Abstract
Background
The prognosis of diffuse intrinsic pontine glioma (DIPG) remains poor, with no drug proven to be effective.
Methods
Patients with clinically and radiologically confirmed, centrally reviewed DIPG, who had failed standard first-line therapy were eligible for this multicenter phase II trial. The anti-epidermal growth factor receptor (EGFR) antibody, nimotuzumab (150 mg/m2), was administered intravenously once weekly from weeks 1 to 7 and once every 2 weeks from weeks 8 to 18. Response evaluation was based on clinical and MRI assessments. Patients with partial response (PR) or stable disease (SD) were allowed to continue nimotuzumab.
Results
Forty-four patients received at least one dose of nimotuzumab (male/female, 20/24; median age, 6.0 years; range, 3.0–17.0 years). All had received prior radiotherapy. Treatment was well tolerated. Eighteen children experienced serious adverse events (SAEs). The majority of SAEs were associated with disease progression. Nineteen patients completed 8 weeks (W8) of treatment: There were 2 PRs, 6 SDs, and 11 progressions. Five patients completed 18 weeks (W18) of treatment: 1 of 2 patients with PR at W8 remained in PR at W18, and 3 of 6 children with SD at W8 maintained SD at W18. Time to progression following initiation of nimotuzumab for the 4 patients with SD or better at W18 was 119, 157, 182 and 335 days, respectively. Median survival time was 3.2 months. Two patients lived 663 and 481 days from the start of nimotuzumab.
Conclusions
Modest activity of nimotuzumab in DIPG, which has been shown previously, was confirmed: A small subset of DIPG patients appeared to benefit from anti-EGFR antibody treatment.
Keywords: children, diffuse intrinsic pontine glioma, epidermal growth factor receptor, nimotuzumab, phase II trial
The dismal prognosis for children with diffuse intrinsic pontine glioma (DIPG) is well documented. Ninety percent or more of affected children will succumb to their disease within 2 years, and the median overall survival in prospective trials is 8–12 months.1,2 Focal radiation (RT) is the only proven treatment, but it does not prevent the inevitable tumor recurrence.3 Following RT, the majority of children will show clinical benefit with improvement, or even disappearance of neurological symptoms, and general well-being. However, once DIPG children show recurrence of their symptoms, their average life span is usually about 3 months. Over the last 3 decades, numerous clinical trials have been conducted and failed to show any significant survival benefit.4–11
Nimotuzumab is a monoclonal antibody, which is produced in Cuba, and is directed against the human epidermal growth factor receptor (EGFR). It was originally intended to be used for adult cancers of epithelial origin, either alone or in combination with radiation and chemotherapy.12 In 2005, a phase II German study of nimotuzumab described 3 partial responses at week 21, suggesting promising activity in children with recurrent high-grade glioma and DIPG.13 The aim of our collaborative study was to confirm these findings in a larger population of children with progressive DIPG.
Patients and Methods
Inclusion and Exclusion Criteria
Children aged between 3 and 18 years with progressive or recurrent DIPG following standard of care first-line therapy were eligible. Strict eligibility criteria included radiologically verified DIPG with pontine epicenter involving at least two-thirds the pons, presence of 2 of the 3 classical clinical signs or symptoms (cranial nerve and corticospinal tract deficits and ataxia) at initial diagnosis, and duration of initial symptoms <6 months.
Standard of care first-line therapy was defined as focal RT ≥54 Gy with or without chemotherapy or biological agents. Patients with evidence of either clinical and/or radiological progression (in the case of chemotherapy) during or following first-line therapy (radiotherapy completed ≥12 weeks prior) were eligible. Progression was defined by the occurrence of new symptoms and/or worsening of existing symptoms (cranial nerve deficit, ataxia, long tract signs) on 2 consecutive clinic visits. If such progression was associated with acute neurological deterioration, then its presence at one clinic visit was sufficient for study entry. Clinical progression was also sufficient, even in the absence of confirmatory imaging changes. Radiological progression, however, did not qualify for enrollment if there were no accompanying clinical signs or symptoms of progression.
