Abstract
Primary progressive aphasia (PPA) is caused by selective neurodegeneration of the language-dominant cerebral hemisphere; a language deficit initially arises as the only consequential impairment and remains predominant throughout most of the course of the disease. Agrammatic, logopenic and semantic subtypes, each reflecting a characteristic pattern of language impairment and corresponding anatomical distribution of cortical atrophy, represent the most frequent presentations of PPA. Such associations between clinical features and the sites of atrophy have provided new insights into the neurology of fluency, grammar, word retrieval, and word comprehension, and have necessitated modification of concepts related to the functions of the anterior temporal lobe and Wernicke’s area. The underlying neuropathology of PPA is, most commonly, frontotemporal lobar degeneration in the agrammatic and semantic forms, and Alzheimer disease (AD) pathology in the logopenic form; the AD pathology often displays atypical and asymmetrical anatomical features consistent with the aphasic phenotype. The PPA syndrome reflects complex interactions between disease-specific neuropathological features and patient-specific vulnerability. A better understanding of these interactions might help us to elucidate the biology of the language network and the principles of selective vulnerability in neurodegenerative diseases. We review these aspects of PPA, focusing on advances in our understanding of the clinical features and neuropathology of PPA and what they have taught us about the neural substrates of the language network.
Introduction
Language is a uniquely human faculty that enables the communication and elaboration of thoughts and experiences through the mediation of arbitrary signs and symbols. Acquired abnormalities of language are known as aphasias. Nearly all right-handed individuals and many left-handed people exhibit severe aphasia only after injury to a specific set of regions in the left hemisphere of the brain, leading to the conclusion that language is under the control of an asymmetrically distributed large-scale neural network (Box 1). According to contemporary concepts, the Sylvian fissure divides this neural network into dorsal and ventral components.1–5 The dorsal components, including Broca’s area in the inferior frontal gyrus (IFG), display a relative specialization for phonological encoding, fluency, and grammatical structure, whereas the ventral components, located predominantly in the temporal lobe, display a relative specialization for lexicosemantic associations that link words to their meaning;1–5 however, these distinctions of functional anatomy are far from absolute.
Box 1. Properties of distributed large-scale neural networks.
The term ‘large-scale distributed neural network’ was introduced to designate computationally plausible and anatomically anchored substrates of cognitive and behavioural domains.97 For the language domain, network components are located in the perisylvian cortex and surrounding parts of the frontal, parietal and temporal lobes of the language-dominant (usually left) hemisphere of the brain.15 The following principles apply to the use of the term ‘network’ in this Review.160
-
▪
Network components can operate as critical hubs or ancillary nodes, both of which contribute to the function of the relevant domain, but only damage to critical hubs causes clinically relevant and sustained impairments. Critical hubs have the properties of transmodal cortex,86 and bind disseminated information into integrated representations
-
▪
The hubs and nodes function collaboratively, but are not interchangeable, and each displays relative specializations for separate components of the relevant cognitive and behavioural domain. The output represents an emergent property of the network, rather than the additive product of its components
-
▪
Critical hubs within a particular network are interconnected and are usually coactivated in the course of mediating the relevant cognitive and behavioural domain. Damage to one hub or its connections will, therefore, cause at least partial disruption in the functionality of the others
-
▪
Hubs and nodes are not dedicated to a unique network. Thus, damage confined to a single hub or node, or its connections, can also trigger perturbations in the function of intersecting networks
-
▪
Partial damage to a network component can give rise to minimal or transient deficits if other modules of the network undergo compensatory reorganization
Following the seminal work of Paul Broca,6 Carl Wernicke7 and their contemporaries in the 19th century, research into the neurology of language was largely focused on aphasias caused by focal cerebrovascular accidents. This perspective has now broadened to include language disorders associated with neurodegenerative brain diseases. In particular, the syndrome of primary progressive aphasia (PPA), in which the language-dominant (usually left) cerebral hemisphere is the selective target of progressive neurodegeneration, leading to aphasia in the absence of marked impairment in other cognitive and behavioural domains, has emerged as a new area of fruitful research. Indeed, the rapidly expanding literature relating to PPA is providing new insights into the neural components of the language network and its vulnerability to neurodegenerative diseases. In this article, we review the advances that have been made to date with regard to PPA, encompassing diagnosis and subclassification of the syndrome, its various clinical and neuropathological manifestations, potential aetiological factors, and disease trajectory and treatment. We also highlight how increased knowledge of PPA has advanced our understanding of language processing in the brain, necessitating revision of some traditional concepts.
Historical recognition of the PPA syndrome
In the late 19th and early 20th centuries, Pick,8 Sérieux,9 Franceschi10 and Rosenfeld11 were among the first to describe aphasias caused by neurodegenerative rather than cerebrovascular lesions. However, many of the patients involved in the studies by these researchers would have failed to meet the current diagnostic criteria for PPA, as the language disturbances reported had emerged in conjunction with other salient cognitive and behavioural impairments. For example, Pick8 noted that the patient he studied additionally displayed progressive memory decline and had threatened his wife with a knife. The patient reported by Sérieux demonstrated a different pattern of neurological impairment; she presented at the age of 47 years with progressive loss of word comprehension, but preserved memory and ‘intelligence’.9 In this woman, the language disorder progressed to a state of Wernicke aphasia over the 8-year period up to the patient’s death in 1897. This case is the earliest historical report of an aphasia syndrome that can be singled out as the prototype for what came to be known as PPA.
The syndrome of PPA as a distinct entity was introduced into the modern literature in the 1980s and was gradually delineated from other dementia syndromes.12,13 This syndrome is characterized by a language disorder of neurodegenerative aetiology, which emerges in relative isolation with respect to other neurological manifestations during the first 1–2 years after onset, and remains predominant during much of the disease course.14,15 Onset usually occurs before 65 years of age, with approximately equal prevalence across sexes. The one common denominator among patients with this syndrome is asymmetric damage to the language-dominant cerebral hemisphere, which manifests as cortical atrophy, hypoperfusion or hypometabolism.16,17 Sites of peak neuronal loss within the language network vary between patients and determine the pattern of aphasic impairment.18
Diagnosis and classification of PPA
Criteria for the root diagnosis of PPA
The root diagnosis of PPA is justified if three criteria are met (Box 2).17 The first two criteria—the presence of a prominent language disorder that developed gradually, and its neurodegenerative nature—can be ascertained through clinical history, tests of language function, and neuroimaging procedures. The third criterion, that is, the relatively isolated emergence of aphasia as the principal impediment to the pursuit of customary activities, is the most challenging to establish. Even experienced clinicians can have difficulty deciding whether a progressive aphasia is truly ‘primary’. It is preferable to be conservative and avoid a PPA diagnosis when disruptions in other cognitive and behavioural domains are prominent early in the course of the disease, even if a progressive (but nonprimary) aphasia is part of the overall syndrome (Box 3).
Box 2. The three criteria for the root diagnosis of primary progressive aphasia.
Gradual impairment of language
The impairment of language should have developed gradually. Common manifestations include frequent word-finding pauses followed by uninformative filler words (such as ‘thing’ and ‘stuff’), ungrammatical and impoverished sentences (“words in the my head and cut up,” for example), the inability to name parts of objects (for instance, the strap of a watch, the stem of a flower, the lid of a jar), impaired repetition of phrases and sentences, failure to understand words (such as strap, stem, lid), and spelling errors of recent onset. Dysarthric or apraxic speech alone is insufficient to fulfil this criterion.
Confirmed neurodegenerative aetiology
Diagnostic brain imaging procedures should establish a neurodegenerative process as the only plausible cause by ruling out stroke, neoplasm or other potential causes of an acquired language disorder.
Disproportionate salience of aphasia
Aphasia should initially arise in relative isolation as the most prominent (that is, primary) impairment and should remain the principal factor underlying the disruption of daily living activities for at least 1–2 years. To fulfil this criterion, it is necessary to ascertain (through an informant, medical records, or clinical examination) that episodic memory, visuospatial skills, executive functions, face and object recognition, comportment, and motor function were mostly preserved during an initial period of 1–2 years after symptom onset. Preferably, nonverbal standardized tests should be used to evaluate other cognitive and behavioural domains, such as the Visual Verbal Test for executive functions, the Three Words-Three Shapes Test for memory, and the Judgement of Line Orientation Test for visuospatial perception.15,161,162 However, excessive reliance on test results must be avoided. If a patient with a prominent progressive inability to find words also has subnormal scores in memorizing a word list or in a sustained attention test, but without corresponding impairments of daily activities (such as forgetfulness of recent events, inability to multitask, or reduced initiative), a primary progressive aphasia diagnosis is justified.
Box 3. Nonprimary progressive aphasias.
Progressive aphasias can arise in patients whose principal and initial impairment is an amnestic dementia of the Alzheimer-type, the posterior cortical atrophy syndrome, the behavioural variant of frontotemporal dementia, the corticobasal degeneration syndrome, the progressive supranuclear palsy syndrome, apraxia of speech, or motor neuron disease. No patient with a progressive aphasia that arises as a secondary component of any of these syndromes would qualify for a primary progressive aphasia (PPA) diagnosis because the language impairments constitute ancillary features. Such patients can be described as having progressive aphasia in conjunction with the dominant syndrome.
Some patients display a combination of aphasia and nonverbal associative agnosia at the early stages of disease. This syndrome, known as semantic dementia, is also inconsistent with a diagnosis of PPA if the associative agnosia is initially prominent and responsible for consequential impairments, such as an inability to recognize familiar faces or the misuse of objects.
Nomenclature and subtyping—a work in progress
More than 100 years of pivotal contributions from key figures in the history of neurology led to the development of a classification system for aphasias of sudden onset. Much of this historical research relates to patients with cerebrovascular accidents (stroke), a setting in which grey and white matter is abruptly and indiscriminately destroyed in at least part of the core lesion site. By contrast, neurodegenerative diseases can selectively target specific layers and regions of the cerebral cortex. Even within sites of peak cortical atrophy in patients with PPA, neuronal destruction is never complete, and the remaining neurons continue to participate in language function, albeit with distorted patterns of neural network connectivity (Figure 1).19,20 The partial and gradual neuronal loss sets the stage for extensive reorganization of cerebral circuitry, which might be at least partially compensatory.21 These partial and progressive perturbations of the underlying neural network induce subtle dissociations of language function that are less likely to arise in patients with cerebrovascular disease. In addition, PPA can be associated with the selective neurodegeneration of regions of the brain—such as those within the anterior temporal lobe (ATL)—that are not vulnerable to isolated cerebrovascular accidents. For these reasons, the language disturbances in PPA do not fit syndromic patterns identified in stroke-induced aphasias, thus necessitating the development of a novel nomenclature and classification system.
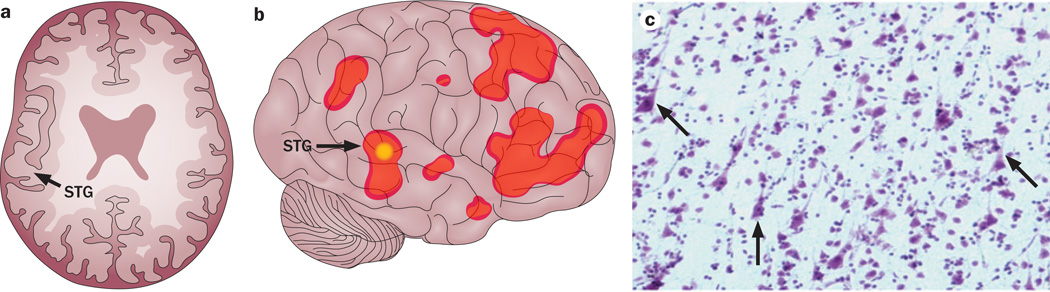
Figure 1.
The unusual complexity of clinicopathological correlations in PPA. a | Drawing of an axial section of a structural MRI scan from a 57-year-old familial left-handed man with right-hemisphere language dominance and logopenic PPA;163 the right cerebral hemisphere is on the left-hand side. Areas of substantial atrophy can be observed in the right perisylvian cortex, including the posterior STG (arrow). b | Illustration of findings based on fMRI evaluation of the same patient performed soon after the structural scan, revealing that the atrophic area of the right hemisphere was activated (arrow) during a task in which the patient was asked to determine whether two words had the same meaning. c | The patient died 8 years later; pathology of autopsy tissue demonstrated features consistent with frontotemporal lobar degeneration with TAR DNA-binding protein 43 pathology. In particular, despite the severe atrophy at the time of the structural MRI scan and progressive neuronal loss over the subsequent 8 years before death, the photomicrograph of a cresyl violet-stained region of the right STG demonstrates identifiable neurons (arrows), some of which might have contributed to the activation of this region during the fMRI assessment. Abbreviations: fMRI, functional MRI; PPA, primary progressive aphasia; STG, superior temporal gyrus.
At the time when the features of PPA were initially being delineated, the descriptive term ‘logopenia’—derived from Greek words ‘λόγος’ (logos), meaning ‘word’, and ‘πενία’ (penia), meaning ‘deficiency’—was introduced to define a language abnormality that seemed distinctive to PPA.12,22 The abnormality was characterized by word-retrieval deficits that led to intermittent loss of verbal fluency but without disorders of motor speech, grammar or comprehension.12,22 These initial observations were not accompanied by specific diagnostic or classification criteria. The first widely accepted classification relevant to PPA was published in 1998,23 in the form of a consensus report that aimed to capture the clinical spectrum of frontotemporal lobar degeneration (FTLD). The report assigned the definition ‘progressive nonfluent aphasia’ (PNFA) to most cases with impaired verbal fluency, and designated ‘semantic dementia’ as a syndrome with both word comprehension impairments (that is, aphasia) and object recognition impairments (associative agnosia), but preserved verbal fluency.
