Abstract
When cells undergo apoptosis, or programmed cell death, they expose phosphatidylserine (PS) on their surface. Macrophages that efficiently phagocytose apoptotic cells also express PS on their surface, although at a lower level. The PS exposed on both cells is required for phagocytosis, because uptake is inhibited by masking PS on either cell with annexin V, a PS-binding protein. The inhibition is not additive, suggesting that the exposed PS molecules on the two cells participate in a common process. We asked whether this dual requirement reflects bridging of the target cell and macrophage by bivalent, PS-binding annexins. Monoclonal antibodies (mAbs) against annexins I or II stained a variety of live phagocytes. Apoptotic Jurkat T lymphocytes and human peripheral T lymphocytes, but not apoptotic thymocytes, were stained by anti-annexin I but not II. Phagocytosis of apoptotic targets was inhibited by mAbs to annexins I or II, or by pretreatment of macrophages with the same mAbs. Pretreatment of apoptotic thymocytes had no effect, whereas pretreating Jurkat cells with anti-annexin I or removing annexin I with EGTA was inhibitory. Annexin bridging is vectorial, because annexin is bound to PS molecules on targets but not on macrophages, suggesting annexins serve as both ligand and receptor in promoting phagocytosis.
INTRODUCTION
In vivo, apoptosis, or programmed cell death, is a process of orderly cell removal. Apoptotic cells are recognized and phagocytosed by neighboring cells and professional phagocytes in a process that suppresses autoimmune and inflammatory reactions (Fadok et al., 1998). Specific removal of an apoptotic cell requires that its surface differs recognizably from that of its healthy neighbors. Exposure of phosphatidylserine (PS) on the cell surface is one difference that contributes to phagocytosis (McEvoy et al., 1986; Fadok et al., 1992a; Schlegel and Williamson, 2001). Normal, healthy cells restrict PS to the inner leaflet of the plasma membrane, whereas cells undergoing apoptosis move PS to the outer leaflet (Verhoven et al., 1995). The ability of the PS-specific binding protein annexin V to inhibit phagocytosis by masking PS on the surface of apoptotic lymphocytes demonstrates that PS itself is a signal for phagocytosis (Krahling et al., 1999).
Macrophages can differ in the mechanism by which they recognize apoptotic lymphocytes. Some macrophages, such as human monocyte-derived macrophages or the murine macrophage cell line J774, require integrin interactions to recognize apoptotic targets (Fadok et al., 1992b; Pradhan et al., 1997), whereas other macrophages, such as elicited peritoneal (EP) macrophages require interactions with a lectin-like receptor to recognize the same targets (Duvall et al., 1985; Pradhan et al., 1997). Indeed, a variety of protein receptors have been identified that contribute to recognition, but the range of macrophages and/or apoptotic targets over which they apply is generally unknown (Schlegel and Williamson, 2001). One recognition signal, however, is common to both classes of macrophages (Pradhan et al., 1997; Krahling et al., 1999) and is found on apoptotic cells of all types (van den Eijnde et al., 1997), namely, PS. A putative PS receptor (PSR), expressed at high levels in activated macrophages, may recognize PS on apoptotic cells (Fadok et al., 2000). It has been proposed that ligation of PSR on phagocytes induces macropinocytosis and ensuing phagocytosis of apoptotic cells, although the PS-PSR interaction is of low avidity and probably does not contribute to the tethering of apoptotic cells to macrophages, which is the first stage of uptake (Hoffmann et al., 2001).
The possibility that PS serves as a signaling molecule on only the apoptotic cell surface is belied by the remarkable fact that both integrin-dependent (J774) and lectin-like receptor-dependent (EP) macrophages constitutively express low levels of PS on their surface (Callahan et al., 2000). Moreover, this PS plays a functional role in the engulfment of PS-expressing targets, because masking PS on the macrophage surface by using annexin V inhibits phagocytosis of apoptotic lymphocytes as effectively as masking PS on the target cell surface (Callahan et al., 2000). This requirement for PS on both phagocyte and target cell may not be symmetrical; for example, the PSR is not expressed by lymphocytes, indicating that the presence of PS on macrophages could not be sensed by this molecule. Moreover, the inhibition of phagocytosis produced by pretreating either phagocyte or target with annexin V is not additive, suggesting that the expressed PS molecules on the two cells participate in a common process. We therefore considered the possibility that a bivalent PS-binding protein might bridge the PS on the two cell types, playing a central role in the engulfment process.
Annexin V, commonly used to detect apoptotic cells, is only one of a family of 12 vertebrate proteins that bind negatively charged phospholipids in a Ca2+-dependent manner (Gerke and Moss, 2002). All annexins share a conserved core domain made up of four homologous repeats (eight, in the case of annexin VI) with phospholipid and Ca2+ binding sites restricted to the convex side of the molecule. However, annexin I and annexin II mediate membrane vesicle aggregation, suggesting that these molecules are capable of cross-linking two membranes. Several models have been proposed to account for this bivalency, all based on exposure of the previously buried N-terminal domain of the molecule upon engagement of the primary, core, membrane-binding domain (Rosengarth et al., 2001; Gerke and Moss, 2002). In one model, the exposed N-terminal domain binds to a second membrane (de la Fuente and Parra, 1995; Bitto et al., 2000; Rosengarth et al., 2001). In a second model, two annexin I or annexin II molecules bound to different membranes by their primary binding domain may interact with each other via their N-terminal domains (Liu, 1999; Lambert et al., 1997). Finally, both annexin I and annexin II form heterotetramers by interaction of their N terminus with small dimeric S100 family proteins (Mailliard et al., 1996; Seemann et al., 1996; Lewit-Bentley et al., 2000; Gerke and Moss, 2002), thus producing a complex capable of cross-linking two membranes or cells if each primary binding site binds to a different membrane. Whatever the mechanism, the fact that annexin I and II are potential bivalent PS-binding proteins raises the question of whether they act as a bridge between the PS on macrophages and apoptotic cells. We address that question here.
MATERIALS AND METHODS
Materials
Dexamethasone, phorbol myristate acetate (PMA), phytohemagglutinin (PHA), Arg-Gly-Asp-Ser (RGDs) N-acetylglucosamine, RPMI 1640 medium, anti-glycophorin A, B (clone 3), purified rabbit IgG, purified mouse IgG1 (clone MOPC 21), and IgG2a (clone UPC-10) monoclonal antibody (mAb) isotype controls, R-phycoerythrin (PE)-conjugated goat anti-mouse IgG, and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG were purchased from Sigma-Aldrich (St. Louis, MO). Anti-human Fas (clone CH-11, mouse IgM) was from Immunotech (Westbrook, ME). Anti-mouse Fas (clone Jo-2, hamster IgG2) was from BD PharMingen (San Diego, CA). IOTest 3 mixture of anti-CD3-energy-coupled dye (ECD) (clone UCHT1, mouse IgG1), anti-CD4-PE (clone 13B8.2, mouse IgG1), and anti-CD8-FITC (clone B9.11, mouse IgG1) was from Beckman Coulter (Miami, FL). Peroxidase-conjugated goat anti-mouse antibody was from Jackson ImmunoResearch Laboratories (catalog no. 115-035-003; West Grove, PA). Camptothecin was from MP Biomedicals (Irvine, CA). FITC-conjugated annexin V was from Molecular Probes (Eugene, OR). Diff-Quik staining reagents were purchased from Baxter (Deerfield, IL). Immobilon-P membrane was from Millipore (Billerica, MA). SuperSignal West Pico chemiluminescent kit was from Pierce Chemical (Rockford, IL). The J774A.1 mouse macrophage cell line and the U937 human monocytic cell line were obtained from the American Type Culture Collection (Manassas, VA). Jurkat cells were a gift from Dr. Andrew Henderson (Penn State University, University Park, PA). Purified bovine lung annexin I, annexin II (heterotetramer), and annexin IV were purchased from Biodesign International (Saco, ME). Recombinant human annexin V was purified as described previously (Krahling et al., 1999).