Further eligibility criteria included a Lansky or Karnofsky performance status >40%, radiologically measurable tumor in at least 2 dimensions, and adequate hematological, renal, and hepatic function. For female patients of childbearing age, a negative pregnancy test was required. For both sexes, if applicable, use of effective contraception was required throughout participation in the study. Patients with disseminated CNS disease, neurofibromatosis type 1, or known contraindications to monoclonal antibodies were excluded.
Each participating center had to obtain institutional review board approval of the study's protocol prior to enrolling patients. Written informed consent and/or assent from patients or their legal representatives was obtained for all participants.
Study Conduct
The baseline brain MRI included axial T1, without and with gadolinium, axial T2 or fluid-attenuated inversion recovery (FLAIR) weighted images, and a sagittal sequence. Follow-up brain MRIs were repeated at weeks 9 and 19 and thereafter as clinically indicated. Spinal MRI was optional, depending upon clinical symptoms. Neurological status and standard blood studies (CBC and comprehensive metabolic panel) were monitored at study entry and at the same intervals as MRI.
Drug Preparation, Administration, and Treatment Plan
Nimotuzumab was supplied by YM Biosciences as an injectable solution in single-use, 10 mL vials containing 5 mg/mL of product. It required dilution in 0.9% NaCl to a final concentration of 1 mg/mL. The dose and volume of the infused study drug were calculated basedupon the participant's body surface area at 150 mg/m2. During the induction phase, participants received nimotuzumab infusions over 30–60 minutes using a low protein binding 0.22 µm in-line filter once weekly for 8 weeks. During the following consolidation phase, nimotuzumab infusions were given once every 2 weeks for 10 weeks (5 infusions). Continuation of treatment beyond these phases was permitted at the discretion of the investigator and the sponsor/designee until progression of disease or occurrence of unacceptable toxicity.
Toxicity and Dose Adjustments
Participants were monitored for adverse events (AEs) at each study visit. As a protocol requirement, participants were to be observed for any potential AE from the start of the nimotuzumab infusion until at least 1 hour after the end of the infusion. Toxicities were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0 of the National Cancer Institute. Grade 5 (death) toxicity includes death not otherwise specified (NOS) and disease progression NOS.
Criteria for Response and Progression
All participants who received at least one dose of nimotuzumab were evaluable for objective response rate. Baseline scans were required no later than 14 days prior to the start of treatment. Initial response evaluation was performed at the completion of the induction phase after 8 weeks and at the completion of the consolidation phase after 18 weeks, as applicable. Tumor response assessments were based on 3-dimensional (3D) MRI measurements. Two-dimensional (2D) measurements were allowed only in the rare circumstance when the length of a lesion could not be determined. Assessments were performed by both the investigator and an independent tumor assessment board (ITAB) unaware of the participant's clinical condition. Tumor response criteria were determined by changes in size using width (W), transverse (T), and length (L) measurements on either T2 or FLAIR-weighted MRIs. Complete response (CR) was defined as disappearance of all measurable disease. Partial response (PR) was defined in 3D response criteria as ≥65% decrease, and progressive disease (PD) was defined as ≥40% increase of the 3 perpendicular diameters. When the length of the lesion was not determinable, comparison of the product of the longest diameter and its longest perpendicular diameter (TxW) was used. 2D measurement response criteria defined PR as ≥50% decrease and PD as ≥25% increase in the products of the 2 perpendicular diameters. Objective response (OR) was defined as all patients with either PR or CR. Stable disease (SD) was defined as the absence of CR, PR, or PD. Treatment was discontinued in case of global deterioration in a participant's physical or neurological condition, regardless of the radiological assessment. Hence, clinical progression was considered as tumor progression (PD).
Trial Design and Statistical Methods
The study population was defined as the intent-to-treat population including all study patients who received at least one dose of nimotuzumab. Those participants were evaluated for safety, response, and progression-free survival (PFS). A safety monitoring committee was established and conducted regular safety reviews.
