Abstract
Rho GTPases control cell dynamics during growth and development. They are activated by guanine nucleotide exchange factors and inactivated by GTPase-activating proteins (GAPs). Many GAPs exist with various protein modules, the functions of which largely remain unknown. We recently cloned and identified BPGAP1 as a novel RhoGAP that coordinately regulates pseudopodia and cell migration via the interplay of its BNIP-2 and Cdc42GAP homology, RhoGAP, and the proline-rich domains. To further elucidate the molecular mechanism underlying cell dynamics control by BPGAP1, we used protein precipitations and matrix-assisted laser desorption/ionization mass spectrometry and identified cortactin, a cortical actin binding protein as a novel partner of BPGAP1 both in vitro and in vivo. Progressive deletion studies confirmed that cortactin interacted directly and constitutively with the proline-rich motif 182-PPPRPPLP-189 of BPGAP1 via its Src homology 3 domain. Together, they colocalized to periphery and enhanced cell migration. Furthermore, substitution of prolines at 184 and 186 with alanines abolished their interaction. Consequently, this BPGAP1 mutant failed to facilitate translocation of cortactin to the periphery, and no enhanced cell migration was observed. These results provide the first evidence that a RhoGAP functionally interacts with cortactin and represents a novel determinant in the regulation of cell dynamics.
INTRODUCTION
Small guanine nucleotide triphosphatases (GTPases) constitute a large superfamily of molecular switches that regulate important signaling networks in cell growth, cell dynamics, and tissue/organ development. For examples, the Ras family members are involved in cell proliferation, Rho family as regulators of cell dynamics, Arf family on intracellular trafficking, whereas Ran family controls nucleus export/import (Bishop and Hall, 2000; Etienne-Manneville and Hall, 2002). These pathways are activated by certain classes of guanine nucleotide exchange factors and inactivated by GTPase-activating proteins (GAPs).
GAP domain catalyzes the conversion of the active GTP-bound form of small GTPases to their inactive GDP-bound state through a canonical “arginine finger” motif (Sprang, 1997). Thus far, at least 53 distinct proteins harboring the GAP domain have been identified from the human genome database (Moon and Zheng, 2003). To date, there is no specific GAP for a single GTPase; instead, there exists a GAP that recognizes more than one GTPases, and a single GTPase can be a target of multiple GAPs. Furthermore, in vitro substrate profile can vary compared with the in vivo results (Ridley et al., 1993). Although it has been well established that GAP-containing proteins usually function to negatively regulate their cognate GTPase substrates, some are believed to function as an effector; for example, the RasGAP Neurofibromatosis 1 (Yunoue et al., 2003) and TcGAP that is involved in insulin-stimulated glucose transport (Chiang et al., 2003), whereas others potentiate the action of the protein they reside in, such as the RhoGAP domain in the regulatory subunits of phosphatidylinositol 3-kinase, p85, which interacts specifically with Rac1 and Cdc42 to stimulate its kinase activity in vitro, without detectable GAP activity (Zheng et al., 1994). Furthermore, GAPs can be subjects of signaling cross talk by providing multiple signaling modules linked to other signaling pathways such as tyrosine kinase, phosphoi-nositites, and serine/threonine kinases. The recent findings that p190-B RhoGAP unexpectedly regulates body size of mice by affecting insulin-mediated CREB transcriptional signaling and that Rho GTPases regulate a switch between adipogenesis and myogenesis immediately open up a wealth of new and exciting prospects for testing functions of other GAPs in vivo (Sordella et al., 2002, 2003). All these point to the complexity in the nature of GAP and small GTPases regulation and highlighting the need to address the function of GAPs (and other regulators) in totality, including the roles of other domains they carry. Being the key signal transducers with multiple motifs and differential substrate specificities, GAPs are poised to coordinately interact with numerous molecules to converge or diverge signals inside the cells.
We have recently identified a novel RhoGAP, termed BPGAP1, that harbors three distinctive protein domains, the BNIP-2 and Cdc42GAP homology (BCH) domain that we first described (Low et al., 1999, 2000a,b), a proline-rich region (PRR) and a functional GAP domain (Shang et al., 2003). BPGAP1 induces the formation of pseudopodia at the cell periphery in a process that required its BCH domain and the GAP domain. Furthermore, we previously showed that these two domains collaborate with the PRR to bring about enhanced cell motility. These results suggest that changes in cell morphology are coupled to determinant(s) in cell migration.
To further elucidate the molecular mechanism underlying cell dynamics control by BPGAP1, we used protein precipitations and matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry and identified cortactin, a cortical actin binding protein as a bona fide partner of BPGAP1. Cortactin has been shown to be involved in various intracellular signaling leading to membrane ruffling, endocytosis, and motility via reorganization of actin cytoskeleton (Weed and Parsons, 2001). In vitro and in vivo protein interaction studies confirmed that cortactin interacted directly and specifically with BPGAP1 in a constitutive manner that required its Src homology (SH)3 domain binding to the proline-rich motif of BPGAP1 and such interaction facilitated translocation of cortactin to the membrane periphery for enhanced cell migration. These results provide the first evidence that a RhoGAP functionally interacts with cortactin and represents a novel determinant in the regulation of cell dynamics.
MATERIALS AND METHODS
Identification of BPGAP1 Interacting Partners
Cells were lysed in lysis buffer [50 mM HEPES, pH 7.4, 150 mM sodium chloride, 1.5 mM magnesium chloride, 5 mM EDTA, 10% (vol/vol) glycerol, 1% (vol/vol) Triton X-100, a mixture of protease inhibitors (Roche Diagnostics, Indianapolis, IN)], 5 mM sodium orthovanadate, and 25 mM glycerol phosphate (Sigma-Aldrich, St. Louis, MO). The glutathione S-transferase (GST)-BPGAP1 proteins, coupled to glutathione beads, were incubated with precleared cell lysates. The bound proteins were resolved by SDS-PAGE and were visualized by silver-staining (Bio-Rad, Hercules, CA). The unique bands were excised and digested with trypsin (Shevchenko et al., 1996). Mass spectra were acquired with a matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometer (Voyager STR BioSpectrometry work station; Applied System) operating in the delayed-extraction reflectron mode. Peptide mass fingerprints of the tryptic peptides from MALDI-TOF mass spectrometric data were used to search the National Center for Biotechnology Information protein database with the programs MS-Fit (http://prospector.ucsf.edu/ucsfhtml4.0/msfit.htm) and Mascotsearch engine (http://www.matrixscience.com).
Plasmid Construction
To obtain cortactin cDNA (GenBank NP_005222; Schuuring et al., 1992), 5 μg of total RNA isolated from HeLa cells (RNeasy; QIAGEN, Valencia, CA) was subjected to first-strand cDNA synthesis with avian myeloblastosis virus reverse transcriptase (Promega, Madison, WI) primed with oligo(dT) (QIAGEN Operon, Alameda, CA) and amplified by high fidelity, long-template DyNAzyme (Finnzymes, Espoo, Finland) by using specific primers. Various domains were generated from the full-length template by using specific polymerase chain reaction primers with BamHI (forward) and PstI (reverse) sites for cloning into FLAG epitope-tagged pXJ40 vector (Dr. E. Manser, Institute of Molecular and Cell Biology, Singapore) and maltose binding protein (MBP)-tagged pMAL-c2 × (Dr .T.S. Sim, National University of Singapore, Singapore) or with EcoRI site (reverse) into pGEX-4T1 vector (Amersham Biosciences, Piscataway, NJ). The BPGAP1 deletion or point mutants were generated by site-directed mutagenesis as described previously (Low et al., 2000a). The hemagglutinin (HA)-tagged BPGAP1 constructs were generated after subcloning with appropriate restriction enzymes. Clones were verified after sequencing entirely in both directions and propagated in Escherichia coli strain XL1-blue and DH5α. All plasmids were purified using miniprep or midiprep kit (QIAGEN) for subsequent use in transfection experiments. Reagents used were of analytical grade, and standard protocols for molecular manipulations and media preparation were as described previously (Sambrook and Russell, 2001).
