Abstract
Background
Several variables are associated with the likelihood of isocitrate dehydrogenase 1 or 2 (IDH1/2) mutation in gliomas, though no guidelines yet exist for when testing is warranted, especially when an R132H IDH1 immunostain is negative.
Methods
A cohort of 89 patients was used to build IDH1/2 mutation prediction models in World Health Organization grades II–IV gliomas, and an external cohort of 100 patients was used for validation. Logistic regression and backward model selection with the Akaike information criterion were used to develop prediction models.
Results
A multivariable model, incorporating patient age, glioblastoma multiforme diagnosis, and prior history of grade II or III glioma, was developed to predict IDH1/2 mutation probability. This model generated an area under the curve (AUC) of 0.934 (95% CI: 0.878, 0.978) in the external validation cohort and 0.941 (95% CI: 0.918, 0.962) in the cohort of The Cancer Genome Atlas. When R132H IDH1 immunostain information was added, AUC increased to 0.986 (95% CI: 0.967, 0.998). This model had an AUC of 0.947 (95% CI: 0.891, 0.995) in predicting whether an R132H IDH1 immunonegative case harbored a less common IDH1 or IDH2 mutation. The models were also 94% accurate in predicting IDH1/2 mutation status in gliomas from The Cancer Genome Atlas. An interactive web-based application for calculating the probability of an IDH1/2 mutation is now available using these models.
Conclusions
We have integrated multiple variables to generate a probability of an IDH1/2 mutation. The associated web-based application can help triage diffuse gliomas that would benefit from mutation testing in both clinical and research settings.
Keywords: glioma, IDH1, IDH2
Mutations in isocitrate dehydrogenase types 1 and 2 (IDH1/2) are present in the majority of grades II and III astrocytomas, oligodendrogliomas, and mixed oligoastrocytomas, as well as about 10% of grade IV glioblastoma multiforme (GBM).1 With rare exceptions, these heterozygous point mutations focus on codon 132 of IDH1 and 172 of IDH2, producing mutant enzymes that reduce α-ketoglutarate to D-2-hydroxyglutarate (D-2-HG).2 Because mutant tumors tend to be far less aggressive than their grade-matched wild-type counterparts, and because the presence of a mutation can help differentiate infiltrative gliomas from nonneoplastic mimickers and noninfiltrative tumors,3–5 IDH1/2 testing has become part of the routine workup of any lesion even suspected of being a diffusely infiltrative glioma.
Overall, nearly 90% of gliomas that have an IDH1/2 mutation will contain the R132H IDH1 variant, which prompted the development of an R132H IDH1–specific monoclonal antibody that works very well as a rapid, specific, inexpensive screen.6–8 But while a positive immunohistochemical result does not need confirmatory sequencing, sometimes testing for less common IDH1 and IDH2 mutations is warranted. This is also relevant for any retrospective brain tumor studies that utilize tissues predating routine clinical IDH1/2 testing, because immunostaining and reflex sequencing of all archived tumors would not be cost-effective.
To date, no formal evidence-based guidelines exist for when full IDH1/2 testing is recommended in the case of an unequivocally infiltrative glioma. And while several variables are known to influence the likelihood of a mutation, including patient age, World Health Organization (WHO) grade, and prior history of grades II–III glioma,1 no models have yet been published integrating all these variables into a single pretest probability. In this paper, we have developed statistical models that can accurately predict mutation likelihood, both before R132H IDH1 immunostaining and in the event of a negative immunostain result. We have further implemented our models in a user-friendly web-based application.
Methods
Cohorts
The original cohort for model development included 123 untreated WHO grades II–IV gliomas, patient age 16+ years, from the University of Pittsburgh Medical Center who had been tested for IDH1 and IDH2 mutations by R132H IDH1 immunohistochemistry (Dianova) and follow-up sequencing. The original validation cohort included 100 untreated WHO grades II–IV gliomas, age 16+ years, from the University of Kentucky Markey Cancer Center who had also been tested for IDH1/2 mutations using the same approach. The variables initially considered for predicting IDH1/2 mutations included patient age, tumor location, GBM diagnosis, prior history of grade II or III glioma, and R132H IDH1 immunohistochemistry results. Patients with complete data on these variables were used for the development and validation of the prediction models.
