Abstract
Purpose
To assess the efficacy of two different approaches to neoadjuvant chemo-radiation for distal rectal cancers.
Materials and Methods
106 patients with T3/T4 distal rectal cancers were randomized in a phase II study. Patients received either CVI 5-FU, 225 mg/m2 per day, 7 days per week plus pelvic hyperfractionated radiation (HRT) 45.6 Gy at 1.2 Gy bid plus a boost of 9.6 - 14.4 Gy for T3 or T4 cancers (Arm 1) or CVI 5-FU 225 mg/m2 per day Monday to Friday, plus Irinotecan 50 mg/m2 once weekly x 4 plus pelvic RT 45 Gy at 1.8 Gy per day and a boost of 5.4 Gy for T3 and 9 Gy for T4 cancers (Arm 2). Surgery was performed 4 to 10 weeks later.
Results
All eligible patients (n=103) are included in this analysis; excluded were 2 patients who were ineligible and 1 patient withdrew consent. 98 of 103 patients (95%) underwent resection . 4 patients did not undergo surgery for either disease progression or patient refusal and 1 patient died during induction chemotherapy. The median time of follow-up is 6.4 years in Arm 1 and 7.0 years in Arm 2. The pCR rate was 30% in Arm 1 and 26% in Arm 2. Loco-regional recurrence was 16% in Arm 1 and 17% in Arm 2. Five-year survival was 61% and 75% and Disease Specific Survival was 78% and 85% for Arm1 and Arm 2 respectively. Five second primaries occurred in patients on Arm 1, and one second primary occurred in Arm 2.
Conclusions
High rate of Disease Specific Survival was seen in each arm. Overall survival appears effected by the development of unrelated second cancers. The high pCR rates with 5-FU and higher dose radiation in T4 cancers provides opportunity for increased R0 resections and improved survival.
Keywords: Rectal cancer, Neoadjuvant, Chemoradiation
Introduction
Rectal cancer management continues to evolve with new approaches to combined modality treatments especially for advanced and distal cancers. Several studies have reported high rates of pathological complete response (pCR) of tumor (10-30%) using neoadjuvant chemotherapy with radiation (1-4). There is, however, no consensus as to patient selection criteria, use of cytotoxic agents and radiation dose/time schedules. Reporting of treatment toxicity has also been variable making it difficult to assess the most appropriate regimen of neoadjuvant therapy for this disease.
This RTOG-0012 study was undertaken to compare neoadjuvant 5-Fluorouracil (5-FU) infusion and radiation dose intensification (55-60 Gy), using a hyperfractionated schedule to a chemotherapy intensification approach using Irinotecan and 5-FU with a standard radiation schedule (50-54 Gy). The primary endpoint of the study was the pathological complete response (pCR) and toxicity of treatment. The details of the rationale, study design and results of the primary endpoints have been previously reported (5). The secondary endpoints, survival and patterns of failure, are the subject of this report.
Materials and Methods
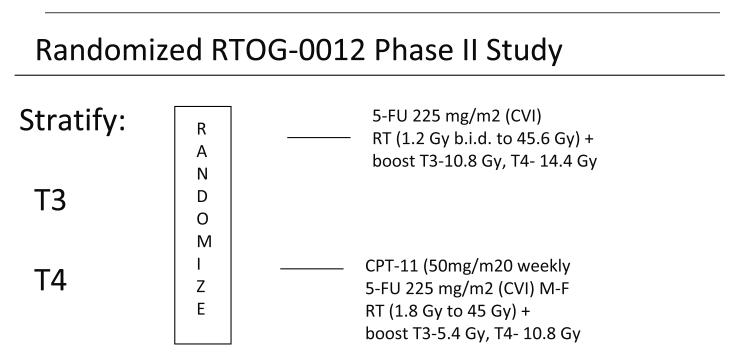
Patients with clinical T3-T4 distal rectal cancers located 0-9 centimeters from the dentate line (3-12 cm from the anal verge- to exclude tumors involving the anal canal) were randomized in a phase II study to receive neoadjuvant chemo-radiation followed by surgical resection. The treatment schema is shown in figure 1. Patients were stratified by clinical stage and received either continuous venous infusion (CVI) 5-F.U. 225 mg/m2 per day, 7 days per week plus hyperfractionated pelvic radiation therapy to a dose of 45.6 Gy at 1.2 Gy BID ( ≥ 6 hour interval between fractions) and a boost of 9.6 Gy for T3 and 14.4 Gy for T4 cancers, (Arm 1) or CVI 5-F.U. 225 mg/m2 per day Monday through Friday (120 hours per week) plus Irinotecan 50 mg/m2 once weekly x 4, plus pelvic radiation (45 Gy 1.8 Gy per day with a boost to the tumor of 5.4 Gy for T3 and 9 Gy for T4 cancers), (Arm 2). Surgery was performed 4-10 weeks following completion of assigned treatment. While Total Mesorectal Excision (TME) was recommended, it was not required as this was not standardized and validated amongst surgeons in the US at the time of this study.
