Abstract
Survival for patients with glioblastoma, the most common high-grade primary CNS tumor, remains poor despite multiple therapeutic interventions including intensifying cytotoxic therapy, targeting dysregulated cell signaling pathways, and blocking angiogenesis. Exciting, durable clinical benefits have recently been demonstrated for a number of other challenging cancers using a variety of immunotherapeutic approaches. Much modern research confirms that the CNS is immunoactive rather than immunoprivileged. Preliminary results of clinical studies demonstrate that varied vaccine strategies have achieved encouraging evidence of clinical benefit for glioblastoma patients, although multiple variables will likely require systematic investigation before optimal outcomes are realized. Initial preclinical studies have also revealed promising results with other immunotherapies including cell-based approaches and immune checkpoint blockade. Clinical studies to evaluate a wide array of immune therapies for malignant glioma patients are being rapidly developed. Important considerations going forward include optimizing response assessment and identifiying correlative biomarkers for predict therapeutic benefit. Finally, the potential of complementary combinatorial immunotherapeutic regimens is highly exciting and warrants expedited investigation.
Keywords: glioblastoma, immune checkpoint, immunosuppression, immunotherapy, vaccine
Outcome for patients diagnosed with glioblastoma (GBM), the most common malignant primary tumor of the central nervous system (CNS), remains dismal despite clinical investigation of varied therapeutic strategies including intensification of chemotherapy,1 targeting dysregulated cell signaling pathways,2 and most recently antiangiogenic therapy.3,4 Innovative and novel therapeutic approaches are clearly needed.
The efficacy of immunotherapy for cancer has been recently validated by U.S.FDA approval of sipuleucel-T, a dendritic cell (DC) cancer vaccine, as well as ipilimumab, a humanized monoclonal antibody against the cytotoxic T-lymphocyte antigen-4 (CTLA-4) immune checkpoint.5,6 In addition, other immune-based therapies, including blockade of programmed death 1 (PD-1) and its ligand PD-L1 as well as bioengineered chimeric antigen-receptor T cells, have achieved dramatic antitumor benefit for solid tumor and leukemia patients, respectively.7–10 These exciting results have fueled current efforts to evaluate such approaches in neuro-oncology. Interest in exploiting immune responses has been heightened by 2 intriguing historical observations. The first of these, initially noted in the 18th century, was the association linking infection and cancer regression.11 Formal investigations, as exemplified by Coley's toxins, also generated encouraging early results.12 Similarly, anecdotal reports have linked improved outcome with postoperative infection in brain cancer patients,13 and a recent retrospective analysis has documented a 2-fold survival benefit in GBM patients who developed perioperative infections.14 The second observation derives from epidemiologic studies noting an inverse relationship between allergy and glioma risk.15–17 Although mechanisms underlying this relationship remain to be elucidated, it is hypothesized that enhanced immunoreactivity associated with atopy may provide collateral antitumor benefit. These separate observations raise the possibility that harnessed and directed immunoreactivity could provide a therapeutic opportunity to improve outcome for GBM patients.
In this review, we will discuss the current status of active vaccine, cellular, and modulatory immunotherapy approaches for GBM patients (Table 1). We will highlight potential benefits, risks, and limitations of these approaches and conclude with future considerations.
Table 1.
Immunotherapeutic strategies for cancer therapy
| Immunotherapeutic Approach | Class of Agent | Representative Therapeutic | Citations |
|---|---|---|---|
| Cellular | Adoptive T-cell transfer | 70–73 | |
| Chimeric antigen receptor-engineered T cell | CTL019 | 9,10,78–83 | |
| Vaccination | Tumor-associated antigen vaccine | ICT-107; IMA950 | 100,101 |
| Tumor-specific antigen vaccine | Rindopepimut (EGFRvIII) | 103–109 | |
| Whole tumor lysate vaccine | DCVax | 98,99,126–136 | |
| HSP96 vaccine | HSPPC-96 | 147,148 | |
| Tumor-specific mutation vaccine | 125 | ||
| Immunomodulation | Anti-CTLA-4 MAb | Ipilimumab; tremelimumab | 6,173,198,199–207,213–216,231,250 |
| Anti-PD-1 MAb | Nivolumab; labrolizumab; pidilizumab | 8,208,250 | |
| Anti-PD-L1 MAb | BMS-936559; MPDL3280A; MEDI4736 | 7 | |
| Treg-depleting reagent | Dacluzimab | 240,241 |
Organization of the Immune System
The normal immune system is divided into innate (pattern-recognition based) and adaptive (acquired) components. The evolutionarily older innate arm provides plants and higher species with immediate responses against invading pathogens. The innate immune system comprises macrophages, neutrophils, natural killer (NK) cells, basophils, eosinophils, and complement and provides a first line of defense against predetermined, germ-line pathogen-associated molecular patterns (PAMPS) via interaction with Toll-like receptors (TLRs) and other pattern recognition receptors (PRRs).18 Innate immune cells can also present antigens to the adaptive immune system. The adaptive immune system, comprising T and B lymphocytes, evolved phylogenetically with vertebrate species. Adaptive immune responses are highly specific and long lived, resulting in immunological memory. B cells provide humoral immunity, while T cells, which are classified primarily as cytotoxic (CD8+), helper (CD4+), and regulatory (CD4+FoxP3+), generate cell-mediated responses.
The major histocompatibility complex (MHC) displays epitopes of self or nonself proteins degraded intracellularly on the cell surface for T-cell interaction. MHC class 1 molecules are expressed on all nucleated cells and present epitopes to specific cytotoxic CD8+ T-cell receptor (TCR) molecules. CD8+ T-cell–mediated killing ensues when a primary signal, composed of TCR interacting with its specific antigen presented by an MHC class I molecule, is generated along with a secondary signal triggered by binding of CD28 on the CD8+ cell to its ligands (B7-1, B7-2) expressed by the antigen-presenting cell. Primary and secondary signaling also leads to clonal expansion of antigen-specific CD8+ cells. MHC class II molecules are conditionally expressed by all cell types but normally occur on professional antigen-presenting cells, including DCs, macrophages and B cells. Interaction of a T-cell receptor (TCR) with its cognate antigen presented by an MHC class II protein leads to activation of specific and long-lived effector CD4+ T helper cells and immunoregulatory T cells. CD4+ T helper cells establish and optimize several effector programs including: (i) Th1 responses, which are mediated by interferon-gamma (IFN-γ) to activate bactericidal macrophages and production of opsonizing and complement-fixing antibodies by B cells; (ii) Th2 responses, which are mediated by interleukin-4 to activate humoral B-cell responses; and (iii) Th17 responses, which are mediated by interleukin-17 and -22 to provide protection from infections at mucosal surfaces. In contrast, activation of regulatory T cells (Tregs), following binding to antigen presented by MHC II proteins, suppresses immune responses to limit immune-mediated damage and prevent autoimmune reactions.
Immune Privilege of the Central Nervous System: Fact, Fallacy, or In Between
The CNS has traditionally been perceived as being immunologically privileged, implying that the immune system is inactive in the CNS and fails to interact effectively with the systemic immune system. This dogma, which originated from experimental data demonstrating prolonged survival of tissues grafted in the CNS that were rapidly rejected upon grafting elsewhere,19 has been further advocated historically by: (i) limitations of leukocyte entry imposed by the blood brain barrier;20 (ii) lack of CNS lymphatics; and (iii) absence of native T cells in the CNS.21,22 In reality, privilege of the CNS is somewhat limited, and growing evidence argues that the CNS is immunocompetent and interacts dynamically with the systemic immune system.23 The blood-brain barrier is disrupted by tissue injury and inflammation, including that associated with malignant gliomas,24,25 thereby facilitating entry of immune cells into the CNS. In addition, CNS antigens and T cells access cervical lymphatic tissue via drainage through Virchow-Robin spaces.26–28 Furthermore, activated peripheral T cells and antibodies can circumvent the CNS to interact with target antigens,22,29,30 while antigen-specific T cells acquire effector function, proliferate, and are retained in the CNS tumor microenvironment.31 In addition, the resident macrophage population of the CNS, known as microglia, can express MHC class II antigens and T-cell costimulatory cytokines when activated32,33 and are capable of presenting tumor-associated antigens to T lymphocytes.34–36 Finally, active immune responses are well documented in experimental autoimmune encephalitis37 as well as common clinical entities including multiple sclerosis, neurodegenerative conditions, paraneoplastic syndromes, stroke, and autism.
Suppression of Immune Activation: Systemic and Local Factors
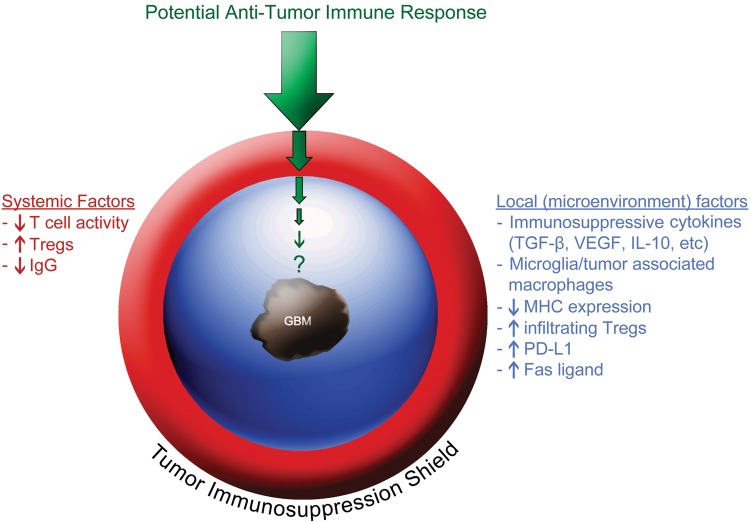
Many cancers, including glioblastoma, exhibit multilayered protective responses that suppress endogenous antitumor immunoreactivity and may limit immunotherapy approaches.38–40 Adaptive local and systemic immunosuppressive forces generated by tumors provide a protective shield to avoid immune rejection that instead fosters immune tolerance (Fig. 1). These forces are typically classified into systemic and local factors. Systemic factors, which act to diminish overall immune reactivity in GBM patients, include decreased T-cell responsiveness, immunoglobulin levels, and monocyte/DC function as well as increased circulating CD4+/CD25+/FoxP3+ Tregs.40–44 In addition, corticosteroids, which are used to diminish cerebral edema, are immunosuppressive, while temozolomide chemotherapy ± radiotherapy can trigger prolonged lymphopenia.45
Fig. 1.
Systemic and local (microenvironment) immunosuppressive adaptations elicited by glioblastoma tumors generating a protective shield against potential antitumor immune responses.
In addition, local immunosuppressive factors with complementary actions can act as a perimeter of immune defense within the GBM microenvironment and include: (i) MHC molecule down regulation;36,46,47 (ii) production of immunoinhibitory cytokines such as transforming growth factor-β (TGF-β),48 vascular endothelial growth factor,49 prostaglandin E2,50 interleukin-10,51 and lectin-like transcript-1;36,52–54 (iii) infiltration of immunosuppressive regulatory T cells (Tregs)36,52,55–58; (iv) polarization of glioma-infiltrating microglia/tumor associated macrophages which can account for up to 40% of glioma mass, toward the immunosuppressive M2 (alternatively activated macrophages) phenotype;59,60 and (v) impairment of T cell function due to hostile physical factors such as hypoxia.61 In addition, apoptosis of activated T cells can be promoted by Fas ligand expressed by malignant glioma cells.62,63
Many cancers, including GBM, also increase expression of immune checkpoint regulators such as programmed death-1 ligand (PD-L1).64 Inhibitory immune checkpoint mediators are normally activated to attenuate appropriate immune responses in order to prevent autoimmunity and local tissue damage.
Cellular Immunotherapeutic Approaches for Glioblastoma
Adoptive T-cell transfer, a therapeutic strategy aimed at infusing T cells with high avidity against tumor antigens, originated from observation of a graft-versus-leukemia effect65 and the use of donor lymphocyte infusions to achieve remission in patients undergoing allogeneic stem cell transplant.66 Subsequent studies successfully utilized Epstein-Barr virus–specific T cells to treat posttransplant lymphoproliferative disease,67 while Rosenberg et al pioneered the administration of expanded tumor-infiltrating lymphocytes to achieve durable responses in a subset of melanoma patients.68 A follow-up study demonstrated that the coadministration of a lymphodepleting preparative regimen enhanced efficacy.69
Early adoptive transfer approaches for malignant glioma patients involved administration of T cells isolated from draining lymph nodes following subcutaneous injection of irradiated tumor cells with granulocyte-macrophage colony-stimulating factor (GM-CSF)70,71 or ex vivo expanded autologous T cells cocultured with tumor cells.72 More recently, the feasibility of expanding autologous, cytomegalovirus (CMV)-specific T cells from CMV-seropositive GBM patients has been reported,73 and a phase I/II clinical trial is ongoing to evaluate the safety and antitumor activity of administering these cells to GBM patients (NCT01205334).
