Abstract
Plasma triglyceride concentration is a biomarker for circulating triglyceride-rich lipoproteins and their metabolic remnants. Common mild-to-moderate hypertriglyceridaemia is typically multigenic, and results from the cumulative burden of common and rare variants in more than 30 genes, as quantified by genetic risk scores. Rare autosomal recessive monogenic hypertriglyceridaemia can result from large-effect mutations in six different genes. Hypertriglyceridaemia is exacerbated by non-genetic factors. On the basis of recent genetic data, we redefine the disorder into two states: severe (triglyceride concentration >10 mmol/L), which is more likely to have a monogenic cause; and mild-to-moderate (triglyceride concentration 2–10 mmol/L). Because of clustering of susceptibility alleles and secondary factors in families, biochemical screening and counselling for family members is essential, but routine genetic testing is not warranted. Treatment includes management of lifestyle and secondary factors, and pharmacotherapy. In severe hypertriglyceridaemia, intervention is indicated because of pancreatitis risk; in mild-to-moderate hypertriglyceridaemia, intervention can be indicated to prevent cardiovascular disease, dependent on triglyceride concentration, concomitant lipoprotein disturbances, and overall cardiovascular risk.
Introduction
The complex causes and classification of hyper triglyceridaemia frequently make diagnosis and management a challenge to many clinicians of diverse specialties. Hyper triglyceridaemia is usually diagnosed when the fasting plasma concentration of triglyceride exceeds a threshold value (eg, >1·7 mmol/L [>150 mg/dL]). Severe hypertriglyceridaemia is often diagnosed when plasma triglyceride concentration is >10 mmol/L (>885 mg/dL).1–7 Proposed definitions of hyper tri glyceridaemia vary (table 1), and none predominates in clinical use. Traditional classification schemes for hypertriglyceridaemia have used terms such as familial hypertriglyceridaemia and familial combined hyperlipidaemia, which imply a single gene or monogenic cause. However, most cases of hyper triglyceridaemia are the result of many genetic factors—ie, they are multigenic or polygenic, with accumulations of both common DNA variants with small effect size, and rare DNA variants with large effect size.4 Hyper triglyceridaemia in susceptible individuals is further exacerbated by exposure to non-genetic secondary factors,4 including lifestyle factors such as being over weight and alcohol use.
Table 1.
Clinical definitions for hypertriglyceridaemia
| Plasma triglyceride concentration (mmol/L) | |
|---|---|
| 2011 ESC/EAS guidelines6,7 | |
| Normal | <1·7 |
| Hypertriglyceridaemia | 1·7-9·9 |
| Severe hypertriglyceridaemia | ≥10 |
| 2001 NCEP ATP III guidelines5 | |
| Normal | <1·7 |
| Hypertriglyceridaemia | |
| Borderline high | 1·7-2·3 |
| High | 2·3-5·6 |
| Very high | >5·6 |
| 2012 Endocrine Society guidelines1 | |
| Normal | <1·7 |
| Hypertriglyceridaemia | |
| Mild | 1·7-2·3 |
| Moderate | 2·3-11·2 |
| Severe hypertriglyceridaemia | |
| Severe | 11·2-22·4 |
| Very severe | >22·4 |
ESC=European Society of Cardiology. EAS=European Atherosclerosis Society.
NCEP ATP III=National Cholesterol Education Program Adult Treatment Panel III.
Although prospective and case-control studies have identified high plasma concentration of triglyceride as an independent risk factor for cardiovascular disease,8,9 uncertainty remains about the specific role of triglyceride-rich lipoproteins in atherogenesis.1–3 Furthermore, findings from intervention studies aimed at reducing triglyceride concentrations have shown inconsistent effects for cardiovascular disease outcomes, and no effect on stroke and all-cause mortality.3 Therefore, mild-to-moderate hypertriglyceridaemia is often viewed as a mere marker of cardiovascular disease risk, whereas severe hypertriglyceridaemia remains a well known risk factor for acute pancreatitis.4 Although the need to intervene in an individual with severe hyper-triglyceridaemia is un disputed, the appropriate response for mild-to-moderate hypertriglyceridaemia is less clear. In this Review, we recommend redefinition of hyper triglyceridaemia using a two-group classification to simplify the diagnosis and clinical management of hyper tri glyceridaemia states.
