Abstract
Epithelial cells in vivo form tight cell-cell associations that spatially separate distinct apical and basolateral domains. These domains provide discrete cellular processes essential for proper tissue and organ development. Using confocal imaging and selective plasma membrane domain activation, the type I and type II transforming growth factor-β (TGFβ) receptors were found to be localized specifically at the basolateral surfaces of polarized Madin-Darby canine kidney (MDCK) cells. Receptors concentrated predominantly at the lateral sites of cell-cell contact, adjacent to the gap junctional complex. Cytoplasmic domain truncations for each receptor resulted in the loss of specific lateral domain targeting and dispersion to both the apical and basal domains. Whereas receptors concentrate basolaterally in regions of direct cell-cell contact in nonpolarized MDCK cell monolayers, receptor staining was absent from areas of noncell contact. In contrast to the defined basolateral polarity observed for the TGFβ receptor complex, TGFβ ligand secretion was found to be from the apical surfaces. Confocal imaging of MDCK cells with an antibody to TGFβ1 confirmed a predominant apical localization, with a stark absence at the basal membrane. These findings indicate that cell adhesion regulates the localization of TGFβ receptors in polarized epithelial cultures and that the response to TGFβ is dependent upon the spatial distribution and secretion of TGFβ receptors and ligand, respectively.
INTRODUCTION
The formation of tissues and organs results from the spatiotemporal integration of various cell and environmental signals (Wollner and Nelson, 1992; Vleminckx and Kemler, 1999). Mammalian epithelial cells exemplify these coordinated functions in the formation of highly polarized structures with distinct apical and basolateral plasma membrane domains, characterized by distinct sets of membrane lipids, transmembrane proteins, and associated cortical proteins (Odorizzi and Trowbridge, 1997; Wodarz, 2002). This cell polarity establishes and maintains functionally specialized regions in the plasma membrane and cytoplasm, facilitating cellular processes as diverse as localized membrane growth, directional cell migration, and vectorial transport of molecules across cell layers (Drubin and Nelson, 1996).
To maintain epithelial cell polarity and compensate for protein turnover at the cell surface, newly synthesized or recycled proteins are sorted in the trans-Golgi network (TGN) and endosomes before delivery to either the apical or basolateral membranes (Drubin and Nelson, 1996). Sorting occurs postsynthetically and is regulated by functionally and spatially distinct apical and basolateral sorting signals (Wandinger-Ness et al., 1990). Basolateral trafficking of integral membrane proteins is mediated by short cytosolic amino acid motifs, many of which are similar to and/or colinear with tyrosine- or dileucine-based endocytic motifs (Simmen et al., 1999, 2002). Truncations in these domains result in random sorting in both biosynthetic and resorting pathways (Bresciani et al., 1997). Conversely, apical transport occurs in the absence of a functional basolateral sorting signal and often involves N- or O-linked carbohydrate moieties in the proteins ectodomains or as yet unspecified information in the transmembrane anchoring domains (Gibson et al., 1998). These default features are thought to drive segregation of the proteins into glycolipid rafts present in the TGN or endosomes that are incorporated into apical transport vehicles (Ohka et al., 2001).
Maintenance of cell polarity is not solely a function of the cells internal sorting machinery, but it is strategically molded by the extracellular environment, linking individual cells to the organism as a whole (Giancotti and Ruoslahti, 1999; Vleminckx and Kemler, 1999). Cells reside in a protein network, the extracellular matrix (ECM), which they secrete and mold into the extracellular space (Giancotti and Ruoslahti, 1999). Epithelial cells are linked to each other at the tight junctions, which lie at the apical apex of the lateral cortex, forming a diffusion barrier in the plane of the membrane separating the apical and basolateral domains (Wodarz, 2002). Transmembrane adhesion primarily occurs at the zonula adherens, positioned immediately below the tight junctions, with molecules of the cadherin superfamily commonly mediating cell-cell contacts (Tepass et al., 2000; Wodarz, 2002). Epithelial cell-cell adhesion is regulated principally by E-cadherin, which induces the localized assembly of cytoskeletal and signaling networks (Drubin and Nelson, 1996). Although no defined signaling motifs have been defined in the cytoplasmic domains of cadherins, crucial links with the cytoskeleton are achieved through associations with a number of cytoplasmic molecules, specifically β-catenin (Vleminckx and Kemler, 1999; Drubin and Nelson, 1996). Formation of complexes between cadherin-catenin and the cytoskeleton strengthens cell adhesion and provides a scaffold for the generation of various signaling networks (Drubin and Nelson, 1996).
Transforming growth factor-β (TGFβ) is a pleiotropic protein involved in a wide range of cellular functions, including regulating cellular growth and development, inflammation, wound healing, fibrosis, and host immunity (Blobe et al., 2000). The biological activity of TGFβ is greatly dependent on the cellular context. Although TGFβ stimulates proliferation in fibroblasts and other mesenchymal cells, it acts as a potent growth inhibitor in a variety of cell types, including epithelial, hematopoietic, and endothelial cells (Howe et al., 1991; Serini and Gabbiani, 1999; Bissell, 2001; Yue and Mulder, 2001). Three mammalian TGFβ isoforms have been described, termed TGFβ-1, -2, and -3, that generally exhibit similar overall effects in vitro, yet have distinct activity in vivo (Hartsough and Mulder, 1997; Kulkarni et al., 2002). Each isoform is secreted as a latent precursor complexed with a latency-associated protein that inhibits binding of TGFβ to the receptors (Khalil, 2001). Dissociation of active TGFβ from the complex may be accomplished by a number of environmental triggers, including heat, shear forces, pH extremes, and proteolysis (Munger et al., 1997) or through cellular association with the extracellular matrix scaffold (Clark and Coker, 1998).
In general, the majority of mammalian cells express three TGFβ binding species referred to as the type I, type II, and type III (betaglycan) receptors, of which the type III receptor is relatively poorly characterized and its role in signaling is unclear (Laiho et al., 1990, 1991; Lopez-Casillas et al., 1991; Wang et al., 1991; Chen et al., 2003). The type I and type II TGFβ receptors are single pass, transmembrane serine/threonine kinases of 53 and 75 kDa, respectively (Bassing et al., 1994; Lin et al., 1992). Whereas homomeric complexes occur on the cell surface, TGFβ signaling is primarily regulated through heteromeric interactions between the type I and type II receptors (Anders and Leof, 1996). The type II TGFβ receptor is a constitutively active kinase that upon ligand binding, recruits and transphosphorylates the type I receptor in the juxtamembrane GS domain (Wrana et al., 1992, 1994). The activated type I receptor serves as a docking site for the receptor-associated Smads (R-Smads), termed Smad2 and Smad3, that after phosphorylation dissociate from the receptor and complex with the common-mediator Smad4. The R-Smad/Smad4 complex subsequently translocates to the nucleus where it can function as a comodulator of transcription (ten Dijke et al., 2002; Shi and Massagué, 2003). Although the Smad pathway has been shown to be critical for many aspects of TGFβ signaling, Smad-independent responses also have been documented (Hocevar et al., 1999; Wilkes et al., 2003).