Cell Culture and Transfection
293T or MCF7 cells were grown in RPMI 1640 medium supplemented with 10% (vol/vol) fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (all from Hyclone Laboratories, Logan, UT), and maintained at 37°C in a 5% CO2 atmosphere, whereas HeLa cells were grown in DMEM (high glucose). They were starved for 18–24 h in serum-free medium before treatment with 10 ng/ml epidermal growth factor or platelet-derived growth factor (PDGF) (Sigma-Aldrich) for 10 min. The 293T and MCF7 cells were transfected using FuGENE 6 (Roche Diagnostics), whereas HeLa cells were transfected with Superfect (QIAGEN) according to the manufacturer's instructions.
Precipitation/“Pulldown,” Direct Binding Studies and Western Blot Analyses
Control cells or cells transfected with expression plasmids were lysed in 1 ml of lysis buffer as described above. Lysates were directly analyzed, either as whole-cell lysates (25 μg) or aliquots (500 μg) used in affinity precipitation/pulldown experiments with various GST fusion proteins (10 μg), or M2 anti-FLAG agarose beads (Sigma-Aldrich) as described previously (Low et al., 2000a). For in vitro direct binding studies, immobilized GST fusion proteins were incubated with 60 ng of purified MBP-fusion proteins in 250 μl of lysis buffer at 4°C for 1 h. Samples were run in SDS-PAGE gels followed by Western blotting, and signals detected using the enhanced chemiluminescence system (Amersham Biosciences). Antibodies used were anti-FLAG (monoclonal and polyclonal; both from Sigma-Aldrich), polyclonal anti-HA (Zymed Laboratories, South San Francisco, CA), monoclonal anti-MBP (New England Biolabs, Beverly, MA), and cortactin antibodies (Cell Signaling Technology, Beverly, MA).
Immunofluorescence
HeLa cells on sterilized glass coverslips were transfected with FLAG-BPGAP1 or FLAG-cortactin and HA-BPGAP1 plasmids, and then made quiescent or treated with PDGF (10 ng/ml) for 10 min. They were washed with cold phosphate-buffered saline (PBS) supplemented with 10 mM calcium chloride and 10 mM magnesium chloride (PBSCM) and fixed with 3% paraformaldehyde for 30 min at 4°C. Fixed cells were washed twice with PBSCM, twice with PBSCM containing 50 mM NH4Cl, and twice again with PBSCM, followed by permeabilization with 0.1% saponin (Sigma-Aldrich) (room temperature, 15 min) and incubation at room temperature for 1 h with 50 μl (0.5 μg) of anti-FLAG, anti-cortactin (BD Transduction Laboratories, Lexington, KY) or anti-HA polyclonal antibodies [in 2% (vol/vol) fetal bovine serum, 2% (wt/vol) bovine serum albumin in PBSCM]. Samples were washed three times (2 min) in 0.1% saponin-containing PBSCM before incubation with rhodamine-conjugated goat anti-mouse IgG (Chemicon International, Temecula, CA) or fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) or Alexa Fluor 350-conjugated goat anti-rabbit IgG (Molecular Probes, Eugene, OR). Filamentous actin was identified by staining with rhodamine-phalloidin (Sigma-Aldrich). After the final wash (5 times in 0.1% saponin containing PBSCM), coverslips were mounted with FluorSave (Calbiochem, San Diego, CA) and examined by confocal fluorescence microscopy (LSM510; Carl Zeiss, Jena, Germany). All images were captured with a 60× objective lens and presented by Microsoft PowerPoint software (Microsoft 2002).
Cell Migration Assay
Migration assays were carried out with six-well cluster plates (Transwell; Costar, Cambridge, MA) by using polycarbonate filters (8.0-μm pore size) coated with 0.5 μg of fibronectin (Sigma-Aldrich). Transfected cells were trypsinized and resuspended as 2.5 × 105 cell/ml in RPMI 1640 medium containing 0.2% bovine serum albumin. Cell suspension (1.5 ml) was added to the upper well and the lower wells of the chamber were filled with 2.6 ml of RPMI 1640 medium alone or 10 ng/ml PDGF in RPMI 1640 medium. After 12 h, migrated and nonmigrated cells from Transwell insert membranes were trypsinized, permeabilized, and labeled as described above, except that R-phycoerythrin–conjugated goat anti-mouse IgG (DakoCytomation California, (Carpinteria, CA) were used instead of rhodamine-conjugated goat antimouse IgG. Stained cells were suspended in PBS containing 1% paraformaldehyde and subjected to analyses by using fluorescence-activated cell sorting (FACSVantage SE). Each assay was done in at least duplicates, and the experiment was repeated at least twice. Statistical significance was analyzed with analysis of variance test. Data are means ± standard deviation (p < 0.005) and expressed as fold-stimulation over the control cells.
