20 cells) under a 40× objective from 2 platings per condition. (E) The intensity of mGFP-γCaMKII in the distal dendrite was measured upon 40 mM K+ stimulation. The ROI used for the measurement is marked in a red box in the schematic SCG neuron (upper row). The other panels show epifluorescence images captured at time points 0 s, 60 s, and 240 s after onset of stimulation. The GFP signal is presented in the union jack format, with color changing from dark blue to white to red as intensity increases. Scale bar, 2 μm. (F) Time-lapse imaging revealed that γCaMKII in distal dendrites decreased progressively following 40 mM K+ stimulation (n=7). (G) SCG neurons were stimulated with 40 mM K+ for different durations, immediately fixed, and pCREB response was measured. The pCREB response rose rapidly and reached maximum after 40 mM K+ stimulation for 30 s. (H) γCaMKII shRNA was packaged into lentivirus and overexpressed in SCG neurons. Compared to non-silencing control shRNA, γCaMKII shRNA reduced γCaMKII mRNA levels by ~80% in SCG neurons. GAPDH, Ppp1ca and β-actin were used as internal references for RT-PCR. The shRNA against γCaMKII had no detectable effect on mRNA levels of the other isoforms of CaMKII (data not shown). (I, J) Western blot to examine γCaMKII protein levels in SCG neurons following shRNA knockdown. In SCG neurons transduced with γCaMKII shRNA, γCaMKII protein levels were only ~20% of that found in non-silencing control shRNA-transduced neurons; right lane and bar demonstrate rescue by overexpressed γCaMKII shRNA resistant γCaMKIIR. Quantification based on 7 platings per condition. (K) Lentiviral-transduced SCG neurons expressing γCaMKII shRNA or nonsilencing control shRNA were stimulated with 20 mM K+ for 300 s (using 20 mM K+ instead of 40 mM K+ to avoid saturating pCREB response) in the presence of PD98059 (50 μm), a specific inhibitor for Ras/mitogen-activated protein kinase kinase (MEK). This was a precaution against the possibility that γCaMKII knockdown might exert indirect effects on MAPK signaling to CREB phosphorylation, via CaMKIV (Enslen et al., 1996; Wu et al., 2001). γCaMKII knockdown prevented the pCREB response. Quantification was based on ≥24 fields (n≥30 cells) under a 63× objective from 3–6 platings per condition. (L) SCG neurons transduced as in (K) were stimulated with 40 mM K+ for 300 s. The calcium transient upon stimulation was assessed by monitoring the Fura-2 emission ratio (340/380). No difference in amplitude or time course of the rise in cytoplasmic Ca2+ (n≥20). (M) c-fos mRNA levels in SCG neurons transduced as in (K) and stimulated with 40 mM K+ for 300 s, followed by 40 min incubation in normal culture medium to allow time for novel transcription. γCaMKII knockdown prevented transcription of c-fos. Data are normalized against three housekeeping genes and normalized to c-fos levels in the control stimulation condition with non-silencing control shRNA. For RT-PCR, N = 3–4 experiments done in triplicate. *, p<0.001 as determined by Student’s t-test. Data are represented as mean +/− SEM.
(A, B) SCG neurons were mock-stimulated with 5 mM K+ or stimulated with 40 mM K+ and stained for pCaMKII (Thr-286/287). Representative confocal images (A) show a significant change of pCaMKII distribution (B). Quantification was based on ≥16 fields (n≥20 cells) under a 40x objective from 2 platings per condition. (C) pCaMKII redistribution triggered by 40 mM K+ stimulation was abolished by applying the CaV1 specific blocker Nim (10 μM). (D) With calcium influx from CaV1 and CaV2 channels distinguished by the CaV1 specific blocker Nim (10 μm), pCaMKII distribution ratio was plotted against the depolarized membrane potential level (membrane potential data from Wheeler et al., 2012). Compared to CaV2 signaling (gray line), calcium influx through CaV1 channels (black line) was more potent in changing the pCaMKII distribution. (E) SCG neurons treated as in (A) were co-stained with antibodies against pCaMKII and γCaMKII. Representative images show nuclear translocation of γCaMKII is accompanied by pCaMKII redistribution. (F) SCG neurons treated as in (A) were costained with antibodies against γCaMKII and CaM. Representative images show nuclear translocation of γCaMKII is accompanied by CaM translocation. (G) SCG neurons treated as in (A) were co-stained with antibodies against pCaMKII and CaM. Representative images show nuclear translocation of pCaMKII is accompanied by CaM redistribution. (H) Single-cell correlation of nuclear: cytoplasmic intensity ratio between CaM and pCaMKII, in response to 40 mM K+ stimulation for 300 s. The red solid line is a linear fit of the data (R=0.8). (I, J) Lentiviral transduced SCG neurons expressing γCaMKII shRNA with γCaMKII shRNA resistant γCaMKIIR or γCaMKIIR 287A were stimulated with 40 mM K+ for 300 s and co-stained for γCaMKII and CaM. CaM translocation was rescued by shRNA resistant γCaMKIIR but not by γCaMKIIR 287A (I), even though both γCaMKIIR and γCaMKIIR 287A can translocate to the nucleus upon the stimulation (J). Scale bar, 10 μm. *, p<0.001 as determined by Student’s t-test. Data are represented as mean +/− SEM.