Anti-Annexin mAbs
Anti-annexin I (clone 29, isotype IgG1), generated against bovine annexin I, and anti-annexin II (clone 5, isotype IgG1), generated against bovine annexin II, were purchased from BD Transduction Laboratories (Lexington, KY). Their specificity was verified against purified bovine annexins I, II, and IV and human recombinant annexin V by both Western analysis and enzyme-linked immunosorbent assay; each reacted only with the annexin against which it was generated (our unpublished data). According to the manufacturer, anti-annexin II reacts with mouse annexin II, and anti-annexin I reacts with annexin I in whole cell lysates of mouse 3T3 cells. Anti-annexin I also reacts with a band at 38 kDa in whole cell lysates of mouse peritoneal macrophages (our unpublished data). Their reactivities with mouse annexins are also verified by staining of mouse J774 and mouse peritoneal macrophages in RESULTS. Anti-annexin V (clone VAA-33, isotype IgG2a), generated against human recombinant annexin V, was purchased from A.G. Scientific (San Diego, CA). Its specificity was verified by enzyme-linked immunosorbent assay by using purified bovine annexin I, II, and IV and human recombinant annexin V; it reacted only with annexin V (our unpublished data).
Animals
Male CBA/J mice, 4 wk old, were purchased from Jackson Labs and maintained on food and water ad libitum in accordance with the institutional guidelines of the Animal Care and Use Committee.
Macrophages
Inflammatory macrophages were elicited in the peritoneal cavity of 6- to 8-wk-old mice by intraperitoneal injection of 1 ml of 3% Brewer's thioglycollate. Cells were harvested 5 d later by peritoneal lavage by using 10 ml of ice-cold RPMI 1640 medium. The collected cells were washed in RPMI 1640 medium and suspended at a concentration of 4 × 106 cells/ml in RPMI 1640 medium containing 10% fetal bovine serum (FBS). Cell suspension (150 μl) was pipetted onto 18-mm bicarbonate-treated glass coverslips. After 2 h at 37°C, nonadherent cells were removed by aspiration. Then, 150 μl of fresh RPMI 1640 medium containing 10% FBS was added to each coverslip, and cultures were incubated overnight at 37°C in 5% CO2 before phagocytosis assays. J774A.1 macrophages were grown in RPMI 1640 medium supplemented with 10% FBS at 37°C in 5% CO2. Coverslip cultures of 3 × 105 J774A.1 macrophages were prepared 1 d before phagocytosis assays. Monocytic U937 cells were maintained in the same way as J774A.1 macrophages. Differentiation of U937 cells was induced by incubation with 10 ng/ml PMA at 37°C for 1 or 2 d, and coverslip cultures of 3 × 105 differentiated cells used in phagocytosis assays.
Apoptotic Targets
Thymuses were removed from 4- to 6-wk-old mice and dissociated in RPMI 1640 medium. Cells were washed in 17 mM Tris-Cl, pH 7.2, 140 mM NH4Cl to lyse erythrocytes, centrifuged, and resuspended at 106 cells/ml in RPMI 1640 medium. Apoptosis was induced by addition of 10-6 M dexamethasone, and cells were incubated for 4–6 h at 37°C in 5% CO2; by addition of 2 μg/ml anti-mouse Fas, and incubation for 15 h; or by addition of 4 μg/ml camptothecin, and incubation for 15 h. Jurkat T lymphocytes were maintained in RPMI 1640 medium supplemented with 10% FBS at 37°C in 5% CO2. Apoptosis was induced by addition of anti-human Fas mAb at 60 ng/ml to cells at 3 × 105/ml, and incubation for 15 h at 37°C in 5% CO2; or addition of 4 μg/ml camptothecin, and incubation for 15 h. Fresh human venous blood was obtained from volunteers according to institutional guidelines, and the mononuclear cell fraction was isolated as described previously (Callahan et al., 2003). PHA was added at 5 μg/ml to the mononuclear cells in RPMI 1640 medium containing 10% FBS, and cultures were incubated at 37°C overnight. Nonadherent cells were collected and changed to fresh RPMI 1640 medium containing 10% FBS and 5 μg/ml PHA. After an additional 2 d of incubation, camptothecin was added to 4 μg/ml, and cultures were incubated for 15 h.
Erythrocytes
Erythrocytes were isolated from fresh human venous blood obtained from volunteers according to institutional guidelines. PS-expressing erythrocytes were prepared by lysis and resealing in the presence of Ca2+ as described previously (Schlegel et al.,1987; Krahling et al., 1999). To prepare opsonized targets, erythrocytes were pretreated with 1.25 μg/ml anti-glycophorin mAb for 20 min at room temperature.
Phagocytosis Assays
All assays were performed in triplicate. Thymocytes (106) or erythrocytes (15 × 106) in 150 μl of RPMI 1640 medium were overlaid onto coverslip cultures of 3 × 105 macrophages in the presence or absence of anti-annexin mAbs. In some experiments, either the target cells or the macrophages were first incubated with 5 μg/ml anti-annexin mAbs in RPMI for 30 min at room temperature and then washed twice with phosphate-buffered saline (PBS) before targets were overlaid onto macrophages. In other experiments, apoptotic Jurkat cells were incubated in buffer containing EGTA (EGTA buffer: 10 mM HEPES, pH 7.4, 140 mM NaCl, 10 mM EGTA) for 5 min at room temperature, washed three times in EGTA buffer, and then resuspended in RPMI 1640 medium and added to macrophages. In other experiments, 106 thymocytes were resuspended in 150 μl of RPMI 1640 medium containing 1 mM RGDS or 20 mM N-acetylglucosamine before overlaying onto U937 macrophages. After 30 min at 37°C in 5% CO2, coverslips were washed vigorously in PBS, fixed in 1.8% formaldehyde for 5 min, and finally submerged in PBS until counted. In assays with erythrocyte targets, after incubation, coverslips were washed in ammonium chloride to lyse nonphagocy-tosed erythrocytes, fixed in acidic methanol for 2 min and then transferred to PBS until counted. Just before counting, coverslips with fixed cells were stained with Diff-Quik, and then counted to determine phagocytosis as described in detail previously (Pradhan et al., 1997).
mAb Staining
For staining with anti-annexin I, anti-annexin II, and IgG1 isotype control, 106 macrophages were preincubated in 50 μl of staining buffer (10 mM HEPES, pH 7.4, 140 mM NaCl, 2 mM CaCl2) containing 1% FBS (+ FBS) for 15 min on ice. For staining with anti-annexin V and IgG2a isotype control, macrophages were preincubated in 50 μl of staining buffer containing rabbit IgG at 2 mg/ml for 15 min on ice. Anti-annexin mAbs, or isotype control antibodies, were then added to the suspension at a final concentration of 10 μg/ml, and incubated for 15 min on ice. The cells were washed twice with ice-cold staining buffer + FBS, and then incubated in 50 μl of FITC-conjugated goat anti-mouse IgG at 10 μg/ml in staining buffer + FBS for 15 min on ice. After washing with ice-cold staining buffer + FBS, the cells were resuspended in 500 μl of staining buffer + FBS and analyzed by flow cytometry. In some experiments, macrophages were washed twice at room temperature with EGTA buffer, and then stained with anti-annexin I or II in EGTA buffer as described above, without preincubation. After washing in EGTA buffer, secondary antibody staining was performed in staining buffer containing Ca2+ as described above. Mouse thymocytes (106), Jurkat cells (2 × 105) or peripheral T lymphocytes (2 × 105) were stained as described above for macrophages, except that preincubations were omitted and in the case of Jurkat cells and peripheral T lymphocytes PE-conjugated goat anti-mouse IgG secondary antibody was used instead of FITC-conjugated goat anti-mouse IgG. In some experiments, apoptotic Jurkat cells and apoptotic peripheral T lymphocytes were incubated in EGTA buffer for 5 min at room temperature, washed three times in EGTA buffer, and then stained in staining buffer containing Ca2+. Alternatively, apoptotic Jurkat cells were stained as normal, resuspended to 500 μl in EGTA buffer and analyzed immediately by flow cytometry. To identify activated T lymphocytes, cultures were stained with 5 μl of a mixture of anti-CD3-ECD, anti-CD4-PE and anti-CD8-FITC mAbs for 5 min on ice. In all cases, propidium iodide (PI) was added to a final concentration of 10 μg/ml immediately before flow cytometric analysis.
