Abstract
Background
Half of all glioblastoma patients are at least 65 years old. The frequency and duration of hospitalization from disease- and treatment-related morbidity in this population are unknown.
Methods
We performed a retrospective cohort study among patients aged 65 years and older with glioblastoma diagnosed between 1999 and 2007 using SEER–Medicare linked data. Diagnoses and procedures were identified using administrative claims data. Logistic regression was performed to identify predictors of high hospitalization burden.
Results
Among the 5029 patients in the cohort, 52% were ages 65–74, and 52% were male. Twenty-six percent of patients underwent extensive resection, 72% received radiotherapy, and 18% received temozolomide. Median survival was 4.9 months. Among all patients, 21% were hospitalized at least 30 cumulative days between diagnosis and death, and 22% of all patients spent at least one-fourth of their remaining lives as inpatients. Higher comorbidity score (adjusted hazard ratio [AHR], 1.72; 95% CI, 1.42–2.07) and black race (AHR, 1.56; 95% CI, 1.11–2.18) were associated with an increased risk of being hospitalized for at least 25% of remaining life, whereas radiation (AHR, 0.49; 95% CI, 0.42–0.58), temozolomide (AHR, 0.31; 95% CI, 0.23–0.42), and extensive surgery (AHR, 0.83; 95% CI, 0.69–0.99) were associated with a decreased risk.
Conclusions
These data highlight the burden of hospitalization faced by a large proportion of older glioblastoma patients. In the setting of short survival, strategies to reduce the amount of time these patients spend hospitalized are urgently needed, to help maintain quality of life at the end of life.
Keywords: elderly, glioblastoma, hospitalization, survival, temozolomide
Glioblastoma multiforme (GBM) is the most common malignant primary brain tumor and is associated with a dismal prognosis, especially among the elderly. The median age at diagnosis is ∼65 years,1 and while patients may be told that the median survival for GBM is ∼15 months, this figure comes from a randomized trial of selected patients with good performance status and a median age of 56 years, and excluded patients over age 70.2 In contrast, population-based data from the Surveillance, Epidemiology, and End Results (SEER) registries in the United States show that median survival time for GBM patients 65 years and older is only 4–5 months from diagnosis,3,4 with only 20% of elderly patients surviving 1 year.
Standard treatment for GBM includes maximal safe resection followed by 6 weeks of adjuvant radiotherapy and, since 2005, concurrent and adjuvant temozolomide,2 but these treatments are not always recommended for or administered to elderly patients,5–10 variously defined as age ≥60, ≥65, or ≥70, in the context of shorter survival and potential for toxicity. Disease- and treatment-related morbidity may be especially pronounced among the elderly due to comorbidities and/or reduced physiologic reserve and can result in inpatient hospitalization during the course of the disease.
Hospitalization is associated with reduced quality of life among patients with advanced or incurable cancer,11 who generally prefer to be at home as much as possible.12 In one study of high-grade glioma patients whose preferences were elicited by physicians, <10% of patients were in favor of hospital admission near the end of life.13 In the setting of such short survival for elderly GBM patients, we sought to better understand the burden of hospitalization in this patient population, by focusing on the percentage of overall survival time patients spend hospitalized between diagnosis and death. One Canadian study from 200114 reported this endpoint among patients of all ages but did not explore associated factors. While patients may be more accepting of hospitalizations during the treatment of a curative disease, the majority of patients with advanced cancer indicate that inpatient admission is particularly burdensome.11 Thus we investigated both the aggregate and the proportion of time elderly GBM patients spent in the hospital, as an important dimension of quality of life at the end of life. This patient-centered information could allow improved physician counseling and patient decision making, with eventual goals of reducing unnecessary admissions and improving resource allocation. We hypothesized that many elderly patients spend a high proportion of their remaining lives hospitalized after being diagnosed as having GBM.
Materials and Methods
Data Sources
Our GBM study sample was drawn from linked SEER–Medicare data. SEER is a consortium of 17 population-based US cancer registries sponsored by the National Cancer Institute that collects incident cancer cases, including GBM, covering ∼28% of the US population.15 SEER collects data regarding patient demographics, tumor histology and grade, disease location and extent, and primary surgical and radiation treatment.
Medicare is the primary health insurer for ∼97% of Americans age 65 years and older and covers inpatient hospital care, outpatient services, and other medical care. Medicare files document use of health-care services by patients enrolled in the Medicare fee-for-service (FFS) plan. Combined SEER–Medicare data link SEER data to Medicare claims files for inpatient hospitalization services, outpatient services, and physician and other professional services to provide complete follow-up information on treatments, expenditures, access to care, source of care, and outcomes for Medicare FFS patients after diagnosis of GBM. This study was deemed exempt from review by the institutional review board at the Harvard School of Public Health.