Next generation T-cell transfer therapy includes genetically modified T cells engineered for enhanced reactivity against tumor antigens. Chimeric antigen receptor (CAR) T cells are reprogrammed to express monoclonal antibody-binding domains that trigger T-cell activation and effector function upon tumor antigen binding.74 CARs typically comprise an antigen-binding domain, an extracellular-spacer/hinge region, a transmembrane domain, and an intracellular-signaling domain. Second and third generation CAR T cells are under development that incorporate additional modular genetic modifications to enhance replicative potential, effector function, and in vivo persistence.75,76 Theoretically, CARs can be designed to target any antigen. They also bypass MHC restriction based on their inherent direct antigen-binding capability. CAR T cells thus provide an attractive strategy to overcome MHC downregulation, an immunoevasive mechanism associated with many cancers including GBM.47 In addition, by combining antigen-binding capability and T-cell activation function into one entity, CAR T cells may generate robust immune responses against tumor-associated antigens that are typically abrogated by central tolerance. Enhanced immunoreactivity may also trigger reactions against normal tissues, expressing target antigens as exemplified by a fatal cytokine reaction in a patient treated with ERBB-2 targeting CARs.77 Nonetheless, dramatic proof-of-concept validation of CAR T-cell therapy has recently been achieved in refractory lymphoid leukemia patients.9,10 CAR T cells also offer promise for CNS tumor patients.78 Preclinical studies have shown benefit with CAR T cells engineered to target IL13Rα2l,79,80 Her2,81 and EphA2-positive82 glioma tumors. Currently, clinical trials of CARs targeting EGFRvIII83 (NCT01454596) and HER2 (NCT01109095) are ongoing for GBM patients.
Bispecific T-cell engagers (BiTEs) generate an immunological synapse between polyclonal cytotoxic T cells and tumor cells.84 Composed of a single-chain variable antibody fragment (scFv) to the T-cell activation ligand CD3and linked to a tumor antigen-specific scFv, BiTEs are capable of mediating highly potent tumor cell lysis independent of traditional costimulatory molecules and MHC peptide recognition as well as clonal T-cell expansion and persistence.85 Several BiTEs are in clinical development including blinatumomab,84 a CD19/CD3 BiTE, that has induced durable cellular and molecular remissions in refractory acute lymphoblastic leukemia patients.86 Systemic administration of a BiTE-targeting EGFRvIII has been recently shown in preclinical studies to effectively treat well-established EGFRvIII-positive intracranial GBM tumors.87
Vaccination Therapy for Glioblastoma
Vaccinations sensitize the immune system against target antigens and have successfully prevented or eradicated diseases such as tetanus, polio, smallpox, diphtheria, and pertussis by generating robust, yet specific, immune responses including immunological memory to trigger reactivation upon future antigen exposure. Nonetheless, only modest, inconsistent immune responses and antitumor benefits have been reported about cancer vaccines to date.
A major determinant of vaccine immunogenicity is antigen choice, and a wide array of antigens has been explored in cancer vaccine trials. Elucidation of factors optimizing vaccine immunogenicity will likely include careful evaluation of both quality and quantity of incorporated antigens. Tumor vaccine antigens are broadly classified as tumor associated and tumor specific (Table 2). Tumor-associated antigens (TAAs) are preferentially derived from native proteins or selectively expressed by tumor cells, but they may also be found on normal cells. Native proteins typically generate relatively weak immune responses due to central tolerance, which is the naturally occurring mechanism preventing immune reactivity against normal cells. In contrast, tumor-specific antigens (TSAs) are exclusive to tumor cells and thus typically elicit robust immune responses in a manner analogous to microbial antigens incorporated by infectious disease vaccines.
Table 2.
Antigen categories for antitumor vaccination
| Tumor-associated Antigens | Tumor-specific Antigens | |
|---|---|---|
| Examples | EphA2; Her-2; IL13Rα2; GP100; Mage-1; survivin; hTERT; Trp-1; Aim-2 | EGFRvIII |
| Advantages | Multitude characterized | Elicit potent, robust, and specific immune responses |
| Available for most patients | ||
| Ability to combine multiple targets into cocktail vaccine | ||
| Lowered risk of immune escape | ||
| Disadvantages | Reduced immune response due to central tolerance if expressed by normal tissues | Available for subset of patients |
| Possible immune escape (growth of tumor cells that lack antigen expression) |
Tumor-associated antigens offer several advantages. First, TAAs are prevalent and frequently expressed by gliomas.88–99 Second, targeting TAAs can be done using off-the-shelf synthesized peptides for most patients, given the prevalent expression of these antigens by glioma tumors. In addition, cocktails of such peptides can be administered to elicit broad antitumor reactivity that reduces immune escape due to growth of nonantigen-expressing tumor cells. Finally, synthetic peptides can be designed that exhibit increased binding affinity for MHC molecules relative to the parent, tumor-derived molecules. Limitations of TAA vaccines include the generation of less robust immune responses compared with TSAs and human leukocyte antigen (HLA) restriction. Nonetheless, initial reports of synthetic TAA peptide vaccines in GBM patients have demonstrated encouraging findings. Nearly 60% of HLA-A2 recurrent GBM patients demonstrated positive ELISPOT or tetramer reactions following vaccination with α-type 1 polarized DCs loaded with synthetic glioma-associated antigen peptides specific for ephrinA2, interleukin-13 receptor-α2, YKL-40, and gp100 when combined with poly-ICLC as an immunoadjuvant.100 There were no associated grade 3 or 4 adverse events (AEs) and no evidence of autoimmune reactivity in this study. Furthermore, radiographic responses were observed in 2 patients (9%), and progression-free survival (PFS) for at least 12 months was noted in 41% of patients. Further data evaluating a cocktail of tumor-associated antigens has been recently reported.101 In this study, median overall survival in HLA-A1/A2-positive newly diagnosed GBM patients who were vaccinated with autologous DCs pulsed with 6 different TAA peptides (HER2, TRP-2, gp100, MAGE-1, IL13Rα2, and AIM-2) was 38.4 months. Of note, tumors from all patients expressed at least 3 target TAAs by reverse transcription (RT)-PCR, while 75% expressed all 6 TAAs. A single-arm phase I/II study evaluating a variation of this vaccine approach is expected to initiate accrual of recurrent GBM patients in mid 2014 (NCT02078648). A randomized, placebo-controlled phase II study of a different TAA-based vaccine (referred to as ICT 107; Immunocellular Therapeutics) for newly diagnosed GBM patients has recently completed accrual (NCT01280552). An 11-TAA peptide vaccine (IMA950; Immatics Biotechnologies GmbH) is also in phase I/II clinical trials for newly diagnosed GBM patients (NCT01403285 and NCT01920191).
Vaccines that incorporate TSAs offer the advantage of generating highly potent immune responses, but these antigens are challenging to exploit because they are either patient specific or expressed by only a subset of patients. Currently, 2 TSA vaccine strategies are undergoing clinical evaluation for malignant glioma patients, while a third is in development. The mutated epidermal growth factor receptor variant III (EGFRvIII), formed by an 801 base pair, inframe deletion of exons 2 to 7, is a prototypic tumor-specific antigen that is expressed by ∼30% of GBM tumors.102 Rindopepimut (Celldex Therapeutics) is a synthetic 14 amino acid peptide mapping to the EGFRvIII-specific splice site, conjugated to the immune adjuvant keyhole limpet hemocyanin, and administered with GM-CSF.103 In preclinical studies, EGFRvIII vaccination demonstrated survival benefit as well as EGFRvIII-specific humoral and cellular immune responses.104 Subsequent clinical trials have confirmed that rindopepimut is well tolerated in EGFRvIII-positive GBM patients, and encouraging antitumor benefit has been observed (although treated patients had good performance status and minimal tumor burden).105–109 In these trials, EGFRvIII-specific immune responses were detected and correlated with improved outcome. Importantly, coadministered temozolomide and its associated lymphopenia did not appear to abrogate the activity of rindopepimut. Of note, the majority of progressive tumors following rindopepimut vaccination no longer expressed EGFRvIII,106,108 thereby providing proof-of-principle that tumor-specific antigen vaccination can generate an immune response capable of eradicating its intended GBM target population. A placebo-controlled, randomized phase III study of rindopepimut is ongoing for newly diagnosed EGFRvIII-positive GBM patients (Clinicaltrials.gov NCT01480479), while encouraging preliminary results have recently reported from a randomized phase II study evaluating the addition of rindopepimut to bevacizumab for recurrent GBM patients (Clinicaltrials.gov NCT01498328).110
Evaluation of a second TSA vaccination strategy involves targeting human cytomegalovirus (HCMV), a β-herpesvirus. DNA as well as HCMV proteins, but not infectious virions, have been demonstrated in most if not all GBM tumors, as well as the majority of grade II and III gliomas, but not in adjacent non-cancerous brain tissue.111–114 Of note, HCMV has also been detected in several other malignancies.115–118 Several immunotherapeutic strategies exploiting the presence of CMV in GBM tumors are underway and include vaccination with HCMV pp65-LAMP-loaded DCs with and without autologous T-cell transfer (Clinicaltrials.gov NCT00639639), a phase I vaccination study of HCMV peptide antigen for newly diagnosed GBM patients (Clinicaltrials.gov NCT01854099, and generation of polyclonal CMV-specific T cells for adoptive transfer (Clinicaltrials.gov NCT01205334).73 Enthusiasm for targeting CMV as a therapeutic strategy for GBM has been heightened by demonstration of a potent pp-65 CD8+ T cell response following whole tumor lysate vaccination113 and by recent data demonstrating prolonged survival in some newly diagnosed GBM patients treated with valganciclovir,119 although these preliminary results require prospective validation.
A third approach utilizing tumor-specific antigens for vaccination is in development and exploits the presence of individual, tumor-specific mutations that generate unique mutant proteins.120,121 With this approach, next-generation DNA sequencing of individual patient tumor and normal DNA is used to identify antigens derived from tumor-specific mutated proteins,122,123 which are then classified based on predicted binding ability to patient-specific, class I HLA molecules.124 Preclinical evaluation of this approach confirmed specific immunogenicity of mutant peptides as well as survival benefit following vaccination in a melanoma model.125 A clinical trial evaluating this approach in advanced melanoma patients was recently initiated (NCT01970358), and similar strategies are in development in the United States and Europe for malignant glioma patients.
Vaccinations derived from tumor lysate preparations represent an approach that offers the advantage of incorporating both TAAs and TSAs on a patient-specific basis.98,99,126–136 This approach is not restricted by HLA subclass and therefore could theoretically be available for every GBM patient with a resectable tumor. On the other hand, this approach requires surgery for vaccine generation plus several weeks for vaccine preparation. In addition, whole tumor lysates could theoretically trigger autoimmune reactions against normal host cells, thereby contaminating the vaccine product.
Preclinical studies demonstrating the feasibility and antitumor activity of tumor-lysate vaccination approaches137,138 have led to a series of clinical trials98,99,126–136 confirming the overall safety of this approach. Specifically, the most commonly observed toxicities included mild local reactions at vaccination site, low-grade fever, and some headache, while autoimmune reactions were not observed. Modest clinical benefit and variable degrees of tumor antigen immunogenicity have also been observed. A randomized phase III, placebo-controlled study evaluating DCVax-L (Northwest Biotherapeutics), a vaccine strategy consisting of autologous DCs pulsed with whole tumor lysate for newly diagnosed GBM patients, is ongoing (NCT00045968).
Two modifications of the tumor lysate vaccination strategy are currently under active investigation for GBM patients. The first adaptation incorporates vaccination with lysates or mRNA derived from glioma stem cells isolated from resected tumors. Targeting glioma stem cells is attractive because this tumor subpopulation fosters tumor growth,139 resistance to chemotherapy and radiation therapy,140,141 and immunosuppression.142,143 In addition, glioma stem cells may express higher levels of some tumor-associated antigens than non-stem cells.143,144 Preclinical studies have demonstrated the feasibility and antitumor activity of glioma stem cell vaccination strategies.144,145 Based on these findings, several ongoing clinical trials are evaluating autologous DCs pulsed with glioma stem cell lysate or mRNA (NCT00890032, NCT00846456, NCT01171469, and NCT01567202).
Another innovative modification of the tumor lysate approach involves vaccination with tumor antigens bound to the 96 KD heat shock protein complex (HSP96). Heat shock proteins are upregulated by stress and are regarded as natural adjuvants based on their inherent ability to augment immune responses.146 Preliminary evidence, which has demonstrated the immunogenicity of this approach and encouraged evidence of antitumor benefit in recurrent GBM patients147,148 has led to a randomized phase II study evaluating HSP96 vaccination with bevacizumab for recurrent GBM patients (NCT01814813).