Although the 1998 criteria were not designed to characterize the spectrum of PPA syndromes as a whole, their use for this purpose had important consequences. First, the logopenia pattern—impaired word retrieval in speech but with preserved grammaticality and word comprehension—was not recognized as a distinct entity. Second, the semantic dementia designation enabled the inclusion of patients with a disease phenotype inconsistent with a PPA diagnosis, owing to concomitant prominent associative agnosia. Third, patients with PPA attributable to non-FTLD pathologies seemed to be implicitly excluded. These issues were addressed in international guidelines for the classification of PPA, published in 2011.24 The 2011 guidelines divided PNFA into agrammatic and logopenic components, required the semantic variant to fulfil the root diagnosis of PPA (thus excluding patients with prominent associative agnosia), and made no assumptions concerning the nature of the underlying neuropathology.24 These guidelines, and their classification of PPA into agrammatic (PPA-G), logopenic (PPA-L) and semantic (PPA-S) variants,24 have received broad acceptance and were cited nearly 100 times within the first year after their publication. Nonetheless, challenges have been recognized, including unclassifiable forms of PPA and language impairment patterns that simultaneously fulfil the criteria for more than one subtype of PPA. 25–30 The 2011 guidelines also added impaired repetition of phrases and sentences, a feature that was not part of the original definition of logopenia, as a core feature of the PPA-L variant; thus, the term ‘logopenic’ can be used in more than one sense in relation to PPA, representing a potential source of inconsistency in the literature.
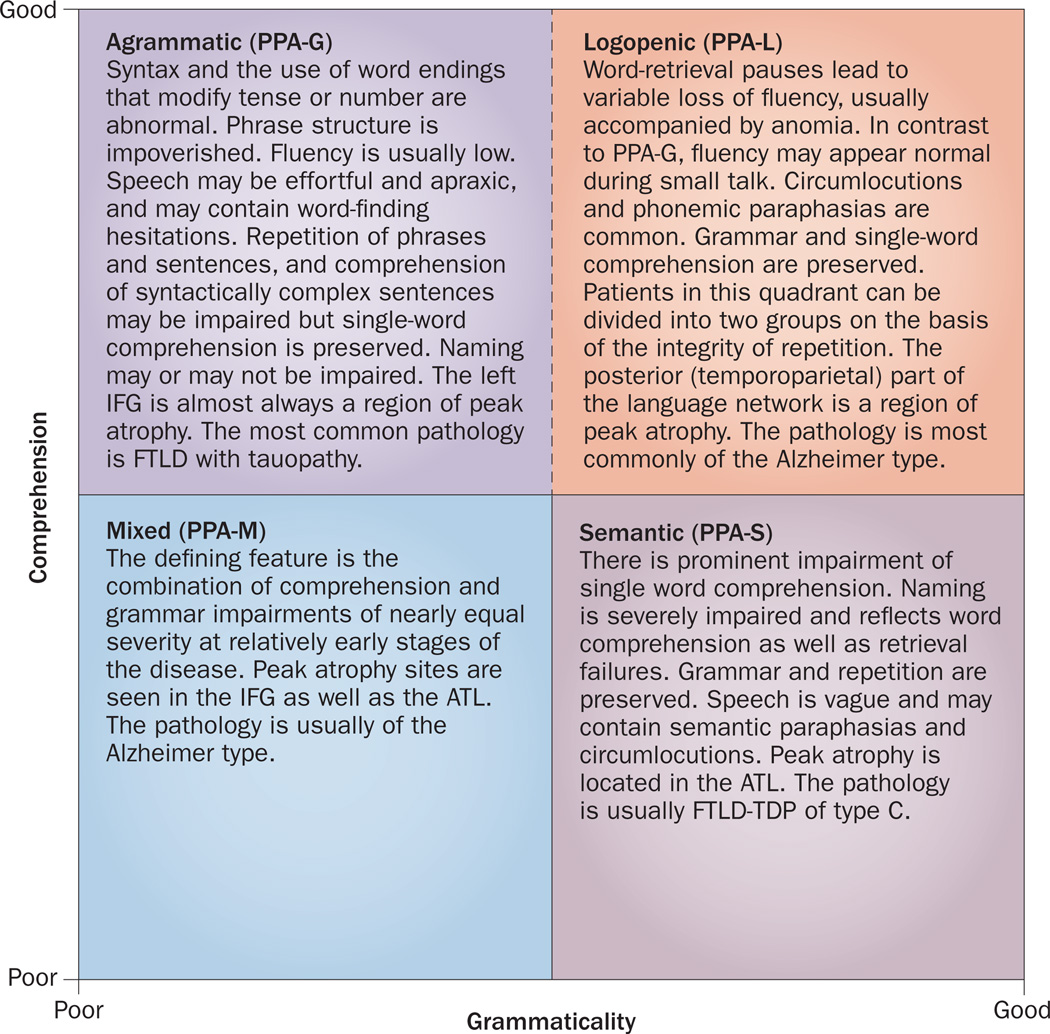
With a few modifications, the 2011 guidelines could offer a more robust classification system for PPA.28 However, the 2011 guidelines also require the assessment of at least 10 different aspects of language function; a more practical but less rigorous alternative classification system focuses on the status of only grammatical ability and single-word comprehension in patients who have already met the PPA diagnostic criteria (Box 2).26,31 Using these criteria, a two-dimensional template comprising four quadrants is generated that defines different subtypes of PPA (Figure 2). The upper-left and lower-right quadrants capture PPA-G and PPA-S, respectively, on the basis of abnormalities of grammaticality and word comprehension. The upper-right quadrant specifies the absence, rather than presence, of deficits in both of these key features of language and, therefore, incorporates the most heterogeneous patient population. In our experience, this quadrant is populated predominantly by patients who display the clinical features of logopenia (word-retrieval pauses with preserved grammar and comprehension), and who may or may not have additional repetition impairment. Some of these patients will maintain the logopenic pattern of aphasia throughout most of the disease course, whereas others will later develop characteristic features of PPA-G or PPA-S.26 The few purely anomic patients who have no word-finding hesitations, agrammatism or word-comprehension impairment will also fit into this quadrant, but are likely to progress to PPA-L or PPA-S.26
Figure 2.
Template approach to the classification of PPA. This simplified approach is based on the integrity of single-word comprehension and grammaticality of sentences. The patient must fulfil the root diagnosis of PPA before undergoing classification according to this system. The performance of the classification system is optimal if conducted neither too early nor too late in the course of the disease. The individual tests and cut-off scores must be determined empirically, and depend on the severity of the aphasia.26,31 The broken boundary line between the PPA-G and PPA-L quadrants represents the challenge that is frequently faced in differentiating these two aphasia subtypes. The upper right quadrant can potentially contain the most heterogeneous population. In practice, however, it becomes populated almost exclusively by patients who are clinically logopenic, either with or without phrase and sentence repetition impairments. Abbreviations: ATL, anterior temporal lobe; FTLD, frontotemporal lobar degeneration; FTLD-TDP, FTLD with TAR DNA-binding protein 43 pathology; IFG, inferior frontal gyrus; PPA, primary progressive aphasia; PPA-G, agrammatic PPA; PPA-L, logopenic PPA; PPA-M, mixed PPA; PPA-S, semantic PPA.
This template approach does not allow patients to fulfil criteria for more than one subtype, introduces a more inclusive definition of PPA-L, and recognizes patients with ‘mixed’ PPA (PPA-M) who present with combined grammar and comprehension impairments (Figure 2). However, classification of PPA according to this template is time-sensitive:26 if performed too early, patients at the prodromal stages of PPA-G or PPA-S will have disease classified as PPA-L; if conducted too late in the course of the disease, patients will fit the PPA-M classification owing to increased aphasia severity. The boundary between PPA-L and PPA-G is particularly indistinct, principally because grammaticality is difficult to quantitate. Another major challenge is the need to choose appropriate difficulty levels of tests, cut-off scores, normative values, and modes of assessment that are appropriate for the severity and nature of the aphasia.26,31
A universally applicable classification system for PPA is unlikely to be found, given the complexity of syndromic characterization in aphasia, the heterogeneity in the sites of peak atrophy, and the progressive nature of the disorder. One way to circumvent the impact of this challenge on clinicopathological correlations is to base relevant analyses on readily quantifiable specific parameters such as grammar, fluency, word retrieval, comprehension and repetition rather than on parameter clusters that define discrete subtypes. Although the language impairment patterns in PPA are not identical to the classic patterns identified in patients with stroke, PPA-G has features that are analogous to Broca’s aphasia of classic aphasiology, while PPA-L resembles conduction aphasia or anomic aphasia, PPA-S resembles sensory transcortical aphasia, and PPA-M resembles global aphasia.
PPA-G—dissociating grammar and fluency
The core feature of PPA-G is disrupted sentence production, characterized by abnormal word order (syntax), poor phrase structure, and misuse of grammatical morphemes (such as word endings that modify tense or number).32–35 The patient can successfully communicate intent in short ungrammatical statements that generally contain fewer verbs than nouns.36–40 According to our clinical observations, in mild cases of PPA-G, agrammatism might be detected only in writing samples, including emails. Most agrammatic patients also have low verbal fluency caused in part by long word-retrieval pauses that precede almost every utterance. Although loss of verbal fluency can occur in the absence of articulatory abnormalities, patients can also show variable degrees of dysarthria, phonological disintegration, laboured pronunciation, and apraxia of speech.41 Object naming is abnormal in some—but not all—patients with agrammatism.
In PPA-G, comprehension is typically well-preserved for single words, but not for grammatically complex sentences, indicating that the neurological dysfunction encompasses decoding, as well as encoding, of grammatical structure. For example, a patient who can correctly match canonical sentences such as “The dog is chasing the cat” with depictions of the corresponding action might fail similar tasks that use noncanonical sentences such as “The cat was chased by the dog.” Even more striking is the phenomenon of grammatical alexia that we have occasionally observed in the advanced stages of PPA, in which patients can correctly recognize written forms of nouns such as ‘table’ or ‘alligator’, but not of pronouns and conjunctions such as ‘he’, ‘but’ or ‘it’.
Compared with other aspects of language, grammatical ability is difficult to assess and requires specifically designed tests, including linguistic analysis of narrative discourse.36,42,43 Assessment of grammaticality in patients with severely diminished language production represents a particular challenge. In such circumstances, the use of an anagram task, in which the patient is asked to order single words, each printed on a separate card, to form sentences with varying syntactic complexity, can be informative.44,45
One of the most distinctive and common features of PPA-G is the presence of sites of peak atrophy within the dominant (usually left) IFG, where Broca’s area is located (Figure 3a). This association is consistent with the vast literature demonstrating that the role of the left IFG within the language network is to serve as a critical hub for processes related to syntax, morphology, and comprehension of grammatically complex statements.46–52 In many patients with PPA-G, brain atrophy might also encompass more-dorsal parts of the posterior frontal lobe as well as patches of the temporoparietal junction (TPJ) and lateral temporal lobe. To a lesser extent, atrophy can also affect the corresponding parts of the contralateral hemisphere.
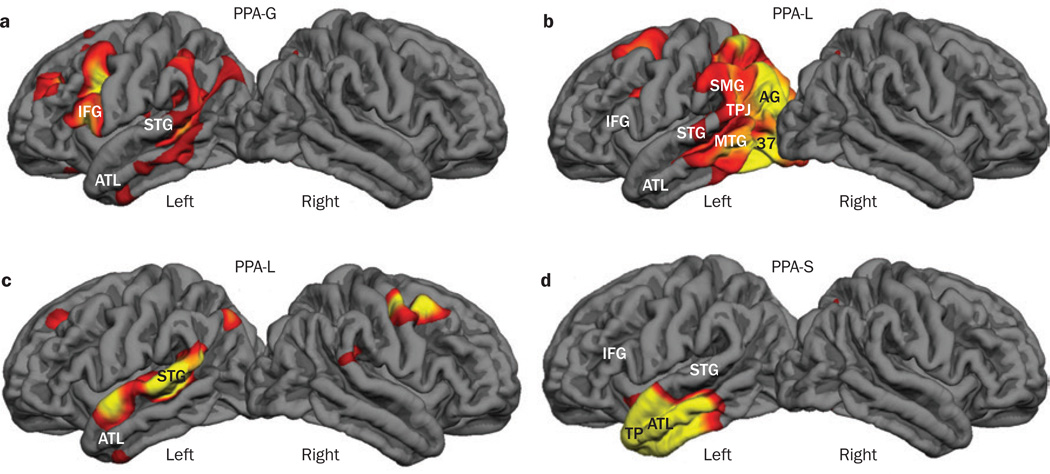
Figure 3.