RESULTS
Identification of Cortactin as a Novel BPGAP1 Interacting Partner
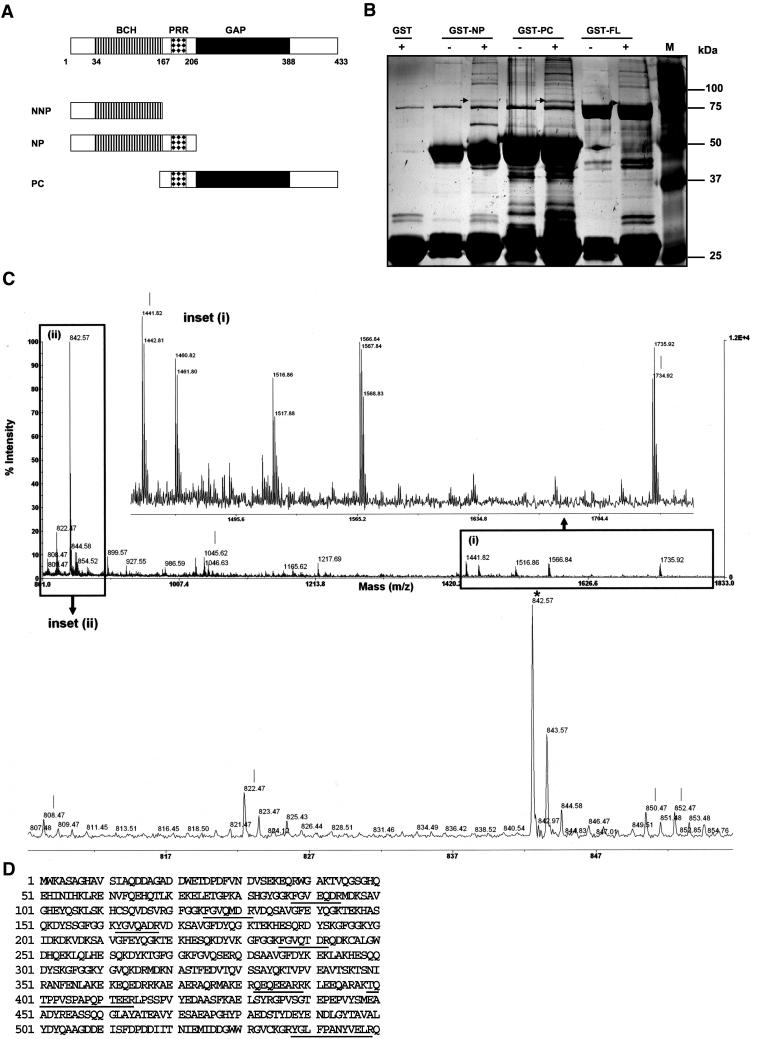
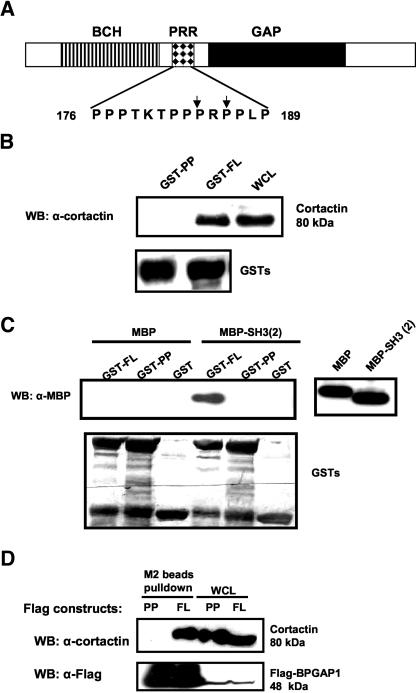
To better define the role of BPGAP1 in the control of cell dynamics, we used GST pulldown assays and MALDI-TOF to identify its cellular interacting partners. In addition to the full-length (FL) BPGAP1, one fragment carrying the BCH domain at the N terminus and the middle proline-rich region (NP fragment), whereas the other that harbored the same PRR together with the GAP domain at the distal C terminus (PC fragment) were used to isolate their putative targets (Figure 1A). Fragments containing PRR were used to maximize the chance of isolating any proteins that target to this region. These GST fusion proteins were coupled to glutathione-Sepharose beads and incubated with precleared lysate from the epithelial human cervical cancer, the HeLa cells. When GST-NP, GST-PC, or GST-FL were used for pulldown assays, several unique bands were consistently observed, including one with an apparent molecular weight of 80 kDa (Figure 1B, arrow), albeit with low amount. This band was excised from the gel and digested in-gel with trypsin. After this, the generated peptide mixture was analyzed by MALDI-TOF as described in MATERIALS AND METHODS. Based on their mass spectra, seven fragments could be clearly identified to be part of cortactin (Figure 1, C and D), a cortical actin binding protein known to be an important regulator of cytoskeleton. Cortactin was also identified when the same experiments were performed using lysates from the human embryonic kidney epithelial 293T cells and human breast epithelial MCF7 cells (our unpublished data).
Figure 1.
Identification of BPGAP1 interacting partner cortactin via protein precipitation and MALDI-TOF. (A) Schematic diagram of BPGAP1 constructs used for identifying their interaction partners. The NP domain (amino acid 1–206), PC domain (amino acid 167–433), and full-length BPGAP1 were expressed as GST-recombinants in E. coli and affinity purified with glutathione-Sepharose beads. NNP, N terminus with BNIP-2 and Cdc42GAP homology domain (BCH); NP, N terminus with BCH domain and proline-rich region (PRR); PC, C terminus with GTPase-activating protein domain (GAP) and PRR. (B) GST-fusion proteins coupled to glutathione beads were incubated with either lysis buffer (-) or HeLa cell lysates (+), which had been precleared with GST-beads to remove nonspecific binding. Bound proteins were resolved by SDS-PAGE and revealed by silver staining. M, molecular weight marker, in kilodaltons. A unique band at 80-kDa (indicated by arrow) was subjected to trypsin digestion followed by MALDI-TOF analyses as described in MATERIALS AND METHODS. (C) Mass spectrum (MS) from MALDI analyses of various peptides derived from the 80-kDa protein band. Insets i and ii show the enlarged views of MS spectrum of monoisotopic peaks (corresponding to the masses of generated tryptic peptides) with lines indicating the matched peptide and * indicating peak for trypsin. (D) Protein sequence of cortactin with sequence coverage of 11% (underlined) over the protein.
BPGAP1 Interacts with Cortactin as a Constitutive Complex via Its Proline-rich Region
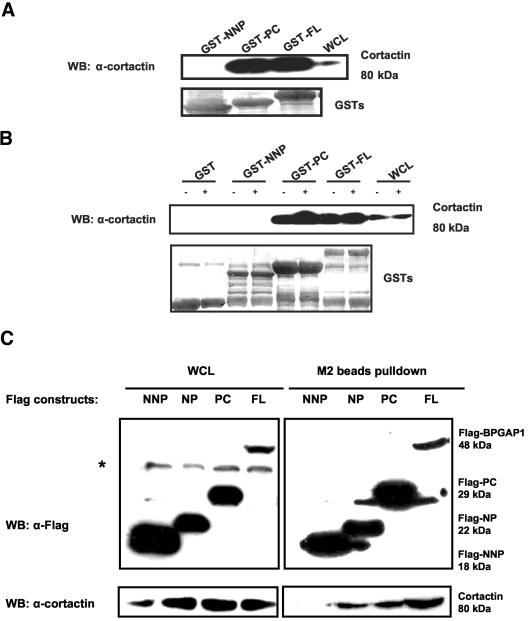
To further confirm that cortactin was indeed a bona fide partner of BPGAP1, we used in vitro and in vivo protein interaction studies to examine the interaction of endogenous cortactin with various BPGAP1 domains that were prepared as bacterially expressed GST recombinants (Figure 2, A and B) or FLAG-tagged proteins expressed in mammalian cells (Figure 2C). Because the earlier proteomics-based pulldown assays detected cortactin in both NP and PC fragments that contained the proline-rich region (176-PPPTKTPPPRPPLP-189) as the common entity, it implied that this region could be involved in the interaction. To test this hypothesis, the N terminus without the PRR (NNP) was constructed for use in further binding studies. Figure 2A shows that when lysates prepared from HeLa cells maintained in culture condition were subjected to pulldown assays, the full-length BPGAP1 and the PC fragment interacted very strongly with endogenous cortactin, as indicated by marked enrichment compared with the cell lysates. The NNP fragment, however, did not reveal any binding to cortactin at all. Similar results were observed when MCF7 cells were used or when cortactin was overexpressed (our unpublished data). To further examine whether the interaction between BPGAP1 and cortactin is subjected to stimulation of cells by growth factors, HeLa cells were made quiescent by serum-deprivation for 24 h followed by treatment with or without PDGF for 10 min. Cell lysates were prepared and used for similar pulldown experiments. The result in Figure 2B again shows that BP-GAP1 interacted strongly and specifically via its fragment that contained the proline-rich region and that their binding did not depend on the activation of cells by PDGF.
Figure 2.