Figure 1.
Treatment Schema for RTOG 00-12
NEOADJUVANT THERAPY FOR RECTAL CANCER
All patients were without evidence of distant metastasis and had a zubrod performance status of 0-1. Patients were required to have a WBC count > 4000/ml, platelet count > 130,000 per ml, satisfactory liver and renal function tests and a bilirubin ≤ 1.5 times the upper normal limit. All patients were required to have a chest x-ray and CT scan of the abdomen and pelvis to determine the location, extent and size of the pretreatment disease. An endorectal ultra sound (TRUS) was performed for TNM staging of mobile cancers. An MRI scan of the pelvis for corroboration of TNM staging was optional. Patients were required to sign the study specific informed consent prior to randomization. The distribution of patients is shown in Table 1.
Table 1.
Distribution of Patients
| 5-FU & RT (n=50) |
5-FU/CPT-11 & RT (n=53) |
p-value* | |
|---|---|---|---|
|
| |||
| Age | |||
| Median | 56 | 58 | |
| Range | 26-78 | 22-80 | |
| Gender | |||
| Male | 38 (76%) | 29 (55%) | 0.024 |
| Female | 12 (24%) | 24 (45%) | |
| Race | 0.89† | ||
| White (not of Hispanic origin) | 42 (84%) | 45 (85%) | |
| Hispanic | 3 (6%) | 2 (4 %) | |
| Black (not of Hispanic origin) | 5 (10%) | 4 (8%) | |
| Asian | 0 | 2 (4 %) | |
| Zubrod | 0.19 | ||
| 0 | 35 (70%) | 43 (81%) | |
| 1 | 15 (30%) | 10 (19%) | |
| T-Stage | 0.53 | ||
| T3 | 34 (68%) | 39 (74%) | |
| T4 | 16 (32%) | 14 (26%) | |
| N-Stage | 0.98 | ||
| N0 N+ |
31 (62%) 19 (38%) |
33 (62%) 20 (38%) |
|
From Chi-Square Test
Comparing White (not of Hispanic origin) vs. Otherwise
The initial radiation treatment was delivered to the whole pelvis. All patients were treated with megavoltage radiation (≥ 6MV) using AP/PA and two lateral fields. The superior border of the treatment volume was at the L5S1 junction with the inferior border a minimum of 5cm inferior to the distal most extent of the tumor or the anal verge as identified by a marker on simulation. Laterally the field extended 2cm lateral to the bony pelvis at its widest point. The anterior border of the lateral fields covered the lower common and external iliac nodes to 1cm anterior to the symphysis for anterior wall lesions and to the mid symphysis for posterior lesions. The posterior border included the entire sacrum with a 1cm margin posteriorly. The boost fields were planned with a 3cm margin around the tumor but included the whole of the sacral hallow. Chemotherapy was started on day 1 of the radiation treatment and continued until the completion of radiation therapy.
Chemotherapy and radiation were interrupted if Grade > 3 toxicity was encountered. Chemotherapy and radiation were restarted when toxicity had resolved to Grade 1. A dose modification of chemotherapy was undertaken for subsequent cycles. The dose of 5-F.U. was decreased by 25% and Irinotecan to 40 mg/m2. Ninety eight of the 103 patients (95%) underwent resection with negative margins. Twenty one patients (42%) in Arm1 and 19 (36%) in Arm 2 underwent an abdomino-perineal resection. The remaining patients had a low anterior or other radical resection.