(A) The organization of functional domains of the CaMKII protein. γA′CaMKII has a nuclear localization signal (KKRK) in the variable domain, followed by four serines. (B, C) SCG neurons were stimulated with 40 mM K+ for 300 s in the absence or presence of the broad spectrum phosphatase inhibitor PhosStop (PS, 1 tablet in 10 ml Tyrode solution). The γCaMKII translocation (B) and nuclear pCaMKII increase (C) were both inhibited by applying PS. (D) Calcium transients (Fura-2, 340/380 ratio) were measured when SCG neurons were stimulated with 40 mM K+ for 10 s in the absence or presence of the broad phosphatase inhibitor PhosStop (PS, 1 tablet in 10 ml Tyrode solution). After stimulation, SCG neurons were rinsed with 5 K+ Tyrode solution. (E) SCG neurons were stimulated as in (B), in the absence or presence of OA (2 μM, sufficient to block both PP2A and PP1) (Cohen et al., 1990; Nagao et al., 1995) or CaN inhibitor FK506 (1 μM). After stimulation, neurons were stained for γCaMKII. FK506 but not OA prevented γCaMKII translocation. (F) Cytoplasm was isolated from cultured cortical neurons (Thermo Scientific) and Western blot analysis was performed to probe for γCaMKII. 40 mM K+ stimulation of intact cells induced what appeared to be a ~10% decrease in cytosolic levels of γCaMKII (p=0.23), which seemed to be prevented by the CaV1 blocker Nim (p=0.56) (n=4). Lamin B is a nuclear marker to assess the purity of the isolated cytoplasm and GAPDH is a loading control. *p<0.001, as determined by student’s t-test. Data are represented as mean +/− SEM.
(A) Lentiviral-transduced SCG neurons expressing HA-tagged γA′CaMKII were stimulated with 40 mM K+ for different durations. The maximum translocation of γA′CaMKII was observed with a stimulation for 300 s. (B) Lentiviral transduced SCG neurons expressing HA-tagged γA′CaMKII were stimulated with 40 mM K+ for 300 s in the presence or absence of OA (2 μM), FK506 (1 μM), CsA (50 nM) or Nim (10 μM). As in the case of endogenous γCaMKII, the translocation of overexpressed γA′CaMKII was prevented by 52 the CaN inhibitors FK506 or CsA, the CaV1 blocker Nim, but not by OA. (C) Lentiviral transduced SCG neurons expressing HA-tagged γA′CaMKII S334E were stimulated with 40 mM K+ for 60 s and stained with antibodies against the HA tag on γA′CaMKII S334E and against CaN. Arrows indicate sites at which γA′CaMKII S334E puncta colocalize with CaN. In the upper row, the focal plane was shifted upwards along the z-axis by ~0.37 μm, away from the coverslip, yet colocalized puncta could nevertheless be observed. Scale bar, 5 μm. (D) To test whether γA′CaMKII must bind CaM before it is mobilized to the surface checkpoint, we overexpressed γA′CaMKII S334E and stimulated SCG neurons with 40 mM K+ in the presence of KN-93 (4 μM), an agent that interferes with CaMKIICaM interactions (Sumi et al., 1991), or KN-92 its inactive congener. Representative images; Scale bar, 1 μm. (E) γA′CAMKII S334E puncta weight was calculated as described in Methods. KN-93 prevented γA′CaMKII S334E puncta formation at the cell surface whereas KN-92 did not. (F, G) Representative images of SCG neurons overexpressing γA′CaMKII A303R (deficient in CaM binding) or γA′CaMKII A303R, S334E upon 40 mM K+ stimulation. Scale bar is 10 μm for (F) and 5 μm for (G). γA′CaMKII A303R does not translocate to the nucleus upon 40 mM K+ stimulation (F). Unlike γA′CaMKII, γA′CaMKII A303R, S334E does not form puncta on the cell surface upon the stimulation (G). (H, I) With endogenous γCaMKII knocked down, γA′CaMKIIR A303R failed to translocate to the nucleus (H) or to rescue CaM translocation (I). (J1–J4) Representative images of SCG neurons overexpressing γA′CaMKII T287E (mimicking the phosphorylation at Thr-287) or γA′CaMKII T287E, S334E upon 40 mM K+ stimulation. Scale bar is 10 μm for (J1) and 5 μm for (J2). γA′CaMKII T287E does not translocate to the nucleus upon 40 mM K+ stimulation (J3). Unlike γA′CaMKII S334E, γA′CaMKII T287E S334E does not form puncta on the cell surface upon the stimulation (J4). (K, L) pCaMKII levels in the cytoplasm after stimulation. Following γA′CaMKII S334E overexpression, cytosolic pCaMKII levels increased significantly upon stimulation. (M) Schematic figure indicating that, in order to translocate to the nucleus, γ A′CaMKII must bind CaM in a dephosphorylated (Thr-287) state to be recruited to a checkpoint where CaN is localized. *, p<0.001 as determined by Student’s t-test. Data are represented as mean +/− SEM.
