Abstract
Background
Currently, there are no known effective treatments for recurrent glioblastoma once patients have progressed on a bevacizumab-containing regimen. We examined the efficacy of adding nitrosoureas to bevacizumab in patients who progressed while on an initial bevacizumab-containing regimen.
Methods
In this retrospective study, we identified adult patients with histologically confirmed glioblastoma (WHO grade IV) who were treated with lomustine or carmustine in combination with bevacizumab as a second or third regimen after failing an alternative initial bevacizumab-containing regimen. Response rate (RR), 6-month progression free survival (PFS6), and progression-free survival (PFS) were assessed for each treatment.
Results
Forty-two patients were identified (28 males) with a median age of 49 years (range, 24–78 y). Of 42 patients, 28 received lomustine (n = 22) or carmustine (n = 6) with bevacizumab as their second bevacizumab-containing regimen, and 14 received lomustine (n = 11) or carmustine (n = 3) as their third bevacizumab-containing regimen. While the median PFS for the initial bevacizumab-containing regimen was 16.3 weeks, the median PFS for the nitrosourea-containing bevacizumab regimen was 6.3 weeks. Patients had an RR of 44% and a PFS6 rate of 26% during the initial bevacizumab regimen and an RR of 0% and a PFS6 rate of 3% during the nitrosourea-containing bevacizumab regimen. There was increased grade 3–4 toxicity (45% vs 19%, P = .010) during the nitrosourea-containing bevacizumab regimen relative to the initial bevacizumab regimen. Median overall survival was 18.7 weeks from initiation of the nitrosourea-containing bevacizumab regimen.
Conclusion
The addition of lomustine or carmustine to bevacizumab after a patient has already progressed on a bevacizumab-containing regimen does not appear to provide benefit for most patients and is associated with additional toxicity with the doses used in this cohort.
Keywords: bevacizumab, malignant glioma, nitrosoureas, recurrent glioblastoma
Glioblastoma (GBM) is the most common primary malignant brain tumor in adults, with an annual incidence of 3–4 cases per 100 000.1 Despite the best available treatment, the prognosis for patients with recurrent GBM remains dismal, with a median survival of 25–40 weeks.2–4 Bevacizumab is a humanized antibody against vascular endothelial growth factor that received accelerated approval by the Food and Drug Administration in 2009 for the treatment of recurrent GBM.5,6 Despite prolonged progression-free survival (PFS) and high response rates following treatment in comparison with historical controls,3,7,8 more than half of patients fail to demonstrate response, and the observed response is often short-lived.9,10 Due to a lack of effective alternate therapies, a common practice at disease progression has been to continue bevacizumab with a change in concurrent chemotherapy.11,12 Although there is a lack of prospective data, a retrospective pooled analysis has indicated that the continuation of bevacizumab may be associated with a modest improvement in overall survival (OS).13
Prior to the advent of temozolomide, nitrosoureas were the primary chemotherapeutic agents for treating newly diagnosed primary adult brain tumors, with some encouraging results in meta-analyses.14,15 A large randomized trial of a nitrosourea-containing regimen, however, did not demonstrate a survival benefit.16 While temozolomide has become a standard component of first-line therapy for newly diagnosed GBM,17 prior and ongoing studies have indicated possible benefits from the combination of bevacizumab and nitrosoureas in recurrent GBM.18,19 Given the lack of clear options after a patient fails initial bevacizumab therapy, we explored the efficacy of adding nitrosoureas to bevacizumab for patients who progressed on an initial bevacizumab-containing regimen with respect to response rate (RR), progression-free survival (PFS), and OS.
Materials and Methods
Patients
This retrospective study was approved by the institutional review board at Dana-Farber/Harvard Cancer Center. We retrospectively reviewed records at Dana-Farber/Harvard Cancer Center between June 2005 and December 2011. During this period, a pharmacy list indicated that 427 patients were initiated on a bevacizumab-containing regimen. We included only cases that met the following criteria: (1) pathologically proven glioblastoma (WHO Grade IV) or variants; (2) failed therapy on an initial bevacizumab-containing regimen; and (3) received a nitrosourea agent in addition to bevacizumab as the second or third bevacizumab-containing regimen after progressing on a prior bevacizumab regimen.