Despite significant progress in determining the cellular signaling pathways that are activated by TGFβ, little is known about the trafficking and membrane environment of the receptors. In that regard, the lack of high-specificity antibodies to the extracellular receptor domains coupled with the relatively low levels of endogenous cell surface TGFβ receptors makes analysis of receptor trafficking and localization problematic. To address these issues, chimeric TGFβ receptors consisting of the ligand binding domains of granulocyte macrophage-colony stimulating factor (GM-CSF) α or β receptors (Gearing et al., 1989; Hayashida et al., 1990) fused to the transmembrane and cytoplasmic domains of the type I and type II TGFβ receptors have been used (Anders and Leof, 1996; Anders et al., 1997, 1998). High-affinity GM-CSF binding and subsequent signaling occurs through the formation of α/β heterodimers, in a manner analogous to the endogenous TGFβ receptors. Use of that system has demonstrated distinct signaling and trafficking behavior/requirements of heteromeric (type I/type II) and homomeric (type I/type I or type II/type II) TGFβ receptors in various cell types (Anders et al., 1997, 1998; Doré et al., 2001; Garamszegi et al., 2001; Yao et al., 2002). However, the spatial distribution and activity of the receptor complex had not been addressed. It was to that end that the present study was undertaken.
We present here a detailed analysis of the localization and signaling of the type I and type II TGFβ receptors in polarized Madin-Darby canine kidney (MDCK) cells. The results show 1) Smad2 and 3 activation primarily occurs through ligand addition to the basolateral surface; 2) TGFβ receptors traffic to the basolateral domain, adjacent to the junctional complex; 3) truncation of the type I or type II receptors' cytoplasmic domain results in a loss of basolateral targeting; 4) cell-cell contact is required for TGFβ receptor localization; and 5) TGFβ ligand is predominantly secreted apically. Thus, polarized epithelial cells regulate TGFβ signaling by expressing the receptors and secreting the ligand in spatially defined locales.
MATERIALS AND METHODS
Materials
Human TGFβ was purchased from R&D Systems (Minneapolis, MN) or Austral Biologicals (San Ramon, CA), whereas recombinant GM-CSF was purchased from the Mayo Medical Pharmacy (Rochester, MN). Cell culture medium and geneticin (G418 sulfate) were from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was obtained from Summit (Fort Collins, CO) and hygromycin B was from Roche Diagnostics (Indianapolis, IN). Unless specifically noted, all other reagents were from Sigma-Aldrich (St. Louis, MO). Transwell 12-mm (#3402) and 24-mm (#3410) polycarbonate membrane plates were purchased from Costar (Cambridge, MA); all other tissue culture materials were from Corning Glassworks (Corning, NY).
Cells and Plasmid Constructs
MDCK cells were maintained in DMEM supplemented with 10% (vol/vol) FBS. The MDCK cell clone MD-1, expressing the chimeric αI and βII receptors, was constructed in a two-step process by using the cDNA constructs described previously (Anders et al., 1996). The designations αI and βII refer to the extracellular domains of the human GM-CSF receptor α or β subunits coupled to the transmembrane and cytoplasmic domains of the TGFβ type I or type II receptor, respectively. The βII chimeric receptor was initially transfected into MDCK cells by using the LipofectAMINE transfection reagent (Roche Diagnostics), and clones were selected in DMEM/10%FBS supplemented with 500 μg/ml geneticin. The αI chimeric receptor was subsequently transfected into a high-expressing MDCK βII clone and αI/βII clones selected in DMEM/10%FBS supplemented with 300 μg/ml hygromycin B and 100 μg/ml geneticin. Clones were screened by fluorescence activated cell sorting for membrane expression of αI and/or βII chimeric receptors as described previously (Anders et al., 1996). Truncated α1ΔC and αIIΔC chimeric TGFβ receptors containing the transmembrane domains and just 17 and 13 amino acid residues of the cytoplasmic domains of the type I and type II TGFβ receptors, respectively, fused to the extracellular domain of the GM-CSF receptor α-chain were described previously (Garamszegi et al., 2001). Stable MDCK cell clones MDα1ΔC-βII and MDα1IΔC-βI were generated expressing the truncated α1ΔC and αIIΔC receptors together with the full-length GM-CSF receptor β-chain chimeric, βII and β1, respectively, as outlined for the MD-1 cell line described above.
Polarized Monolayer Cell Culture
Parental MDCK or MD-1 epithelial cells were plated in 12- or 24-mm Costar Transwell polycarbonate membrane plates at densities of 0.5 × 105 or 2 × 105 cells/well, respectively, in DMEM/10%FBS. Formation of tight junctions and integrity of the monolayers were determined by serial measurement of transepithelial resistance. Fully polarized monolayers were achieved 72–96 h post-cell plating. The peak transmembrane resistance, corrected for background, was typically in the range of 150–200 Ω/cm2.
Immunofluorescence Microscopy
For surface receptor staining of polarized monolayers, MDCK cells were plated at a density of 0.5 × 105 cells/12-mm Transwell. Polarized MDCK cell monolayers were washed three times with ice-cold phosphate-buffered saline (PBS)/0.1 mM CaCl2/1 mM MgCl2/0.2% bovine serum albumin (BSA) pH 7.4, before addition of primary antibody diluted in PBS/0.2% BSA/5% normal donkey serum (NDS) for 1 h on ice. The monolayer containing membranes were subsequently washed with three 10-min incubations in ice cold PBS/0.2%BSA before a final 5-min wash with PBS. Cells were fixed for 30 min at room temperature with 2% formaldehyde containing PBS/0.1 mM CaCl2/1 mM MgCl2, washed once with PBS/0.2% BSA, and treated for 10 min on ice with 50 mM NH4Cl in PBS to block background autofluorescence. For internal staining, after fixing the cells were permeablized for 1 min at room temperature with 0.25% Triton X-100 in PBS. Incubation with primary antibody and blocking was performed as detailed above. Cells were subsequently washed twice with PBS/0.2% BSA, and secondary antibody diluted in PBS/0.2% BSA/5% NDS was added for 30 min in the dark. Nuclear staining (blue) was performed by incubation for 10 min in the dark with 300 nM 4,6-diamidino-2-phenylindole (DAPI) diluted in PBS/0.2% BSA/5% NDS. Cells were then washed three times with PBS/0.2% BSA, mounted with Vectasheild, and the membranes viewed at 40× using a LSM 510 confocal microscope. Applied primary antibody concentrations were as follows: human TGFβ type I receptor antibody (1:20, sc9048; Santa Cruz biotechnology, Santa Cruz, CA), GM-CSF receptor antibodies to the α (sc458; Santa Cruz Biotechnology) and β (sc457; Santa Cruz Biotechnology) chains were applied at 1:50, β-catenin (1:400, #06-734; Upstate Biotechnology, Lake Placid, NY), and TGFβ1 ligand antibody (1:50, sc146; Santa Cruz Biotechnology). Secondary anti-mouse Cy3 (715-165-150; Jackson ImmunoResearch Laboratories, West Grove, PA) and anti-rabbit Alexa 488 (A-11008; Molecular Probes, Eugene, OR) were each used at concentrations of 1:200.
Western Blotting
MDCK or MD-1 cells were plated on 24-mm Transwell polycarbonate membranes at densities of 2 × 105 cells/well. The medium was changed daily until cells were fully polarized as assessed by resistance recordings across the monolayers. Ligand stimulations were performed by addition of serum-free DMEM containing TGFβ2 (10 ng/ml) or GM-CSF (10 ng/ml) to the upper (apical) or lower (basolateral) reservoirs. After incubation for the indicated times, cells were washed twice with cold PBS at 4°C and carefully scraped from the membranes in 0.5 ml of cold PBS. Cells were pelleted at 5000 × g and lysed in 100 μl of lysis buffer; 50 mM Tris, pH7.4, 1% NP-40, 0.25% deoxycholate, 50 mM NaCl, 1 mM EGTA, 1 mM Na3VO4, 1 mM NaF, and protease Complete inhibitor cocktail (Roche Diagnostics). The cell debris was removed by centrifugation at 21,000 × g, and equivalent supernatant protein was separated on an 8% SDS-PAGE. Total and phospho-Smad2 antibodies were from Upstate Biotechnology (#06-654 and #06-829, respectively), whereas the total Smad3 antibody was from Zymed Laboratories (South San Francisco, CA) (#51-1500). The rabbit anti-phospho-Smad3 antibody was generated in our laboratory to the peptide COOH-GSPSIRCSpSVpS.