Considerations for measurement of triglyceride concentrations
In most countries, triglyceride concentration is established by direct laboratory analysis of plasma (usually) or serum after a 10–12 h fasting period. Indeed, clinicians routinely measure plasma triglyceride, because it is usually required for the Friedewald calculation of LDL cholesterol concentration. Modern methods for measurement of plasma triglyceride establish the free glycerol concentration after specific lipase action, which is the sum of the glycerol formed from the triglyceride plus the original free glycerol. However, the value for free glycerol is usually ignored because of the low plasma concentration of this molecule. Therefore, hyper-triglyceridaemia can be incorrectly diagnosed in rare patients with glycerol kinase deficiency who have high baseline concentrations of plasma glycerol.10 The only procedure that reliably differentiates the specific triglyceride-rich lipoprotein fractions is ultracentrifugation followed by electrophoresis, which is done in some specialised lipid centres.
Most people—certainly in high-income countries—are in the non-fasting or postprandial state for most of the day. Although recent guidelines1–3,5–7 unequivocally recommend measurement of fasting triglyceride concentrations, the importance of measurement of non-fasting triglyceride and remnant cholesterol is an emerging aspect of stratification for cardiovascular disease risk, because these measures partly show the capacity of the individual to clear postprandial lipids. Findings from population studies show that despite postprandial increases in triglyceride, quantitative changes in other lipids, lipoproteins, and apolipoproteins seem to be negligible in response to the average meal intake in most individuals.11,12 By contrast, for patients with dyslipidaemia with or without insulin resistance, the postprandial area under the curve for triglyceride-rich lipoproteins can be up to four times larger than for patients without dyslipidaemia, with pronounced modification of lipoprotein remodelling leading to an increase in the potentially atherogenic cholesterol load.12
High concentration of non-fasting triglyceride is also strongly associated with increased risk of myocardial infarction, ischaemic stroke, and early death.13,14 Evidence suggests that non-fasting triglyceride-rich lipoproteins or their remnants predispose to ischaemic heart disease and myocardial infarction.15,16 Non-fasting concentrations of total cholesterol, triglyceride, HDL cholesterol, non-HDL cholesterol, LDL cholesterol, APOB, APOA1, the ratio of total cholesterol to HDL cholesterol, and the ratio of APOB to APOA1, are also associated with increased risk of cardiovascular disease.11 These findings suggest that, compared with profiles for fasting lipids, profiles for non-fasting lipids are not only useful but also perhaps equally or more informative for risk prediction of cardiovascular disease. This approach has already been used clinically in some Scandinavian countries (eg, Denmark).17
Triglycerides and risk of cardiovascular disease
High plasma concentrations of triglyceride and triglyceride-rich lipoproteins have a role in cardiovascular disease.1–3 The magnitude of the contribution of triglyceride to cardiovascular disease risk and the exact mechanisms by which triglyceride-rich lipoproteins exert their effects on the vascular wall are incompletely established. That non-fasting triglyceride concentrations are relevant to cardiovascular disease risk is evident from large, long-term prospective studies13,18 in the general population, thereby corroborating the original hypothesis proposed by Zilversmit19 that atherosclerosis, at least partly, occurs postprandially. Indeed, triglyceride-rich lipoproteins (eg, intermediate-density lipoprotein and very low-density lipoprotein) could be particularly prone to entrapment within the arterial wall, whereas nascent chylomicrons and very large particles of very low-density lipoprotein are too large to penetrate.19–23 Consistent with this notion, findings from Mendelian randomisation studies suggest that lifelong high plasma concentrations of triglyceride-rich lipoproteins or their remnants are causally associated with increased risk of ischaemic heart disease,15,16 independent of subnormal concentrations of HDL cholesterol.13
The relative risk of cardiovascular disease from an increase of 1 mmol/L in plasma triglyceride concentrations ranges from 1·14 to 1·80, dependent on sex and race, after adjustment for established risk factors (eg, HDL cholesterol).3 Other studies in various cohorts compared the top versus the bottom tertile or quintile for triglyceride concentrations, and reported adjusted odds ratios (ORs) of 1·2–4·0 for increased risk of cardiovascular disease.2 The Emerging Risk Factors Collaboration24 assessed 302 430 people from Europe and North America without cardiovascular disease at baseline in 68 prospective studies. The hazard ratio (HR) for cardiovascular disease was 1·37 per standard deviation of triglyceride (95% CI 1·31–1·42), after adjustment for non-lipid risk factors. However, this risk was essentially lost after adjustment for both HDL and non-HDL cholesterol (HR 0·99, 95% CI 0·94–1·05);24 HDL cholesterol alone weakened, but did not abolish, the association (Ray KK, unpublished). Importantly, even for non-fasting samples, triglyceride was not independently associated with cardiovascular disease risk after adjustment for non-HDL cholesterol and HDL cholesterol. Although this finding suggests that HDL cholesterol drives the association with cardiovascular disease, re-examination of the putative atheroprotective role of HDL25 suggests that triglyceride-related mechanisms should be reconsidered as part of the pathophysiology of cardiovascular disease. Finally, findings from Mendelian randomisation analyses suggest a fairly direct causal association between triglyceride and triglyceride-rich lipoproteins, and risk of coronary heart disease, with similar ORs to those reported for prospective studies.15,16,26 Findings from a recent genetic study27 that accounted for effects on multiple components of the lipid profile similarly supported a causal role for triglycerides in development of coronary artery disease. On the basis of the available epidemiological and genetic data, a randomised clinical trial of a specific triglyceride-lowering drug should show a causal association between plasma triglyceride concentrations and morbidity and mortality from cardiovascular disease. However, findings from clinical trials of available drugs to reduce plasma triglycerides, which also affect other components of the lipid profile, have shown little effect for cardiovascular disease outcomes.28–31 One reason for this finding might be that trials with cardiovascular outcomes have mainly enrolled individuals without clinically relevant hypertriglyceridaemia, making the assessment of the effects of specific interventions challenging.