There was no limit on the number of prior treatment regimens or recurrences allowed. A chart review was conducted on the 42 cases that met the above criteria. Patient, treatment, pathological characteristics, and outcomes were reviewed, including O6-methylguanine–DNA methyltransferase (MGMT) status as determined by methyl-specific polymerase chain reaction if available. MRI images with contrast were obtained every 8 weeks or earlier at the provider's discretion. Laboratory tests, including complete blood count, serum electrolytes, and liver function, were assessed every 2–3 weeks or at shorter intervals depending upon results. These were graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE), version 4.0 (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf).
Treatment Response and Progression Assessment
Previously published Response Assessment in Neuro-Oncology (RANO) criteria, including parameters for changes in the T1-weighted gadolinium-enhancing lesion and nonenhancing T2/FLAIR progression, were used to assess treatment response.20 Imaging was reviewed independently of clinical data to determine radiographic response and radiographic progression. As defined by the RANO criteria, the 2-D measurements were the sum of the products of the largest diameters and their maximum perpendicular diameters in the axial plane. In order to quantitatively assess nonenhancing disease, abnormal T2/FLAIR signal intensity was also measured as the sum of the products of the largest diameter(s) and their maximum perpendicular diameter(s). The pattern of progression was categorized as local recurrence of contrast-enhancing disease, distant recurrence of contrast-enhancing disease, or nonenhancing progression by the imaging reviewer. Clinical decline, according to provider notes, was also used as a criterion for progression. If a patient died due to presumed progressive disease in the absence of radiographic evidence of progression, the death date was used as the date of progression. PFS and OS were calculated with respect to the date of bevacizumab-therapy initiation. PFS6 was defined as the percentage of patients alive and progression-free at 24 weeks.
Statistical Analysis
The primary outcome measures for this study were RR, PFS, and PFS6. The Kaplan-Meier method was used to provide median point estimates and time-specific rates. The Cox proportional hazards model was used in univariable and multivariable settings to identify significant factors associated with PFS and OS. Differences in PFS were analyzed by log-rank testing, and differences in RR and PFS6 were analyzed by chi-square testing. Appropriate subanalyses were planned to account for significant clinical variables. All statistical analyses were performed using STATA, version 12.0.
Results
Patient Characteristics
From the initial pharmacy database, 42 cases were selected using the screening criteria. Patient characteristics are summarized in Table 1. There were 28 males (14 females), and the median patient age was 49 years (range 24–78 y). Thirty-eight patients (90.5%) were diagnosed with primary GBM, while the remainder had secondary GBM: 3 patients (7.1%) whose tumor arose from low-grade glioma and one patient (2.4%) whose tumor arose from anaplastic oligoastrocytoma. At the time of GBM diagnosis, 36 patients (85.7%) underwent surgical resection, and 6 patients (14.3%) underwent biopsy. Of 25 patients (60%) with known MGMT status, the tumors of 15 were unmethylated, 9 were methylated, and one was partially methylated.
Table 1.
Patient characteristics
| Characteristics | Median (range) or n (%) |
|---|---|
| Age, years | 49 (24–78) |
| KPS score | |
| Prior to first bevacizumab-containing regimen (available for n = 28) | 80 (60–100) |
| Prior to nitrosourea-containing bevacizumab regimen (available for n = 22) | 80 (50–100) |
| Sex, n (%) | |
| Males | 28 (66.7%) |
| Females | 14 (33.3%) |
| Histology, n (%) | |
| GBM | 38 (90.5%) |
| GBM arising from oligoastrocytoma (grade II) | 2 (4.8%) |
| GBM arising from anaplastic astrocytoma (grade III) | 1 (2.4%) |
| GBM arising from diffuse astrocytoma (grade II) | 1 (2.4%) |
| Number of chemotherapy regimens prior to first bevacizumab containing regimen for recurrence of GBM | |
| No prior regimens | 29 (69.0%) |
| 1 prior regimen | 9 (21.4%) |
| 2 prior regimens | 4 (9.5%) |
| MGMT Status n (%), | |
| Unmethylated | 15 (36%) |
| Methylated | 9 (21%) |
| Partially methylated | 1 (2%) |
| Unknown | 17 (40%) |
Abbreviation: GBM, glioblastoma.