Patients and Initial Treatment
All subjects were Medicare beneficiaries aged 65 years and older with diagnoses of GBM in SEER regions between January 1999 and December 2007 who had continuous enrollment in Medicare Parts A and B from diagnosis through death. We used Medicare data from January 1, 1999 to December 31, 2009, to allow all GBM patients to have a minimum of 2 years of follow-up after diagnosis. GBM was defined according to the International Classification of Diseases for Oncology, third edition (ICD-O-3; SEER codes 9440–9444). Patients without a known diagnosis month, who were diagnosed at autopsy, or who were diagnosed without histologic evidence from a biopsy or resection were excluded, as were patients with prior cancers, except for nonmelanoma skin cancers. Date of diagnosis was based on date of biopsy or resection per Medicare Part A claims (defined below) and was cross-referenced against the diagnosis month provided in the SEER database. Patients enrolled in a health maintenance organization at the time of diagnosis were excluded, because those services are not itemized on Medicare claims forms. In addition, patients were excluded if they were not enrolled in Medicare FFS for 12 months before diagnosis, in order to determine comorbidities.16,17
Treatment-related Medicare claims were identified principally with the following codes, based on ICD-9, Current Procedural Terminology (CPT), and National Drug Code (NDC) classifications: surgical biopsy, 0113–0114 (ICD-9) and 61304–61305 (CPT); surgical resection, 0153–0159 (ICD-9); radiotherapy, V580, V661, V671, and 9921–9929 (ICD-9) and 77499, 77261–77431, and 77750–77797 (CPT); temozolomide, J8700 (CPT) and 000851XXXXX, 000853XXXXX, 545695XXXXX, and 548685XXXXX (NDC); other chemotherapy, 9925, V581, V662, and V672 (ICD-9) and 51720, 96400–96549, J9000–J9999, Q0083–Q0085, J0640, J7150, J8530, J8600, J8610, J8999, J8510, J8520, and J8521 (CPT). Temozolomide was assigned a dedicated “J” billing code in January 2001 and was analyzed separately from other chemotherapies given that it became the standard-of-care initial chemotherapy for GBM in 2005, with no clear standard chemotherapy prior to temozolomide. Extent of resection was dichotomized as limited, defined as either subtotal resection (SEER coding) or biopsy (SEER or Medicare coding); or extensive, defined as a gross total or radical resection (SEER coding).
Hospitalization was defined as inpatient admission according to Medicare Part A claims. Patient admissions to skilled nursing facilities, intermediate care facilities, rehabilitation units or hospitals, or long-term care institutions were not considered to be inpatient hospitalizations for the purposes of hospitalization analyses. Hospital demographic characteristics for the index hospitalization, including number of beds, nonprofit status, National Cancer Institute–designated cancer center status, teaching hospital status, and surrounding population size, were obtained from the American Hospital Association's 2010 Annual Survey Database.
Clinical Characteristics
Baseline patient demographics and treatment parameters were characterized including age at diagnosis, sex, race/ethnicity, marital status, comorbidity, area median income, SEER region, year of diagnosis, tumor location, extent of surgery, receipt of radiotherapy, and receipt of temozolomide or other chemotherapy. The Deyo16 adaptation of the Charlson17 comorbidity index was used to measure severity of comorbid diseases, with modification to exclude cancer diagnoses. This method was applied to Medicare inpatient, outpatient, and physician claims during the 12-month period prior to GBM diagnosis.
Outcomes
In the context of known short median survival in this patient population, the amount of time spent hospitalized is an important patient-centered outcome, as described above. We analyzed cumulative days spent hospitalized, not necessarily consecutive days, from the date of GBM diagnosis until death. In addition, we calculated the proportion of remaining life spent hospitalized by dividing the cumulative days spent hospitalized by the cumulative days of overall survival (OS) from the time of diagnosis. We determined the proportion of remaining life spent hospitalized and used the dichotomized variable of <25% versus ≥25% of remaining life spent hospitalized as a primary endpoint, as an illustration of hospitalization burden.14 For all stratified analyses, we have included only those patients who were discharged alive after their index hospitalization (n = 4897, representing 97.4% of the entire GBM cohort), as these patients were at risk to experience a hospital readmission. We also report OS, in days, from diagnosis to death.
Statistical Analysis
We report descriptive statistics for baseline patient and initial treatment characteristics and the results of a chi-square test to compare these characteristics according to proportion of life spent hospitalized (<25% vs ≥25%). Logistic regression was used to evaluate the relationship between patient and initial treatment characteristics, with the odds of spending at least 25% of remaining life as an inpatient stratified according to treatment era. Treatment era was defined according to the publication month of the March 2005 landmark trial by the European Organisation for Research and Treatment of Cancer/National Cancer Institute of Canada (EORTC/NCIC),2 which established concurrent temozolomide as the standard of care for patients up to age 70. Patient and treatment characteristics in the model included age, sex, race, marital status, comorbidity status, income, diagnosis year, SEER region, tumor size, extent of surgery, receipt of radiotherapy, receipt of temozolomide or other chemotherapy, and initial hospitalization discharge status. An additional model was tested that also included the hospital-level characteristics of number of hospital beds, nonprofit status, National Cancer Institute–designated cancer center status, teaching hospital status, and surrounding population size. The receiver operating characteristic c-statistic was calculated for logistic regression models. Survival was estimated using the Kaplan–Meier method. SAS software version 9.3 64-bit was used for all analyses. Statistical significance was set at P < .05, and all tests were 2-tailed.
Results
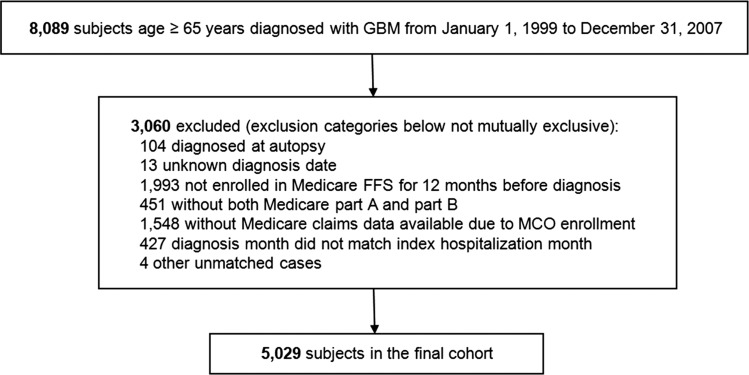
We identified 8089 patients age 65 years or older with histologic diagnosis of GBM between 1999 and 2007 using the SEER–Medicare linkage (Fig. 1). After exclusion criteria were applied as described in the Methods section, the final cohort consisted of 5029 patients. Approximately half of all patients (51.6%) were age 65 to 74, half (51.6%) were male, and most were white/non-Hispanic ethnicity (88.3%) (Table 1). Sixty-one percent had no comorbid illness, and over one-third (36%) were diagnosed in 2005 or later. One-quarter of patients (25.7%) underwent extensive resection, 72.3% received adjuvant radiotherapy, and 17.5% received adjuvant temozolomide.