Historical classification of hypertriglyceridaemia phenotypes
Phenotypic heterogeneity among patients with hypertriglyceridaemia was defined in the past by qualitative and quantitative differences in plasma lipoproteins. In the pre-genomic era, the Fredrickson classification of hyperlipoproteinaemia phenotypes (included in the WHO International Classification of Diseases32) was based on the electrophoretic patterns of lipoprotein fractions (table 2). Five of the six phenotypes described by this classification include hyper triglyceridaemia in their definitions, the only exception being familial hypercholesterolaemia (hyper-lipo proteinaemia type 2A).33,34
Table 2.
Summary of classic hyperlipoproteinaemia phenotypes
| WHO ICD number |
Fredrickson hyper- lipoproteinaemia phenotype |
OMIM number | Main lipid change | Primary lipoprotein change |
Genetics | |
|---|---|---|---|---|---|---|
| Familial hyperchylomicronaemia | E78.3 | Type 1 | 238600 | ↑Triglyceride | ↑Chylomicrons | Monogenic; autosomal recessive due to two mutant alleles of LPL, APOC2, APOA5, LMF1, GPIHBP1, or GPD1; presentation mainly paediatric or early adulthood |
| Familial hypercholesterolemia | E78.0 | Type 2A | 143890 | ↑Total cholesterol | ↑LDL | Monogenic; autosomal codominant; heterozygous form results from one mutant allele of LDLR, APOB, or PCSK9; homozygous form results from two mutant alleles of these genes or of LDLRAP1 |
| Combined hyperlipoproteinaemia | E78.2, E78.4 | Type 2B | 144250 | ↑Total cholesterol, ↑triglyceride | ↑VLDL, ↑LDL | Polygenic; high GRS for hypertriglyceridaemia; excess of rare variants in hypertriglyceridaemia-associated genes; high GRS for LDL cholesterol |
| Dysbetalipoproteinaemia | E78.2 | Type 3 | 107741 | ↑Total cholesterol, ↑triglyceride | ↑IDL | Polygenic; high GRS for hypertriglyceridaemia; excess of rare variants in hypertriglyceridaemia-associated genes; APOE ε2/ε2 homozygosity, or heterozygous rare mutation in APOE |
| Primary or simple hypertriglyceridaemia | E78.1 | Type 4 | 144600 and 145750 | ↑Triglyceride | ↑VLDL | Polygenic; high GRS for hypertriglyceridaemia; excess of rare variants in hypertriglyceridaemia-associated genes |
| Mixed hypertriglyceridaemia | E78.3 | Type 5 | 144650 | ↑Total cholesterol, ↑triglyceride | ↑VLDL, ↑chylomicrons | Polygenic; high GRS for hypertriglyceridaemia; excess of rare variants in hypertriglyceridaemia-associated genes, with higher burden of risk alleles than for hyperlipoproteinaemia type 4 |
GRS was created by unweighted tallying of risk alleles from single nucleotide polymorphisms associated with increased plasma concentrations of triglyceride and hypertriglyceridaemia. Adapted from Hegele (2009).33 ICD=International Classification of Diseases. OMIM=Online Mendelian Inheritance in Man database. VLDL=very low-density lipoprotein. GRS=polygenic genetic risk score. IDL=intermediate-density lipoprotiein.
