Abstract
There are opposing views about the status of layer IV in primary motor cortex (area 4). Cajal described a layer IV in area 4 of adult humans. In contrast, Brodmann found layer IV in development but not in adult primates and called area 4 ‘agranular’. We addressed this issue in rhesus monkeys using the neural marker SMI-32, which labels neurons in lower layer III and upper V, but not in layer IV. SMI-32 delineated a central unlabeled cortical stripe in area 4 that corresponds to layer IV, which was populated with small interneurons also found in layer IV in ‘granular’ areas (such as area 46). We distinguished layer IV interneurons from projection neurons in the layers above and below using cellular criteria. The commonly used term ‘agranular’ for area 4 is also used for the phylogenetically ancient limbic cortices, confusing areas that differ markedly in laminar structure. This issue pertains to the systematic variation in the architecture across cortices, traced from limbic cortices through areas with increasingly more elaborate laminar structure. The principle of systematic variation can be used to predict laminar patterns of connections across cortical systems. This principle places area 4 and agranular anterior cingulate cortices at opposite poles of the graded laminar differentiation of motor cortices. The status of layer IV in area 4 thus pertains to core organizational features of the cortex, its connections and evolution.
Keywords: agranular cortex, motor cortex, limbic cortex, cortical type, glial cells
Introduction
It is widely believed that area 4, the primary motor cortex in primates, does not have a granular layer IV [e.g. (Parent, 1996; Amaral, 2000)]. There are two ways to interpret this statement. The first is that area 4 lacks layer IV altogether, and is one explicitly or implicitly harbored by many (Bailey & von Bonin, 1951; Matelli et al., 1991; Geyer et al., 2000). The second is that area 4, also known as M1, has a layer IV but the neurons are not granular, a description applied for the small-sized excitatory or inhibitory interneurons found mostly in layer IV of sensory and association cortices. This interpretation implies that the neurons in layer IV of area 4 are bigger than in the sensory areas situated behind it. The closest sensory neighbor of area 4 is the somatosensory cortex. Further front are the premotor areas, which are also regarded by most as agranular, and in front of them is the prefrontal cortex. In many accounts the prefrontal cortex has been called the ‘granular’ frontal cortex to distinguish it from its premotor neighbors [e.g., (Akert & Warren, 1964; Preuss & Goldman-Rakic, 1991)].
The significance of the issue of whether area 4 has layer IV is based on core organizational features of the cerebral cortex. First, it pertains to the centrality of layer IV as the recipient of pathways from the thalamus. If area 4 has no layer IV, where do thalamic pathways terminate? Second, the term ‘agranular’ groups area 4 with the agranular limbic areas. The phylogenetically ancient limbic cortices differ markedly in architecture from area 4. They either lack, or have a rudimentary layer IV and their superficial layers (II–III) and deep layers (V–VI) are not distinct and cannot be subdivided into individual layers. Third, if area 4 lacks layer IV, what is its connectional relationship with surrounding areas? The latter poses a particular dilemma in view of the known laminar patterns of origin and termination of corticocortical connections seen in other cortical systems, such as the visual [for discussion see (Shipp, 2005)]. Here we approach the issue of the status of layer IV in area 4 from a historical perspective and employ experimental analyses to help conclude that area 4 indeed has a layer IV. We discuss the significance of this issue in the context of thalamocortical connections, and from the perspective of the organization of cortical architecture, the patterns of corticocortical connections and cortical evolution.
The status of layer IV in area 4: historical perspective
The father of brain histology, Santiago Ramón y Cajal, was the first to provide a detailed cytoarchitectonic description of the human primary motor cortex using the Nissl technique that stains the bodies of neurons and glia. Cajal also summarized earlier studies on the physiology of the motor cortex and clarified the apparent confusion of the primary motor area with the more caudally situated somatosensory area (Ramón y Cajal, 1899).
Cajal used a seven layer system for the primary motor cortex. In Table 1 we provide the equivalent layers to the six layer system proposed by Bevan Lewis (1880) for the isocortex, which was used later by Brodmann (1909/1999) and is still used today. For Cajal, the granule zone (layer 5 in his terminology) of the human primary motor cortex was rudimentary, discontinuous and hard to discern. But Cajal stressed that the granule zone is invaded by large neurons of the ‘deep pyramidal formation’ composed of layers 4, 5, and 6. Accordingly, the transition from the primary somatosensory to the primary motor cortex is accompanied by loss of a compact central (inner) granular layer that is characteristic of sensory cortices. Using the Golgi method, Cajal described several types of neurons with short axons in the inner granular zone of the motor cortex, some of which have the morphological features of granule neurons found in the primary somatosensory and the primary visual cortex. Accordingly, Cajal concluded that there is a layer IV in the precentral motor region of the human brain and indicated that the granule cells are interspersed within larger pyramidal neurons that protrude from the neighboring layers above and below (Ramón y Cajal, 1899).
Table I.
Layers of the human primary motor cortex according to Cajal and Brodmann
| Cajal, 1899 | Brodmann, 1909/1999 | ||
|---|---|---|---|
| Capa plexiforme (Plexiform layer) | 1 | Lamina zonalis | I |
| Capa de las pequeñas pirámides (Small pyramid layer) | 2 | Lamina granularis externa | II |
| Capa de las medianas pirámides (Medium pyramid layer) | 3 | Lamina pyramidalis | III |
| Capa de las grandes pirámides externas (External large pyramid layer) | 4 | ||
| Capa de las pequeñas pirámides y células estrelladas (Small pyramid and stellate cell layer) | 5 | Lamina granularis interna (present only in fetal life) | IV |
| Capa de las pirámides grandes profundas (Deep large pyramidal zone) | 6 | Lamina ganglionaris | V |
| Capa de las pirámides medianas y los corpúsculos triangulares (Medium pyramidal and triangular corpuscle layer) | 7 | Lamina multiformis | VI |
Cajal also disambiguated another important issue, the fact that not all cortical regions have the same number of layers or possess an inner granular zone that corresponds to layer IV by today’s terminology. He found, for example, that in the inferior cingulate gyrus there are fewer layers than in other cortices, and there is no central granule zone, where layer IV—when present—ought to reside. He suggested that the inferior part of the cingulate gyrus in humans is homologous to the interhemispheric cortex of rodents (Ramón y Cajal, 1901–1902). Based on such observations, Cajal proposed that there is a simplification in the laminar structure of the cerebral cortex from the human and other gyrencephalic mammals to rodents and other animals with simpler cortices, which lack an inner granular layer (Ramón y Cajal, 1904/2002). Among the three principles listed above, this is the most significant because it pertains to the organization of the entire cortex, its connections and evolution.
However, it did not take long to complicate matters with the publication of Korbinian Brodmann’s map of the architecture of the entire human cerebral cortex and other mammalian species. In his widely known monograph on the cerebral cortex, Brodmann sharply criticized Cajal’s evolutionary ideas for the cerebral cortex. Brodmann proposed, instead, that the paradigmatic mammalian cerebral cortex has six layers that are visible either permanently or temporarily during development in all mammals. He called these areas ‘homogenetic’. He called ‘heterogenetic’ rudimentary areas that have fewer layers during the embryonic stage, like the rhinecephalon. According to Brodmann, most areas have six layers both in embryonic and adult stages (homogenetic homotypical). But some areas only show six layers during development and in the adult stage either gain layers (duplication of layer IV in primary visual cortex) or lose them (loss of layer IV in agranular cortices); Brodmann called these areas ‘homogenetic heterotypical’ (Brodmann, 1909/1999). The problem was that Brodmann, and others after him (Table II), included area 4 with areas such as the anterior cingulate as belonging to the same (heterotypical) cortical type, which is an error in fact and concept, as shown in our findings and discussed below.
Table II.
