Abstract
OBJECTIVES/HYPOTHESIS
Perform a longitudinal description of swallowing function following transoral laser microsurgery (TLM) +/−adjuvant therapy for advanced-stage oropharyngeal cancer (OPC) and identify factors associated with persistently poor swallowing function.
STUDY DESIGN
Retrospective analysis and descriptive study of swallowing outcomes.
METHODS
Patients treated with TLM for AJCC stage III-IV OPC at Washington University from 1996 to 2008 were included. A search of medical records and direct patient contact were performed to obtain swallowing function at multiple time points. Persistently poor swallowing at 2-years after surgery was the primary outcome measure.
RESULTS
118 patients met criteria for the study (median follow-up 53.9 months). T-stages were T1–44;T2–41;T3–23;T4–10. 55% received radiotherapy and 48% received chemoradiotherapy. 98% underwent neck dissection. Patients tolerated TLM well with 82% enjoying good swallowing at 1-month after surgery. This dropped to 55% at 3-months, during adjuvant therapy. At 1 and 2-years after TLM, 89% and 88% of patients had good swallowing function, respectively. At 2-years, 9 patients had persistently poor swallowing function. T-stages 3 or 4 were significantly associated with persistently poor swallowing function(P=0.019). Preexisting comorbidities and conversion to an open procedure were associated with delayed return of swallowing function, but not with persistently poor swallowing. 7 patients developed late-onset swallowing dysfunction. 2-year swallowing outcomes stratified by tumor site and T-stage are presented.
CONCLUSION
Treatment of advanced stage OPC with TLM+/−adjuvant therapy results in excellent swallowing outcomes. T-stages 3 or 4 were associated with persistently poor swallowing. A detailed, longitudinal swallowing profile is presented to assist in preoperative counseling.
Keywords: Transoral laser microsurgery, TLM, tonsil, base of tongue, oropharynx, advanced stage oropharyngeal cancer, chemoradiotherapy, FOSS, dysphagia, swallow
INTRODUCTION
Oropharyngeal (OP) cancer has been increasing in prevalence over the past decade.1 There are two main treatment options for advanced stage OP cancer: 1) surgical +/− adjuvant treatment or 2) definitive chemoradiotherapy. In order to avoid surgical resections, the treatment paradigm for OP cancer has shifted towards primary chemoradiotherapy. However, minimally invasive surgical approaches, including transoral laser microsurgery (TLM), have been gaining momentum in Europe and North America, based on excellent oncologic and functional outcomes.2,3 Transoral laser microsurgery utilizes intraoperative magnification to visualize tumor-host interfaces and facilitates full resection of malignant tissue while minimizing excision of normal, functioning tissue. It therefore avoids dismantling the musculoskeletal structure of the neck, oral cavity, and pharynx to provide surgical access. For these reasons, it is postulated that patients treated with TLM will retain swallowing function.
However, for oncologic survival reasons, patients with advanced OP cancer appear to also require adjuvant therapy,4 which introduces further treatment interventions known to acutely5 and chronically6 decrease swallowing ability. To date there are limited data on swallowing function following TLM plus adjuvant therapy for advanced stage OP cancers. The objective of this study was to longitudinally analyze swallowing function, identify risk factors associated with persistently poor swallowing function, and provide data for preoperative counseling.
MATERIALS & METHODS
Recently we published survival outcomes and preliminary functional data for 84 patients undergoing TLM +/− adjuvant therapy for advanced OP cancer.2 This current study describes in detail the longitudinal swallowing function of those patients, as well as an additional 34 patients who reached the 2 year postoperative time point since that publication and have 2 years of swallowing data. As previously documented,2 patients were included in the study who had biopsy proven advanced stage OP cancer (American Joint Committee on Cancer [AJCC] stages III and IV), had not previously received definitive treatment, and were treated with TLM +/− adjuvant therapy with curative intent at Washington University between June 1996 and June 2008. Patients were included who had undergone diagnostic excisional neck biopsy, diagnostic tonsillectomy, or prior neck dissection by a department member, and were then referred to the senior author for definitive TLM management. Patients with a prior history of head and neck aerodigestive tract cancer or evidence of distant metastasis at presentation were excluded from the study. Prior approval from the Human Research Protection Office at Washington University School of Medicine was obtained.
Transoral Laser Microsurgery
Primary tumor site resection was performed using TLM techniques as previously described.2 In brief, access to the tumor was accomplished using modified laryngoscopes or gag style retraction devices. Magnification of the operative field was achieved with either an operating microscope or rod telescopes. If needed, the tumor was first debulked using monopolar cauterization. Then using a CO2 laser, the tumor was transected at multiple points to allow visualization of tumor depth/extension and the tumor/host interface. The tumor was then resected in specimens with at least a 1 cm margin. Frozen sections were utilized as needed to verify complete tumor extirpation. The majority of resection beds were allowed to heal by secondary intent, however larger defects were reconstructed using local flaps or microvascular free flaps at the surgeons' discretion.
Neck Dissections and Adjuvant Therapy
Neck dissections were performed based on the presence or clinical suspicion of cervical metastases. Levels 2 through 4 were removed in all dissections, with extended dissections performed as needed. Administration and localization of adjuvant therapy was determined at a multidisciplinary tumor board, based on pathologic and intraoperative findings. Adjuvant radiotherapy (RT) was administered as either two dimensional (2D) or intensity modulated radiation therapy (IMRT).
Post Operative Care
Most patients were allowed to take a liquid diet at postoperative days 1 or 2. Nasogastric tube (NGT) feeds were initiated for those who failed oral feeds or if there was a high clinical suspicion that the patient was at aspiration risk. Nasogastric tubes were removed at subsequent postoperative visits as patients were able to tolerate oral feeds. Criteria for placement of gastrostomy tube (G-tube) were prolonged dysphagia for > 2 weeks or loss of > 10% of initial body weight.
Evaluation of Swallowing Function
The Functional Outcome Swallowing Scale (FOSS)7 was used to quantify swallowing function (Table I). FOSS classifies swallowing function from 0 to 5, with 0 being normal function and 5 representing complete dependence on non-oral feeding. Stages 1 and 2 represent compensated swallow function with only episodic or daily symptoms of dysphagia, and are considered acceptable. Stages 3 to 5 represent decompensated swallowing function with varying degrees of non-oral feeding requirements. Medical records were searched for swallowing data and feeding tube usage throughout the entire treatment course and subsequent follow up period for each patient. Patients were also directly contacted to provide up-to-date swallowing function data. These data were then translated into FOSS stages. For analyses purposes, each swallowing data point was translated into binary values:
“Good swallowing,” if FOSS stages from 0-2, or
“Poor swallowing,” if FOSS stages from 3-5
Table I.