Cell Growth Inhibition Analysis
MDCK cells were plated in 24-mm Transwell plates at 5 × 105 cells/well. At 48 h, the conditioned medium was removed from the apical and basolateral reservoirs, cleared of cell debris by centrifugation at 5000 × g, and stored on ice. The media were divided into two equal fractions, and one-half of each was acid treated to pH 2–3 with 6 M HCl and incubated at room temperature for 30 min before neutralizing back to pH 7–8 by using 6 M NaOH. Different volumes of same day, unfrozen, treated, and untreated conditioned media were added to Mv1Lu cells plated in DMEM/10%FBS at 4 × 104 cells/well in 24-well culture plates. After 24-h incubation with the conditioned medium, the cells were pulsed for 2 h with 1 μCi/ml [3H]thymidine, and trichloracetic acid-precipitatable counts were determined (Shipley et al., 1984).
RESULTS
TGFβ Receptor Activation Occurs from Distinct Plasma Membrane Domains in Polarized MDCK Cell Monolayers
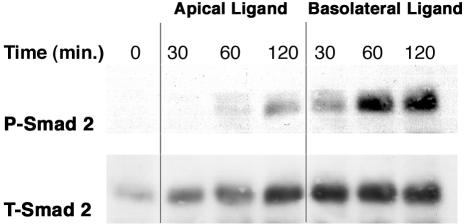
To determine whether TGFβ signaling was differentially regulated by ligand exposure to the apical or basolateral membrane surfaces, MDCK cells were plated in Transwell plates and allowed to form polarized monolayers. Fully polarized cultures were then exposed to TGFβ2 ligand either at the apical or basolateral surfaces, and the degree of TGFβ receptor activation was assessed by Western blot analysis for phospho-Smad2 (Figure 1). Smad2 phosphorylation (P-Smad2) was observed predominantly upon exposure of TGFβ2 to the basolateral surface. Maximal P-Smad2 was observed after 60-min basolateral stimulation, whereas the equivalent apical activation was insignificant. Slight P-Smad2 activity was observed at 120-min ligand exposure from the apical surface, possibly reflecting a small number of apical TGFβ receptors or diffusion of the ligand across the membrane with time. However, the degree of phosphorylation was insignificant relative to the activity observed after basolateral ligand addition. The data clearly demonstrate that the TGFβ receptors on MDCK cells grown as polarized epithelial monolayers are selectively activated upon ligand exposure from the basolateral surface.
Figure 1.
TGFβ receptor activation occurs upon selective ligand exposure to the basolateral domains in polarized MDCK monolayers. MDCK cells were plated at 2 × 105 cells/24-mm Transwell as described in MATERIALS AND METHODS. After complete polarization, duplicate wells were incubated for 30, 60, or 120 min with TGFβ2 (10 ng/ml) exposed to either the upper apical or lower basolateral domains. Cells were lysed, cleared of cell debris and 100 μg of proteins separated by SDS-PAGE. Membranes were probed with a phospho-Smad2 antibody (P-Smad2) to determine the degree of Smad2 activation and a total-Smad2 antibody (T-Smad2) to control for protein loading.
TGFβ Receptors Localize Predominantly at the Basolateral Surface of Polarized MDCK Cell Monolayers
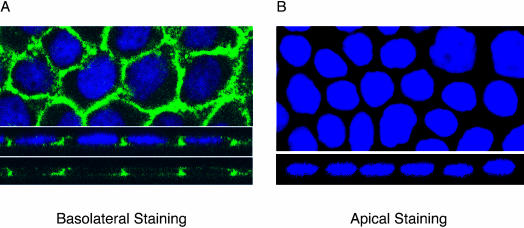
Figure 1 demonstrates that Smad2 phosphorylation primarily occurs when TGFβ is added to the basolateral surfaces of polarized MDCK cells. To determine whether this reflected either an inability of apical receptors to engage the signaling machinery or receptor trafficking to defined plasma membrane domains, immunohistochemical staining of endogenous TGFβ receptors was performed. Horizontal (XY) sections revealed a honeycomb expression pattern of surface type I TGFβ receptors (Figure 2A). When sections perpendicular (XZ) to the monolayer were examined (Figure 2A, bottom), distinct basolateral expression was observed with minimal evidence of apical localization. Moreover, when receptor antibody was applied solely to the apical surface of the cell monolayer, no receptor staining was detected (Figure 2B), whereas control antibody to the MRP2 receptor (which has a well-established apical localization in MDCK cells; Nies et al., 2002) showed predominant apical staining (our unpublished data). Thus, the type I TGFβ receptor primarily localizes to the basolateral membrane in MDCK cells.
Figure 2.
Localization of endogenous type I TGFβ receptors in polarized MDCK monolayers. MDCK cells were plated in 12-mm Transwells and allowed to polarize >72 h. Receptors were imaged upon selective basolateral (A) or apical (B) immunohistochemical staining with an endogenous type I TGFβ receptor primary rabbit antibody coupled to an antirabbit Alexa 488 secondary antibody (green). XY (horizontal) sections are in the top image and XZ (vertical) sections are shown in the lower image. Nuclei were stained with DAPI.
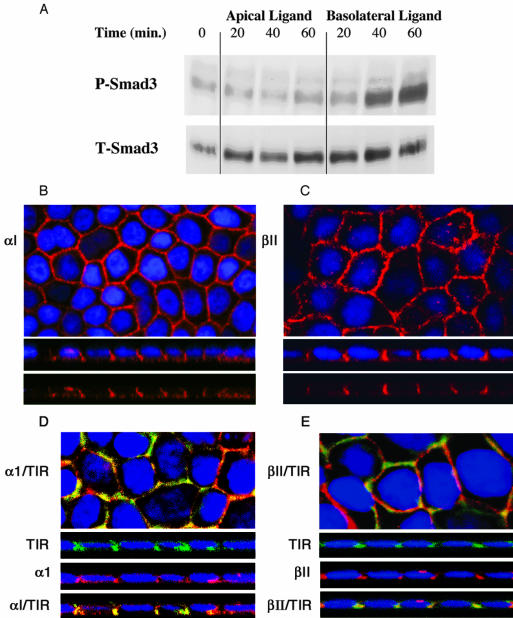
Staining for the TGFβ type II receptor was hindered by the lack of a suitable antibody to detect the endogenous canine type II receptor. Although the type I receptor is restricted to the basolateral domain (Figure 2), because TGFβ ligand requires expression of both type I and II receptors to signal, this presented the possibility that signaling could still occur if the type II receptor was distributed equally over both apical and basolateral surfaces. To investigate expression of the type II receptor (and further confirm type I receptor localization), we used the GM-CSF chimeric TGFβ receptor model because high-specificity antibodies are available to the external domains of both receptors (Anders and Leof, 1996; Anders et al., 1998). The chimeric receptors were generated by fusing the cytoplasmic and transmembrane domains of the TGFβ type I and II receptors with the extracellular ligand binding domains of the α and β GM-CSF receptors, respectively, termed α1 and βII (Anders and Leof, 1996). High-affinity ligand binding requires dimerization of α and β chains, creating a two receptor signaling mechanism analogous to the native TGFβ receptor system (Anders and Leof, 1996). Because the signals required for basolateral localization have, to date, been solely localized to the cytoplasmic domains in all basolateral proteins studied (Simmen et al., 1999, 2002), sorting of the chimeric receptors would be expected to faithfully follow the endogenous homologues. MDCK cells were stably transformed (clone MD-1) with chimeric αI and βII receptors and surface expression confirmed by fluorescence activated cell sorting analysis (our unpublished data). Consistent with our previous data in Figure 1, the MD-1 clone phosphorylated Smad3 and Smad2 in response to both GM-CSF and TGFβ in a basolateral specific manner (Figure 3A; our unpublished data). Moreover, confocal imaging revealed classical honeycomb surface staining and Z-sectioning confirmed the predominantly basolateral localization for both chimeric receptors (Figure 3, B and C).