By Kaplan-Meier estimates, the median overall survival (mOS) of the cohort from the day of diagnosis was 92.4 weeks. Univariable analysis indicated that tumor size (bidimensional product of contrast-enhancing tumor) at the time of bevacizumab initiation was a significant predictor of OS (continuous, P = .002). On the other hand, age (P = .805), sex (P = .144), original diagnosis (GBM vs low-grade glioma, P = .307), number of recurrences prior to first bevacizumab regimen (P = .663), steroid dose (P = .185), and Karnofsky performance score (KPS) at the time of bevacizumab initiation (P = .658) were not significant in the Cox proportional hazards model for OS. At the time of analysis, 38 of the 42 patients had died.
First Bevacizumab-containing Regimen
During the initial bevacizumab regimen, 21 patients (50%) received monotherapy, 13 patients received irinotecan with bevacizumab, 6 patients received temozolomide with bevacizumab, and 2 patients received panobinostat with bevacizumab. At the time of initiation, patients with available data (n = 27) had a median KPS of 80% (range, 60%–100%). The median number of recurrences at the time of bevacizumab initiation was one (range 1–3). In terms of bevacizumab dosage, 37 (85.7%) patients received 10 mg/kg every 2 weeks, 4 (9.5%) patients received 15 mg/kg every 3 weeks, and one (2.4%) patient received 5 mg/kg every 2 weeks. In our analysis, 39 of the 42 patients progressed on their initial bevacizumab regimen. One patient was discontinued from bevacizumab monotherapy regimen to pursue more aggressive therapy. One patient was discontinued from the initial regimen due to intractable nausea and gastrointestinal symptoms. Finally, one patient was receiving bevacizumab and irinotecan, and irinotecan was discontinued for suspicion of inducing failure to thrive. All 3 cases were censored appropriately; they would each progress on their second bevacizumab-containing regimen.
Sixteen patients (38.1%) did not require steroids at the time of initiation of their first bevacizumab-containing regimen. Fifteen of these 16 patients were not on steroids at the termination date of their regimen, while one patient was receiving 8 mg of dexamethasone daily. Among the remaining 26 patients (61.9%), median daily dexamethasone dose was 4 mg (range, 0.125–24 mg) at the time of initiation. In this group receiving steroids, 20 patients had a decreased dose at the time of regimen termination (median decrease, 4 mg; range, 0.125–20 mg decrease), 3 patients had a stable dose, and 3 patients had an increase in steroid dose. Median OS was 39.0 weeks from initiation of initial bevacizumab.
Nitrosourea-containing Bevacizumab Regimen
Per selection criteria, each patient failed initial bevacizumab therapy and received a nitrosourea with bevacizumab as either a second or third bevacizumab-containing regimen. Of the 42 patients, 28 received lomustine (n = 22) or carmustine (n = 6) as their second bevacizumab-containing regimen, while 14 received lomustine (n = 11) or carmustine (n = 3) as their third bevacizumab-containing regimen. Lomustine or carmustine was administered once every 6 weeks. Doses were determined by the provider. For those patients who received lomustine, 19 received 110 mg/m2, 6 received 130 mg/m2, 3 received 100 mg/m2, 3 received 90 mg/m2, and one received 120 mg/m2. The lomustine dose for one patient was prescribed through a local institution, and dosage was not available. For those who received carmustine, 4 patients received 200 mg/m2, 2 patients received 175 mg/m2, 2 patients received 150 mg/m2, and one patient received 180 mg/m2. Of note, one patient received erlotinib in addition to bevacizumab and lomustine. At time of regimen initiation, patients with available data (n = 22) had a median KPS of 80% (range, 50%–100%). Of the 42 patients, 36 had imaging available to determine RR. It should be noted that 2 patients died prior to their first posttreatment scan, and one patient was discontinued due to toxicity prior to the first posttreatment scan. Among the 8 patients who received at least 2 cycles of a nitrosourea agent, 5 (62.5%) required a dose reduction during treatment due to toxicity. Two patients had lomustine dose reduced to 90 mg/m2 (from 110 and 130 mg/m2), 2 patients had lomustine dose reduced to 80 mg/m2 (from 110 mg/m2), and one patient had carmustine dose reduced from 200 to 150 mg/m2.
Twelve patients (28.6%) did not require steroids at the time of initiation of their nitrosourea-containing bevacizumab regimen, and none of them were receiving steroids at the termination date of their regimen. Among the remaining 30 patients (71.4%), median daily dexamethasone dose was 4 mg (range, 0.125–24 mg). Of those patients receiving steroids, 5 patients had a decreased dose at time of regimen termination (median decrease, 3 mg; range, 1–8 mg decrease), 10 patients had a stable dose, and 15 patients had an increase in steroid dose (median increase, 4 mg; range, 2–12 mg increase) at the time of regimen termination.