Determinants of Vaccine Immunogenicity: Additional Considerations
Several variables beyond tumor antigen choice may impact vaccine immunogenicity, and studies designed to optimize these variables in malignant glioma patients remain to be performed. A potentially important variable among vaccine components is the use of harvested autologous DCs. Dendritic cells are considered the most potent antigen-presenting cells of the immune system due to their high level of major histocompatibility complex and costimulatory molecule expression and their ability to induce tumor-specific effector T-cell responses.149 Pheresed, autologous DCs can be readily activated ex vivo using cytokines such as GM-CSF and IL-4 and can then process tumor antigens generated by exposure to tumor cell lysates, mRNA, or purified antigens prior to administration back into the patient. Immune adjuvants induce DC maturation, which then demonstrate greater migration to draining lymph nodes and more potent upregulation of immunostimulatory molecules compared with immature DCs.131,150 Several vaccine approaches incorporate ex vivo antigen sensitization of harvested autologous DCs, while others administer tumor antigen(s) with adjuvant directly. It remains unclear whether the additional labor, cost, and time required for pheresis and ex vivo processing of autologous DCs are required to optimize vaccine immunogenicity.
Another important vaccine variable is immune adjuvance, which augments vaccine-induced immune responses via several mechanisms.151–154 In the absence of adjuvant-induced activation of DCs, immune tolerance may be promoted by Tregs.155,156 Although several immune adjuvants have been evaluated in malignant glioma vaccine trials to date, the optimal immune adjuvant or combination remains unclear. TLR agonists such as imiquimod, polylysine, and carboxymethyl-cellulose (poly-ICLC) are mimics of pathogen danger signals that trigger DC activation and T-cell antitumor immunoreactivity.157–160 cytosine:guanine nucleotide base-pairing, a synthetic oligodeoxynucleotide with unmethylated cytosine: guanine nucleotides motifs mimics microbial DNA and activates TLR9.158 Keyhole limpet hemocyanin, a protein derived from the sea mollusk Megathura crenulata, is an active adjuvant that has been incorporated safely into active vaccination studies for GBM patients.107,109,161 In addition, GM-CSF, a myeloid cytokine that can enhance DC maturation and function,162 has been coadministered with vaccines and via gene-transduced tumor cells.163–165 GM-CSF has improved outcome in some studies but has shown a detrimental effect in others.164,166 One possible explanation for these mixed responses could be differential dose effects because low doses of GM-CSF can promote immune responses, while higher doses may enhance immunosuppression.166,167 Another possibility is the dual role of GM-CSF in enhancing both effector and regulatory T-cell responses. Coadministration of a second signal, such as a TLR agonist, skews GM-CSF–elicited immunity toward tumor protection.168
Additional vaccine variables that have not been systemically investigated include site and frequency of vaccination. Data from a preclinical GBM model suggested that the vaccination site is important because the number and function of antigen-specific T cells increased with increasing distance of vaccination site from intracranial tumor.169
Patient-related variables that may also impact vaccine immunogenicity include age, degree of prior treatment, status of underlying tumor, size of tumor burden, and concurrent use of corticosteroids, although the latter 2 factors are frequently associated. Preliminary data suggest that younger patients respond more favorably.127,128,170,171 In addition, minimal tumor burden has been associated with improved outcome, which may be due to immunosuppressive factors associated with tumor bulk.127,128,171–172 For example, elevated systemic Treg cell levels at diagnosis decrease substantially following resection but then return toward baseline at tumor recurrence.43,57,173 Future clinical trials should prospectively evaluate the impact of vaccine and patient-related factors on immunogenicity and overall outcome.
Finally, in addition to careful elucidation of factors contributing to vaccine immunogenicity, vaccine-induced tumor-specific immune responses must overcome substantive systemic and local immunosuppressive factors, which have been previously discussed in this review, if they are to generate meaningful antitumor benefit and improve patient outcome.
Immune Checkpoint Targeting Therapy for Glioblastoma
Underlying Biology
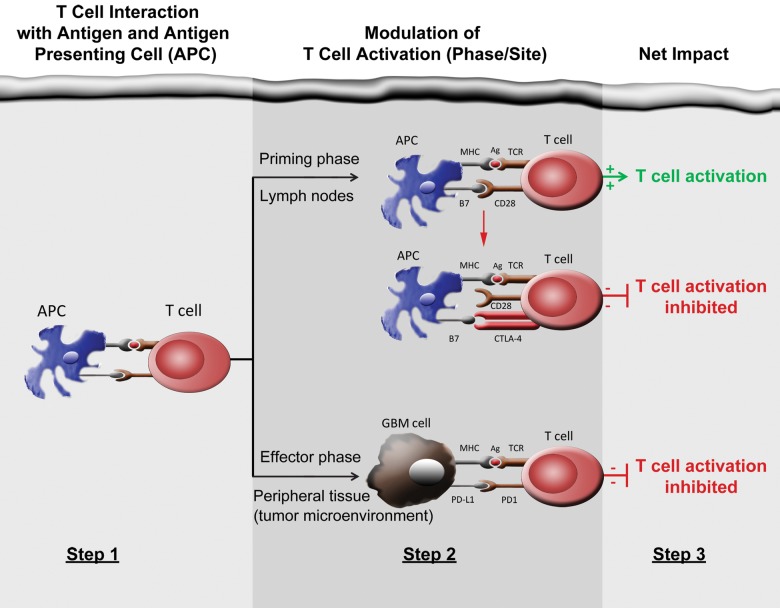
Cellular immunity, critically mediated by antigen-specific T cells, is temporally and spatially regulated through a complex series of sequential steps including: (i) clonal selection; (ii) activation and proliferation in secondary lymphoid tissues; (iii) mobilization to sites of inflammation; (iv) initiation of effector functions at target sites; and (v) recruitment and activation of additional immune cells through cytokines and other signaling molecules. Both costimulatory/agonistic and antagonistic/inhibitory members of the B7/CD28 family modulate this process at key points referred to as immune checkpoints (Fig. 2). Immune checkpoint mediators function to optimize appropriate, normal T-cell immune responses while simultaneously maintaining self-tolerance that minimizes the risk of autoimmune reactions and potential collateral damage to normal tissues. Inhibitory checkpoint mediators function as brakes to attenuate normal T-cell immune responses (Fig. 3). Therapeutic blockade of inhibitory checkpoints, including cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and programmed cell death protein 1(PD-1) or its ligands PD-L1 and PD-L2, have recently demonstrated exciting antitumor benefit against several cancers, including some such as metastatic melanoma,6,8,174 which have historically responded poorly to conventional therapies.
Fig. 2.
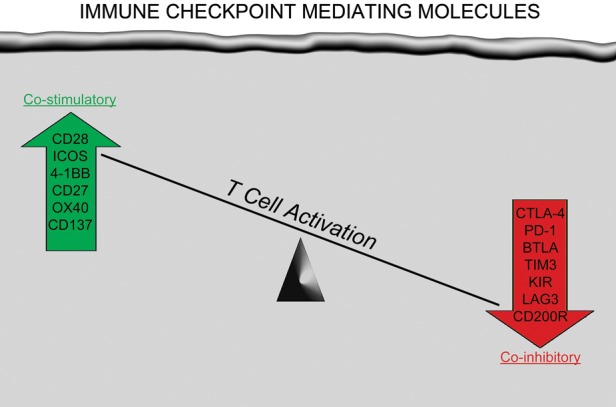
Immunomodulatory molecules expressed on T cells that provide either a costimulatoryeffect to enhance T-cell activation or a coinhibitory effect to attenuate T-cell activation following interaction of antigen/MHC complex on antigen-presenting cells with the T-cell receptor.
Fig. 3.
Steps involved with T-cell activation as well as its negative modulation by CTLA-4 and PD-1/PD-L1. Note that CTLA-4 and PD-1/PD-L1 act by complementary mechanisms to inhibit T-cell activation. Monoclonal antibody blockade of CTLA-4 or PD-1/PD-L1 relieves immune checkpoint inhibition, thereby restoring T-cell activation.
CTLA-4 is solely expressed by T cells and inhibits early steps of the T-cell activation localized primarily within secondary lymphoid tissues. Surface expression of CTLA-4 normally upregulates on T cells upon antigen binding, with the degree of expression commensurate to the strength of T-cell receptor stimulation.175 CTLA-4 outcompetes CD28, a costimulatory molecule required for augmenting T-cell activation, for its activating ligands CD80 (also referred to as B7.1) and CD86 (also referred to as B7.2).176 Interruption of ligand binding blocks CD28 signaling and thus dampens antigen-specific T-cell activation. CTLA-4 can also suppress immune reactivity by decreasing helper T-cell activity and augmenting myeloid-derived suppressor cells as well as regulatory T cells, in which CTLA-4 is constitutively expressed.177–179 The critical modulatory suppression of immune activation by CTLA-4 is underscored by lethal autoimmunity observed in Ctla-4-knockout mice.180
PD-1 also functions to suppress T-cell activation but does so in a distinct, yet complementary, manner relative to CTLA-4. Specifically, PD-1 primarily blocks T-cell activation and effector function at later stages and typically does so within peripheral organs and local sites of inflammation. However, PD-1 may also play an important regulatory role during T-cell priming by DCs. Inflammatory cytokines, particularly interferons, enhance PD-L1 expression. Analogous to CTLA-4, activated T cells also upregulate PD-1 expression.181,182 In addition, chronic antigen exposure, which typically occurs with chronic viral infection or cancer, may trigger persistent PD-1 expression and lead to anergy or exhaustion of antigen-specific T cells.183 Within the inflammatory or tumor microenvironment, PD-1 binding to PD-L1 suppresses T-cell effector activity by inhibiting signaling through interaction with the SHP2 phosphatase.184 Of note, PD-1 promotes other aspects of immune suppression including proliferation of Tregs and attenuation of B cell and natural killer (NK) cell responses; thus, therapeutic targeting of PD-1 may enhance multiple aspects of immune reactivity associated with cytotoxic T cells, B cells, Tregs, and NK cells.185,186
Cancers notoriously subvert normal physiological processes to promote their survival. Among malignant gliomas, markedly upregulated angiogenesis and overexpression of the ubiquitous DNA repair enzyme methyl-guanine methyltransferase are 2 well-established examples of such adaptive behavior. Analogously, many malignancies overexpress the PD-L1 immune checkpoint ligand to counteract antitumor T-cell responses and proinflammatory cytokines.187 Tumor cell PD-L1 expression can occur as an adaptive immune reaction in response to interferons, especially IFNγ, which are secreted by tumor cells or associated stroma.188 In addition, increased PD-L1 expression has been linked to dysregulated oncogenic cell signaling pathways, including inactivating mutation of the phosphatase and tensin homolog (PTEN) tumor suppressor.189 Among gliomas, PD-L1 expression correlates with grade190 and has been demonstrated in established GBM cell lines,191 primary tumors,190–194 and CD133+ GBM stem cells.190 In addition, a high percentage of tumor-infiltrating lymphocytes from malignant gliomas express PD-1.194
Preclinical Studies
CTLA-4 blockade led to 80% long-term survival among immunocompetent VM/Dk mice with established SMA-560 intracranial tumors.195 CTLA-4 therapy was well tolerated with no evidence of autoimmune toxicity. Furthermore, anti-CTLA-4 therapy led to normalized CD4+ T cell counts and decreased CD4+CD25+FoxP3+ Treg cells. In a separate study, intratumoral IL-12 therapy combined with systemic CTLA-4 blockade led to long-term survival in the majority of GL-261 tumor-bearing mice.196 In addition, long-term surviving mice exhibited evidence of immunological memory when reinoculation of tumor into the contralateral cerebral hemisphere failed to generate tumor growth.
Limited preclinical data evaluating PD-1/PD-L1 blockade for GBM currently exist. Administration of an anti-PD-1 MAb improved median survival from 26 days to 52 days among C57BL/6 mice with GL-261 intracranial tumors when combined with a 10 Gy dose of radiation.197 A cohort of long-term survivors was apparently cured; tumor growth was not observed upon flank rechallenge, which indicated that immunological memory had prevented recurrence. In addition, combination therapy led to increased tumor infiltration of cytotoxic T cells and decreased Treg infiltrate.