BPGAP1 interacts with cortactin as a constitutive complex via its proline-rich region. GST-recombinants of various domain constructs (depicted in Figure 1A) were prepared and used for pulldown assays with HeLa cell lysates as described in MATERIALS AND METHODS. Cells were either maintained in normal culture condition (A) or serum starved for 24 h followed by treatment with (+) or without (-) 10 ng/ml PDGF for 10 min before lysed (B). Blots were stripped and stained with amido black to reveal loading of GST-recombinants. Bound proteins were detected by cortactin antibody. (C) 293T cells were transfected with FLAG-tagged BPGAP1 domains for 24 h. Cells were lysed and subjected to pulldown with M2 anti-FLAG beads. The beads were washed and processed for Western analysis by using FLAG antibody (top) to detect bound domains or cortactin antibody (bottom) to reveal the bound endogenous cortactin. Beads from the pulldown experiments were washed and processed for Western analysis as described in MATERIALS AND METHODS. * denotes nonspecific band from whole-cell lysates (WCL).
To verify that BPGAP1 could indeed associate with endogenous cortactin in vivo, immunoprecipitation assays were carried out using lysates prepared from MCF7 cell transfected with FLAG-tagged BPGAP1 FL, NNP, NP, and PC fragments. The result shows that BPGAP1 FL, NP, and PC fragments, but not the NNP fragment, could be precipitated with endogenous cortactin (Figure 2C). Together, all these results confirm that BPGAP1 associates specifically and constitutively with cortactin in vitro and in vivo through its PRR.
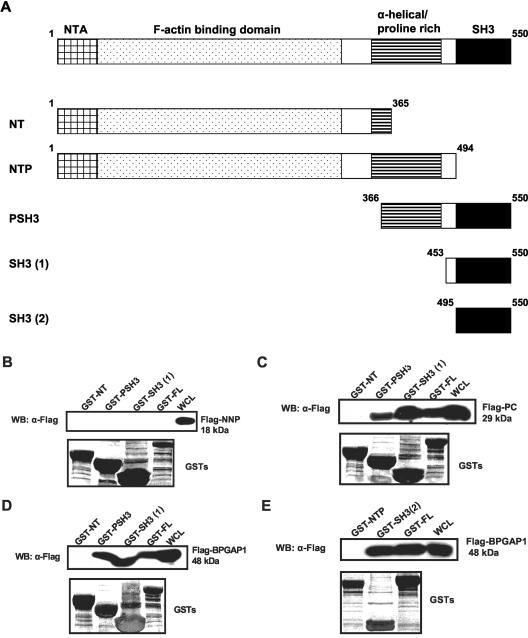
Cortactin Interacts with BPGAP1 via Its SH3 Domain
Depending on the context of sequence motif, PRR could serve as cognate ligands for specific interaction with three independent protein modules, namely, SH3, WW, and Enabled/VASP homology domains (Zarrinpar et al., 2003). Indeed, cortactin contains an SH3 domain at its C terminus that could well be the functional interactive domain for BPGAP1's proline-rich region. To confirm our hypothesis, various deletion constructs of cortactin were made as described in Figure 3A. BPGAP1 FL, NNP, and PC fragments were expressed as FLAG-tagged proteins in 293T and subjected to pulldown assays with cortactin GST recombinants of either the full-length, or its NT, PSH3, or SH3(1) domains. As shown in Figure 3, B–D, FLAG-tagged BPGAP1 NNP fragment that lacked the proline-rich region did not bind to any of the cortactin constructs, whereas FLAG-tagged PC or BPGAP1 full-length interacted with full-length cortactin, SH3(1), or PSH3 but not the NT fragment that did not have the SH3 domain. To confirm that the flanking region (amino acid 453–494) of SH3(1) domain was not partly responsible for the binding, another set of GST recombinant proteins were made, namely, the NTP (amino acid 1–494) and SH3(2) (amino acid 495–550). These GST recombinants were used for pulldown with cells transfected with FLAG-tagged BP-GAP1 FL. The result in Figure 3E strongly suggested that the SH3 domain (amino acid 495–550) was indeed sufficient to mediate its interaction with BPGAP1. Hence, it can be concluded that cortactin associates specifically with BPGAP1 through its SH3 domain.
Figure 3.
Cortactin binds to BPGAP1 via its SH3 domain. (A) Schematic diagram of cortactin domains used in binding studies. NTA, amino-terminal acidic region; F-actin, filamentous actin. (B–E) GST-recombinants of various domain constructs of cortactin were prepared as Sepharose beads and used for pulldown assays on lysates prepared from 293T cells that expressed FLAG-tagged versions of BPGAP1 NNP domain (B), PC domain (C), or full-length BPGAP1 (D and E). Beads from the pulldown experiments were washed and processed for Western analyses by using the FLAG antibody. Blot was stripped and stained with amido black to reveal loading of GST-recombinants.
The PRR of BPGAP1 Constitutes a Novel Target Sequence for Cortactin SH3 Domain
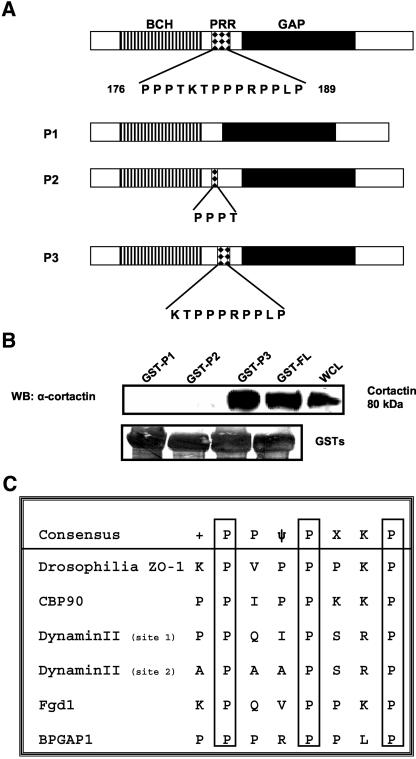
The PRR of BPGAP1 (176-PPPTKTPPPRPPLP-189) harbors various potential consensus target sequence motif (PXXP) for SH3 binding. To further delineate the specific motif within this region, various internal deletion (as opposed to truncation mutants used in earlier experiments) or point mutants were made as GST recombinant constructs and subjected to pulldown assays by using lysates isolated from HeLa cells that expressed endogenous cortactin (Figure 4). As shown in Figure 4B, deletion of the entire proline rich region (P1 mutant) or the KTPPPRPPLP sequence in P2 mutant both abolished the binding between BPGAP1 and cortactin, suggesting that this stretch of sequence is essential for interacting with cortactin SH3 domain. Consistent with this was the observation that P3 mutant, which still retained the sequence KTPPPRPPLP, could indeed pull down endogenous cortactin. This target sequence is unique because it differs from the consensus binding site (+PPΨPxKP) (where + and Ψ indicate basic and hydrophobic amino acid residues, respectively) (Sparks et al., 1996) for many partners of cortactin SH3 domain identified to date (Figure 4C). To further assess which proline residues within this region were important in mediating this interaction, proline residues at 184 and 186 were substituted with alanines (termed PP mutant) because they conform best to the consensus core motif PXXP (Figure 5A). The PP mutant was expressed as FLAG-tagged and GST-tagged recombinants for further analyses. Pulldown assays were performed, and the results show that unlike the wild-type full-length BPGAP1, the GST-PP mutant failed to interact with endogenous cortactin from HeLa cell lysates (Figure 5B) or with MBP-cortactin SH3(2) in the direct binding assays (Figure 5C). Consistent with these is the lack of coimmunoprecipitation of endogenous cortactin with FLAG-tagged PP mutant (Figure 5D).