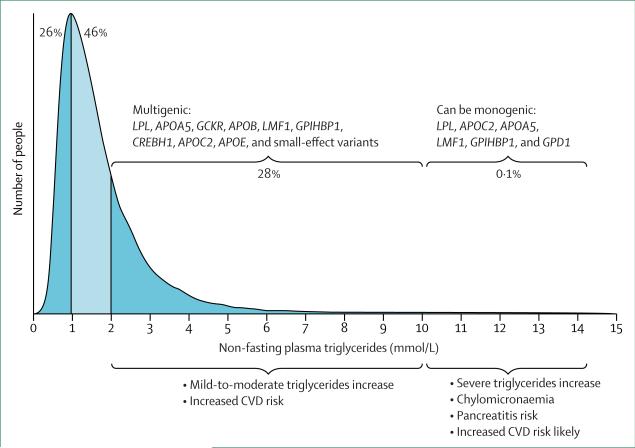
The different hypertriglyceridaemia-associated pheno-types are defined by the specific class or classes of accumulated triglyceride-rich lipoprotein particles, including chylomicrons, and very low-density lipoproteins and their remnants (table 2).33 Often, excess of triglyceriderich lipoproteins coexists with other lipoprotein disturbances; for instance, patients with all forms of hypertriglyceridaemia often have decreased concentrations of HDL cholesterol. Implicit in this classification system was the idea that differences between hyper-triglyceridaemia-associated phenotypes were due to genetic differences; however, recent data suggest that this scenario is typically not the case.35–39 As a result, this classification system has neither improved scientific insight nor been clinically useful to direct therapy or predict hard outcomes (eg, cardiovascular mortality). We suggest that triglyceride concentration itself (figure 1), together with the presence of other risk factors, should be the main driver of clinical management.
Figure 1. Redefinition of hypertriglyceridaemic states on the basis of new genetic data.
Triglyceride concentrations of more than 10 mmol/L, especially in young patients, are more likely to be due to monogenic causes combined with secondary factors, whereas patients with triglyceride concentrations of 2–10 mmol/L represent a single group, based on the interplay of several genes (both heterozygous mutations of large effect, and the cumulative burden of small-effect variants, causing a high genetic risk score; figure 2), together with secondary factors. Plasma triglyceride concentrations and approximate population percentages are based on data for more than 70 000 adults (>20 years of age) from the Copenhagen General Population Study.40
Complex genetic basis for hypertriglyceridaemia
For several decades, the word familial has been used in the definitions and classification of disorders of plasma triglyceride metabolism. However, the constant reinforcement of this terminology has a misleading effect. Colloquially, familial often implies a single-gene problem, as in the case of familial hypercholesterolaemia, a monogenic disorder characterised by increased concentrations of LDL cholesterol, xanthelasma palpebrarum, arcus cornealis, tendon xanthomata, and accelerated atherosclerosis.34 Familial hyperchol esterolaemia is usually due to loss-of-function mutations in LDLR, which encodes the LDL receptor, and in other genes encoding proteins that interact with LDLR such as APOB or PCSK9. A clear monogenic cause can be established in more than 80% of patients with a strong clinical diagnosis of familial hypercholesterolaemia, whereas in the remainder, high LDL cholesterol is a polygenic trait due to an increased burden of common risk variants.41 By stark contrast, more than 95% of patients with hyper triglyceridaemia have a multigenic susceptibility component.3,35–39
Multigenic hypertriglyceridaemia has a complex cause, consisting of an excess burden of common small-effect variants (appendix), in addition to rare heterozygous large-effect variants in genes either directly or indirectly associated with plasma triglyceride concentration. Familial should not be thought of as synonymous with monogenic; most cases of hypertriglyceridaemia are familial or inherited, but they are not monogenic.35,36
Monogenic hypertriglyceridaemia
Monogenic hypertriglyceridaemia in patients with severe hypertriglyceridaemia (triglyceride concentrations >10 mmol/L) displays classic autosomal recessive inheritance, with a population prevalence of about one in 1 000 000. Typically, the disorder is first evident in childhood and adolescence. Affected individuals are often homozygous or compound heterozygous for large-effect loss-of-function mutations in genes that regulate catabolism of triglyceride-rich lipoproteins (eg, LPL, APOC2, APOA5, LMF1, GPIHBP1, and GPD1; table 2).37–39 Patients with monogenic disorders have substantially increased fasting concentrations of chylomicrons, but usually do not develop premature atherosclerosis, probably because of size exclusion that limits the ability of chylomicrons to traverse the vascular endothelial barrier.19–23 In the 20th century, a diagnosis of LPL deficiency was established biochemically by the absence of LPL activity in plasma obtained after intravenous injection of heparin.42 At present, the diagnosis can be made by DNA sequence analysis, which shows mutations in both LPL alleles causing complete LPL deficiency; mutations in the other genes can also be detected by resequencing.43