Figure 3.
Expression and signaling of chimeric TGFβ receptors in polarized MDCK cells. (A) Smad3 signaling was assessed on polarized MD-1 monolayers with GM-CSF ligand (10 ng/ml) exposed selectively to the apical or basolateral domains. After specified incubation times the cells from duplicate 24-mm Transwells were collected, lysed, and 125 μg of protein was separated by SDS-PAGE and probed with phospho-Smad3 (P-Smad3) and total-Smad3 (T-Smad3) antibodies. Chimeric αI (B and D) or βII (C and E) TGFβ receptors were visualized either alone (B and C) or together with the endogenous type I receptor (D and E) as described in MATERIALS AND METHODS. Nuclei were all additionally DAPI stained. Bottom panels for each represent XZ confocal images documenting basolateral expression. Colocalization (yellow) of the chimeric and endogenous TGFβ receptors is shown in the bottom XZ panels labeled αI/TIR and βII/TIR for D and E, respectively.
We have previously determined that the chimeric receptors do not form heteromeric complexes with native TGFβ receptors (Anders et al., 1998), indicating that the two systems traffic independently. Because both receptor complexes localize to the basolateral surface, however, it might be expected that significant colocalization of endogenous and chimeric TGFβ receptors would be observed. To address that question double labeling of each chimeric receptor and the endogenous type I receptor (αI/TIR and βII/TIR, respectively) was performed. As shown in Figure 3, D and E, both the type I and type II chimeric receptors colocalize with endogenous type I TGFβ receptors at the lateral surfaces of the basolateral membrane. Thus, both native and chimeric TGFβ receptors traffic to and signal from the same membrane locale in polarized MDCK cells (Figures 1, 2, 3).
Mutant Chimeric TGFβ Receptors with Cytoplasmic Domain Truncations Lose Their Basolateral Targeting Functions
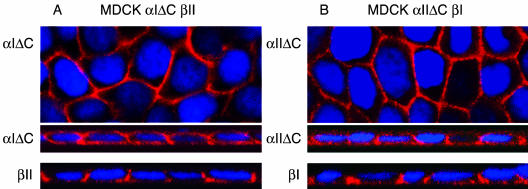
To address whether the observed lateral membrane targeting was a function of basolateral targeting signals contained in the receptor's cytoplasmic tail, type I and type II receptor truncation mutants were generated as described in MATERIALS AND METHODS. Stable MDCK cell clones expressing the truncated α1ΔC or αIIΔC receptor together with a full-length βII or βI receptor (MDα1ΔC-βII and MDα1IΔC-βI, respectively), were imaged for membrane localization. Figure 4 demonstrates that both the α1ΔC and αIIΔC truncated receptors no longer maintain specific basolateral retention, significant staining was observed on both the apical and basal membrane domains. Conversely, the coexpressed βI or βII chimeric receptor maintained the lateral targeting properties demonstrated in Figures 2 and 3. Selective exposure of receptor antibodies solely to the apical surfaces stained both the C-terminally truncated α1ΔC and αIIΔC receptor, no significant staining was observed for the partnering full-length receptor (our unpublished data). These results indicate that lateral delivery of the TGFβ receptor complex is mediated through defined basolateral targeting signals and deletion of these cytoplasmic sequences results in significant receptor miss-sorting to the apical surface.
Figure 4.
Localization of C-terminal–deleted chimeric TGFβ receptors in polarized MDCK cells. Truncated α1ΔC (A) and αIIΔC (B) chimeric receptors, together with the full-length partnering chimeric receptors (βII and β1), were imaged in MDCK cell clones MDα1ΔC-βII and MDα1IΔC-βI, respectively. The truncated α1ΔC (A, expresses 17 of 355 cytoplasmic amino acids) and αIIΔC (B, expresses 13 of 378 cytoplasmic amino acids) receptors are shown as flat XY and horizontal XZ images, above the respective full-length partnering chimeric receptor βII (A) and β1. (B) Horizontal XZ images from parallel cultures. Nuclei were stained with DAPI.
TGFβ Receptors Localize Adjacent to the Zonula Adherens Complex, Predominantly at Regions of Cell-Cell Contact
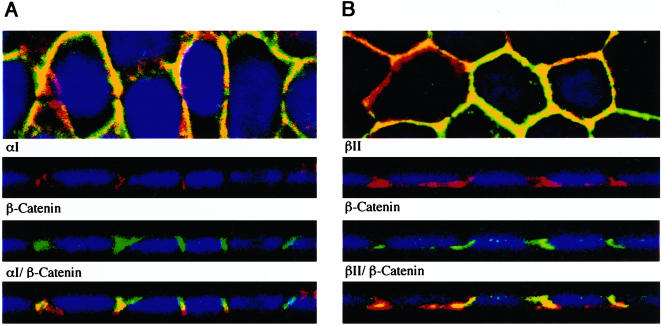
Recently, Tian and Phillips (2002) reported that type II TGFβ receptors could be coimmunoprecipitated with E-cadherin and β-catenin. Because the results in Figures 2 and 3 demonstrate that TGFβ receptors predominantly localize at the lateral interfaces of cell-cell contact, we wished to more carefully define this association with zonula adheren proteins. Dual staining of the type I and type II chimeric receptors with β-catenin was performed on polarized MDCK monolayers (Figure 5). Although some overlap was apparent, the predominant staining pattern reflected two species in proximity. Thus, although the type I and type II chimeric TGFβ receptors are localized near β-catenin, they predominantly reside at the lateral membranes adjacent to the zonula adherens complex.
Figure 5.
Costaining of TGFβ chimeric receptors with the adherens junction marker β-catenin. Polarized MD-1 cells were stained for surface expression of the chimeric αI (A) and βII (B) receptors, fixed, and permeablized before incubation with a β-catenin antibody as described in MATERIALS AND METHODS. The monolayers were subsequently tagged with Cy3 for the chimeric receptors (red) and Alexa 488 secondary for β-catenin (green). Nuclei were stained with DAPI. Images are represented as the horizontal XY flat sections above lower perpendicular XZ cross-sectional images. Costaining of the receptors and β-catenin is shown in the bottom XZ panels labeled αI/β-catenin and βII/β-catenin for A and B, respectively.