Types of human cerebral cortex according to several authors in the XX century
| Brodmann (1909/1999) | von Economo (1927/2009) | Bailey & von Bonin (1951) | Yakovlev (1959) |
|---|---|---|---|
| Heterogenetic | Allocortex | Allocortex | Entocortex |
| Homogenetic | Isocortex | Isocortex | Mesocortex |
| heterotypical agranular | heterotypical agranular | heterotypical agranular | agranular |
| (limbic and motor areas) | (limbic and motor areas) | (limbic and motor areas) | (limbic and motor areas) |
| heterotypical visual | heterotypical visual | heterotypical granular | granular |
| homotypical | homotypical | eulaminate | Ectocortex |
| (frontal, parietal, polar) |
The status of layer IV in area 4 in the adult primate brain: experimental evidence
To investigate whether there is a layer IV in area 4 with similar features as in other (granular) cortices, we conducted architectonic analysis in brain tissue sections through the motor cortex in a total of seven normal adult rhesus monkeys (Macaca mulatta). For comparison we conducted the same analysis in prefrontal area 46 because it is a good exemplar of granular cortex in the frontal lobe (Barbas & Pandya, 1989). The animals had previously been used for tract tracing experiments unrelated to the present analyses. Perfusion methods (4% parformaldehyde), tissue preparation, Nissl staining and immunohistochemical procedures were according to those described previously (Ghashghaei & Barbas, 2001; Medalla & Barbas, 2006).
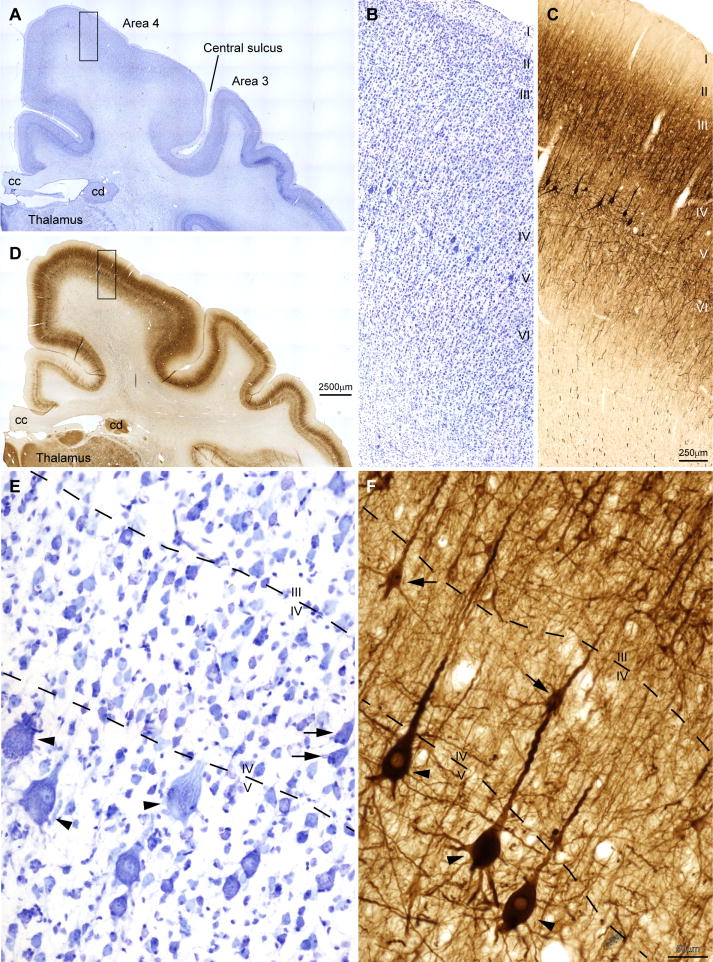
We first identified the areal and laminar borders of area 4 in Nissl-stained sections (n=4 cases) using the architectonic descriptions in maps from previous studies (Gatter & Powell, 1978; Barbas & Pandya, 1987; Morecraft et al., 2012), as well as for area 46 (Barbas & Pandya, 1989) (Figs. 1 and 2). Area 4 is easily identified in Nissl stained sections by the characteristic clusters of giant pyramidal neurons, or Betz cells, in the upper part of layer V. The anterior border of area 4 abuts caudal area 6, which has scattered large pyramidal neurons that are not as large as Betz cells. At the posterior border of area 4 lies somatosensory area 3 which does not have large pyramidal neurons as in the motor cortex (Fig. 1A).
Figure 1.
Laminar architecture of area 4. (A) Low power view of the dorsal frontal and parietal cortex in a Nissl stained section. (B) A higher power view of area 4 stained for Nissl, corresponding to the rectangular inset in (A) shows a thick layer I and small pyramids closely packed in layer II. Layer III pyramids are arranged in columns and are larger in the deep part of the layer. Layer IV is identified as a pale band that is sparsely populated with neurons; the upper limit of layer V is identified by clusters of large pyramids, the Betz cells. (C) SMI-32 labeled neurons are prominent in layers III and V, as seen at higher magnification of the rectangular inset in (D); layer IV can be distinguished as a non-labeled band of cortex below layer III and above the clusters of big pyramids of Betz in layer V. (D) Adjacent section to (A) stained for SMI-32; label is denser in area 4 than in the cingulate cortex, situated above the corpus callosum (cc) and somatosensory area 3. (E) High power view of (B) shows some large Betz cells (black arrowheads); layer IV is populated with neurons of several sizes and shapes, including some large and deeply stained pyramids (black arrows) which are displaced layer III or V pyramids. (F) Some of these large pyramids in layer IV (black arrows) found above the Betz cells (black arrowheads) are stained for SMI-32. Cd: caudate nucleus.
Figure 2.
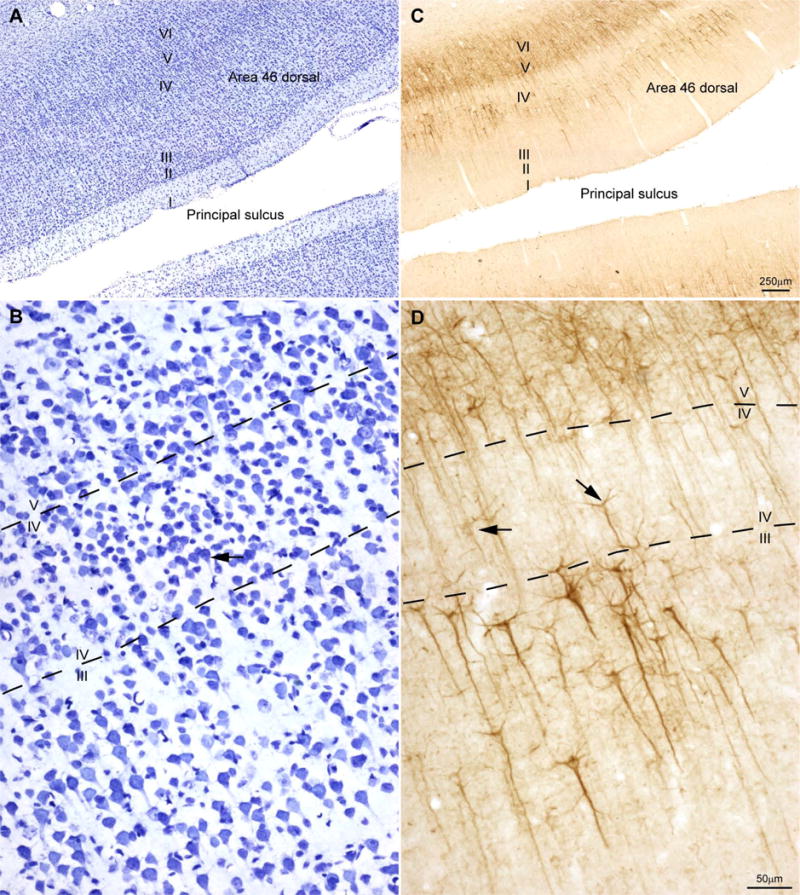
Laminar architecture of area 46. (A) Area 46 is eulaminate cortex with a well-developed granular layer IV (Nissl stain). (B) A higher power view of (A); neurons in layer IV appear densely packed and most are granular, but some are larger pyramids (black arrow). (C) SMI-32 labeled neurons are seen in the deep part of layer III and in layers V and VI. (D) Some large pyramids in layer IV are stained for SMI-32 (black arrows).