In our analysis, 36 patients had progressed on their nitrosourea-containing bevacizumab regimen. Six patients did not progress on their regimen but had treatment terminated due to toxicity; 4 patients had myelosuppression, and one patient had proteinuria. One patient had treatment terminated because surgical intervention was needed for a nonhealing wound. The median OS was 18.7 weeks from time of nitrosourea-containing bevacizumab regimen initiation.
Clinical Outcomes of Initial Bevacizumab Regimen and Subsequent Nitrosourea-containing Bevacizumab Regimen
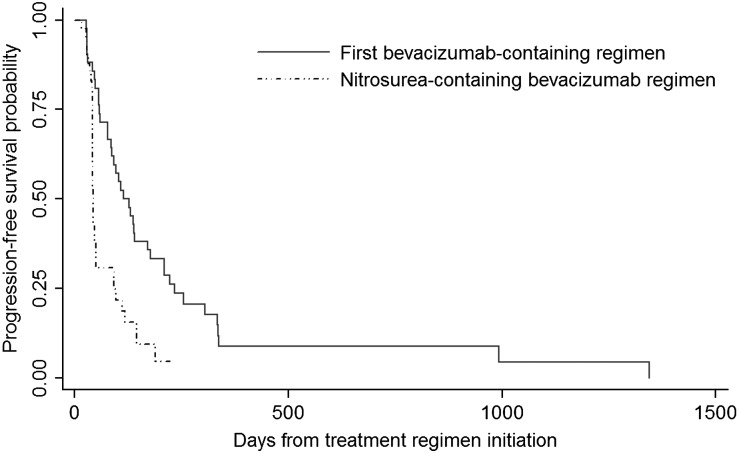
Results of clinical outcomes are presented in Table 2. As shown in Fig. 1, patients had a mPFS of 16.3 weeks during initial bevacizumab-containing regimen and a mPFS of 6.3 weeks during nitrosourea-containing bevacizumab regimen as their second or third bevacizumab regimen. Among patients who received a nitrosourea-containing bevacizumab regimen as their second bevacizumab regimen specifically (n = 28), mPFS was 6.6 weeks.
Table 2.
Summary of primary endpoints
| Endpoint | During First Bevacizumab-Containing Regimen | During Nitrosourea-Containing Bevacizumab Regimen | P value |
|---|---|---|---|
| RR | 44% | 0 | <.001a |
| PFS6 | 26.2% | 2.7% | .038a |
| Grade 3–5 toxicity event | 19.0% | 45.2% | .010a |
| Median PFS | 16.3 weeks | 6.3 weeks | <.001b |
Abbreviations: PFS, progression-free survival; PFS6, 6-month progression-free survival; RR, response rate.
aBy chi-square testing.
bBy log-rank testing.
Fig. 1.
Kaplan-Meier progression-free survival graph comparing the first bevacizumab-containing regimen (solid line) with the subsequent nitrosourea-containing bevacizumab regimen (dashed line). Progression-free survival was significantly decreased during the subsequent nitrosourea-containing bevacizumab regimen (P value = .0001 by log-rank test).
Response assessment stratified by regimen is presented in Table 3. RR during the initial bevacizumab regimen was 44% and 0% during the nitrosourea-containing bevacizumab regimen. PFS6 rate during the initial bevacizumab regimen was 26.2% versus 2.7% during the nitrosourea-containing bevacizumab regimen. While there was a 19% incidence of grade 3 or 4 toxicity during the initial bevacizumab regimen, there was a 45.2% incidence of grade 3 or 4 toxicity during the nitrosourea-containing bevacizumab regimen. Toxicity events are further detailed in Table 4.
Table 3.
Radiographic response rates
| Best Radiographic Responsea | During First Bevacizumab-Containing Regimen (Scans Available in 39 Patients), n (%) | During Nitrosourea-Containing Bevacizumab Regimen (Scans Available in 36 Patients), n (%) |
|---|---|---|
| Complete response | 0 | 0 |
| Partial response | 17 (44%) | 0 |
| Stable disease | 13 (33%) | 9 (25%) |
| Progressive disease | 9 (23%) | 27 (75%) |
aDefined by RANO criteria.