Atrophy maps of the brain in PPA subtypes. Atrophy maps of the brain were generated by comparing MRI-based cortical thickness data from a single patient with ‘normal’ measurements derived from a control group of 27 healthy individuals using FreeSurfer software, which presents the peak atrophic sites in red and yellow.26,31 The maps were produced using a threshold false discovery rate of P <0.05. a | Map based on an MRI scan from a right-handed man with PPA-G aged 59 years, 3 years after symptom onset at the age of 56 years. b | Atrophy map associated with PPA-L in a right-handed woman with symptom onset at the age of 60 years; the scan was obtained 5 years after symptom onset. c | Atrophy map of a 72-year-old right-handed woman with PPA-L, 4 years after symptom onset. d | Map of atrophy in a right-handed woman with PPA-S based on a scan performed 2 years after symptom onset at the age of 59 years. Abbreviations: 37, Brodmann area 37; AG, angular gyrus; ATL, anterior temporal lobe; IFG, inferior frontal gyrus; MTG, middle temporal gyrus; PPA, primary progressive aphasia; PPA-G, agrammatic PPA; PPA-L, logopenic PPA; PPA-M, mixed PPA; PPA-S, semantic PPA; SMG, supramarginal gyrus; STG, superior temporal gyrus; TP, temporopolar cortex; TPJ, temporoparietal junction.
In stroke-based aphasiology, the terms ‘Broca’s aphasia’, ‘agrammatic aphasia’ and ‘nonfluent aphasia’ tend to be used interchangeably, leading to the implication that grammar and fluency share a common neural substrate. Studies in PPA, however, indicate that dissociations can arise between these two aspects of language.36 For example, one of our patients with PPA-G, who wrote exceedingly agrammatic sentences such as “I am for the speech therapy for year,” had nearly normal verbal fluency (110 words per minute compared with a normative value of 130 ± 20 words per minute), at a time when only 62% of the sentences she produced during connected speech were grammatically correct. In keeping with such observations, quantitative imaging in a group of 31 patients with PPA showed that the sites of peak IFG atrophy associated with impairments of either grammar or verbal fluency are mostly nonoverlapping;53 impairments of grammar correlated with atrophy within the main body of the IFG, whereas atrophy that was associated with diminished verbal fluency was confined to a dorsal rim of the IFG and also extended further dorsally into the premotor cortex.53 Furthermore, studies based on diffusion tensor imaging demonstrated that impaired grammar was associated with abnormality of the arcuate fasciculus, which interconnects the TPJ with the IFG, whereas impaired verbal fluency was associated with abnormality of the aslant tract, which connects the IFG with more dorsal premotor areas of the brain.2,54 Thus, although the majority of agrammatic patients do have decreased verbal fluency, investigations in PPA have shown that the two types of impairment can be dissociated from each other.
PPA-L—insights into word retrieval
Abnormal repetition, an indicator of phonological loop dysfunction, was included as a necessary criterion for the diagnosis of the logopenic variant of PPA (PPA-L) in the 2011 classification guidelines.24 However, the ability to repeat phrases and sentences can remain intact in many patients who otherwise have the clinical features of logopenia. Such patients are unclassifiable using the 2011 guidelines.26,31,55 Furthermore, some patients with PPA-L simultaneously fulfil PPA-G or PPA-S criteria defined in the 2011 guidelines, thus complicating classification. These difficulties can be resolved by redefining and subdividing the PPA-L classification to include two forms, one with and one without repetition impairment, and by making the absence of major grammar and word-comprehension abnormalities necessary features, as prescribed by the template approach (Figure 2).26
The two forms of PPA-L distinguished according to the presence or absence of repetition impairment have peak atrophy within similar regions of the brain, and also have comparable neuropathological correlates of PPA.28,29,55 Both forms are characterized by word-retrieval impairments on a background of relatively intact grammatical abilities, word comprehension, and motor components of speech.18,22,56 Comprehension of long or noncanonical sentences might be compromised,26 probably due to limitations in auditory working memory. In contrast to patients with PPA-G, who seem to require additional effort and time for retrieval of most words, the word-finding pauses observed in patients with PPA-L are variable, being generally more prominent for nouns than for verbs.57 Patients with PPA-L might seem verbally fluent during small talk, but often start to display frequent word-finding hesitations when access to specific or infrequently used words becomes necessary. Many patients circumvent these word-retrieval blocks through circumlocution, simplification and substitution, using terms such as ‘thing’ or ‘stuff’.
Object anomia, due to word-retrieval failures, is usually present and tends to elicit phonological paraphasias (abnormal utterances that approximate the sound of the intended word). However, some patients with frequent word-finding pauses during spontaneous speech have nearly intact object-naming abilities, suggesting that different mechanisms underlie word-retrieval impairment depending on whether word selection is guided by external sensory percepts or internal ideas. For example, one of our patients with PPA-L and low verbal fluency (mean length of utterance of 5.46 words compared with a normative value of 11.11 ± 0.56) correctly named 83% of the items on the Boston Naming Test, indicating much greater impairment of word recall triggered by internal than by external ideas. Another patient with PPA-L and a low verbal fluency of 50 words per minute correctly named 97% of the items, offering an even more striking dissociation. These two examples illustrate that word-selection impairments can arise from multiple mechanisms, and suggest that studies in PPA might provide new insights into their differential anatomical substrates.
Patients with PPA-G or PPA-L frequently state that they ‘know’ the word but cannot ‘say’ it. However, object-naming failures in these patients were associated with an abnormal N400 component in EEG event-related potentials, even when the patient recognized the meaning of the word.58 This observation indicates that retrieval anomia also has an associative basis, perhaps at the level of accessing a hypothetical lemma.59,60 The impaired lemma in these patients might be sufficient to support word–object matching (that is, recognizing the meaning of the word), but not the task of scanning the internal thesaurus and selecting the corresponding noun. Although word comprehension in PPA-L is generally well-preserved, subtle chronometric abnormalities of semantic and taxonomic interference have been reported in this syndrome, as well as in PPA-G.61–63 These characteristics highlight the fact that dysfunction centred on one component of the language network can influence the functionality of the others, in keeping with the principles of neural network organization (Box 1).
The cortical atrophy associated with PPA-L is usually more pronounced in the posterior and temporal components of the language network (Figure 3b – c). The sites of greatest atrophy almost always include posterior temporal cortex and the TPJ. For example, Brodmann area 37, which is implicated in naming, and the posterior superior temporal gyrus, which is implicated in repetition, are frequently atrophic.31,53,64 Atrophy in patients with PPA-L can also extend into other parts of the left temporal lobe and patches of the right hemisphere. Although the atrophy in some patients may encroach on the ATL, the area of peak atrophy rarely covers the temporal pole.
PPA-S—insights into word comprehension
The core features of PPA-S include profound impairments in object naming and word comprehension, on a back-ground of preserved grammatical structure and fluency. At the initial stages of this syndrome, the successful comprehension of casual conversation (due in part to the patient’s ability to recognize contextual cues) contrasts sharply with the severe deficits in understanding nouns that denote objects, especially animals, fruits and vegetables. This anomia is often associated with circumlocution, simplification, and semantic paraphasias, but usually without the prolonged word-finding pauses seen in other PPA variants. In PPA-L or PPA-G, a patient who fails to name an object can nonetheless match it to the corresponding noun, indicating that the anomia is based on a word-retrieval deficit, rather than a lack of comprehension of the word. This pattern of anomia has been defined as a ‘one-way’ naming deficit.17 By contrast, patients with PPA-S who cannot name an object also frequently fail to match the item to its corresponding noun, even when the nature of the object is recognized, indicating that such anomic errors are caused by a failure to recognize (that is, comprehend) the meaning of the relevant word. This pattern has been defined as a ‘two-way’ naming deficit.17
Initially, patients with PPA–S seem to understand words at a generic level (for example, that the word ‘grape’ refers to something edible), but not at the specific level (that the word ‘grape’ refers to a type of fruit distinct from a ‘strawberry’, for instance).65–67 This intra-categorical blurring of distinctions in word usage and interpretation, also known as taxonomic interference, provides a potential mechanism for the vagueness of verbal output and the propensity for semantic paraphasias that are key characteristics of PPA-S. As the disease progresses, the comprehension impairment might encompass the generic level of meaning and, therefore, interferes with the ability to understand all kinds of verbal communication.
Regions of the brain that are most atrophic in patients with PPA-S are concentrated within the anterior and polar cortices of the left temporal lobe (Figure 3d). Although a small proportion of patients with considerable left ATL atrophy have preserved single-word comprehension, nearly all patients with severe word-comprehension impairments turn out to have sites of peak atrophy centred within the ATL of the language-dominant hemisphere. In addition, the uncinate fasciculus, which interconnects the ATL with the frontal lobes, often displays structural abnormalities that correlate with the word-comprehension impairments in patients with PPA-S.54
Left anterior temporal lobe function
The ATL is not considered part of the classic neural network that mediates language. Despite being associated with certain clinical features reminiscent of sensory transcortical aphasia, such as poor comprehension in the presence of intact repetition, PPA-S has anatomical correlates that do not conform to the anatomy of lesion sites described in sensory transcortical aphasia by classic aphasiology.68,69 Although observations relating to herpes simplex infection, temporal lobectomy and intraoperative cortical stimulation have revealed language-related functions of the ATL,69–72 investigations in semantic dementia and PPA have offered the most compelling evidence for the profound importance of this region of the brain to language function.66,67,73–77
One such investigation involved 11 right-handed patients with PPA, who were selected solely because the greatest degree of brain atrophy was localized either exclusively or predominantly in the left ATL, including the temporal polar region.66 In these patients, cortical volume within sites of peak atrophy in the left ATL had been reduced to approximately 50% of normative values, whereas cortical volume in the other parts of the left cerebral hemisphere remained at 90% of normative values.66 Severe impairments of word comprehension (mean accuracy 52%) and object naming (mean accuracy 21%) were the most obvious and consistently shared features of disease among these patients.66 By contrast, the patients demonstrated over 80% accuracy in picture–picture matching tasks that were sensitive to nonverbal object associations.66 Furthermore, computerized tasks with parallel designs revealed that the taxonomic interference caused by atrophy in the left ATL selectively undermined verbal, but not nonverbal, associations of objects.66,67
These and similar observations in PPA indicate that the classic aphasiology paradigm is incomplete, and that the left ATL should be considered as part of the language network, with a critical role in honing the acuity of object naming and word comprehension. This conclusion is consistent with the findings of a study in aphasic patients with stroke,60,78 which attributed an essential role to the left ATL in mediating refined taxonomic distinction of word selection during object naming. The proposed role of the ATL in word comprehension is also in keeping with functional imaging data that have demonstrated activation of the left lateral ATL in tasks of verbal semantic judgement,79,80 and in tasks that require high levels of taxonomic specificity during lexical retrieval.81,82 Additional support for the selective role of the left ATL in language came from task-free functional imaging studies,83 which showed that the left lateral ATL was functionally interconnected with neural hubs of the language network located in the left IFG and TPJ, and to a lesser extent with the same regions of the contralateral hemisphere (Figure 4), whereas the right ATL had no comparable connectivity with analogous regions of the right or left hemisphere.83
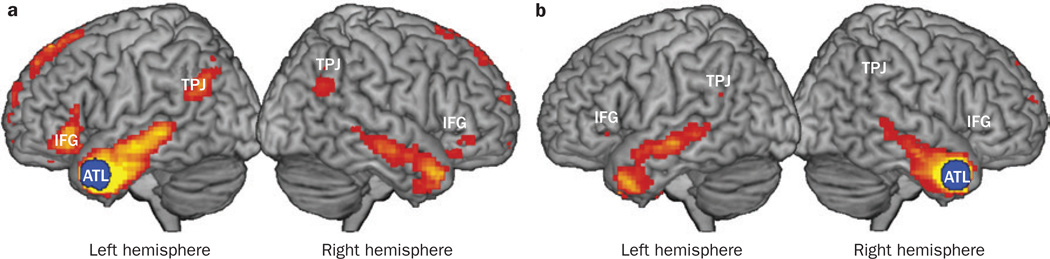
Figure 4.
Resting state functional connectivity of the ATL. 33 right-handed neurologically intact patients underwent MRI, and the task-free functional connectivity of a 10 mm seed in the ATL (blue circle) with all other voxels in the brain was computed by correlating the averaged haemodynamic time series data.83,164 Areas in red and yellow contain voxels that have statistically significant functional connectivity with the seed area at a false discovery rate threshold of P <0.001.83,164 a | The left ATL has functional connectivity with the IFG and TPJ in the ipsilateral and, to a lesser extent, in the contralateral hemisphere. The TPJ area includes parts of the inferior parietal lobule and posterior tip of the superior temporal gyrus. b | The right ATL has almost no significant functional connectivity at this threshold with the IFG or TPJ area in either hemisphere. The ATL is, therefore, asymmetrically organized. Abbreviations: ATL, anterior temporal lobe; IFG, inferior frontal gyrus; TPJ, temporoparietal junction.