Clinical Experience
There are currently 2 fully human, CTLA-4 monoclonal antibodies under clinical evaluation for cancer patients including ipilimumab (Yervoy; Bristol-Myers Squibb) and tremilimumab (MedImmune LLC). Ipilimumab, an IgG1isotype, was approved for advanced, unresectable melanoma based on improved survival noted in a randomized phase III study.6 The outcome for metastatic melanoma patients has historically been dismal due to failure to improve survival significantly by a variety of investigated therapeutic approaches.198 In this study of 676 patients, median survival for those treated with either ipilimumab alone or ipilimumab plus gp100 peptide vaccine was 10 months, compared with only 6 months for those treated with vaccine alone (hazard ratio, 0.66; P = .0026). In addition, investigator-assessed radiographic response rate was 10.9% for patients treated with ipilimumab compared with only 1.5% for those treated with vaccine. A second randomized phase III study also noted a survival benefit in patients treated with ipilimumab plus dacarbazine compared with dacarbazine plus placebo.174 Notably, the durability of antitumor benefit has been unprecedented, even though it was limited to a subset of patients. Recent long-term follow-up of 177 advanced melanoma patients, who were treated in early clinical trials of ipilimumab, revealed that the median duration of tumor response was 88 months.199 Although it has not been approved by the FDA, antitumor benefits have also been observed in advanced melanoma patients treated with tremelimumab, an IgG2 CTLA-4 blocking MAb. Specifically, median overall survival was 12.8 months in tremelimumab recipients compared with 10.7 months for either temozolomide or dacarbazine chemotherapy recipients.200 In addition, 10.7% of tremelimumab recipients achieved a radiographic response, which was durable for a median of 35.8 months. Of note, encouraging evidence of antitumor activity is emerging for both ipilimumab and tremelimumab among other solid tumors including lung,201 prostate,202,203 breast,204 colorectal,205 renal,206 and pancreatic cancers207 as well as mesothelioma.208
Dramatic evidence of antitumor benefit has also been observed with therapeutics blocking PD-1/PD-L1 signaling. In an initial phase I study of advanced solid-tumor patients treated with BMS-936559, a fully human IgG4 mAb that blocks PD-L1 binding to either PD-1 or CD80, a maximum tolerated dose was not reached, and 9% of patients experienced grade 3-4 treatment-related AEs that led to discontinuation of treatment for 6% of patients.7 There were no treatment-related deaths. Evidence of meaningful antitumor benefit was observed at biweekly doses ≥1 mg/kg and was durable in an encouraging subset of patients; however, frequency of response varied by tumor type. Specifically, responses were noted in patients with melanoma (17%) as well as lung (10%), ovarian (6%), and renal cell cancers (12%) but were not observed in patients with colorectal or pancreatic cancers (although only small numbers of these latter tumor types have been published to date). In a simultaneously reported phase I study of nivolumab, a fully human IgG4 PD-1 blocking MAb, a maximum tolerated dose was also not defined despite dose escalation from 0.1 to 10 mg/kg biweekly.8 Grade 3-4 drug-related AEs occurred in 14% of patients, while 5% of patients discontinued therapy due to treatment-related AEs. In addition, 3 deaths from pneumonitis were noted. Highly encouraging evidence of antitumor activity was again noted despite a significant degree of pretreatment in enrolled patients; however, benefit was also restricted by tumor type. Specifically, durable radiographic responses and improved PFS-6 rates were observed in melanoma, renal cell and lung cancer patients, but no radiographic responses were observed for prostate and colorectal cancer patients, although relatively small numbers of these tumors have been evaluated. In addition, a higher rate of radiographic response was noted in patients with PD-L1–expressing archival tumor specimens. Significant single-agent activity was recently reported in advanced melanoma patients treated with lambrolizumab, a humanized, IgG4-kappa isotype, PD-1 blocking MAb, in a single-arm phase II study.209 Three different dosing schedules were evaluated including 2 mg/kg every 3 weeks and 10 mg/kg every 2 or every 3 weeks. Specifically, 38% of all patients achieved a radiographic response by central review with a median PFS > 7 months. Radiographic response rates were higher in patients treated at 10 mg/kg every 2 weeks (32%), compared with 10 mg/kg every 3 weeks (15%) and 2 mg/kg every 3 weeks (3%). Of note, responses were also observed in patients who had progressed on prior ipilimumab therapy. In this study, 13% of patients reported grade 3-4 treatment-related AEs, and one patient died. The incidence of treatment-related AEs was more common in patients treated with 10 mg/kg every 2 weeks.
With regard to toxicity, immune checkpoint blockade is associated with a diverse spectrum of well-characterized, immune-related adverse events (irAEs) including rash, colitis, hypophysitis, hepatitis, pancreatitis, iridocyclitis, lymphadenopathy/sarcoid-like syndrome, neuropathy, and nephritis.210 Although most irAEs are mild to moderate, particularly if recognized early and treated appropriately, severe reaction have occurred including life-threatening toxic epidermal necrolysis and fatal colitis and pneumonitis. Similar irAEs have been noted in patients treated with CTLA-4 as well as PD-1/PD-L1 blockade, although the frequency and severity of irAEs appear to be more prominent following anti-CTLA-4 therapy that are presumably related to earlier and less specific inhibition of T cell activation.211 AEs of any grade have been reported in 75% of patients on ipilimumab, and 15%–30% have experienced grade ≥ 3 irAEs.6,174,210,212 Of note, a characteristic pattern of timing has emerged for irAEs. Specifically, dermatologic events typically occur first and are noted most commonly within 2–3 weeks of treatment initiation, while gastrointestinal and hepatic events are most common after 6–7 weeks of therapy. Endocrinologic AEs typically evolve after 9 or more weeks.210,212 The frequency and severity of irAEs appear to be dose related.212,213 Guidance for early recognition and appropriate intervention of irAEs has proven to be highly valuable for reducing their overall severity.210,212
Clinical evaluation of CTLA-4 blocking MAbs for neuro-oncology patients is thus far limited to patients with CNS metastases of systemic cancers. Ipilimumab has demonstrated encouraging benefit for patients with melanoma and brain metastases as a single-agent therapy214 and in combination with radiotherapy administered using standard fractionated delivery or stereotactic radiosurgery.215–217 Of note, concurrent corticosteroid use was associated with diminished activity in one study.214 Clinical trials evaluating ipilimumab are in advanced development for both recurrent and newly diagnosed GBM patients (NCT02017717).
Several clinical trials evaluating PD-1/PD-L1immune checkpoint inhibitors are also in development for malignant glioma patients. Accrual has recently initiated to a phase II study which randomizes recurrent glioblastoma patients to receive nivolumab, nivolumab plus ipilimumab, or bevacizumab (NCT02017717). A phase I/II study is also expected to begin accrual soon for evaluating pidilizumab (CurTech), a humanized anti-PD-1 MAb, in patients with either recurrent malignant glioma or diffuse intrinsic pontine glioma (NCT01952769).
Combinatorial Approaches
To derive optimal patient benefit, immune-based therapies will need to generate potent, specific, and durable antitumor immune responses that are able to overcome systemic and local immunosuppressive factors. In order to achieve this goal, combinatorial approaches may be required. Intuitively, combining cytotoxic therapy with immunotherapy appears counterproductive because cytotoxic therapies can diminish overall immune function. In addition, recent data have associated radiation and alkylating chemotherapeutics, including temozolomide with selective lymphocyte toxicity, that have led to profound and persistent lymphopenia.45,218–221 Paradoxically, growing data have also demonstrated that cytotoxic therapies may augment the magnitude, quality, and efficacy of tumor-specific T-cell responses via several mechanisms. First, dying tumor cells release tumor-specific antigens that can in turn initiate T-cell activation.222,223 Second, lymphodepleting chemotherapeutics can induce homeostatic proliferation that preferentially enhances restoration of cytotoxic T cells faster than Tregs, leading to an increase in the CD8+:Treg ratio and improved outcome in both preclinical models and patients.106,224,225 Third, some chemotherapeutic agents, including low-dose cyclophosphamide and gemcitabine, may deplete immunosuppressive Tregs and myeloid-derived suppressor cells.226–228 Fourth, chemotherapeutics, including temozolomide, can induce intratumoral expression of immunostimulatory cytokines and chemokines that promote antitumor immune responses.224 Finally, cytotoxic therapy may induce stress or danger signals that increase the susceptibility of tumor cells to immune attack.229–231 Based on these considerations, several clinical trials combining cytotoxic therapy with glioma vaccination have been conducted, and encouraging preliminary efficacy has been reported.101,105,107,132,133,135,136 Limited yet intriguing data have also demonstrated that cytotoxic therapy and immune checkpoint blockade may generate enhanced antitumor benefit; a recent report described an abscopal effect (defined as regression of metastatic tumor distant from sites of local radiation) in a patient with advanced melanoma treated with ipilimumab and focal radiotherapy.232 Additional studies have suggested that immune checkpoint blockade, combined with chemotherapy, enhances antitumor benefit.174,201 Immunologic benefit may vary between chemotherapeutic agents and may also depend on administration schedule.201,233 Only one study evaluating combinatorial cytotoxic therapy and checkpoint blockade has been reported for GBM. In this recent preclinical study, significantly prolonged survival in an immunocompetent murine GBM model was achieved following combinatorial anti-PD-1 MAb plus stereotactic radiation.197
There is also a rationale for combining immunotherapy approaches with agents that block VEGF signaling. VEGF contributes to tumor immunosuppression by several mechanisms including: (i) inhibition of DC maturation and antigen presentation; (ii) induction of CD8+ T-cell apoptosis; (iii) promotion of Treg activity; and (iv) restriction of T-cell migration across tumor vascular endothelium into tumors.234–237 Indeed, inhibition of VEGF signaling has been shown to augment antitumor immune responses.49,238–240 Based on these considerations, a randomized phase II study evaluating rindopepimut with bevacizumab in recurrent GBM patiets is ongoing (Clinicaltrials.gov: NCT01498328).
A third combinatorial approach includes integrating immunotherapies with potentially synergistic mechanisms of antitumor activity. The FDA approval of sipuleucel-T for metastatic prostate cancer and ipilimumab for advanced melanoma are noteworthy proof-of-principle achievements highlighting the value of immunotherapies for oncology. Nevertheless, the overall clinical benefit of each of these modalities is modest in that sipuleucel-T improves survival by only 4.1 months, and durable antitumor responses are achieved in only a minority of patients treated with immune checkpoint blockade. Such results underscore the likely need to combine complementary immune-based therapies in order to achieve broad and durable antitumor benefit. Fortunately, the spectrum of immune-based treatment strategies offers many exciting opportunities for combinatorial approaches in neuro-oncology. For example, based on preclinical antitumor benefit associated with either Treg depletion241,242 or inhibition of Treg activation via IL-2 blockade,241,243 a phase I/II clinical trial has initiated evaluating dacluzimab (Zenapax; Hoffman-La Roche Incl, Nutley, NJ), an IL-2 receptor MAb administered with EGFRvIII vaccination (Clinicaltrials.gov NCT00626015). (Clinicaltrials.gov NCT00626015). CMV antigen vaccination, combined with adoptive transfer of expanded CMV-specific T cells, is also currently under evaluation (Clinicaltrials.gov NCT 00693095).
Integration of tumor vaccination with immune checkpoint blockade has also been shown to enhance long-term survival in preclinical studies including GBM,244–246 although optimal benefit may be schedule dependent.247 Recent clinical studies in other cancer indications confirm the safety of combining immune checkpoint blockade with cancer vaccines.202,248,249
Finally, an exciting approach currently under wide clinical evaluation includes combination of immune checkpoint inhibitors. The rationale for such an approach is based on differences in timing and localization of T-cell activation regulated by CTLA-4 and PD-1/PD-L1 signaling.211 Initial preclinical studies have revealed synergistic antitumor benefit following combined CTLA-4 and PD-1 blockade.244,250 A recent clinical trial confirmed greater antitumor activity when CTLA-4 blockade was combined with anti-PD-1 therapy.251 A clinical trial evaluating combined CTLA-4 and PD-1 blockade is underway for recurrent GBM patients (NCT02017717).
Additional Considerations
The ability to accurately assess response remains a significant challenge in neuro-oncology. The Response Assessment in Neuro-oncology (RANO) criteria were recently adopted because they address both pseudoprogression and pseudoresponse.252 Complex imaging patterns of response have also been observed following immunotherapy and have included pseudoprogression due to inflammation associated with antitumor immune responses that led to enlargement of pre-existing lesions or the appearance of new lesions. In addition, tumors may initially grow, including the appearance of new lesions prior to the generation of antitumor immune responses.253 To address these phenomena, immune-related response criteria have been integrated into immunotherapy clinical trials.254,255 Critical considerations of these criteria include: (i) the ability of patients who are adequately tolerating therapy to remain on treatment beyond initial progression; and (ii) measurement of overall tumor burden rather than the appearance of new lesions to define progressive disease. A multidisciplinary working group is currently integrating immune response criteria into RANO for use in neuro-oncology patients undergoing immunotherapy clinical trials.
The holy grail of all cancer therapies, including immunotherapy, is the identification of correlative biomarkers to identify patients most likely to benefit.256 Myriad assays of immune response have demonstrated variable association with survival benefit in GBM patients treated in immunotherapy clinical trials.98–101,105–109,126–136,148,257 One proposed innovative use of immune parameters includes hierarchical clustering to predict response to immunotherapy treatments.132,136 Recognition that significant variability in methodology and characterization of assay response poses a significant limitation to effective use of these data has led to a multinational effort to harmonize immunologic monitoring efforts across immunotherapy trials.258
Additional correlative data may be derived from tumor analyses. For example, a preliminary report has suggested that GBM tumors exhibiting a mesenchymal gene expression profile benefit more from tumor lysate vaccination than other subtypes due to induction of higher levels of CD3+ and CD8+ tumor-infiltrating lymphocytes.133 Higher levels of response to anti-PD-1 therapy have been associated with tumor PD-L1 expression, although this result has not been validated by other studies.8 Finally, because PTEN deficiency has been linked with increased PD-L1 expression, some investigators have suggested that GBM tumors exhibiting PTEN loss may be more likely to derive benefit from PD-1/PD-L1 blockade.259 It will be imperative that hypotheses evaluating potential biomarkers be investigated as the clinical trials advance for immune-based therapies in malignant glioma patients.