Institutional review boards from the University of Pittsburgh and the University of Kentucky approved this study prior to intramural case collections. Data from The Cancer Genome Atlas (TCGA) on grades II–IV gliomas were downloaded through the University of California, Santa Cruz Cancer Browser at https://genome-cancer.ucsc.edu.9
Statistical Methods
We first considered using patient age and clinical characteristics, including GBM status, tumor location, and prior history of grade II or III glioma to predict the likelihood of IDH1/2 mutation. A multivariable logistic regression model was built using backward model selection with the Akaike information criterion that ended up eliminating location in the final model, which is referred to as model A.
We also considered using R132H IDH1 immunostain status in addition to age and the above clinical characteristics to predict the likelihood of IDH1/2 mutation. Let R denote R132H IDH1 immunostain status (1 = positive, 0 = negative) and X denote age and clinical characteristics. Based on the law of total probability and the fact that the predicted probability of IDH1/2 mutation is given a positive R132H IDH1 immunostain result, some simple algebra yields that given R132H IDH1 immunostain status and X, the predicted probability of IDH1/2 mutation is equal to R + (1 – R)*(p1 – p2)/(1 – p2), where p1 = the predicted probability of IDH1/2 mutation given X, and p2 = the predicted probability of R132H IDH1 immunopositivity given X. Here p1 was obtained by model A. To obtain p2, we built a multivariable logistic regression model for predicting R132H IDH1 immunostain status using a similar method for building model A. The above method for predicting IDH1/2 mutation is referred to as model B.
Model B therefore works in the following way. If a patient is R132H IDH1 positive, then he/she will be classified as IDH1/2 mutated; if a patient is R132H IDH1 negative, then the predicted probability for that patient to have an IDH1/2 mutation is calculated by p = (p1 – p2)/(1 – p2). This formula was derived by the law of total probability: p1 = 1*p2 + p*(1 – p2). In model building, we used data from all patients to calculate p1 and p2 because they are probabilities conditional on only clinical factors but not conditional on R132H IDH1 immunostain status. Although one could use only R132H IDH1–negative patients to directly calculate p, our approach is likely more powerful, since we utilized more patient data.
The prediction models were 10-fold cross-validated on the development cohort and externally validated on the validation cohorts. The discriminative ability of the developed models was studied by receiver operating characteristic (ROC) curves. The area under the ROC curve (AUC) was calculated. The bootstrap method with 500 bootstrap samples was used to obtain the 95% confidence interval (CI) of the AUC.
The statistical analyses were performed by R software version 3.0.1. Particularly, the step function was used for backward model selection on the development cohort, and the ROCR package was used to calculate the AUCs.
The web-based application was developed using an iterative process working closely with the investigators and biostatisticians to validate the results. It is a self-contained client-side Javascript application utilizing the Ext JS framework version 4.2.1 (Sencha). The University of Kentucky Office of Legal Counsel reviewed the website and approved its implementation.
Results
Patient Characteristics
Among 123 patients in the development cohort, 89 had complete data on the variables considered and were used to develop prediction models. All 100 patients in the validation cohort had complete data and were used to validate the developed prediction models. Table 1 summarizes the characteristics of patients in the development and validation cohorts. The general characteristics for both cohorts (patient age, WHO grade frequencies, tumor location, etc) are broadly representative of the general adult glioma population. The 7 categories of tumor location were combined into 4 categories (frontal or not otherwise specified; temporal; parietal; and occipital, basal ganglia, or posterior fossa/spine) due to small numbers of observations in some categories.
Table 1.
Characteristics of patients in the development and validation cohorts
| Characteristic | Development Cohort (n = 89), No. of Patients (%) | Validation Cohort (n = 100), No. of Patients (%) | P* |
|---|---|---|---|
| IDH1/2 mutation | .502 | ||
| Mutated | 39 (43.8) | 39 (39) | |
| Wild type | 50 (56.2) | 61 (61) | |
| R132H IDH1 immuno status | .594 | ||
| Positive | 29 (32.6) | 29 (28.7) | |
| Negative | 60 (67.4) | 71 (71.3) | |
| Age | .115 | ||
| Median (SD; range) | 58 (16.6; 22–85) | 55 (16.7; 20–84) | |
| GBM status | .333 | ||
| Yes | 49 (55.1) | 62 (62) | |
| No | 40 (44.9) | 38 (38) | |
| Location | .290 | ||
| Frontal | 42 (47.2) | 49 (49) | |
| Temporal | 25 (28.1) | 27 (27) | |
| Parietal | 11 (12.4) | 15 (15) | |
| Occipital | 4 (4.5) | 2 (2) | |
| Basal ganglia | 2 (2.2) | 6 (6) | |
| Posterior fossa/spine | 1 (1.1) | 1 (1) | |
| Not otherwise specified | 4 (4.5) | ||
| Prior grade II or III glioma | .450 | ||
| Yes | 18 (20.2) | 16 (16) | |
| No | 71 (79.8) | 84 (84) |
*P-values were calculated based on a 2-sample t-test, Fisher's exact test, or chi-squared test as appropriate.