How to identify a layer that many believe does not exist
The challenge is to identify layer IV in area 4 for analysis. In Nissl stained sections through area 4, in between the largest pyramidal neurons in layer III and the clusters of Betz cells in layer V there is a thin and pale band of tissue that corresponds to layer IV (Fig. 1B) (Gatter & Powell, 1978).
To clearly delineate layer IV we also used tissue stained for SMI-32 (n=3 cases), an antibody for a non-phosphorylated intermediate neurofilament protein (Sternberger Monoclonals; Lutherville, MD, USA). In the primate cortex SMI-32 labels a subset of pyramidal projection neurons in layers III and V, and to a lesser extent pyramidal neurons in layers II and VI (Campbell & Morrison, 1989). Labeled neurons are especially prominent at the bottom part of layer III and the upper part of layer V. The selective labeling of pyramidal projection neurons allows easy delineation of cortical layers, and in particular layer IV, where neurons do not stain for SMI-32 (Fig. 1C). This band of unstained tissue in area 4 is continuous with the similarly unstained band in ‘granular’ area 3 (Fig. 1D). The central band of cortex with unstained neurons is invaded focally by stained pyramidal neurons from the adjacent layers (Fig. 1E and F).
We also identified the borders of prefrontal area 46 for comparison in the same cases. Area 46 lies within the banks of the principal sulcus and adjacent cortex (Fig. 2A) and has six well-delineated layers, including a dense granular layer IV (Fig. 2B) (Barbas & Pandya, 1989). SMI-32 labels pyramidal neurons in layers III, V and VI and clearly delineates layer IV (Fig. 2C). The clear zone in area 46 is also invaded focally by stained pyramidal neurons from the adjacent layers, as in area 4, though to a lesser extent (Fig. 2D).
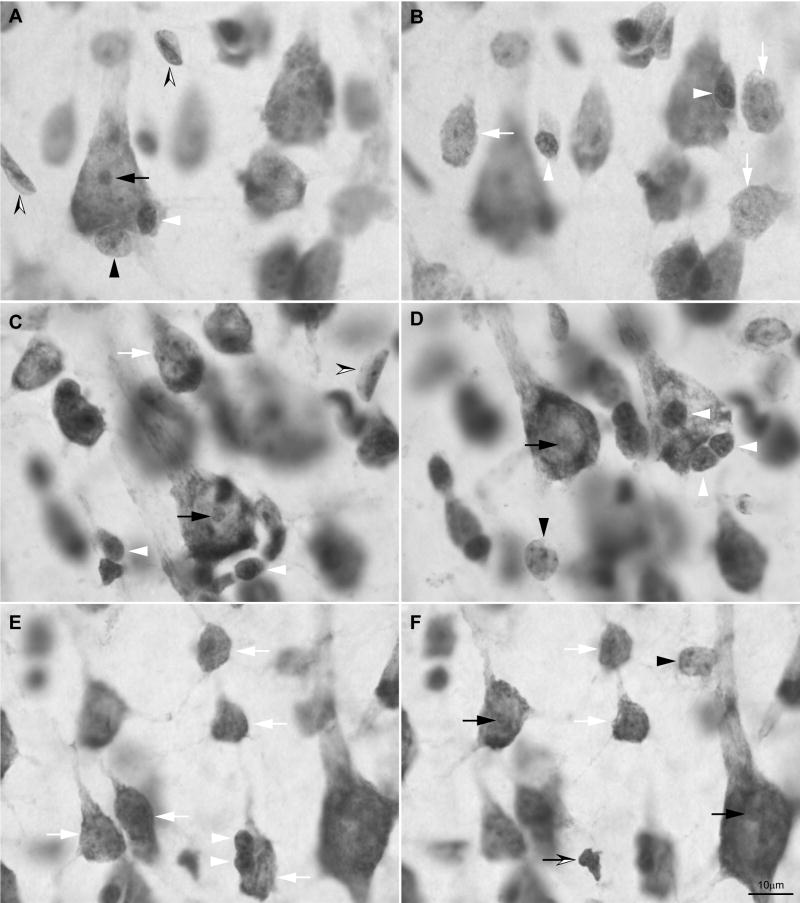
We used the above criteria to identify layer IV in areas 4 and 46. But there is yet another way to identify clearly neurons that reside in layer IV in the cortex. The basis for this identification dates back to classical studies, which apparently have been forgotten by most. In his classic studies, Cajal distinguished the nuclear features of large projection neurons from small pyramids and other interneurons (Ramón y Cajal, 1896; 1899/2002). Briefly, large pyramidal neurons have a single, large, rounded and well-defined nucleolus. In contrast, small neurons, including small pyramids and small inhibitory neurons, have several nucleoli, or have two or more rod-like clumps of chromatin that may coalesce in the center of the nucleus. But small neurons do not show a clearly differentiated nucleolus. The cytoplasm of large (projection) and small (local) neurons is always stained. In the latter, the cytoplasm appears as a thin stripe around the nucleus but the pyramidal or oval shape of the cell can always be identified (Fig. 3).
Figure 3.
Morphological features of neurons and glial cells in layer IV of area 4 stained for Nissl showing two planes of focus in three different fields (A–B, C–D, E–F). Large pyramids (projection neurons) have a single rounded nucleolus (black arrows in A, C, D & F). Small neurons (local interneurons) have several clumps of chromatin (white arrows in B, C, E & F). Astrocytes have an oval-shape nucleus with slightly irregular nuclear membrane (black arrowheads in A, D & F); some are neuron satellites, as in (A). Oligodendrocytes have a deeply stained rounded nucleus with clumps of chromatin (white arrowheads in A, B, C, D & E), they are smaller than astrocytes, and have a smooth nuclear membrane; some are neuron satellites (A, D & E). The nuclei of microglial cells are deeply stained and can be polylobulated or have the shape of a coma (black and white arrow in F). Endothelial cells have rectangular to ovoid nuclei with smooth nuclear membrane, and two to four nucleoli. Endothelial nuclei are usually curved to the shape of the blood vessels with which they are associated (black and white arrowheads in A & C).
We distinguished two types of neurons using these classical cellular criteria to determine the composition of the clear central zone that we identified as layer IV in area 4, which was aided by tissue stained for SMI-32. We identified small and large neurons in Nissl stained tissue. One series of sections was labeled for SMI-32 and also counterstained for Nissl to label and sort out neurons in the inner ‘granular’ layer.
We also identified types of glia according to the classical descriptions of Cajal (1896; 1899/2002/1913) and del Río-Hortega (1919; 1921; 1928; 1932) as well as more recent workers (Ling et al., 1973; O’Kusky & Colonnier, 1982a; Gabbott & Stewart, 1987). Studies in the electron microscope in the monkey primary visual cortex yield estimates of neurons and glial cell types comparable to those obtained with the optical microscope without immunohistochemical markers (O’Kusky & Colonnier, 1982a; Peters et al., 1991). Astrocytes have rounded to oval nuclei, homogeneous nucleoplasm and several rounded clumps of heterochromatin, some of which are located under the slightly irregular nuclear membrane. Frequently, the cytoplasm has pigmented granular inclusions close to the nucleus. Oligodendrocytes have a smooth nuclear membrane and a few clumps of heterochromatin and round and darkly stained nuclei, which usually are smaller than in astrocytes. Microglia have darkly stained elongated or polylobular nuclei without visible heterochromatin and the cytoplasm does not stain; in some cases there are granular inclusions close to the lobulations of the nucleus. Finally, we distinguished endothelial cells, which are not glia; they have rectangular to ovoid nuclei, mold to the shape of blood vessels, have smooth nuclear membrane and two to four nucleoli, one usually larger than the rest (Fig. 3).
