Abstract
Background
The presentation, management and outcome of patients with primary cardiac sarcomas are not well defined. Furthermore, the role of adjuvant therapy has not been delineated in the management of primary cardiac sarcomas.
Methods
Patients with primary cardiac sarcoma and non-cardiac sarcoma, diagnosed between 1988 and 2005, were identified in the Surveillance, Epidemiology, and End Results (SEER) database. Clinical characteristics and outcomes of primary cardiac sarcoma were defined and compared to the characteristics of non-cardiac sarcomas. Univariate and multivariate methods were used to identify factors associated with primary cardiac sarcoma survival.
Results
Compared to non-cardiac sarcomas, primary cardiac sarcomas were found to occur in a younger age group and were more likely to present with advanced disease. Primary cardiac sarcomas were ten times more likely to be vessel-derived (e.g. angiosarcoma), comprising almost half of all cases. Median overall survival for cardiac sarcoma patients was 6 months while that of non-cardiac sarcoma patients was significantly longer at 93 months (p < .001). Furthermore, cardiac sarcoma patients who underwent surgery had a median survival of 12 months while those who did not undergo surgery had a median survival of one month (p < .001).
Conclusions
Cardiac sarcomas are a distinct, rare subset of soft tissue sarcomas with a very poor prognosis. Surgery continues to be the central component of successful management. Future clinical efforts should be directed at developing approaches to permit safe radical excision and, potentially, developing effective adjuvant therapy.
Keywords: cardiac sarcoma, cardiac tumors
INTRODUCTION
At an incidence of 0.001% to 0.03% in autopsy series, primary cardiac tumors are extremely rare entities. In fact, metastatic tumors to the heart are 30 to 50 times more common (1, 2, 3). In adults, approximately one fourth of primary cardiac tumors are malignant with sarcomas representing the most common histology (4). Primary cardiac sarcomas are aggressive tumors that generally do not produce symptoms until they are locally advanced. These delayed manifestations are nonspecific and include dyspnea, chest pain, congestive heart failure secondary to obstruction of blood flow as well as systemic symptoms.
In the absence of unequivocal clinical evidence, management of cardiac sarcomas has largely been guided by small retrospective series and non-cardiac sarcoma management principles. Thus, complete surgical resection, when feasible, is considered the mainstay of cardiac sarcoma therapy. Unfortunately, definitive surgical therapy is often not offered due to frank unresectability, anticipated morbidity, the presence of metastatic disease or concerns of only modest improvements in survival with a large operative procedure (5). Roles for (neo)adjuvant therapy have not been well defined. Consequently, primary cardiac sarcoma patients continue to have a poor outcome.
Thus far, there have been few retrospective studies analyzing the presentation, management and outcome of patients with primary cardiac sarcomas. These studies are severely limited by small patient numbers. Prospective studies are not plausible given the rarity of these tumors. Thus, to better characterize cardiac sarcomas and identify factors associated with survival, we used the Surveillance, Epidemiology, and End Results (SEER) database to define clinical characteristics of cardiac sarcomas and identify patient, tumor and treatment factors related to outcome.
MATERIALS AND METHODS
Study Population
The SEER-17 registries data set of the US National Cancer Institute (April 2008 release) was used to identify patients diagnosed with primary cardiac sarcomas and non-cardiac sarcomas between January 1,1988, to December 31, 2005. Unspecified neoplasms, epithelial neoplasms, squamous cell carcinomas, adenomas, adenocarcinomas, paragangliomas, glomus tumors, and gliomas were excluded. Additional inclusion criteria consisted of known age, sex, race, and tumor stage. A total of 210 patients met the inclusion criteria for cardiac sarcomas while 24,404 patients met the criteria for non-cardiac sarcomas. Informed consent by the study participants and approval of an ethics committee were unnecessary to perform the analyses in this study because all of the information from the SEER database is de-identified.