Figure 4.
BPGAP1 presents novel target sequence for cortactin SH3 domain. (A) Schematic diagram of the three deletion mutants of BPGAP1. P1, devoid of the entire PRR; P2, retaining sequence PPPT; P3, retaining sequence KTPPPRPPLP. (B) GST-recombinants of these deletion constructs were prepared as Sepharose beads and used for pulldown assays by using HeLa cell lysates expressing endogenous cortactin. Beads from the pulldown experiments were washed and processed for Western analysis by using cortactin antibody. Blot was stripped and stained with amido black to reveal equal loading of GST-recombinants. WCL, whole-cell lysates. (C) Comparison of various cortactin-SH3 domain binding sequences with that of BPGAP1. Indicated consensus sequence is derived from the peptide phage display library screening of Sparks et al., 1996. Sequences used are from Takahisa et al., 1996 (ZO-1), Ohoka and Takai, 1998 (CBP90), McNiven et al., 2000 (Dynamin), Hou et al., 2003 (Fgd1), and Shang et al., 2003 (BPGAP1).
Figure 5.
PXXP core motif within the PRR of BPGAP1 is indispensable for binding cortactin. (A) Schematic diagram of BPGAP1 PP mutant, indicating substitution of the proline residues at 184 and 186 with alanines (arrows). (B) GST-recombinants of BPGAP1 FL and point mutant (PP) were used for pulldown assays by using lysates prepared from HeLa cells. Beads were washed and processed for Western analysis by using cortactin antibody. (C) Purified SH3(2) fusion of maltose binding protein, MBP-SH3(2), or the MBP control were incubated with Sepharose beads conjugated with purified GST recombinant proteins of either the full-length wild type or PP mutant of BPGAP1 or GST control, and bound targets revealed by Western blot analyses by using MBP antibody. Purified MBP and MBP-SH3(2) were analyzed with MBP antibody and revealed intact targets used in the direct binding assays. The lower apparent molecular weight for MBP-SH3(2) compared with the MBP alone was due to the removal of internal lacZ coding sequence upon cloning of the target insert. Blot was stripped and stained by amido black to verify loading of equal amounts of GSTs. (D) Cells were transfected with either the wild-type FL or PP mutant of FLAG-tagged BPGAP1 and immunoprecipitated with anti-FLAG M2 beads, washed, and analyzed for bound endogenous cortactin by using cortactin antibody. WCL, whole-cell lysates.
Together, all these results confirm that PXXP core motif within the PRR of BPGAP1 is indispensable for directly targeting BPGAP1 to cortactin at its SH3 domain to form a stable constitutive complex. Furthermore, the target sequence represents a novel recognition motif for the binding of cortactin SH3 domain. Delineation of these interaction domains/motifs should allow us to probe for their possible roles in regulating cellular process as described in the following section.
BPGAP1 Facilitates Cortactin Translocation to Cell Periphery
We recently observed that BPGAP1 induced pseudopodia via its BCH and GAP domain independently of the PRR. However, this PRR was necessary to facilitate cell migration upon changes in cell morphology induced by both BCH and GAP domains (Shang et al., 2003). Because cortactin is known to be a key regulator for membrane dynamics (Weed and Parsons, 2001), we hypothesized that BPGAP1 could act in concert with cortactin to regulate cell migration.
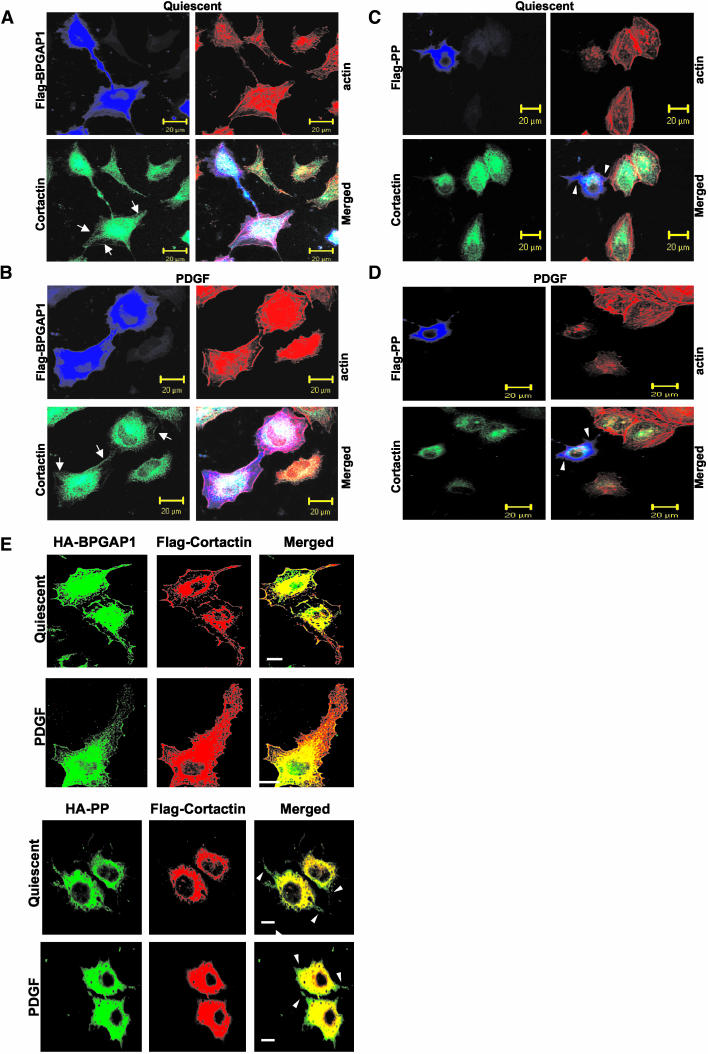
We first set out to examine the subcellular localization of endogenous cortactin and the disposition of filamentous actin in HeLa cells expressing either the wild-type BPGAP1 or the PP mutant. These cells were made quiescent by serum removal or stimulated for 10 min with PDGF, followed by indirect immunofluorescence studies. Figure 6, A and B, show that under both types of condition, BPGAP1 and cortactin were mainly colocalized within cytoplasm and also with presence on the membrane periphery along with the filamentous actin (indicated by arrows). However, cells without BPGAP1 overexpression showed a strong absence of cortactin as well as reduced actin disposition on the membrane periphery. Consistent with this, cells expressing BPGAP1 PP mutant that did not interact with cortactin failed to display the presence of cortactin and actin on the membrane periphery or the protruding ends, despite the PP mutant being localized to those areas (Figure 6, C and D, arrowheads). Furthermore, we revealed that the actin staining in the protrusions formed by the PP mutant was also reduced compared with those formed by the wild-type counterpart. Similarly, when more cortactin was introduced upon cotransfection with wild-type BPGAP1, it was also markedly localized to the cell periphery with BPGAP1. However, such disposition was lost when the PP mutant was expressed in the cells (Figure 6E, arrows). These results suggest that the binding of BPGAP1 to cortactin facilitates the translocation of cortactin to the cell periphery and the assembly of actin there, in a manner independent of growth factor stimulation.