The OR rate was defined as the number of participants experiencing an OR at week 8 plus the number of participants who were assessed as SD at that time but demonstrated OR after the consolidation phase, divided by the number of enrolled participants who received at least one dose of nimotuzumab.
Based on the hypothesis of a 15% response rate with treatment (vs 0% without) and an a priori determined 1-sided significance level of.025 with a 90% power, a total of 44 participants were needed for study recruitment, considering an expected 10% dropout rate.
Results
Study Cohort
Forty-four of the 46 participants enrolled into the trial from February 2008 to June 2010 received at least one course of nimotuzimab and were considered eligible and evaluable for toxicity and response. The median age of the study population was 6.0 years (range, 3.0–17.0 years). Twenty-four participants (54.5%) were female, and 25 (56.8%) were of Caucasian descent. Thirty-two children had a Karnofsky/Lansky score > 60% at the time of study enrollment (Table 1). All participants had prior radiotherapy, and 25 (57%) had received prior chemotherapy. The most commonly used chemotherapy agents at the time of initial treatment were temozolomide (n = 17; 38.7%), etoposide (n = 13; 29%), cisplatin (n = 8; 18.2%), and vincristine (n = 7; 15.9%). Four participants (9.1%) had received bevacizumab as part of their first-line treatment. Thirty-five participants (79.5%) were receiving dexamethasone at the time of study enrollment.
Table 1.
Patient characteristics
| No. | % | |
|---|---|---|
| ITT population | 44 | 100 |
| Age (years) | ||
| >3 ≤ 12 | 39 | 88.6 |
| >12 | 5 | 11.4 |
| Gender | ||
| Female | 24 | 54.5 |
| Male | 20 | 45.5 |
| Race | ||
| Caucasian | 25 | 56.8 |
| Hispanic/Latin American | 6 | 13.6 |
| Asian | 4 | 9.1 |
| African American | 3 | 6.8 |
| Other | 6 | 13.6 |
| Lansky/Karnofsky status | ||
| 100%–91% | 6 | 13.6 |
| 90%–81% | 9 | 20.5 |
| 80%–61% | 17 | 38.4 |
| 60%–41% | 12 | 27.3 |
| Prior surgery | ||
| Biopsy | 2 | 4.5 |
| Ventriculostomy | 1 | 2.3 |
| Shunt insertion | 6 | 13.6 |
Abbreviation: ITT, intention to treat.
Toxicity and Safety
A total of 320 nimotuzumab infusions were given, and all but one participant had at least one adverse event (AE). The majority of AEs was mild to moderate (grade 1–2) in severity and were unrelated/unlikely related to the study drug but were rather due to CNS dysfunction and disease progression. A total of 24 participants (54.5%) reported 54 study drug-related AEs, all of which were grade 1–2 except for 4 participants. Three of those 4 participants experienced grade 3 lymphopenia, neutropenia, and hypokalemia. Eighteen participants experienced serious adverse events (SAEs), including 3 children who experienced 4 grade 5 SAEs. Two SAEs occurred after the ninth injection in one participant. This participant's MRI revealed both intracranial tumor hemorrhage and tumor necrosis, each considered as separate SAEs. This participant died 12 days after the last dose of nimotuzumab. Two other participants died due to progressive neurologic dysfunction and cardiac arrest; both events were considered unrelated SAEs.
Radiological and Clinical Response
Nineteen participants completed the induction phase and underwent clinical and MRI assessment at week 8. Five participants completed the consolidation phase, 3 of whom continued to receive nimotuzumab beyond the 18th week.
Independent central radiological review was conducted on all participants. 3D measurements were available for all participants except for 8 in whom only 2D measurements were reliably feasible. Radiological responses observed at week 8 included 2 PRs, 6 SD, and 11 progressions (PDs). One of 2 participants with PR at week 8 remained in PR at week 18, and 3 of 6 participants (13.6% of the original cohort) with SD at week 8 maintained stable disease at week 18. The time to progression following initiation of nimotuzumab treatment for the 3 children with SD and the one with PR at week 18 was 119, 157, 335, and 182 days, respectively. Eventually, all tumors progressed. The 2 longest study survivors lived for 663 and 481 days after the start of nimotuzumab.