An extensive literature on semantic dementia has characterized the ATL as an amodal (or pan-modal) hub that is critical for all domains of semantic representation: verbal as well as nonverbal.76 This description might appropriately define the aggregate functionality of the ATL cortices in both hemispheres; however, observations in PPA also reveal a differentiated and asymmetric internal organization of this brain region, according to which the left lateral ATL is critically important for verbal associations of objects, a role that is not shared by the right ATL. Studies of associative agnosias in patients with various neurological lesions have shown that nonverbal representations of objects are sustained through a distinct inferotemporal face and object recognition network, the critical components of which are located either bilaterally or predominantly in the right hemisphere.66,84,85 Interaction between the language and object-recognition networks through transmodal nodes, which are sites of partial convergence and hubs for binding of distributed information (Box 1), integrates the verbal, functional and experiential attributes of the object and also gives meaning to the word. Through this extended neural network, different parts of the temporal lobe, including the anterior temporal cortex, mediate different aspects of the object-recognition and word comprehension processes.86
On the basis of this model, representations of objects should be impaired principally in the verbal domain after left ATL damage, as the intact right ATL is predicted to be sufficient to sustain relevant nonverbal associations, unless the task is unusually difficult or unfamiliar. A truly amodal impairment of both verbal and nonverbal associations would, therefore, require bilateral damage to the ATL. In keeping with these hypotheses, the impaired naming of famous faces (a verbal association task) in PPA was found to correlate with atrophy of the left ATL, whereas additional difficulty in recognizing the identity of the depicted person (an object recognition task) correlated with bilateral atrophy of the ATL.87 Thus, the pan-modal functionality attributed to the anterior temporal cortex might reflect, at least in part, the inclusion of patients with extensive damage to the ATL in both cerebral hemispheres.88,89 This conclusion is also consistent with a more recent ‘graded connectivity’ model of the ATL.90 In summary, rather than a pan-modal semantic hub, the ATL seems to contain an overlapping mosaic of transmodal areas,86 each displaying domain-selective specializations, with a strong leftward asymmetry for verbal representation.
The diversity of opinion on the functional affiliations of the ATL is not surprising considering the architectural and hodological (pathway) heterogeneity revealed by experimental neuroanatomical studies in monkeys.91 Developments in postmortem chemoarchitectonic analyses,92 and in vivo diffusion tensor imaging combined with functional MRI,93–95 are beginning to unravel analogous principles of organization in the human ATL.
Where, if anywhere, is Wernicke’s area?
According to established teaching, the destruction of a region of the brain known as Wernicke’s area causes severe impairments in both word and sentence comprehension.69,96–98 The cortical area with the functionality attributed to Wernicke’s area has been located variably in the superior temporal gyrus, inferior parietal lobule and, more recently, in the posterior portion of the middle temporal gyrus.98–105 Most of the literature on the location of Wernicke’s area is related to studies of patients with cerebrovascular lesions; however, clinicopathological correlations in PPA are difficult to reconcile with this literature.
The main challenge comes from PPA-L, in which atrophy sites can encompass nearly all proposed locations of Wernicke’s area,26,106 but without causing substantial impairments of single-word comprehension (Figure 3b – c). Word-comprehension impairments reminiscent of Wernicke aphasia arise in patients with PPA only when the sites of peak atrophy include the left ATL, a region that has never been proposed as a potential location for Wernicke’s area. Conceivably, the preservation of word comprehension in PPA-L could be attributed to partial neuronal preservation at atrophy sites (Figure 1) and to compensatory functional reorganization. Nonetheless, clinicopathological correlations in PPA also raise the alternative possibility that the neural bottleneck for word comprehension might occupy a more anteriorly located part of the temporal lobe than previously envisaged. This possibility is supported by evidence from voxel-based lesion–symptom-mapping studies in groups of aphasic patients with cerebrovascular lesions.60
Such an alternative organization of the language network would centre on the rostrally directed sensory-fugal synaptic hierarchies known to exist in the temporal lobe and on a Bayesian account of their top-down regulation.86,107,108 According to this view, auditory and visual word forms, encoded in the superior temporal gyrus109,110 and inferotemporal cortex,111 respectively, would trigger widespread activations of the temporal cortex extending all the way into the temporal polar region. In the left hemisphere, this entire neural matrix would be engaged in linking the word form to the distributed associations that collectively give the word meaning. Within this matrix, the rostrally directed synaptic hierarchies are expected to mediate increasing refinement of such associations.86,112,113 Furthermore, the anterior (downstream) parts of the sensory-fugal synaptic chain are hypothesized to generate contextually relevant top-down signals that are based on prior knowledge and that guide the progression of word representations from generic to increasingly specific forms.108
According to this hypothetical model, even partial neuronal loss in the left temporal lobe would initially impair the overt naming of objects at a stage when the computationally simpler task of word recognition— tested by word–object matching—remains intact. This account could potentially explain why one-way retrieval anomia is the most common language abnormality observed in neurological diseases affecting the left temporal lobe.64,66,68,114–116 When neuronal loss in the anterior and polar components of the left temporal lobe exceeds a certain threshold, the top-down projections that mediate the gradual honing of word representations might be undermined. Consequently, word recognition (comprehension) in PPA-S is initially impaired at the most computationally demanding, specific level of representation at early stages of disease, and eventually at coarser generic levels as the disease progresses and left anterior temporal lobe components of the language network are increasingly compromised. Sentence comprehension, based on the ability to interpret grammatical words and structure, can remain relatively preserved in this context if the constituent nouns can be decoded even at a generic level. In some patients with PPA-S, the impairments can eventually encompass sentence comprehension and become as severe as those described in Wernicke aphasia. However, repetition tends to remain preserved in PPA-S, as the initial sites of peak atrophy rarely extend into the posterior parts of the superior temporal gyrus and TPJ, areas commonly damaged by cerebrovascular accidents associated with Wernicke aphasia.
According to this model, and as supported by clinic-pathological correlations in PPA-L, cortical atrophy confined to the posterior temporal lobe locations traditionally assigned to Wernicke’s area might not be enough to cause the severe word-comprehension impairment associated with Wernicke aphasia, probably because the remaining parts of the temporal lobe can continue to supply the ATL with sufficient stimulus to trigger the top-down guidance of word recognition. This hypothesis is consistent with experimental evidence that residual verbal comprehension abilities in patients with Wernicke aphasia is sustained by ATL activity.80,117 Although loss of neurons in the posterior temporal lobe regions does disrupt the encoding of word meaning, as indicated by excessive semantic interference effects in PPA-L,61 the resultant word-comprehension difficulties are substantially less severe than the impairments associated with Wernicke aphasia. The cause of this apparent discrepancy may lie in the differential involvement of the white matter. White matter abnormalities are common in patients with PPA, but are never as severe as those in patients with vascular disease. Perhaps, therefore, lesions in traditional locations of Wernicke’s area lead to severe word-comprehension impairments only if underlying white matter pathways are damaged (as commonly occurs in cerebrovascular lesions), such that the ATL becomes massively disconnected from posterior regions of the temporal lobe.75,117 Such disconnection would reduce functional effectiveness of the ATL either as the downstream platform for relevant synaptic hierarchies, or as the source of key top-down projections.
In summary, word meaning seems to be subserved by a neuronal matrix—unimodal as well as transmodal— that is spread throughout the left temporal lobe. Minimal dysfunction at any point within this matrix causes retrieval anomia, whereas progressive damage to its anterior and polar components interferes with critical top-down refinement of associations and leads to gradually intensifying word-comprehension impairments. Thus, the notion of Wernicke’s area as a cortical hub of the posterior temporal lobe with a critical role in both word and sentence comprehension will probably need considerable revision.
Neuropathology of PPA and its asymmetry
The aphasic presentation and sparing of explicit memory in PPA led to the assumption that the pathological basis of this syndrome would not be Alzheimer disease (AD). However, numerous autopsy series have provided no support for this expectation.25,32,118–126 In fact, a report of 58 consecutive autopsies revealed AD pathology in 45% of PPA cases;29 the remaining 55% of cases had evidence of FTLD, distributed approximately equally between TAR DNA-binding protein 43 (TDP-43) proteinopathy (FTLD-TDP) and tauopathy (FTLD-tau). The most common feature of PPA in this study was the asymmetrically greater atrophy, neuronal loss and disease-specific proteinopathy in the language-dominant cerebral hemisphere, which was predominantly the left hemisphere in right-handed individuals (Figure 5); conversely, in most of the left-handed patients, neurodegeneration tended to be more severe in the right hemisphere.
Figure 5.
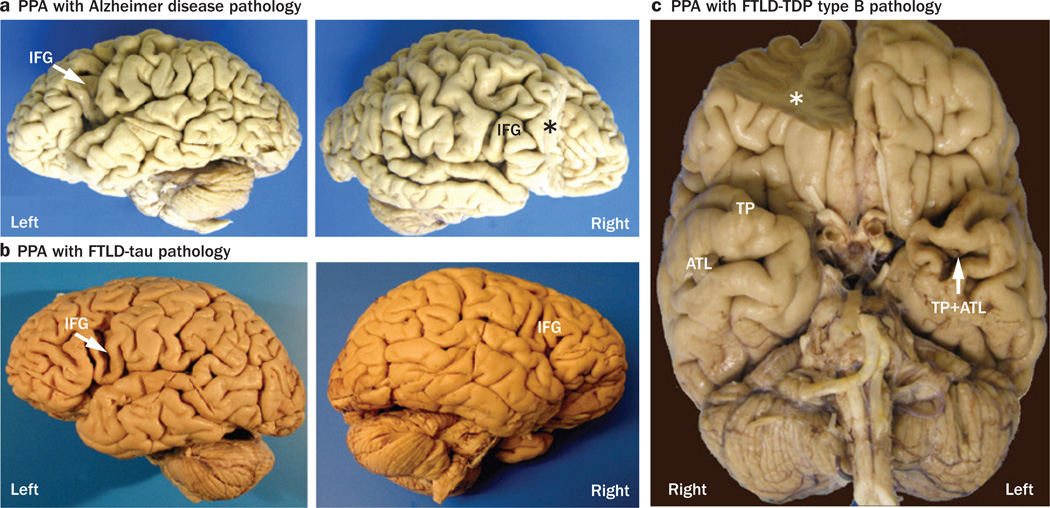
Asymmetry of brain atrophy in PPA. These photographs demonstrate the asymmetric atrophy of the left-hemisphere language network associated with three different types of neuropathology in three right-handed patients with PPA.29 a | Alzheimer disease pathology in a woman who died at the age of 80 years, 7 years after the onset of PPA; the left IFG (arrow) is atrophic, but the right IFG is not. b | FTLD-tau pathology of the corticobasal degeneration type in a woman with PPA onset at the age of 72 years, who subsequently died at the age of 78 years; again, the left IFG (arrow) is atrophic, but the right IFG is not. c | Type B FTLD-TDP in a man with onset of PPA at the age of 66 years, who died 6 years later; the left TP and ATL are atrophic, but the homologous areas of the right hemisphere are not. Asterisks indicate brain regions that were removed for biochemical analyses. Abbreviations: ATL, anterior temporal lobe; FTLD-tau, frontotemporal lobar degeneration with tau protein pathology; FTLD-TDP, frontotemporal lobar degeneration with TAR DNA-binding protein 43 pathology; IFG, inferior frontal gyrus; PPA, primary progressive aphasia; TP, temporal pole. Permission obtained from Oxford University Press © Mesulam, M.-M. et al. Brain 137, 1176–1192 (2014).
Though surprisingly common, Alzheimer disease (AD) pathology in PPA displays atypical features. In ‘typical’ AD, neurodegeneration is most extensive in the hippocampo-entorhinal region, a pattern that provides the basis for the Braak staging of AD pathology and explains the characteristic impairment of memory function.127 Furthermore, in typical AD, no consistent hemispheric asymmetry of neuropathology is present, the apolipoprotein E ε4 (APOE ε4) allele is a major risk factor, symptoms usually emerge after the age of 65 years, and a female preponderance tends to be observed. The AD pathology in PPA has a completely different set of associations: the Braak pattern of hippocampo-entorhinal prominence becomes tilted in favour of the neocortex; hemispheric asymmetry is evident, with more-intense neurofibrillary pathology in the language-dominant hemisphere (Figure 6); the APOE ε4 allele is not a risk factor; symptoms usually emerge before 65 years of age; and the disease is slightly more prevalent in males. The neuropathology of PPA has, therefore, shown that AD is not a uniform entity, and that it has distinct biological subtypes, one of which accounts for nearly half of all PPA cases.29,128,129
Figure 6.