Functional Outcome Swallowing Scale (FOSS)7
| Stage | Symptoms |
|---|---|
| 0 | Normal function and asymptomatic. |
| 1 | Normal function with episodic or daily symptoms of dysphagia. |
| 2 | Compensated abnormal function manifested by considerable dietary modifications or prolonged mealtime (without weight loss or aspiration). |
| 3 | Decompensated abnormal function with weight loss of 10% of body weight over 6 months owing to dysphagia; or daily cough, gagging, or aspiration during meals. |
| 4 | Severely decompensated abnormal function with weight loss of 10% of body weight over 6 months owing to dysphagia; or severe aspiration with bronchopulmonary complications. Non-oral feeding for most nutrition. |
| 5 | Non-oral feeding for all nutrition. |
Two analyses were performed in this study: 1) longitudinal analyses of swallowing and 2) univariate and multivariate analyses of factors associated with persistently poor swallowing function.
Longitudinal Analyses of Swallowing
In order to provide a longitudinal overview of swallowing function, FOSS scores were evaluated at or near time points of 1, 3, 6, and 12 months after surgery, then followed by annual assessments up to 5 years. Swallowing function was categorized as good (FOSS 0 – 2) or poor (FOSS 3 – 5) for patients who were both living and had available swallowing data at a given time point. The percentage of good swallowing was calculated as the number of patients at a given time point with “good swallowing” (FOSS 0 - 2), divided by all patients with available data for that time point.
Patients were divided into three groups based on their swallowing function during the first 2 years after TLM:
Group 1: “Consistently good” always had FOSS stages 0 - 2 during first 2 years.
Group 2: “Recovered good swallowing” developed dysphagia (FOSS 3 – 5) during surgery or adjuvant therapy, but then recovered good swallowing (FOSS 0 – 2) within 2 years after surgery.
Group 3: “Persistently poor” always had FOSS stages 3 - 5 during first 2 years.
A fourth subset of patients included “late-onset swallowing dysfunction” which was defined as development of late dysphagia in patients who had maintained good swallowing function during or following cancer treatment. Patients with “persistently poor swallowing” were not considered late-onset.
Univariate/Multivariate Analyses of Swallowing
Univariate analyses were performed to assess the associations of various factors with patients' swallowing during the first 2 years following surgery. Two years after surgery was chosen as a time point because there appeared to be a stabilization of swallowing function by 2 years, and it largely avoided confounding factors due to late-onset swallowing dysfunction. Patients were divided into the previously described groups 1 – 3 for these analyses.
To evaluate the impact of variables on persistently poor swallowing function, group 3 was compared against groups 1 and 2. Significant and clinically relevant variables were then included in a multivariate analysis. Fisher's exact and Kruskal-Wallis tests were utilized as appropriate. All statistical tests were 2-tailed and a P value of 0.05 or less was considered significant. Odds ratios were calculated based on logistic regression models.
Comorbidities at the time of cancer diagnosis were captured using the Adult Comorbidity Evaluation 27 (ACE-27). The ACE-27 is a validated instrument that classifies preexisting comorbidities as either 0 (none), grade 1 (mild), grade 2 (moderate), or grade 3 (severe). A patient's overall status is defined as the highest single ranking ailment, except in the case where two or more grade 2 ailments occur in different organ systems, wherein grade 3 (severe) is designated.8,9,10
Tumor stage (T stage) and nodal stage (N stage) were determined by pathologic assessment. p16 status was determined by immunohistochemical staining as previously described.2
RESULTS
Patient Characteristics
One hundred and eighteen patients met criteria for analysis in the study. Of these, 115 were treated surgically by the senior author (BHH) and 3 were treated by another surgeon on faculty. The mean and median follow-up were 53.9 month and 47.5 months (range 2 - 138), respectively. One hundred and five patients (89%) had follow-up of 24 months or greater. Patient characteristics are described in Table II.
Table II.
Patient characteristics
| Characteristic | All Patients (N = 118) | Patients in Univariate/Multivariate Analyses* (N = 112) | ||
|---|---|---|---|---|
| Consistently Good (N = 65) | Recovered Swallowing (N = 38) | Persistently Poor (N = 9) | ||