Of the nineteen participants who completed the induction phase and underwent clinical and MRI assessment at week 8, 6 children were not on steroids at both inclusion and week 8. The steroid dose for the remaining 13 children remained unchanged (n = 5), decreased (n = 5), increased (n = 2), and unknown (n = 1). Mean changes in performance status (increases and decreases) from baseline were noted and fluctuated over the course of the study. No clinically meaningful changes were documented.
Survival
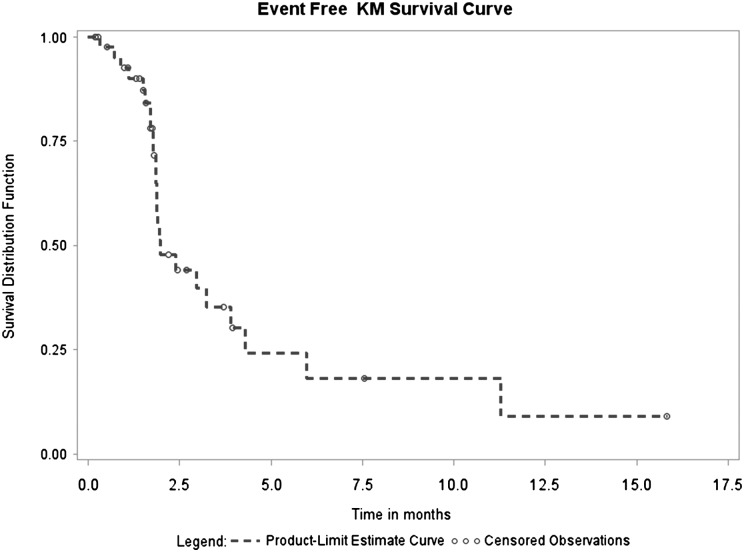
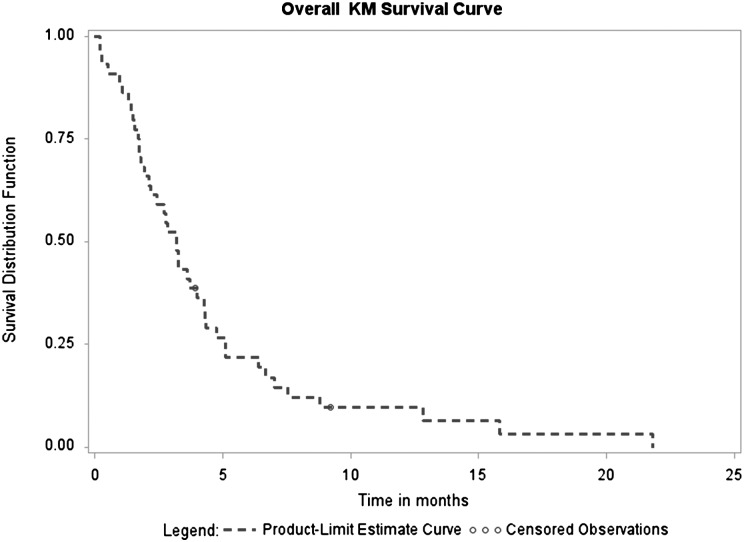
Median time to progression was 1.7 months (95% CI, 1.4–1.9 months) with a range of 0.2–11.0 months. Median survival time for the entire cohort was 3.2 months (95% CI, 2.2–4.3 months) with a range 0.2–21.8 months (Figs 1 and 2).
Fig. 1.
Progression-free survival distribution.
Fig. 2.
Overall survival.
The mean overall survival of participants who completed induction therapy and showed progressive disease after completion of induction therapy (n = 11) was 146 days (range, 87–268 days), which did not differ significantly compared with the overall survival of 282 days (range, 85–663 days) in participants with SD/PR (n = 8) (P = .06).