Table 4.
Grade 3 or 4 toxicities during treatment
| Toxicity | During First Bevacizumab-Containing Regimen, n (%) | During Nitrosourea-Containing Bevacizumab Regimen, n (%) |
|---|---|---|
| Neutropenia | 0 | 4 (9.5%) |
| Thrombocytopenia | 0 | 12 (28.6%) |
| Pulmonary embolism/venous thrombosis | 3 (7.1%) | 1 (2.4%) |
| Intracranial hemorrhage | 0 (0%) | 1 (2.4%) |
| Hypertension | 3 (7.1%) | 3 (7.1%) |
| Elevation in aspartate transaminase and/or alanine transaminase | 1 (2.4%) | 0 |
| Fatigue | 1 (2.4%) | 0 |
| Diarrhea | 1 (2.4%) | 0 |
| Nonhealing wound | 0 | 1 (2.4%) |
| Total number of patients requiring termination of treatment due to toxicity | 2 (4.8%) | 6 (14.3%) |
Subanalysis: Examining PFS in Second Bevacizumab-containing Regimen
Given that the majority of patients receiving a nitrosourea-containing bevacizumab regimen progressed on the first posttreatment scan (Table 3), we examined how patients receiving a nitrosourea as their second bevacizumab-containing regimen (n = 28) compared with patients receiving a different bevacizumab-containing regimen (n = 14) with respect to PFS. These 14 patients were heterogeneous: 5 patients received irinotecan, 4 patients received carboplatin, one patient received dose-dense temozolomide, one patient received erlotinib in addition to bevacizumab, and 3 patients received bevacizumab monotherapy as their second bevacizumab-containing regimen. Patients receiving a nitrosourea in their second bevacizumab-containing regimen (mPFS, 6.6 weeks) did not have a significantly different PFS compared with patients receiving an alternate regimen (mPFS, 12.0 weeks) by log-rank test (P = .1641).
Subanalysis: Patients with Stable Disease at Posttreatment Imaging During Nitrosourea-containing Bevacizumab Regimen
None of the patients receiving a salvage nitrosourea-containing bevacizumab regimen achieved complete response or partial response. However, 9 patients achieved stable disease (SD), which may still represent a clinically meaningful outcome for this patient population. Among these 9 patients, median age was 57 years (range 31–78 y). When the first bevacizumab regimen was initiated, median KPS was 85% (n = 4 available), and all 9 patients were at first recurrence. Finally, 6 of these 9 patients received a nitrosourea as part of their second bevacizumab-containing regimen, while 3 patients received it as part of their third bevacizumab-containing regimen. By Kaplan-Meier estimates for these 9 patients, the mPFS was 16.9 weeks, and the mOS was 58.3 weeks from the time of initiation of nitrosourea-containing bevacizumab regimen.
Table 5 summarizes the characteristics of patients exhibiting SD or progressive disease (PD) as their best RR with the nitrosourea-containing bevacizumab regimen. Prior to initiating the nitrosourea-containing bevacizumab regimen, the median bidimensional contrast-enhancing tumor size of the 9 patients who eventually exhibited SD was 9.0 cm2, which was not significantly different from the median tumor size of patients with PD (median, 15.3 cm2, P = .206 by t test). The tumors of 4 patients with SD were MGMT methylated or partially methylated, and the tumors of 2 patients with SD were unmethylated. At the time of progression during initial bevacizumab regimen among patients with SD, 7 patients (78%) exhibited local recurrence of contrast-enhancing disease, and 2 patients (22%) exhibited nonenhancing progression.
Table 5.