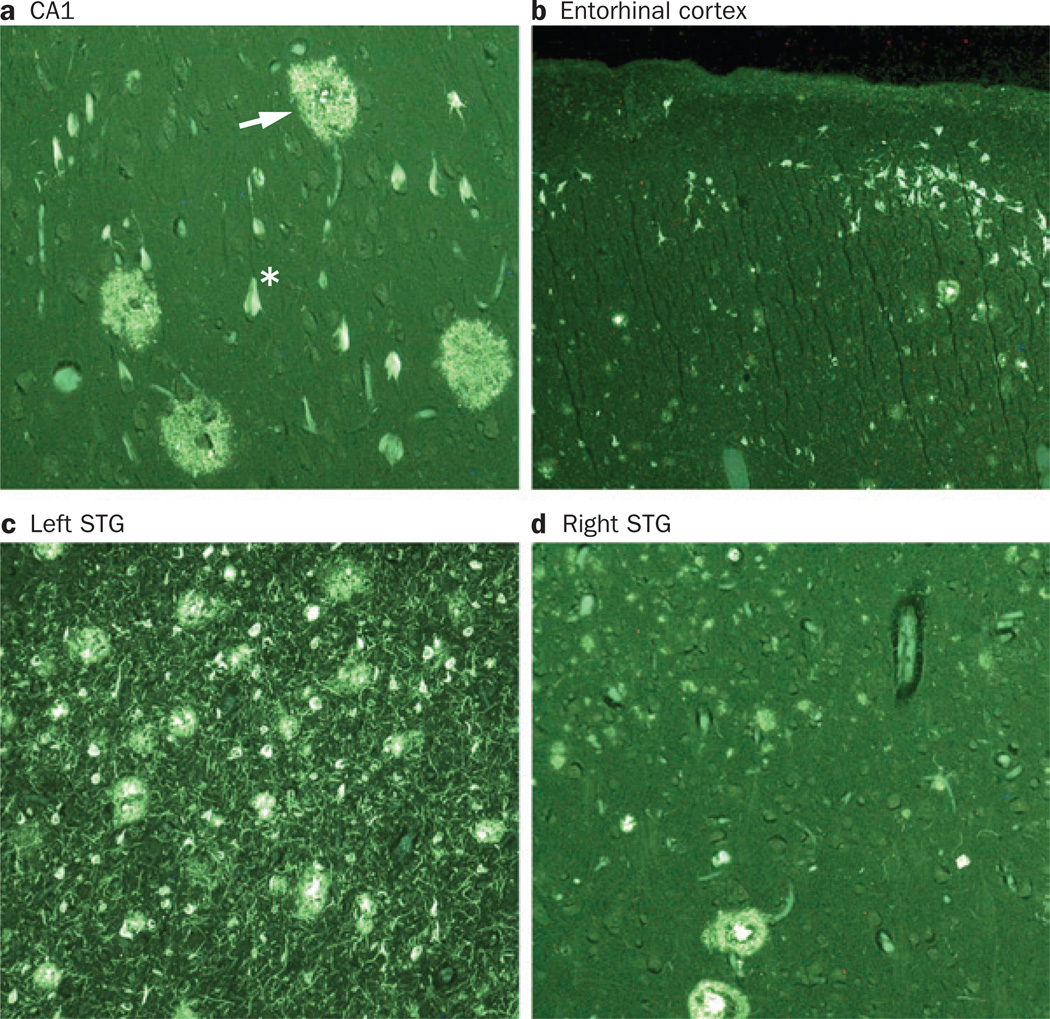
Atypical distribution of AD pathology in PPA. Photomicrographs show fluorescent thioflavin-S-stained tissue from different regions of the brain of a man with PPA, with onset at age 61 years. The patient died aged 71 years and the autopsy revealed AD pathology. Plaques (arrow) and neurofibrillary tangles (asterisk) were detected in the a | CA1 area of the hippocampus, b | entorhinal cortex, c | STG of the left hemisphere and d | STG of the right hemisphere. Panels a and b show that the lesion density is lower in the CA1 and entorhinal cortex than in neocortical areas such as the STG. This pattern of lesion distribution does not conform to the principles of Braak staging in Alzheimer disease.127 Panels c and d demonstrate a higher density of plaques and neurofibrillary tangles in the STG of the left hemisphere than in the homologous region on the right side. Magnification was 100×, except in the entorhinal cortex (40× magnification). Abbreviations: AD, Alzheimer disease; PPA, primary progressive aphasia; STG, superior temporal gyrus. Permission obtained from Oxford University Press © Mesulam, M.-M. et al. Brain 137, 1176–1192 (2014).
Each type of pathology encountered in the series of 58 autopsies of patients with PPA displayed characteristic—but not invariant—clinical presentations.29 The most common aphasic manifestation was the logopenic form in patients with AD pathology, and the agrammatic form in patients with various forms of FTLD-tau pathology.29 Moreover, the progressive supranuclear palsy form of FTLD-tau was frequently associated with prominent speech abnormalities together with agrammatism, and FTLD-TDP type C frequently caused selective left ATL atrophy and PPA-S.29 However, no clinical pattern was found to be pathognomonic of a specific type of neuropathology. The diagnosis of the underlying neuropathology in living patients with PPA, therefore, requires the use of biomarkers. At present, amyloid-β imaging by PET and the quantitation of tau and amyloid-β in cerebrospinal fluid can determine the likelihood of AD pathology in patients with PPA (Figure 7). In the near future, tau imaging using PET will probably also facilitate differentiation of FTLD-tau from FTLD-TDP pathologies in patients with PPA who are found to be negative for biomarkers of AD pathology.
Figure 7.
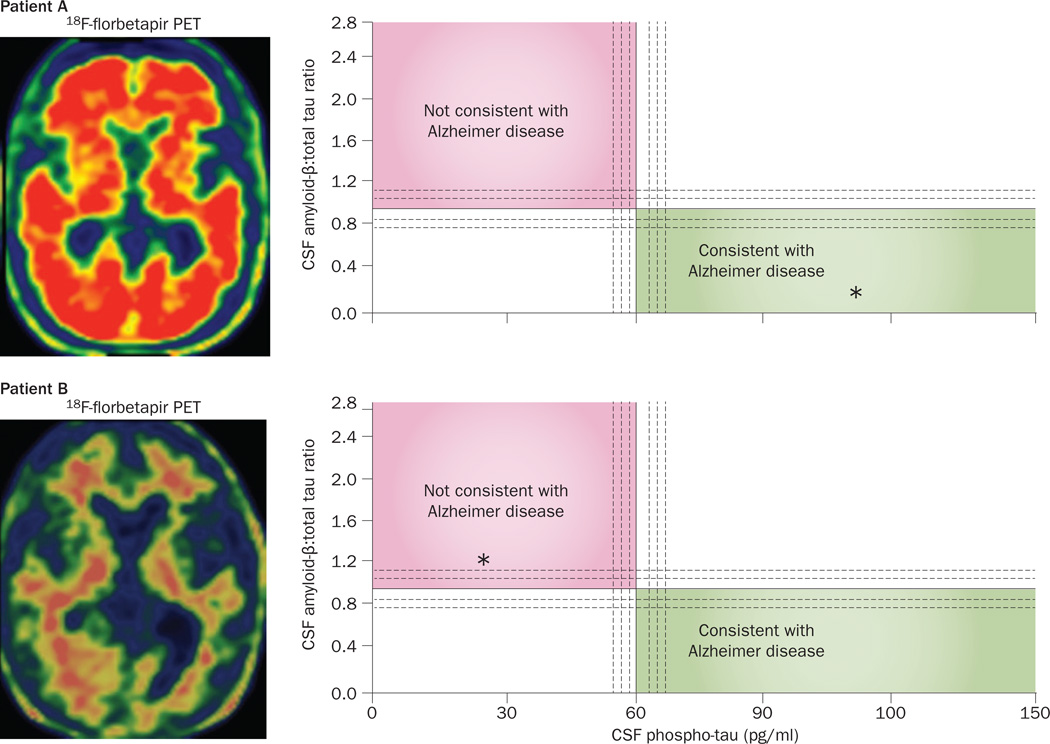
Biomarker assessments to gauge the likelihood of Alzheimer pathology in PPA. Data from biomarker evaluations in two patients with a logopenic pattern of PPA at the initial clinical visit. Axial scans on the left show the results of PET imaging using an Aβ-binding tracer, 18F-florbetapir. Intense red areas on scan from Patient A are indicative of a positive PET result for Aβ plaques, indicating AD pathology, whereas the scan from Patient B demonstrates a negative result of this biomarker test. Right-hand panels show CSF levels of phospho-tau on the x-axis and a proprietary ratio of CSF Aβ:total tau on the y-axis (test performed by Athena Diagnostics). The diagnostic implications of values plotted on this graph have been determined empirically.165 Asterisks in the right-hand panels denote these values measured in CSF samples from the two patients. Patient A had high CSF levels of phospho-tau and a low CSF Aβ:total tau ratio, consistent with AD pathology, confirmed at postmortem examination. Both the CSF and PET results for Patient B are inconsistent with AD pathology and lead us to predict that the autopsy will reveal a form of frontotemporal lobar degeneration underlying PPA in this individual. Abbreviations: Aβ, amyloid-β; AD, Alzheimer disease; CSF, cerebrospinal fluid; PPA, primary progressive aphasia.
Support for the agrammatic–logopenic split
The validity of delineating a logopenic variant of PPA has been questioned.27,130 In our series of 58 autopsied patients with PPA, the status of grammatical ability divided the group of 49 patients who would have fulfilled the PNFA designation into two subsets (Figure 8). In the agrammatic group (PPA-G), FTLD-tau neuropathology was three times more prevalent than either Alzheimer or FTLD-TDP pathologies; in the absence of agrammatism (PPA-L with or without repetition impairment), AD pathology was observed around twice as frequently as FTLD-tau or FTLD-TDP pathologies.29 These findings, together with differential patterns of brain atrophy (Figure 3) and disparate trajectories of clinical progression,34 support the biological validity of the agrammatic–logopenic distinction in PPA.
Figure 8.
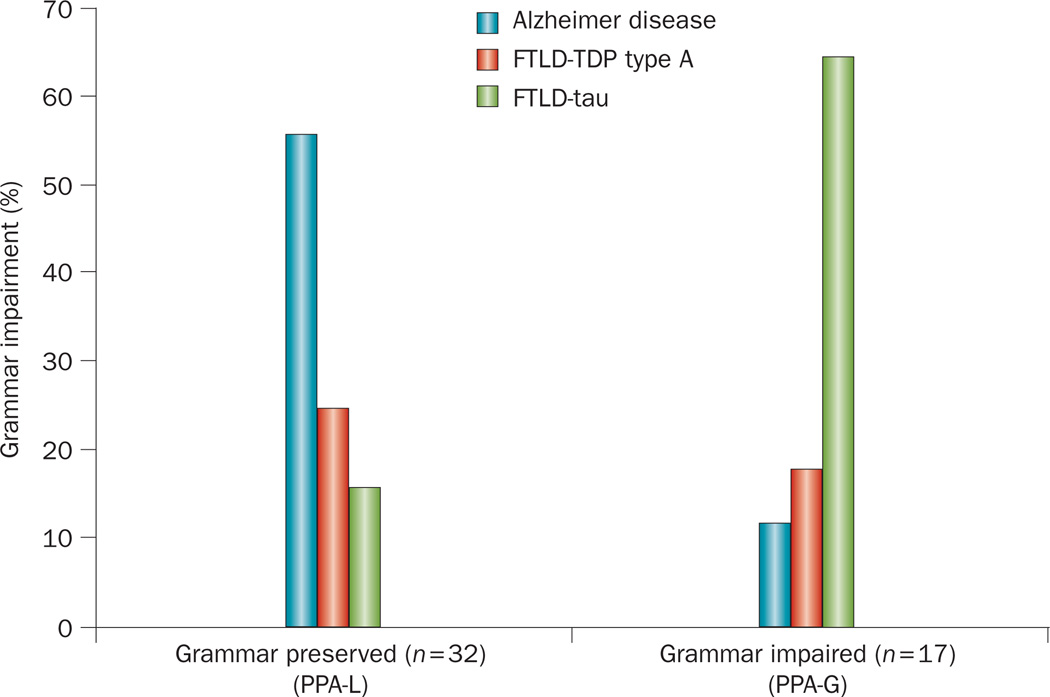
Differential patterns of neuropathology in PPA-G and PPA-L. 49 PPA patients without word comprehension impairments, most of whom would fit the ‘progressive nonfluent aphasia’ designation, were divided into two groups on the basis of grammaticality. The two groups had significantly different proportions of neuropathology types (Fisher’s exact test, P = 0.001). The group of patients with preserved grammar (consisting of PPA-L patients with and without impairment in repetition of phrases and sentences) mostly had Alzheimer pathology, whereas the group with impaired grammar (PPA-G) had mostly FTLD-tau pathology. Abbreviation: FTLD-tau, frontotemporal lobar degeneration with tau protein pathology; FTLD-TDP, frontotemporal lobar degeneration with TAR DNA-binding protein 43 pathology; PPA, primary progressive aphasia; PPA-G, agrammatic PPA; PPA-L, logopenic PPA.
PPA susceptibility factors
Asymmetry of neurodegeneration in PPA persists until the time of death (Figure 5). This asymmetry cannot be attributed to the cellular or molecular pathogenesis of the underlying disease, as a lack of symmetry in neurodegeneration is observed for all types of PPA neuropathology. Furthermore, each of the neuropathological mechanisms encountered in PPA can also cause nonaphasic syndromes such as behavioural variants of frontotemporal dementia (FTD) or amnestic dementias in other patients. Indeed, PPA was one of the first clinical entities to highlight the general principle that disease manifestations reflect the anatomical distribution rather than the cellular nature of the underlying neurodegenerative disease.131,132 Current evidence suggests that the distribution of neuronal loss in any neurodegenerative entity is likely to reflect the outcome of complex interactions among patient-specific factors that delineate anatomical loci of least resistance, network-specific factors that constrain pathways of progression, and disease-specific factors that determine the cellular nature of the pathology.133–135 These factors probably explain why PPA results from a range of neurodegenerative pathologies, why each of these processes leads to predominant but not invariant aphasia subtypes, and why the disease spreads preferentially (although not exclusively) within the language network.136
The patient-specific factors that cause multiple disease pathologies to be expressed asymmetrically in the language-dominant hemisphere of patients with PPA remain to be identified;137 however, a potentially important finding in this regard was that patients with PPA had a higher frequency of personal or family history of learning disability, including dyslexia, compared with healthy individuals or patients with other dementia syndromes.138,139 This association with learning disabilities that included dyslexia stimulated speculation that at least some cases of PPA could reflect the tardive manifestation of a developmental or genetic vulnerability of the language network. This susceptibility can remain compensated during much of adulthood, but might eventually provide a locus of least resistance for the expression of an independently arising neurodegenerative process. The same vulnerability could potentially be expressed as developmental dyslexia in some family members, but as an involutionary process that leads to PPA in others. Autoimmunity has been implicated as another potential factor influencing patient-specific vulnerabilities to PPA.140,141
In most patients with PPA, the underlying neurodegenerative disease arises sporadically; however, in a few cases, PPA has been reported in families with disease-causing mutations in the MAPT, GRN, C9orf72 or PSEN1 genes, which encode tau, granulin, protein C9orf72 and presenilin 1, respectively.142–145 In these families, some members develop PPA, whereas others develop nonaphasic dementias. Exceptions to this pattern have been described in two families with GRN mutations and FTLD-TDP neuropathology, in which all affected members (three of four siblings in one kindred, and two of three in the other) had a clinical picture of PPA.146
Of all the dominant mutations associated with PPA, those within GRN are by far the most prevalent. Interestingly, asymptomatic carriers of a disease-causing GRN mutation (Thr272fsX10) seem to display asymmetric distortions of resting state frontoparietal connectivity in the left hemisphere of the brain when compared with family members who do not carry the mutation.147 Such asymmetries, if also found to be associated with other GRN mutations, might account for the high prevalence of PPA in these kindreds. Nevertheless, a different explanation is needed to account for the observation that identical GRN mutations lead to behavioural variant FTD (bvFTD) in some individuals and PPA in other members of the same family. A potential clue came from the finding that levels of RAP1GAP mRNA were significantly lower in patients with the GRN Thr272fsX10 mutation who developed bvFTD than in mutation carriers who developed PPA (P = 0.049).148 Although the majority of PPA cases arise in the absence of known mutations, these results justify the search for potential molecular factors that underlie the selective vulnerability of the language-dominant cerebral hemisphere in this syndrome.