Lentiviral-transduced SCG neurons expressing βCaMKII shRNA or non-silencing control shRNA were stimulated with 40 mM K+ for 300 s and stained for pCaMKII, co-stained for γCaMKII and CaM, or stained for pCREB. βCaMKII knock-down prevented the increase in nuclear pCaMKII levels (A), CaM translocation (B) and pCREB response (C), but did not affect γCaMKII translocation (D). (E) mGFP-tagged βCaMKII or βCaMKII shRNA-resistant mGFP-βCaMKIIR was overexpressed in HEK 293 cells with or without the presence of βCaMKII shRNA. In the presence of βCaMKII shRNA, only ~3% of basal βCaMKII was expressed (left two lanes, p<0.001, n=4), while βCaMKII shRNA spared the expression of βCaMKIIR (right two lanes, p>0.5, n=4). GAPDH was used as a loading control. (F–I) Lentiviral-transduced SCG neurons expressing βCaMKII shRNA with βCaMKII shRNA resistant βCaMKIIR were stimulated with 40 mM K+ for 300 s (F, G, H) or 10 s (I) and stained for pCaMKII, CaM, pCREB or γCaMKII. Nuclear increase of pCaMKII (F), CaM translocation (G), and pCREB response (H) were effectively rescued by shRNA-resistant βCaMKIIR, while γCaMKII translocation was not affected (I). *, p<0.001 as determined by Student’s t-test. All the intensity ratios were normalized to the ratio with 5 mM K+ mockstimulation in control neurons. Quantification was based on ≥24 fields (n≥30 cells) under a 63x objective from 3–6 platings per condition. Data are represented as mean +/− SEM.
(A) Representative images for SCG neurons stimulated with 40 mM K+ for 300 s and stained for pCaMKIV (phospho-Thr-196) and CaMKIV. Scale bar, 10 μm. (B–C) SCG neurons were treated as in (A), and the nuclear levels of pCaMKIV (B) and CaMKIV (C) were measured. While nuclear pCaMKIV levels increased significantly upon stimulation (B), no significant change in the level of nuclear CaMKIV was observed (C). (D) Lentiviral transduced SCG neurons expressing CaMKIV shRNA or non-silencing control shRNA were stained with anti-CaMKIV antibody. After CaMKIV knockdown, less than 1% CaMKIV immunoreactivity remained in the nucleus. (E) Lentiviral transduced SCG neurons expressing CaMKIV shRNA or non-silencing control shRNA were stimulated with 40 mM K+ for 10 s and stained for pCREB. CaMKIV knockdown prevented the pCREB response in the nucleus. (F, G) SCG neurons were stimulated with 40 mM K+ for 10 s in the absence and presence of 3 μm STO-609, a specific inhibitor for CaMKK. Increases in nuclear pCaMKIV (F) and pCREB (G) response were abolished in the presence of the CaMKK inhibitor. (H) Testing for involvement of PP2A in dephosphorylation of nuclear pCaMKII. SCG neurons were stimulated with 40 mM K+ in the absence or presence of 20 nM okadaic acid (OA) to specifically inhibit PP2A. Nuclear pCaMKII level after stimulation was plotted against the nuclear γCaMKII level. Linear regression lines were compared between the groups with (red) and without OA (black). The slope for the OA group is more than two-fold steeper than for the group without OA, indicating that more γCaMKII was in a Thr-287 phosphorylated form after nuclear import with PP2A inhibited by OA. (I) Comparison of nuclear pCaMKII and γCaMKII values from (H). OA increased significantly the nuclear pCaMKII level after stimulation, without changing the γCaMKII and CaM level; notably, nuclear CREB phosphorylation was significantly diminished. These data are consistent with a scenario in which PP2A serves to dephosphorylate T287-phosphorylated γCaMKII, thus liberating trapped CaM and driving the nuclear CaMK cascade; OA would oppose such an action. *p<0.001, as determined by Student’s t-test. Data are represented as mean +/− SEM.
(A–B) Lentiviral transduced SCG neurons expressing γCaMKII shRNA or non-silencing control shRNA were stimulated with 40 mM K+ for 10 s. NLS-Nrgn (but not NLS-Nrgn S36D) was able to restore the pCREB response, consistent, respectively, with its ability 55 (or inability) to release free CaM upon the rise in nuclear Ca2+. Under basal conditions, overexpressing NLS-Nrgn or NLS-Nrgn S36D does not affect pCREB levels. Scale bar, 10 μm. N.S. connotes p>0.05 as determined by Student’s t-test. Quantification was based on ≥24 fields (n≥30 cells) under a 63× objective from 3 platings per condition. Error bars represent SEM. (C) Lentiviral transduced SCG neurons expressing γCaMKII shRNA were stimulated with 40 mM K+ for 10 s. NLS-Nrgn restored the pCREB response and the restoration was prevented by specific CaMKK inhibitor STO-609, consistent with the idea that CaM delivery operates upstream of the activation of nuclear CaMKK-CaMKIV-pCREB cascade.