Fig. 1.
Assembly of the study cohort. MCO, managed care organization.
Table 1.
Baseline patient characteristics (n = 5029) and hospitalization burden
| Characteristic | No. (%) | Percent Patients Spending ≥25% of Remaining Life Hospitalized |
|---|---|---|
| All patients | 5029 (100) | 21.7 |
| Age at diagnosis, y | ||
| 65 to ≤74 | 2597 (51.6) | 18.2 |
| 75 to ≤84 | 2038 (40.5) | 25.8 |
| 85+ | 394 (7.8) | 24.4 |
| Gender | ||
| Male | 2595 (51.6) | 21.7 |
| Female | 2434 (48.4) | 21.7 |
| Race/ethnicity | ||
| White, non-Hispanic | 4441(88.3) | 21.1 |
| Hispanic | 280 (5.6) | 23.6 |
| Black | 187 (3.7) | 33.7 |
| Asian | 67 (1.3) | 19.4 |
| Other | 54 (1.1) | 24.1 |
| Marital status | ||
| Married | 3044 (60.5) | 20.3 |
| Unmarried | 638 (12.7) | 23.4 |
| Unknown | 1347 (26.8) | 24.1 |
| Modified Charlson comorbidity score (13) | ||
| 0 | 3077 (61.2) | 18.7 |
| 1 | 1280 (25.5) | 22.7 |
| 2+ | 672 (13.4) | 33.8 |
| Year of diagnosis | ||
| 1999–2001 | 1383 (27.5) | 21.7 |
| 2002–2004 | 1835 (36.5) | 21.6 |
| 2005–2007 | 1811 (36.0) | 21.9 |
| Census tract income level | ||
| Lower income (<median) | 1733 (34.5) | 22.3 |
| Higher income (≥median) | 3296 (65.5) | 21.4 |
| SEER registry region | ||
| Northeast | 364 (7.2) | 20.9 |
| South | 520 (10.3) | 19.0 |
| Midwest | 804(16.0) | 24.1 |
| West | 3341(66.4) | 21.7 |
| Tumor location | ||
| 1 brain lobe | 3752 (74.6) | 21.0 |
| 2+ brain lobes | 941 (18.7) | 24.8 |
| Other | 336 (6.7) | 21.7 |
| Surgery | ||
| Extensive (GTR) | 1293 (25.7) | 17.1 |
| Limited (biopsy/STR) | 3736 (74.3) | 23.3 |
| Radiotherapy delivered | ||
| Yes | 3637 (72.3) | 16.3 |
| No | 1392 (27.7) | 36.0 |
| Temozolomide delivered | ||
| Yes | 880 (17.5) | 6.1 |
| Before March 2005 | 402 (8.0) | |
| March 2005 or later | 478 (9.5) | |
| No | 4149 (82.5) | 25.0 |
| Other chemotherapy delivered | ||
| Yes | 1022 (20.3) | 10.5 |
| No | 4007 (79.7) | 24.6 |
| Discharge disposition after index hospitalization for GBM diagnosis | ||
| Home | 2300 (45.7) | 11.1 |
| Home with services | 588 (11.7) | 14.6 |
| Rehabilitation | 483 (9.6) | 33.1 |
| ICF/SNF | 921 (18.3) | 30.6 |
| Hospice | 182 (3.6) | 36.3 |
| Expired | 132 (2.6) | 100.0 |
| Cumulative days hospitalized, including index hospitalization and all rehospitalizations, median (IQR) | 15 (8–26) | 25 (16–39) |
Abbreviations: GTR, gross total resection; STR, subtotal resection; ICF, intermediate care facility; SNF, skilled nursing facility; IQR, interquartile range.
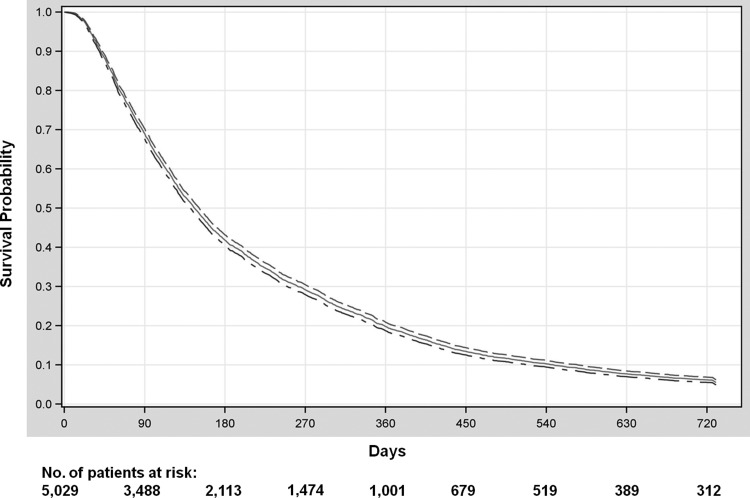
Median survival in the cohort was 4.9 months (interquartile range, 2.5–10.2 mo; Fig. 2). Overall survival at 1 year was 19.3% (95% CI, 18.2%–20.4%), OS at 2 years was 5.5% (95% CI, 4.9%–6.2%), and OS at 3 years was 2.4% (95% CI, 2.0%–2.9%). Among all patients, 53.9% (n = 2709) were hospitalized for at least 14 cumulative days between GBM diagnosis and death, and 21% (n = 1052) were hospitalized for at least 30 cumulative days.