Data from TCGA included 444 grades II–IV cases, with a median patient age of 57 years (SD = 15.6, range = 18–89). Among these patients, 149 (33.6%) had IDH1/2 mutation, 276 (62.2%) had GBM, and 6 (1.4%) had a reported prior history of grade II or III glioma.
Prediction Models
The predictors identified in model A (see Methods) included age, GBM status, and prior history of grade II or III glioma. Under model A, the predicted probability of IDH1/2 mutation (p1) = 1/{1 + exp(−L1)}, where L1 = 11.2746 − 0.1756*V1 − 4.3305*V2 + 1.6161*V3, and V1 = age, V2 = 1 if tumor is glioblastoma and 0 if not, and V3 = 1 if with prior grade II or III glioma and 0 if not.
The predictors in model B include R132H IDH1 immunohistochemical status in addition to those in model A. Under model B, the predicted probability of IDH1/2 mutation is R + (1 − R)*(p1 − p2)/(1 − p2), where R = 1 if R132H IDH1 is immunopositive and 0 if negative, p1 is defined as above, and p2 = 1/{1 + exp(−L2)}, where L2 = 1.9441 − 0.0387*V1 − 2.3990*V2 + 1.1468*V3 and V1, V2, and V3 are defined as above. In principle, p1 ≥ p2 and thus the predicted probability of IDH1/2 mutation is ≥0. However, in practice, it is possible that p1 < p2. In this case, p1 − p2 is set to 0.
Based on models A and B, a publicly accessible interactive web-based application was created and is hosted by the Markey Cancer Center's Cancer Research Informatics Shared Resource Facility (http://www.kcr.uky.edu/webapps/IDH/app.html). The application accepts input parameters for patients including age, GBM status, prior history of grade II or III glioma, and status of R132H IDH1 testing. Based upon the parameter inputs, the application selects and applies the appropriate model to calculate and display the predicted probability of IDH1/2 mutation. The application also provides an option to display the details of the calculation, including L1, p1, L2, p2, and the selected model.
Validation
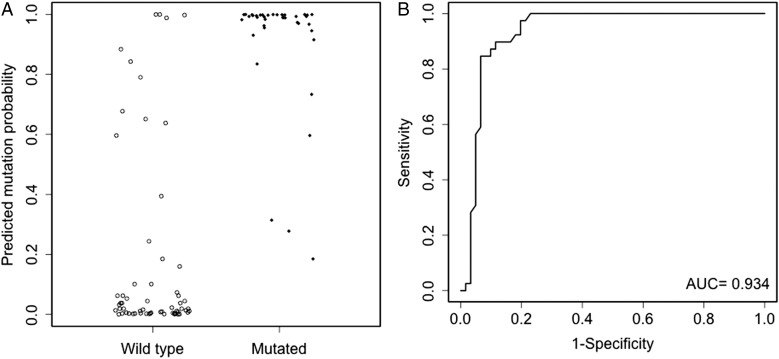
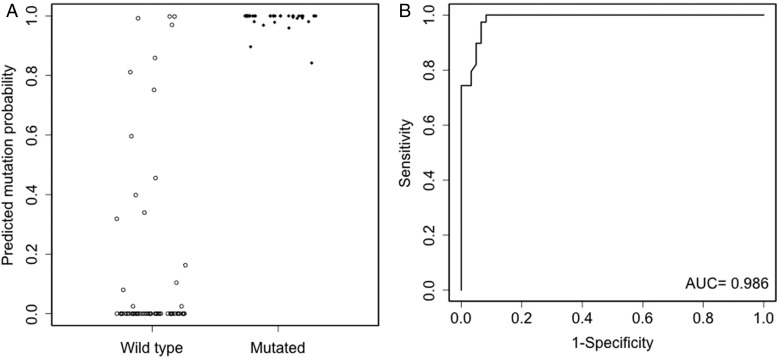
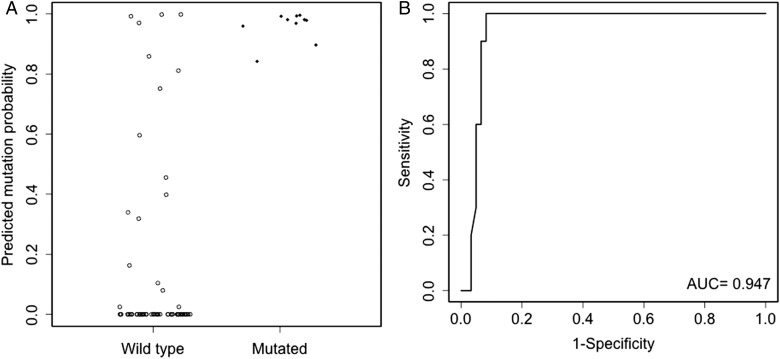
Model A had an AUC of 0.938 for the 10-fold cross-validation in the development cohort. External validation of model A in the validation cohort had an AUC of 0.934 (95% CI: 0.878, 0.978). The corresponding ROC curve is shown in Fig. 1 along with the predicted probabilities of IDH1/2 mutation for mutated and wild-type patients in the validation cohort. With the inclusion of R132H IDH1 immunonegative status, model B had increased AUCs of 0.988 and 0.986 (95% CI: 0.967, 0.998) for the 10-fold cross-validation and external validation, respectively. The