| Age (years) | ||||
| Mean (range) | 55.8 (35-81) | 56.1 (35-81) | 54.5 (40-70) | 58.1 (48-74) |
| Sex (%) | ||||
| Male | 104 (88) | 55 (85) | 36 (95) | 8 (89) |
| Female | 14 (12) | 10 (15) | 2 (5) | 1 (11) |
| Tumor subsite (%) | ||||
| Base of tongue | 60 (51) | 34 (52) | 16 (42) | 6 (67) |
| Tonsil | 58 (49) | 31 (48) | 22 (58) | 3 (33) |
| AJCC stage (%) | ||||
| III | 19 (16) | 12 (18) | 4 (11) | 1 (11) |
| IV | 99 (84) | 53 (82) | 34 (89) | 8 (89) |
| T stage (%) | ||||
| T1 | 44 (37) | 33 (51) | 11 (29) | 0 |
| T2 | 41 (35) | 23 (35) | 15 (39) | 1 (11) |
| T3 | 23 (19) | 9 (14) | 7 (18) | 3 (33) |
| T4 | 10 (8) | 0 | 5 (13) | 5 (56) |
| N stage (%) | ||||
| N0 | 4 (3) | 2 (3) | 1 (3) | 0 |
| N1 | 16 (14) | 10 (15) | 3 (8) | 2 (22) |
| N2 | 92 (78) | 51 (78) | 31 (82) | 7 (78) |
| N3 | 6 (5) | 2 (3) | 3 (8) | 0 |
| Neck dissection (%) | ||||
| Unilateral | 101 (86) | 61 (94) | 32 (84) | 5 (56) |
| Bilateral | 15 (13) | 3 (5) | 6 (16) | 4 (44) |
| None | 2 (2) | 1 (2) | 0 | 0 |
| Converted to open+ (%) | ||||
| Yes | 5 (4) | 0 | 4 (11) | 1 (11) |
| Re-resections++ (%) | ||||
| Yes | 9 (8) | 6 (9) | 0 | 1 (11) |
| CN XII injury (%) | ||||
| Yes | 3 (3) | 0 | 0 | 2 (22) |
| Adjuvant therapy | ||||
| Radiotherapy | 55 (47) | 38 (58) | 15 (39) | 1 (11) |
| Chemoradiotherapy | 48 (41) | 17 (26) | 23 (61) | 7 (78) |
| None | 15 (13) | 10 (15) | 0 | 1 (11) |
| Type of radiation | ||||
| 2D | 13 (11) | 8 (12) | 3 (8) | 1 (11) |
| IMRT | 90 (76) | 47 (72) | 35 (92) | 7 (78) |
| No radiation | 15 (13) | 10 (15) | 0 | 1 (11) |
| Comorbidity scale** | ||||
| 0 (none) | 57 (48) | 36 (55) | 15 (39) | 4 (44) |
| 1 (mild) | 44 (37) | 24 (37) | 14 (37) | 3 (33) |
| 2 (moderate) | 9 (8) | 3 (5) | 5 (13) | 0 |
| 3 (severe) | 8 (7) | 2 (3) | 4 (11) | 2 (22) |
| Locoregional recurrence (%) | ||||
| Yes | 6 (5) | 2 (3) | 1 (3) | 2 (22) |
| p16 | ||||
| Positive | 103 (87) | 56 (86) | 35 (92) | 8 (89) |
| Negative | 6 (5) | 2 (3) | 2 (5) | 1 (11) |
| Unknown | 9 (8) | 7 (11) | 1 (3) | 0 |
| Tobacco use (%) | ||||
| Never | 41 (35) | 24 (37) | 14 (37) | 3 (33) |
| Ever | 74 (63) | 40 (62) | 22 (58) | 6 (67) |
| Unknown | 3 (3) | 1 (2) | 2 (5) | 0 |
| Alcohol consumption (ounces/week) (%) | ||||
| 0 - 32 | 49 (42) | 27 (42) | 15 (39) | 6 (67) |
| > 32 | 67 (57) | 37 (57) | 23 (61) | 2 (22) |
| Unknown | 2 (2) | 1 (2) | 0 | 1 (11) |
() represents % of that specific group. Not all groups add up to 100% due to averaging. T stage = tumor stage, N stage = nodal stage, CN = cranial nerve, 2D = two dimensional, IMRT = intensity modulated radiation therapy
6 patients excluded in uni- and multivariate analysis due to early death or lost to follow-up
per ACE-27 instrument, see text for explanation
conversion of the initial surgery to an open procedure for cancer clearance
second look surgery for revision of margins
Neck Dissection and Adjuvant Therapy
Neck dissections were performed in 116 patients (98%), with 86% of these patients undergoing bilateral neck dissections. Fifty-five patients received postoperative RT alone and 48 patients received postoperative chemoradiotherapy, for a total of 87% (N = 103) that received adjuvant therapy. The majority of patients undergoing RT received IMRT (87%). Ninety-three patients received RT to the primary site and bilateral necks, 8 patients received RT to the primary site and ipsilateral neck, and 2 received RT to the ipsilateral neck only. The median dosages to both the primary tumor and ipsilateral neck were 6600 cGy (range 4400 to 7000). The median dosage to the contralateral neck was 5600 cGy (range 3608 to 6600). Only 6 patients received an increased dosage of approximately 7000 cGy: to the primary site only in 4 patients, to both the primary site and ipsilateral neck in 1 patient, and to the ipsilateral neck in 1 patient. The main chemotherapeutic agent was cisplatin, with 2 patients receiving carboplatin, 2 receiving taxol, and 2 receiving cetuximab.
Longitudinal Analyses of Swallowing
Overview
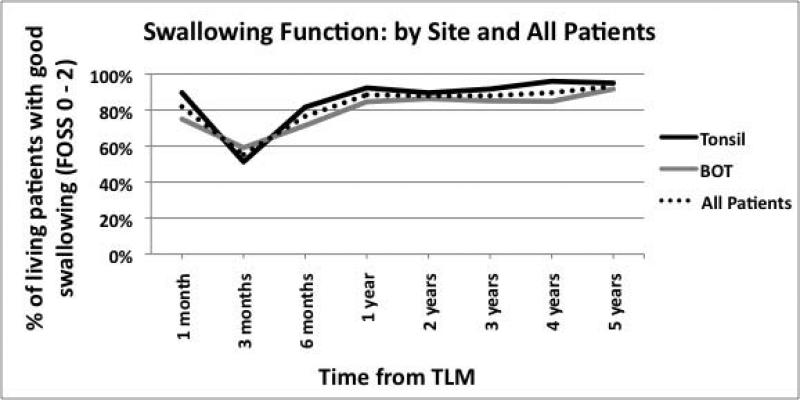
All 118 patients were included in the longitudinal analyses of swallowing. At 1 month following TLM (with or without neck dissection), 97 patients (82%) had good swallowing. At 3 months postoperatively, which coincides with the administration of adjuvant therapy, the percentage of patients with good swallowing dropped to 55% and rose to 89% by the end of 12 months. At 2, 3, 4, and 5 years after surgery, the percentages of good swallowing were 88%, 88%, 90%, and 93%, respectively (Figure 1A).
Fig. 1.