Conclusion
Effective antitumor activity by the immune system offers the ability to both eradicate existing tumors and prevent future recurrence by generating immune memory responses. The CNS interacts in a effective manner with the peripheral immune system to generate meaningful immune responses. A wide array of exciting therapeutic approaches have been developed to generate effective antitumor immune responses and have demonstrated highly encouraging benefit for patients following active vaccination, immune checkpoint blockade, and adoptive cellular therapies. Nonetheless, several challenges exist including better understanding of immunosuppressive systemic and local adaptations frequently invoked by malignancies including GBM to foster immunotolerance. In addition, in order to maximize therapeutic benefit, systematic investigation of potential variables that may impact the optimal activity of immunotherapies and the evaluation of potentially informative biomarkers will be required. Finally, it is likely that combinatorial regimens with complementary mechanisms of action will be required to achieve broad and durable antitumor benefit.
Funding
None declared.
Conflict of interest statement. John H. Sampson has a consultancy, honoraria and research funding with Celldex Therapeutics. Gordon Freeman has a consultancy with CoStim Pharmaceuticals and grants/patents/pending royalties with Bristol-<yers Squibb/Medarex, Roche/Genetech, Merck, EMD-Serono, Boehringer-Ingelheim, GlaxoSmithKline and CoStim Pharmaceuticals. None of the other authors have any conflict of interest with the subject matter or materials discussed in the manuscript.
References
- 1.Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. doi: 10.1200/JCO.2013.49.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao H, Lebrun DG, Yang J, et al. Deregulated signaling pathways in glioblastoma multiforme: molecular mechanisms and therapeutic targets. Cancer Invest. 2012;30(1):48–56. doi: 10.3109/07357907.2011.630050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 6.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119(12):2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hobohm U. Fever and cancer in perspective. Cancer Immunol Immunother. 2001;50:391–396. doi: 10.1007/s002620100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coley WB. The treatment of malignant tumors by repeated inoculations of Erysipelas, with a report of ten original cases. Am J Med Sci. 1893;105:487–511. [PubMed] [Google Scholar]
- 13.Margolis J, West D. Spontaneous regression of malignant disease: report of three cases. J Am Geriatr Soc. 1967;15(3):251–253. doi: 10.1111/j.1532-5415.1967.tb01134.x. [DOI] [PubMed] [Google Scholar]
- 14.De Bonis P, Albanese A, Lofrese G, et al. Postoperative infection may influence survival in patients with glioblastoma: simply a myth? Neurosurg. 2011;69(4):864–868. doi: 10.1227/NEU.0b013e318222adfa. discussion 868–869. [DOI] [PubMed] [Google Scholar]
- 15.Wiemels JL, Wilson D, Patil C, et al. IgE, allergy, and risk of glioma: update from the San Francisco Bay Area Adult Glioma Study in the temozolomide era. Int J Cancer. 2009;125(3):680–687. doi: 10.1002/ijc.24369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linos E, Raine T, Alonso A, et al. Atopy and risk of brain tumors: a meta-analysis. J Natl Cancer Inst. 2007;99(20):1544–1550. doi: 10.1093/jnci/djm170. [DOI] [PubMed] [Google Scholar]
- 17.Wrensch M, Wiencke JK, Wiemels J, et al. Serum IgE, tumor epidermal growth factor receptor expression, and inherited polymorphisms associated with glioma survival. Cancer Res. 2006;66(8):4531–4541. doi: 10.1158/0008-5472.CAN-05-4032. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Zeng G. Cancer and innate immune system interactions: translational potentials for cancer immunotherapy. J Immunother. 2012;35(4):299–308. doi: 10.1097/CJI.0b013e3182518e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medawar P. Immunity to hemologous grafted skin: III. The fate of skin hemografts transplanted to the brain, to subcutaneous tissue, and toe the anterior chamber of the eye. Br J Exp Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J Clin Invest. 2010;120(5):1368–1379. doi: 10.1172/JCI41911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barker CF, Billingham RE. Immunologically privileged sites. Adv Immunol. 1977;25:1–54. [PubMed] [Google Scholar]
- 22.Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28(2):254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 23.Carson MJ, Doose JM, Melchior B, et al. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies DC. Blood-brain barrier breakdown in septic encephalopathy and brain tumours. J Anat. 2002;200(6):639–646. doi: 10.1046/j.1469-7580.2002.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rascher G, Fischmann A, Kroger S, et al. Extracellular matrix and the blood-brain barrier in glioblastoma multiforme: spatial segregation of tenascin and agrin. Acta Neuropathol. 2002;104(1):85–91. doi: 10.1007/s00401-002-0524-x. [DOI] [PubMed] [Google Scholar]
- 26.van Zwam M, Huizinga R, Melief MJ, et al. Brain antigens in functionally distinct antigen-presenting cell populations in cervical lymph nodes in MS and EAE. J Mol Med (Berl) 2009;87(3):273–286. doi: 10.1007/s00109-008-0421-4. [DOI] [PubMed] [Google Scholar]
- 27.Goldmann J, Kwidzinski E, Brandt C, et al. T cells traffic from brain to cervical lymph nodes via the cribroid plate and the nasal mucosa. J Leuk Biol. 2006;80(4):797–801. doi: 10.1189/jlb.0306176. [DOI] [PubMed] [Google Scholar]
- 28.Cserr HF, Harling-Berg CJ, Knopf PM. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol. 1992;2(4):269–276. doi: 10.1111/j.1750-3639.1992.tb00703.x. [DOI] [PubMed] [Google Scholar]
- 29.Prins RM, Shu CJ, Radu CG, et al. Anti-tumor activity and trafficking of self, tumor-specific T cells against tumors located in the brain. Cancer Immunol Immunother. 2008;57(9):1279–1289. doi: 10.1007/s00262-008-0461-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Odoardi F, Sie C, Streyl K, et al. T cells become licensed in the lung to enter the central nervous system. Nature. 2012;488(7413):675–679. doi: 10.1038/nature11337. [DOI] [PubMed] [Google Scholar]
- 31.Masson F, Calzascia T, Di Berardino-Besson W, et al. Brain microenvironment promotes the final functional maturation of tumor-specific effector CD8+ T cells. J Immunol. 2007;179(2):845–853. doi: 10.4049/jimmunol.179.2.845. [DOI] [PubMed] [Google Scholar]
- 32.Kulprathipanja NV, Kruse CA. Microglia phagocytose alloreactive CTL-damaged 9L gliosarcoma cells. J Neuroimmunol. 2004;153(1–2):76–82. doi: 10.1016/j.jneuroim.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 33.Carson MJ, Sutcliffe JG, Campbell IL. Microglia stimulate naive T-cell differentiation without stimulating T-cell proliferation. J Neurosci Res. 1999;55(1):127–134. doi: 10.1002/(SICI)1097-4547(19990101)55:1<127::AID-JNR14>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 34.Tambuyzer BR, Ponsaerts P, Nouwen EJ. Microglia: gatekeepers of central nervous system immunology. J Leukoc Biol. 2009;85(3):352–370. doi: 10.1189/jlb.0608385. [DOI] [PubMed] [Google Scholar]
- 35.Karman J, Ling C, Sandor M, et al. Initiation of immune responses in brain is promoted by local dendritic cells. J Immunol. 2004;173(4):2353–2361. doi: 10.4049/jimmunol.173.4.2353. [DOI] [PubMed] [Google Scholar]
- 36.Hussain SF, Yang D, Suki D, et al. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006;8(3):261–279. doi: 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Handel AE, Lincoln MR, Ramagopalan SV. Of mice and men: experimental autoimmune encephalitis and multiple sclerosis. Eur J Clin Inv. 2011;41(11):1254–1258. doi: 10.1111/j.1365-2362.2011.02519.x. [DOI] [PubMed] [Google Scholar]
- 38.Jackson C, Ruzevick J, Phallen J, et al. Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. Clin Develop Immunol. 2011;2011:732413. doi: 10.1155/2011/732413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomez GG, Kruse CA. Mechanisms of malignant glioma immune resistance and sources of immunosuppression. Gene Ther Mol Biol. 2006;10(A):133–146. [PMC free article] [PubMed] [Google Scholar]
- 40.Waziri A. Glioblastoma-derived mechanisms of systemic immunosuppression. Neurosurg Clin NA. 2010;21(1):31–42. doi: 10.1016/j.nec.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Avril T, Vauleon E, Tanguy-Royer S, et al. Mechanisms of immunomodulation in human glioblastoma. Immunotherapy. 2011;3(4 Suppl):42–44. doi: 10.2217/imt.11.39. [DOI] [PubMed] [Google Scholar]
- 42.Dix AR, Brooks WH, Roszman TL, et al. Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol. 1999;100(1–2):216–232. doi: 10.1016/s0165-5728(99)00203-9. [DOI] [PubMed] [Google Scholar]
- 43.Fecci PE, Mitchell DA, Whitesides JF, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66(6):3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 44.Kempuraj D, Devi RS, Madhappan B, et al. T lymphocyte subsets and immunoglobulins in intracranial tumor patients before and after treatment, and based on histological type of tumors. Int J Immunopath Pharmacol. 2004;17(1):57–64. doi: 10.1177/039463200401700108. [DOI] [PubMed] [Google Scholar]
- 45.Grossman SA, Ye X, Lesser G, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17(16):5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schartner JM, Hagar AR, Van Handel M, et al. Impaired capacity for upregulation of MHC class II in tumor-associated microglia. Glia. 2005;51(4):279–285. doi: 10.1002/glia.20201. [DOI] [PubMed] [Google Scholar]
- 47.Facoetti A, Nano R, Zelini P, et al. Human leukocyte antigen and antigen processing machinery component defects in astrocytic tumors. Clin Cancer Res. 2005;11(23):8304–8311. doi: 10.1158/1078-0432.CCR-04-2588. [DOI] [PubMed] [Google Scholar]
- 48.Constam DB, Philipp J, Malipiero UV, et al. Differential expression of transforming growth factor-beta 1, -beta 2, and -beta 3 by glioblastoma cells, astrocytes, and microglia. J Immunol. 1992;148(5):1404–1410. [PubMed] [Google Scholar]
- 49.Gabrilovich DI, Ishida T, Nadaf S, et al. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin Cancer Res. 1999;5(10):2963–2970. [PubMed] [Google Scholar]
- 50.Sawamura Y, Diserens AC, de Tribolet N. In vitro prostaglandin E2 production by glioblastoma cells and its effect on interleukin-2 activation of oncolytic lymphocytes. J Neurooncol. 1990;9(2):125–130. doi: 10.1007/BF02427832. [DOI] [PubMed] [Google Scholar]
- 51.Huettner C, Paulus W, Roggendorf W. Messenger RNA expression of the immunosuppressive cytokine IL-10 in human gliomas. Am J Pathol. 1995;146(2):317–322. [PMC free article] [PubMed] [Google Scholar]
- 52.Tran Thang NN, Derouazi M, Philippin G, et al. Immune infiltration of spontaneous mouse astrocytomas is dominated by immunosuppressive cells from early stages of tumor development. Cancer Res. 2010;70(12):4829–4839. doi: 10.1158/0008-5472.CAN-09-3074. [DOI] [PubMed] [Google Scholar]
- 53.Hishii M, Nitta T, Ishida H, et al. Human glioma-derived interleukin-10 inhibits antitumor immune responses in vitro. Neurosurg. 1995;37(6):1160–1166. doi: 10.1227/00006123-199512000-00016. discussion 1166–1167. [DOI] [PubMed] [Google Scholar]
- 54.Roth P, Mittelbronn M, Wick W, et al. Malignant glioma cells counteract antitumor immune responses through expression of lectin-like transcript-1. Cancer Res. 2007;67(8):3540–3544. doi: 10.1158/0008-5472.CAN-06-4783. [DOI] [PubMed] [Google Scholar]
- 55.Heimberger AB, Abou-Ghazal M, Reina-Ortiz C, et al. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res. 2008;14(16):5166–5172. doi: 10.1158/1078-0432.CCR-08-0320. [DOI] [PubMed] [Google Scholar]
- 56.El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro Oncol. 2006;8(3):234–243. doi: 10.1215/15228517-2006-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crane CA, Ahn BJ, Han SJ, et al. Soluble factors secreted by glioblastoma cell lines facilitate recruitment, survival, and expansion of regulatory T cells: implications for immunotherapy. Neuro Oncol. 2012;14(5):584–595. doi: 10.1093/neuonc/nos014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacobs JF, Idema AJ, Bol KF, et al. Prognostic significance and mechanism of Treg infiltration in human brain tumors. J Neuroimmunol. 2010;225(1–2):195–199. doi: 10.1016/j.jneuroim.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 59.Badie B, Schartner J. Role of microglia in glioma biology. Microscopy Res Tech. 2001;54(2):106–113. doi: 10.1002/jemt.1125. [DOI] [PubMed] [Google Scholar]
- 60.Li W, Graeber MB. The molecular profile of microglia under the influence of glioma. Neuro Oncol. 2012;14(8):958–978. doi: 10.1093/neuonc/nos116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei J, Wu A, Kong LY, et al. Hypoxia potentiates glioma-mediated immunosuppression. PloS One. 2011;6(1):e16195. doi: 10.1371/journal.pone.0016195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walker DG, Chuah T, Rist MJ, et al. T-cell apoptosis in human glioblastoma multiforme: implications for immunotherapy. J Neuroimmunol. 2006;175(1–2):59–68. doi: 10.1016/j.jneuroim.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 63.Jansen T, Tyler B, Mankowski JL, et al. FasL gene knock-down therapy enhances the antiglioma immune response. Neuro Oncol. 2010;12(5):482–489. doi: 10.1093/neuonc/nop052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Ann Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Molldrem J, Riddell S. Understanding and enhancing the graft-versus-leukemia effect after hematopoietic stem cell transplantation. Ca Treat Res. 2009;144:187–208. doi: 10.1007/978-0-387-78580-6_8. [DOI] [PubMed] [Google Scholar]