Discussion
The epidermal growth factor receptor (EGFR) has been implicated in the development of high-grade astrocytic tumors. In the adult population, more than 40% of glioblastomas harbor EGFR amplification, mutation, or overexpression.14 By contrast, EGFR amplification is uncommon in pediatric high-grade gliomas and DIPGs, although EGFR overexpression is often detected without gene amplification.15 In a study performed on postmortem tissue, Zarghooni et al described EGFR immunopositivity in 7 of 11 (64%) DIPGs and copy number gain in one.16 The UK and French group conducted a trial of the EGFR inhibitor erlotinib in children with DIPG and recurrent high-grade gliomas. In that trial, tissue collection was mandated for inclusion. Overexpression of EGFR (assessed by immunohistochemistry) was found in 8 of 20 (40%) brainstem tumors and 6 of 8 (75%) supratentorial lesions.17 Since EGFR overexpression or level of activity has been associated with responsiveness to EGFR inhibitors in solid tumors, in particular lung cancer, the use of EGFR inhibitors has raised significant hope for the management of high-grade gliomas.14
The primary aim of this phase II study of nimotuzumab was to confirm early data from Fleischhack et al, who reported promising responses in pediatric patients with recurrent high-grade gliomas and DIPG.13 In that trial, 20 children with recurrent DIPG and high-grade glioma were treated with a weekly IV infusion of 150 mg/m2 nimotuzumab for 6 weeks. Those who did not show evidence of progression were eligible for a consolidation phase consisting of 4 150 mg/m2 infusions given at a 3-week interval. Six of 17 eligible participants demonstrated partial response or stable disease at the end of the 6-week induction phase, including 4 with recurrent DIPG. The authors subsequently updated these results and reported 1 partial response and 9 SD at the end of the 8-week induction period in a group of 21 participants with recurrent DIPG. At week 21, they described 3 partial responses including a sustained response in a participant who received the drug for 14.5 months.13 Our results confirm these results: we observed 2 partial responses in our study cohort. The difference in the response rate is within the range of variations expected in such clinical trials, and the fact that 2 independent studies done in 2 different continents showed similar results adds validity to the finding that a small proportion of patients with progressive/recurrent DIPG responds to the anti-EGFR antibody nimotuzumab.
Nimotuzumab was well tolerated, without severe skin toxicities or adverse gastrointestinal effects. The majority of adverse events were attributed to disease progression. One study patient suffered intratumoral hemorrhage and tumor necrosis. While a causal relationship to the treatment with nimotuzumab cannot be ruled out with certainty, the occurrence of spontaneous intratumoral bleeds in the disease course of DIPGs is reported in nearly 20% of patients and is most commonly located in the necrotic areas.18
Based on promising results in recurrent tumors, an international study of radiation plus nimotuzumab was conducted in newly diagnosed DIPG patients. Forty-one patients were enrolled in this study. The median progression-free survival in this trial was 5.5 ± 0.2 months, and the median overall survival was 9.6 ± 1 months, revealing no significant improvement over historical controls. Unfortunately, the hopes that the promising results observed in recurrent tumors could be reproduced in newly diagnosed patients did not come true.19 This difference between the promising results of the phase II study in the recurrent setting and the disappointing outcome observed in the frontline study confirms the fact that only a small subset of patients (in the range of 5%–10%) seems to benefit from this antibody. Therefore, it is not surprising that the results of the phase II study did not translate into a survival benefit in the context of an upfront study in an unselected group of DIPG patients. Notwithstanding these results, a pilot study was initiated combining nimotuzumab with vinorelbine and radiation. At a median follow-up of 9 months, 8 of ten children were alive without progressive disease.20
Interpretation of these data is complex, as the main limitation of all these trials is the lack of tissue material, which precludes analysis of molecular correlates of clinical response. At the time this study was conducted, the data on safety of biopsies at initial diagnosis of DIPGs had not yet matured,21,22 and the requirement for a biopsy at the time of recurrence as an eligibility criterion was considered unethical. In our experience, as in the original phase II study, it appears that some patients benefitted from the administration of nimotuzumab, as demonstrated by the evidence of partial responders in both studies. Survival times longer than 6 months are exceptional following DIPG progression, and both studies reported patients with sustained tumor control. However, in the absence of molecular data, it is impossible to know whether the responders had tumors that exhibited EGFR overexpression or amplification.