Statistical Analyses
Overall survival was evaluated with respect to multiple patient, tumor, and treatment characteristics (age, sex, race, tumor grade, SEER stage (localized, regional, distant and unstaged), histology) using Kaplan-Meier survival analyses. Patients were censored at either death or date of last follow-up. Two-sided Mantel-Cox log-rank tests were used to assess the significance of differences between survival curves. Multivariate Cox proportional hazards models were also used to determine hazard ratios associated with categoric variables of interest. A forward stepwise approach was used with variables entered into the model that met statistical significance (P < 0.05). SEER*STAT software version 6.4.4 (Surveillance Research Program, NCI, Bethesda, MD) was used to extract case level data from the SEER public-use database. All analyses were conducted using the Statistical Package for the Social Sciences (SPSS, V14.0).
RESULTS
The study population consisted of 210 patients diagnosed with primary cardiac sarcoma and 24,404 patients with non-cardiac sarcoma. Patient characteristics, tumor grade, SEER stage and histology are shown for both cardiac sarcoma and non-cardiac sarcoma patients in Table 1. Though both cancers predominated in patients younger than 65, primary cardiac sarcoma patients were far more likely to present at a younger age. At the time of diagnosis, an equal number of primary cardiac sarcoma patients are found to have localized and distant disease while the majority of non-cardiac sarcoma patients present with only local disease. Furthermore, primary cardiac sarcoma patients are more likely to present with higher grade tumors in comparison to non-cardiac sarcomas. Angiosarcomas represent almost half of all cardiac sarcomas, while comprising less than five percent of non-cardiac sarcomas. No gender or racial differences between cardiac and non-cardiac sarcomas were detected.
Table 1.
Characteristics of patients with cardiac sarcoma and non-cardiac sarcoma.
| Cardiac sarcoma | Non-cardiac sarcoma | P value* | |
|---|---|---|---|
| Number of patients | 210 | 24,404 | |
| Age (years) | < 0.001 | ||
| 20-39 | 78 (37.1) | 4999 (20.5) | |
| 40-54 | 63 (30.0) | 5762 (23.6) | |
| 55-64 | 31 (14.8) | 4235 (17.4) | |
| 65-74 | 22 (10.5) | 4285 (17.6) | |
| ≥75 | 16 (7.6) | 5123 (21.0) | |
| Sex | 0.703 | ||
| Male | 117 (55.7) | 13,275 (54.4) | |
| Female | 93 (44.3) | 11,129 (45.6) | |
| Race | 0.145† | ||
| White | 167 (79.5) | 20,032 (82.1) | |
| Black | 20 (9.5) | 2510 (10.3) | |
| Asian/ Pacific Islander | 22 (10.5) | 1459 (6.0) | |
| American Indian/Alaska Native | 1 (0.5) | 126 (0.5) | |
| Unknown | 0 | 277 (1.1) | |
| Tumor grade | < 0.001† | ||
| Well differentiated | 1 (0.5) | 2871 (11.8) | |
| Moderately | 12 (5.7) | 3234 (13.3) | |
| Differentiated Poorly differentiated | 34 (16.2) | 3266 (13.4) | |
| Undifferentiated/Anaplastic | 50 (23.8) | 4411 (18.1) | |
| Unknown | 113 (53.8) | 10,622 (43.5) | |
| SEER stage | < 0.001† | ||
| Localized | 75 (35.7) | 14,024 (57.5) | |
| Regional | 50 (23.8) | 4994 (20.5) | |
| Distant | 76 (36.2) | 3173 (13.0) | |
| Unstaged | 9 (4.3) | 2213 (9.1) | |
| Histology | < 0.001† | ||
| Soft tissue tumors and sarcomas, NOS | 40 (19.0) | 3412 (14.0) | |
| Fibromatous neoplasms | 26 (12.4) | 7432 (30.5) | |
| Myxomatous neoplasms | 6 (2.9) | 130 (0.5) | |
| Lipomatous neoplasms | 3 (1.4) | 4759 (19.5) | |
| Myomatous neoplasms | 29 (13.8) | 3917 (16.1) | |
| Complex mixed and stromal neoplasms | 1 (0.5) | 299 (1.2) | |
| Synovial-like neoplasms | 6 (2.9) | 1290 (5.3) | |
| Blood vessel tumors | 97 (46.2) | 1029 (4.2) | |
| Lymphatic vessel tumors | 0 | 31 (0.1) | |
| Osseus and chondromatous neoplasms | 2 (1.0) | 450 (1.8) | |
| Giant cell tumors | 0 | 46 (0.2) | |
| Miscellaneous bone tumors | 0 | 143 (0.6) | |
| Odontogenic tumors | 0 | 2 (0) | |
| Nerve sheath tumors | 0 | 1328 (5.4) | |
| Granular cell tumors and alveolar soft tumors | 0 | 134 (0.5) |
Chi-square value.