Annexin V Staining
FITC-conjugated annexin V (0.7 μl) was added to 106 mouse thymocytes, 2 × 105 Jurkat cells, 2 × 105 peripheral T lymphocytes, or 106 differentiated U937 cells in 50 μl of staining buffer. After 15 min on ice, the volume was brought to 500 μl by staining buffer. PI was added to a final concentration of 10 μg/ml immediately before flow cytometric analysis.
Double Staining with Anti-Annexin mAbs and Annexin V
Apoptotic Jurkat cells (2 × 105) were stained with anti-annexin mAbs and PE-conjugated goat anti-mouse IgG as described above. After washing with ice-cold staining buffer, the cells were stained with FITC-conjugated annexin V as described above, and then brought to 500 μl in staining buffer. PI was added to a final concentration of 10 μg/ml immediately before flow cytometric analysis.
Flow Cytometry
Cells (104) were analyzed using an EPICS-XL-MCL flow cytometer (Coulter Electronics, Hialeah, FL) with excitation at 488 nm. FITC staining was monitored at 525 nm. PE fluorescence was monitored at 575 nm. ECD fluorescence was monitored at 610 nm. PI fluorescence was monitored at 610 nm, and PI-positive cells gated out of all profiles. Forward and side light scatter properties were used to gate for morphologically normal thymocytes and Jurkat cells, which include both nonapoptotic and early apoptotic cells. In double-staining experiments, uncompensated data were collected at the cytometer, and color compensation was carried out offline by using Winlist 4.0 (Verity Software House, Topsham, ME). After activation, peripheral T lymphocytes were identified by their characteristic light scatter profile and gated. To verify the identification, the gated population (98% viable by PI staining) was stained with a mixture of anti-CD3-ECD, anti-CD4-PE and anti-CD8-FITC; 90% of the gated population stained positive for CD3. Fluorescence of the gated population was measured after staining with anti-annexin I, II, or isotype control mAbs.
Western Analysis
Apoptotic Jurkat cells, induced by anti-Fas, were washed with buffer with or without EGTA as described above, and the pellet was resuspended in SDS sample buffer. Mouse thymocytes, induced by dexamethasone, were washed and the pellet resuspended in SDS sample buffer. Cell equivalents (105) or 30 ng of purified bovine annexin I was resolved using an SDS 12% polyacrylamide gel and transferred (25 V for 50 min at room temperature) onto an Immobilon-P membrane. The membrane was blocked by incubating in 150 mM NaCl, 10 mM Tris-Cl, pH 7.5, containing 3% bovine serum albumin (blocking buffer) at 4°C overnight, and then incubated with anti-annexin I mAb (0.15 μg/ml in blocking buffer) at room temperature for 1 h, washed three times with 500 mM NaCl, 20 mM Tris-Cl, pH 7.5, 0.05% Tween 20, and 0.2% Triton X-100 (wash buffer), and then incubated with peroxidase-conjugated goat anti-mouse secondary antibody (1:20,000 dilution in blocking buffer) at room temperature for 1 h, washed three times with wash buffer, visualized by SuperSignal West Pico chemiluminescent kit, and developed on Kodak BioMax MR film.
RESULTS
Immunogold labeling and Western analysis have suggested that annexins I, II, III, IV, and V can all be found on the plasma membrane and phagosomes of J774 macrophages (Diakonova et al., 1997), but these studies did not determine on which side of the membrane the annexins were located. To address the question of whether annexins are present on the extracellular side of the plasma membrane, live J774 macrophages were stained with anti-annexin antibodies. Because macrophages express surface Fc receptors which bind antibodies, they pose challenges in staining studies not faced with other cells types; for instance, IgG antibodies present in normal rabbit sera bind with high avidity to macrophage Fc receptors. Therefore, it was necessary to identify mouse mAbs that were not only specific for annexins but also of an isotype for which Fc receptors have low avidity. The mAbs finally selected and the tests of their specificity are described in MATERIALS AND METHODS. As shown in Figure 1A, anti-annexins I and II stained J774 macrophages, whereas their IgG1 isotype control mAb, for which Fc receptors have low avidity, did not. Although anti-annexin V mAb also stained J774 macrophages (Figure 1B), the level of staining was no greater than its IgG2a isotype control mAb, for which Fc receptors have high avidity, suggesting that if any annexin V is present on the macrophage surface, its staining is obscured by mAb binding to Fc receptors. To establish that the expression of annexins on the macrophage surface is not restricted to integrin-dependent macrophages such as J774 macrophages, and to confirm that it is not just a property of cell lines, lectin-dependent, primary mouse EP macrophages were examined. Anti-annexin I and II mAbs stained the cells (Figure 1C), although not as intensely as J774 macrophages.
Figure 1.
Binding of anti-annexin mAbs to live J774 and EP macrophages. (A and B) J774 macrophages were stained with anti-annexin I, II, or mouse IgG1 isotype control mAbs (A), or anti-annexin V or mouse IgG2a isotype control mAbs (B). (C) EP macrophages were stained with anti-annexin I, II, or mouse IgG1 isotype control mAbs. (D and E) J774 macrophages were washed twice with buffer containing 10 mM EGTA (thick line) or 2 mM Ca2+ (thin line), and then stained with anti-annexin I or mouse IgG1 isotype control mAbs (D), or anti-annexin II or mouse IgG1 isotype control mAbs in the same buffer (E). Cells were analyzed by flow cytometry after secondary staining with goat antimouse IgG-FITC.
Because macrophages constitutively express PS on their surface, an initial model would propose that annexins on the macrophage surface are bound to PS. A first indication otherwise came from studies of human U937 cells. U937 is a monocytic cell line that can be induced to differentiate to macrophages upon treatment with the phorbol ester, PMA; upon induction, the cells become adherent, express the Mac-1 differentiation antigen, develop the low level of PS exposure characteristic of macrophages (Figure 2D), and, at the same time, develop the ability to efficiently engulf apoptotic cells (Callahan et al., 2003). As shown in Figure 2, differentiated U937 cells are stained by anti-annexin I and II mAbs, but not by anti-annexin V mAb, consistent with the results obtained with mouse macrophages. Undifferentiated U937 cells, which do not express surface PS, were also analyzed; surprisingly, undifferentiated cells are also stained by anti-annexin I and II, but not anti-annexin V mAbs. The latter result argues that the annexin on the surface of macrophages is not bound to exposed PS. To test that possibility, advantage was taken of the fact that annexin binding to PS is reversibly dependent on the presence of Ca2+. J774 macrophages were washed with EGTA and stained in the presence of EGTA. As shown in Figure 1, D and E, EGTA did not significantly decrease staining by anti-annexin I or II mAbs, suggesting that these annexins are not bound to PS on the macrophage surface.