Fig. 2.
Overall survival of older glioblastoma patients, from date of diagnosis, with accompanying 95% CI lines.
With regard to our primary endpoint, we found that 21.7% of all patients (n = 1083) spent at least 25% of their remaining lives as inpatients (Table 1) after diagnosis of GBM. When stratified according to baseline characteristics, patient factors associated with higher proportion of remaining life spent hospitalized included older age (P < .001), black race (P = .005), unmarried status (P = .002), higher comorbidity burden (P < .001), and tumor involving multiple brain lobes (P = .046) (Table 2). Of note, treatment era based on year of diagnosis was not associated with hospitalization burden (P = .816). Analysis of treatment factors revealed an association of extensive surgery (P < .001), receipt of radiation (P < .001), receipt of temozolomide (P < .001), and receipt of other chemotherapy (P < .001) with a lower proportion of remaining life spent hospitalized.
Table 2.
Distribution of patients according to hospitalization burden after index hospitalization*
| Characteristic | <25% of Remaining Life Spent Hospitalized (n = 3906) | 25%–100% of Remaining Life Spent Hospitalized (n = 991) | P |
|---|---|---|---|
| Age at diagnosis, y | <.001 | ||
| 64 to ≤74 | 54.1 | 43.5 | |
| 75 to ≤84 | 38.4 | 48.1 | |
| 85+ | 7.6 | 8.4 | |
| Gender | .967 | ||
| Male | 51.5 | 51.5 | |
| Female | 48.5 | 48.5 | |
| Race/ethnicity | .005 | ||
| White, non-Hispanic | 88.9 | 85.8 | |
| Hispanic | 5.5 | 6.2 | |
| Black | 3.2 | 5.9 | |
| Asian | 1.5 | 1.1 | |
| Other | 0.9 | 1 | |
| Marital status | .002 | ||
| Married | 61.6 | 56.6 | |
| Unmarried | 12.1 | 12.7 | |
| Unknown | 26.3 | 30.7 | |
| Comorbidity score | <.001 | ||
| 0 | 63.5 | 52.6 | |
| 1 | 25.2 | 27.1 | |
| 2+ | 11.4 | 20.3 | |
| Year of diagnosis | .816 | ||
| 1999–2001 | 27.4 | 27.1 | |
| 2002–2004 | 36.5 | 36.4 | |
| 2005–2007 | 36.1 | 36.4 | |
| Census tract income | .856 | ||
| Lower income (<median) | 34.2 | 35.5 | |
| Higher income (>median) | 65.8 | 64.5 | |
| SEER registry region | .06 | ||
| Northeast | 7.3 | 6.4 | |
| South | 10.7 | 8.9 | |
| Midwest | 15.5 | 18.4 | |
| West | 66.5 | 66.4 | |
| Tumor extent | .046 | ||
| 1 brain lobe | 75.4 | 71.9 | |
| 2+ brain lobes | 18 | 21.3 | |
| Unknown | 6.6 | 6.9 | |
| Surgery | <.001 | ||
| Extensive (GTR) | 27.4 | 19.4 | |
| Limited (biopsy/STR) | 72.6 | 80.6 | |
| Radiotherapy delivered | <.001 | ||
| Yes | 77.9 | 57.8 | |
| No | 22.1 | 42.2 | |
| Temozolomide delivered | <.001 | ||
| Yes | 21.2 | 5.5 | |
| No | 78.8 | 94.5 | |
| Other chemotherapy delivered | <.001 | ||
| Yes | 23.3 | 10.5 | |
| No | 76.7 | 89.5 | |
| Discharged home after index hospitalization | <.001 | ||
| Yes | 52.4 | 25.7 | |
| No | 47.6 | 74.3 | |
| Cumulative days hospitalized after index hospitalization, ** median (IQR) | 13 (7–22) | 26 (17–41) | <.001 |
Abbreviations: GTR, gross total resection; STR, subtotal resection; IQR, interquartile range.
*Excluding the 132 patients who did not survive their initial hospitalization for glioblastoma diagnosis. All values represent percentages.
**Among all patients surviving index hospitalization.
Patients with higher burden of hospitalization (≥25% of remaining life hospitalized) had longer median length of stay, in days, during initial hospitalization when the diagnosis of GBM was made (10 vs 5 d) as well as cumulative length of stay for all hospitalizations combined (median 31 vs 17 d) compared with those with lower hospitalization burden (Table 3). The median number of outpatient visits was lower among those with higher hospitalization burden (10 vs 16) compared with those with lower hospitalization burden. Of note, approximately one-third of all patient discharges after any hospitalization were to some form of intermediate care facility (including rehabilitation hospitals, long-term care facilities, skilled nursing facilities, etc.), as opposed to home (granular data not shown).
Table 3.