The P value given excludes cases for which the category of interest is unknown (race, tumor grade, or SEER stage).
Table 2 shows the use of surgery and radiotherapy by SEER stage for cardiac and non-cardiac sarcomas. Among primary cardiac sarcoma patients, surgery was performed in the majority (73.6%) of patients with localized and regional disease while forty percent of patients with distant disease also underwent surgery. Radiotherapy was far less frequently employed. Patients with regional and distant disease were more likely to receive radiotherapy than patients with localized disease. In contrast, both surgery and RT were more likely to be employed for the treatment of non-cardiac sarcoma patients regardless of stage.
Table 2.
Utilization of surgery and radiotherapy by SEER stage.
| Localized | Regional | Distant | All patients* | |
|---|---|---|---|---|
| Cardiac Sarcoma | ||||
| Number of Patients | 75 | 50 | 76 | 210 |
| Surgery | ||||
| Cancer-directed surgery performed | 58 (77.3) | 34 (68.0) | 31 (40.8) | 125 (59.5) |
| No surgery | 16 (21.3) | 16 (32.0) | 42 (55.3) | 81 (38.6) |
| Unknown | 1 (1.3) | 0 | 3 (3.9) | 4 (1.9) |
| Radiotherapy | ||||
| RT given | 13 (17.3) | 15 (30.0) | 20 (26.3) | 50 (23.8) |
| No RT | 61 (81.3) | 35 (70.0) | 56 (73.7) | 159 (75.7) |
| Unknown | 1 (1.3) | 0 | 0 | 1 (0.5) |
| Non-cardiac sarcoma | ||||
| Number of Patients | 14,024 | 4,994 | 3,173 | 24,404 |
| Surgery | ||||
| Cancer-directed surgery performed | 13,336 (95.1) | 4364 (87.4) | 1506 (47.5) | 20,330 (83.3) |
| No surgery | 601 (4.3) | 593 (11.9) | 1602 (50.5) | 3677 (15.1) |
| Unknown | 87 (0.6) | 37 (0.7) | 65 (2.0) | 397 (1.6) |
| Radiotherapy | ||||
| RT given | 5583 (39.8) | 2310 (46.3) | 1077 (33.9) | 9449 (38.7) |
| No RT | 8166 (58.2) | 2574 (51.5) | 2041 (64.3) | 14,348 (58.8) |
| Unknown | 275 (2.0) | 110 (2.2) | 55 (1.7) | 607 (2.5) |
In addition to the categories shown, patients with unknown SEER stage are included (cardiac sarcoma: n = 9; non-cardiac sarcoma: 2213).
Multivariate analysis of overall survival of the 93 cardiac sarcoma patients with complete data is shown in Table 3. Patients with missing data were excluded. Tumor grade (poorly differentiated) and surgical resection were found to be important prognostic factors, whereas age, sex, race, SEER stage, histology and use of radiotherapy were not associated with outcome. The hazard ratio for the group that did not undergo surgery exceeded 3, while that for patients with poorly differentiated tumors was 9.3. In a separate multivariate analysis of all 210 cardiac sarcoma patients (with unknown status used for each categoric variable when necessary), SEER stage and surgical resection were found to be significant prognosticators while grade was not found to be associated with survival (data not shown).
Table 3.