Characteristics stratified by best radiographic response on nitrosourea-containing bevacizumab regimen
| Stable Disease (n = 9) | Progressive Disease (n = 27) | |
|---|---|---|
| KPS prior to initial bevacizumab regimen, median (range) | 85% (60%–90%)a | 80% (70%–100%)b |
| Age (years), median (range) | 57 (31–78) | 49 (29–69) |
| MGMT Status, n (%) | ||
| Methylated | 3 (33%) | 6 (22%) |
| Partially methylated | 1 (11%) | 0 (0) |
| Unmethylated | 2 (22%) | 10 (37%) |
| Unknown | 3 (33%) | 11 (41%) |
| Recurrences prior to initial bevacizumab regimen, n (%) | ||
| 0 | 9 (100%) | 16 (59%) |
| ≥1 | 0 (0) | 11 (41%) |
| Pattern of progression on initialb bevacizumab regimen, n (%) | ||
| Local recurrence of contrast-enhancing disease | 7 (78%) | 23 (85%) |
| Distant recurrence of contrast-enhancing disease (>3 cm from initial lesion) | 0 | 2 (7%) |
| Nonenhancing (T2/FLAIR) progression of disease | 2 (22%) | 2 (7%) |
| Baseline 2-D tumor size during initiation of nitrosourea-containing bevacizumab regimen, median (range) | 9.0 cm2 (1.8–28.1) | 15.3 cm2 (1.4–44.0) |
aAvailable for 4 of 9 patients.
bAvailable for 8 of 27 patients.
cIf patient did not progress on first bevacizumab regimen (n = 3), pattern of progression on second bevacizumab regimen was utilized.
Discussion
There is currently no effective therapy for patients with recurrent GBM once they progress on a bevacizumab-containing regimen. The present study specifically examined the efficacy of adding a nitrosourea agent (lomustine or carmustine) to bevacizumab for patients failing their first bevacizumab-containing regimen. After progression on bevacizumab, patients receiving a nitrosourea in combination with bevacizumab exhibited a RR of 0%, a PFS6 rate of 2.7%, and a mPFS of 6.6 weeks. Our findings are consistent with prior studies examining other salvage therapies following bevacizumab failure.3,12,21 The lack of objective radiographic response and the poor clinical outcomes in this cohort of 42 patients underscores the lack of antitumor activity when a nitrosourea is added to bevacizumab after bevacizumab failure.
While other studies have demonstrated the inefficacy of alternative bevacizumab regimens at progression,3,11,12,22 they have not specifically examined the addition of a nitrosourea agent to bevacizumab. Two studies have examined patients primarily switching from bevacizumab with concurrent irinotecan to carboplatin at progression,11,12 while 2 other studies examined patients who were switched from bevacizumab monotherapy to bevacizumab combined with a cytotoxic agent.3,22 Our results would indicate that bevacizumab with a nitrosourea is similarly ineffective. With a relative dearth of literature on third- or fourth-line therapy for recurrent GBM, the study indicates that the cohort receiving continued bevacizumab with a nitrosourea after bevacizumab failure has a dismal prognosis with a median OS of 18.7 weeks.
With respect to steroid requirement, 15 of 30 (50%) of patients on steroids required an increased steroid dose during their nitrosourea-containing bevacizumab regimen. While this was likely attributable to progressive disease, all 12 patients who were steroid-free at the time of initiating nitrosourea-containing bevacizumab regimen were maintained without steroids at the time of regimen termination, which highlights the anti-edema effect of bevacizumab that has been part of the rationale for continuing it at progression.13
Our study does not address the use of nitrosoureas in combination with bevacizumab as the initial bevacizumab-containing regimen. There is growing evidence that nitrosoureas, when combined with bevacizumab, may be effective for patients with recurrent GBM who are bevacizumab- naïve, including the preliminary results from the Dutch BELOB study.18 This randomized phase II trial in bevacizumab- naïve patients with recurrent GBM at first relapse suggests a potential PFS and OS benefit from the combination of lomustine and bevacizumab compared with bevacizumab alone or lomustine alone. Our findings suggest that the timing of initiating nitrosourea with bevacizumab may be critical to the effectiveness of this regimen since it was not effective for the vast majority of our cohort of patients. Although speculative, it appears that genetic and molecular changes to glioblastoma, which may lead to progression in spite of bevacizumab, may also make the tumor more resistant to a combination of nitrosourea and bevacizumab. Wiestler et al investigated the sequential therapy of bevacizumab and nitrosourea in recurrent glioma and demonstrated that earlier treatment with bevacizumab was not associated with better outcome.23 That study, however, did not specifically examine the combination of bevacizumab and a nitrosourea.