Inpatient and outpatient visit characteristics among older glioblastoma patients*
| Characteristic | Percent of Remaining Life Spent Hospitalized |
|
|---|---|---|
| <25% (n = 3906) | 25–100% (n = 991) | |
| Length of stay, index hospitalization for GBM diagnosis, median n days (range) | 5 (1–58) | 10 (1–97) |
| No. (%) of patients with ≥1 hospitalization after index hospitalization | 2697 (69.0) | 716 (72.3) |
| Cumulative length of stay, all | 17 (2–170) | 31 (5–229) |
| rehospitalizations, | ||
| median n days (range) | ||
| Total n hospitalizations per patient, | 2 (1–17) | 3 (1–18) |
| median (range) | ||
| No. (%) of patients with ≥1 outpatient visit after index hospitalization | 3820 (97.8) | 930 (93.8) |
| Total n outpatient visits per patient, median (range) | 16 (1–318) | 10 (1–184) |
*Excluding the 132 patients who did not survive their initial hospitalization for GBM diagnosis.
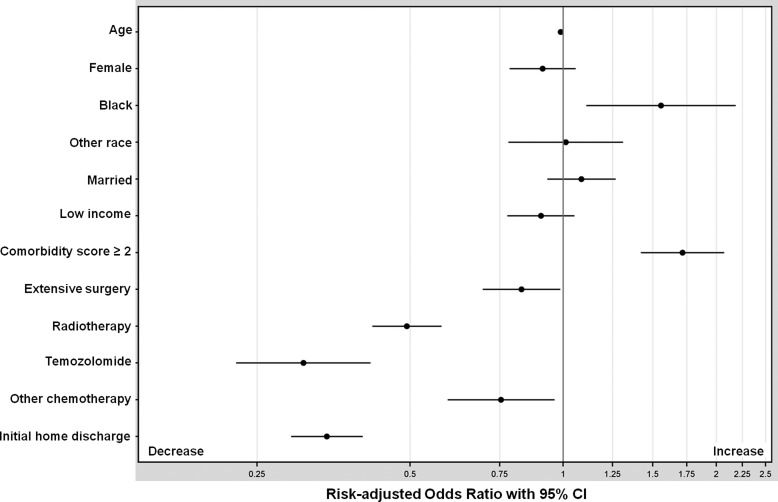
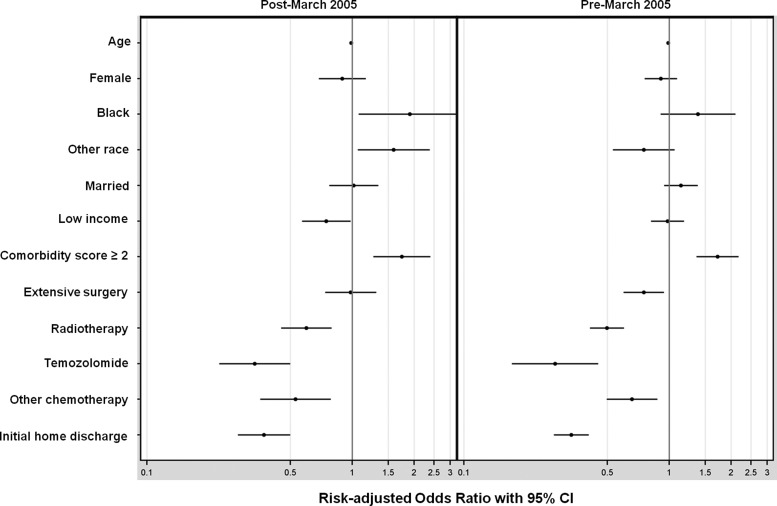
Logistic regression revealed that higher comorbidity score (adjusted hazard ratio [AHR], 1.72; 95% CI, 1.42–2.07) and black race (AHR, 1.56; 95% CI, 1.11–2.18) were independently associated with an increased risk of being hospitalized for at least 25% of remaining life (Fig. 3). Receipt of radiotherapy (AHR, 0.49; 95% CI, 0.42–0.58), of temozolomide (AHR, 0.31; 95% CI, 0.23–0.42), of other chemotherapy (AHR, 0.76; 95% CI, 0.59–0.96), and of extensive surgery (AHR, 0.83; 95% CI, 0.69–0.99) were independently associated with a decreased risk of being hospitalized for at least 25% of remaining life. In addition, decreased risk was observed among patients discharged to home after the index hospitalization (AHR, 0.34; 95% CI, 0.29–0.40). The c-statistic for the regression model was 0.73. When stratified according to treatment era, extensive surgery remained associated with decreased hospitalization burden in the pre–March 2005 period but not in the post–March 2005 period (Fig. 4).
Fig. 3.
Risk-adjusted odds ratios for spending ≥25% of remaining life hospitalized.
Fig. 4.
Risk-adjusted odds ratios for spending ≥25% of remaining life hospitalized, stratified by treatment era. X-axis displayed on a logarithmic scale.
With regard to the characteristics of the 704 hospitals in the study sample for the index hospitalization, 65.5% were nonprofit hospitals, 21.5% were major teaching hospitals, 3.1% were National Cancer Institute–designated teaching hospitals, 3.6% were located in large urban areas, and the median number of beds was 290 (25th to 97th percentile, 167 to 436). When these 5 hospital characteristics were added into the regression model, none was significantly associated with spending at least 25% of remaining life hospitalized (P > .25 for all).
Discussion
Elderly patients with GBM have a very poor prognosis, with median survival of <6 months from the time of diagnosis for patients aged 65 and older,3–10 and population-based data so far have not suggested major survival gains among these patients in the temozolomide era.18 In the context of such short survival among the elderly, a focus on quality of life during a patient's remaining life becomes a primary goal. Given that avoiding hospitalizations is one of the chief concerns among advanced cancer patients in general11 and among high-grade glioma patients specifically,13 plus the growing call to reduce hospital readmissions to improve quality of patient care,19 we analyzed the frequency, duration, and factors associated with hospital admissions among elderly GBM patients in the United States. We found an extraordinary burden of hospitalization experienced by a large proportion of elderly patients despite a median survival of <5 months, with 21% of all patients hospitalized for at least 30 days between diagnosis and death, and 22% of all patients spending at least one-fourth of their remaining lives as inpatients.