Figure 2.
Binding of anti-annexin mAbs to live U937 cells. Undifferentiated U937 cells (thin line) or U937 cells treated with PMA for 2 d (thick line) were stained with anti-annexin I or mouse IgG1 isotype control mAbs (A), anti-annexin II or mouse IgG1 isotype control mAbs (B), or anti-annexin V or IgG2a isotype control mAbs (C), and cells were examined by flow cytometry after secondary staining with goat anti-mouse IgG-FITC. (D) Cells treated with PMA for 2 d were stained with FITC-annexin V in the presence (thick line) or absence (thin line) of Ca2+ to distinguish Ca2+-dependent, PS-specific binding, and analyzed by flow cytometry.
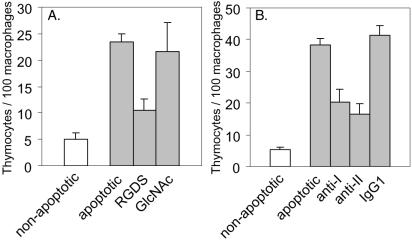
To investigate the functional contributions of these cell surface annexins, the ability of the anti-annexin mAbs to inhibit phagocytosis of apoptotic cells by macrophages was examined, using primary mouse apoptotic thymocytes as targets. As shown in Figure 3A, phagocytosis by J774 macrophages was substantially inhibited when phagocytosis assays were performed in the presence of mAbs to annexins I and II, whereas anti-annexin V and IgG1 isotype control mAbs had no effect. To confirm the generality of this effect, lectin-dependent, primary mouse EP macrophages were also examined. As shown in Figure 3B, these cells behaved like the integrin-dependent, immortal J774 macrophages: anti-annexin I and II mAbs inhibited phagocytosis, whereas anti-annexin V and isotype control mAbs had no effect. As expected if the effect of the mAbs results from specific and stoichiometric binding to a cell surface molecule, inhibition was dose dependent (Figure 3, C and D). To test for additivity, phagocytosis was compared in the presence of saturating levels (5 μg/ml, because 10 μg/ml was no more inhibitory) of anti-annexin I, anti-annexin II, or both mAbs. As shown in Figure 3E, the combination of anti-annexin I and II mAbs did not inhibit phagocytosis any more than either mAb alone, suggesting that anti-annexin I and II mAbs block the same step in the engulfment process. Because undifferentiated U937 cells express annexin I and II on their surface but do not efficiently phagocytose apoptotic thymocytes (Callahan et al., 2003), it was important to determine whether the annexins on the surface of differentiated U937 cells are functional in phagocytosis of apoptotic cells. The mechanism by which differentiated U937 macrophages phagocytose apoptotic cells has not been previously investigated; in particular, it has not been established whether these cells use the lectin-dependent or the integrin-dependent mechanism to recognize PS-presenting cells. As shown in Figure 4A, RGDS inhibited phagocytosis of apoptotic thymocytes by differentiated U937 macrophages, whereas N-acetylglucosamine did not, indicating that U937 cells use the integrin-dependent mechanism. As shown in Figure 4B, phagocytosis of apoptotic targets was also inhibited by both anti-annexin I and II mAbs, indicating their involvement in the process. However, as noted above, annexins I and II are present on the surface of undifferentiated U937 cells, which cannot efficiently phagocytose apoptotic cells, indicating that the expression of these molecules is not sufficient to confer the ability to engulf PS-expressing cells.
Figure 3.
Phagocytosis of apoptotic mouse thymocytes in the presence of anti-annexin mAbs. Mouse thymocytes, either untreated or treated with 10-6 M dexamethasone for 6 h to induce apoptosis, were presented to J774 (A and E) or EP (B–D) macrophages in the presence of various anti-annexin and IgG1 isotype control mAbs (A and B), different concentrations of anti-annexin I mAb (C) or anti-annexin II mAb (D), or a combination of anti-annexin I and II mAbs (E).
Figure 4.
Phagocytosis of apoptotic mouse thymocytes by differentiated U937 macrophages. U937 cells were treated with PMA for 1 d to induce differentiation, and presented with mouse thymocytes, either untreated or treated with 10-6 M dexamethasone for 6 h to induce apoptosis, in the presence of RGDS or N-acetylglucosamine (GlcNAc) (A) or anti-annexin I, II or IgG1 isotype control mAbs (B).
To test whether the inhibitory effects were specific to PS-expressing target cells, nonapoptotic target cells expressing or not expressing PS were examined. When the cytosolic level of Ca2+ in erythrocytes is artificially elevated, PS previously restricted to the inner leaflet of the plasma membrane is exposed on the surface of the cells (Williamson et al., 1992). As shown in Figure 5A, phagocytosis of these PS-expressing erythrocytes by EP macrophages was inhibited by anti-annexin I and II mAbs. In contrast, Fc-mediated phagocytosis of erythrocytes opsonized with IgG was insensitive to the presence of anti-annexins I and II mAbs (Figure 5B). These results suggest that exposure of PS on the target cell surface is sufficient to engage the annexin-dependent engulfment process and that the involvement of annexins in phagocytosis is restricted to PS-expressing targets.
Figure 5.
Phagocytosis of PS-expressing or opsonized erythrocytes by EP macrophages in the presence of anti-annexin mAbs. (A) PS expressing, lipid-symmetric (LS), or PS-nonexpressing, lipid-asymmetric (LA) erythrocytes or (B) erythrocytes opsonized with antiglycophorin antibody were presented to EP macrophages in the presence of anti-annexin mAbs.
Given the sufficiency of inert, PS-expressing erythrocytes as targets, inhibition of phagocytosis by anti-annexin mAbs likely results from targeting the annexin molecules on the macrophage surface. To test this possibility, either apoptotic thymocyte target or macrophage was separately incubated with mAbs, washed to remove unbound antibody, and then targets and macrophages were combined in phagocytosis assays. As shown in Figure 6, A and B, preincubation of J774 macrophages with either anti-annexin I or anti-annexin II mAbs inhibited phagocytosis, whereas preincubation of apoptotic thymocyte targets with either antibody had no effect, suggesting that the macrophage is in fact the target of the mAbs in inhibiting phagocytosis of apoptotic thymocytes.
Figure 6.
Phagocytosis of apoptotic thymocytes or apoptotic Jurkat cells after pretreatment of either macrophages or target cells with anti-annexin mAbs, or after treatment of Jurkat cells with EGTA. (A and B) Mouse thymocytes (T), either untreated or treated with 10-6 M dexamethasone for 6 h to induce apoptosis, or (C) Jurkat T lymphocytes (T), either untreated or treated with anti-Fas mAb for 15 h at 37°C to induce apoptosis, were presented to J774 macrophages. Either macrophages or target cells were incubated with anti-annexin mAbs for 30 min at 37°C, washed, and then used in phagocytosis assays. Coincubation of macrophages and targets with anti-annexin mAbs, as done in Figure 3, is shown for comparison. One sample of apoptotic Jurkat cells was treated with EGTA to remove annexin I before presenting to macrophages.