While it was ineffective for most patients in our cohort, it is unclear whether there is a subset of patients who may benefit from adding nitrosourea to bevacizumab after bevacizumab failure. To further examine this subset of patients, we characterized the 9 patients with SD on early posttreatment imaging (Table 5). These patients tended to have a higher KPS (median, 85%) with fewer prior recurrences. All 9 patients were at first recurrence when they initiated their first bevacizumab regimen. This subset of 9 patients had a mPFS of 16.9 weeks and mOS of 58 weeks, which underscores the need for better predictors to identify patients likely to benefit from continued bevacizumab-containing regimens. In comparison with patients who exhibited PD, there was no clear association between patients with SD and MGMT status or patterns of progression on initial bevacizumab regimen, although the insufficient sample size of these subanalyses makes it difficult to draw conclusions. Of note, 22% of the patients with SD (vs 7% of patients with PD) exhibited nonenhancing progression during their initial bevacizumab regimen. Given recent advancements in our understanding of nonenhancing progression and subsequent outcome,24 future research should evaluate this further.
Our results indicated that increased treatment toxicity resulted from the addition of a nitrosourea agent to bevacizumab. Five patients required termination during the nitrosourea-containing bevacizumab regimen due to toxicity. Furthermore, 45% of patients experienced grade 3 or 4 toxicity during their nitrosourea-containing bevacizumab regimen, which was mostly attributable to an increased incidence of expected neutropenia and thrombocytopenia (Table 4). This safety profile, except for thromboembolic events and hypertension attributable to bevacizumab, appears to be comparable with the data published on lomustine monotherapy toxicity in recent phase III trials of recurrent glioblastoma patients using lomustine doses ranging from 100 to 130 mg/m2.25,26 The Dutch BELOB study has demonstrated that a lower dose of lomustine (90 mg/m2) is generally well tolerated and demonstrates favorable activity.18 In our cohort, only 3 patients initially received 80 or 90 mg/m2 lomustine, while the remaining patients received a higher dose. Therefore, the toxicity seen in our study is likely not reflective of the safety profile that would be seen with lower drug doses such as those used in the Dutch BELOB study. Future research should continue to investigate a lower dose of lomustine, given its favorable safety profile and comparable efficacy.
On the whole, our study supports prior findings that the continuation of bevacizumab with concurrent chemotherapy is not an effective salvage therapy. Nonetheless, the continuation of bevacizumab has been associated with possible benefits in survival and quality of life.13,27 Considering the dismal prognosis of patients with recurrent GBM who have progressed on a bevacizumab-containing regimen, the continuation of bevacizumab after progression may have a role in a subset of patients. Above all, however, there is a clear need for the development of better therapies, as well as comparative prospective studies investigating treatment options for patients who have progressed on initial bevacizumab therapy.
Limitations
As with any retrospective study, there are inherent limitations to our study. In addition to possible selection bias, other limitations include the fact that imaging for determining radiographic response was not available for central review of all cases. Other limitations include a heterogeneous number of prior regimens; some patients were heavily pretreated (3 patients received their first bevacizumab regimen on their third overall recurrence). We also included clinical deterioration as a criterion for progression, as determined by the patient's provider. This determination can be somewhat subjective; however, progression by clinical deterioration without radiographic findings was rare and unlikely to affect our outcome metrics. The patients in our study were treated with various doses of lomustine and carmustine. The number of recurrences prior to treatment with a nitrosourea-containing bevacizumab regimen was also variable in our patient sample. In order to account for the effect of prior recurrences, we performed a subanalysis examining only patients who received a nitrosourea-containing agent as their second overall bevacizumab-containing regimen. The subanalysis demonstrated similar results, and therefore we do not feel that the number of recurrences significantly affected our overall results.
Conclusion
While bevacizumab, possibly in conjunction with a nitrosourea, may be an effective treatment for bevacizumab-naïve patients with recurrent GBM, the practice of adding a nitrosourea to bevacizumab in patients who have already failed bevacizumab does not appear to be an effective salvage therapy for the majority of patients and carries the risk of additional toxicity.
Funding
This study was supported with institutional funds.
Acknowledgments
None.
Conflict of interest statement: Drs Wen and Reardon have research support and have served on an advisory board for Genentech.
References
- 1.2000. Central Brain Tumor Registry of the United States: Statistical report: Primary brain tumors in the United States. Available at: http://www.cbtrus.org/reports/2007-2008/2007report.pdf .
- 2.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17(8):2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 3.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norden AD, Drappatz J, Muzikansky A, et al. An exploratory survival analysis of anti-angiogenic therapy for recurrent malignant glioma. J Neurooncol. 2009;92(2):149–155. doi: 10.1007/s11060-008-9745-8. [DOI] [PubMed] [Google Scholar]
- 5.2012. Genentech, Inc. Avastin (Highlights of Prescribing Information). Available at http://www.gene.com/gene/products/information/pdf/avastin-prescribing.pdf .