Little is known about factors that may be associated with hospitalization among patients with brain tumors, and thus we investigated patient, treatment, and hospital-level characteristics among elderly GBM patients and their related hospitalizations. While one might anticipate that more aggressive treatments such as extensive surgery, radiotherapy, and chemotherapy could increase the proportion of remaining life that patients spend hospitalized, we found that these treatments were actually associated with a lower proportion of life spent hospitalized. A common limitation of using retrospective medical records to ascertain treatment and outcome associations, however, is the potential for some degree of confounding bias. Specifically, it is possible that patients selected by physicians to receive active treatments may have had better baseline performance status and as a result were less likely to require hospitalization in the first place. It is therefore impossible to assign causation, and in our data we can demonstrate only an association, even though we have attempted to mitigate this bias by adjusting for patient comorbidity score. Prior retrospective and prospective data have demonstrated improved survival with surgery, radiation, and chemotherapy among elderly patients with GBM,9,10,14,20 and this report demonstrates that they are also associated with decreased hospitalization burden, independent of comorbidity. The French trial by ANOCEF (Association des Neuro-Oncologues d'Expression Française) showed no increase in symptoms among elderly GBM patients receiving radiotherapy compared with supportive care alone, but it did not evaluate hospitalizations.9 Based on our findings, it is plausible that patients who do not receive active treatments may be more likely to develop tumor-related symptoms that lead to inpatient admission; yet as outlined above, it is possible that patients with higher performance status may have been less likely to be hospitalized, independent of the treatments received. Similarly, when we examined hospitalization burden according to treatment era, the association between extensive surgery and lower hospitalization burden was lost in the temozolomide era. It is unclear whether temozolomide itself or other factors may be lessening the advantages of further debulking surgery from a hospitalization standpoint, as this was a nonrandomized setting and unknown confounders may influence the results.
In addition to treatment-related factors, we found that age was not associated with hospitalization burden, whereas increasing patient comorbidity was associated. This suggests that even patients in their 70s or 80s are not necessarily at higher risk of spending much of their remaining lives in the hospital after a diagnosis of GBM simply based on age, as long as they otherwise have minimal to no comorbid disease. We also observed that independent of treatment, elderly black patients were more likely to spend a larger proportion of their remaining lives admitted to the hospital compared with other races. One previous report found that black patients with GBM were significantly less likely to receive upfront surgical resection,3 but other reports have not found significant overall differences in survival between races.4 One possibility is that black patients were more likely to pursue aggressive care near the end of life, as has been shown elsewhere among patients with advanced cancers,21 although definitive conclusions cannot be reached with our dataset, which is based on administrative claims and not patient survey data.
Unfortunately there have been few advances in the past 3 decades that have substantially extended the lives of patients with GBM, particularly among the elderly, who are half of the GBM population. The EORTC/NCIC trial reported by Stupp et al.2 found an OS advantage by adding temozolomide to radiotherapy, but this study excluded patients over age 70, and a subgroup analysis in this trial demonstrated no survival benefit to adding temozolomide among the 83 patients who were ages 65 to 70.22,23 Thus the optimal treatment strategy for GBM among the elderly remains unclear. An ongoing EORTC/NCIC/TROG (Trans Tasman Radiation Oncology Group) randomized phase III trial (EORTC 26062–22061), which adds concurrent and adjuvant temozolomide to 40 Gy hypofractionated radiotherapy, is currently being tested among patients age 65 and older with favorable performance status and will hopefully define a subset of elderly patients who may benefit from combined modality therapy. European and Canadian trials have demonstrated that abbreviated courses of radiotherapy alone or temozolomide alone appear to be reasonable treatment options among patients who are at least 60 years of age5–8 and are superior to supportive care alone.8,9 In particular, temozolomide monotherapy may be a reasonable option for patients whose tumors harbor O6-DNA methylguanine-methyltransferase promoter methylation, which confers greater sensitivity to temozolomide.5 However, none of these regimens has been compared in a randomized fashion with concurrent chemoradiation as per the Stupp regimen, and in the United States there may be a tendency among physicians to still recommend aggressive, full-course combined modality treatments more frequently for elderly patients,18 as opposed to monotherapy treatment options, which are typically viewed as palliative. Despite this, the high hospitalization burden we observed among elderly GBM patients may not be particular to the US health-care system. A population-based study of Canadian patients in the 1980s and 1990s found that after a diagnosis of GBM, approximately 1 out of every 4 patients aged 60 years or older spent over half of their remaining lives in the hospital, a rate that was 4 to 5 times higher than for patients under age 40.14
Our findings demonstrate that among GBM patients with a high hospitalization burden, the location of medical care shifts more to the inpatient as opposed to the outpatient setting, which is logical. However, given the poorer quality of life that patients with advanced cancers associate with hospital admission,11,13 and the recent finding that hospital admissions are the biggest driver of health-care cost variability in the escalating costs of cancer care in the United States,24 alternative strategies for caring for elderly GBM patients are warranted. Pace et al.25 have recently reported outcomes from a pilot study in Italy of home-based outpatient care for patients with brain tumors, 72% of whom had GBM, involving a care team of a neurologist, nurses, physical therapists, psychologists, and a social worker. The mean length of home assistance was 5.5 months, averaging ∼35 total visits per patient. Importantly, they demonstrated that the rehospitalization rate in the last 2 months of life was reduced by more than half (38% for standard care vs 17% for supportive home care, incidence rate ratio = 0.35, P = .001). It is unclear what primary treatments these patients received, such as surgical resection, radiotherapy, and/or chemotherapy, and ongoing study is required to understand if this care model may fit into more widespread practice. In addition, reorganizing care delivery for GBM patients according to standardized national guidelines and increasing multidisciplinary team referral have been shown to reduce hospital length of stay by nearly half and reduce emergency admissions in England,26 suggesting another process improvement that may decrease hospitalization burden. Similarly, involvement of a palliative care physician may also be hypothesized to be associated with reduced hospitalization among GBM patients, yet we were unable to investigate this in our current study, as it was not until late 2009 that palliative care physicians were recognized with a distinct subspecialty physician code within Medicare.27