We estimated the number and density of neurons and glial cells in layer IV of area 4 and area 46 in Nissl-stained sections (n=4 cases) using the unbiased stereological method of the optical fractionator (Gundersen, 1986; Howard & Reed, 1998) with the aid of a commercial system (StereoInvestigator; MicroBrightField, Inc.), as described in previous studies (Barbas et al., 2005; Medalla & Barbas, 2006; García-Cabezas & Barbas, 2013). Briefly, we analyzed a minimum of four evenly spaced brain sections for each area per case using systematic random sampling of layer IV. The stereological data included volume calculation for layer IV, which takes into consideration the sampled area and thickness of each section. We used as guard zones the top and bottom of each section (minimum 2 μm for 15 μm sections after shrinkage) and measured the actual mounted section thickness using the program software at each counting site. The counting frame/disector size (area= 50×50 μm; height=5 μm) and grid spacing (150×150 μm) were set to employ a fraction to yield a coefficient of error of < 10%, as recommended (Gundersen, 1986; Howard & Reed, 1998). For the sparse microglial cells, the coefficient of error was < 20% as recommended for the monkey cerebral cortex (Peters et al., 2008). More than 400 cells were counted in each area analyzed per case.
Measures of neurons and glia in layer IV of areas 4 and 46
Figure 4 summarizes neuron density, glia/neuron ratio, proportions of types of glial cells, proportions of neuron types and their major transverse diameter in layer IV of areas 4 and 46 in four cases stained for Nissl (± standard deviation, sd). The density of neurons in layer IV (including both pyramidal neurons and interneurons) was considerably lower in area 4 than in area 46 (Fig. 4A) and the glia/neuron ratio was higher in area 4 than in area 46 (Fig. 4B). Among glial types, oligodendroglia predominated in layer IV of area 4 (50% ± 1.9 sd) over astroglia (41% ± 3.7 sd) and microglia (11% ± 0.8 sd). In layer IV of area 46 the most abundant glial type was astroglia (62% ± 4.6 sd), followed by oligodendroglia (22% ± 1.5 sd), and microglia (16% ± 2.9 sd; Fig. 4C). In one case processed for SMI-32 and counterstained for Nissl we estimated the number of neurons and glial cells. The densities of neuron and glia types in layer IV obtained with the aid of SMI-32 parcellation were in the range of the Nissl stained cases (data not shown in Fig. 4). In layer IV of area 4, neurons with several nucleoli (small neurons) made up more than half (57% ± 3 sd) of the total and the rest (41% ± 3.5 sd) had one nucleolus, which is characteristic of large pyramidal neurons (Fig. 4D). In area 46, the majority of layer IV neurons had several nucleoli (79% ± 6.4 sd), whereas larger neurons with one nucleolus made up a smaller proportion of the total (21% ± 6.4 sd; Fig. 4D).
Figure 4.
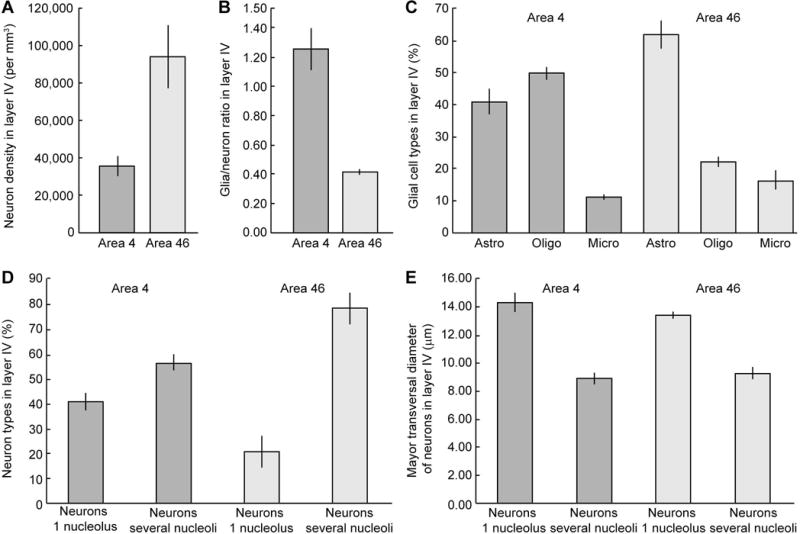
Neurons and glia in layer IV of areas 4 and 46. (A) Neuron density in layer IV of area 4 is less than half than in area 46. (B) Glia/neuron ratio is three fold higher in layer IV of area 4 than in area 46. (C) Oligodendrocytes are the most abundant type of glial cell in layer IV of area 4, while astrocytes are the most abundant type in layer IV of area 46. (D) Neurons with several clumps of chromatin or nucleoli (interneurons) are more frequent in layer IV of areas 4 and 46 than projection neurons, which have one big rounded nucleolus. In area 46 interneurons constitute the large majority in layer IV. (E) Projection neurons have larger major transverse diameter than interneurons in both areas. The parameter of dispersion in the graphs is standard deviation (sd). Astro, astrocytes; Micro, microglia; Oligo, oligodendrocytes.
We also measured the major transverse diameter of neurons. In layer IV of area 4, small neurons with several nucleoli had a smaller transverse diameter (8.88μm ± 0.41 sd) than larger pyramidal neurons with one nucleolus (14.27μm ± 0.69 sd). By comparison, in layer IV of area 46 the mean transverse diameter of small neurons with several nucleoli was a bit larger (9.2μm ± 0.42 sd), and neurons with a single nucleolus were a bit smaller (13.36μm ± 0.25 sd) than in area 4, but overall these figures were comparable (Fig. 4E).
Classical and modern methods help see the forest for the trees
The controversy as to whether area 4 has layer IV in primates did not begin and end with Cajal or Brodmann, but persists to modern times, with some authors considering the area to be agranular [e.g., (Bailey & von Bonin, 1951; Matelli et al., 1991)] while others describe a layer IV (Gatter & Powell, 1978; Meyer, 1987). Some authors propose that area 4 is secondarily agranular because it loses layer IV postnatally (Zilles, 1991). Here we provide evidence that area 4 has layer IV using unbiased quantitative methods. This was achieved using classical criteria to differentiate small neurons found in layer IV from larger neurons that protrude into layer IV from the layers above and below. In addition, the process of sorting out neurons that belong to layer IV was aided with the use of the molecular marker SMI-32. This marker labels pyramidal projection neurons especially in the deep part of layer III and the upper part of layer V, and thus brings into high relief the clear space occupied by unstained small neurons in layer IV. Most neurons in layer IV of area 4 are local neurons; they have short axons and form connections with neurons in the layers above or below, or in nearby columns, but their axons do not enter the white matter below (Ramón y Cajal, 1899). Layer IV is composed of small pyramidal neurons as well as inhibitory neurons, all of which are local neurons and collectively are called interneurons.
Thalamic input to area 4: more than just layer IV
One of the key principles concerning the status of layer IV in area 4 pertains to projections from the thalamus. It is generally assumed that layer IV is the key recipient of pathways from the thalamus, or the only recipient as text books frequently describe (Parent, 1996). The presumption that area 4 is agranular even led some to propose that area 4 does not receive strong projections from the thalamus (Amaral, 2000). But this idea is not supported by the general pattern of thalamocortical connections, or the projections to area 4.
The widely held view that layer IV is the sole target of thalamic projections emerged in part by analogy with the primary visual cortex, which receives pathways from the thalamus that target the highly specialized layer IV that has subdivisions, recognized in classical and modern studies (Wilson & Cragg, 1967; Hubel & Wiesel, 1972; Lund, 1973; Tigges et al., 1977; Livingstone & Hubel, 1982). But the primary visual cortex (V1 or area 17) is not the paradigmatic cortex, as is widely espoused. Experimental findings suggest that V1 is an exception rather than the rule in more ways than one. To begin with, in areas other than V1 (sensory, high-order association, or motor areas alike) thalamic pathways target not only layer IV, but also the bottom part of layer III and the upper part of layer V. These layers can be called for short the middle layers of the cortex. This pattern is consistent for the large majority of cortical areas in primates [see (Jones, 2007)]. With regard to area 4, in particular, studies show a strong projection from motor thalamic nuclei to layer III with patches in layer V (Jones, 1975; Sloper & Powell, 1979; McFarland & Haber, 2002). The thalamic pathway impinging on neurons in the upper part of layer V is thought to exert rapid and strong influence on neurons that give rise to the pyramidal tract and to the striatum (Jones et al., 1977; Jones & Wise, 1977).