increase in AUC comparing model B versus model A for external validation was 0.053 (95% CI: 0.018, 0.095), which was statistically significant. Of particular importance, model B had an AUC of 0.947 (95% CI: 0.891, 0.995) in the R132H IDH1 immunonegative subgroup of the validation cohort. The corresponding ROC curves along with predicted mutation probabilities in the validation cohort and the R132H IDH1 immunonegative subgroup of the validation cohort are shown in Figs. 2 and 3, respectively. In the R132H IDH1 immunonegative subgroup of the validation cohort, the predicted mutation probabilities are very well separated between the mutated and wild-type patients: 100% (10/10) mutated patients have predicted probabilities >0.84, but only 8.2% (5/61) wild-type patients have predicted probabilities >0.84.
Fig. 1.
Evaluation of model A in the validation cohort. The left panel (A) shows model A predicted mutation probabilities for mutated and wild-type patients. The right panel (B) shows the ROC curve of model A. The dots have been jittered for better visualization.
Fig. 2.
Evaluation of model B in the validation cohort. The left panel (A) shows model B predicted mutation probabilities for mutated and wild-type patients. The right panel (B) shows the ROC curve of model B. The dots have been jittered for better visualization.
Fig. 3.
Evaluation of model B in the R132H IDH1 immunonegative subgroup of the validation cohort. The left panel (A) shows model B predicted mutation probabilities for mutated and wild-type patients. The right panel (B) shows the ROC curve of model B. The dots have been jittered for better visualization.
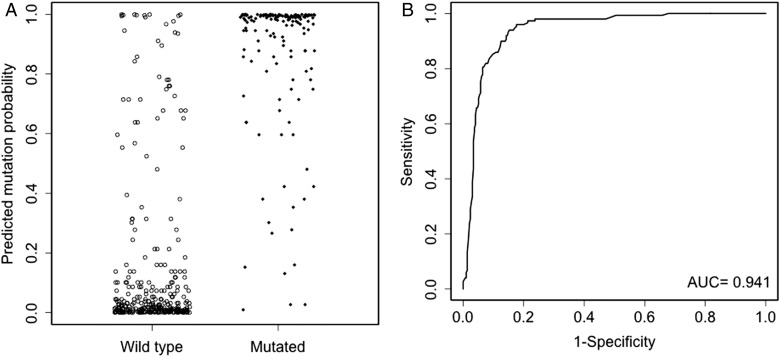
Model A was further validated by TCGA data, which yielded an AUC of 0.941 (95% CI: 0.918, 0.962). The corresponding ROC curve is shown in Fig. 4 along with the predicted probabilities of IDH1/2 mutation for mutated and wild-type patients in the cohort from TCGA. This model proved superior to a case-by-case prediction by the principal investigator (C.H.), who correctly predicted IDH mutation status in 374/444 cases (84%) based on the same variables (patient age, GBM diagnosis, prior history of grades II–III glioma). Model B could not be validated by TCGA data because no information on R132H IDH1 immunostain status was available in the dataset.
Fig. 4.
Evaluation of model A in the cohort of TCGA. The left panel (A) shows model A predicted mutation probabilities for mutated and wild-type patients. The right panel (B) shows the ROC curve of model A. The dots have been jittered for better visualization.