At face value, this result seems at odds with a recent report that apoptotic Jurkat cells, an immortal human T lymphocyte line, present annexin I on their surface after induction of apoptosis by anti-Fas treatment (Arur et al., 2003). This finding was confirmed using Jurkat cells induced to undergo apoptosis by treatment with anti-Fas mAb. As shown in Figure 7A, when morphologically normal cells were gated by their light scatter characteristics, annexin V stained a substantial number of the cells, indicating that PS is exposed on their surface. A similar fraction of the cells stained with anti-annexin I mAb (Figure 7B), but not with anti-annexin II mAb (Figure 7C). Double staining with annexin V and anti-annexin I mAb showed that annexin V-negative, nonapoptotic cells were not stained by anti-annexin I mAb (Figure 7D). In contrast, most (but not all) of the annexin V-positive, apoptotic cells with PS expressed on their surface also stained with anti-annexin I mAb (Figure 7D). Shrunken apoptotic cells with reduced forward light scatter and increased side light scatter, including those that had lost membrane integrity (PI positive), all stained with both annexin V and anti-annexin I mAb (our unpublished data). No staining was observed with isotype control mAb (Figure 7E). These results indicate that normal Jurkat cells that do not express PS on their surface also do not have annexin I on their surface, that most morphologically normal, early apoptotic cells that express PS on their surface also have annexin I on their surface, and that shrunken late apoptotic cells (PI negative) with PS exposed on their surface have all also acquired annexin I on their surface.
Figure 7.
Binding of anti-annexin mAbs to apoptotic Jurkat cells. Jurkat T lymphocytes treated with anti-Fas mAb for 15 h at 37°Cto induce apoptosis were stained with annexin V-FITC (A), anti-annexin I (B) or anti-annexin II mAb (C), followed by staining with PE-conjugated goat anti-mouse IgG. Jurkat T lymphocytes were also double stained with anti-annexin I (D) or IgG1 isotype control (E) followed by goat anti-mouse IgG-PE staining, and after washing, with annexin V-FITC. Thin line, mAb staining of gated annexin V-negative cells; thick line, mAb staining of gated annexin V-positive cells. (F) Jurkat T lymphocytes were washed with buffer containing 10 mM EGTA before antiannexin I mAb staining.
The observation that annexin I colocalizes on Jurkat cells with regions of the membrane enriched in PS (Arur et al., 2003) and occurs on the cell surface soon after PS is expressed suggests that annexin I is binding to PS. To determine whether this is so, cells were washed with EGTA, as described above for macrophages, to release annexin I bound to PS, and then stained with anti-annexin I mAb. As shown in Figure 7F, annexin I staining was abolished. Staining was also abolished if cells were not exposed to EGTA until after they already had been stained with anti-annexin I, just before analysis (our unpublished data). These results suggest that annexin I is binding to PS exposed on the surface of apoptotic Jurkat cells.
Two tests of the functionality of the annexin I on apoptotic Jurkat cells were performed. First, apoptotic targets were washed with EGTA to remove annexin I and presented to macrophages. As shown in Figure 6C, removal of annexin I inhibited phagocytosis. Second, apoptotic targets (or macrophages) were preincubated with anti-annexin I or II mAbs, washed, and combined in phagocytosis assays. As was the case for primary thymocytes, pretreatment of macrophages but not pretreatment of targets with anti-annexin II inhibited phagocytosis. In contrast to primary thymocytes, however, pretreatment of apoptotic Jurkat cells with anti-annexin I inhibited phagocytosis the same as pretreatment of the macrophages. These combined results indicate that the annexin I on the surface of apoptotic Jurkat cells is functional in phagocytosis. These results also suggest that either primary apoptotic thymocytes do not have annexin I (or annexin II) on their surface, or if they do, it is not involved in their phagocytosis. To distinguish these possibilities, primary thymocytes were stained with anti-annexin mAbs. As shown in Figure 8A, uninduced populations contained a small number of spontaneously apoptotic cells, and induced populations contained a much larger number, as judged by binding of annexin V. Despite the expression of PS on the cell surface, however, the apoptotic cells were not stained by anti-annexin I (or anti-annexin II) mAb (Figure 8B). When thymocytes were induced to undergo apoptosis in the presence of 10% serum, as was done with Jurkat cells, apoptotic cells still were not stained by anti-annexins.
Figure 8.
Expression of annexins by apoptotic thymocytes and apoptotic Jurkat cells. (A) Mouse thymocytes, either untreated or treated with 10-6 M dexamethasone for 4 h, were stained with annexin V-FITC to confirm the presence of apoptotic cells. (B) Apoptotic mouse thymocytes were stained with anti-annexin I, II, V, or mouse IgG1, IgG2a isotype control mAbs, and analyzed by flow cytometry after secondary staining with goat anti-mouse IgG-FITC. (C) Whole cell lysates of apoptotic Jurkat cells, washed with buffer without EGTA (J) or with EGTA (J+E), or whole cell lysates of apoptotic primary thymocytes (T) were resolved by SDS-PAGE, and the blot of the gel probed with anti-annexin I mAb. I, purified annexin I.
To determine whether the expression of annexin I on apoptotic Jurkat cells but not on apoptotic primary thymocytes was a consequence of the agent used to induce apoptosis, primary thymocytes were treated with anti-Fas, the reagent used to induce apoptosis in Jurkat cells. After induction under the conditions presented in MATERIALS AND METHODS, apoptotic thymocytes induced by anti-Fas were not stained by anti-annexins (our unpublished data). The converse experiment could not be done because Jurkat cells are refractory to induction of apoptosis by dexamethasone, the agent used to induce apoptosis in primary thymocytes. Accordingly, to test whether an inducing agent other than anti-Fas resulted in surface expression of annexin I, Jurkat cells were treated with the topoisomerase inhibitor, camptothecin, to induce apoptosis (Callahan et al., 2003). The results were virtually identical to those presented in Figure 7, with a similar fraction of apoptotic cells produced, and similar staining of annexin V-positive cells but not annexin V-negative cells by anti-annexin I (Figure 7D) (our unpublished data). Finally, primary thymocytes were treated with camptothecin to induce apoptosis; once again, apoptotic cells induced by this treatment were not stained by antiannexin I or anti-annexin II (our unpublished data). These results indicate that the difference in cell surface expression of annexin I is an inherent difference between the Jurkat cell line and primary thymocytes, and not a consequence of differences in the way in which apoptosis is induced.
This difference between Jurkat cells and primary thymocytes could reflect either a difference in cellular expression of annexin I by the two cell types, or a difference in the display of expressed annexin on the surface. To distinguish between these possibilities, western analysis was performed on whole cell extracts of apoptotic thymocytes and apoptotic Jurkat cells, the latter either washed or not washed with EGTA to estimate the amount of total cellular annexin expressed on the surface. As shown in Figure 8C, the intensity of bands at 38 kDa, the molecular weight of annexin I, were similar whether whole cell extracts were prepared from unwashed apoptotic Jurkat cells or the same number of Jurkat cells washed with EGTA, indicating that surface annexin I can be no more than a small fraction of total cellular annexin. In contrast, no band was seen at 38 kDa in whole cell extracts of thymocytes, indicating that annexin I is not displayed on the surface of apoptotic thymocytes because it is not expressed and present in the cell.
Jurkat cells were originally derived from the circulation of a patient with acute lymphoblastic leukemia. To test whether their normal cell counterparts behave similarly with respect to expression of annexin I on their surface, human peripheral blood T lymphocytes, induced to proliferate with PHA, were examined. PHA-activated peripheral T lymphocytes were induced with camptothecin to undergo apoptosis (Ferraro et al., 2000) and analyzed for surface expression of annexins. As shown in Figure 9A, annexin V staining indicated that a large fraction of T lymphocytes were apoptotic. As shown in Figure 9B, anti-annexin I staining produced a subpopulation of cells with increased fluorescence, seen as a shoulder on the fluorescence profile. Because the fraction of cells stained with annexin V was greater than the fraction stained with anti-annexin I, not all apoptotic cells had annexin I on their surface, as was also the case for Jurkat cells. Neither anti-annexin II nor IgG control mAb stained the cells (Figure 9, C and D). To determine whether annexin I was binding to PS on the apoptotic cell surface, cells were washed with EGTA before anti-annexin I staining. As shown in Figure 9E, EGTA largely eliminated annexin I staining.