There is also another thalamic pathway that runs in parallel to the pathway that terminates in the middle layers. The parallel pathway, which is often ignored in discussions of thalamic pathways, terminates expansively in layer I, but frequently also in the adjacent layer II and the upper part of layer III. This neglected but important thalamic pathway innervates widely the superficial band of cortex (Zikopoulos & Barbas, 2007). A comprehensive study that included not only thalamic pathways to the motor cortex but also to premotor cortices, showed that in macaque monkeys the most extensive thalamic terminations in the motor cortex are in layer I as well as layer III (McFarland & Haber, 2002). Thalamic input to the motor cortex and the adjacent premotor areas is thus unusually robust to the superficial band of cortex.
Taken together, these findings suggest that thalamic projections to the motor cortex are robust. It would indeed be surprising if the motor nuclei of the thalamus, which receive the output of the large structures of the basal ganglia and the cerebellum [reviewed in (Barbas et al., 2013)], did not project robustly to the primary motor cortex. Indeed, motor thalamic nuclei that receive the output of the cerebellum and the basal ganglia project strongly to area 4 (Holsapple et al., 1991; Hoover & Strick, 1999).
The inner granular layer IV in area 4 is prominent in development
Brodmann observed and described a prominent layer IV in development, which he strongly supported with photographic evidence (Fig. 5), a finding later confirmed by others (Conel, 1951; Marin-Padilla, 1970; Amunts et al., 1995). Layer IV appears in area 4 around the seventh month of gestation in humans and is well developed at birth. But in the first postnatal months, layer IV is invaded by pyramidal neurons from the adjacent layers III and V (Marin-Padilla, 1970; Amunts et al., 1995). Brodmann stated that all cortical areas start out with six layers, but some areas lose or gain layers later in development, and he provided the example of area 4 as a star case. Brodmann’s pronouncement that all cortices start out with six layers was likely influenced by the prevailing belief that ‘ontogeny recapitulates phylogeny’, which has since been questioned and discredited in modern evolutionary thought (Gould, 1977; Richardson et al., 1997).
Figure 5.
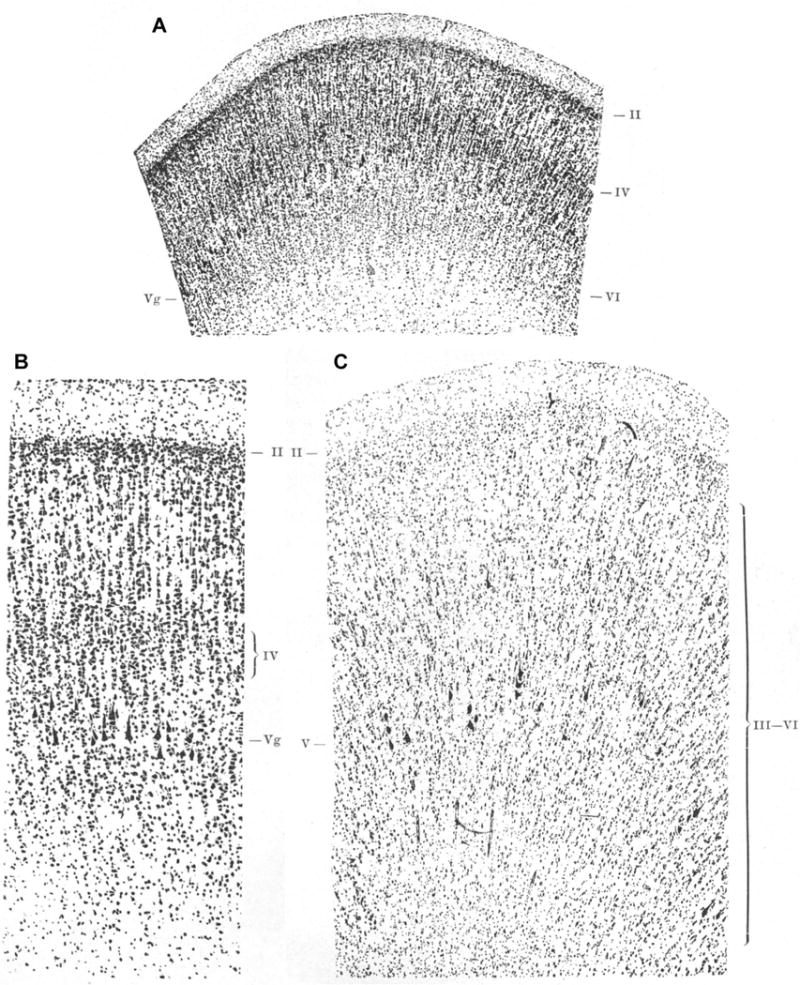
Area 4 has layer IV in the human brain during fetal life and disappears postnatally according to Brodmann. The photomicrographs appeared as Figures 5 (A, here), 6 (B, here), and 7 (C, here) in Brodmann’s monograph (1909); A and B show the primary motor cortex of a human fetus at 8 months of gestation shows an inner granular layer IV. According to Brodmann, layer IV disappears in postnatal life as is shown in C. [The figures were reproduced from the original stored at the Center for the History of Medicine in Francis A. Countway Library of Medicine, Harvard University, Boston, MA (Brodmann, 1909)].
The observations of Brodmann and other investigators–that there are changes in cortical layers in development–reflect cortical specialization. The primary motor cortex has a large and highly specialized layer V, as do neighboring premotor cortices, all of which contribute fibers to the pyramidal tract that innervates motor neurons (Dum & Strick, 2002). Our findings show that ‘granular’ area 46 indeed has a higher density of neurons in layer IV than the primary motor cortex, highlighting the specialization of this high-order association area. The prominence of layer IV is exaggerated in sensory areas, like V1 (area 17 of Brodmann), which has a highly specialized layer IV with several subdivisions. The primary sensory cortices receive input from peripheral organs through pathways from sensory relay thalamic nuclei.
The specialization of areas in postnatal life takes many forms: one is enrichment of the dendritic tree (Conel, 1951; Koenderink et al., 1994; Koenderink & Uylings, 1995), another is enlargement of cell size in some areas (Petanjek et al., 2008), which is prominently reflected in the giant pyramidal (Betz) neurons in layer V of area 4 (Marin-Padilla, 1970; Amunts et al., 1995). The normal postnatal development of neurons in area 4 is likely based on use, as suggested by a persistent and prominent granular layer IV in the brains of children with cerebral palsy, a pattern correlated with the severity of motor problems (Amunts et al., 1997). The developmental and pathological data suggest that after birth layer IV granule neurons of the primary motor cortex are pushed by the growth of pyramidal neurons and are obscured as they become interspersed between neurons of layers III and V, as described by Cajal (1899) and supported and expanded here. It is not known whether neurons in layer IV of area 4 undergo elimination at a higher rate than in other layers in development. Cell death during postnatal development is seen in some areas, such as the primary visual cortex, where it affects some layers more than others (O’Kusky & Colonnier, 1982b).
The cortex in evolution: not all areas have six layers
Brodmann’s first error on factual grounds was in dismissing the existence of layer IV in area 4 of adult primates. Brodmann’s second error was to view area 4 as agranular along with the anterior cingulate cortex, the retrosplenial cortex and the anterior insula (Brodmann, 1909/1999), an error in concept that led astray even astute investigators of cortical architecture (von Economo, 1927/2009; Beck, 1949; Bailey & von Bonin, 1951; Yakovlev, 1959). With the exception of area 4, the other areas are truly agranular–they belong to the group of limbic cortices that are ancient in phylogeny and have simpler laminar structure. Brodmann does not provide evidence that these areas too have a granular layer IV at any point in development.