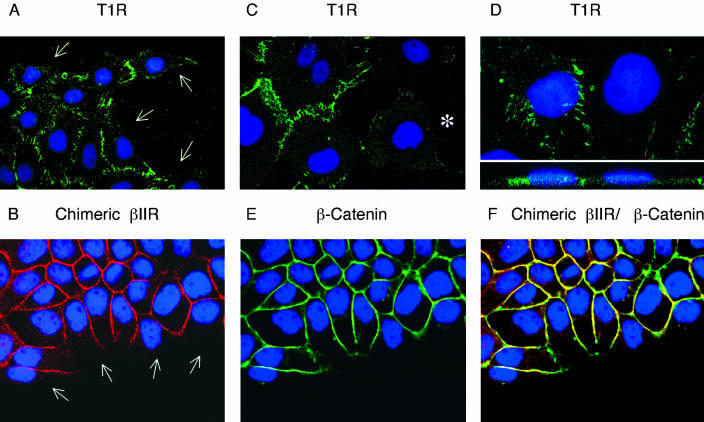
To determine whether specific receptor localization reflected a consequence of cellular polarization, per se, or an intrinsic association of TGFβ receptors to regions of cell-cell contact, receptor expression was determined in nonpolarized MDCK cells. Cultures were plated at lower densities and allowed to establish isolated colonies on Transwell membranes >72 h. Cells were then stained for either chimeric or endogenous TGFβ receptors. Distinct staining profiles were observed dependent upon the degree of cell-cell contact (Figure 6). For example, both the type I and type II receptors selectively localized at sites of cell adhesion as intense fluorescent foci (Figure 6, A and B). Conversely, at regions of non-(Figure 6, A and B, white arrows) or negligible (Figure 6C, star) cell contact, there was a relative absence of receptor staining. This latter finding indicates that TGFβ receptors are either predominantly cytoplasmic, or alternatively (and more likely), the receptors are dispersed over the entire cell surface and localize to the basolateral membrane domain in response to cell adhesion. In support of this hypothesis, Z-sectioning of these nonpolarized MDCK cells demonstrated that the selective lateral localization of the type I and type II receptors was retained despite the lack of complete basolateral and apical domain segregation (Figure 6D and our unpublished data, respectively). Additional staining for the zonula adherens marker β-catenin demonstrated an identical pattern of expression, with minimal staining at nonadherent regions (Figure 6E) and extensive colocalization with TGFβ receptors at sites of cell-cell contact (Figure 6F).
Figure 6.
Localization of TGFβ receptors in nonpolarized MDCK cells. MDCK cells were plated in 12-mm Transwell plates at 5 × 103 cells/well and allowed to propagate for 72 h. (A, C, and D) The endogenous type I TGFβ receptor (TIR) was visualized using anti-rabbit Alexa 488 secondary antibody (green) as described in MATERIALS AND METHODS. (B) Chimeric βII receptors were stained and subsequently tagged with anti-mouse Cy3 secondary antibody (red). (E) β-Catenin was visualized using anti-rabbit Alexa 488 secondary antibody (green) and the degree of colocalization (yellow) with chimeric βII receptors (B) is presented as a superimposed image in F. The monolayers were all additionally DAPI stained. White arrows represent example areas of noncell contact (A and B) and the white star in C exemplifies an isolated MDCK cell with minimal surrounding neighbor contact. The image in D represents a horizontal XY flat section above a lower perpendicular XZ cross-sectional image.
TGFβ Receptor Activation and Ligand Secretion Occur from Distinct Plasma Membrane Domains in Polarized MDCK Cell Monolayers
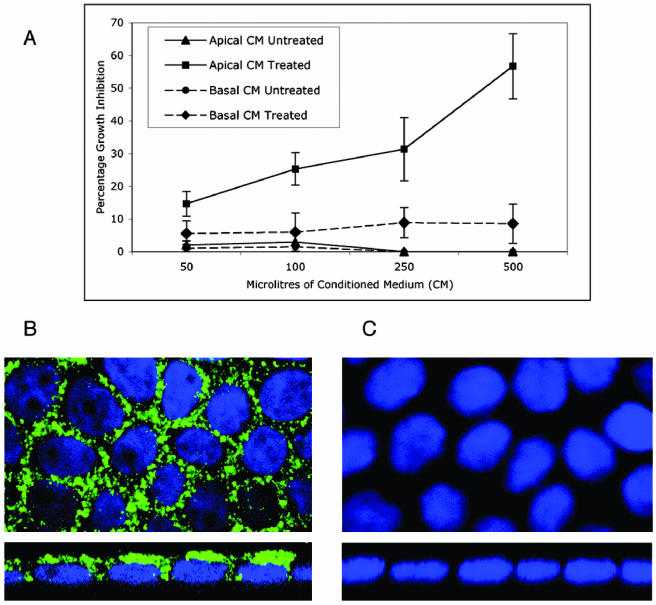
Because essentially all cells in culture or primary tissues in vivo secrete TGFβ or TGFβ family members, the finding of a distinct polarity to TGFβ receptor expression/activation (Figures 1, 2, 3) raises important questions concerning the autocrine and/or paracrine role(s) of secreted ligand. To investigate whether secretion of endogenous TGFβ was directionally regulated, conditioned medium was collected from the apical and basolateral reservoirs of fully polarized MDCK cell cultures. Because TGFβ is primarily secreted in an inactive form that requires cleavage to liberate the active ligand, the conditioned media were assessed for both active and latent TGFβ activity. The results of Figure 7A demonstrate that latent TGFβ ligand is selectively secreted from the apical surface, in stark contrast to receptor activation occurring from the basolateral domain (Figures 1 and 3A). Acid-treated apical conditioned medium was demonstrated to contain TGFβ activity (∼0.1–0.2 ng/ml) capable of inhibiting the growth of MulLv epithelial cells in a dose-dependent manner. The acid-treated basolateral medium, however, demonstrated minimal TGFβ activity at levels comparable with those of the untreated conditioned media from both surfaces. To further address whether basolaterally secreted TGFβ ligand was being trapped in lateral domain complexes or was unable to pass freely through the transwell membranes, confocal imaging of latent TGFβ1 was performed. Imaging of permeablized MDCK cells showed TGFβ1 staining as discrete cytoplasmic vesicular granules characteristic of a secretary molecule (Figures 7B, top). Perpendicular Z-sections, however, revealed the locale predominantly at the apical cytoplasmic domains of the cells, with staining starkly absent from the lower basal domains (Figures 7B, bottom). Although cytoplasmic TGFβ1 was also evident adjacent to the upper lateral domains (Figure 7B), surface staining of nonpermeablized cells revealed negligible ligand association with the external cellular domains or with the Transwell membrane itself (Figure 7C). In addition, to address the possibility that small amounts of secreted active TGFβ might obscure apical receptor staining, acid treatment of apical membranes similarly showed no receptor expression (our unpublished data). These results demonstrate that polarized MDCK cell monolayers predominantly secrete latent TGFβ apically (Figure 7), spatially distinct from the basolateral membrane locale of the type I and type II TGFβ receptor (Figures 1, 2, 3, 4, 5, 6).
Figure 7.
TGFβ ligand secretion occurs from the apical domains in polarized MDCK epithelial cell monolayers. (A) Fresh conditioned medium was collected from the apical (▴ and ▪) and basolateral (• and ♦) reservoirs of polarized MDCK monolayers as described in MATERIALS AND METHODS. The indicated volumes of either acid treated (▪ and ♦) or untreated (▴ and •) medium were applied to Mv1Lu cells, and growth inhibition was assessed by [3H]thymidine incorporation. Results represent the mean of four separate experiments ± SE. (B and C) Cellular localization of TGFβ1 ligand was determined in polarized MDCK cultures. Permeablized (B) or nonpermeabolized (C) cells were immunohistochemically stained with a primary rabbit antibody coupled to an anti-rabbit Alexa 488 secondary antibody (green) to latent TGFβ1. XY (horizontal) sections are in the top image and XZ (vertical) sections are shown in the lower image. Nuclei were stained with DAPI.