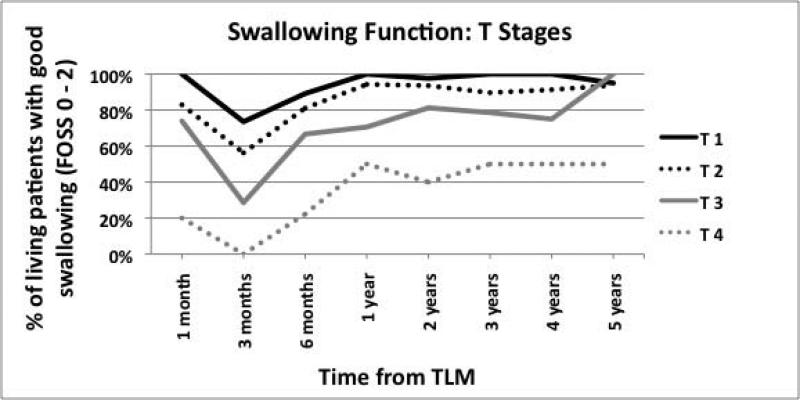
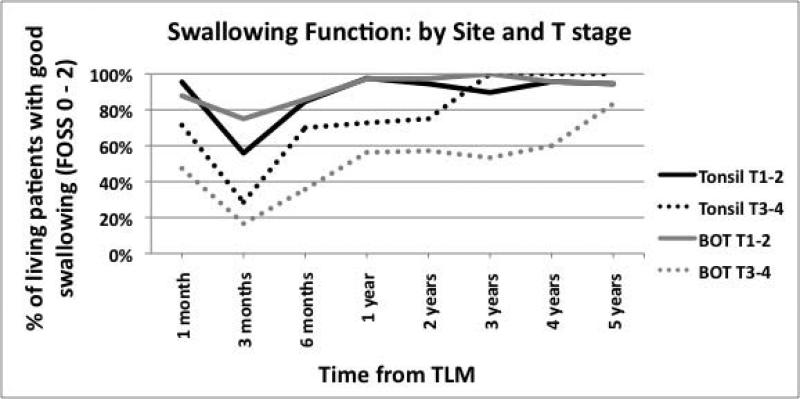
(A) Swallowing function of all patients and by tumor site (B) Swallowing function stratified by T stage (C) Swallowing function stratified by both site and T state.
Consistently Good Swallowing Function
Sixty-five patients (55%) maintained consistently good swallowing function at all time points during and immediately after their cancer treatment (see Table II for demographics of this group).
Recovered Swallowing Function
Thirty-eight patients (32%) had poor swallowing function during cancer treatment and then recovered to good swallowing function. Ten patients had poor swallowing immediately following surgery, which persisted through adjuvant therapy before improving, and 28 patients had good swallowing function following surgery and then developed poor swallowing function during adjuvant therapy administration, which eventually recovered. The mean time to recovering good swallow function was 7.2 months following surgery. At 12 months after surgery, 37 of the 38 patients had recovered swallowing function; with 1 outlying patient who recovered well swallowing function at 24 months.
When compared to patients who had consistently good swallowing, the recovered group tended to have more advanced tumor stages (T stage 3 – 4: 32% vs. 14%), more frequent conversions to an open procedure during the initial surgery (11% vs. 0%), greater rate of chemotherapy administration (61% vs. 26%), and more moderate to severe preexisting comorbidities (24% vs. 8%) (Table II).
Persistently Poor Swallowing Function
Nine patients (8%) had persistently poor swallowing function throughout the length of this study (Table III). Six of these patients experienced dysphagia immediately following surgery, which never improved; and 3 patients swallowed well after surgery, then developed dysphagia at 2, 3, and 11 months postoperatively, which never improved. Seven of the 9 patients received adjuvant chemoradiotherapy. Clinically intuitive reasons for persistent dysphagia were multifactorial for most patients, and included: radiation fibrosis (N = 3), trismus (N = 3), surgical pharyngeal scarring (N = 2), cancer recurrence (N = 2), cranial nerve XII injury (N = 2), RT-induced complete esophageal stricture (N = 1), extensive radionecrosis of the parapharyngeal space with resultant stroke (N = 1), and large treatment field requiring microvascular free flap reconstruction (N = 1).
Table III.
Characteristics of patients with persistently poor swallowing function and late-onset swallowing dysfunction
| Patient | Dysphagia type | Tumor site | Tumor stage | Nodal stage | Adjuvant therapy | Onset after TLM (mos) | Presumptive reasons for dysphagia |
|---|---|---|---|---|---|---|---|
| 1 | persistent | BOT | 4 | 2 | CRT | immediate | Post RT esophageal stricture |
| 2 | persistent | BOT | 4 | 2 | CRT | immediate | Surgical pharyngeal scarring, trismus |
| 3 | persistent | BOT | 3 | 2 | CRT | immediate | CN XII injury, radiation fibrosis, cancer recurrence |
| 4 | persistent | BOT | 4 | 1 | RT | immediate | Large treatment field requiring MFFR |
| 5 | persistent | BOT | 3 | 2 | CRT | immediate | Trismus, CN XII paralysis |
| 6 | persistent | BOT | 4 | 2 | CRT | immediate | Surgical and radiation induced scarring of pharynx, trismus |
| 7 | persistent | tonsil | 4 | 2 | CRT | 2 | Radiation fibrosis |
| 8 | persistent | tonsil | 2 | 2 | CRT | 3 | Extensive radionecrosis of parapharyngeal space, stroke |
| 9 | persistent | tonsil | 3 | 1 | CRT* | 11 | Cancer recurrence |
| 10 | delayed | tonsil | 4 | 2 | RT | 18 | Radiation fibrosis |
| 11 | delayed | BOT | 1 | 3 | RT | 22 | Radiation fibrosis |
| 12 | delayed | tonsil | 2 | 2 | CRT | 24 | Radiation fibrosis, laryngeal edema |
| 13 | delayed | BOT | 3 | 2 | None | 32 | Parkinson's disease |
| 14 | delayed | tonsil | 2 | 2 | RT | 41 | Distant cancer recurrence |
| 15 | delayed | BOT | 2 | 2 | RT | 45 | 2nd H&N primary requiring PLx and MFFR, initial cancer recurrence, large treatment field |
| 16 | delayed | tonsil | 1 | 2 | RT | 60 | 2nd H&N primary, post RT esophageal stricture |
BOT = base of tongue, RT = radiotherapy, CRT = chemoradiotherapy, CN = cranial nerve, H&N = head and neck, PLx = pharyngolaryngectomy, MFFR = microvascular free flap reconstruction, TLM = transoral laser microsurgery
received palliative CRT
Late-onset Swallowing Dysfunction
Seven patients (6%) developed late-onset swallowing dysfunction. The mean time to dysphagia was 34.3 months (range 18 to 60) (Table III). Of these patients, 4 had good swallowing during their cancer treatment and then developed late-onset dysfunction; and 3 had poor swallowing during treatment, recovered good swallowing function, then developed late-onset dysfunction. Presumptive reasons for late-onset dysphagia were often multifactorial and included: cancer recurrence (N = 2), development of second head and neck primary malignancy (N = 2), RT induced esophageal stricture (N = 1), diffuse RT fibrosis (N = 3), and Parkinson's disease (N = 1).