Brodmann criticized Cajal for stating that some areas in primates resemble the simpler cortex of rodents. But this is precisely the point–the changes in cortical architecture can be seen within one brain and they are systematic. Since the classic work of Cajal, other students of cortical architecture observed variation in the structure of the cortex, and questioned Brodmann’s idea of a primitive six-layered cortex. Abbie, for example, suggested, as Dart did earlier (Dart, 1934), that the origin of the cortex can be traced to the hippocampus and to the primary olfactory cortex, which were thought to be the antecedent of the six-layered granular cortex (Abbie, 1940; 1942). The departure from Brodmann’s six-layer paradigm for the cortex was questioned by several other authors, who also noted changes in the number of layers across the cortical landscape [e.g. (Filimonoff, 1947; 1949/1965)].
A chief modern architect of the organization of cortical structure from an evolutionary perspective is Sanides, who extended and formalized the earlier work of Abbie and Dart. Sanides noted progressive laminar differentiation trends traced from the hippocampus and the primary olfactory areas through neighboring cortices that have fewer than six layers, and then to areas with progressively more elaborate laminar structure and an accentuated granular layer IV (Sanides, 1964; 1970). Perhaps because of the obscure writings of Sanides many dismissed the idea and essentially threw the baby out with the bath water. But Sanides was not alone in these observations [reviewed in (Pandya et al., 1988)].
As laminar structure becomes more elaborate so does myelination
There are other features that are consistent with the idea of gradual changes in the laminar structure of the cortex. A feature that has been used in classical and modern studies is the amount of myelin, which also increases along the axis of progressive lamination. Myelin is lightest in areas with the fewest layers, such as the limbic cortices, and highest in areas with the most elaborate laminar structure (Sanides, 1964; 1970). The primary motor cortex has a high myelin content, a far cry from the agranular insula and the anterior cingulate, which are poorly myelinated (Barbas & Pandya, 1987). Consistent with the pattern of myelination in area 4 is our finding of the predominance of oligodendrocytes among glial types, which myelinate neurons. Architectonic analysis shows that area 4 has the most specialized structure in the premotor/motor system. Area 4 is found at the other end of the spectrum than the anterior cingulate cortex, a limbic region that lies at the foot of laminar differentiation of the motor cortical system (Barbas & Pandya, 1987; Morecraft et al., 2012).
Confusing areas of different types: apples and oranges in one basket
The idea of systematic variation in cortical architecture is significant because it applies to all cortical systems—sensory and association areas alike. The fundamental idea is that the phylogenetically ancient limbic areas lie at the foot of every cortical system, and the gradual changes in architecture are observed in every system, including the cortical premotor-motor system (Sanides, 1964; Sanides & Krishnamurti, 1967; Sanides, 1970; Barbas & Pandya, 1987; Morecraft et al., 2012). The cortical motor system can be traced to the cingulate gyrus through areas now known as cingulate motor areas, and then through successive steps to medial and then lateral premotor areas, culminating in the primary motor cortex, or area 4.
The systematic variation in cortical lamination makes it possible to identify types of cortices rather than rely only on their specific features. Agranular limbic areas have three layers because they lack a granular layer IV and their superficial (II–III) and deep (V–VI) layers are not distinct. These areas lie near the primary olfactory areas and the hippocampus, as the classical students of architecture observed. Near the agranular areas, there is another type of limbic cortex characterized by a poorly developed layer IV—the dysgranular cortices. Nearby areas that have six layers belong to the general type of eulaminate cortex. But the latter too, show differences in laminar structure, with some areas having a specialized layer IV with subdivisions, as Brodmann and others observed for the primary visual cortex. Brodmann made a critical error in stating that area 4 is agranular along with the anterior cingulate. He grouped together areas of vastly different types, i.e. areas that are at the opposite spectrum with respect to cortical structure. In concluding that some areas differ in the number of layers they have, with some having more layers (e.g. the visual) or fewer in others (e.g. the anterior insula), Brodmann saw variation locally but missed the global principle, namely that the changes in cortical structure are systematic.
The concept of cortical type has provided the basis to predict the pattern in the laminar origin and termination of corticocortical connections, as first postulated for primates (Barbas, 1986; Barbas & Rempel-Clower, 1997; Rempel-Clower & Barbas, 2000; Barbas et al., 2005), and later shown that it applies to other species (Hilgetag & Grant, 2010). By analogy with the visual system, corticocortical connections have been called ‘feedforward’ when they originate in the upper layers (mostly layer III) of one area and terminate in the middle layers (including layer IV) of another area [reviewed in (Felleman & Van Essen, 1991)]. Viewed in the context of cortical type and the systematic variation in cortical architecture, ‘feedforward’ connections simply describe those that originate in an area with more elaborate laminar structure than the area of termination (Barbas & Rempel-Clower, 1997). Corticocortical connections have been called ‘feedback’ when they originate in the deep layers (V and VI) of one area and terminate in the upper layers (mostly layer I, but often also in layer II and the upper part of III) of another area. ‘Feedback’ connections more parsimoniously describe those that originate in an area with less elaborate laminar structure than the area of termination (Barbas & Rempel-Clower, 1997). Connections that link areas of the same type, whether they are neighboring or distant, involve most layers [they originate in all layers (except layer IV) of one area and terminate in all layers (including layer IV) of another area]. This principle, which we call the structural model for connections, is relational and based on the magnitude of the differences in laminar structure between linked cortices.
Within the cortical motor system, which includes area 6 (also known as the dorsal and ventral premotor areas), connections often involve several layers because the differences in structure among premotor areas are not large. The subtlety of the laminar patterns of connections within the motor-premotor system has led to approximate description more than thorough study [for review see (Shipp, 2005)]. But pathways from the premotor cortices to area 4 target more prominently layer I, consistent with the idea that area 4 is at the pinnacle of the graded changes in laminar structure of the cortical motor system (Barbas & Pandya, 1987). In conclusion, including area 4 in the same cortical type as agranular areas that never had a layer IV is a conceptual error and more consequential than failing to find a layer that is, after all, hidden in the rich forest of neuropil of the neurons above and below.
Acknowledgments
Protocols describing procedures were approved by the Institutional Animal Care and Use Committee at Harvard Medical School and Boston University School of Medicine in accordance with NIH guidelines (DHEW Publication no. [NIH] 80-22, revised 1996, Office of Science and Health Reports, DRR/NIH, Bethesda, MD, USA).
This work was supported by National Institutes of Health grants from the National Institute of Neurological Disorders and Stroke (R01NS024760) and the National Institute of Mental Health (R01MH057414); and by the Center of Excellence for Learning in Education, Science and Technology (CELEST), a National Science Foundation Science of Learning Center (NSF SBE-0354378). M. Á. García-Cabezas received a fellowship (Grant for Research in Foreign Universities and Centers) from Fundación Alfonso Martín Escudero (Spain).