Multigenic hypertriglyceridaemia
Role of rare variants
Hypertriglyceridaemia, with or without concomitant lipid or lipoprotein disturbances, tends to cluster in families. Although hypertriglyceridaemia usually does not result from strong single-gene effects that show Mendelian inheritance, it still has a genetic basis, albeit one that is more complex in nature. This complexity was suggested by findings from pre-molecular-era family studies of obligate heterozygous parents of patients with complete LPL or APOC2 deficiency44,45 and unrelated heterozygous carriers of disease-causing mutations from the general population.46 These study findings showed that heterozygous carriers of disease-causing mutations had a very wide range of triglyceride phenotypes, ranging from normotriglyceridaemia to severe hypertriglyceridaemia,44–46 probably because of chance co-inheritance of different numbers of common triglyceride-raising variants. Similarly, triglyceride concentrations range from normal to very high in heterozygous carriers of APOA5 mutations.47 Overall, mean triglyceride concentrations in carriers of the various heterozygous mutations are higher than in normal family-based or population-based controls, but many mutation carriers have normal triglyceride concentrations.
DNA resequencing has shown that individuals with triglyceride concentrations of more than 3·3 mmol/L (>95th percentile in the USA and Canada) as a group have a clinically significantly increased—about 2·5 times higher—frequency of rare, heterozygous loss-of-function mutation in one of several genes governing triglyceride metabolism compared with normotriglyceridaemic controls.48,49 Most of these variants have confirmed loss-of-function effects in vitro or predicted harmful effects in silico.48,49 Although these rare mutations are strongly associated with hypertriglyceridaemia in patient groups, they are not necessarily associated with hyper-triglyceridaemia in individual patients. Even within families, carriers of the same mutation show a wide range of triglyceride concentrations from normal to severe hypertriglyceridaemia, with inconsistent vertical transmission of triglyceride concentrations in mutation carriers across generations.50 Such findings emphasise that hypertriglyceridaemia is not a dominantly inherited trait in most families with a hypertriglyceridaemia proband.
Role of common variants
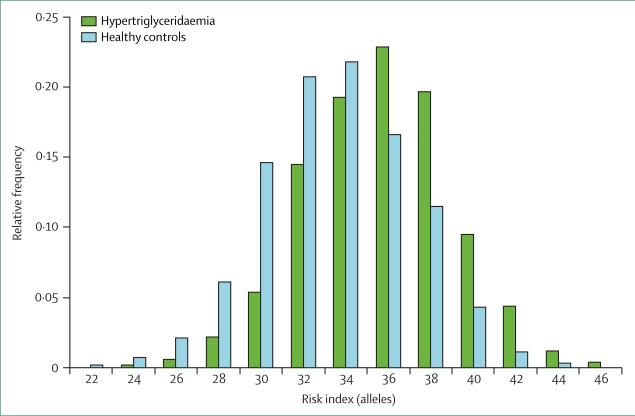
Findings from genome-wide association studies of hypertriglyceridaemia show that common variants in several genes (eg, APOA5, GCKR, LPL, and APOB) are strongly associated with susceptibility to hyper triglyceridaemia.51 In fact, common variations in 32 triglyceride-associated genes, identified by the Global Lipids Genetics Consortium,37–39 are robustly associated with hypertriglyceridaemia; the same loci are also associated with small variations in plasma triglyceride concentrations within the normal range in healthy people (appendix). A genetic risk score, constructed by unweighted tallying of carrier status for triglyceride-raising alleles at the 32 triglyceride-associated loci, was on average higher for patients with hypertriglyceridaemia than for healthy controls (figure 2).36,51 Thus, as for mutation-negative patients with familial hyper-cholesterolaemia and LDL-cholesterol raising variants,41 an increased burden of triglyceride-raising alleles contributes to hypertriglyceridaemia susceptibility.48,49
Figure 2. Genetic risk scores for triglyceride-associated risk alleles.