- 66.Kolb HJ, Mittermuller J, Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76(12):2462–2465. [PubMed] [Google Scholar]
- 67.Heslop HE, Slobod KS, Pule MA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115(5):925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosenberg SA, Lotze MT, Muul LM, et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- 69.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23(10):2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Plautz GE, Barnett GH, Miller DW, et al. Systemic T cell adoptive immunotherapy of malignant gliomas. J Neurosurg. 1998;89(1):42–51. doi: 10.3171/jns.1998.89.1.0042. [DOI] [PubMed] [Google Scholar]
- 71.Plautz GE, Miller DW, Barnett GH, et al. T cell adoptive immunotherapy of newly diagnosed gliomas. Clin Cancer Res. 2000;6(6):2209–2218. [PubMed] [Google Scholar]
- 72.Tsuboi K, Saijo K, Ishikawa E, et al. Effects of local injection of ex vivo expanded autologous tumor-specific T lymphocytes in cases with recurrent malignant gliomas. Clin Cancer Res. 2003;9(9):3294–3302. [PubMed] [Google Scholar]
- 73.Ghazi A, Ashoori A, Hanley PJ, et al. Generation of polyclonal CMV-specific T cells for the adoptive immunotherapy of glioblastoma. J Immunother. 2012;35(2):159–168. doi: 10.1097/CJI.0b013e318247642f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jena B, Dotti G, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116(7):1035–1044. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park TS, Rosenberg SA, Morgan RA. Treating cancer with genetically engineered T cells. Trends Biotechnol. 2010;29(11):550–557. doi: 10.1016/j.tibtech.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gilham DE, Debets R, Pule M, et al. CAR-T cells and solid tumors: tuning T cells to challenge an inveterate foe. Trends Mol Med. 2012;18(7):377–384. doi: 10.1016/j.molmed.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 77.Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18(4):843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johnson LA. Model T muscle CARs can treat brain tumors. Clin Cancer Res. 2012;18(21):5834–5836. doi: 10.1158/1078-0432.CCR-12-2627. [DOI] [PubMed] [Google Scholar]
- 79.Brown CE, Starr R, Aguilar B, et al. Stem-like tumor-initiating cells isolated from IL13Ralpha2 expressing gliomas are targeted and killed by IL13-zetakine-redirected T Cells. Clin Cancer Res. 2012;18(8):2199–2209. doi: 10.1158/1078-0432.CCR-11-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kong S, Sengupta S, Tyler B, et al. Suppression of human glioma xenografts with second-generation IL13R-specific chimeric antigen receptor-modified T cells. Clin Cancer Res. 2012;18(21):5949–5960. doi: 10.1158/1078-0432.CCR-12-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jin J, Joo KM, Lee SJ, et al. Synergistic therapeutic effects of cytokine-induced killer cells and temozolomide against glioblastoma. Oncol Rep. 2011;25(1):33–39. [PubMed] [Google Scholar]
- 82.Chow KK, Naik S, Kakarla S, et al. T cells redirected to EphA2 for the immunotherapy of glioblastoma. Mol Ther. 2013;21(3):629–637. doi: 10.1038/mt.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morgan RA, Johnson LA, Davis J, et al. Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Hum Gen Ther. 2012;23:1043–1053. doi: 10.1089/hum.2012.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Frankel SR, Baeuerle PA. Targeting T cells to tumor cells using bispecific antibodies. Curr Opin Chem Biol. 2013;17(3):385–392. doi: 10.1016/j.cbpa.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 85.Choi BD, Cai M, Bigner DD, et al. Bispecific antibodies engage T cells for antitumor immunotherapy. Exp Opin Biol Ther. 2011;11(7):843–853. doi: 10.1517/14712598.2011.572874. [DOI] [PubMed] [Google Scholar]
- 86.Topp MS, Kufer P, Gokbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29(18):2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 87.Choi BD, Kuan CT, Cai M, et al. Systemic administration of a bispecific antibody targeting EGFRvIII successfully treats intracerebral glioma. Proc Natl Acad Sci USA. 2013;110(1):270–275. doi: 10.1073/pnas.1219817110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jarboe JS, Johnson KR, Choi Y, et al. Expression of interleukin-13 receptor alpha2 in glioblastoma multiforme: implications for targeted therapies. Cancer Res. 2007;67(17):7983–7986. doi: 10.1158/0008-5472.CAN-07-1493. [DOI] [PubMed] [Google Scholar]
- 89.Bigner SH, Humphrey PA, Wong AJ, et al. Characterization of the epidermal growth factor receptor in human glioma cell lines and xenografts. Cancer Res. 1990;50(24):8017–8022. [PubMed] [Google Scholar]
- 90.Libermann TA, Nusbaum HR, Razon N, et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985;313(5998):144–147. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- 91.Tchirkov A, Rolhion C, Kemeny JL, et al. Clinical implications of quantitative real-time RT-PCR analysis of hTERT gene expression in human gliomas. Brit J Cancer. 2003;88(4):516–520. doi: 10.1038/sj.bjc.6600754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Imaizumi T, Kuramoto T, Matsunaga K, et al. Expression of the tumor-rejection antigen SART1 in brain tumors. Int J Cancer. 1999;83(6):760–764. doi: 10.1002/(sici)1097-0215(19991210)83:6<760::aid-ijc11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 93.Renkvist N, Castelli C, Robbins PF, et al. A listing of human tumor antigens recognized by T cells. Cancer Immunol Immunother. 2001;50(1):3–15. doi: 10.1007/s002620000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scarcella DL, Chow CW, Gonzales MF, et al. Expression of MAGE and GAGE in high-grade brain tumors: a potential target for specific immunotherapy and diagnostic markers. Clin Cancer Res. 1999;5(2):335–341. [PubMed] [Google Scholar]
- 95.Liu G, Ying H, Zeng G, et al. HER-2, gp100, and MAGE-1 are expressed in human glioblastoma and recognized by cytotoxic T cells. Cancer Res. 2004;64(14):4980–4986. doi: 10.1158/0008-5472.CAN-03-3504. [DOI] [PubMed] [Google Scholar]
- 96.Okano F, Storkus WJ, Chambers WH, et al. Identification of a novel HLA-A*0201-restricted, cytotoxic T lymphocyte epitope in a human glioma-associated antigen, interleukin 13 receptor alpha2 chain. Clin Cancer Res. 2002;8(9):2851–2855. [PubMed] [Google Scholar]
- 97.Chakravarti A, Noll E, Black PM, et al. Quantitatively determined survivin expression levels are of prognostic value in human gliomas. J Clin Oncol. 2002;20(4):1063–1068. doi: 10.1200/JCO.2002.20.4.1063. [DOI] [PubMed] [Google Scholar]
- 98.Yu JS, Liu G, Ying H, et al. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64(14):4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 99.Yu JS, Wheeler CJ, Zeltzer PM, et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. 2001;61(3):842–847. [PubMed] [Google Scholar]
- 100.Okada H, Kalinski P, Ueda R, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29(3):330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Phuphanich S, Wheeler CJ, Rudnick JD, et al. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol Immunother. 2013;62:125–135. doi: 10.1007/s00262-012-1319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wong AJ, Ruppert JM, Bigner SH, et al. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc Natl Acad Sci USA. 1992;89(7):2965–2969. doi: 10.1073/pnas.89.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Del Vecchio CA, Li G, Wong AJ. Targeting EGF receptor variant III: tumor-specific peptide vaccination for malignant gliomas. Exp Rev Vac. 2012;11(2):133–144. doi: 10.1586/erv.11.177. [DOI] [PubMed] [Google Scholar]
- 104.Heimberger AB, Crotty LE, Archer GE, et al. Epidermal growth factor receptor VIII peptide vaccination is efficacious against established intracerebral tumors. Clin Cancer Res. 2003;9(11):4247–4254. [PubMed] [Google Scholar]
- 105.Heimberger AB, Sun W, Hussain SF, et al. Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: case study. Neuro Oncol. 2008;10(1):98–103. doi: 10.1215/15228517-2007-046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sampson JH, Aldape KD, Archer GE, et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro Oncol. 2011;13(3):324–333. doi: 10.1093/neuonc/noq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sampson JH, Archer GE, Mitchell DA, et al. An epidermal growth factor receptor variant III-targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Mol Cancer Ther. 2009;8(10):2773–2779. doi: 10.1158/1535-7163.MCT-09-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sampson JH, Heimberger AB, Archer GE, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28(31):4722–4729. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schmittling RJ, Archer GE, Mitchell DA, et al. Detection of humoral response in patients with glioblastoma receiving EGFRvIII-KLH vaccines. J Immunol Meth. 2008;339(1):74–81. doi: 10.1016/j.jim.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 110.Reardon DA, Li G, Recht L, et al. ReACT.: A Phase II Study of Rindopepimut Vaccine (CDX-110) Plus Bevacizumab in Relapsed Glioblastoma (GBM). 4th Quadrennial Meeting of the World Federation of Neuro-Oncology/18th Annual Meeting of the Society for Neuro-Oncology; San Francisco, CA. Oxford Press; 2013. [Google Scholar]
- 111.Cobbs CS, Harkins L, Samanta M, et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002;62(12):3347–3350. [PubMed] [Google Scholar]
- 112.Mitchell DA, Xie W, Schmittling R, et al. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro-oncol. 2008;10(1):10–18. doi: 10.1215/15228517-2007-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Prins RM, Cloughesy TF, Liau LM. Cytomegalovirus immunity after vaccination with autologous glioblastoma lysate. New Engl J Med. 2008;359(5):539–541. doi: 10.1056/NEJMc0804818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lucas KG, Bao L, Bruggeman R, et al. The detection of CMV pp65 and IE1 in glioblastoma multiforme. J Neurooncol. 2011;103(2):231–238. doi: 10.1007/s11060-010-0383-6. [DOI] [PubMed] [Google Scholar]
- 115.Harkins LE, Matlaf LA, Soroceanu L, et al. Detection of human cytomegalovirus in normal and neoplastic breast epithelium. Herpesviridae. 2010;1(1):8. doi: 10.1186/2042-4280-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Melnick M, Sedghizadeh PP, Allen CM, et al. Human cytomegalovirus and mucoepidermoid carcinoma of salivary glands: cell-specific localization of active viral and oncogenic signaling proteins is confirmatory of a causal relationship. Exp Mol Pathol. 2012;92(1):118–125. doi: 10.1016/j.yexmp.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 117.Samanta M, Harkins L, Klemm K, et al. High prevalence of human cytomegalovirus in prostatic intraepithelial neoplasia and prostatic carcinoma. J Urol. 2003;170(3):998–1002. doi: 10.1097/01.ju.0000080263.46164.97. [DOI] [PubMed] [Google Scholar]
- 118.Baryawno N, Rahbar A, Wolmer-Solberg N, et al. Detection of human cytomegalovirus in medulloblastomas reveals a potential therapeutic target. J Clin Invest. 2011;121(10):4043–4055. doi: 10.1172/JCI57147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Soderberg-Naucler C, Rahbar A, Stragliotto G. Survival in patients with glioblastoma receiving valganciclovir. N Engl J Med. 2013;369(10):985–986. doi: 10.1056/NEJMc1302145. [DOI] [PubMed] [Google Scholar]
- 120.Hacohen N, Fritsch EF, Carter TA, et al. Cancer Immunology at the Crossroads: Functional Genomics Getting Personal with Neoantigen-Based Therapeutic Cancer Vaccines. Cancer Immunol Res. 2013;1:11–15. doi: 10.1158/2326-6066.CIR-13-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Matsushita H, Vesely MD, Koboldt DC, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482(7385):400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lennerz V, Fatho M, Gentilini C, et al. The response of autologous T cells to a human melanoma is dominated by mutated neoantigens. Proc Natl Acad Sci USA. 2005;102(44):16013–16018. doi: 10.1073/pnas.0500090102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rajasagi M, Shukla S, DeLuca D, et al. Systemic identification of personal mutated tumor-specifc neoantigens in CLL. 54th ASH Annual Meeting and Exposition.2012. [Google Scholar]
- 124.Zhang GL, Ansari HR, Bradley P, et al. Machine learning competition in immunology - Prediction of HLA class I binding peptides. J Immunol Methods. 2011;374(1–2):1–4. doi: 10.1016/j.jim.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 125.Castle JC, Kreiter S, Diekmann J, et al. Exploiting the mutanome for tumor vaccination. Cancer Res. 2012;72(5):1081–1091. doi: 10.1158/0008-5472.CAN-11-3722. [DOI] [PubMed] [Google Scholar]
- 126.Liau LM, Black KL, Martin NA, et al. Treatment of a patient by vaccination with autologous dendritic cells pulsed with allogeneic major histocompatibility complex class I-matched tumor peptides. Case Report. Neurosurg Focus. 2000;9(6):e8. doi: 10.3171/foc.2000.9.6.9. [DOI] [PubMed] [Google Scholar]