EGFR antibodies, such as nimotuzumab, bind to the extracellular domain and result in a blockade of ligand binding and receptor activation. EGFR can also be targeted by inhibitors of receptor tyrosine activity (so-called “small molecules”), which bind to ATP in a reversible fashion, inhibit ATP, and stop subsequent downstream signaling.12 Several clinical trials with these small molecule EGFR inhibitors have been conducted in patients with DIPG (Table 2). In these studies, as in ours, there appears to be a subset of patients who may benefit from these agents. The Pediatric Brain Tumor Consortium conducted 2 studies of gefitinib in combination with radiation in children with DIPG. The phase I study reported 1-year overall and event-free survival rates of 48% and 16%, respectively.23 The results of the phase II study reported an overall 1-year survival of 56%, and 3 of 43 patients remained progression free with 36 months of follow-up.24 In the UK-French phase I study of erlotinib, histological confirmation of the diagnosis of brainstem gliomas was mandated for eligibility, allowing correlation of tumor biology with response. This trial reported a median survival of 12 months and a trend towards improved progression-free survival (P = .058) among the group of patients with EGFR overexpression.17 Finally, a phase I study of the small-molecule inhibitor of VEGFR2 and EGFR, vandetanib, given in combination with radiation, reported a 1-year survival rate of 37.5%.25 However, that trial also described the unusual occurrence of 3 patients who were free of disease progression for more than 2 years. In summary, these data suggest that not all DIPGs are the same and that a small subgroup of patients, for whom EGFR inhibition might be beneficial, needs to be identified up front; otherwise, the small response rates and rare prolongations of life will appear insignificant among the much larger number of patients without benefit.26–28 These results underscore the importance of biopsy-driven targeted therapies for DIPG patients.
Table 2.
Phase I/II trials with epidermal growth factor receptor small molecules in diffuse intrinsic pontine glioma
| Drug | Trial | No. of Patients | 1-year EFS | 1-year OS | Median EFS in Months | Median OS in Months | Number of Patients alive >2 years | Reference |
|---|---|---|---|---|---|---|---|---|
| Gefitinib | Phase I | 20 | 16.1 ± 7.4% | 48 ± 11% | 24 | |||
| Gefitinib | Phase II | 44 | 20.9 ± 5.6% | 56.4 ± 7.6% | 7.4 | 3 (at 3 years) | 25 | |
| Erlotinib | Phase I | 21 | 8 | 12 | NA | 18 | ||
| Vandetanib | Phase I | 21 | 21.4% ± 11% | 37.5% ± 10.5% | 3 | 26 | ||
| Vandetanib & dasatinib | Phase I | 25 | 52% ± 10% | 2 | 29 |
Abbreviations: EFS, event-free survival; NA, not applicable; OS, overall survival; SE, standard error.
In conclusion, this trial confirmed the results observed in an earlier report of nimotuzumab in children with progressive DIPG: The 2 partial responses observed and the sustained tumor control experienced by one patient are noteworthy and intriguing. Undoubtedly, this phase II trial of nimotuzumab adds to the ongoing debate regarding the need to develop targeted strategies based on molecular characterization based on stereotactic biopsies in DIPG trials. The feasibility of this approach is currently assessed in cooperative studies (http://clinicaltrials.gov/ct2/show/NCT01182350). These studies will probably confirm the large molecular heterogeneity of DIPGs that precludes a “one drug fits all” approach and the need to explore the feasibility and relevance of tailored strategies to improve the outcome of this devastating condition.
Conflict of interest statement. None declared.
Funding
YM Biosciences Inc.