Unweighted risk scores composed of risk alleles at 32 triglyceride-associated loci were summed across individuals and compared between patients with hypertriglyceridaemia and controls. The minimum unweighted risk score is 0, whereas the maximum unweighted risk score is 64, but most scores in the population range between 22 and 46. Compared with healthy controls, the relative frequency distribution of triglyceride genetic risk scores was significantly increased in 504 patients with hypertriglyceridaemia (p=1·6×10−53). Figure reproduced from Johansen and colleagues39 by permission of Elsevier.
Common plus rare variants
Hypertriglyceridaemia susceptibility is thus established by combinations of common small-effect and rare large-effect variants in genes governing production or catabolism, or both, of triglyceride-rich lipoproteins.35–38,48,49 People with average triglyceride concentrations could have a balance of protective and harmful alleles. On the basis of studies of 765 individuals for whom nine hypertriglyceridaemia-associated genes were resequenced, common and rare genetic variants together accounted for about 25% of total variation (and about 50% of attributable variation) in hypertriglyceridaemia susceptibility.44,45 Because of the wide range of triglyceride concentrations and severity of hypertriglyceridaemia phenotypes within families and among carriers of the same genotype, genetic testing is not recommended. Finally, the classic Fredrickson phenotypes characterised by hypertriglyceridaemia closely resemble each other at the genetic level, with similar accumulations of common and rare genetic variants despite different biochemical phenotypes.33,35–38,48,49 Among these phenotypes, hyperlipoproteinaemia type 3 (dysbetalipoproteinaemia) is unique in that a single gene (APOE) can force the expression of hypertriglyceridaemia and hypercholesterolaemia because of accumulation of remnant particles; the cumulative effects of polygenic predisposition are compounded by either homozygosity for the binding-defective E2 isoform of APOE, or heterozygosity for a rare dysfunctional APOE mutation.36
Secondary causes
Hypertriglyceridaemia is often associated with other disorders that independently increase plasma triglyceride concentrations, such as type 2 diabetes, obesity, alcohol overuse, hypothyroidism, pregnancy, hepatosteatosis, renal failure, or concomitant drug use (panel 1).1,4,6,7 When one of these factors is present, hypertriglyceridaemia is termed secondary. However, secondary hyper-triglyceridaemia often also has a genetic component, because some secondary factors are frequently, but not universally, associated with hypertriglyceridaemia. This genetic component suggests that people who develop dyslipidaemia might carry inherited defects that confer susceptibility, which becomes clinically expressed in the presence of an external or secondary stress.4 For example, abdominal obesity, metabolic syndrome, and non-alcoholic fatty liver disease are associated with increased risk of hypertriglyceridaemia and are becoming increasingly common in adults, adolescents, and even children. Whether there is a strong secondary factor underlying dyslipidaemia needs to be established, because this knowledge would guide intervention. Furthermore, the severity of secondary hypertriglyceridaemia in an individual is probably determined by their genetic susceptibility component. Finally, some secondary causes, such as obesity, metabolic syndrome, non-alcoholic fatty liver disease, and diabetes, have their own genetic susceptibility components.
Redefinition of hypertriglyceridaemic states
On the basis of new genetic data, we recommend a redefinition of hypertriglyceridaemia states (panel 2). First, triglyceride concentrations of more than 10 mmol/L are likely to have a monogenic basis (especially in young patients), together with interacting secondary factors. However, even in this group, in many instances (particularly in adults) no monogenic cause can be established; in these cases, there is marked polygenic susceptibility compounded by significant exposure to secondary factors. Thus, except in children and adolescents with severe hypertriglyceridaemia, we do not recommend routine genetic testing, even for adults with triglyceride concentrations of more than 10 mmol/L. Second, people with triglyceride concentrations of 2–10 mmol/L should be considered as a single group, irrespective of concomitant lipoprotein disturbances (eg, increased LDL cholesterol), with increased triglyceride caused by interaction of several genetic effects and secondary factors (figure 1). For example, individuals with hyper lipo proteinaemia type 2B (often called familial combined hyperlipidaemia; table 2) have the same genetic risk score as do individuals with similar triglyceride concentrations who have isolated or hyperlipoproteinaemia type 4 hypertriglyceridaemia; they differ in that individuals with hyperlipoproteinaemia type 2B have a higher genetic burden of alleles associated with hypercholesterolaemia.36 Again, we do not recommend routine genetic testing in any individuals with triglyceride concentrations of 2–10 mmol/L, and recommend testing in severe triglyceridaemia only for paediatric and adolescent patients.