References
- Abbie AA. Cortical lamination in the monotremata. J Comp Neurol. 1940;72:429–467. [Google Scholar]
- Abbie AA. Cortical lamination in a polyprotodont marsupial, perameles nasuta. J Comp Neurol. 1942;76:509–536. [Google Scholar]
- Akert K, Warren JM. The Frontal Granular Cortex and Behavior. McGraw-Hill; New York: 1964. [Google Scholar]
- Amaral DG. The Anatomical Orzanization of the Central Nervous System. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. McGraw-Hill; 2000. pp. 317–336. [Google Scholar]
- Amunts K, Istomin V, Schleicher A, Zilles K. Postnatal development of the human primary motor cortex: a quantitative cytoarchitectonic analysis. Anat Embryol (Berl) 1995;192:557–571. doi: 10.1007/BF00187186. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Zilles K. Persistence of layer IV in the primary motor cortex (area 4) of children with cerebral palsy. J Hirnforsch. 1997;38:247–260. [PubMed] [Google Scholar]
- Bailey P, von Bonin G. The Isocortex of Man. University of Illinois Press; Urbana: 1951. [Google Scholar]
- Barbas H. Pattern in the laminar origin of corticocortical connections. J Comp Neurol. 1986;252:415–422. doi: 10.1002/cne.902520310. [DOI] [PubMed] [Google Scholar]
- Barbas H, García-Cabezas MA, Zikopoulos B. Frontal-thalamic circuits associated with language. Brain Lang. 2013;126:49–61. doi: 10.1016/j.bandl.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Medalla M, Alade O, Suski J, Zikopoulos B, Lera P. Relationship of prefrontal connections to inhibitory systems in superior temporal areas in the rhesus monkey. Cereb Cortex. 2005;15:1356–1370. doi: 10.1093/cercor/bhi018. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. J Comp Neurol. 1987;256:211–218. doi: 10.1002/cne.902560203. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Barbas H, Rempel-Clower N. Cortical structure predicts the pattern of corticocortical connections. Cereb Cortex. 1997;7:635–646. doi: 10.1093/cercor/7.7.635. [DOI] [PubMed] [Google Scholar]
- Beck E. A cytoarchitectural investigation into the boundaries of cortical areas 13 and 14 in the human brain. J Anat. 1949;83:147–157. [PubMed] [Google Scholar]
- Bevan Lewis W. Researches on the Comparative Structure of the Cortex Cerebri. Phil Trans R Soc Lond. 1880;171:35–64. [Google Scholar]
- Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Verlag von Johann Ambrosius Barth; Leipzig: 1909. [Google Scholar]
- Brodmann K. Brodmann’s Localisation in the Cerebral Cortex. Translated from German by Laurence J Garey. Imperial College Press; London: 1909/1999. [Google Scholar]
- Campbell MJ, Morrison JH. Monoclonal antibody to neurofilament protein(SMI-32) labels a subpopulation of pyramidal neurons in the human and monkey neocortex. J Comp Neurol. 1989;282:191–205. doi: 10.1002/cne.902820204. [DOI] [PubMed] [Google Scholar]
- Conel JL. The Postnatal Development of the Human Cerebral Cortex IV The Cortex of the Six-Month Infant. Harvard University Press; Cambridge, MA: 1951. [Google Scholar]
- Dart RA. The dual structure of the neopallium: Its history and significance. J Anat. 1934;69:3–19. [PMC free article] [PubMed] [Google Scholar]
- del Río-Hortega P. El tercer elemento de los centros nerviosos. I La microglía en estado normal. Bol Soc Esp Biol. 1919;9:68–169. [Google Scholar]
- del Río-Hortega P. Estudios sobre la neuroglía. La glía de escasas radiaciones (oligodendroglía) Bol R Soc Esp Hist Nat. 1921;21:63–92. [Google Scholar]
- del Río-Hortega P. Tercera aportación al conocimiento morfológico e interpretación funcional de la oligodendroglía. Mem R Soc Esp Hist Nat. 1928;14:5–122. [Google Scholar]
- del Río-Hortega P. Microglia. In: Penfield W, editor. Cytology and Cellular Pathology of the Nervous System. Paul B. Hoebner Inc.; New York: 1932. pp. 481–534. [Google Scholar]
- Dum RP, Strick PL. Motor areas in the frontal lobe of the primate. Physiol Behav. 2002;77:677–682. doi: 10.1016/s0031-9384(02)00929-0. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Filimonoff IN. A rational subdivision of the cerebral cortex. Arch Neurol Psychiat. 1947;58:296–311. doi: 10.1001/archneurpsyc.1947.02300320047002. [DOI] [PubMed] [Google Scholar]
- Filimonoff IN. Comparative anatomy of the cerebral cortex of mammals. Paleocortex, archicortex and interstitial cortex. Translated from Russian by Victor Dukoff. U. S. Department of Commerce, Clearinghose for Federal and Technical Information, Joint Publications Research Service; Washington, D. C.: 1949/1965. [Google Scholar]
- Gabbott PL, Stewart MG. Distribution of neurons and glia in the visual cortex (area 17) of the adult albino rat: a quantitative description. Neuroscience. 1987;21:833–845. doi: 10.1016/0306-4522(87)90040-6. [DOI] [PubMed] [Google Scholar]
- García-Cabezas MA, Barbas H. A direct anterior cingulate pathway to the primate primary olfactory cortex may control attention to olfaction. Brain Struct Funct. 2013 doi: 10.1007/s00429-013-0598-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatter KC, Powell TPS. The intrinsic connections of the cortex of area 4 of the monkey. Brain. 1978;101:513–541. doi: 10.1093/brain/101.3.513. [DOI] [PubMed] [Google Scholar]
- Geyer S, Matelli M, Luppino G, Zilles K. Functional neuroanatomy of the primate isocortical motor system. Anat Embryol (Berl) 2000;202:443–474. doi: 10.1007/s004290000127. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Neural interaction between the basal forebrain and functionally distinct prefrontal cortices in the rhesus monkey. Neuroscience. 2001;103:593–614. doi: 10.1016/s0306-4522(00)00585-6. [DOI] [PubMed] [Google Scholar]
- Gould SJ. Ontogeny and Phylogeny. The Belknap Press of Harvard University Press; Cambridge MA: 1977. [Google Scholar]
- Gundersen HJ. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc. 1986;143(Pt 1):3–45. [PubMed] [Google Scholar]
- Hilgetag CC, Grant S. Cytoarchitectural differences are a key determinant of laminar projection origins in the visual cortex. Neuroimage. 2010;51:1006–1017. doi: 10.1016/j.neuroimage.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Holsapple JW, Preston JB, Strick PL. The origin of thalamic inputs to the “hand” representation in the primary motor cortex. J Neurosci. 1991;11:2644–2654. doi: 10.1523/JNEUROSCI.11-09-02644.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover JE, Strick PL. The organization of cerebellar and basal ganglia outputs to primary motor cortex as revealed by retrograde transneuronal transport of herpes simplex virus type 1. J Neurosci. 1999;19:1446–1463. doi: 10.1523/JNEUROSCI.19-04-01446.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard CV, Reed MG. Unbiased Stereology, Three-dimensional Measurement in Microscopy. BIOS Scientific Publishers Limited; Oxford: 1998. [Google Scholar]
- Hubel DH, Wiesel TN. Laminar and columnar distribution of geniculo-cortical fibers in the macaque monkey. J Comp Neurol. 1972;146:421–450. doi: 10.1002/cne.901460402. [DOI] [PubMed] [Google Scholar]
- Jones EG. Lamination and differential distribution of thalamic afferents within the sensory-motor cortex of the squirrel monkey. J Comp Neurol. 1975;160:167–203. doi: 10.1002/cne.901600203. [DOI] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. Cambridge University Press; New York: 2007. [Google Scholar]
- Jones EG, Coulter JD, Burton H, Porter R. Cells of origin and terminal distribution of corticostriatal fibers arising in the sensory-motor cortex of monkeys. J Comp Neurol. 1977;173:53–80. doi: 10.1002/cne.901730105. [DOI] [PubMed] [Google Scholar]
- Jones EG, Wise SP. Size, laminar and columnar distribution of efferent cells in the sensory-motor cortex of monkeys. J Comp Neurol. 1977;175:391–438. doi: 10.1002/cne.901750403. [DOI] [PubMed] [Google Scholar]
- Koenderink MJ, Uylings HB. Postnatal maturation of layer V pyramidal neurons in the human prefrontal cortex. A quantitative Golgi analysis. Brain Res. 1995;678:233–243. doi: 10.1016/0006-8993(95)00206-6. [DOI] [PubMed] [Google Scholar]