Discussion
In this era of heightened cost-consciousness for both research and clinical endeavors, effective ways of triaging cases that would benefit from targeted molecular testing are valuable. IDH1/2 screening is critical for the clinical care of brain tumor patients and is also necessary for many investigations that utilize archival paraffin-embedded or snap-frozen tissue samples. Although several variables are known to associate with the likelihood of a mutation, many cases will have IDH-related factors that seem to contradict each other. For example, a grade II astrocytoma in a 35-year-old who presented with seizures should certainly be screened for less common IDH1 and IDH2 mutations if the R132H IDH1 immunostain is negative. But it is less obvious what to do with an immunonegative GBM in a 26-year-old, or an immunonegative glioma that appears grade III but is in a 64-year-old patient with de novo presentation of the tumor. Furthermore, on rare occasions the R132H IDH1 antibody is falsely negative. The web-based application described here, which utilizes advanced statistical modeling to produce an integrated mutation probability with and without R132H IDH1 immunostain data, should therefore be useful to clinicians, pathologists, and researchers.
A large meta-analysis revealed that mutation frequencies vary between studies depending on whether tumors were only screened via R132H IDH1, IDH1 sequencing, or combined IDH1 and IDH2 sequencing, with the highest frequencies reported when both genes were fully screened.1 In that analysis, about 11% of grades II–III astrocytomas had a mutation that would not have been detected by R132H IDH1 immunohistochemistry. Likewise, 3% and 14% of mutations in grades II and III oligodendrogliomas would have also been missed by immunostaining. Thus, any workup of gliomas that uses only R132H IDH1 immunohistochemistry will miss some mutations. Such an approach would thus adversely skew prognosis and perhaps even clinical trial eligibility in a subset of patients. Yet, adjusting for the fact that the most common infiltrative glioma, GBM, is also the least likely to have a mutation, automatic reflex sequencing of all immunonegative gliomas, or sequencing all gliomas while skipping the immunostain, is probably excessive. This web-based application can therefore help focus additional studies on tumors that have a reasonable chance of being mutated.
Use of this application comes with some caveats. This is not meant for pre- or perioperative workup of a patient, but rather to assist with postsurgical evaluation of the specimen, as well as to help researchers studying retrospective tumor collections. We did not include astrocytic versus oligodendroglial morphology as a criterion because overall mutation rates among grades II and III astrocytomas and oligodendrogliomas are similar.1 Because IDH1/2 mutations are more associated with late adolescent and adult gliomas, the development and validation cohorts excluded pediatric cases, so this application cannot be used in that age group. This application is also not meant for cases in which the biopsy was suboptimal and a firm diagnosis of infiltrative glioma cannot be made. In such a setting, the R132H IDH1 immunostain has a better chance of detecting rare scattered infiltrating glioma cells than can even pyrosequencing (but of course not if the tumor is a less common IDH1/2 variant). This is also not intended for other neoplasms in which IDH1/2 mutations are seen, such as acute myeloid leukemias, intrahepatic cholangiocarcinomas, and chondroid tumors. Finally, we cannot recommend a specific percent cutoff above which a tumor should always be screened for a mutation. For now, that will depend on the judgment of each user.
In the future, additional variables may be added to further improve the predictive accuracy of this application. For example, absent nuclear staining for alpha thalassemia/mental retardation syndrome X-linked is strongly associated with an IDH1/2 mutation in astrocytomas.10 Likewise, loss of expression of branched chain amino-acid transaminase 1 could help identify R132H IDH1 immunonegative cases requiring additional sequencing.11 And specialized radiologic imaging to detect elevated levels of mutant IDH1/2-associated D-2-HG may someday be routine,12–16 but as of now it is not, since it requires advanced expertise and techniques. Similarly, next-generation sequencing could eventually replace evidence-based triaging, though currently next-generation sequencing is cost-prohibitive for routine use in all cases.
In summary, we have developed a novel web-based interactive means to obtain an accurate estimation of IDH1/2 mutation probability in a glioma, both before R132H IDH1 immunohistochemistry and in the event of a negative immunostain result. This application, which is analogous to online tests in colorectal carcinomas for Lynch syndrome (http://premm.dfci.harvard.edu/) and microsatellite instability (http://sitemaker.umich.edu/gruber.lab/files/msi_pre.htm), is part of a trend toward more sophisticated algorithms and statistical modeling to improve the screening of patients likely to have actionable genetic alterations. We anticipate the development of more and better ways of screening for additional mutations, so as to maximize cost-efficiency while maintaining a high standard of patient care.