MVA. Analysis of cardiac sarcomas by tumor and patient characteristics.
| Hazard Ratio (95% C.I.) | P-value | |
|---|---|---|
| Grade | 0.034 | |
| Well Differentiated | 1.0 (ref) | |
| Moderately differentiated | 3.8 (0.4 – 33.4) | 0.229 |
| Poorly differentiated | 7.2 (0.9 - 57.3) | 0.062 |
| Undifferentiated/Anaplastic | 9.3 (1.2 - 73.7) | 0.034 |
| Surgery | < 0.001 | |
| Surgery | 1.0 (ref) | |
| No Surgery | 3.4 (2.0 – 6.0) | < 0.001 |
Median overall survival for cardiac sarcoma patients was 6 months while that of non-cardiac sarcoma patients was significantly longer at 93 months (p < 0.001) (Figure 1). Cumulative survival based on stage and grade is shown in Figures 2 and 3. Furthermore, cardiac sarcoma patients who underwent surgery had a median survival of 12 months while those who did not undergo surgery had a median survival of merely one month (Figure 4). RT use was associated with a median survival of 11 months, compared to 4 months for not using RT, though the association of RT use with outcome was not statistically significant.
Figure 1.
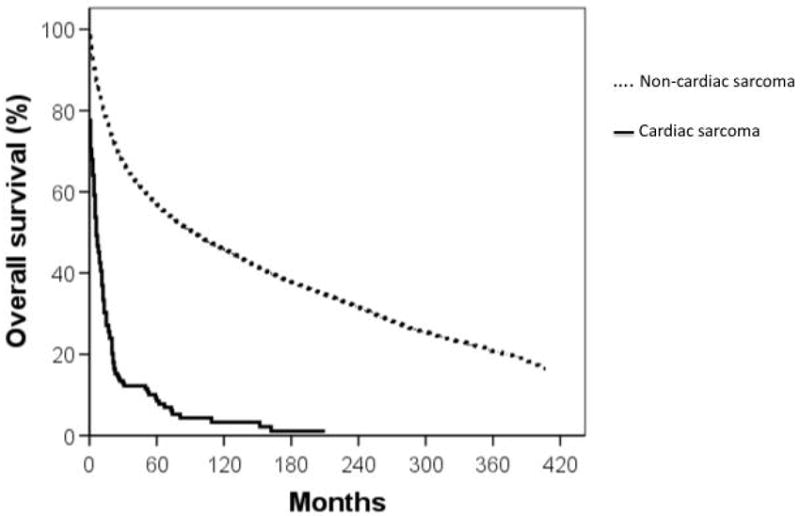
Comparison of cumulative survival (in months) of primary cardiac sarcoma patients and non-cardiac sarcoma patients. Solid line: cardiac sarcoma; dashed line: non-cardiac sarcoma.
Figure 2.
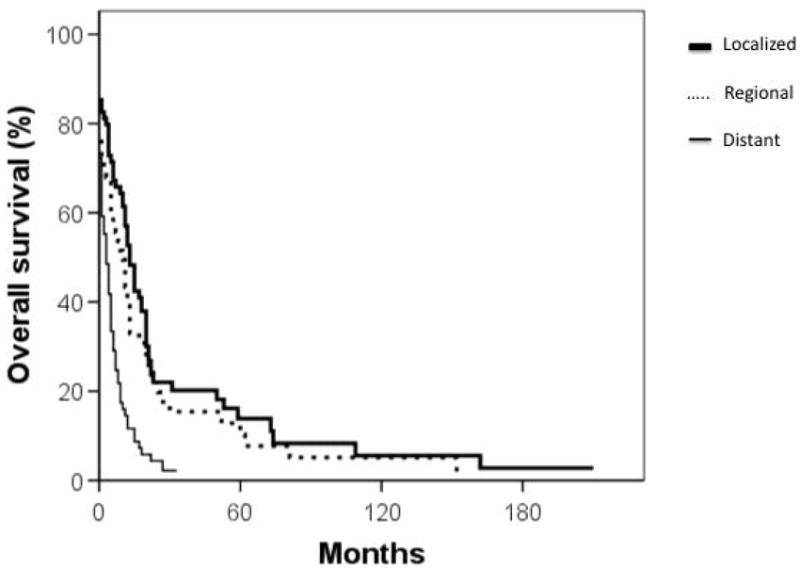
Cumulative survival (in months) of primary cardiac sarcoma patients by stage of disease. Thick, solid line: localized disease; dashed line: regional disease; thin, solid line: distant disease.