- Koenderink MJ, Uylings HB, Mrzljak L. Postnatal maturation of the layer III pyramidal neurons in the human prefrontal cortex: a quantitative Golgi analysis. Brain Res. 1994;653:173–182. doi: 10.1016/0006-8993(94)90387-5. [DOI] [PubMed] [Google Scholar]
- Ling EA, Paterson JA, Privat A, Mori S, Leblond CP. Investigation of glial cells in semithin sections. I Identification of glial cells in the brain of young rats. J Comp Neurol. 1973;149:43–71. doi: 10.1002/cne.901490104. [DOI] [PubMed] [Google Scholar]
- Livingstone M, Hubel DH. Thalamic inputs to cytochrome oxidase-rich regions in monkey visual cortex. Proc Natl Acad Sci USA. 1982;79:6098–6101. doi: 10.1073/pnas.79.19.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund JS. Organization of neurons in the visual cortex, area 17, of the monkey (Macaca mulatta) J Comp Neurol. 1973;147:455–496. doi: 10.1002/cne.901470404. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Prenatal and early postnatal ontogenesis of the human motor cortex: A golgi study. I The sequential development of the cortical layers. Brain Res. 1970;23:167–183. doi: 10.1016/0006-8993(70)90037-5. [DOI] [PubMed] [Google Scholar]
- Matelli M, Luppino G, Rizzolatti G. Architecture of superior and mesial area 6 and the adjacent cingulate cortex in the macaque monkey. J Comp Neurol. 1991;311:445–462. doi: 10.1002/cne.903110402. [DOI] [PubMed] [Google Scholar]
- McFarland NR, Haber SN. Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. J Neurosci. 2002;22:8117–8132. doi: 10.1523/JNEUROSCI.22-18-08117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalla M, Barbas H. Diversity of laminar connections linking periarcuate and lateral intraparietal areas depends on cortical structure. Eur J Neurosci. 2006;23:161–179. doi: 10.1111/j.1460-9568.2005.04522.x. [DOI] [PubMed] [Google Scholar]
- Meyer G. Forms and spatial arrangement of neurons in the primary motor cortex of man. J Comp Neurol. 1987;262:402–428. doi: 10.1002/cne.902620306. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Stilwell-Morecraft KS, Cipolloni PB, Ge J, McNeal DW, Pandya DN. Cytoarchitecture and cortical connections of the anterior cingulate and adjacent somatomotor fields in the rhesus monkey. Brain Res Bull. 2012;87:457–497. doi: 10.1016/j.brainresbull.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Kusky J, Colonnier M. A laminar analysis of the number of neurons, glia, and synapses in the visual cortex (area 17) of adult macaque monkeys. J Comp Neurol. 1982a;210:278–290. doi: 10.1002/cne.902100307. [DOI] [PubMed] [Google Scholar]
- O’Kusky J, Colonnier M. Postnatal changes in the number of neurons and synapses in the visual cortex (area 17) of the macaque monkey: a stereological analysis in normal and monocularly deprived animals. J Comp Neurol. 1982b;210:291–306. doi: 10.1002/cne.902100308. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B, Barbas H. Input-output organization of the primate cerebral cortex. In: Steklis HD, Erwin J, editors. Comparative Primate Biology, Vol 4: Neurosciences. Alan R. Liss; New York: 1988. pp. 39–80. [Google Scholar]
- Parent A. Carpenter’s human neuroanatomy. Williams & Wilkins; Baltimore: 1996. [Google Scholar]
- Petanjek Z, Judas M, Kostovic I, Uylings HB. Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: a layer-specific pattern. Cereb Cortex. 2008;18:915–929. doi: 10.1093/cercor/bhm124. [DOI] [PubMed] [Google Scholar]
- Peters A, Josephson K, Vincent SL. Effects of aging on the neuroglial cells and pericytes within area 17 of the rhesus monkey cerebral cortex. Anat Rec. 1991;229:384–398. doi: 10.1002/ar.1092290311. [DOI] [PubMed] [Google Scholar]
- Peters A, Verderosa A, Sethares C. The neuroglial population in the primary visual cortex of the aging rhesus monkey. Glia. 2008;56:1151–1161. doi: 10.1002/glia.20686. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. Myelo- and cytoarchitecture of the granular frontal cortex and surrounding regions in the strepsirhine primate Galago and the anthropoid primate Macaca. J Comp Neurol. 1991;310:429–474. doi: 10.1002/cne.903100402. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Estructura del protoplama nervioso. Rev Trim Microg. 1896;1:1–30. [Google Scholar]
- Ramón y Cajal S. Estudios sobre la corteza cerebral humana. II La corteza motriz del hombre y mamíferos superiores. Rev Trim Microg. 1899;4:117–200. [Google Scholar]
- Ramón y Cajal S. Textura del sistema nervioso del hombre y de los vertebrados. Tomo I. Gobierno de Aragón. Departamento de Cultura y Turismo; Zaragoza: 1899/2002. [Google Scholar]
- Ramón y Cajal S. Estudios sobre la corteza cerebral humana. IV Estructura de la corteza cerebral olfativa del hombre y mamíferos. Trab Lab Invest Biol. 1901–1902;1:1–140. [Google Scholar]
- Ramón y Cajal S. Textura del sistema nervioso del hombre y de los vertebrados. Tomo II. Gobierno de Aragón. Departamento de Cultura y Turismo; Zaragoza: 1904/2002. [Google Scholar]
- Ramón y Cajal S. Contribución al conocimiento de la neuroglia del cerebro humano. Trab Lab Invest Biol. 1913;11:255–315. [Google Scholar]
- Rempel-Clower NL, Barbas H. The laminar pattern of connections between prefrontal and anterior temporal cortices in the rhesus monkey is related to cortical structure and function. Cereb Cortex. 2000;10:851–865. doi: 10.1093/cercor/10.9.851. [DOI] [PubMed] [Google Scholar]
- Richardson MK, Hanken J, Gooneratne ML, Pieau C, Raynaud A, Selwood L, Wright GM. There is no highly conserved embryonic stage in the vertebrates: implications for current theories of evolution and development. Anat Embryol (Berl) 1997;196:91–106. doi: 10.1007/s004290050082. [DOI] [PubMed] [Google Scholar]
- Sanides F. The Cyto-myeloarchitecture of the Human Frontal Lobe and its Relation to Phylogenetic Differentiation of the Cerebral Cortex. J Hirnforsch. 1964;7:269–282. [PubMed] [Google Scholar]
- Sanides F. Functional architecture of motor and sensory cortices in primates in the light of a new concept of neocortex evolution. In: Noback CR, Montagna W, editors. The Primate Brain: Advances in Primatology. Appleton-Century-Crofts Educational Division/Meredith Corporation; New York: 1970. pp. 137–208. [Google Scholar]
- Sanides F, Krishnamurti A. Cytoarchitectonic subdivisions of sensorimotor and prefrontal regions and of bordering insular and limbic fields in slow loris (Nycticebus coucang coucang) J Hirnforsch. 1967;9:225–252. [PubMed] [Google Scholar]
- Shipp S. The importance of being agranular: a comparative account of visual and motor cortex. Philos Trans R Soc Lond B Biol Sci. 2005;360:797–814. doi: 10.1098/rstb.2005.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloper JJ, Powell TP. An experimental electron microscopic study of afferent connections to the primate motor and somatic sensory cortices. Philos Trans R Soc Lond B Biol Sci. 1979;285:199–226. doi: 10.1098/rstb.1979.0005. [DOI] [PubMed] [Google Scholar]
- Tigges J, Tigges M, Perachio AA. Complementary laminar terminations of afferents to area 17 originating in area 18 and in the lateral geniculate nucleus in squirrel monkey. J Comp Neurol. 1977;176:87–100. doi: 10.1002/cne.901760106. [DOI] [PubMed] [Google Scholar]
- von Economo C. The Cytoarchitectonics of the Human Cerebral Cortex. Translated from German by Lazaros C Thriarhou. Karger; Basel: 1927/2009. [Google Scholar]
- Wilson ME, Cragg BG. Projections from the lateral geniculate nucleus in the cat and monkey. J Anat. 1967;101:677–692. [PMC free article] [PubMed] [Google Scholar]
- Yakovlev PI. Pathoarchitectonic studies of cerebral malformations. III Arrhinencephalies (holotelencephalies) J Neuropathol Exp Neurol. 1959;18:22–55. doi: 10.1097/00005072-195901000-00003. [DOI] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Parallel driving and modulatory pathways link the prefrontal cortex and thalamus. PLoS One. 2007;2:e848. doi: 10.1371/journal.pone.0000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K. Cortex. In: Paxinos G, editor. The Human Nervous System. Academic Press, Inc.; San Diego: 1991. pp. 757–802. [Google Scholar]