Figure 9.
Binding of anti-annexin mAbs to apoptotic human peripheral T lymphocytes. PHA-activated T lymphocytes treated with camptothecin for 15 h at 37°C to induce apoptosis were stained with annexin V-FITC (A), anti-annexin I mAb (B), anti-annexin II mAb (C), or IgG1 isotype control mAb (D), followed by staining with PE-conjugated goat anti-mouse IgG. (E) Camptothecin-treated T lymphocytes were washed with buffer containing 10 mM EGTA before anti-annexin I mAb staining.
DISCUSSION
By injecting labeled annexin V into the circulation of mouse embryos, van den Eijnde et al. (1997) demonstrated that virtually all normal cells in the body restrict PS to the inner leaflet of the plasma membrane. Just as important, these elegant studies established that all apoptotic cells, regardless of cell type or lineage, expose PS on their surface. One exception to this universal rule was the site of contact between macrophages and apoptotic cells, where annexin V seemed to bind to the macrophage as well as the apoptotic cell membrane. These sites of contact thus seem to represent the juxtaposition of PS on the macrophage with PS on the apoptotic cell. Among the normal cells not stained by annexin V were blood cells, confirming previous studies (McEvoy et al., 1988) suggesting that blood cells do not express PS on their surface. Among these nonstaining blood cells were monocytes, the precursors of macrophages, suggesting that surface expression of PS may occur as monocytes differentiate to macrophages. This sequence has been experimentally confirmed (Callahan et al., 2003), and the surface PS has been shown to be functional, because masking it with annexin V inhibits phagocytosis of apoptotic targets (Callahan et al., 2000, 2003).
The existence of juxtaposed PS on the two cell types begs the question of whether PS plays a similar role on the two cell types. Because the PSR is expressed on macrophages, but not on apoptotic lymphocytes, any PSR-mediated signaling would be unidirectional. Nevertheless, PS on the two surfaces might still play reciprocal roles in tethering, with the most simple and direct model being that some molecule, capable of binding two PS-containing membranes simultaneously, cross-links the two cells at sites of contact. Because annexins I and II are known to have this property, the primary goal of our studies was to elucidate the role, if any, of these annexins in phagocytosis of apoptotic lymphocytes. Our results demonstrate that macrophages have both annexin I and annexin II on their surface. Although mAbs to these annexins block phagocytosis of apoptotic thymocytes, the surface annexins are not part of the general phagocytosis process, because the mAbs do not block Fc-mediated phagocytosis. Rather, the annexins are specific to engulfment of PS-expressing cells, such as apoptotic targets, because the mAbs inhibit phagocytosis of even erythrocytes with PS on their surface.
Although these investigations were initiated on the premise that bivalent binding of annexins I and II might provide the raison d'etre for PS expression on macrophages, the data do not favor this model. The fact that the annexins cannot be removed from the macrophage surface by using EGTA to reverse Ca2+-dependent binding of annexins to PS strongly suggests that the annexins are not bound to PS on the macrophage surface. That conclusion is also supported by the fact that the annexins can be observed on the surface of U937 cells before they are induced to differentiate to macrophages, and before they develop PS on their surface. If the annexins are not bound to macrophage PS, then the second and third models described in INTRODUCTION, which require annexin binding to PS at the surface of both cells, are strongly discounted. The first model invokes crosslinking through PS-specific binding to just one surface. In this case, the PS would be on the surface of the apoptotic cell, with the N terminus of the annexin molecule interacting with the macrophage surface in some way.
Annexins do not contain transmembrane domains, so it is unlikely that the annexins on the macrophage surface are anchored in the membrane bilayer, raising the possibility that the N terminus of the annexin molecules is bound to some receptor on the macrophage surface. Annexin I binding studies indicate that human monocytes have trypsin-sensitive, saturable annexin I binding sites on their surface (Goulding et al., 1996), but endogenous annexin I on the surface was not analyzed. Annexin II has been described as a profibrinolytic coreceptor for tissue plasminogen activator and plasminogen on the surface of endothelial cells (Hajjar and Acharya, 2000) and the RAW264.7 mouse macrophage cell line (Falcone et al., 2001). However, in marked contrast to the results presented here, the annexin II in this case could be removed with EGTA.
Interestingly, the serum protein β2-glycoprotein I has been reported to bind to annexin II on the endothelial cell surface (Ma et al., 2000). Because β2-glycoprotein I binds PS and enhances phagocytosis of apoptotic targets by macrophages (Balasubramanian and Schroit, 1998), it is possible that annexin II on macrophages might actually bind β2-glycoprotein I, which itself is bound to PS on apoptotic cells, rather than annexin II binding directly to PS on the apoptotic cells. However, macrophages bind β2-glycoprotein I only after it is bound to its lipid ligand (Balasubramanian and Schroit, 1998). Because serum is not used in our phagocytosis assays, this mechanism would only be applicable if targets acquired β2-glycoprotein I from serum subsequent to PS exposure. In the experiments reported here, neither thymocyte targets nor lipid-symmetric erythrocytes were exposed to serum during or subsequent to induction of PS exposure.
It is not clear how annexins I and II reach the cell surface, because annexins do not have secretion signal sequences. Were they to be already bound to the membrane inside vesicles that fuse to become part of the plasma membrane, they still would have to have a secretion signal to cross into the lumen of the vesicle from the cytosol. Nonetheless, extracellular annexin I (Vergnolle et al., 1995) and annexin II (Tressler et al., 1993; Siever and Erickson, 1997; Faure et al., 2002) have both been reported. Clearly, much remains to be resolved concerning how annexins I and II reach the macrophage surface, the identity of their receptor on macrophages, if they have one, and whether other molecules besides PS are ligands of the annexins.
Our results indicate that annexins I and II are deployed by phagocytes as receptors for the recognition of apoptotic cells. Recently, it was proposed that during apoptosis, target cells display annexin I on their surface, a conclusion based largely on the demonstration that annexin I occurs on the surface of apoptotic Jurkat cells (Arur et al., 2003). In contrast to J774 macrophages, however, annexin I could be removed from Jurkat cells with EGTA, indicating it is bound directly or indirectly to PS on the Jurkat cell surface, similar to the binding of annexin II to RAW264.7 macrophages (see above). The results presented here show that targets need not display annexin in this manner to be engulfed. Lipid-symmetric erythrocytes are essentially inert cells, unable to synthesize or secrete molecules that would make them attractive to macrophages. Because these PS-expressing target cells are readily engulfed by macrophages, and because their engulfment is inhibited by anti-annexin I and II antibodies, it seems that surface expression of PS is sufficient to engage annexin-dependent uptake and that annexins are not required on the target cell surface for efficient phagocytosis.
Although target cell annexins are not required for engulfment, surface expression of annexin could still be part of the apoptotic program. This seems to be the case for Jurkat cells and primary T lymphocytes, but it cannot be generally true, however, because annexins are not expressed on the surface of apoptotic primary thymocytes (or Epstein-Barr virus-transformed B lymphocytes; Fan, Williamson, and Schlegel, unpublished data). The proportion of total cellular annexins that are expressed at the surface of either apoptotic target or engulfing macrophage is not easily determined. In a careful immunogold analysis performed on J774 macrophages (Diakonova et al., 1997), approximately one-half of annexins I and II were found associated with the plasma membrane, and the other one-half was associated with early endosomes and phagosomes. Unfortunately, immunogold labeling cannot distinguish the fraction of the annexins associated with the plasma membrane on the outside from the fraction on the inside. Purification of a plasma membrane fraction followed by western analysis is similarly unable to determine the fraction on the outside. However, in the case of Jurkat cells, such an estimate can be made by comparing the amount of annexin I present in extracts of apoptotic Jurkat cells that have or have not been washed with EGTA buffer to remove annexin on the surface. As demonstrated here, the fraction of total cellular annexin expressed on the surface cannot be large.