Effect of Tumor Stage and Site on Longitudinal Swallowing Function
Dividing patients by tumor site did not reveal much difference in swallowing function over time (Figure 1A). However, when patients were stratified by T stages, each incremental stage demonstrated worse swallowing function (Figure 1B). When stratified by both tumor site and T stage, patients with advanced BOT tumors had worse swallowing over time (Figure 1C).
Two-Year Swallowing Outcomes
The percentage of patients with good swallowing at 2 years after surgery was calculated and tabulated by tumor site and tumor stage, in order to facilitate preoperative counseling with patients regarding post-treatment swallowing expectations (Table IV). Patients with T stages 1 – 2 (both BOT and tonsil) and T stage 3 tonsil had high percentages of good swallowing at 2 years (86% to 100%). Patients with T stage 4 tumors, both BOT and tonsil, had the lowest percentages of good swallowing at 2 years, with 43% and 33%, respectively.
Table IV.
Percentage of living patients with good swallowing at 2 years after surgery – stratified by tumor site and tumor stage
| BOT | Tonsil | |
|---|---|---|
| T1 | 95 | 100 |
| T2 | 100 | 86 |
| T3 | 71 | 89 |
| T4 | 43 | 33 |
BOT = base of tongue, T = tumor stage
Analysis of Factors Affecting Swallow Function
Univariate Analysis
Patients were allocated to one of three groups based on their swallowing function during the first 2 years after TLM as described in the Materials and Methods section (characteristics of each group are detailed in Table II). Six patients who had less than 1 year of swallowing data were excluded (lost to follow-up N=2; death N=4), leaving 112 patients for analysis.
Group 1: Consistently good swallowing (65 patients, 58%),
Group 2: Recovered good swallowing (38 patients, 34%).
Group 3: Persistently poor swallowing (9 patients, 8%).
In order to identify factors associated with persistently poor swallowing function, patients from Group 3 were compared against all other patients using univariate analyses. Advanced T stage (3 or 4), undergoing bilateral neck dissections, and the administration of chemotherapy were significantly associated with persistently poor swallowing function (Table V). Age at time of surgery, tumor subsite (BOT vs tonsil), N stage, second look surgery for revision of margins, nor p16 positivity were associated with persistently poor swallowing function.
Table V.
Univariate and multivariate analyses for persistently poor swallowing
| Variable | Univariate | Multivariate | |
|---|---|---|---|
| P | OR (95% CI) | P | |
| Age | 0.40 | ||
| Bilateral neck dissections (yes vs. no) | 0.011 | 2.07 (0.29 - 14.57) | 0.47 |
| Chemotherapy received (yes vs. no) | 0.034 | 5.53 (0.45 - 68.66) | 0.18 |
| Comorbidity scale (2-3 vs 0-1)** | 0.61 | 1.67 (0.19 - 14.81) | 0.64 |
| Converted to open (yes vs. no)+ | 0.35 | 5.61 (0.17 - 187.80) | 0.34 |
| Cranial nerve XII injury (yes vs. no) | * | ||
| Free flap (yes vs. no) | * | ||
| Locoregional recurrence (yes vs. no) | 0.051 | 12.48 (0.61 - 255.57) | 0.10 |
| Re-resections (yes vs. no)++ | 0.45 | ||
| N stage (N2-3 vs N0-1) | 0.64 | ||
| p16 (positive vs negative) | 0.37 | ||
| RT modality (IMRT vs 2D) | >0.99 | ||
| RT to primary site (yes vs. no) | >0.99 | ||
| RT to ipsilateral neck (yes vs. no) | >0.99 | ||
| RT to contralateral neck (yes vs. no) | >0.99 | ||
| RT to all 3 sites (yes vs. no) | >0.99 | ||
| RT dosage to primary site | 0.67 | ||
| RT dosage to ipsilateral neck | 0.13 | ||
| RT dosage to contralateral neck | 0.14 | ||
| Tumor subsite (BOT vs tonsil) | 0.49 | 1.91 (0.27 - 13.46) | 0.52 |
| T stage (T3-4 vs. T1-2) | < 0.0001 | 15.38 (1.57 - 150.90) | 0.019 |
OR = odds ratio, BOT = base of tongue, RT = radiation therapy
Not sufficient events for accurate analysis T stage = tumor stage, N stage = nodal stage, CN = cranial nerve, 2D = two dimensional, IMRT = intensity modulated radiation therapy
per ACE-27 instrument, see text for explanation
conversion of the initial surgery to an open procedure for cancer clearance
second look surgery for revision of margins
The type of RT (2D vs IMRT) and dosage to each site (primary tumor, ipsilateral neck, and contralateral neck) were also analyzed and found to have no association with swallowing function. Administration of RT to each site were analyzed as independent variables, as well as RT given to all three sites concurrently, and none were found to be significantly associated with persistently poor swallowing function.
Locoregional recurrence achieved marginal significance (P = 0.051) with persistently poor swallowing. Due to the small numbers of hypoglossal nerve injuries (N = 2) and microvascular free flap reconstructions (during initial tumor resection) (N = 2), reliable statistical models could not be constructed to assess significance.
Conversion of the initial surgery to an open procedure was not associated with persistently poor swallowing function (all 5 cases consisted of transhyoid pharyngotomies to facilitate full cancer clearance). However, when comparing patients who never had difficulty swallowing (Group 1) to all other patients, conversion to open was significant (P = 0.011). This suggests that converting to an open procedure can delay return of normal swallowing function, but is not associated with permanent swallowing dysfunction.
Preexisting moderate or severe comorbidities (grades 2 or 3) were not associated with persistently poor swallowing function either. However, when comparing patients who never had difficulty swallowing (Group 1) to all other patients, having preoperative moderate or severe comorbidities had a significant association (P = 0.028). This suggests that comorbidities may have an effect on the time to return of swallowing function, but are not associated with persistent swallowing dysfunction.
Multivariate Analysis
Variables with significance in univariate analyses and of clinical importance were included in a multivariate analysis of persistently poor swallowing function (Table V). Having tumors of T stages 3 or 4 was significantly associated with persistent swallowing dysfunction at 2 years after surgery, with an odds ratio of 15.38 (P = 0.019; 95% confidence interval, 1.57-150.90).