This study has certain limitations, mostly related to its observational design. Our analysis assumed consistent coding of variables, which may vary regionally or even by hospital. Both SEER registry and Medicare claims data have been shown to contain incomplete information regarding patient characteristics28 and treatment information29,30 for certain patients, and thus our results are only as reliable as the original population-based datasets. This may be particularly true for temozolomide claims. As an oral chemotherapy, it may have incurred out-of-pocket or supplemental insurance expenses that were not captured by Medicare claims,31 and thus our results may underestimate the prevalence of its use among our cohort. Temozolomide was only approved for newly diagnosed GBM in 2005,32 and this report is the first we are aware of to examine its use in GBM patients using Medicare claims. Moreover, national databases do not contain information on O6-DNA methylguanine-methyltransferase promoter methylation status of the tumor, which is a known prognostic factor in GBM, in part because its prognostic importance was not reported until 200533 and it is not tested in all GBM patients. In addition, retrospective examination of adjuvant treatment data can lead to immortal time bias, and this is relevant for the interpretation of our data with regard to radiotherapy and chemotherapy.34 Specifically, patients who receive these adjuvant treatments have by definition already lived long enough to receive them, which could predispose them to improved survival or other outcomes, compared with patients who did not receive those therapies. This was another rationale for focusing on the proportion of remaining life spent hospitalized as an endpoint, since it is a fraction and therefore less sensitive to absolute number of days surviving. In addition, this endpoint may have more valence with patients who are focused on retaining the highest quality of life during their remaining time alive with an incurable disease. Finally, confounding bias related to fitter patients being selected to receive certain therapies could also affect the results as described previously, and associations between therapies and hospitalization burden does not imply causation.
Ongoing study will be required to ascertain the reasons for rehospitalization among elderly GBM patients and associated resource utilization, which were not the focus of the current investigation. A small report on 29 patients with GBM in Austria showed that the most frequent reasons for hospital admission in the last 2–3 months of life were difficulties in home care due to immobility, acute deterioration, and seizure,35 yet little else is known regarding the specific indications for inpatient admission among this population. Neurologic symptomatology related to mass effect in the brain may be more challenging for caregivers to manage in the home compared with pain or other symptoms seen among other advanced cancers, and thus the hospitalization burden in this cohort may be unique, and further study is warranted. The eventual goal of these investigations is to reduce the frequency and duration of hospitalizations among elderly GBM patients, including the prevention of potentially unnecessary admissions to improve quality of life at the end of life.
Funding
This work was supported by grants from the National Cancer Institute of the National Institutes of Health (2P01CA134294-06 to F.D.) and from the Agency for Healthcare Research and Quality (K18 HS21991 to F.D.). Contents of this publication are solely the responsibility of the authors, and no official endorsement by the National Cancer Institute, National Institutes of Health, Agency for Healthcare Research and Quality, or the U.S. Department of Health and Human Services is intended or should be inferred.
Acknowledgments
This study used the linked SEER–Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors.
The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER–Medicare database.
Conflict of interest statement. None declared.
References
- 1.Wrensch M, Minn Y, Chew T, et al. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol. 2002;4(4):278–299. doi: 10.1093/neuonc/4.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Iwamoto FM, Reiner AS, Panageas KS, et al. Patterns of care in elderly glioblastoma patients. Ann Neurol. 2008;64(6):628–634. doi: 10.1002/ana.21521. [DOI] [PubMed] [Google Scholar]
- 4.Barnholtz-Sloan JS, Maldonado JL, Williams VL, et al. Racial/ethnic differences in survival among elderly patients with a primary glioblastoma. J Neurooncol. 2007;85(2):171–180. doi: 10.1007/s11060-007-9405-4. [DOI] [PubMed] [Google Scholar]
- 5.Malmström A, Gronberg BG, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 6.Wick W, Platten M, Meisner C, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. doi: 10.1016/S1470-2045(12)70164-X. [DOI] [PubMed] [Google Scholar]
- 7.Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22(9):1583–1588. doi: 10.1200/JCO.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 8.Gállego Pérez-Larraya J, Ducray F, Chinot O, et al. Temozolomide in elderly patients with newly diagnosed glioblastoma and poor performance status: an ANOCEF phase II trial. J Clin Oncol. 2011;29(22):2050–2055. doi: 10.1200/JCO.2011.34.8086. [DOI] [PubMed] [Google Scholar]
- 9.Keime-Guibert F, Chinot O, Taillandier L, et al. Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356(15):1527–1535. doi: 10.1056/NEJMoa065901. [DOI] [PubMed] [Google Scholar]
- 10.Barnholtz-Sloan JS, Williams VL, Maldonado JL, et al. Patterns of care and outcomes among elderly individuals with primary malignant astrocytoma. J Neurosurg. 2008;108(4):642–648. doi: 10.3171/JNS/2008/108/4/0642. [DOI] [PubMed] [Google Scholar]
- 11.Zhang B, Nilsson ME, Prigerson HG. Factors important to patients' quality of life at the end of life. Arch Intern Med. 2012;172(15):1133–1142. doi: 10.1001/archinternmed.2012.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Townsend J, Frank AO, Fermont D, et al. Terminal cancer care and patients' preference for place of death: a prospective study. Br Med J. 1990;301(6749):415–417. doi: 10.1136/bmj.301.6749.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sizoo EM, Pasman HR, Buttolo J, et al. Decision-making in the end-of-life phase of high-grade glioma patients. Eur J Cancer. 2012;48(2):226–232. doi: 10.1016/j.ejca.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Paszat L, Laperriere N, Groome P, et al. A population-based study of glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2001;51(1):100–107. doi: 10.1016/s0360-3016(01)01572-3. [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Institute. Surveillance, Epidemiology and End Results. Available at: http://seer.cancer.gov/about/overview.html. Accessed February 11, 2014.