The presence of both annexins I and II on the surface of macrophages, only annexin I on apoptotic Jurkat cells and peripheral T lymphocytes, and neither annexin on apoptotic thymocytes raises the question of what determines which annexins will be present and able to contribute to recognition. Our evidence and results in the literature all suggest that surface expression is a reflection of intracellular expression. Western analysis of total thymus homogenates by using polyclonal antibody to annexin I readily detected the protein (De et al., 1986), although annexin I was not detected in isolated thymocytes (Fava and Piltch, 1987). This apparent discrepancy was reconciled by immunohistochemical staining of the thymus, which revealed strong expression of annexin I in thymic macrophages and endothelial cells, but no expression in thymocytes (Fava et al., 1989); alveolar macrophages also strongly express annexin I. On the other hand, peripheral blood lymphocytes, as well as granulocytes and monocytes, express annexin I (Morand et al., 1995; Dreier et al., 1998). However, expression is restricted to T lymphocytes, with B lymphocytes not expressing the protein, and stimulation of T lymphocytes with PHA not significantly increasing expression levels (Morand et al., 1995). These observations are in general agreement with our observations of annexin I expression in Jurkat cells and primary circulating T cells, as well as immature and mature macrophages, but not in thymocytes or B cell lines. With respect to expression of annexin II, as noted above, J774 macrophages express this annexin. However, neither thymocytes nor peripheral blood lymphocytes, granulocytes or monocytes are reported to express it (Dreier et al., 1998). Thus, surface expression of annexins I or II on macrophages, thymocytes and peripheral T lymphocytes correlates with their general levels of expression of the proteins, with one exception: Jurkat cells are reported to express annexin II (Arur et al., 2003). However, its expression seems to be down-regulated in apoptotic Jurkat cells, consistent with it not being expressed on the surface of apoptotic Jurkat cells.
Overall, these findings are a reminder that the details of the apoptotic program differ from cell type to cell type. They also suggest that apoptosis of activated T lymphocytes may be specialized to expose this critical molecule, to enhance their clearance from the circulation or after extravasation into tissues. Such specialization may not be confined to circulating T lymphocytes. When neutrophils extravasate from the circulation and infiltrate sites of inflammation, they secrete cytokines that serve as chemoattractants for monocytes to leave the circulation and differentiate into macrophages, and presumably express PS on their surface. Annexin I has been reported to be stored within gelatinase granules in neutrophils and released from the cell upon adhesion to endothelial cells (Perretti et al., 2000). Thus, neutrophils could be a source of annexin I at sites of inflammation, opsonizing resident apoptotic cells, including T lymphocytes and neutrophils themselves, for clearance by macrophages in an annexin-dependent manner. When annexin I (or potentially annexin II) is already bound to the target cell via the PS-binding surface, the N-terminal domain is made available to bind to the annexin receptor on the macrophage surface, facilitating target cell engulfment. Thus, whether provided on apoptotic targets or macrophages, annexins may play a pivotal role at sites of tissue damage in resolving the inflammatory episode and preventing autoimmune responses.
Acknowledgments
We thank Elaine Kunze for assistance with flow cytometry and Bethsebah Gekonge for U937 cell staining. This study was supported by National Institutes of Health grant R01 AI46261.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-09-0670. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-09-0670.
References
- Arur, S., Uche, U.E., Rezaul, K., Fong, M., Scranton, V., Cowan, A.E., Mohler, W., and Han, D. (2003). Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev. Cell 4, 587-598. [DOI] [PubMed] [Google Scholar]
- Balasubramanian, K., and Schroit, A.J. (1998). Characterization of phosphatidylserine-dependent beta (2)-glycoprotein I macrophage interactions: implications for apoptotic cell clearance by phagocytes. J. Biol. Chem. 273, 29272-29277. [DOI] [PubMed] [Google Scholar]
- Bitto, E., Li, M., Tikhonov, A.M., Schlossman, M.L., and Cho, W. (2000). Mechanism of annexin I-mediated membrane aggregation. Biochemistry 39, 13469-13477. [DOI] [PubMed] [Google Scholar]
- Callahan, M.K., Halleck, M.S., Krahling, S., Henderson, A.J., Williamson, P., and Schlegel, R.A. (2003). Phosphatidylserine expression and phagocytosis of apoptotic thymocytes during differentiation of monocytic cells. J. Leukoc. Biol. 74, 846-856. [DOI] [PubMed] [Google Scholar]
- Callahan, M.K., Williamson, P., and Schlegel, R.A. (2000). Surface expression of phosphatidylserine on macrophages is required for phagocytosis of apoptotic thymocytes. Cell Death Differ. 7, 645-653. [DOI] [PubMed] [Google Scholar]
- De, B.K., Misono, K.S., Lukas, T.J., Mroczkowski, B., and Cohen, S. (1986). A calcium-dependent 35-kilodalton substrate for epidermal growth factor receptor/kinase isolated from normal tissue. J. Biol. Chem. 261, 13784-13792. [PubMed] [Google Scholar]
- de la Fuente, M., and Parra, A.V. (1995). Vesicle aggregation by annexin I: role of a secondary membrane binding site. Biochemistry 34, 10393-10399. [DOI] [PubMed] [Google Scholar]
- Diakonova, M., Gerke, V., Ernst, J., Liautard, J.P., Van Der Vusse, G., and Griffiths, G. (1997). Localization of five annexins in J774 macrophages and on isolated phagosomes. J. Cell Sci. 110, 1199-1213. [DOI] [PubMed] [Google Scholar]
- Dreier, R., Schmid, K.W., Gerke, V., and Riehemann, K. (1998). Differential expression of annexins I, II, and IV in human tissues: an immunohistochemical study. Histochem. Cell Biol. 110, 137-148. [DOI] [PubMed] [Google Scholar]
- Duvall, E., Wyllie, A.H., and Morris, R.G. (1985). Macrophage recognition of cells undergoing programmed cell death (apoptosis). Immunol. 56, 351-358. [PMC free article] [PubMed] [Google Scholar]
- Fadok, V.A., Bratton, D.L., Konowal, A., Freed, P.W., Westcott, J.Y., and Henson, P.M. (1998). Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Investig. 101, 890-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok, V.A., Bratton, D.L., Rose, D.M., Pearson, A., Ezekewitz, R.A.B., and Henson, P.M. (2000). A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 405, 85-90. [DOI] [PubMed] [Google Scholar]
- Fadok, V.A., Savill, J.S., Haslett, C., Bratton, D.L., Doherty, D.E., Campbell, P.A., and Henson, P.M. (1992b). Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J. Immunol. 149, 4029-4035. [PubMed] [Google Scholar]