DISCUSSION
With the recent rise in incidence of OP cancers,1 it is incumbent upon otolaryngologists to accurately diagnose, counsel, and treat patients with this disease. Given the unique anatomical location of these cancers in the upper aerodigestive tract, functional outcomes of treatments (such as swallowing) become nearly as important as survival outcomes. Poor swallowing function is a significant morbidity that affects many aspects of a patient's life, and the presence of a G-tube has been shown to strongly correlates with decreased quality of life.11 It is therefore essential for physicians to maximize good swallowing function following treatment.
Traditionally advanced OP cancers were treated with large, open surgical procedures that dismantled the musculoskeletal infrastructure of the oral cavity and pharynx, resulting in potential disfigurement and swallowing dysfunction. The 1991 Veterans Affairs Laryngeal Cancer Study Group12 and the Radiation Therapy Oncology Group (RTOG) 91-11 study13 generated popular interest in the non-surgical treatment of laryngeal cancer. Such “organ sparing” treatment paradigms soon spread to OP tumors in order to limit the aforementioned surgical comorbidities. However, with regards to advanced stage OP cancer, there have not been any significant, randomized studies comparing surgery and non-surgical treatments. Thus, it is still not know which treatment provides superior survival and functional outcomes. In addition, the concept of “organ sparing,” as it has now been applied to the larynx, has little similarity or application with oropharyngeal structures. Nevertheless, concurrent chemoradiotherapy has now become the main treatment of OP cancers in many centers. To add further complexity, human papilloma virus (HPV) related OP cancer is increasing in prevalence. This disease entity has been shown to have different tumor biology, treatment responses (to both surgical and non-surgical treatments), and survivorship when compared to non-HPV related OP cancers.2,14-16 Thus, primary chemoradiotherapy can not be assumed to be the “standard of care” for OP cancers at this time.
Transoral laser microsurgical approaches to upper aerodigestive tract tumors, such as described by Panje et al.17 in 1989 in the United States, have been performed for many years. Expansion of these techniques to treat larger laryngeal and OP tumors was popularized in Europe by Wolfgang Steiner and others.3 Transoral laser microsurgery is an endoscopic approach that utilizes the operating microscope or endoscopes to provide detailed visualization of the tissues. Our prior study and others show that TLM +/− adjuvant therapy for advanced OP cancer provides excellent survival outcomes and local control.2,18,19
It can be postulated that by not dismantling the musculoskeletal architecture, as in TLM, swallowing function should be less affected. However, there is limited data on swallowing function following TLM for advanced stage OP cancers. This study is novel in that it focuses on swallowing function in this particular population, details early and late swallowing function throughout the whole treatment process, and has long-term follow-up. Swallowing function at 2 years after surgery was chosen as the primary outcome for this study. Even though swallowing function changes considerably during treatment of OP cancers, it is the long term swallowing function that is most important clinically and to patients.
We show that, in general, resection of oropharyngeal tumors with TLM, neck dissections, and +/− adjuvant therapy results in good swallowing function. Specifically, patients' swallowing function was minimally affected by surgical resection of the primary tumor and neck dissections, as evidenced by 82% of patients enjoying good swallowing at 1 month post operative. Rather, it was the administration of adjuvant therapy which most severely affected swallowing function, as shown by a drop in good swallowing function to 55% at 3 months. However, most patients were able to recover from their treatments and at 1 year 89% of patients had good swallowing function.
The combined therapy of TLM plus adjuvant therapy is tolerated modestly well, as evidenced by 55% of patients never experiencing swallowing dysfunction. An additional 32% of patients experienced a temporary episode of poor swallowing, requiring feeding tube supplementation; but then returned to normal swallowing at a median of 7.2 months. Only 8% of patients were left with poor swallowing function at 2 years of follow up.
The pathogenesis of dysphagia and G-tube dependency following treatment of OP cancers is multifactorial, and is often derived from a combination of both the surgical and adjuvant therapies. Surgical resection of mucosa and/or nerves, especially at the BOT, results in insensate tissue surfaces. These tissues cannot sense the presence of food boluses nor initiate appropriate swallowing reflexes. In addition, deep resection of the BOT causes loss of tongue volume and potential difficulty propelling food boluses past the oropharynx.
Radiation therapy can cause acute complications that affect oral intake, such as mucositis, edema, xerostomia, altered taste, and contracture of denuded/inflamed mucosal surfaces. Delayed and progressive complications like pharyngeal or esophageal strictures, pharyngeal muscle dysfunction, diffuse fibrosis, and trismus also contribute to poor swallowing function. Radiation therapy also causes obliteration of the microvascularity of tissues, which predisposes to radionecrosis (i.e. of mandible or soft tissue). Extensive parapharyngeal space radionecrosis of one patient in our study resulted in thrombosis of the internal carotid artery and stroke, as well as significant tissue destruction necessitating free flap reconstruction. These effects are often accentuated with the addition of chemotherapy, resulting in more extensive side effects to surrounding normal tissue.20
In an attempt to identify pre-disposing factors that might be associated with poorer swallowing function, univariate and multivariate analyses were performed. In multivariate analysis, T stages 3 or 4 were found to be significantly associated with persistently poor swallowing at 2 years after surgery (odds ratio of 15.38 when compared to T stages 1 or 2). The confidence interval was quite wide at 1.57 to 150.90. This finding is clinically intuitive and is most likely due to resection of greater surface area and of important structures with higher stage tumors. Boosting RT to the primary site of these aggressive tumors and adding concurrent chemotherapy also plays a role in swallowing dysfunction.
Intuition would dictate that resection of BOT tumors would result in higher rates of swallowing dysfunction than resection of tonsil tumors. Nevertheless, tumor site was not associated with persistently poor swallowing in univariate or multivariate analysis.
Interestingly, age at the time of surgery was not associated with permanent swallowing dysfunction. The oldest patient in this series was an 81 year-old man who underwent TLM for a T stage 3 BOT cancer and maintained good swallowing postoperatively. Older patients tend to tolerate TLM better than chemoradiotherapy; thus in select elderly patients, surgery as a unimodality treatment may be considered.
Returning to the operating room for endoscopic re-resection to obtain negative margins was not associated with persistently poor swallowing outcomes. Conversion of the initial surgery to an open procedure (transhyoid pharyngotomies in this study) to obtain negative margins was associated with a delay in return to normal swallowing function. However, conversion to an open procedure was not associated with persistent swallowing dysfunction in the univariate or multivariate analyses. Thus, surgeons can be reassured that expanding the surgical resection to obtain negative margins, either by reresection or pharyngotomy, is not necessarily associated with persistently poor swallowing function for the patient.