DISCUSSION
Although there are many reports concerning the signaling pathways through which TGFβ inhibits and/or stimulates cell proliferation (Wrana et al., 1992, 1994; Mehra and Wrana, 2002), the trafficking and membrane localization of the TGFβ receptor complex is relatively unknown. To that end, the current study was designed to address whether TGFβ receptors localize and signal through defined membrane domains in polarized epithelial cells. Addition of TGFβ to the basolateral, not apical, surfaces of MDCK cell monolayers selectively stimulated Smad2 and Smad3 phosphorylation, demonstrating domain specific TGFβ receptor signaling in polarized epithelial cells (Figures 1 and 3A). As selective basolateral signaling could indicate either an inability of apical receptors to engage the signaling machinery, or receptor trafficking to defined membrane domains, immunocytochemical staining of the endogenous type I receptor demonstrated the latter hypothesis to be operative (Figure 2). Similar results were obtained with an MDCK cell clone (MD-1) constitutively expressing chimeric TGFβ receptors (Figure 3). The findings in MD-1 cells would be expected as basolateral sorting signals have been exclusively reported (to date) to be localized in the cytoplasmic domains of all basolateral proteins studied (Wandinger-Ness et al., 1990; Bresciani et al., 1997; Simmen et al., 1999, 2002), which are conserved in the chimeric receptors. Although the sorting sequences regulating TGFβ receptor trafficking are currently unknown, deletion of the majority of the intracellular domains of either the type I or type II chimeric receptor resulted in miss sorting to the apical surfaces (Figure 4). These results are strongly indicative of undetermined basolateral targeting signals within the cytoplasmic domains of each receptor. Projects are currently underway to further define and characterize these sequences.
Initial epithelial cell adhesion is mediated by transmembrane E-cadherin molecules on adjoining cells binding to the extracellular domains of neighboring E-cadherin dimers with high affinity (Vleminckx and Kemler, 1999). E-cadherin contacts occur along the entire lateral membrane and subsequently coalesce at the apicolateral region of the plasma membrane as a belt-like adhesive contact encircling the apex of each epithelial cell (Drubin and Nelson, 1996; Rajasekaran et al., 1996; Yeaman et al., 1999; Fleming et al., 2000; Sheth et al., 2000; Wodarz, 2002). After these initial cues of gap junction formation, a number of cellular proteins (including β-catenin) are recruited to these domains to establish and maintain the polarized cell phenotype (Drubin and Nelson, 1996). Because immunocytochemical staining demonstrated that both the type I and II TGFβ receptors predominantly localized at the lateral sites of cell-cell contact (Figures 2A, 3, B and C, and 4), we next investigated whether receptor localization might be coupled to, or explained by, direct interaction with the cell adhesion machinery. E-cadherin, and its intracellular binding partner β-catenin, represent definitive markers of the zonula adherens complex and hence the physical regions of direct cell-cell adhesion. Although E-cadherin and β-catenin could be coimmunoprecipitated with an antibody to the TGFβ type II receptor (our unpublished data; Tian and Phillips, 2002), immunocytochemical staining with β-catenin revealed that although overlap was present, the predominant staining pattern reflected two species in proximity (Figure 5).
To further examine this possible adherens junction association, TGFβ receptor localization also was investigated in nonpolarized MDCK cell monolayers. Although chimeric and native TGFβ receptors both reproduced the honeycomb staining pattern observed around the periphery of fully polarized MDCK cells, this was only discerned at sites of direct cell-cell contact (Figure 6). Interestingly, at sites of noncell-cell contact, no significant receptor staining was observed (Figure 6). These results indicate that the basolateral localization of TGFβ receptors is primarily dependent on cell adhesion, in contrast to membrane polarization per se. Moreover, the data support a model of polarized TGFβ targeting in epithelial cells whereby an initial spatial cue (E-cadherin interactions on adhering cells) provides the primary signal for cell-cell adhesion. E-cadherin contact and clustering would subsequently support the local assembly of an intracellular framework designed to initiate the separation of apical and basolateral domains on the plasma membrane. Although complete polarization does not technically occur on the single cell level until all neighboring cellular interactions are resolved, the polarized scaffold seems to assemble simultaneously with adhesion events at sites of cell-cell contact. Thus, the machinery regulating polarized plasma membrane trafficking would be established concurrently with advancing cell-cell adhesions and be fully operational subsequent to full polarization. Accordingly, in nonepithelial or sparse epithelial cells TGFβ receptors would be randomly distributed over the cell surface due to the absence of discrete polar targeting cues, whereas in confluent epithelial cultures TGFβ receptors would traffic along the axis of developing polarity and coalesce at the lateral membrane interface (Drubin and Nelson, 1996).
TGFβ is produced by most tissues and cells in vivo and in vitro. This has resulted in a number of reports discussing potential autocrine or paracrine roles for the secreted ligand. Because we have found TGFβ receptors to be predominantly localized to the basolateral surface in polarized MDCK cells (Figures 2 and 3), this provided an ideal opportunity to directly investigate the possibility of autocrine and/or paracrine signaling. To address the relation between TGFβ receptor localization and ligand secretion, conditioned media from the apical and basolateral reservoirs of polarized MDCK cells were assayed for TGFβ activity. In contrast to receptor signaling that occurred at the basolateral domain (Figures 1 and 3A), latent TGFβ activity was detected predominantly in the apical media (Figure 7A). This observation was further supported upon confocal imaging of TGFβ1 ligand within polarized MDCK monolayers, revealing cytoplasmic staining predominantly in apical adjacent locales (Figure 7B). A distinct absence of TGFβ1 containing secretory vesicles was observed at the basal domains, and negligible ligand staining was observed at the cell surface (Figure 7, B and C). Thus, there seems to be (at least) three levels of control to TGFβ action in polarized epithelia. First, the receptors are on the basolateral surface; second, the ligand is secreted apically; and third, the ligand is latent. Similar spatial segregation of receptor and ligand was recently reported by Vermeer et al. (2003) for erb-B2-4 and its ligand heregulin-α in differentiated human epithelial cells. Whereas heregulin-α was present exclusively in the apical membrane and the overlaying airway surface liquid, erb-B2-4 segregated to the basolateral membrane. This physical separation would prevent potential autocrine stimulation unless the epithelial barrier became disrupted. Hence, the authors present a credible wound repair model whereby the growth induction properties of heregulin-α are restricted to times of disruption of epithelial polarity.
Although the necessity to restrict autocrine TGFβ activity has similarities to that described above for heregulin-α, the spatial segregation of TGFβ receptors and ligand suggests additional levels of control are necessary for tissue integrity. For example, although apically secreted TGFβ acting upon a breached epithelial monolayer would be detrimental to reepithelialization, this would be modulated by ligand latency. Because latent TGFβ would also prevent growth inhibition of newly forming epithelial tissues during early development, the necessity for this additional level of receptor/ligand segregation beyond the protection it provides from unscheduled or “leaky” ligand activation is not readily obvious. One possibility is suggested by the ability of TGFβ to positively regulate its own synthesis (Kelley et al., 2000) through basolateral receptor activation and apical secretion. This would provide a situation whereby internal tissue signals could promote 1) mesenchymal-epithelial signaling and 2) ligand production for easy dissemination and paracrine/endocrine stimulation of distal TGFβ receptors. For example, a recent publication by Bhowmick et al. (2004) proposed a mechanism whereby TGFβ signaling in stromal fibroblasts could modulate the growth and oncogenic potential of adjacent epithelia. Thus, these findings support a physiological scenario whereby the role(s) of stromal-derived TGFβ on epithelial tissue functions under normal and pathological conditions can be investigated.