- 16.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Darefsky AS, King JT, Jr, Dubrow R. Adult glioblastoma multiforme survival in the temozolomide era: a population-based analysis of Surveillance, Epidemiology, and End Results registries. Cancer. 2012;118(8):2163–2172. doi: 10.1002/cncr.26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams MV. A requirement to reduce readmissions: take care of the patient, not just the disease. JAMA. 2013;309(4):394–396. doi: 10.1001/jama.2012.233964. [DOI] [PubMed] [Google Scholar]
- 20.Vuorinen V, Hinkka S, Färkkilä M, et al. Debulking or biopsy of malignant glioma in elderly people—a randomised study. Acta Neurochir. 2003;145(1):5–10. doi: 10.1007/s00701-002-1030-6. [DOI] [PubMed] [Google Scholar]
- 21.Smith AK, McCarthy EP, Paulk E, et al. Racial and ethnic differences in advance care planning among patients with cancer: impact of terminal illness acknowledgment, religiousness, and treatment preferences. J Clin Oncol. 2008;26(25):4131–4137. doi: 10.1200/JCO.2007.14.8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stupp R. Elderly GBM patients—temozolomide or radiotherapy? A perspective. Presented at EORTC EANO Conference, Trends in Central Nervous System Malignancies; Bucharest, Romania. 2011. Available at: http://eccamsterdam2013.ecco-org.eu/Home/Events/Past-conferences/EORTC-EANO/Presentations.aspx . Accessed February 11, 2014. [Google Scholar]
- 23.Laperriere N, Weller M, Stupp R, et al. Optimal management of elderly patients with glioblastoma. Cancer Treat Rev. 2013;39(4):350–357. doi: 10.1016/j.ctrv.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Brooks GA, Li L, Sharma DB, et al. Regional variation in spending and survival for older adults with advanced cancer. J Natl Cancer Inst. 2013;105(9):634–642. doi: 10.1093/jnci/djt025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pace A, Di Lorenzo C, Capon A, et al. Quality of care and rehospitalization rate in the last stage of disease in brain tumor patients assisted at home: a cost effectiveness study. J Palliat Med. 2012;15(2):225–227. doi: 10.1089/jpm.2011.0306. [DOI] [PubMed] [Google Scholar]
- 26.Guilfoyle MR, Weerakkody RA, Oswal A, et al. Implementation of neuro-oncology service reconfiguration in accordance with NICE guidance provides enhanced clinical care for patients with glioblastoma multiforme. Br J Cancer. 2011;104(12):1810–1815. doi: 10.1038/bjc.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Medicare & Medicaid Services. New Physician Specialty Code for Hospice and Palliative Care. Available at: http://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/R1715CP.pdf . Accessed February 11, 2014.
- 28.Song Y, Skinner J, Bynum J, et al. Regional variations in diagnostic practices. N Engl J Med. 2010;363(2):45–53. doi: 10.1056/NEJMsa0910881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jagsi R, Abrahamse P, Hawley ST, et al. Underascertainment of radiotherapy receipt in Surveillance, Epidemiology, and End Results registry data. Cancer. 2012;118(2):333–341. doi: 10.1002/cncr.26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du X, Freeman JL, Warren JL, et al. Accuracy and completeness of Medicare claims data for surgical treatment of breast cancer. Med Care. 2000;38(7):719–727. doi: 10.1097/00005650-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Davidoff AJ, Shaffer T, Erten MZ, et al. Use and spending on antineoplastic therapy for Medicare beneficiaries with cancer. Med Care. 2013;51(4):351–360. doi: 10.1097/MLR.0b013e3182726ceb. [DOI] [PubMed] [Google Scholar]
- 32.Cohen MH, Johnson JR, Pazdur R. Food and Drug Administration Drug approval summary: temozolomide plus radiation therapy for the treatment of newly diagnosed glioblastoma multiforme. Clin Cancer Res. 2005;11(19 Pt 1):6767–6771. doi: 10.1158/1078-0432.CCR-05-0722. [DOI] [PubMed] [Google Scholar]
- 33.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 34.Park HS, Gross CP, Makarov DV, et al. Immortal time bias: a frequently unrecognized threat to the validity in the evaluation of postoperative radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83(5):1365–1373. doi: 10.1016/j.ijrobp.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 35.Oberndorfer S, Lindeck-Pozza E, Lahrmann H, et al. The end-of-life hospital setting in patients with glioblastoma. J Palliat Med. 2008;11(1):26–30. doi: 10.1089/jpm.2007.0137. [DOI] [PubMed] [Google Scholar]