PPA temporal trajectory and treatments
Gradual intensification of language impairment is the hallmark of PPA.15,149,150 Some patients develop additional cognitive and behavioural impairments within a few years after clinical onset of PPA, whereas language impairment remains the only conspicuous obstacle to customary daily activities over many years in other patients (Box 4). Even as language functions deteriorate, many patients continue to enjoy complex nonverbal activities.151,152 Examples that we have come across include woodworking, breeding rare birds, gardening, sculpting, and photography. Some of our patients started painting after being diagnosed with PPA, and produced works of considerable artistry (Figure 9); others became devoted to master-level Sudoku, or developed a passion for 1,000–3,000-piece jigsaw puzzles.
Box 4. Progression trajectories of PPA.
PPA in general
-
▪
During the initial stages of disease, at which time the patient’s symptoms simultaneously fulfil the designation of both nonamnestic mild cognitive impairment and PPA, most daily living activities remain unaffected
-
▪
At advanced stages of PPA, prominent impairments of additional cognitive domains and motor function can emerge; in this setting, the designation of PPA-plus (PPA+) can be used to indicate that although the disease started as PPA, the aphasia is no longer the only salient feature
PPA-S
-
▪
In PPA-S, the spread of atrophy to the anterior insula, posterior orbitofrontal cortex and contralateral anterior temporal lobe can lead to the emergence of aberrant behaviours and associative agnosias; the addition of agnosia to the aphasia adheres to the syndromic definition of semantic dementia. In this context, the semantic dementia pattern represents a PPA+ stage
-
▪
Other patients, who present with the semantic dementia pattern in the initial disease stages, do not fulfil the criteria for a PPA diagnosis (Box 3); thus, syndromic classification is sensitive to the temporal pattern of symptom emergence, a pattern that reflects the anatomical trajectory of disease progression
PPA-G
-
▪
In PPA-G, the spread of atrophy to dorsolateral prefrontal cortex, motor areas and basal ganglia can lead to the emergence of impaired executive functions, motor speech abnormalities, or movement disorders that are characteristic of dysexecutive, speech apraxia, corticobasal degeneration, and progressive supranuclear palsy syndromes, as additional manifestations of PPA+
PPA-L
-
▪
The trajectory of PPA-L is the most variable of all the PPA subtypes
-
▪
In some patients, the PPA-L pattern represents a prodromal stage of PPA-G or PPA-S
-
▪
In other patients, the spread of atrophy into the medial temporal lobe leads to episodic memory impairments as part of the PPA+ stage
-
▪
A few patients show a persistent PPA-L pattern for many years; this indolent form of PPA-L seems to have the closest association with Alzheimer pathology
Abbreviation: PPA, primary progressive aphasia; PPA-G, agrammatic PPA; PPA-L, logopenic PPA; PPA-S, semantic PPA.
Figure 9.
Artwork by R.S., a patient with agrammatic primary progressive aphasia. She was 60 years of age at the time of diagnosis and started painting 2 years later. Image reproduced with permission of the artist.
Treatment in PPA can be divided into symptomatic and aetiological components. The symptomatic approach necessitates thorough evaluation by a knowledgeable speech–language pathologist, who can subsequently work with the patient and their family to maximize communicative effectiveness, and also assess the potential usefulness of assistive software and hardware.153 Education and life-enrichment interventions tailored to fit the individual patient’s strengths and weaknesses are important aspects of patient care.154 The aetiological component of patient care in PPA revolves around the judicious use of clinical subtyping and biomarker information to surmise the nature of the underlying disease process. On the basis of this information, the clinician can decide whether to use AD medications, or to channel the patient into clinical trials relevant to AD or FTLD. Depression is frequently observed in patients with PPA, and should be treated with appropriate interventions.155
One of the most distinctive aspects of PPA is the prolonged resilience of the nondominant (usually right) cerebral hemisphere and its potentially compensatory participation in language tasks.21 Thus, whether the application of direct-current stimulation or repetitive transcranial magnetic stimulation can somehow promote beneficial neuroplasticity within the language network is a pertinent question. Encouraging effects of these interventions have been reported in isolated case studies, and now need to be replicated in larger systematic investigations.156–158
Conclusions
Although the frequency of the diagnosis is increasing, PPA remains a relatively rare syndrome; therefore, patients, clinicians and families might have limited access to appropriate information and assistance. A website dedicated to PPA, the International PPA Connection (IMPPACT),159 has been established to serve as a registry for patients and resources. The purpose of this online resource is to improve the access of patients and clinicians to relevant local resources around the world and to facilitate the implementation of collaborative clinical trials when promising therapeutic interventions become available in the future. The site also provides demonstrations of key diagnostic features and a section on frequently asked questions. A major long-term goal in PPA research is the development of effective treatments. In the meantime, the heterogeneous language disturbances associated with PPA, and their complex anatomical correlates, continue to provide new insights into core aspects of aphasia research. The selective degeneration of the language-dominant cerebral hemisphere also offers unique opportunities for exploring the biology of selective vulnerability and hemispheric asymmetry in the human brain.
Key points.
-
▪
Primary progressive aphasia (PPA) is a clinical syndrome caused by selective neurodegeneration of the language-dominant cerebral hemisphere, thus affecting the language network
-
▪
The language disorder manifest in patients with PPA can take the form of agrammatic, logopenic or semantic aphasia, depending on the anatomical distribution of cortical atrophy
-
▪
PPA can be caused by several types of neuropathology, including Alzheimer disease and frontotemporal lobar degeneration; these diseases tend to be associated with specific variants of PPA
-
▪
Concepts relating to Wernicke’s area and anterior temporal lobe function need to be revised on the basis of the relationships identified between the clinical characteristics and neuroanatomy of peak atrophy sites in PPA
-
▪
PPA susceptibility, aetiology and pathogenesis, and the asymmetry of cerebral atrophy in particular, are poorly understood and require further elucidation
-
▪
Effective PPA treatments are urgently needed; development of such treatments should be considered a research area of importance
Review criteria.
A literature review was conducted, based on the authors’ personal libraries of historical and contemporary works on language, aphasia and neurodegeneration. In addition, PubMed and Google Scholar databases were searched for relevant publications, using key terms including “primary progressive aphasia”, “language”, “dementia” and “neurodegeneration” in various combinations. No restrictions were used regarding article type, language or publication date.
Acknowledgements
The work of the authors is supported by the National Institute on Deafness and Communication Disorders (grants DC008552 to M.-M.M. and DC01948 to C.K.T.), the NIH National Institute on Aging (grant AG13854 for the Alzheimer Disease Centre to M.-M.M.), and the NIH National Institute on Neurological Disease and Stroke (grant NS075075 to E.J.R.) Imaging was performed at the Northwestern University Centre for Translational Imaging.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
All authors researched literature for the article and reviewed and/or edited the manuscript before submission. M.-M.M. wrote the article.
References
- 1.Hickok G, Poeppel D. The cortical organization of speech processing. Nat. Rev. Neurosci. 2007;8:293–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- 2.Wilson SM, et al. Syntactic processing depends on dorsal language tracts. Neuron. 2011;72:397–403. doi: 10.1016/j.neuron.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz MF, Faseyitan O, Kim JG, Coslett HB. The dorsal stream contribution to phonological retrieval in object naming. Brain. 2012;135:3799–3814. doi: 10.1093/brain/aws300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiang H-D, Fonteijn HM, Norris DG, Hagoort P. Topographical functional connectivity pattern in the perisylvian language networks. Cereb. Cortex. 2010;20:549–560. doi: 10.1093/cercor/bhp119. [DOI] [PubMed] [Google Scholar]
- 5.Saur D, et al. Ventral and dorsal pathways for language. Proc. Natl Acad. Sci. USA. 2008;105:18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broca P. Localisations des fonctions cérébrales. Siège du langage articulé [French] Bulletin de la Sociét d’Anthropologieé. 1863;4:200–204. [Google Scholar]
- 7.Wernicke C. Der Aphasische Symptomencomplex: Eine Psychologische Studie Auf Anatomischer Basis. Cohn and Weigert; 1874. [Google Scholar]
- 8.Pick A. Über die Beziehungen der senilen Hirnatrophie zur Aphasie [German] Prager Medizinische Wochenschrift. 1892;17:165–167. [Google Scholar]
- 9.Sérieux P. Sur un cas de surdité verbale pure [French] Revue de Medecine. 1893;13:733–750. [Google Scholar]
- 10.Franceschi F. Gliosi perivasculare in un caso de demenza afasica [Italian] Annali di Neurologia. 1908;26:281–290. [Google Scholar]
- 11.Rosenfeld M. Die partielle Grosshirnatrophie [German] Journal of Psychology and Neurology. 1909;14:115–130. [Google Scholar]
- 12.Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann. Neurol. 1982;11:592–598. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- 13.Mesulam MM. Primary progressive aphasia —differentiation from Alzheimer’s disease. Ann. Neurol. 1987;22:533–534. doi: 10.1002/ana.410220414. [DOI] [PubMed] [Google Scholar]
- 14.Mesulam M-M, Weintraub S. Ch. 54. In: Duyckaerts C, Litvan I, editors. Handbook of Clinical Neurology: Dementias. 3rd edn. Vol. 89. Elsevier; 2008. pp. 573–587. [DOI] [PubMed] [Google Scholar]
- 15.Weintraub S, Rubin NP, Mesulam MM. Primary progressive aphasia. Longitudinal course, neuropsychological profile, and language features. Arch. Neurol. 1990;47:1329–1335. doi: 10.1001/archneur.1990.00530120075013. [DOI] [PubMed] [Google Scholar]
- 16.Chawluk JB, et al. Slowly progressive aphasia without generalized dementia: studies with positron emission tomography. Ann. Neurol. 1986;19:68–74. doi: 10.1002/ana.410190112. [DOI] [PubMed] [Google Scholar]
- 17.Mesulam M-M. Primary progressive aphasia. Ann. Neurol. 2001;49:425–432. [PubMed] [Google Scholar]
- 18.Gorno-Tempini ML, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann. Neurol. 2004;55:335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonty SP, et al. Primary progressive aphasia: PPA and the language network. Ann. Neurol. 2003;53:35–49. doi: 10.1002/ana.10390. [DOI] [PubMed] [Google Scholar]
- 20.Sonty SP, et al. Altered effective connectivity within the language network in primary progressive aphasia. J. Neurosci. 2007;27:1334–1345. doi: 10.1523/JNEUROSCI.4127-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vandenbulcke M, Peeters R, Van Hecke P, Vandenberghe R. Anterior temporal laterality in primary progressive aphasia shifts to the right. Ann. Neurol. 2005;58:362–370. doi: 10.1002/ana.20588. [DOI] [PubMed] [Google Scholar]
- 22.Mesulam M-M, Weintraub S. Ch. 6. In: Rossor MN, editor. Unusual Dementias. Vol. 1. Baillière Tindall; 1992. pp. 583–609. [Google Scholar]