Acknowledgments
This work was supported by Public Health Service grants GM-54200 and GM-55816 from the National Institute of General Medical Sciences (E.B.L.) and CA-46413 from the National Cancer Institute (R.J.C.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-02-0097. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-02-0097.
Abbreviations used: ECM, extracellular matrix; GM-CSF, granulocyte macrophage-colony stimulating factor; MDCK, Madin-Darby canine kidney; T1R, type I TGFβ receptor, T2R, type II TGFβ receptor; TGN, trans-Golgi network; TGFβ, transforming growth factor-β.
References
- Anders, R.A., Arline, S.L., Doré, J.J., and Leof, E.B. (1997). Distinct endocytic responses of heteromeric and homomeric transforming growth factor beta receptors. Mol. Biol. Cell 8, 2133-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders, R.A., Doré, J.J., Jr., Arline, S.L., Garamszegi, N., and Leof, E.B. (1998). Differential requirement for type I and type II transforming growth factor beta receptor kinase activity in ligand-mediated receptor endocytosis. J. Biol. Chem. 273, 23118-23125. [DOI] [PubMed] [Google Scholar]
- Anders, R.A., and Leof, E.B. (1996). Chimeric granulocyte/macrophage colony-stimulating factor/transforming growth factor-beta (TGF-beta) receptors define a model system for investigating the role of homomeric and heteromeric receptors in TGF-beta signaling. J. Biol. Chem. 271, 21758-2166. [DOI] [PubMed] [Google Scholar]
- Bassing, C.H., Yingling, J.M., and Wang, X.F. (1994). Receptors for the TGF-beta ligand family. Vitam. Horm. 48, 111-115. [DOI] [PubMed] [Google Scholar]
- Bhowmick, N.A., Chytil, A., Pleith, D., Gorska, A.E., Dumont, N., Shappell. S., Washington, M.K., Neilson, E.G., and Moses, H.L. (2004). TGFβ signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303, 848-851. [DOI] [PubMed] [Google Scholar]
- Bissell, D.M. (2001). Chronic liver injury, TGF-beta, and cancer. Exp. Mol. Med. 33, 179-190. [DOI] [PubMed] [Google Scholar]
- Blobe, G.C., Schiemann, W.P., and Lodish, H.F. (2000). Role of transforming growth factor beta in human disease. N. Engl. J. Med. 342, 1350-1358. [DOI] [PubMed] [Google Scholar]
- Bresciani, R., Denzer, K., Pohlmann, R., and von Figura, K. (1997). The 46 kDa mannose-6-phosphate receptor contains a signal for basolateral sorting within the 19 juxtamembrane cytosolic residues. Biochem. J. 327, 811-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W., Kirkbride, K.C., How, T., Nelson, C.D., Mo, J., Frederick, J.P., Wang, X.F., Lefkowitz, R.J., and Blobe, G.C. (2003). beta-Arrestin 2 mediates endocytosis of type III TGF-beta receptor and down-regulation of its signaling. Science 301, 1394-1397. [DOI] [PubMed] [Google Scholar]
- Clark, D.A., and Coker, R. (1998). Transforming growth factor-beta (TGF-beta). Int. J. Biochem. Cell Biol. 30, 293-298. [DOI] [PubMed] [Google Scholar]
- ten Dijke, P., Goumans, M.J., Itoh, F., and Itoh, S. (2002). Regulation of cell proliferation by Smad proteins. J. Cell. Physiol. 191, 1-16. [DOI] [PubMed] [Google Scholar]
- Doré, J.J., Jr., Yao, D., Edens, M., Garamszegi, N., Sholl, E.L., and Leof, E.B. (2001). Mechanisms of transforming growth factor-beta receptor endocytosis and intracellular sorting differ between fibroblasts and epithelial cells. Mol. Biol. Cell 12, 675-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin, D.G., and Nelson, W.J. (1996). Origins of cell polarity. Cell 84, 335-344. [DOI] [PubMed] [Google Scholar]
- Fleming, T.P., Papenbrock, T., Fesenko, I., Hausen, P., and Sheth, B. (2000). Assembly of tight junctions during early vertebrate development. Semin. Cell Dev. Biol. 11, 291-299. [DOI] [PubMed] [Google Scholar]
- Garamszegi, N., Doré, J.J., Jr., Penheiter, S.G., Edens, M., Yao, D., and Leof, E.B. (2001). Transforming growth factor beta receptor signaling and endocytosis are linked through a COOH terminal activation motif in the type I receptor. Mol. Biol. Cell 12, 2881-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing, D.P., King, J.A., Gough, N.M., and Nicola, N.A. (1989). Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 8, 3667-3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti, F.G., and Ruoslahti, E. (1999). Integrin signaling. Science 285, 1028-1032. [DOI] [PubMed] [Google Scholar]
- Gibson, A., Futter, C.E., Maxwell, S., Allchin, E.H., Shipman, M., Kraehenbuhl, J.P., Domingo, D., Odorizzi, G., Trowbridge, I.S., and Hopkins, C.R. (1998). Sorting mechanisms regulating membrane protein traffic in the apical transcytotic pathway of polarized MDCK cells. J. Cell Biol. 143, 81-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartsough, M.T., and Mulder, K.M. (1997). Transforming growth factor-beta signaling in epithelial cells. Pharmacol. Ther. 75, 21-41. [DOI] [PubMed] [Google Scholar]
- Hayashida, K., Kitamura, T., Gorman, D.M., Arai, K., Yokota, T., and Miyajima, A. (1990). Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF): reconstitution of a high-affinity GM-CSF receptor. Proc. Natl. Acad. Sci. USA 87, 9655-9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocevar, B.A., Brown, T.L., and Howe, P.H. (1999). TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 18, 1345-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe, P.H., Draetta, G., and Leof, E.B. (1991). Transforming growth factor beta 1 inhibition of p34cdc2 phosphorylation and histone H1 kinase activity is associated with G1/S-phase growth arrest. Mol. Cell. Biol. 11, 1185-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley, J., Shull, S., Walsh, J.J., Cutroneo, K.R., and Absher, M. (1993). Auto-induction of transforming growth factor-beta in human lung fibroblasts. Am. J. Resp. Cell Mol. Biol. 8, 417-24. [DOI] [PubMed] [Google Scholar]
- Khalil, N. (2001). Post translational activation of latent transforming growth factor beta (L-TGF-beta): clinical implications. Histol. Histopathol. 16, 541-551. [DOI] [PubMed] [Google Scholar]
- Kulkarni, A.B., Thyagarajan, T., and Letterio, J.J. (2002). Function of cytokines within the TGF-beta superfamily as determined from transgenic and gene knockout studies in mice. Curr. Mol. Med. 2, 303-227. [DOI] [PubMed] [Google Scholar]
- Laiho, M., Weis, F.M., Boyd, F.T., Ignotz, R.A., and Massagué, J. (1991). Responsiveness to transforming growth factor-beta (TGF-beta) restored by genetic complementation between cells defective in TGF-beta receptors I and II. J. Biol. Chem. 266, 9108-9112. [PubMed] [Google Scholar]