Figure 3.
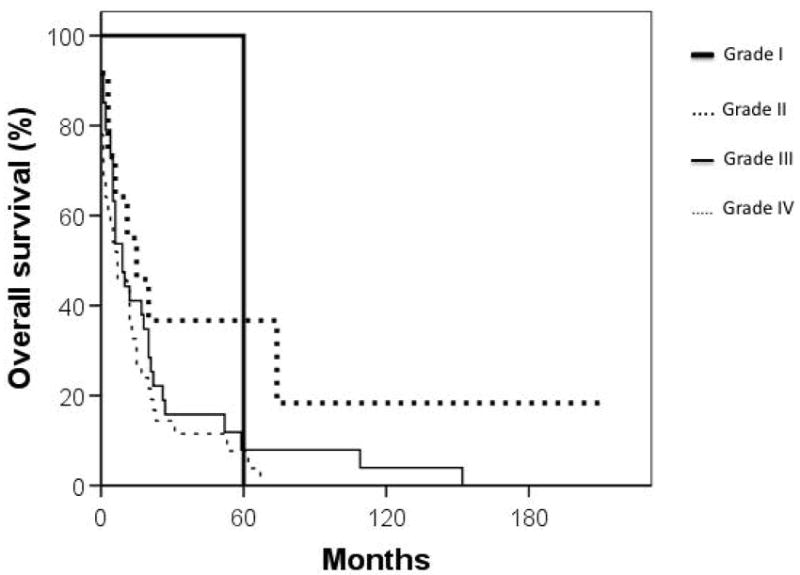
Cumulative survival (in months) of primary cardiac sarcoma patients by grade. Thick, solid line: well differentiated; thick, dashed line: moderately differentiated; thin, solid line: poorly differentiated; thin, dashed line: anaplastic/ undifferentiated.
Figure 4.
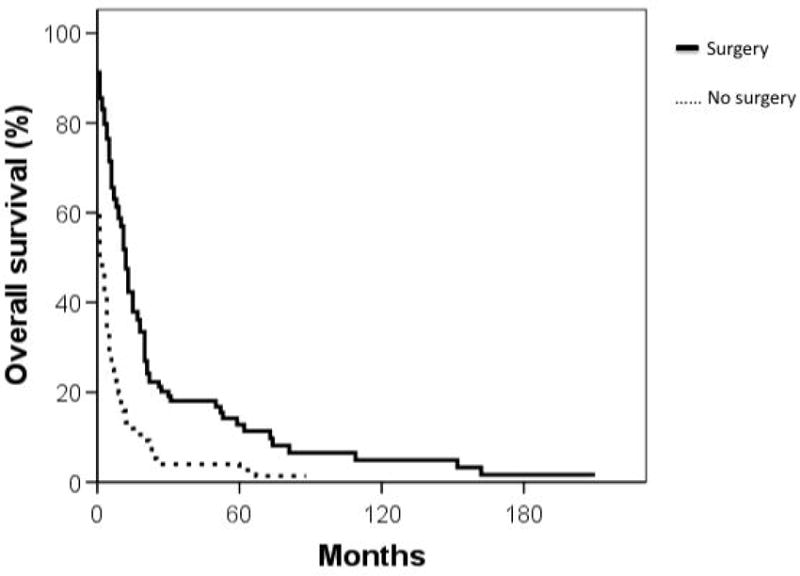
Comparison of cumulative survival (in months) of patients by surgical treatment. Solid line: surgery performed; dashed line: no surgery.
COMMENT
Though primary cardiac sarcomas occur over a wide age range, they are more likely to present in patients less than 65 years old. Previous retrospective series have also found that the median age of cardiac sarcoma patients was in the 40s with the majority of cases diagnosed in the 4th and 5th decades of life (6-12). In terms of gender, we found a slight male predominance. Most previous studies have found that malignant cardiac tumors are equally prevalent in both genders (6-12).