- 23.Neary D, et al. Frontotemporal lobar degeneration. A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 24.Gorno-Tempini ML, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris JM, et al. Classification and pathology of primary progressive aphasia. Neurology. 2013;81:1–8. doi: 10.1212/01.wnl.0000436070.28137.7b. [DOI] [PubMed] [Google Scholar]
- 26.Mesulam M-M, Wieneke C, Thompson C, Rogalski E, Weintraub S. Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain. 2012;135:1537–1553. doi: 10.1093/brain/aws080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sajjadi SA, Patterson K, Arnold RJ, Watson PC, Nestor PJ. Primary progressive aphasia: a tale of two syndromes and the rest. Neurology. 2012;78:1670–1677. doi: 10.1212/WNL.0b013e3182574f79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mesulam M, Weintraub S. Is it time to revisit the classification of primary progressive aphasia? Neurology. 2014;82:1108–1109. doi: 10.1212/WNL.0000000000000272. [DOI] [PubMed] [Google Scholar]
- 29.Mesulam M-M, et al. Asymmetry and heterogeneity of Alzheimer and frontotemporal pathology in primary progressive aphasia. Brain. 2014;137:1176–1192. doi: 10.1093/brain/awu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wicklund MR, et al. Quantitative application of the primary progressive aphasia consensus criteria. Neurology. 2014 doi: 10.1212/WNL.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mesulam M, et al. Quantitative template for subtyping primary progressive aphasia. Arch. Neurol. 2009;66:1545–1551. doi: 10.1001/archneurol.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grossman M. The non-fluent/agrammatic variant of primary progressive aphasia. Lancet Neurol. 2012;11:545–555. doi: 10.1016/S1474-4422(12)70099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson CK, et al. Syntactic and morphosyntactic processing in stroke-induced and primary progressive aphasia. Behav. Neurol. 2013;26:35–54. doi: 10.3233/BEN-2012-110220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson CK, Ballard KJ, Tait ME, Weintraub S, Mesulam M. Patterns of language decline in non-fluent primary progressive aphasia. Aphasiology. 1997;11:297–321. [Google Scholar]
- 35.Wilson SM, et al. Neural correlates of syntactic processing in the nonfluent variant of primary progressive aphasia. J. Neurosci. 2010;30:16845–16854. doi: 10.1523/JNEUROSCI.2547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson CK, et al. Dissociations between fluency and agrammatism in primary progressive aphasia. Aphasiology. 2012;26:20–43. doi: 10.1080/02687038.2011.584691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hillis AE, Tuffiash E, Caramazza A. Modality-specific deterioration in naming verbs in nonfluent primary progressive aphasia. J. Cogn. Neurosci. 2002;14:1099–1108. doi: 10.1162/089892902320474544. [DOI] [PubMed] [Google Scholar]
- 38.Hillis AE, Oh S, Ken L. Deterioration of naming nouns versus verbs in primary progressive aphasia. Ann. Neurol. 2004;55:268–275. doi: 10.1002/ana.10812. [DOI] [PubMed] [Google Scholar]
- 39.Thompson CK, Lukic S, King MC, Mesulam M-M, Weintraub S. Noun and verb production and comprehension in stroke-induced and primary progressive aphasia: an introduction to the Northwestern Naming Battery. Aphasiology. 2012;26:632–655. doi: 10.1080/02687038.2012.676852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson CK, Mack JE. Grammatical impairments in PPA. Aphasiology. 2014;28:1018–1037. doi: 10.1080/02687038.2014.912744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Josephs KA, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129:1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson CK. Northwestern Assessment of Verbs and Sentences (NAVS). Unpublished developmental version. Flintbox. 2011 [online], http://northwestern.flintbox.com/public/project/9299/
- 43.Wilson SM, et al. Connected speech production in three variants of primary progressive aphasia. Brain. 2010;133:2069–2088. doi: 10.1093/brain/awq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weintraub S, et al. The Northwestern anagram test: measuring sentence production in primary progressive aphasia. Am. J. Alzheimers Dis. Other Dem. 2009;24:408–416. doi: 10.1177/1533317509343104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson CK, Weintraub S, Mesulam M-M. The Northwestern Anagram Test (NAT) Flintbox. 2012 [online], http://flintbox.com/public/project/19927/
- 46.Grodzinsky Y. The neurology of syntax: language use without Broca’s area. Behav. Brain Sci. 2000;23:1–17. doi: 10.1017/s0140525x00002399. [DOI] [PubMed] [Google Scholar]
- 47.Meltzer JA, McArdle JJ, Schafer RJ, Braun AR. Neural aspects of sentence comprehension: syntactic complexity, reversibility and reanalysis. Cereb. Cortex. 2010;20:1853–1864. doi: 10.1093/cercor/bhp249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papoutsi M, Stamatakis EA, Griffiths J, Marslen-Wilson WD, Tyler LK. Is left frontotemporal connectivity essential for syntax? Effective connectivity, tractography and performance in left-hemisphere damaged patients. NeuroImage. 2011;58:656–664. doi: 10.1016/j.neuroimage.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 49.Koester D, Schiller NO. The functional neuroanatomy of morphology in language production. NeuroImage. 2011;55:732–741. doi: 10.1016/j.neuroimage.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 50.Ben-Shachar M, Hendler T, Kahn I, Ben-Bashat D, Grodzinsky Y. The neural reality of syntactic transformations. Psychol. Sci. 2003;14:433–440. doi: 10.1111/1467-9280.01459. [DOI] [PubMed] [Google Scholar]
- 51.Davis C, et al. Speech and language functions that require a functioning Broca’s area. Brain Lang. 2008;105:50–58. doi: 10.1016/j.bandl.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 52.Sahin NT, Pinker S, Cash SS, Schomer DL, Halgren E. Sequential processing of lexical, grammatical, and phonological information within Broca’s area. Science. 2009;326:445–449. doi: 10.1126/science.1174481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rogalski E, et al. Anatomy in language impairments in primary progressive aphasia. J. Neurosci. 2011;31:3344–3350. doi: 10.1523/JNEUROSCI.5544-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Catani M, et al. A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain. 2013;136:2619–2628. doi: 10.1093/brain/awt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sajjadi SA, Patterson K, Nestor PJ. Logopenic, mixed, or Alzheimer-related aphasia? Neurology. 2014;82:1127–1131. doi: 10.1212/WNL.0000000000000271. [DOI] [PubMed] [Google Scholar]
- 56.Gorno-Tempini ML, et al. The logopenic/ phonological variant of primary progressive aphasia. Neurology. 2008;71:1227–1234. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mack JE, et al. Word-finding pauses in primary progressive aphasia (PPA): effects of lexical category; Presented at the 43rd Clinical Aphasiology Conference. [Google Scholar]
- 58.Hurley RS, et al. Electrophysiology of object naming in primary progressive aphasia. J. Neurosci. 2009;16:15762–15769. doi: 10.1523/JNEUROSCI.2912-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Indefrey P, Hellwig F, Herzog H, Seitz RJ, Hagoort P. Neural responses to the production and comprehension of syntax in identical utterances. Brain Lang. 2004;89:312–319. doi: 10.1016/S0093-934X(03)00352-3. [DOI] [PubMed] [Google Scholar]
- 60.Schwartz MF, et al. Anterior temporal involvement in semantic word retrieval: voxel-based lesion-symptom mapping evidence from aphasia. Brain. 2009;132:3411–3427. doi: 10.1093/brain/awp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thompson C, et al. Semantic interference during object naming in agrammatic and logopenic primary progressive aphasia (PPA) Brain Lang. 2012;120:237–250. doi: 10.1016/j.bandl.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vandenberghe RR, et al. Paradoxical features of word finding difficulty in primary progressive aphasia. Ann. Neurol. 2005;57:204–209. doi: 10.1002/ana.20362. [DOI] [PubMed] [Google Scholar]
- 63.Rogalski E, Rademaker A, Mesulam M, Weintraub S. Covert processing of words and pictures in nonsemantic variants of primary progressive aphasia. Alzheimer Dis. Assoc. Dis. 2008;21:343–351. doi: 10.1097/WAD.0b013e31816c92f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DeLeon J, et al. Neural regions essential for distinct cognitive processes underlying picture naming. Brain. 2007;130:1408–1422. doi: 10.1093/brain/awm011. [DOI] [PubMed] [Google Scholar]
- 65.Mesulam M-M, et al. Neurology of anomia in the semantic subtype of primary progressive aphasia. Brain. 2009;132:2553–2565. doi: 10.1093/brain/awp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mesulam M-M, et al. Words and objects at the tip of the left temporal lobe in primary progressive aphasia. Brain. 2013;136:601–618. doi: 10.1093/brain/aws336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hurley RS, Paller K, Rogalski E, Mesulam M-M. Neural mechanisms of object naming and word comprehension in primary progressive aphasia. J. Neurosci. 2012;32:4848–4855. doi: 10.1523/JNEUROSCI.5984-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benson F, Geschwind N. Ch. 5. In: Mesulam M-M, editor. Principles of Behavioral Neurology. 1st ed. F. A. Davis; 1985. pp. 193–238. [Google Scholar]
- 69.Damasio AR. Aphasia. N. Eng. J. Med. 1992;326:531–539. doi: 10.1056/NEJM199202203260806. [DOI] [PubMed] [Google Scholar]
- 70.Heilman KM. Anomic aphasia following anterior temporal lobectomy. Trans. Am. Neurol. Assoc. 1972;97:291–293. [Google Scholar]
- 71.Ojemann GA. Brain organization for language from the perspective of electrical stimulation. Behav. Brain Sci. 1983;6:189–230. [Google Scholar]
- 72.Warrington EK, Shallice T. Category specific semantic impairments. Brain. 1984;107:829–854. doi: 10.1093/brain/107.3.829. [DOI] [PubMed] [Google Scholar]
- 73.Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- 74.Snowden JS, Goulding PJ, Neary D. Semantic dementia: a form of circumscribed atrophy. Behav. Neurol. 1989;2:167–182. [Google Scholar]
- 75.Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain. 2006;129:2132–2147. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- 76.Patterson K, Nestor P, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat. Rev. Neurosci. 2007;8:976–988. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- 77.Rogers TT, et al. Structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychol. Rev. 2004;111:205–235. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- 78.Walker GM, et al. Support for anterior temporal involvement in semantic error production in aphasia: new evidence from VLSM. Brain Lang. 2011;117:110–122. doi: 10.1016/j.bandl.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gitelman DR, Nobre AC, Sonty S, Parrish TB, Mesulam M-M. Language network specializations: an analysis with parallel task design and functional magnetic resonance imaging. Neuroimage. 2005;26:975–985. doi: 10.1016/j.neuroimage.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 80.Robson H, et al. The anterior temporal lobes support residual comprehension in Wernicke’s aphasia. Brain. 2014;137:931–943. doi: 10.1093/brain/awt373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR. A neural basis for lexical retrieval. Nature. 1996;380:499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- 82.Grabowski TJ, et al. A role for left temporal pole in the retrieval of words for unique entities. Hum. Brain Mapp. 2001;13:199–212. doi: 10.1002/hbm.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hurley RS, Wang X, Mesulam M-M. Asymmetric physiology of the perisylvian language network: evidence from resting state fMRI. Cognitive Neuroscience Society. 2013 [online], http://www.cogneurosociety.org/wordpress/wp-content/uploads/2013/04/CNS2013_Program.pdf.