References
- 1.Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7(3):241–248. doi: 10.1016/S1470-2045(06)70615-5. [DOI] [PubMed] [Google Scholar]
- 2.Jansen MH, van Vuurden DG, Vandertop WP, Kaspers GJ. Diffuse intrinsic pontine gliomas: a systematic update on clinical trials and biology. Cancer Treat Rev. 2012;38(1):27–35. doi: 10.1016/j.ctrv.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Bartels U, Hawkins C, Vezina G, Kun L, Souweidane M, Bouffet E. Proceedings of the diffuse intrinsic pontine glioma (DIPG) Toronto Think Tank: advancing basic and translational research and cooperation in DIPG. J Neurooncol. 2011;105(1):119–125. doi: 10.1007/s11060-011-0704-4. [DOI] [PubMed] [Google Scholar]
- 4.Jenkin RD, Boesel C, Ertel I, et al. Brain-stem tumors in childhood: a prospective randomized trial of irradiation with and without adjuvant CCNU, VCR, and prednisone. A report of the Childrens Cancer Study Group. J Neurosurg. 1987;66(2):227–233. doi: 10.3171/jns.1987.66.2.0227. [DOI] [PubMed] [Google Scholar]
- 5.Packer RJ, Boyett JM, Zimmerman RA, et al. Outcome of children with brain stem gliomas after treatment with 7800 cGy of hyperfractionated radiotherapy. A Childrens Cancer Group Phase I/II Trial. Cancer. 1994;74(6):1827–1834. doi: 10.1002/1097-0142(19940915)74:6<1827::aid-cncr2820740628>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 6.Allen JC, Siffert J. Contemporary chemotherapy issues for children with brainstem gliomas. Pediatr Neurosurg. 1996;24(2):98–102. doi: 10.1159/000121024. [DOI] [PubMed] [Google Scholar]
- 7.Bouffet E, Raquin M, Doz F, et al. Radiotherapy followed by high dose busulfan and thiotepa: a prospective assessment of high dose chemotherapy in children with diffuse pontine gliomas. Cancer. 2000;88(3):685–692. doi: 10.1002/(sici)1097-0142(20000201)88:3<685::aid-cncr27>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 8.Lashford LS, Thiesse P, Jouvet A, et al. Temozolomide in malignant gliomas of childhood: a United Kingdom Children's Cancer Study Group and French Society for Pediatric Oncology Intergroup Study. J Clin Oncol. 2002;20(24):4684–4691. doi: 10.1200/JCO.2002.08.141. [DOI] [PubMed] [Google Scholar]
- 9.Wolff JE, Westphal S, Molenkamp G, et al. Treatment of paediatric pontine glioma with oral trophosphamide and etoposide. Br J Cancer. 2002;87(9):945–949. doi: 10.1038/sj.bjc.6600552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korones DN, Fisher PG, Kretschmar C, et al. Treatment of children with diffuse intrinsic brain stem glioma with radiotherapy, vincristine and oral VP-16: a Children's Oncology Group phase II study. Pediatr Blood Cancer. 2008;50(2):227–230. doi: 10.1002/pbc.21154. [DOI] [PubMed] [Google Scholar]
- 11.Cohen KJ, Heideman RL, Zhou T, et al. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children's Oncology Group. Neuro Oncol. 2011;13(4):410–416. doi: 10.1093/neuonc/noq205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam C, Bouffet E, Bartels U. Nimotuzumab in pediatric glioma. Future Oncol. 2009;5(9):1349–1361. doi: 10.2217/fon.09.119. [DOI] [PubMed] [Google Scholar]
- 13.Fleischhack G, Bode U, Buchen S, et al. Phase II study of H-R# monoclonal antibody (Nimotuzumab) against the EGF Receptor in the treatment of resistent or relapsed high-grade gliomas in children and adolescents [abstract] Pediatr Blood Cancer. 2005;45(6):444. [Google Scholar]
- 14.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353(19):2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 15.Pollack IF, Hamilton RL, James CD, et al. Rarity of PTEN deletions and EGFR amplification in malignant gliomas of childhood: results from the Children's Cancer Group 945 cohort. J Neurosurg. 2006;105(5 Suppl):418–424. doi: 10.3171/ped.2006.105.5.418. [DOI] [PubMed] [Google Scholar]