Desirable concentrations of triglyceride and related variables
Hypertriglyceridaemia is arbitrarily defined as a plasma triglyceride concentration of more than 2 mmol/L (>175 mg/dL) on the basis of large prospective observational studies, although plasma triglyceride can start to confer risk or become a marker for cardiovascular disease at even lower concentrations.4,6,7,13,14 Triglyceride concentrations rising above this threshold, due to increased production or decreased clearance of triglyceride-rich lipoproteins from the circulation, are accompanied by changes in the metabolism and composition of other lipoprotein fractions such as LDL and HDL, which might partly explain the increased cardiovascular disease risk.3
According to the joint guidelines from the European Society of Cardiology and the European Atherosclerosis Society for management of dyslipidaemia, and Consensus Panel recommendations from the European Atherosclerosis Society, a triglyceride concentration of less than 1·7 mmol/L (<150 mg/dL) is desirable, especially if HDL cholesterol is less than 1·0 mmol/L (<40 mg/dL) in men or 1·2 mmol/L (<45 mg/dL) in women.3,6,7 In post-hoc subgroup analyses of clinical endpoint studies of fibrates, clinical benefit was shown in people with triglyceride concentrations of more than 2·3 mmol/L (>200 mg/dL) and low HDL cholesterol.28,29 Thus, when lifestyle measures are insufficient, individuals with high risk of cardiovascular disease and increased plasma triglyceride could be considered for drug treatment if triglyceride concentrations exceed 2·3 mmol/L.3,6,7 However, there is inadequate evidence to define treatment targets for plasma triglyceride. Even the triglyceride threshold for diagnosis of hyper-triglyceridaemia is not irrefutable; no high-grade evidence exists to suggest that 2·0 mmol/L is better than 1·7 or 2·3 mmol/L. In this Review, we use 2·0 mmol/L as the diagnostic threshold for hypertriglyceridaemia (panel 3), but expert opinion suggests values of ±0·3 mmol/L around this cutpoint.1,3–7
An emerging focus for reduction of cardiovascular disease risk in patients with hypertriglyceridaemia is the concentration of non-HDL cholesterol (comprising cholesterol in LDL and in remnant triglyceride-rich lipoproteins), which represents the total mass of cholesterol in circulating atherogenic lipoprotein particles.3,13,15,16 This variable has been advocated because LDL cholesterol cannot be estimated by the Friedewald equation when triglyceride concentrations are more than 4·5 mmol/L; additionally, standardised direct measurement of LDL cholesterol is not routinely available in most centres. The desirable concentration of non-HDL cholesterol is less than 2·6 mmol/L (<100 mg/dL) in high-risk individuals, and less than 3·4 mmol/L (<130 mg/dL) in low-risk individuals (panel 3). Again, there is insufficient high-grade evidence to define specific targets for any of these alternative variables, and treatment should be individually tailored.3,6,7
An alternative estimation of atherogenic lipoprotein concentrations uses APOB as a substitute for non-HDL cholesterol. APOB represents the total number of atherogenic APOB-containing lipoprotein particles, and predicts cardiovascular disease risk at least as well as non-HDL cholesterol.6,7 APOB can be reliably measured in the presence of hypertriglyceridaemia and under non-fasting conditions. Some expert panels have therefore recommended APOB as a secondary target in individuals with hypertriglyceridaemia.3,6,7,52 Accordingly, APOB concentrations of more than 1·2 g/L identify individuals at high risk of cardiovascular disease, and the desirable concentration is less than 0·8 g/L.3,6,7 For individuals at very high risk, an APOB target of less than 0·7 g/L might be appropriate, corresponding to a non-HDL cholesterol concentration of less than 2·6 mmol/L (100 mg/dL; panel 3).3,6,7
Management of hypertriglyceridaemia
Treatment for short-term and long-term risks
Treatment of hypertriglyceridaemia has two distinct objectives: immediate prevention of pancreatitis in patients with severe hypertriglyceridaemia (triglyceride concentration >10 mmol/L), and reduction of global cardiovascular disease risk. Because hypertriglyceridaemia is characterised by increased concentrations of remnant triglyceride-rich lipoproteins, concentrations of non-HDL cholesterol or APOB are secondary treatment targets, after LDL cholesterol.53
After secondary causes have been treated, the management of mild-to-moderate hypertriglyceridaemia should follow guideline recommendations,3,6,7 with initial emphasis on diet and exercise. Non-pharmacological therapy is recommended for individuals with triglyceride concentrations of more than 2 mmol/L. The decision to initiate pharmacological therapy depends on the amount of triglyceride elevation. Individuals with triglyceride concentrations of more than 10 mmol/L warrant immediate and aggressive triglyceride reduction to minimise the risk of acute pancreatitis, with use of a strict fat-reduced diet and avoidance of simple carbohydrates; use of fibrates, nicotinic acid, or omega-3 fatty acids could also be considered. In the context of abdominal pain, treatment of severe hyper triglyceridaemia includes hospitalisation, with cessation of oral intake, and supportive measures including fluid replacement, avoidance of glucose infusions, and control of obvious precipitating factors (eg, diabetes). Drugs are less effective in this situation, and substantial interventions—eg, infusions of insulin or heparin, high-dose antioxidants, or plasma exchange—are also probably of little value for most patients.4 As noted earlier, because of the uncertain clinical benefit, practice guidelines are not universal or consistent regarding the management of individuals with triglyceride concentrations of 2–10 mmol/L.