- 84.Damasio AR, Damasio H, Van Hoesen GW. Prosopagnosia: anatomic basis and behavioral mechanisms. Neurology. 1982;32:331–341. doi: 10.1212/wnl.32.4.331. [DOI] [PubMed] [Google Scholar]
- 85.Damasio AR, Tranel D, Rizzo M. Ch. 7. In: Mesulam M-M, editor. Principles of Behavioral and Cognitive Neurology. 2nd edn. Oxford University Press; 2000. pp. 332–372. [Google Scholar]
- 86.Mesulam M-M. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 87.Gefen T, et al. Naming vs knowing faces in primary progressive aphasia. A tale of two hemispheres. Neurology. 2013;81:658–664. doi: 10.1212/WNL.0b013e3182a08f83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Adlam ALR, et al. Semantic dementia and fluent primary progressive aphasia: two sides of the same coin? Brain. 2006;129:3066–3080. doi: 10.1093/brain/awl285. [DOI] [PubMed] [Google Scholar]
- 89.Mummery CJ, et al. A voxel-based morphometry study of semantic dementia: relatiosnhip between temportal lobe atrophy and semantic memory. Ann. Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- 90.Lambon Ralph MA, Cipolotti L, Manes F, Patterson K. Taking both sides: do unilateral anterior temporal lobe lesions disrupt semantic memory? Brain. 2010;133:3243–3255. doi: 10.1093/brain/awq264. [DOI] [PubMed] [Google Scholar]
- 91.Morán MA, Mufson EJ, Mesulam MM. Neural inputs into the temporopolar cortex of the rhesus monkey. J. Comp. Neurol. 1987;256:88–103. doi: 10.1002/cne.902560108. [DOI] [PubMed] [Google Scholar]
- 92.Ding S-L, Van Hoesen GW, Cassell MD, Poremba A. Parcellation of human temporal polar cortex: a combined analysis of multiple cytoarchitectonic, chemoarchitectonic, and pathologic markers. J. Comp. Neurol. 2009;514:595–623. doi: 10.1002/cne.22053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fan L, et al. Connectivity-based parcellation of the human temporal pole using diffusion tensor imaging. Cereb. Cortex. doi: 10.1093/cercor/bht196. http://dx.doi.org/10.1093/cercor/bht196. [DOI] [PubMed]
- 94.Pascual B, et al. Large-scale brain networks of the human left temporal pole: a functional connectivity MRI study. Cereb. Cortex. doi: 10.1093/cercor/bht260. http://dx.doi.org/10.1093/cercor/bht260. [DOI] [PMC free article] [PubMed]
- 95.Warren JE, Crinion JT, Lambon Ralph MA, Wise RJ. Anterior temporal lobe connectivity correlates with functional outcome after aphasic stroke. Brain. 2009;132:3428–3442. doi: 10.1093/brain/awp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Geschwind N. Language and the brain. Sci. Am. 1972;226:76–83. doi: 10.1038/scientificamerican0472-76. [DOI] [PubMed] [Google Scholar]
- 97.Mesulam M-M. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann. Neurol. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- 98.Ogar J, et al. Semantic dementia and persisting Wernicke’s aphasia: linguistic and anatomical profiles. Brain Lang. 2011;117:28–33. doi: 10.1016/j.bandl.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bogen JE, Bogen GM. Wernicke’s region —where is it? Ann. N. Y. Acad. Sci. 1976;280:834–843. doi: 10.1111/j.1749-6632.1976.tb25546.x. [DOI] [PubMed] [Google Scholar]
- 100.Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analysis. Front. Syst. Neurosci. 2011;5:1–20. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lau EF, Phillips C, Poeppel D. A cortical network for semantics: (de)constructing the N400. Nat. Rev. Neurosci. 2008;9:920–933. doi: 10.1038/nrn2532. [DOI] [PubMed] [Google Scholar]
- 102.Ohrui T, et al. Effects of brain-penetrating ACE inhibitors on Alzheimer disease progression. Neurology. 2004;63:1324. doi: 10.1212/01.wnl.0000140705.23869.e9. [DOI] [PubMed] [Google Scholar]
- 103.Robson H, Sage K, Lambon Ralph MA. Wernicke’s aphasia reflects a combination of acoustic-phonological and semantic control deficits: a case-series comparison of Wernicke’s aphasia, semantic dementia and semantic aphasia. Neuropsychologia. 2012;50:266–275. doi: 10.1016/j.neuropsychologia.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 104.Booth JR, et al. Modality independence of word comprehension. Hum. Brain Mapp. 2002;16:251–261. doi: 10.1002/hbm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gow DW., Jr The cortical organization of lexical knowledge: a dual lexicon model of spoken language processing. Brain Lang. 2012;120:174–186. doi: 10.1016/j.bandl.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rohrer JD, et al. Patterns of longitudinal brain atrophy in the logopenic variant of primary progressive aphasia. Brain Lang. 2013;127:121–126. doi: 10.1016/j.bandl.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Friston K. A theory of cortical responses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:815–836. doi: 10.1098/rstb.2005.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mesulam MM. Representation, inference and transcendent encoding in neurocognitive networks of the human brain. Ann. Neurol. 2008;64:367–378. doi: 10.1002/ana.21534. [DOI] [PubMed] [Google Scholar]
- 109.Chang EF, et al. Categorical speech representation in human superior temporal gyrus. Nat. Neurosci. 2011;13:1428–1432. doi: 10.1038/nn.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mesgarani M, Cheung C, Johnson K, Chang EF. Phonetic feature encoding in human superior temporal gyrus. Science. 2014;343:1006–1010. doi: 10.1126/science.1245994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nobre AC, Allison T, McCarthy G. Word recognition in the human inferior temporal lobe. Nature. 1994;372:260–263. doi: 10.1038/372260a0. [DOI] [PubMed] [Google Scholar]
- 112.Grabowski TJ, Damasio H, Tranel D, Boles Ponto LL, Hichwa RD, Damasio AR. A role for left temporal pole in the retrieval of words for unique entities. Hum. Brain Mapp. 2001;13:199–212. doi: 10.1002/hbm.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Damasio AR. The brain binds entities and events by multiregional activation from convergence zones. Neural Comput. 1989;1:123–132. [Google Scholar]
- 114.Antonucci SM, Beeson PM, Labiner DM, Rapcsak SZ. Lexical retrieval and semantic knowledge in patients with left inferior temporal lesions. Aphasiology. 2008;22:281–304. doi: 10.1080/02687030701294491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grossman M, et al. What’s in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer’s disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127:628–649. doi: 10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- 116.Rohrer JD, et al. Word-finding difficulty: a clinical analysis of the progressive aphasias. Brain. 2008;131:8–38. doi: 10.1093/brain/awm251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Crinion JT, Warburton EA, Lambon-Ralph MA, Howard D, Wise RJ. Listening to narrative speech after aphasic stroke: the role of the left anterior temporal lobe. Cereb. Cortex. 2006;16:1116–1125. doi: 10.1093/cercor/bhj053. [DOI] [PubMed] [Google Scholar]
- 118.Deramecourt V, et al. Prediction of pathology in primary progressive language and speech disorders. Neurology. 2010;74:42–49. doi: 10.1212/WNL.0b013e3181c7198e. [DOI] [PubMed] [Google Scholar]
- 119.Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain. 2005;128:1996–2005. doi: 10.1093/brain/awh598. [DOI] [PubMed] [Google Scholar]
- 120.Alladi S, et al. Focal cortical presentations of Alzheimer’s disease. Brain. 2007;130:2636–2645. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- 121.Forman MS, et al. Frontotemporal dementia: clinicopathological correlations. Ann. Neurol. 2006;59:952–962. doi: 10.1002/ana.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rohrer JD, Rossor MN, Warren J. Alzheimer pathology in primary progressive aphasia. Neurobiol. Aging. 2012;33:744–752. doi: 10.1016/j.neurobiolaging.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Ann. Neurol. 2006;59:156–165. doi: 10.1002/ana.20700. [DOI] [PubMed] [Google Scholar]
- 124.Mesulam M, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann. Neurol. 2008;63:709–719. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Grossman M, et al. Longitudinal decline in autopsy-defined frontotemporal lobar degeneration. Neurology. 2008;70:2036–2045. doi: 10.1212/01.wnl.0000303816.25065.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hodges JR, et al. Clinicopathological correlates in frontotemporal dementia. Ann. Neurol. 2004;56:399–406. doi: 10.1002/ana.20203. [DOI] [PubMed] [Google Scholar]
- 127.Braak H, Braak E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol. Scand. 1996;165(Suppl. 1):S3–S12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- 128.Murray ME, et al. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011;10:785–796. doi: 10.1016/S1474-4422(11)70156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gefen T, et al. Clinically concordant variations of Alzheimer pathology in aphasic versus amnestic dementia. Brain. 2012;135:1554–1565. doi: 10.1093/brain/aws076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Knibb JA, Woollams AM, Hodges JR, Patterson K. Making sense of progressive non-fluent aphasia: an analysis of conversational speech. Brain. 2009;132:2734–2746. doi: 10.1093/brain/awp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Weintraub S, Mesulam M. With or without FUS, it is the anatomy that dictates the dementia phenotype. Brain. 2009;132:2906–2908. doi: 10.1093/brain/awp286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Weintraub S, Mesulam M-M. Ch. 8. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. 1st edn. Vol. 8. Elsevier; 1993. pp. 253–281. [Google Scholar]
- 133.Mesulam M-M. Primary progressive aphasia: a language-based dementia. N. Engl. J. Med. 2003;348:1535–1542. doi: 10.1056/NEJMra022435. [DOI] [PubMed] [Google Scholar]
- 134.Warren JD, et al. Molecular nexopathies: a new paradigm of neurodegenerative disease. Trends Neurosci. 2013;36:561–569. doi: 10.1016/j.tins.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rogalski E, et al. Asymmetry of cortical decline in subtypes of primary progressive aphasia. Neurology. doi: 10.1212/WNL.0000000000000824. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rogalski E, Weintraub S, Mesulam M-M. Are there susceptibility factors for primary progressive aphasia? Brain Lang. 2013;127:135–138. doi: 10.1016/j.bandl.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Miller ZA, et al. Handedness and language learning disability differentially distribute in progressive aphasia variants. Brain. 2013;136:3461–3473. doi: 10.1093/brain/awt242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rogalski E, Johnson N, Weintraub S, Mesulam M-M. Increased frequency of learning disability in patients with primary progressive aphasia and their first degree relatives. Arch. Neurol. 2008;65:244–248. doi: 10.1001/archneurol.2007.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Miller ZA, et al. TDP-43 frontotemporal lobar degeneration and autoimmune disease. J. Neurol. Neurosurg. Psychiatry. 2013;84:956–962. doi: 10.1136/jnnp-2012-304644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Weintraub S, et al. Vasectomy in men with primary progressive aphasia. Cogn. Behav. Neurol. 2007;19:190–193. doi: 10.1097/01.wnn.0000213923.48632.ab. [DOI] [PubMed] [Google Scholar]
- 142.Munoz DG, Ros R, Fatas M, Bermejo F, de Yebenes JG. Progressive nonfluent aphasia associated with a new mutation V363I in tau gene. Am. J. Alzheimers Dis. Other Demen. 2007;22:294–299. doi: 10.1177/1533317507302320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Simón-Sánchez J, et al. The clinical and pathological phenotype of C9ORF72 hexanucleotide repeat expansions. Brain. 2012;135:723–735. doi: 10.1093/brain/awr353. [DOI] [PubMed] [Google Scholar]
- 144.Rademakers R, et al. Phenotypic variability associated with progranulin haploinsufficiency in patients with the common 1477C→T (Arg493X) mutation: an international initiative. Lancet Neurol. 2007;6:857–868. doi: 10.1016/S1474-4422(07)70221-1. [DOI] [PubMed] [Google Scholar]
- 145.Godbolt AK, et al. A presenilin 1 R278 mutation presenting with language impairment. Neurology. 2004;63:1702–1704. doi: 10.1212/01.wnl.0000143060.98164.1a. [DOI] [PubMed] [Google Scholar]
- 146.Mesulam M, et al. Progranulin mutations in primary progressive aphasia. Arch. Neurol. 2007;64:43–47. doi: 10.1001/archneur.64.1.43. [DOI] [PubMed] [Google Scholar]
- 147.Premi E, et al. Effect of tMEM106B polymorphism on functional network connectivity in asymptomatic GRN mutation carriers. JAMA Neurol. 2014;71:216–221. doi: 10.1001/jamaneurol.2013.4835. [DOI] [PubMed] [Google Scholar]
- 148.Bonvicini C, et al. Understanding phenotype variability in frontotemporal lobar degeneration due to granulin mutation. Neurobiol. Aging. 2014;35:1206–1211. doi: 10.1016/j.neurobiolaging.2013.10.097. [DOI] [PubMed] [Google Scholar]
- 149.Dickerson BC. Quantitating severity and progression in primary progressive aphasia. J. Mol. Neurosci. 2011;45:618–628. doi: 10.1007/s12031-011-9534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rogalski E, et al. Progression of language impairments and cortical atrophy in subtypes of primary progressive aphasia. Neurology. 2011;76:1804–1810. doi: 10.1212/WNL.0b013e31821ccd3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Seeley WW, et al. Unraveling Boléro: progressive aphasia, transmodal creativity and the right posterior neocortex. Brain. 2008;131:39–49. doi: 10.1093/brain/awm270. [DOI] [PubMed] [Google Scholar]
- 152.Papagno C, Semenza C, Girelli L. Meeting an “impossible challenge” in semantic dementia: outstanding performance in numerical Sudoku and quantitative number knowledge. Neuropsychology. 2013 doi: 10.1037/a0034457. [DOI] [PubMed] [Google Scholar]
- 153.Beeson PM, et al. Positive effects of language treatment for logopenic variant of primary progressive aphasia. J. Mol. Neurosci. 2011;45:724–736. doi: 10.1007/s12031-011-9579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Weintraub S, Morhardt DJ. Treatment, education and resources for non-Alzheimer dementia: one size does not fit all. Alzheimers Care Q. 2005;6:201–214. [Google Scholar]
- 155.Medina J, Weintraub S. Depression in primary progressive aphasia. J. Geriatr. Psychiatr. Neurol. 2007;20:153–160. doi: 10.1177/0891988707303603. [DOI] [PubMed] [Google Scholar]
- 156.Wang J, Wu D, Chen Y, Yuan Y, Zhang M. Effect of transcranial direct current stimulation on language improvement and cortical activation in nonfluent variant primary progressive aphasia. Neurosci. Lett. 2013;9:29–33. doi: 10.1016/j.neulet.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 157.Trebbastoni A, Raccah R, de Lena C, Zangen A, Inghilleri M. Repetitive deep transcranial magnetic stimulation improves verbal fluency and written language in a patient with primary progressive aphasia–logopenic variant (LPPA) Brain Stim. 2013;6:545–553. doi: 10.1016/j.brs.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 158.Finocchiaro C, et al. A case study of primary progressive aphasia: improvement on verbs after rTMS treatment. Neurocase. 2006:317–321. doi: 10.1080/13554790601126203. [DOI] [PubMed] [Google Scholar]
- 159.Welcome to the International PPA Connection. International PPA Connection. 2013 [online], http://www.ppaconnection.org.
- 160.Mesulam M. The evolving landscape of human cortical connectivity: facts and inferences. Neuroimage. 2012;62:2182–2189. doi: 10.1016/j.neuroimage.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wicklund A, Johnson N, Weintraub N. Preservation of reasoning in primary progressive aphasia: further differentiation from Alzheimer’s disease and the behavioral presentation of frontotemporal dementia. J. Clin. Exp. Neuropsychol. 2004;26:347–355. doi: 10.1080/13803390490510077. [DOI] [PubMed] [Google Scholar]
- 162.Weintraub S, et al. Verbal and nonverbal memory in primary progressive aphasia: the Three Words–Three Shapes Test. Behav. Neurol. 2012;26:67–76. doi: 10.3233/BEN-2012-110239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mesulam M-M, Weintraub S, Parrish T, Gitelman DR. Primary progressive aphasia: reversed asymmetry of atrophy and right hemisphere language dominance. Neurology. 2005;64:556–557. doi: 10.1212/01.WNL.0000150545.46351.DE. [DOI] [PubMed] [Google Scholar]
- 164.Song XW, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS ONE. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Blennow K, Hampel H. CSF markers for insipient Alzheimer’s disease. Lancet Neurol. 2003;2:605–613. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]