Figure 6.
Effect of BPGAP1 on localization of cortactin and actin disposition. HeLa cells were transfected with expression plasmids coding for FLAG-tagged BPGAP1 FL (A and B) or PP constructs (C and D). Cells were made quiescent by serum removal for 24 h (A and C), or followed by treatment with 10 ng/ml PDGF for 10 min (B and D). Cells were permeabilized, stained, and visualized under confocal fluorescent microcopy as described in MATERIALS AND METHODS. Bottom right, merged image of endogenous actin (red), endogenous cortactin (green), and overexpressed FLAG-tagged BPGAP1 or PP (blue). Arrows indicate presence of cortactin and actin on cell periphery (A and B), whereas arrowheads highlight lack of staining for cortactin and actin on cell periphery (C and D). (E) HeLa cells were cotransfected with HA-tagged BPGAP1 and FLAG-tagged cortactin and processed for immunofluorescence detection by confocal microscopy. Images from FLAG-tagged cortactin (red) and HA-tagged BPGAP1 FL or PP (green) were merged to show their colocalization as depicted in yellow. Arrowheads indicate lack of cortactin colocalization with BPGAP1 PP mutant on cell periphery or protrusions. The intensities of images were enhanced to capture changes in the cell peripheries, including the cell protrusions. White bars, 10 μm.
BPGAP1 and Cortactin Interact to Enhance Cell Migration
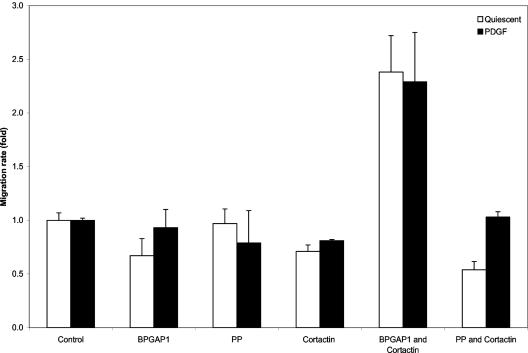
The localization of BPGAP1–cortactin-interacting complex along the membrane periphery strongly suggests a possible function for such complex in regulating cell migration, especially cortactin, which has been implicated in cell motility in both fibroblasts and endothelial cells (Huang et al., 1998; Patel et al., 1998), although little is known for its effects on cell motility in epithelial cells. We sought to further determine the effect of BPGAP1 with cortactin on cell migration in 293T cells, which when compared with HeLa cells, offered greater transfection efficiency required for the expression of both BPGAP1 and cortactin for subsequent reliable detection by FACS. Cells were transfected with the vector control, expression plasmids for HA-tagged wild-type BPGAP1 or the PP mutant, either alone or together with the FLAG-tagged cortactin and assayed for their effects on cell migration as described in MATERIALS AND METHODS. Figure 7 shows that 293T cells expressing BPGAP1–cortactin-interacting complex resulted in a significant twofold increase (p < 0.005) in the cell migration rate when cells were under quiescent or when PDGF was used as a chemoattractant. No effect was seen with the expression of either BPGAP1 or cortactin alone. Consequently, the PP mutant that did not interact with cortactin failed to stimulate any significant changes in the cell motility. These results therefore strongly support the notion that BPGAP1 interaction with cortactin facilitates cortactin translocation to cell periphery, leading to significant effect in the regulation of cell motility in epithelial cells. The significance of this is discussed and a model for such regulation is proposed below (Figure 8).
Figure 7.
Concerted effect of BPGAP1 and cortactin in inducing cell migration. 293T cells were transfected with vector control or expression plasmids coding for HA-tagged BPGAP1 FL or PP constructs or FLAG-tagged cortactin FL either alone or in combinations as indicated. Cell migration through fibronectin-coated polycarbonate filters in response to PDGF as chemoattractant was assayed in Boyden chambers as described in MATERIALS AND METHODS. The fraction of migrated cells in the control was 14.0 ± 0.3% of the total. Data are means ± standard deviations of four replicates from two independent experiments, determined using analysis of variance test. Results are expressed as fold-stimulation over the control.
Figure 8.
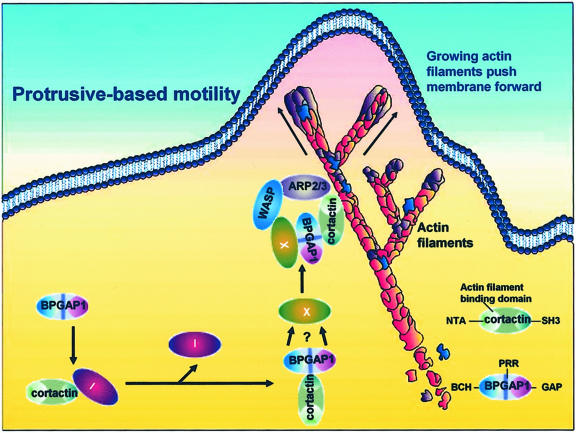
Model for BPGAP1–cortactin action on cell migration. In quiescent state, endogenous cortactin is sequestered within the cytosol. On stimulation (with PDGF or other external stimuli) or its overexpression, it will translocate to the periphery (Weed and Parsons, 2001). Likewise, cortactin mutant lacking the SH3 domain also translocates to the membrane (Weed et al., 1998). This implies a possible inhibitory system, whereby there exists an inhibitor (I) binding to cortactin at its SH3 domain. Such inhibitor can be displaced upon BPGAP1 binding to this domain, allowing the translocation of BPGAP1-cortactin to the periphery to enhance cell migration as reported in this study. This process could involve formation of other complexes (X) necessary for the protrusive-based motility, perhaps through BCH and/or GAP domains of BPGAP1.
DISCUSSION
Our recent study showed that BPGAP1 modulates cell dynamics via an interplay of its multiple protein domains. The formation of pseudopodia by the BCH and GAP domains is coupled to enhanced cell motility that requires an intact proline-rich region. In the present study, we have provided molecular evidence that the cortical actin binding protein cortactin could mediate this missing link.
BPGAP1 as a Novel Integrator of Cortactin and GTPase Signaling
Being a multidomain protein itself, cortactin interacts with various signaling partners in different cell types and locations. It has the potential role as an adaptor/scaffold connecting other signaling proteins to regulate different cellular events. These include interaction with Shank proteins to organize the clustering of receptor complexes within the neuronal postsynaptic sites, binding with dynamin 2 to regulate receptor-mediated endocytosis at the membrane periphery and also involved with ZO-1 in the control of tight junction assembly of epithelial cells (Weed and Parsons, 2001). Besides, cortactin is also involved in the signaling pathway regulated by two small GTPases, Rac1 and Cdc42 (Weed et al., 1998; Vidal et al., 2002). The activation of Rac1 and Cdc42, together with the consequential Arp2/3 activity, is thought to be necessary for protrusive-based cell motility. In NIH3T3 fibroblasts and endothelial cells, cortactin was responsible for enhanced migration (Huang et al., 1998; Patel et al., 1998), whereas Rac1-induced signal transduction is required for the translocation of cortactin to the membrane periphery (Weed et al., 1998). Despite all these observations, and because cortactin itself is not directly associated with these GTPases, the actual molecular mechanisms linking cortactin to them remain unknown.