Post operatively chemotherapy was recommended for patients with pathological residual disease. Patients with pCR could have their postoperative chemotherapy omitted at the discretion of the treating physician. Twenty four patients in Arm 1 and 32 patients in Arm 2 received post op chemotherapy.
Endpoints of the study were measurement of both acute and late toxicity of treatment, as well as pCR rate, which was defined as absence of identifiable cancer cells in the surgical resected specimen. Any residual cancer at surgery or patients not undergoing surgery were considered as having < pCR. Secondary endpoints included survival and local recurrence rates.
Statistical Considerations
This study was designed to evaluate the pCR rate for patients with cancer of the rectum treated with neo-adjuvant chemo-radiation. Since survival and local recurrence were secondary endpoints, the study was not powered for statistical comparison of these and the data is presented as observed. The failure event for overall survival was death due to any cause and was estimated using the Kaplan-Meier method(6). The failure event for disease-specific survival was death due to study cancer or complications of protocol treatment; death due to any other cause was treated as a competing risk. Disease-specific survival rates were estimated using the cumulative incidence method(7). All statistical analyses were performed using SAS®.
Results
Patient Characteristics
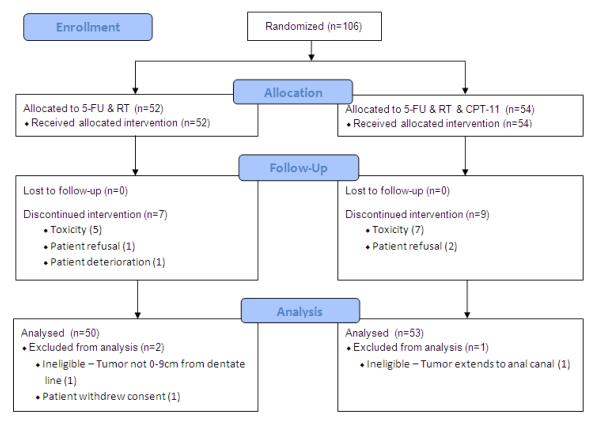
Fifty-two patients were entered in Arm 1 and 54 patients on Arm 2 between February 1, 2001 and January 22, 2003. Two patients were ineligible for the study, one on each Arm, and one patient on Arm 1 withdrew consent (Figure 2). The median follow up is 6.4 years in Arm 1 and 7.0 years in Arm 2. There were more males in Arm 1 than on Arm 2 (76% vs. 55% respectively, p=0.024) but otherwise pretreatment characteristics were well balanced (Table 1).
Figure 2.
RTOG 00-12 CONSORT Flow Diagram
Response Rate
Four patients did not undergo the planned surgery (3 in Arm 1 and 1 in Arm 2) because of either disease progression or patient/physician refusal and one patient in Arm 1 died during induction therapy for an overall resectability rate of 95%. The median time to surgery was 8.1 weeks of all eligible patients who started treatment on Arm 1 and 6.9 weeks on Arm 2 (p=0.059). The pCR rate, as determined on surgical review, was, 15/50 (30%) in Arm 1 and 14/53 (26%) in Arm 2. For T3 cancers, 29% of patients achieved pCR as compared to 31% for T4 cancers on Arm 1. For T3 cancers on Arm 2, 31% achieved pCR as compared to 14% for T4 cancers. The overall tumor down staging rate was 74% in Arm 1 and 75% in Arm 2 (Table 2).
Table 2.
T-Stage Comparison at Study Entry & After Surgery
| After Surgery | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All evaluable pts. |
5-FU & RT (n=50) |
5-FU/CPT-11 & RT (n=53) |
||||||||||
| Pretreatment | T0 | T1 | T2 | T3 | T4 | Unknown | T0 | T1 | T2 | T3 | T4 | Unknown |
| T3 | 10 | 1 | 11 | 9 | 0 | 3 | 12 | 3 | 13 | 10 | 0 | 1 |
| T4 | 5 | 2 | 3 | 5 | 0 | 1 | 2 | 0 | 2 | 8 | 0 | 2 |
| Downstaging | 37/50 (74%) | 40/53 (75%) | ||||||||||
| pCR rate (95% CI) |
15/50 (30%) (0.17, 0.43) |
14/53 (26%) (0.15,0.38) |
||||||||||
Toxicity
In general the treatment was well tolerated. Acute and late toxicity was previously reported (5). Worst overall late RT toxicity ≥ grade 3 was 8% (n=4) on Arm 1 and 13% (n=7) on Arm 2.