- 16.Zarghooni M, Bartels U, Lee E, et al. Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor alpha and poly (ADP-ribose) polymerase as potential therapeutic targets. J Clin Oncol. 2010;28(8):1337–1344. doi: 10.1200/JCO.2009.25.5463. [DOI] [PubMed] [Google Scholar]
- 17.Geoerger B, Hargrave D, Thomas F, et al. Innovative Therapies for Children with Cancer pediatric phase I study of erlotinib in brainstem glioma and relapsing/refractory brain tumors. Neuro Oncol. 2011;13(1):109–118. doi: 10.1093/neuonc/noq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broniscer A, Laningham FH, Kocak M, et al. Intratumoral hemorrhage among children with newly diagnosed, diffuse brainstem glioma. Cancer. 2006;106(6):1364–1371. doi: 10.1002/cncr.21749. [DOI] [PubMed] [Google Scholar]
- 19.Fleischhack G, Siegler N, Zimmermann M, et al. Concomitant therapy of Nimotuzumab and standard radiotherapy for the treatment of newly diagnosed diffuse intrinsic pontine gliomas in children and adolescents [abstract] Neuro Oncol. 2010;12(6):ii9. [Google Scholar]
- 20.Massimino M, Bode U, Biassoni V, Fleischhack G. Nimotuzumab for pediatric diffuse intrinsic pontine gliomas. Expert Opin Biol Ther. 2011;11(2):247–256. doi: 10.1517/14712598.2011.546341. [DOI] [PubMed] [Google Scholar]
- 21.Chassot A, Canale S, Varlet P, et al. Radiotherapy with concurrent and adjuvant temozolomide in children with newly diagnosed diffuse intrinsic pontine glioma. J Neurooncol. 2012;106(2):399–407. doi: 10.1007/s11060-011-0681-7. [DOI] [PubMed] [Google Scholar]
- 22.Puget S, Blauwblomme T, Grill J. Is Biopsy Safe in Children with Newly Diagnosed Diffuse Intrinsic Pontine Glioma? Am Soc Clin Oncol Educ Book. 2012;32:629–633. doi: 10.14694/EdBook_AM.2012.32.59. [DOI] [PubMed] [Google Scholar]
- 23.Geyer JR, Stewart CF, Kocak M, et al. A phase I and biology study of gefitinib and radiation in children with newly diagnosed brain stem gliomas or supratentorial malignant gliomas. Eur J Cancer. 2010;46(18):3287–3293. doi: 10.1016/j.ejca.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollack IF, Stewart CF, Kocak M, et al. A phase II study of gefitinib and irradiation in children with newly diagnosed brainstem gliomas: a report from the Pediatric Brain Tumor Consortium. Neuro Oncol. 2011;13(3):290–297. doi: 10.1093/neuonc/noq199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broniscer A, Baker JN, Tagen M, et al. Phase I study of vandetanib during and after radiotherapy in children with diffuse intrinsic pontine glioma. J Clin Oncol. 2010;28(31):4762–4768. doi: 10.1200/JCO.2010.30.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khuong-Quang DA, Buczkowicz P, Rakopoulos P, et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012;124(3):439–447. doi: 10.1007/s00401-012-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puget S, Philippe C, Bax DA, et al. Mesenchymal transition and PDGFRA amplification/mutation are key distinct oncogenic events in pediatric diffuse intrinsic pontine gliomas. PLoS One. 2012;7(2):e30313. doi: 10.1371/journal.pone.0030313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu G, Broniscer A, McEachron TA, et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44(3):251–253. doi: 10.1038/ng.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broniscer A, Baker SD, Wetmore C, et al. Phase I trial, pharmacokinetics, and pharmacodynamics of vandetanib and dasatinib in children with newly diagnosed diffuse intrinsic pontine glioma. Clin Cancer Res. 2013;19(11):3050–3058. doi: 10.1158/1078-0432.CCR-13-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]