Because most patients received adjuvant RT (87%) in this study, and the range of radiation dosages was very narrow, we did not detect an affect on swallowing by either the dosage of RT or locations of RT administered (primary site, ipsilateral neck, contralateral neck). Interestingly, there was also no difference in swallowing function between patients who received 2D or IMRT.
Chemotherapy acts synergistically with radiotherapy to enhance cancer cell death, but also accentuates the negative effects of radiotherapy to the surrounding normal tissue.20 This detrimental effect was observed in our study as 78% of patients with persistently poor swallowing received adjuvant chemotherapy. Though chemotherapy was significantly associated with poor swallowing function in the univariate analysis (P = 0.034), it was not significant in the multivariate analysis.
Adjuvant RT has been shown to improve survival following TLM,4 and in this study the majority of patients (87%) received adjuvant RT. One may argue that primary surgical resection does not minimize treatment morbidity if most patients still require adjuvant RT or chemoradiotherapy. However, the overall dosages of RT for adjuvant treatment are different that for definitive primary treatment. Most primary chemoradiotherapy protocols apply 7000 cGy to the primary site and involved lymph nodes,13,21,22 whereas in this study the median dosages for adjuvant RT to the primary site and ipsilateral neck were 6600 cGy, and 5600 cGy to contralateral neck. This may seem to be a nominal difference, but the probability of normal tissue complications with increasing RT dosages is sigmoidal rather than linear.23 Thus, decreasing overall RT dosages to adjuvant levels theoretically leads to lower risk of normal tissue complications.
Comparison of swallowing between primary surgery with adjuvant therapy and primary chemoradiotherapy is difficult due to conflicting reports. Tschudi et al.24 showed improved quality of life with surgery, Gillespie et al.25 suggest patients undergoing primary CRT have a better swallowing quality of life, whereas Mowry et al.26 showed no difference between these groups. Published G-tube dependency rates following TLM +/− adjuvant therapy for OP cancers range from 0% to 10%.19,27,28 This is in contrast to G-tube dependency rates of 15% to 25% following primary radiotherapy,29,30 and 18.1% to 51%, following primary chemoradiotherapy.6,29,31 Nevertheless, direct, prospective comparison of surgical and non-surgical treatment for advanced stage oropharyngeal cancers must be performed to clarify this issue.
Preoperative counseling is important to enable patients to make an educated treatment decision. Our findings support that T stage, which is a variable that can be estimated preoperatively, can be used to counsel patients regarding expected swallowing outcomes (Table IV). Based on our institution's experience, patients with BOT cancers of T stages 1 or 2, or with tonsil cancers of T stages 1, 2 or 3, have a high probability of good swallowing function at 2 years after surgery (86% to 100%). Patients with T stage 3 BOT tumors have intermediate chances of good swallowing at 2 years (71%). Lastly, patients with T stage 4 tumors, either BOT or tonsil, have the lowest probability of good swallowing at 2 years (43% and 33%, respectively). Likewise, in terms of preoperative counseling and planning, we found that moderate or severe preexisting comorbidities may predispose a patient to a delayed return in swallowing following cancer treatment. However, most patients with these comorbidities had return of good swallowing function, and there was no association with persistently poor swallowing.
Delayed-onset dysphagia is a significant concern, of which practitioners must be vigilant. Chemoradiotherapy has been shown to produce significant late toxicities 2 – 3 years after treatment.6 In our study, 7 patients (6% of total) developed delayed swallowing dysfunction, with a median time to onset of 34.3 months (range 18 – 60 months). The main causes for this dysphagia were cancer related (N = 3, either primary recurrence or 2nd primary cancer) and late RT effects (N = 4, diffuse radiation fibrosis or esophageal strictures).
The Functional Outcome Swallowing Scale or FOSS was used as the primary measurement instrument in this study. The FOSS has been used extensively at our institution and others because it is simple to use and has been shown to have high interrater reliability.7 We find FOSS measurements to be more sensitive to detect swallow function than using the presence or absence of G-tubes alone. For example, patients' swallowing dysfunction is often preceded by NGT feeding for a period of time. This early dysphagia is not reported if only the presence of G-tubes is used as a measurement of dysphagia. Likewise, some patients maintain a G-tube for many months after return of good swallowing function before the feeding tube is finally removed. Using removal of G-tubes would therefore not be an accurate measurement of return to function.
As with any study, there are limitations that must be addressed. First, this is a retrospective analysis, which has its inherent biases. Also, since most patients received adjuvant therapy, it is difficult to separate the direct effects of TLM or chemo/radiotherapy on swallowing function. A subjective swallowing scale, FOSS, was utilized rather than objective swallowing studies. However, for the intent of assessing the longitudinal swallowing patterns of patients, multiple radiographic swallowing tests would have been overly expensive and impractical. Performance status and patient motivation also have a great impact on swallowing function, and were not assessed in this study. Lastly, there are likely other variables that are independently associated with poor swallow function, but we either did not have either sufficient numbers to detect their effect or did not include them in the analyses. Future research will hopefully elucidate such factors.
Conclusion
In this study we demonstrate that TLM +/− adjuvant therapy results in very good, long term swallowing function. Longitudinal swallowing function shows that 88% of patients enjoy good swallowing at 2 years after surgery. Having advanced T stage 3 or 4 disease was shown to be associated with persistently poor swallowing function.
ACKNOWLEDGEMENTS
The authors wish to acknowledge Bridgette Sims and Parul Sinha; and the support of Kathryn Trinkaus of the Biostatistics Core, Siteman Comprehensive Cancer Center, and NCI Cancer Center Support Grant P30 CA091842.
Footnotes
Financial support/Funding: Private patient donation
Conflict of Interest: None
Presented at the 2010 Annual Meeting at the Combined Otolaryngology Spring Meetings of the Triological Society, Las Vegas, Nevada, USA, April 28 – May 2, 2010.