Survival
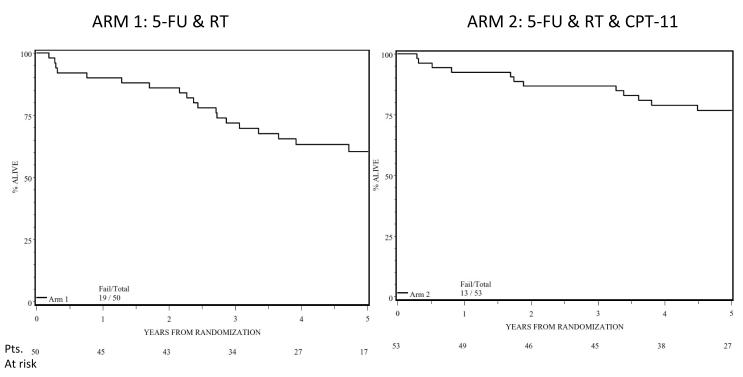
The overall survival at 5 years was 61% (95% CI: 47%, 74%) in Arm 1 and 75% (95%CI: 61%, 85%) in Arm 2 (Figure 3). The 5-years Disease Specific Survival was 78% (95% CI: 66%, 90%) in Arm 1 and 85% (95% CI: 75%, 95%) in Arm 2. Survival for patients with a pCR was 79% and 85% in Arms 1 and 2 respectively, as compared to the survival for patient with less than pCR (54% and 72% respectively). The 5-yr. DSS for T4 cancers was 87.5% (95% CI: 71%,100%) in Arm1 as compared to 71.4% (95%CI: 47%,96%) in Arm 2.
Figure 3.
Survival of all patients in Arm 1 (5-FU /RT) and Arm 2 (5-FU+ CPT-11 /RT)
Patterns of Failure
The patterns of first failure were similar in both arms of the study. Loco-regional recurrence was seen in 8/50 (16%) of all eligible patients in Arm 1 and 9/53 (17%) in Arm 2. This included 4 patients who did not undergo surgery but are considered as local failure of treatment in Arm1 and 1 patient in Arm 2. For patients having surgery, 4 patients (8.6%) in Arm 1 and 8 patients (15%) in Arm 2 developed a loco-regional recurrence. For patients with pCR following surgery, no patients in Arm 1 and 1 patient in Arm 2 developed a loco-regional recurrence. All of the other recurrences were in patients with < pCR. The overall rate of distant metastasis was 8/50 (16%) in Arm 1 and 11/53 (21%) in Arm 2. The rate of distant metastasis for patients undoing surgery was 2/15 (13%) with pCR and 8/31 (26%) in patients with <pCR in Arm 1, and 2/14 (14%) with pCR and 11/38 (29%) in patients with <pCR in Arm 2.
Six second primary cancers developed in these patients, five in Arm 1(Lip sarcoma, melanoma, lung cancer, bladder, and colon) and 1 in Arm 2 (lung cancer). Four of the five second primary cancers in Arm 1 occurred in pCR patients and one in <pCR patients. The one second primary in Arm 2 was in a patient with <pCR.
Discussion
Preoperative radiation for treatment of advanced rectal cancer has historically been used to downstage the disease in an effort to maximize surgical resection of marginal and unresectable tumors. Recent studies have shown that preoperative radiation has resulted in improved local control and survival of patients even with early cancers (1-3). The combined use of chemotherapy with radiation has been shown to further improve downstaging of disease and increase the rate of pathological complete responses than with preoperative radiation alone. 5-fluorouracil has been the mainstay of combined chemotherapy and radiation for gastro-intestinal tumors. Improved outcomes observed with continuous infusion of 5-FU with radiation has been most efficacious in the postoperative adjuvant setting in the Intergroup 0014 trial as compared to bolus 5-FU and this appears to also hold true for neoadjuvant combined modality therapy (8).
While the response of rectal cancer to neoadjuvant chemo-radiation has been impressive, the addition of chemotherapy has also lead to increased GI, mucosal and hematologic toxicity. Many of the studies utilizing combined chemotherapy and radiation have reported GI toxicities ranging from 21 to 44% (≥ Grade 3) (9 -14).