- Laiho, M., Weis, M.B., and Massague, J. (1990). Concomitant loss of transforming growth factor (TGF)-beta receptor types I and II in TGF-beta-resistant cell mutants implicates both receptor types in signal transduction. J. Biol. Chem. 265, 18518-18524. [PubMed] [Google Scholar]
- Lin, H.Y., Wang, X.F., Ng-Eaton, E., Weinberg, R.A., and Lodish, H.F. (1992). Expression cloning of the TGF-beta type II receptor, a functional transmembrane serine/threonine kinase. Cell 68, 775-785. [DOI] [PubMed] [Google Scholar]
- Lopez-Casillas, F., Cheifetz, S., Doody, J., Andres, J.L., Lane, W.S., and Massague, J. (1991). Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-beta receptor system. Cell 67, 785-795. [DOI] [PubMed] [Google Scholar]
- Mehra, A., and Wrana, J.L. (2002). TGF-beta and the Smad signal transduction pathway. Biochem. Cell Biol. 80, 605-622. [DOI] [PubMed] [Google Scholar]
- Munger, J.S., Harpel, J.G., Gleizes, P.E., Mazzieri, R., Nunes, I., and Rifkin, D.B. (1997). Latent transforming growth factor-beta: structural features and mechanisms of activation. Kidney Int. 51, 1376-1382. [DOI] [PubMed] [Google Scholar]
- Nies, A.T., Konig, J., Cui, Y., Brom, M., Spring, H., and Keppler, D. (2002). Structural requirements for the apical sorting of human multidrug resistance protein 2 (ABCC2). Eur. J. Biochem. 269, 1866-1876. [DOI] [PubMed] [Google Scholar]
- Odorizzi, G. and Trowbridge, I.S. (1997). Structural requirements for basolateral sorting of the human transferrin receptor in the biosynthetic and endocytic pathways of Madin-Darby canine kidney cells. J. Cell Biol. 137, 1255-1264, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohka, S., Ohno, H., Tohyama, K., and Nomoto, A. (2001). Basolateral sorting of human poliovirus receptor alpha involves an interaction with the mu1B subunit of the clathrin adaptor complex in polarized epithelial cells. Biochem. Biophys. Res. Commun. 287, 941-948. [DOI] [PubMed] [Google Scholar]
- Rajasekaran, A.K., Hojo, M., Huima, T., and Rodriguez, B.E. (1996). Catenins and zonula occludens-1 form a complex during early stages in the assembly of tight junctions. J. Cell Biol. 132, 451-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serini, G., and Gabbiani, G. (1999). Mechanisms of myofibroblast activity and phenotypic modulation. Exp. Cell Res. 250, 273-283. [DOI] [PubMed] [Google Scholar]
- Sheth, B., Fontaine, J.J., Ponza, E., McCallum, A., Page, A., Citi, S., Louvard, D., Zahraoui, A., and Fleming, T.P. (2000). Differentiation of the epithelial apical junctional complex during mouse preimplantation development: a role for rab13 in the early maturation of the tight junction. Mech. Dev. 97, 93-104. [DOI] [PubMed] [Google Scholar]
- Shi, Y., and Massagué, J. (2003). Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113, 685-700. [DOI] [PubMed] [Google Scholar]
- Shipley, G.D., Childs, C.B., Volkenant, M.E., and Moses, H.L. (1984). Differential effects of epidermal growth factor, transforming growth factor, and insulin on DNA and protein synthesis and morphology in serum-free cultures of AKR-2B cells. Cancer Res. 44, 710-716. [PubMed] [Google Scholar]
- Simmen, T., Honing, S., Icking, A., Tikkanen, R., and Hunziker, W. (2002). AP-4 binds basolateral signals and participates in basolateral sorting in epithelial MDCK cells. Nat. Cell Biol. 4, 154-159. [DOI] [PubMed] [Google Scholar]
- Simmen, T., Nobile, M., Bonifacino, J.S., and Hunziker, W. (1999). Basolateral sorting of furin in MDCK cells requires a phenylalanine-isoleucine motif together with an acidic amino acid cluster. Mol. Cell. Biol. 19, 3136-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass, U., Truong, K., Godt, D., Ikura, M., and Peifer, M. (2000). Cadherins in embryonic and neural morphogenesis. Nat. Rev. Mol. Cell. Biol. 1, 91-100. [DOI] [PubMed] [Google Scholar]
- Tian, Y.C., and Phillips, A.O. (2002). Interaction between the transforming growth factor-beta type II receptor/Smad pathway and beta-catenin during transforming growth factor-beta1-mediated adherens junction disassembly. Am. J. Pathol. 160, 1619-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer, P.D., Einwalter, L.A., Moninger, T.O., Rokhlina, T., Kern, J.A., Zabner, J., and Welsh, M.J. (2003). Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature 422, 322-326. [DOI] [PubMed] [Google Scholar]
- Vleminckx, K., and Kemler, R. (1999). Cadherins and tissue formation: integrating adhesion and signaling. Bioessays 21, 211-220. [DOI] [PubMed] [Google Scholar]
- Wandinger-Ness, A., Bennett, M.K., Antony, C., and Simons, K. (1990). Distinct transport vesicles mediate the delivery of plasma membrane proteins to the apical and basolateral domains of MDCK cells. J. Cell Biol. 111, 987-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X.F., Lin, H.Y., Ng-Eaton, E., Downward, J., Lodish, H.F., and Weinberg, R.A. (1991). Expression cloning and characterization of the TGF-beta type III receptor. Cell 67, 797-805. [DOI] [PubMed] [Google Scholar]
- Wilkes, M.C., Murphy, S.J., Garamszegi, N., and Leof, E.B. (2003). Cell-type specific activation of PAK2 by TGFβ independent of Smad2 and Smad3. Mol. Cell. Biol. 23, 8878-8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz, A. (2002). Establishing cell polarity in development. Nat. Cell Biol. 4, 39-44. [DOI] [PubMed] [Google Scholar]
- Wollner, D.A., and Nelson, W.J. (1992). Establishing and maintaining epithelial cell polarity. Roles of protein sorting, delivery and retention. J. Cell Sci. 102, 185-90. [DOI] [PubMed] [Google Scholar]
- Wrana, J.L., Attisano, L., Carcamo, J., Zentella, A., Doody, J., Laiho, M., Wang, X.F., and Massagué, J. (1992). TGF beta signals through a heteromeric protein kinase receptor complex. Cell 71, 1003-1014. [DOI] [PubMed] [Google Scholar]
- Wrana, J.L., Attisano, L., Wieser, R., Ventura, F., and Massagué, J. (1994). Mechanism of activation of the TGF-beta receptor. Nature 370, 341-347. [DOI] [PubMed] [Google Scholar]
- Yao, D., Ehrlich, M., Henis, Y.I., and Leof, E.B. (2002). Transforming growth factor-beta receptors interact with AP2 by direct binding to beta2 subunit. Mol. Biol. Cell 13, 4001-4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman, C., Grindstaff, K.K., and Nelson, W.J. (1999). New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol. Rev. 79, 73-98. [DOI] [PubMed] [Google Scholar]
- Yue, J., and Mulder, K.M. (2001). Transforming growth factor-beta signal transduction in epithelial cells. Pharmacol. Ther. 91, 1-34. [DOI] [PubMed] [Google Scholar]