Considering that surgical resection is considered the mainstay of definitive therapy and represents an effective means of palliation (5), it is not surprising that regardless of stage, the majority of cardiac sarcoma patients in this series underwent surgery. In fact, 77% of patients with localized and 68% of patients with regional disease were surgically treated. Interestingly, a large proportion (41%) of patients with distant disease also underwent surgical resection. This likely reflects the frequent need for palliative debulking in cardiac sarcoma patients coupled with an appreciation that locoregional progression is often the cause of death even among patients presenting with metastatic disease. These observations echo the trend toward aggressive surgical management advocated by several institutions (6, 8-10, 12).
Historically, cardiac sarcoma studies have been so limited in size that comparisons to non-cardiac sarcomas have not been tenable. Our data suggests that in contrast to non-cardiac sarcomas, cardiac sarcomas tend to occur in younger patients and present with higher grade and more advanced disease. Furthermore, blood vessel neoplasms (e.g. angiosarcomas) represent half of cardiac sarcoma cases while less than 5% of non-cardiac sarcomas share this histology. Thus, cardiac sarcomas comprise a distinct subset of sarcomas.
Due to the surgical challenges presented by cardiac sarcomas and the dearth of efficacious, non-surgical therapeutic options, cardiac sarcoma patients continue to have dismal outcomes. Historically, cardiac sarcoma median overall survival has ranged from 11 to 18 months and 2 year survival rates have ranged from 14% to 26% (6,7,13). The overall survival of the patients in our study is shown in Figure 1. Recently, Bakaeen and colleagues reported a promising 2 year overall survival rate of 62% among 27 primary cardiac sarcoma patients who received aggressive multimodal therapy. In light of the clear survival advantage we identified with surgical management, these promising results are likely attributable to the very high rate of R0/R1 resections (96%) (12). In our analysis of all 210 patients, stage was found to be an important indicator of survival (Figure 2). The latter agrees with a study by Simpson and colleagues, in which patients with metastatic disease had a median survival of 5 months compared to 15 months in patients with non-metastatic disease (9).
Grade was found to be an important factor associated with survival in the 93 patients with complete data. Statistically, only undifferentiated/anaplastic tumors were associated with poor survival (Figure 3 and Table 3) although the trend was that of worse survival with higher grade across the entire spectrum of differentiation. This data contradicts that of a study by Burke and colleagues in which they evaluated the outcome of cardiac sarcoma patients by pathologic parameter and found that differentiated versus undifferentiated histology was not significantly associated with survival (7). The association of tumor grade with survival is somewhat surprising because locoregional progression, rather than distant metastases, has long been recognized as the primary cause of death among cardiac sarcoma patients and grade tends to be more predictive of distant metastases than locoregional progression. Our data raise the possibility that patients with undifferentiated cardiac sarcomas may be a unique subset at risk from death due to distant metastases. Alternatively, this finding may be a statistical anomaly. When all 210 patients were examined, grade was not found to be significantly associated with survival.
Surgical resection is generally felt to be essential for long-term survival of cardiac sarcoma patients (6-12). Several studies have shown that the median overall survival of patients with complete resection is twice as long or longer (17 – 24 months) than the median overall survival of patients with incomplete resection (6 to 10 months) (6, 9, 13). Bakaeen et al. have adopted a particularly aggressive approach to patients with both non-metastatic and metastatic disease. They advocate a multimodal treatment consisting of surgical resection (including resection of lung metastases) with adjuvant chemotherapy and/or radiation therapy. In their study of 27 patients treated in this manner between 1990 and 2005, the one year overall survival was 80.9% and the two year overall survival was 62%. They also found that aggressive treatment of recurrent disease was associated with better survival. Patients with no further treatment had a median survival of 25 months while those with further treatment, whether surgical or non-surgical, had a median survival of 47 months (12). These results are significantly superior to historic norms. However, cautious interpretation is required due to the small patient population, the high rate of R0/R1 resections (96%), and biases inherent in retrospective reviews. Nonetheless, these data strongly support the value of aggressive surgical approaches in an attempt to achieve an R0/R1 resection.