The question therefore remains whether the increased tumor downstaging and pathological complete response rates observed with combined modality treatment at the expense of enhanced toxicity translates into improvement in survival of patients. Reports have now shown that pathological downstaging does lead to improved survival of patients(14-17). In patients with a pCR, survival of patients has ranged from 85 to 100%. It has been shown that radiation dose, even in the presence of chemotherapy, is of significant importance in downstaging of disease(15 ). At the same time, it has been observed that combinations Irinotecan/5-FU also have higher response rates than single agent 5-FU alone(18 ). This study was therefore designed to test two experimental arms using a radiation dose intensification (total dose of 55 to 60 Gy) versus a chemo-intensification using two drugs, 5-FU/CPT-11 combined with the standard radiation dose of 50 to 54 Gy, to establish the most efficacious approach to neoadjuvant therapy in rectal cancer. The results of this study indicate that the rate of tumor downstaging in T3, T4 cancers is high (80%) and similar with both radiation dose intensification or with chemotherapy intensification. The higher dose of radiation did not compromise tumor resectability or increase surgical wound complications.
The pathological complete response of 26-30% in this study appeared higher than most other studies, where a more moderate dose of radiation (45 to 50.4 Gy) has been utilized (Table 3). The pCR rate in Arm 2 in this study was higher than the identical Irinotecan/Capcitabine/Radiation in the subsequent RTOG -0247 study but similar to the Capcitabine/Oxaliplat/Radiation (22%) arm of that study (14). The acute toxicity observed in this study was also similar to that observed in other studies (15 to 40%) but somewhat higher than the non-hematological toxicity in the RTOG-0247 study (24-29%)(table 3). There was a marked difference in pathological complete response for T3 tumors (31%) as compared to T4 tumors (14%) on Arm 2, indicating that tumor volume may be a critical factor in pCR as a function of treatment. Radiation dose may be a significant factor in pCR rates especially compared with results of preoperative chemo-radiation in the German trial were pCR was 10% for T3 cancers and 0% for T4 cancers using a moderate dose of radiation (50.4 Gy) (1 ). In comparison the 31% (5/16) pCR rate for T4 cancers in Arm 1 and 14% (2/14) in Arm 2 is encouraging even though the numbers of patients was relatively small.
Table 3.
Recent results of neoadjuvant therapy for Rectal cancer
| Series | RT | Chemo | pCR | Toxicity | 5 yr Survival |
|---|---|---|---|---|---|
| RTOG - 0247(19) |
50.4 Gy | Cap/Irinotecan Cap/Oxal |
10% 22% |
24% 29% |
Not available |
| German (1) | 50.4 Gy | 5-FU | 8% | 23% | 76% |
| Accord (11) | 45 Gy | 5-FU 5-FU/Oxal |
14% 19% |
25% | Not available |
| Star -01(12) | 50.4 Gy | 5-FU 5-FU/Oxal |
16% 16% |
8% 24% |
Not available |
| NSABP R- 03(13) |
50.4 Gy | 5-FU | 16% | 36% | 75% |
| NSABP R- 04(14) |
50.4 ± 5.4 Gy |
5-FU 5-FU/Oxal |
19% 22% |
35% 40% |
Not available |
| RTOG-0012(19) | 55-60 Gy/bid 50-55 Gy |
5-FU 5-FU/CPT-11 |
33% 28% |
36% 47% |
61% 75% |
The median follow up time in this study is over five years and since most recurrences in rectal cancer occur within the first two years, this data provides a useful perspective on patterns of failure and survival of these patients. The overall survival with 5-FU, radiation (Arm 1) at five years was 61%, and the overall survival at five years in the Irinotecan/5-FU/ radiation arm was 79%. However, the survival in the 5-FU radiation arm could have been compromised with the unanticipated occurrence of five second primary tumors as compared to one in the Irinotecan/5-FU /radiation arm. This survival outcome is similar to the survival reported in other studies (1,13) especially considering that the proportion of T4 cancers was higher in this study (26-32%) than the T4 cancers (6% ) included in the German Trial (1). A disease-specific survival at five years of 78% in 5-FU/radiation and 85% in the Irinotecan/5-FU/ radiation arm is a high cure rate for T3 and T4 distal rectal cancers compared to other reported series and compared to historical results of postoperative adjuvant studies which have reported a survival of 55 to 60% in a resectable group of patients (1,8). This is especially significant as TME was not a requirement of this study.