REFERENCES
- 1.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma increasing trends in the U.S. population ages 20–44 years. Cancer. 2005;103:1843–9. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 2.Rich JT, Milov S, Lewis JS, et al. Transoral laser microsurgery (TLM) +/-adjuvant therapy for advanced stage oropharyngeal cancer: outcomes and prognostic factors. Laryngoscope. 2009;119:1709–1719. doi: 10.1002/lary.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pradier O, Christiansen H, Schmidberger H, et al. Adjuvant radiotherapy after transoral laser microsurgery for advanced squamous carcinoma of the head and neck. Int J Rad Onc Biol Phys. 2005;63:1368–1377. doi: 10.1016/j.ijrobp.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 4.Haughey BH, Hinni ML, Salassa JR. Transoral laser microsurgery as primary treatment of advanced stage oropharynx cancer: a United States multicenter study. Presented at American Head Neck Society meeting 2009, in review, Head and Neck Journal. doi: 10.1002/hed.21669. [DOI] [PubMed] [Google Scholar]
- 5.Cooper JS, Pajak TF, Forastiere A, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 6.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26:3582–3589. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salassa JR. A functional outcome swallowing scale for staging oropharyngeal dysphagia. Digest dis. 1999;17:230–234. doi: 10.1159/000016941. [DOI] [PubMed] [Google Scholar]
- 8.Piccirillo JF, Tierney RM, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 9.Piccirillo JF, Creech CM, Zequeira R, et al. Inclusion of comorbidity into oncology data registries. J Reg Manag. 1999;26:66–70. [Google Scholar]
- 10.Johnston AS, Piccirillo JF, Creech CM, et al. Validation of a comorbidity education program. J Reg Manag. 2001;28:125–131. [Google Scholar]
- 11.Terrell JE, Ronis DL, Fowler KE, et al. Clinical predictors of quality of life in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004;130:401–408. doi: 10.1001/archotol.130.4.401. [DOI] [PubMed] [Google Scholar]
- 12.Wolf GT, et al. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer: the department of veterans affairs laryngeal cancer study group. N Engl J Med. 1991;324:1685–1690. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 13.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 14.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 15.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 16.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panje WR, Scher N, Karnell M. Transoral carbon dioxide laser ablation for cancer, tumors, and other diseases. Arch Otolaryngol Head Neck Surg. 1989;115:681–8. doi: 10.1001/archotol.1989.01860300035012. [DOI] [PubMed] [Google Scholar]
- 18.Hinni ML, Salassa JR, Grant DG, et al. Transoral laser microsurgery for advanced laryngeal cancer. Arch Otolaryngol Head Neck Surg. 2007;133:1198–1204. doi: 10.1001/archotol.133.12.1198. [DOI] [PubMed] [Google Scholar]
- 19.Grant DG, Hinni ML, Salassa JR, et al. Oropharyngeal cancer: a case for single modality treatment with transoral laser microsurgery. Arch Otolaryngol Head Neck Surg. 2009;135:1225–30. doi: 10.1001/archoto.2009.185. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen NP, Moltz CC, Frank C, et al. Dysphagia following chemoradiation for locally advanced head and neck cancer. Annals of Oncology. 2004;15:383–388. doi: 10.1093/annonc/mdh101. [DOI] [PubMed] [Google Scholar]
- 21.Cmelak AJ, Li S, Goldwasser MA, et al. Phase II trial of chemoradiation for organ preservation in resectable stage III or IV squamous cell carcinomas of the larynx or oro- pharynx: results of Eastern Cooperative Oncology Group Study E2399. J Clin Oncol. 2007;25:3971–3977. doi: 10.1200/JCO.2007.10.8951. [DOI] [PubMed] [Google Scholar]
- 22.Denis F, Garaud P, Bardet E, et al. Final results of the 94–01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced stage oropharynx carcinoma. J Clin Oncol. 2004;22:69–76. doi: 10.1200/JCO.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 23.McBride WH, Withers HR. Biologic basis of radiation therapy. In: Halperin EC, Perez CA, Brady LW, editors. Principles and Practice of Radiation Oncology. Lippincott Williams & Wilkins; Philadelphia, PA: 2008. pp. 95–96. [Google Scholar]
- 24.Tschudi D, Stoeckli S, Schmid S. Quality of life after different treatment modalities for carcinoma of the oropharynx. Laryngoscope. 2003;113:1949–1954. doi: 10.1097/00005537-200311000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Gillespie MB, Brodsky MB, Day TA, et al. Swallowing-related quality of life after head and neck cancer treatment. Laryngoscope. 2004;114:1362–1367. doi: 10.1097/00005537-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Mowry SE, Ho A, LoTempio MM, et al. Quality of life in advanced oropharyngeal carcinoma after chemoradiation versus surgery and radiation. Laryngoscope. 2006;116:1589–1593. doi: 10.1097/01.mlg.0000233244.18901.44. [DOI] [PubMed] [Google Scholar]
- 27.Henstrom DK, Moore EJ, Olsen KD, et al. Transoral resection for squamous cell carcinoma of the base of the tongue. Arch Otolaryngol Head Neck Surg. 2009;135:1231–1238. doi: 10.1001/archoto.2009.177. [DOI] [PubMed] [Google Scholar]
- 28.Moore EJ, Henstrom DK, Olsen KD, et al. Transoral resection of tonsillar squamous cell carcinoma. Laryngoscope. 2009;119:508–515. doi: 10.1002/lary.20124. [DOI] [PubMed] [Google Scholar]
- 29.Staar S, Rudat V, Stuetzer H, et al. Intensified hyperfractionated accelerated radiotherapy limits the additional benefit of simultaneous chemotherapy—results of a multicentric randomized German trial in advanced head-and-neck cancer. Int J Rad Onc Biol Phys. 2001;50:1161–1171. doi: 10.1016/s0360-3016(01)01544-9. [DOI] [PubMed] [Google Scholar]
- 30.Mendenhall WM, Stringer SP, Amdur RJ, et al. Is radiation therapy a preferred alternative to surgery for squamous cell carcinoma of the base of tongue? J Clin Oncol. 2000;18:35–42. doi: 10.1200/JCO.2000.18.1.35. [DOI] [PubMed] [Google Scholar]
- 31.Garden AS, Harris J, Trotti A, et al. Long-term results of concomitant boost radiation plus concurrent cisplatin for advanced head and neck carcinomas: a phase II trial of the radiation therapy oncology group (RTOG 99–14). Int J Radiat Oncol Biol Phys. 2008;71:1351–1355. doi: 10.1016/j.ijrobp.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]