The role of adjuvant therapy remains poorly defined in the treatment of cardiac sarcomas. Llombart-Cussac et al. followed 15 cardiac sarcoma patients with an average age of 45 who underwent a doxorubicin-containing regimen of adjuvant chemotherapy within six weeks of surgery. Six of the patients underwent complete resection while the remaining had incomplete resections. Patients with unresectable tumors and low performance status were excluded from the study. Despite the optimal patient population, the median survival in this study was 12 months (13). Case studies of combined heart transplantation and chemotherapy reiterate these unfavorable results (14).
Data regarding radiotherapy in the treatment of cardiac sarcomas are even less abundant. Movsas and colleagues reported on a 51-year-old male with an unresectable high-grade sarcoma involving both the right and left atria as well as the tricuspid, with no evidence of metastatic disease (17). Definitive therapy consisted of hyperfractionated (twice daily) RT to a total of 70.5 Gy with a radiosensitizer (5’-iodode-oxyuridine). At 5 years, the patient remained disease free. Despite this success, our data strongly suggest RT is not a preferred primary treatment modality. Furthermore, our data suggest adjuvant RT does not confer a statistically significant survival advantage as multivariate analyses, accounting for variations in surgical management, failed to identify RT as a predictor of overall survival. Although this finding conforms with the evidence that adjuvant RT provides a local control benefit, but not a survival advantage, in non-cardiac sarcoma patients, it must be cautiously interpreted. As locoregional progression appears to be a major cause of mortality in cardiac sarcoma patients, local control may be more tightly linked to overall survival when compared to non-cardiac sarcoma patients. Supporting this was the trend toward an overall survival benefit identified for RT. Patients that received RT in our series had a median survival of 11 months compared to just 4 months in patients who did not receive RT. Thus, although surgical therapy is clearly the most important local therapy for cardiac sarcomas, our data do not rule out the possibility that RT may confer a small survival advantage. Furthermore, potential late toxicities of RT on cardiac tissue and surrounding structures need to be balanced against the poor outcomes in this population. Although clear benefits of adjuvant therapy in cardiac sarcoma management cannot be identified, the primacy of surgical excision suggests that chemotherapy, RT, or both should be considered in suitable patients presenting with unresectable disease.
In light of the locally aggressive nature of primary cardiac sarcomas, heart transplantation has been suggested as a curative approach to the disease. However, a small number of case studies have shown that cardiac transplantation leads to limited improvement in survival, with patients succumbing to either metastatic disease or transplant related complications within 2 to 4 years (14-16). Uberfuhr et al. combined heart transplantation with either neoadjuvant or adjuvant therapy in an attempt to control hematologic spread in 4 patients. Unfortunately, three of the four patients died from distant metastases within 2 years while the fourth patient died 37 months post-operatively from right heart failure without disease progression (14). Considering the extremely small patient numbers in these case studies, the effectiveness of transplantation cannot be adequately assessed. In light of the paucity of available heart donors, heart transplantation is unlikely to be widely accepted in cardiac sarcoma management.
This study has several limitations. First, though our study has a much larger population than other cardiac sarcoma series, the patient numbers are still small. Secondly, although the SEER database represents a unique tool for investigation of uncommon malignancies, it does not provide all characteristics that may impact survival and treatment decisions. For example, patient co-morbidities and resection margin status may have a profound impact on both outcome and management decisions but are not captured in the SEER database.
In summary, cardiac sarcomas are a rare and distinct subset of sarcomas. Unfortunately, poor survival is the rule. Surgery remains the primary treatment for local control and survival in these patients. However, the use of adjuvant therapies should be considered on an individualized basis particularly in the setting of unresectable disease.
Acknowledgments
None.
Footnotes
Disclosures: There are no conflicts of interest.