The survival of patients clearly is impacted by the level of downstaging even in this study with patients with pCR having a survival of 79% and 85% in Arms 1 and 2, as compared to those with less than pCR of 54% and 72% respectively. These results reflect a similar outcome to other studies where patients having a pCR have excellent outcomes (17).
The high survival observed in T4 cancers ( 55% in Arm1 and 62% in Arm 2) is especially encouraging and may be a reflection of higher downstaging (pCR rates) due to the higher radiation dose which in turn may allow for greater opportunity for R0 resections in these patients leading to improved survival. The differences in the two arms of this study were not compared for statistical significance because this study was not powered for comparison of secondary endpoints. The lower survival in Arm 1 may be a reflection of the slightly higher number of male patients and T4 tumors in this Arm and the high incidence of second primary cancers. The overall survival in these patients is also calculated on an “intent-to-treat” basis and 4/50 (6%) patients on Arm 1 and 1/53 (2%) in Arm 2 did not undo surgery but are included in the survival estimates.
Surprisingly the local recurrence rate was not impacted by the pathological completeness of response. Local recurrence was 16% in the Arm 1 and 17% in Arm 2 as site of first failure. Again, these results appear higher than other recent reported results following neoadjuvant therapy. However, this study was undertaken before the requirement of Total Meso-rectal excision (TME) and also included a significantly higher proportion of T4 cancers in both cohorts compared to most recent randomized studies.(11-14) Patients not able to undergo surgery are also included as having failed with local disease-4 in Arm 1 and 1 in Arm 2. Excluding these patients the effective local recurrence rate would be 8% in Arm 1 and 15% in Arm 2. Distant metastasis was somewhat greater in those patients having less than a pCR (26% versus 7% in Arm 1, and 29% versus 14% in Arm 2). However, these were less than the 30% rate of distant metastasis reported in the German trial(1 ).
Conclusion
Neoadjuvant chemoradiation for advanced rectal cancer is safe with acceptable toxicity. While a high rate of Disease Specific Survival was seen in each arm, overall survival appears to be effected by the development of unrelated second cancers in this study. The high pCR rates and improved DSS with 5-FU and higher dose radiation (55-60 Gy) in T4 cancers provides an opportunity for increased R0 resections and potential for further improvement in survival.
Acknowledgement
This trial was conducted by the Radiation Therapy Oncology Group (RTOG), and was supported by RTOG grant U10 CA21661 and CCOP grant U10 CA37422 from the National Cancer Institute (NCI). This manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Conflict of Interest: NONE
106 rectal cancer patients were randomized to neo-adjuvant 5-FU and hyperfractionated radiation (55-60 Gy) or CPT-11/5-FU and once a day radiation (50-55 Gy). Surgery was performed 4-10 weeks later. DSS was 78% and 85% and 5-year overall survival was 61% and 75% for Arm1 and Arm 2 respectively. A high DSS was observed in both arms but overall survival was compromised by development of second primaries (5 in Arm 1, and 1 in Arm 2.)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 2.Gerard A, Buyse M, Nordlinger B, et al. Preoperative radiotherapy as adjuvant treatment in rectal cancer. Final results of a randomized study of the European Organization for Research and Treatment of Cancer (EORTC) Ann Surg. 208:606–614. doi: 10.1097/00000658-198811000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosset JF, Calais G, Mineur L, et al. Does the addition of Chenotherapy (CT) to preoperative radiotherapy (preopRT) increase the pathological response in patients with resected rectal cancer: Report of the 22921 EORTC phase III trial. J Clin Oncol. 2004;Vol 23:247. [Google Scholar]