- 127.Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11(15):5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 128.De Vleeschouwer S, Fieuws S, Rutkowski S, et al. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res. 2008;14(10):3098–3104. doi: 10.1158/1078-0432.CCR-07-4875. [DOI] [PubMed] [Google Scholar]
- 129.De Vleeschouwer S, Van Calenbergh F, Demaerel P, et al. Transient local response and persistent tumor control in a child with recurrent malignant glioma: treatment with combination therapy including dendritic cell therapy. Case report. J Neurosurg. 2004;100(5 Suppl Pediatrics):492–497. doi: 10.3171/ped.2004.100.5.0492. [DOI] [PubMed] [Google Scholar]
- 130.Rutkowski S, De Vleeschouwer S, Kaempgen E, et al. Surgery and adjuvant dendritic cell-based tumour vaccination for patients with relapsed malignant glioma, a feasibility study. Br J Cancer. 2004;91(9):1656–1662. doi: 10.1038/sj.bjc.6602195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yamanaka R, Homma J, Yajima N, et al. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res. 2005;11:4160–4167. doi: 10.1158/1078-0432.CCR-05-0120. [DOI] [PubMed] [Google Scholar]
- 132.Fadul CE, Fisher JL, Hampton TH, et al. Immune response in patients with newly diagnosed glioblastoma multiforme treated with intranodal autologous tumor lysate-dendritic cell vaccination after radiation chemotherapy. J Immunother. 2011;34(4):382–389. doi: 10.1097/CJI.0b013e318215e300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Prins RM, Soto H, Konkankit V, et al. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17(6):1603–1615. doi: 10.1158/1078-0432.CCR-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wheeler CJ, Black KL, Liu G, et al. Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res. 2008;68(14):5955–5964. doi: 10.1158/0008-5472.CAN-07-5973. [DOI] [PubMed] [Google Scholar]
- 135.Ardon H, Van Gool S, Lopes IS, et al. Integration of autologous dendritic cell-based immunotherapy in the primary treatment for patients with newly diagnosed glioblastoma multiforme: a pilot study. J Neurooncol. 2010;99(2):261–272. doi: 10.1007/s11060-010-0131-y. [DOI] [PubMed] [Google Scholar]
- 136.Ardon H, Van Gool SW, Verschuere T, et al. Integration of autologous dendritic cell-based immunotherapy in the standard of care treatment for patients with newly diagnosed glioblastoma: results of the HGG-2006 phase I/II trial. Cancer Immunol Immunother. 2012;61:2033–2044. doi: 10.1007/s00262-012-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Heimberger AB, Crotty LE, Archer GE, et al. Bone marrow-derived dendritic cells pulsed with tumor homogenate induce immunity against syngeneic intracerebral glioma. J Neuroimmunol. 2000;103(1):16–25. doi: 10.1016/s0165-5728(99)00172-1. [DOI] [PubMed] [Google Scholar]
- 138.Liau LM, Black KL, Prins RM, et al. Treatment of intracranial gliomas with bone marrow-derived dendritic cells pulsed with tumor antigens. J Neurosurg. 1999;90(6):1115–1124. doi: 10.3171/jns.1999.90.6.1115. [DOI] [PubMed] [Google Scholar]
- 139.Prestegarden L, Enger PO. Cancer stem cells in the central nervous system--a critical review. Cancer Res. 2010;70(21):8255–8258. doi: 10.1158/0008-5472.CAN-10-1592. [DOI] [PubMed] [Google Scholar]
- 140.Murat A, Migliavacca E, Gorlia T, et al. Stem cell-related “self-renewal” signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J Clin Oncol. 2008;26(18):3015–3024. doi: 10.1200/JCO.2007.15.7164. [DOI] [PubMed] [Google Scholar]
- 141.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 142.Wu A, Wei J, Kong LY, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro-oncol. 2010;12(11):1113–1125. doi: 10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Di Tomaso T, Mazzoleni S, Wang E, et al. Immunobiological characterization of cancer stem cells isolated from glioblastoma patients. Clin Cancer Res. 2010;16(3):800–813. doi: 10.1158/1078-0432.CCR-09-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xu Q, Liu G, Yuan X, et al. Antigen-specific T-cell response from dendritic cell vaccination using cancer stem-like cell-associated antigens. Stem cells. 2009;27(8):1734–1740. doi: 10.1002/stem.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pellegatta S, Poliani PL, Corno D, et al. Neurospheres Enriched in Cancer Stem-Like Cells Are Highly Effective in Eliciting a Dendritic Cell-Mediated Immune Response against Malignant Gliomas. Cancer Res. 2006;66(21):10247–10252. doi: 10.1158/0008-5472.CAN-06-2048. [DOI] [PubMed] [Google Scholar]
- 146.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5(11):1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 147.Bloch O, Crane CA, Fuks Y, et al. Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a phase II, single-arm trial. Neuro Oncol. 2014;16(2):274–279. doi: 10.1093/neuonc/not203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Crane CA, Han SJ, Ahn B, et al. Individual patient-specific immunity against high-grade glioma after vaccination with autologous tumor derived peptides bound to the 96 KD chaperone protein. Clin Cancer Res. 2013;19(1):205–214. doi: 10.1158/1078-0432.CCR-11-3358. [DOI] [PubMed] [Google Scholar]
- 149.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15(2):138–147. doi: 10.1016/s0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 150.Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117(5):1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.de Veer M, Meeusen E. New developments in vaccine research--unveiling the secret of vaccine adjuvants. Discov Med. 2011;12(64):195–204. [PubMed] [Google Scholar]
- 152.Chiang CL, Kandalaft LE, Coukos G. Adjuvants for enhancing the immunogenicity of whole tumor cell vaccines. Int Rev Immunol. 2011;30(2–3):150–182. doi: 10.3109/08830185.2011.572210. [DOI] [PubMed] [Google Scholar]
- 153.Schijns VE, Lavelle EC. Trends in vaccine adjuvants. Exp Rev Vaccines. 2011;10(4):539–550. doi: 10.1586/erv.11.21. [DOI] [PubMed] [Google Scholar]
- 154.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12(4):265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Ann Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 156.Darrasse-Jeze G, Deroubaix S, Mouquet H, et al. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206(9):1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gnjatic S, Sawhney NB, Bhardwaj N. Toll-like receptor agonists: are they good adjuvants? Cancer J. 2010;16(4):382–391. doi: 10.1097/PPO.0b013e3181eaca65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ursu R, Carpentier AF. Immunotherapeutic approach with oligodeoxynucleotides containing CpG motifs (CpG-ODN) in malignant glioma. Adv Exp Med Biol. 2012;746:95–108. doi: 10.1007/978-1-4614-3146-6_8. [DOI] [PubMed] [Google Scholar]
- 159.Prins RM, Craft N, Bruhn KW, et al. The TLR-7 agonist, imiquimod, enhances dendritic cell survival and promotes tumor antigen-specific T cell priming: relation to central nervous system antitumor immunity. J Immunol. 2006;176(1):157–164. doi: 10.4049/jimmunol.176.1.157. [DOI] [PubMed] [Google Scholar]
- 160.Bode C, Zhao G, Steinhagen F, et al. CpG DNA as a vaccine adjuvant. Exp Rev Vaccines. 2011;10(4):499–511. doi: 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Linn JF, Black P, Derksen K, et al. Keyhole limpet haemocyanin in experimental bladder cancer: literature review and own results. Eur Urol. 2000;37(Suppl 3):34–40. doi: 10.1159/000052390. [DOI] [PubMed] [Google Scholar]
- 162.Zhan Y, Xu Y, Lew AM. The regulation of the development and function of dendritic cell subsets by GM-CSF: more than a hematopoietic growth factor. Mol Immunol. 2012;52(1):30–37. doi: 10.1016/j.molimm.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 163.Salgia R, Lynch T, Skarin A, et al. Vaccination with irradiated autologous tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor augments antitumor immunity in some patients with metastatic non-small-cell lung carcinoma. J Clin Oncol. 2003;21(4):624–630. doi: 10.1200/JCO.2003.03.091. [DOI] [PubMed] [Google Scholar]
- 164.Soiffer R, Hodi FS, Haluska F, et al. Vaccination with irradiated, autologous melanoma cells engineered to secrete granulocyte-macrophage colony-stimulating factor by adenoviral-mediated gene transfer augments antitumor immunity in patients with metastatic melanoma. J Clin Oncol. 2003;21(17):3343–3350. doi: 10.1200/JCO.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 165.Nemunaitis J. Vaccines in cancer: GVAX, a GM-CSF gene vaccine. Exp Rev Vaccines. 2005;4(3):259–274. doi: 10.1586/14760584.4.3.259. [DOI] [PubMed] [Google Scholar]
- 166.Parmiani G, Castelli C, Pilla L, et al. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol. 2007;18(2):226–232. doi: 10.1093/annonc/mdl158. [DOI] [PubMed] [Google Scholar]
- 167.Clive KS, Tyler JA, Clifton GT, et al. Use of GM-CSF as an adjuvant with cancer vaccines: beneficial or detrimental? Exp Rev Vaccines. 2010;9(5):519–525. doi: 10.1586/erv.10.40. [DOI] [PubMed] [Google Scholar]
- 168.Jinushi M, Nakazaki Y, Dougan M, et al. MFG-E8-mediated uptake of apoptotic cells by APCs links the pro- and antiinflammatory activities of GM-CSF. J Clin Invest. 2007;117(7):1902–1913. doi: 10.1172/JCI30966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ohlfest JR, Andersen BM, Litterman AJ, et al. Vaccine injection site matters: qualitative and quantitative defects in CD8 T cells primed as a function of proximity to the tumor in a murine glioma model. J Immunol. 2013;190(2):613–620. doi: 10.4049/jimmunol.1201557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wheeler CJ, Black KL, Liu G, et al. Thymic CD8+ T-cell production strongly influences tumor antigen recognition and age-dependent glioma mortality. J Immunol. 2003;171:4927–4933. doi: 10.4049/jimmunol.171.9.4927. [DOI] [PubMed] [Google Scholar]
- 171.De Vleeschouwer S, Ardon H, Van Calenbergh F, et al. Stratification according to HGG-IMMUNO RPA model predicts outcome in a large group of patients with relapsed malignant glioma treated by adjuvant postoperative dendritic cell vaccination. Cancer Immunol Immunother. 2012;61:2105–2112. doi: 10.1007/s00262-012-1271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pellegatta S, Eoli M, Frigerio S, et al. The natural killer cell response and tumor debulking are associated with prolonged survival in recurrent glioblastoma patients receiving dendritic cells loaded with autologous tumor lysates. Oncoimmunology. 2013;2(3):e23401. doi: 10.4161/onci.23401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sampson JH, Schmittling RJ, Archer GE, et al. A pilot study of IL-2Ralpha blockade during lymphopenia depletes regulatory T-cells and correlates with enhanced immunity in patients with glioblastoma. PloS One. 2012;7(2):e31046. doi: 10.1371/journal.pone.0031046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. New Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 175.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229(1):12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Linsley PS, Greene JL, Brady W, et al. Human B7–1 (CD80) and B7–2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors.[erratum appears in Immunity 1995 Feb;2(2):following 203] Immunity. 1994;1(9):793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 177.Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 178.Peggs KS, Quezada SA, Chambers CA, et al. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206(8):1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liu Y, Yu Y, Yang S, et al. Regulation of arginase I activity and expression by both PD-1 and CTLA-4 on the myeloid-derived suppressor cells. Cancer Immunol Immunother. 2009;58(5):687–697. doi: 10.1007/s00262-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270(5238):985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 181.Keir ME, Freeman GJ, Sharpe AH. PD-1 regulates self-reactive CD8+ T cell responses to antigen in lymph nodes and tissues. J Immunol. 2007;179(8):5064–5070. doi: 10.4049/jimmunol.179.8.5064. [DOI] [PubMed] [Google Scholar]
- 182.Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 184.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Velu V, Titanji K, Zhu B, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458(7235):206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Terme M, Ullrich E, Aymeric L, et al. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res. 2011;71(16):5393–5399. doi: 10.1158/0008-5472.CAN-11-0993. [DOI] [PubMed] [Google Scholar]
- 187.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 188.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13(1):84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 190.Yao Y, Tao R, Wang X, et al. B7-H1 is correlated with malignancy-grade gliomas but is not expressed exclusively on tumor stem-like cells. Neuro Oncol. 2009;11(6):757–766. doi: 10.1215/15228517-2009-014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wintterle S, Schreiner B, Mitsdoerffer M, et al. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63(21):7462–7467. [PubMed] [Google Scholar]