- 6.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 7.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 8.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 9.Jakobsen JN, Hasselbalch B, Stockhausen M-T, et al. Irinotecan and bevacizumab in recurrent glioblastoma multiforme. Expert Opin Pharmacother. 2011;12(5):825–833. doi: 10.1517/14656566.2011.566558. [DOI] [PubMed] [Google Scholar]
- 10.Chamberlain MC. Emerging clinical principles on the use of bevacizumab for the treatment of malignant gliomas. Cancer. 2010;116(17):3988–3999. doi: 10.1002/cncr.25256. [DOI] [PubMed] [Google Scholar]
- 11.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70(10):779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 12.Quant EC, Norden AD, Drappatz J, et al. Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab. Neuro Oncol. 2009;11(5):550–555. doi: 10.1215/15228517-2009-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reardon DA, Herndon JE, 2nd, Peters KB, et al. Bevacizumab continuation beyond initial bevacizumab progression among recurrent glioblastoma patients. Br J Cancer. 2012;107(9):1481–1487. doi: 10.1038/bjc.2012.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stenning SP, Freedman LS, Bleehen NM. An overview of published results from randomized studies of nitrosoureas in primary high grade malignant glioma. Br J Cancer. 1987;56(1):89–90. doi: 10.1038/bjc.1987.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine HA, Dear KB, Loeffler JS, et al. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;71(8):2585–2597. doi: 10.1002/1097-0142(19930415)71:8<2585::aid-cncr2820710825>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 16.Medical Research Council Brain Tumor Working Party. Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high-grade astrocytoma: a Medical Research Council trial. J Clin Oncol. 2001;19(2):509–518. doi: 10.1200/JCO.2001.19.2.509. [DOI] [PubMed] [Google Scholar]
- 17.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 18.Taal W, Oosterkamp HM, Walenkamp AME, et al. A randomized phase II study of bevacizumab versus bevacizumab plus lomustine versus lomustine single agent in recurrent glioblastoma: The Dutch BELOB study. J Clin Oncol. 2013;31 (May 20 suppl):2001. [Google Scholar]
- 19.Soffietti R, Trevisan E, Bertero L, et al. Bevacizumab and fotemustine for recurrent glioblastoma: a phase II study of AINO (Italian Association of Neuro-Oncology) J Neurooncol. 2014;116(3):533–541. doi: 10.1007/s11060-013-1317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 21.Lu-Emerson C, Norden AD, Drappatz J, et al. Retrospective study of dasatinib for recurrent glioblastoma after bevacizumab failure. J Neurooncol. 2011;104(1):287–291. doi: 10.1007/s11060-010-0489-x. [DOI] [PubMed] [Google Scholar]
- 22.Chamberlain MC. Role for cytotoxic chemotherapy in patients with recurrent glioblastoma progressing on bevacizumab: a retrospective case series. Expert Rev Neurother. 2012;12(8):929–936. doi: 10.1586/ern.12.84. [DOI] [PubMed] [Google Scholar]
- 23.Wiestler B, Radbruch A, Osswald M, et al. Towards optimizing the sequence of bevacizumab and nitrosoureas in recurrent malignant glioma. J Neurooncol. 2014;117(1):85–92. doi: 10.1007/s11060-013-1356-3. [DOI] [PubMed] [Google Scholar]
- 24.Nowosielski M, Wiestler B, Goebel G, et al. Progression types after antiangiogenic therapy are related to outcome in recurrent glioblastoma. Neurology. 2014;82(19):1684–1692. doi: 10.1212/WNL.0000000000000402. [DOI] [PubMed] [Google Scholar]
- 25.Wick W, Puduvalli VK, Chamberlain MC, et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28(7):1168–1174. doi: 10.1200/JCO.2009.23.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Batchelor TT, Mulholland P, Neyns B, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–3218. doi: 10.1200/JCO.2012.47.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vredenburgh JJ, Cloughesy T, Samant M, et al. Corticosteroid use in patients with glioblastoma at first or second relapse treated with bevacizumab in the BRAIN study. Oncologist. 2010;15(12):1329–1334. doi: 10.1634/theoncologist.2010-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]