- 4.Mohiuddin M, Regine WF, John WJ, et al. Preoperative chemoradiation in fixed distal rectal cancer: dose time factors for Pathological complete response. Int J. Radiat Oncol Biol Phys. March. 2000;1(4):883–8. doi: 10.1016/s0360-3016(99)00486-1. [DOI] [PubMed] [Google Scholar]
- 5.Mohiuddin M, Winter K, Mitchell E, et al. Randomized phase II study of neoadjuvant combined-modality chemoradiation for distal rectal cancer: Radiation Therapy Oncology Group Trial 0012. J Clin Oncol. 2006;24:650–655. doi: 10.1200/JCO.2005.03.6095. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan E, Meier P. Nonparameteric estimation from incomplete observations. J Amer Stat Assoc. 1958;53:457–481. [Google Scholar]
- 7.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 8.O’Connell MJ, Martenson JA, Wieand HS, et al. Improved combined modality surgical adjuvant therapy for high-risk rectal cancer. NEMJ. 1994;331:502–07. [Google Scholar]
- 9.Roedel C, Becker H, R. Fietkau R, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with 5- flourouracil and oxaliplatin versus 5-flourouracil alone in locally advanced rectal cancer: First results of the German CAO/ARO/AIO-04 randomized Phase III trial. J Clin Oncol. 2011;29 (suppl; abstr LBA3505) [Google Scholar]
- 10.Wong SJ, Moughan J, Meropol NJ, Anne PR, Kachnic LA, Rashid A, Watson JC, Mitchell EP, Pollock J, Haddock MG, Erickson B, Willett CG. Efficacy endpoints of RTOG 0247: A randomized phase II study of neoadjuvant capecitabine (C) and irinotecan (I) or C and oxaliplatin (O) with concurrent radiation therapy (RT) for locally advanced rectal cancer. J Clin Oncol. 2011;29 (suppl; abstr 3517) [Google Scholar]
- 11.Gerard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: Results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28:1638–1644. doi: 10.1200/JCO.2009.25.8376. [DOI] [PubMed] [Google Scholar]
- 12.Aschele C, Cionini L, Loardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: Pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. doi: 10.1200/JCO.2010.34.4911. doi: 10.1200/JCO.2010.34.4911. [DOI] [PubMed] [Google Scholar]
- 13.Roh MS, Colangelo LH, O’Connell MJ, et al. Preoperative Multimodality Therapy Improves Disease-Free Survival in Patients With Carcinoma of the Rectum: NSABP R-03. J Clin Oncol. 2009:5124–5130. doi: 10.1200/JCO.2009.22.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roh MS, Yothers GA, O’Connell MJ, et al. The impact of capecitabine and oxaliplatin in the preoperative multimodality treatment in patients with carcinoma of the rectum: NSABP R-04. J Clin Oncol. 2011;29 (suppl; abstr 3503) [Google Scholar]
- 15.Mohiuddin M, Hayne M, Regine WF, et al. Prognostic significance of postchemoradiation stage following preoperative chemotherapy and radiation for advanced/recurrent rectal cancers. Int J Radiat Oncol Biol Phys. 2000 Nov 1;48(4):1075–80. doi: 10.1016/s0360-3016(00)00732-x. [DOI] [PubMed] [Google Scholar]
- 16.Rodel C, Martus P, Papadoupolos T, Fuzesi L, Klimpfinger M, Fietkau R, Liersch T, Hohenberger W, Raab R, Sauer R, Wittekind C. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005 Dec 1;23(34):8688–96. doi: 10.1200/JCO.2005.02.1329. [DOI] [PubMed] [Google Scholar]
- 17.Mass M, Nelemans PJ, et al. Long-term outcome in patients with a pathologic complete response after chemoradiation for rectal cancer: a pooled analysis of 3105 patients. Lancet Oncology. 2010;11(9):835–44. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell EP. Irinotecan in preoperative combined modality therapy for locally advanced rectal cancer. Oncology. 2000;14:56–59. [PubMed] [Google Scholar]
- 19.Wong SJ, Winter K, Meropol NJ, Anne R, Kachnic LA, Rashid A, Watson JC, Mitchell EP, Pollock J, Lee RJ, Willett CG. RTOG 0247: A randomized phase II study of neoadjuvant capecitabine and irinotecan versus capecitabine and oxaliplatin with concurrent radiation therapy for locally advanced rectal cancer. J Clin Oncol. 2008;26 doi: 10.1016/j.ijrobp.2011.05.027. (May 20 suppl; abstr 4021) [DOI] [PMC free article] [PubMed] [Google Scholar]