To date, no RhoGAP has been implicated in any of the cortactin-mediated signaling, although both have been widely studied for their separate cellular processes. We have established that BPGAP1 functions biochemically as a RhoA inactivator through its GAP domain while exerting its peculiar cell extensions via its BCH and GAP domains in a process that requires both active Cdc42 and Rac1 (Shang et al., 2003). Intriguingly, the BCH domain of BPGAP1, in addition to its ability to form homophilic and heterophilic complex as we previously identified for the BNIP-2 family proteins (Low et al., 1999, 2000a,b), also selectively target Cdc42. In contrast, despite not being a substrate for the GAP domain, Rac1 is still a preferred binding partner for this domain in vitro and in vivo (our unpublished data). Coupled to the requirement of BCH and GAP domains in Cdc42/Rac-1 induced pseudopodia formation and the RhoA inactivation by GAP domain, the current identification of cortactin as the missing link for its induced cell migration would immediately implicate that BPGAP1 could serve to bridge cortactin and GTPases signaling for the concerted regulation of cell dynamics. The intricate nature of such regulation is now being investigated.
BPGAP1 Modulates Cortactin and Actin Disposition in Cells
Cortactin is normally distributed diffusely in the cytoplasm of quiescent cells and upon stimulation or its overexpression, it is translocated to the periphery (McNiven et al., 2000; Weed et al., 2000; Van Rossum et al., 2003). However, we did not observe this translocation phenomenon in our system by using the HeLa cells, despite stimulation with PDGF, unless BPGAP1 also was coexpressed. Likewise, different from previous reports on fibroblasts and endothelial cells where cortactin alone promoted cell motility (Huang et al., 1998; Patel et al., 1998) overexpression of cortactin alone in the epithelial 293T cells did not elicit enhanced cell migration unless BP-GAP1 also was overexpressed. Such cell type specificity is likely due to the absolute requirement of BPGAP1 as a primary inducer for cortactin translocation in these cells, leading to the stimulation on cell migration. Although not measured in the current study, we believe that such inducer of cortactin translocation might already exist in great abundance to readily elicit their effects in fibroblasts and endothelial cells. Interestingly, others have shown that cortactin lacking its SH3 domain was also sufficient to translocate to the periphery upon activation of Rac pathway (Weed et al., 2000). Perhaps, there exists an “inhibitory” system in the cells where unknown protein(s) binding to cortactin at its SH3 domain could prevent it from migrating to the periphery. However, upon overexpression of cortactin, this inhibition could be released simply by the lack of sufficient inhibitors to sequester its translocation. Consistently, in our system where BPGAP1 was overexpressed, it is equally plausible that BPGAP1 could have displaced such unknown protein(s), thus allowing translocation probably by recruiting at the same time other cellular proteins through its BCH and/or even the GAP domain. The BPGAP1-cortactin would then be available for subsequent complex to form, leading to protrusive-based motility (Figure 8, see model). In this regard, it is interesting to note that the PP mutant that still elicited protrusions via BCH and GAP domains failed to promote significant assembly of cortactin and actin on the cell periphery or the leading edge of protrusions. These results suggest that different cytoskeletal networks could be used for cell protrusions leading to different physiological outcomes.
Our findings that cortactin binding to BPGAP1 via its SH3 domain could therefore provide a new mechanism for the translocation of cortactin to the periphery, consistent with the observations that subcellular localization of cortactin may be regulated by mechanisms independent of its tyrosine phosphorylation upon growth factor stimulation (Weed et al., 1998) and further supporting the notion that tyrosine phosphorylation of cortactin itself could not account fully for its role in conferring cell migration (Van Rossum et al., 2003).
BPGAP1 Presents a Novel Target Sequence for Cortactin SH3 Domain
Although the PRR of BPGAP1 (182-PPPRPPLP-189) forms a constitutive complex with cortactin via its SH3 domain, this region is unique compared with the widely regarded cortactin SH3 domain binding consensus sequence +PPΨPXKP (Sparks et al., 1996; Figure 4C). Apart from the conserved prolines at + 2, +5, and +8 positions, the requirement for basic residue in +1, hydrophobic residue in +4 and the lysine residue in +7 are all notably missing. Nonetheless, mutations of the two conserved prolines at +3 and +5 (P184A and P186A, respectively; Figure 5A) were sufficient to abolish the interaction. For comparison, it also had been indicated that the substitution of the alternating two prolines with alanines in cortactin binding protein 1 reduced its binding to the cortactin SH3 domain (Du et al., 1998). Other than that, the BPGAP1 target sequence shows little resemblance to the target sequence of several other “nonconforming” proteins (Figure 4C). For examples, for dynamin both 826PPQIPSRP833 and 849APAAPSRP856 are used (McNiven et al., 2000); CBP90 uses 540PPIPPKKP547 (Ohoka and Takai, 1998) and most recently Fgd1 with 158KPQVPPKPSYL168 (Hou et al., 2003). This implies that the SH3 domain of cortactin could have different degrees of substrate specificity, perhaps useful for its roles as a modulator in multiple signaling pathways.
In summary, by using proteomics, molecular and cellular tools, our current work delineates functional interaction between a novel RhoGAP and cortactin in cell dynamics control. We have identified BPGAP1 as an important regulator of cell dynamics that requires cortactin for a concerted effect on cell migration. The challenge now lies on how the PRR of BPGAP1 and the SH3 domain of cortactin could distinguish between several other known or potential partners in the confines of the living cell. We believe that this issue can be partly resolved by identification of many more other cellular interacting partners in this BPGAP1–cortactin complex or complex where these two proteins exist as mutually exclusive entities. Although many cortactin binding partners have been identified to date, our results provide the first evidence that a RhoGAP functionally interacts with cortactin and represents a novel determinant in the regulation of cell migration. It also could potentially provide a link between the small GTPases and cortactin in regulating the spatial and temporal context of cell dynamics.
Acknowledgments
We thank Sashikant Joshi and Wang Xian Hui at the Protein and Proteomics Centre for technical advice and help on MALDI-TOF analyses; Dr. Mohan K. Balasubramania at Temasek Life Sciences Laboratory for providing the facility for triple-labeling confocal microscopy; Tan Li Hui for technical help in FACS; and Dr. Jan Buschdorf and Soh Fu Ling for reviewing our manuscripts. This work is supported by a Graduate Research Scholarship awarded to B.L.L. and a grant from Academic Research Fund, the National University of Singapore and Biomedical Research Council of Singapore.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-02-0141. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-02-0141.