Funding
C.H. was supported by the National Cancer Institute (K08CA155764), the Peter and Carmen Lucia Buck Training Program in Translational Clinical Oncology, and the University of Kentucky College of Medicine Physician Scientist Program. The University of Kentucky Biospecimen and Tissue Procurement, Biostatistics, and Cancer Research Bioinformatics Shared Resource Facilities are supported by the Markey Cancer Center (P30CA177558).
Acknowledgments
Thanks to Dana Napier of the Markey Biospecimen and Tissue Procurement (BSTP) Shared Resource Facility and Monika Ellis from the Department of Pathology for their excellent histologic expertise. Thanks also to the Markey Cancer Center Research Communications Office for their help in formatting the figures.
Conflict of interest statement. None declared.
References
- 1.Horbinski C. What do we know about IDH1/2 mutations so far, and how do we use it? Acta Neuropathol. 2013;125(5):621–636. doi: 10.1007/s00401-013-1106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horbinski C, Kofler J, Yeaney G, et al. Isocitrate dehydrogenase 1 analysis differentiates gangliogliomas from infiltrative gliomas. Brain Pathol. 2011;21(5):564–74. doi: 10.1111/j.1750-3639.2011.00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camelo-Piragua S, Jansen M, Ganguly A, et al. A sensitive and specific diagnostic panel to distinguish diffuse astrocytoma from astrocytosis: chromosome 7 gain with mutant isocitrate dehydrogenase 1 and p53. J Neuropathol Exp Neurol. 2011;70(2):110–115. doi: 10.1097/NEN.0b013e31820565f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camelo-Piragua S, Jansen M, Ganguly A, et al. Mutant IDH1-specific immunohistochemistry distinguishes diffuse astrocytoma from astrocytosis. Acta Neuropathol. 2010;119(4):509–511. doi: 10.1007/s00401-009-0632-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capper D, Reuss D, Schittenhelm J, et al. Mutation-specific IDH1 antibody differentiates oligodendrogliomas and oligoastrocytomas from other brain tumors with oligodendroglioma-like morphology. Acta Neuropathol. 2011;121(2):241–252. doi: 10.1007/s00401-010-0770-2. [DOI] [PubMed] [Google Scholar]
- 7.Capper D, Weissert S, Balss J, et al. Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol. 2009;20(1):245–254. doi: 10.1111/j.1750-3639.2009.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capper D, Zentgraf H, Balss J, et al. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol. 2009;118(5):599–601. doi: 10.1007/s00401-009-0595-z. [DOI] [PubMed] [Google Scholar]
- 9.Cline MS, Craft B, Swatloski T, et al. Exploring TCGA pan-cancer data at the UCSC Cancer Genomics Browser. Sci Rep. 2013;3:2652. doi: 10.1038/srep02652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiestler B, Capper D, Holland-Letz T, et al. ATRX loss refines the classification of anaplastic gliomas and identifies a subgroup of IDH mutant astrocytic tumors with better prognosis. Acta Neuropathol. 2013;126(3):443–51. doi: 10.1007/s00401-013-1156-z. [DOI] [PubMed] [Google Scholar]
- 11.Mayers JR, Vander Heiden MG. BCAT1 defines gliomas by IDH status. Nat Med. 2013;19(7):816–817. doi: 10.1038/nm.3263. [DOI] [PubMed] [Google Scholar]
- 12.Elkhaled A, Jalbert LE, Phillips JJ, et al. Magnetic resonance of 2-hydroxyglutarate in IDH1-mutated low-grade gliomas. Sci Transl Med. 2012;4(116):116ra115. doi: 10.1126/scitranslmed.3002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaumeil MM, Larson PE, Yoshihara HA, et al. Non-invasive in vivo assessment of IDH1 mutational status in glioma. Nat Commun. 2013;4:2429. doi: 10.1038/ncomms3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi C, Ganji SK, DeBerardinis RJ, et al. 2-Hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med. 2012;18(4):624–629. doi: 10.1038/nm.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andronesi OC, Kim GS, Gerstner E, et al. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med. 2012;4(116):116ra114. doi: 10.1126/scitranslmed.3002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pope WB, Prins RM, Albert Thomas M, et al. Non-invasive detection of 2-hydroxyglutarate and other metabolites in IDH1 mutant glioma patients using magnetic resonance spectroscopy. J Neurooncol. 2012;107(1):197–205. doi: 10.1007/s11060-011-0737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]