References
- 1.Abraham KP, Reddy V, Gattuso P. Neoplasms metastatic to the heart: review of 3314 consecutive autopsies. Am J Cardiovasc Pathol. 1990;3:195–198. [PubMed] [Google Scholar]
- 2.Lam KY, Dickens P, Chan AC. Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med. 1993;117:1027–1031. [PubMed] [Google Scholar]
- 3.Reynen K. Frequency of primary tumors of the heart. Am J Cardiol. 1996:77–107. doi: 10.1016/s0002-9149(97)89149-7. [DOI] [PubMed] [Google Scholar]
- 4.Silverman NA. Primary cardiac tumors. Ann Surg. 1980;191:127–138. doi: 10.1097/00000658-198002000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blondeau P. Prrimary cardiac tumors- French studies of 533 cases. J Thorac Cardiovasc Surg. 1990;38(suppl 2):192–195. doi: 10.1055/s-2007-1014065. [DOI] [PubMed] [Google Scholar]
- 6.Putnam JB, Jr, Sweeney MS, Colon R, et al. Primary cardiac sarcomas. Ann Thorac Surg. 1991;51:906–910. doi: 10.1016/0003-4975(91)91003-e. [DOI] [PubMed] [Google Scholar]
- 7.Burke AP, Cowan D, Virmani R. Primary sarcomas of the heart. Cancer. 1992;69:387–395. doi: 10.1002/1097-0142(19920115)69:2<387::aid-cncr2820690219>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 8.Perchinsky MJ, Lichetenstein SV, Tyers GF. Primary cardiac tumors: forty years’ experience with 71 patients. Cancer. 1997;79:1809–1815. doi: 10.1002/(sici)1097-0142(19970501)79:9<1809::aid-cncr25>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Simpson L, Kumar SK, Okuno S, et al. Malignant Primary Cardiac Tumors Review of a Single Institution. Cancer. 2008;112:2440–2446. doi: 10.1002/cncr.23459. [DOI] [PubMed] [Google Scholar]
- 10.ElBardissi AW, Dearani JA, Daly RC, et al. Survival after resection of primary cardiac tumors: A 48-year experience. Circulation. 2008;118:S7–S15. doi: 10.1161/CIRCULATIONAHA.107.783126. [DOI] [PubMed] [Google Scholar]
- 11.Zhang PJ, Brooks JS, Goldblum JR, et al. Primary cardiac sarcomas: a clinicopathologic analysis of a series with follow-up information in 17 patients and emphasis on long-term survival. Human Pathology. 2008;39:1385–1395. doi: 10.1016/j.humpath.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakaeen FG, Jaroszewski DE, Rice DC, et al. Outcomes after surgical resection of cardiac sarcoma in the multimodality treatment era. J Thorac Cardiovasc Surg. 2009;137:1454–1460. doi: 10.1016/j.jtcvs.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Llombart-Cussac A, Pivot X, Contesso G, et al. Adjuvant chemotherapy for primary cardiac sarcomas: the IGR experience. Br J Cancer. 1998;78:1624–1628. doi: 10.1038/bjc.1998.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uberfuhr P, Meiser B, Fuchs A, et al. Heart Transplantation: An Approach to Treating Primary Cardiac Sarcoma? J Heart Lung Transplant. 2002;21:1135–1139. doi: 10.1016/s1053-2498(02)00409-6. [DOI] [PubMed] [Google Scholar]
- 15.Aravot DJ, Banner NR, Madden B, et al. Primary cardiac tumours- is there a place for cardiac transplantation? Eur J Cardio-thorac Surg. 1989;3:521–524. doi: 10.1016/1010-7940(89)90112-7. [DOI] [PubMed] [Google Scholar]
- 16.Talbot SM, Taub RN, Keohan M, et al. Combined heart and lung transplantation for unresectable primary cardiac sarcoma. J Thorac Cardiovaxc Surg. 2002;124:1145–1148. doi: 10.1067/mtc.2002.126495. [DOI] [PubMed] [Google Scholar]
- 17.Movsas B, Terya-Feldstein J, Smith J, Glatstein E, Epstein AH. Primary cardiac sarcoma: a novel treatment approach. Chest. 1998;114:648–652. doi: 10.1378/chest.114.2.648. [DOI] [PubMed] [Google Scholar]