References
- Bishop, A.L., and Hall, A. (2000). Rho GTPases and their effector proteins. Biochem. J. 348, 241-255. [PMC free article] [PubMed] [Google Scholar]
- Chiang, S.H., Hwang, J., Legendre, M., Zhang, M., Kimura, A., and Saltiel, A.R. (2003). TCGAP, a multidomain Rho GTPase-activating protein involved in insulin-stimulated glucose transport. EMBO J. 2, 2679-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, Y., Weed, S.A., Xiong, W.C., Marshall, T.D., and Parsons, J.T. (1998). Identification of a novel cortactin SH3 domain-binding protein and its localization to growth cones of cultured neurons. Mol. Cell. Biol. 18, 5838-5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville, S., and Hall, A. (2002). Rho GTPases in cell biology. Nature 420, 629-635. [DOI] [PubMed] [Google Scholar]
- Hou, P., Estrada, L., Kinley, A.W., Parsons, J.T., Vojtek, A.B., and Gorski, J.L. (2003). Fgd1, the Cdc42 GEF responsible for faciogenital dysplasia, directly interacts with cortactin and mAbp1 to modulate cell shape. Hum. Mol. Genet. 15, 1981-1993. [DOI] [PubMed] [Google Scholar]
- Huang, C., Liu, J., Haudenschild, C.C., and Zhan, X. (1998). The role of tyrosine phosphorylation of cortactin in the locomotion of endothelial cells. J. Biol. Chem. 273, 25770-25776. [DOI] [PubMed] [Google Scholar]
- Low, B.C., Lim, Y.P., Lim, J., Wong, E.S., and Guy, G.R. (1999). Tyrosine phosphorylation of the Bcl-2-associated protein BNIP-2 by fibroblast growth factor receptor-1 prevents its binding to Cdc42GAP and Cdc42. J. Biol. Chem. 274, 33123-33130. [DOI] [PubMed] [Google Scholar]
- Low, B.C., Seow, K.T., and Guy, G.R. (2000a). The BNIP-2 and Cdc42GAP homology domain of BNIP-2 mediates its homophilic association and heterophilic interaction with Cdc42GAP. J. Biol. Chem. 275, 37742-37751. [DOI] [PubMed] [Google Scholar]
- Low, B.C., Seow, K.T., and Guy, G.R. (2000b). Evidence for a novel Cdc42GAP domain at the carboxyl terminus of BNIP-2. J. Biol. Chem. 275, 14415-14422. [DOI] [PubMed] [Google Scholar]
- McNiven, M.A., Kim, L., Krueger, E.W., Orth, J.D., Cao, H., and Wong, T.W. (2000). Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J. Cell Biol. 151, 187-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon, S.Y., and Zheng, Y. (2003). Rho GTPase-activating proteins in cell regulation. Trends Cell Biol. 13, 13-22. [DOI] [PubMed] [Google Scholar]
- Ohoka, Y., and Takai, Y. (1998). Isolation and characterization of cortactin isoforms and a novel cortactin-binding protein, CBP90. Genes Cells 3, 603-612. [DOI] [PubMed] [Google Scholar]
- Patel, A.S., Schechter, G.L., Wasilenko, W.J., and Somers, K.D. (1998). Overexpression of EMS1/cortactin in NIH3T3 fibroblasts causes increased cell motility and invasion in vitro. Oncogene 16, 3227-3232. [DOI] [PubMed] [Google Scholar]
- Ridley, A.J., Self, A.J., Kasmi, F., Paterson, H.F., Hall, A., Marshall, C.J., and Ellis, C. (1993). Rho family GTPase activating proteins p190, bcr and rhoGAP show distinct specificities in vitro and in vivo. EMBO J. 12, 5151-5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., and Russell, D.W. (2001). Molecular Cloning: A Laboratory Manual, 3rd ed., Cold Spring Harbor, NY: Cold Spring Harbor Press.
- Schuuring, E., Verhoeven, E., Mooi, W.J., and Michalides, R.J. (1992). Identification and cloning of two overexpressed genes, U21B31/PRAD1 and EMS1, within the amplified chromosome 11q13 region in human carcinomas. Oncogene 7, 355-361. [PubMed] [Google Scholar]
- Shang, X., Zhou, Y.T., and Low, B.C. (2003). Concerted regulation of cell dynamics by BNIP-2 and Cdc42GAP Homology/Sec14p-like, proline-rich and GTPase-activating protein domains of a novel Rho GTPase-activating protein, BPGAP1. J. Biol. Chem. 278, 45903-45914. [DOI] [PubMed] [Google Scholar]
- Shevchenko, A., Wilm, M., Vorm, O., and Mann, M. (1996). Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68, 850-858. [DOI] [PubMed] [Google Scholar]
- Sordella, R., Classon, M., Hu, K.Q., Matheson, S.F., Brouns, M.R., Fine, B., Zhang, L., Takami, H., Yamada, Y., and Settleman, J. (2002). Modulation of CREB activity by the Rho GTPase regulates cell and organism size during mouse embryonic development. Dev. Cell 2, 553-565. [DOI] [PubMed] [Google Scholar]
- Sordella, R., Jiang, W., Chen, G.C., Curto, M., and Settleman, J. (2003). Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell 113, 147-158. [DOI] [PubMed] [Google Scholar]
- Sparks, A.B., Rider, J.E., Hoffman, N.G., Fowlkes, D.M., Quilliam, L.A., and Kay, B.K. (1996). Distinct ligand preferences of Src homology 3 domains from Src, Yes, Ab1, Cortactin, p53bp2, PLCγ, Crk, and Grb2. Proc. Natl. Acad. Sci. USA 93, 1540-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprang, S.R. (1997). G proteins, effectors and GAPs: structure and mechanism. Curr. Opin. Struct. Biol. 7, 849-856. [DOI] [PubMed] [Google Scholar]
- Takahisa, M., Togashi, S., Suzuki, T., Kobayashi, M., Murayama, A., Kondo, K., Miyake, T., and Ueda, R. (1996). The Drosophila tamou gene, a component of the activating pathway of extramacrochaetae expression, encodes a protein homologous to mammalian cell-cell junction-associated protein ZO-1. Genes Dev. 10, 1783-1795. [DOI] [PubMed] [Google Scholar]
- Van Rossum, A.G., De Graaf, J.H., Schuuring-Scholtes, E., Kluin, P.M., Fang, Y.X., Zhan, X., Moolenaar, W.H., and Schuuring, E. (2003). Alternative splicing of the actin-binding domain of human cortactin affects cell migration. J. Biol. Chem. 278, 45672-45679. [DOI] [PubMed] [Google Scholar]
- Vidal, C., Geny, B., Melle, J., Jandrot-Perrus, M., and Fontenay-Roupie, M. (2002). Cdc42/Rac1-dependent activation of the p21-activated kinase (PAK) regulates human platelet lamellipodia spreading: implication of the corticalactin binding protein cortactin. Blood 100, 4462-4469. [DOI] [PubMed] [Google Scholar]
- Weed, S.A., Du, Y., and Parsons, J.T. (1998). Translocation of cortactin to the cell periphery is mediated by the small GTPase Rac1. J. Cell Sci. 111, 2433-2443. [DOI] [PubMed] [Google Scholar]
- Weed, S.A., Karginov, A.V., Schafer, D.A., Weaver, A.M., Kinley, A.W., Cooper, J.A., and Parsons, J.T. (2000). Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J. Cell Biol. 151, 29-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed, S.A., and Parsons, J.T. (2001). Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene 20, 6418-6434.s [DOI] [PubMed] [Google Scholar]
- Yunoue, S., et al. (2003). Neurofibromatosis type I tumor suppressor neurofibromin regulates neuronal differentiation via its GAP function toward Ras. J. Biol. Chem. 278, 26958-26969. [DOI] [PubMed] [Google Scholar]
- Zarrinpar, A., Bhattacharyya, R.P., and Lim, W.A. (2003). The structure and function of proline recognition domains. Sci. STKE 2003, RE8. [DOI] [PubMed] [Google Scholar]
- Zheng, Y., Bagrodia, S., and Cerione, R.A. (1994). Activation of phosphoinositide 3-kinase activity by Cdc42Hs binding to p85. J. Biol. Chem. 269, 18727-18730. [PubMed] [Google Scholar]