- 192.Avril T, Saikali S, Vauleon E, et al. Distinct effects of human glioblastoma immunoregulatory molecules programmed cell death ligand-1 (PDL-1) and indoleamine 2,3-dioxygenase (IDO) on tumour-specific T cell functions. J Neuroimmunol. 2010;225(1–2):22–33. doi: 10.1016/j.jneuroim.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 193.Wilmotte R, Burkhardt K, Kindler V, et al. B7-homolog 1 expression by human glioma: a new mechanism of immune evasion. Neuroreport. 2005;16(10):1081–1085. doi: 10.1097/00001756-200507130-00010. [DOI] [PubMed] [Google Scholar]
- 194.Jacobs JF, Idema AJ, Bol KF, et al. Regulatory T cells and the PD-L1/PD-1 pathway mediate immune suppression in malignant human brain tumors. Neuro Oncol. 2009;11(4):394–402. doi: 10.1215/15228517-2008-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fecci PE, Ochiai H, Mitchell DA, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer Res. 2007;13(7):2158–2167. doi: 10.1158/1078-0432.CCR-06-2070. [DOI] [PubMed] [Google Scholar]
- 196.Vom Berg J, Vrohlings M, Haller S, et al. Intratumoral IL-12 combined with CTLA-4 blockade elicits T cell-mediated glioma rejection. J Exp Med. 2013;210(13):2803–2811. doi: 10.1084/jem.20130678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zeng J, See AP, Phallen J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86(2):343–349. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wolchok J. How recent advances in immunotherapy are changing the standard of care for patients with metastatic melanoma. Ann Oncol. 2012;23(Suppl 8):viii15–21. doi: 10.1093/annonc/mds258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Prieto PA, Yang JC, Sherry RM, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18(7):2039–2047. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ribas A, Kefford R, Marshall MA, et al. Phase III Randomized Clinical Trial Comparing Tremelimumab With Standard-of-Care Chemotherapy in Patients With Advanced Melanoma. J Clin Oncol. 2013;31:616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30(17):2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 202.Madan RA, Mohebtash M, Arlen PM, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(5):501–508. doi: 10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.McNeel DG, Smith HA, Eickhoff JC, et al. Phase I trial of tremelimumab in combination with short-term androgen deprivation in patients with PSA-recurrent prostate cancer. Cancer Immunol Immunother. 2012;61(7):1137–1147. doi: 10.1007/s00262-011-1193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Vonderheide RH, LoRusso PM, Khalil M, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res. 2012;16(13):3485–3494. doi: 10.1158/1078-0432.CCR-10-0505. [DOI] [PubMed] [Google Scholar]
- 205.Chung KY, Gore I, Fong L, et al. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol. 2010;28(21):3485–3490. doi: 10.1200/JCO.2010.28.3994. [DOI] [PubMed] [Google Scholar]
- 206.Yang JC, Hughes M, Kammula U, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30(8):825–830. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33(8):828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Calabro L, Morra A, Fonsatti E, et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2013;14(11):1104–1111. doi: 10.1016/S1470-2045(13)70381-4. [DOI] [PubMed] [Google Scholar]
- 209.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 211.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 Blockade: New Immunotherapeutic Modalities with Durable Clinical Benefit in Melanoma Patients. Clin Cancer Res. 2013;19(19):5300–5309. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 212.Weber JS, Dummer R, de Pril V, et al. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119(9):1675–1682. doi: 10.1002/cncr.27969. [DOI] [PubMed] [Google Scholar]
- 213.Feng Y, Roy A, Masson E, et al. Exposure-response relationships of the efficacy and safety of ipilimumab in patients with advanced melanoma. Clin Cancer Res. 2013;19(14):3977–3986. doi: 10.1158/1078-0432.CCR-12-3243. [DOI] [PubMed] [Google Scholar]
- 214.Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459–465. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- 215.Mathew M, Tam M, Ott PA, et al. Ipilimumab in melanoma with limited brain metastases treated with stereotactic radiosurgery. Melanoma Res. 2013;23(3):191–195. doi: 10.1097/CMR.0b013e32835f3d90. [DOI] [PubMed] [Google Scholar]
- 216.Silk AW, Bassetti MF, West BT, et al. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med. 2013;2(6):899–906. doi: 10.1002/cam4.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hodi FS, Oble DA, Drappatz J, et al. CTLA-4 blockade with ipilimumab induces significant clinical benefit in a female with melanoma metastases to the CNS. Nature clinical practice. Oncology. 2008;5(9):557–561. doi: 10.1038/ncponc1183. [DOI] [PubMed] [Google Scholar]
- 218.Litterman AJ, Zellmer DM, Grinnen KL, et al. Profound impairment of adaptive immune responses by alkylating chemotherapy. J Immunol. 2013;190(12):6259–6268. doi: 10.4049/jimmunol.1203539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Campian J, Sarai G, Ye X, et al. The association between severe treatment-related lymphopenia and progression free survival in patients with newly diagnosed squamous cell head and neck cancer. Head Neck. 2013 doi: 10.1002/hed.23535. doi:10.1002/hed.23535. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Campian JL, Ye X, Brock M, et al. Treatment-related lymphopenia in patients with stage III non-small-cell lung cancer. Cancer Invest. 2013;31(3):183–188. doi: 10.3109/07357907.2013.767342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wild AT, Ye X, Ellsworth SG, et al. The Association Between Chemoradiation-related Lymphopenia and Clinical Outcomes in Patients With Locally Advanced Pancreatic Adenocarcinoma. Am J Clin Oncol. 2013 doi: 10.1097/COC.0b013e3182940ff9. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8(3):151–160. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 223.Apetoh L, Tesniere A, Ghiringhelli F, et al. Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res. 2008;68(11):4026–4030. doi: 10.1158/0008-5472.CAN-08-0427. [DOI] [PubMed] [Google Scholar]
- 224.Sanchez-Perez LA, Choi BD, Archer GE, et al. Myeloablative temozolomide enhances CD8(+) T-cell responses to vaccine and is required for efficacy against brain tumors in mice. PLoS One. 2013;8(3):e59082. doi: 10.1371/journal.pone.0059082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.McCoy MJ, Lake RA, van der Most RG, et al. Post-chemotherapy T-cell recovery is a marker of improved survival in patients with advanced thoracic malignancies. Br J Cancer. 2012;107(7):1107–1115. doi: 10.1038/bjc.2012.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56(5):641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Suzuki E, Kapoor V, Jassar AS, et al. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11(18):6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 228.Bruchard M, Mignot G, Derangere V, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 2013;19(1):57–64. doi: 10.1038/nm.2999. [DOI] [PubMed] [Google Scholar]
- 229.Fine JH, Chen P, Mesci A, et al. Chemotherapy-induced genotoxic stress promotes sensitivity to natural killer cell cytotoxicity by enabling missing-self recognition. Cancer Res. 2010;70(18):7102–7113. doi: 10.1158/0008-5472.CAN-10-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hong M, Puaux AL, Huang C, et al. Chemotherapy induces intratumoral expression of chemokines in cutaneous melanoma, favoring T-cell infiltration and tumor control. Cancer Res. 2011;71(22):6997–7009. doi: 10.1158/0008-5472.CAN-11-1466. [DOI] [PubMed] [Google Scholar]
- 231.Demaria S, Pilones KA, Formenti SC, et al. Exploiting the stress response to radiation to sensitize poorly immunogenic tumors to anti-CTLA-4 treatment. Oncoimmunology. 2013;2(3):e23127. doi: 10.4161/onci.23127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. New Engl J Med. 2012;366(10):925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lesterhuis WJ, Salmons J, Nowak AK, et al. Synergistic effect of CTLA-4 blockade and cancer chemotherapy in the induction of anti-tumor immunity. PLoS One. 2013;8(4):e61895. doi: 10.1371/journal.pone.0061895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Gabrilovich D, Ishida T, Oyama T, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92(11):4150–4166. [PubMed] [Google Scholar]
- 235.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2(10):1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 236.Johnson BF, Clay TM, Hobeika AC, et al. Vascular endothelial growth factor and immunosuppression in cancer: current knowledge and potential for new therapy. Exp Opin Biol Ther. 2007;7(4):449–460. doi: 10.1517/14712598.7.4.449. [DOI] [PubMed] [Google Scholar]
- 237.Tartour E, Pere H, Maillere B, et al. Angiogenesis and immunity: a bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev. 2011;30(1):83–95. doi: 10.1007/s10555-011-9281-4. [DOI] [PubMed] [Google Scholar]
- 238.Shrimali RK, Yu Z, Theoret MR, et al. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70(15):6171–6180. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Manning EA, Ullman JG, Leatherman JM, et al. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin Cancer Res. 2007;13(13):3951–3959. doi: 10.1158/1078-0432.CCR-07-0374. [DOI] [PubMed] [Google Scholar]
- 240.Huang Y, Goel S, Duda DG, et al. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013;73(10):2943–2948. doi: 10.1158/0008-5472.CAN-12-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mitchell DA, Cui X, Schmittling RJ, et al. Monoclonal antibody blockade of IL-2 receptor {alpha} during lymphopenia selectively depletes regulatory T cells in mice and humans. Blood. 2011;118(11):3003–3012. doi: 10.1182/blood-2011-02-334565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Fecci PE, Sweeney AE, Grossi PM, et al. Systemic anti-CD25 monoclonal antibody administration safely enhances immunity in murine glioma without eliminating regulatory T cells. Clin Cancer Res. 2006;12(14 Pt 1):4294–4305. doi: 10.1158/1078-0432.CCR-06-0053. [DOI] [PubMed] [Google Scholar]
- 243.Cheng G, Yu A, Malek TR. T-cell tolerance and the multi-functional role of IL-2R signaling in T-regulatory cells. Immunol Rev. 2011;241(1):63–76. doi: 10.1111/j.1600-065X.2011.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Duraiswamy J, Kaluza KM, Freeman GJ, et al. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013;73(12):3591–3603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Agarwalla P, Barnard Z, Fecci P, et al. Sequential immunotherapy by vaccination with GM-CSF-expressing glioma cells and CTLA-4 blockade effectively treats established murine intracranial tumors. J Immunother. 2012;35(5):385–389. doi: 10.1097/CJI.0b013e3182562d59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Williams EL, Dunn SN, James S, et al. Immunomodulatory monoclonal antibodies combined with peptide vaccination provide potent immunotherapy in an aggressive murine neuroblastoma model. Clin Cancer Res. 2013;19(13):3545–3555. doi: 10.1158/1078-0432.CCR-12-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wada S, Jackson CM, Yoshimura K, et al. Sequencing CTLA-4 blockade with cell-based immunotherapy for prostate cancer. J Transl Med. 2013;11:89. doi: 10.1186/1479-5876-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.van den Eertwegh AJ, Versluis J, van den Berg HP, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(5):509–517. doi: 10.1016/S1470-2045(12)70007-4. [DOI] [PubMed] [Google Scholar]
- 249.Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31(34):4311–4318. doi: 10.1200/JCO.2013.51.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Curran MA, Montalvo W, Yagita H, et al. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107(9):4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 253.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 254.Wolchok JD, Chapman PB. Phase I trial of adoptive immunotherapy with cytolytic T lymphocytes immunized against a tyrosinase epitope. J Clin Oncol. 2002;20(14):3176. doi: 10.1200/JCO.2002.20.14.3176. author reply 3176–3177. [DOI] [PubMed] [Google Scholar]
- 255.Okada H, Pollack IF. Do we need novel radiologic response criteria for brain tumor immunotherapy? Exp Rev Neurotherap. 2011;11(5):619–622. doi: 10.1586/ern.11.49. [DOI] [PubMed] [Google Scholar]
- 256.Disis ML. Immunologic biomarkers as correlates of clinical response to cancer immunotherapy. Cancer Immunol Immunother. 2011;60(3):433–442. doi: 10.1007/s00262-010-0960-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Yamanaka R, Abe T, Yajima N, et al. Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: results of a clinical phase I/II trial. Br J Cancer. 2003;89(7):1172–1179. doi: 10.1038/sj.bjc.6601268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.McNeil LK, Price L, Britten CM, et al. A harmonized approach to intracellular cytokine staining gating: Results from an international multiconsortia proficiency panel conducted by the Cancer Immunotherapy Consortium (CIC/CRI) Cytometry A. 2013;83(8):728–738. doi: 10.1002/cyto.a.22319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Han SJ, Ahn BJ, Waldron JS, et al. Gamma interferon-mediated superinduction of B7-H1 in PTEN-deficient glioblastoma: a paradoxical mechanism of immune evasion. Neuroreport. 2009;20(18):1597–1602. doi: 10.1097/WNR.0b013e32833188f7. [DOI] [PMC free article] [PubMed] [Google Scholar]