- Fadok, V.A., Voelker, D.R., Campbell, P.A., Cohen, J.J., Bratton, D.L., and Henson, P.M. (1992a). Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148, 2207-2216. [PubMed] [Google Scholar]
- Falcone, D.J., Borth, W., Khan, K.M.F., and Hajjar, K.A. (2001). Plasminogen-mediated matrix invasion and degradation by macrophages is dependent on surface expression of annexin II. Blood 97, 774-784. [DOI] [PubMed] [Google Scholar]
- Faure, A.V., Migne, C., Devilliers, G., and Ayala-Sanmartin, J. (2002). Annexin 2 “secretion”accompanying exocytosis of chromaffin cells: possible mechanisms of annexin release. Exp. Cell Res. 276, 79-89. [DOI] [PubMed] [Google Scholar]
- Fava, R.A., McKanna, J., and Cohen, S. (1989). Lipocortin I (p35) is abundant in a restricted number of differentiated cell types in adult organs. J. Cell. Physiol. 141, 284-293. [DOI] [PubMed] [Google Scholar]
- Fava, R.A., and Piltch, A.S. (1987). Histological distribution of the 35-KD protein substrate of the epidermal growth factor receptor/kinase in thymus. J. Histochem. Cytochem. 35, 1309-15. [DOI] [PubMed] [Google Scholar]
- Ferraro, C., Quemeneur, L., Fournel, S., Prigent, A.F., Revillard, J.P., and Bonnefoy-Berard, N. (2000). The topoisomerase inhibitors camptothecin and etoposide induce a CD95-independent apoptosis of activated peripheral lymphocytes. Cell Death Differ. 7, 197-206. [DOI] [PubMed] [Google Scholar]
- Gerke, V., and Moss, S.E. (2002). Annexins: from structure to function. Physiol. Rev. 82, 331-371. [DOI] [PubMed] [Google Scholar]
- Goulding, N.J., Pan, L., Wardwell, K., Guyre, V.V., and Guyre, P.M. (1996). Evidence for specific annexin I-binding proteins on human monocytes. Biochem. J. 316, 593-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar, K.A., and Acharya, S.S. (2000). Annexin II and regulation of cell surface fibrinolysis. Ann. N.Y. Acad. Sci. 902, 265-271. [DOI] [PubMed] [Google Scholar]
- Hoffmann, P.R., deCathelineau, A.M., Ogden, C.A., Leverrier, Y., Bratton, D.L., Daleke, D.L., Ridley, A.J., Fadok, V.A., and Henson, P.M. (2001). Phosphatidylserine (PS) induces PS receptor mediated macropinocytosis and promotes clearance of apoptotic cells. J. Cell Biol. 155, 649-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahling, S., Callahan, M.K., Williamson, P., and Schlegel, R.A. (1999). Exposure of phosphatidylserine is a general feature in the phagocytosis of apoptotic lymphocytes by macrophages. Cell Death Differ. 6, 183-189. [DOI] [PubMed] [Google Scholar]
- Lambert, O., Gerke, V., Bader, M.F., Porte, F., and Brisson, A. (1997). Structural analysis of junctions formed between lipid membranes and several annexins by cryo-electron microscopy. J. Mol. Biol. 272, 42-55. [DOI] [PubMed] [Google Scholar]
- Lewit-Bentley, A., Rety, S., Sopkova-De Oliveira Santos, J., and Gerke. V. (2000). S100-annexin complexes: some insights from structural studies. Cell Biol. Int. 24, 799-802. [DOI] [PubMed] [Google Scholar]
- Liu, L. (1999). Calcium-dependent self-association of annexin II: a possible implication in exocytosis. Cell Signal 11, 317-324. [DOI] [PubMed] [Google Scholar]
- Ma, K.Y., Simantov, R., Zhang, J.C., Silverstein, R., Hajjar, K.A., and McCrae, K.R. (2000). High affinity binding of beta(2)-glycoprotein I to human endothelial cells is mediated by annexin II. J. Biol. Chem. 275, 15541-15548. [DOI] [PubMed] [Google Scholar]
- Mailliard, W.S., Haigler, H.T., and Schlaepfer, D.D. (1996). Calcium-dependent binding of S100C to the N-terminal domain of annexin 1. J. Biol. Chem. 271, 719-725. [DOI] [PubMed] [Google Scholar]
- McEvoy, L., Williamson, P., and Schlegel, R.A. (1986). Membrane phospholipid asymmetry as a determinant of erythrocyte recognition by macrophages. Proc. Natl. Acad. Sci. USA 83, 3311-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy, L., Schlegel, R.A., Williamson, P., and Del Buono, B.J. (1988). Merocyanine 540 as a flow cytometric probe of membrane lipid organization in leukocytes. J. Leukoc. Biol. 44, 337-344. [DOI] [PubMed] [Google Scholar]
- Morand, E.F., Hutchinson, P., Hargreaves, A., Goulding, N.J., Boyce, N.W., and Holdsworth, S.R. (1995). Detection of intracellular lipocortin 1 in human leukocyte subsets. Clin. Immunol. Immunopathol. 76, 195-202. [DOI] [PubMed] [Google Scholar]
- Perretti, M., Christian, H., Wheller, S.K., Aiello, I., Mugridge, K.G., Morris, J.F., Flower, R.J., and Goulding, N.J. (2000). Annexin I is stored within gelatinase granules of human neutrophils and mobilized on the cell surface upon adhesion but not phagocytosis. Cell Biol. Int. 24, 163-174. [DOI] [PubMed] [Google Scholar]
- Pradhan, D., Krahling, S., Williamson, P., and Schlegel, R.A. (1997). Multiple systems for recognition of apoptotic lymphocytes by macrophages. Mol. Biol. Cell 8, 767-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengarth, A., Gerke, V, and Luecke, H. (2001). X-ray structure of full-length annexin I and implications for membrane aggregation. J. Mol. Biol. 306, 489-498. [DOI] [PubMed] [Google Scholar]
- Schlegel, R.A., Reed, J.A., McEvoy, L., Algarin, L., and Williamson, P. (1987). Phospholipid asymmetry of loaded red cells. Methods Enzymol. 149, 281-293. [DOI] [PubMed] [Google Scholar]
- Schlegel, R.A., and Williamson, P. (2001). Phosphatidylserine, a death knell. Cell Death Differ. 8, 551-563. [DOI] [PubMed] [Google Scholar]
- Seemann, J., Weber, K., and Gerke, V. (1996). Structural requirements for annexin 1-S100C complex formation. Biochem. J. 319, 123-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siever, D.A., and Erickson, H.P. (1997). Extracellular annexin II. Int. J. Biochem. Cell Biol. 29, 1219-1223. [DOI] [PubMed] [Google Scholar]
- Tressler, R.J., Updyke, T.V., Yeatman, T., and Nicolson, G.L. (1993). Extracellular annexin II is associated with divalent cation-dependent tumor cell–endothelial cell adhesion of metastatic RAW117 large-cell lymphoma cells. J. Cell Biochem. 53, 265-276. [DOI] [PubMed] [Google Scholar]
- van den Eijnde, S.M., Boshart, L., Reutelingsperger, C.P.M., De Zeeuw, C.I., and Vermeij-Keers, C. (1997). Phosphatidylserine plasma membrane asymmetry in vivo: a pancellular phenomenon which alters during apoptosis. Cell Death Differ. 4, 311-316. [DOI] [PubMed] [Google Scholar]
- Vergnolle, N., Comera, C., and Bueno, L. (1995). Annexin I is overexpressed and specifically secreted during experimentally-induced colitis in rats. Eur. J. Biochem. 232, 603-610. [DOI] [PubMed] [Google Scholar]
- Verhoven, B., Schlegel, R.A., and Williamson, P. (1995). Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J. Exp. Med. 82, 1597-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson, P., Kulick, A., Zachowski, A., Schlegel, R.A., and Devaux, P.F. (1992). Ca2+ induces transbilayer redistribution of all major phospholipids in human erythrocytes. Biochemistry 31, 6355-6360. [DOI] [PubMed] [Google Scholar]