Patients with hypertriglyceridaemia should be assessed and managed for their global risk of cardiovascular disease (table 3), which does not necessarily imply management of their triglyceride concentrations. A positive family history of cardiovascular disease (defined as at least one first-degree relative or at least two second-degree relatives with cardiovascular disease) should be taken into account, even if it is independent of dyslipidaemia. Because many susceptibility alleles, environmental factors, and secondary factors tend to be shared within families, other family members might also have a lipid disorder and assessment for dyslipidaemia and related cardiometabolic risk should be considered. This situation is analogous to that for type 2 diabetes, which clusters in families but is usually not associated with a single monogenic cause.
Table 3.
Treatment strategies for hypertriglyceridaemia by triglyceride concentration
| Moderately high (2–9·9 mmol/L) | High (≥10 mmol/L) | |
|---|---|---|
| Treatment priority | Prevent cardiovascular disease | Prevent acute pancreatitis |
| Primary therapeutic goal | Achieve LDL cholesterol target | Reduce triglyceride concentrations |
| Secondary therapeutic goals | Achieve non-HDL cholesterol target, which is 0·8 mmol/L higher than LDL cholesterol goal, or APOB concentration <0·8 g/L; rule out and treat secondary factors | Goals: achieve LDL cholesterol and non-HDL cholesterol goals once pancreatitis risk is decreased, as described above; rule out and treat secondary factors |
| Non-pharmacological therapeutic strategies | Reduce bodyweight, reduce alcohol intake, reduce simple sugar intake, increase aerobic activity, reduce total carbohydrate intake, replace trans and saturated fats with monounsaturated fats, increase dietary omega-3 fatty acids | Eliminate oral intake during acute pancreatitis with intravenous rehydration, then slowly re-introduce foods with small frequent meals, then longer-term strict fat-reduced diet (<20% of calories as fat), reduce bodyweight, reduce alcohol intake, reduce simple sugar intake, reduce total carbohydrate intake, replace trans and saturated fats with monounsaturated fats; increase dietary omega-3 fatty acids; increase aerobic activity |
| Pharmacological therapeutic strategies | Statins if necessary to control LDL cholesterol; if LDL cholesterol is close to goal, titrate statin dose to achieve both LDL and non-HDL cholesterol targets; if LDL cholesterol is at goal, but non-HDL cholesterol is still high, titrate statin dose or add fibrate, nicotinic acid, or omega-3 fatty acids | Consider fibrate, nicotinic acid, and omega-3 fatty acids |
Increased LDL cholesterol is part of the phenotype of combined hyperlipidaemia, and amplifies cardiovascular disease risk. Thus, family members (particularly first-degree relatives of such patients) should be screened. Irrespective of clinical designation, individuals with combined hyperlipidaemia in particular, and hyper-triglyceridaemia in general, still need to be managed. Risk assessment and continuing care needs baseline and follow-up lipid profiling, especially because hyper-triglyceridaemia can obscure calculation of LDL cholesterol in these instances. Furthermore, measurements of non-HDL cholesterol (or, if available, APOB) concentrations can be helpful for both risk assessment and monitoring of treatment if LDL cholesterol concentrations cannot be measured.
Statins
Statins decrease LDL cholesterol concentrations by up to 55%, leading to a reduction in cardiovascular disease risk of 23% per mmol/L of LDL cholesterol lowered, irrespective of baseline concentrations of LDL cholesterol, triglyceride, or HDL cholesterol.54 The use of these drugs in patients with hypertriglyceridaemia is justifiable because of their proven ability to reduce cardiovascular disease. They also variably reduce plasma triglyceride concentrations by up to 30%,55 with reductions dependent on baseline triglyceride concentration and dose of statin used. To achieve recommended targets, the choice of statin should be based on efficacy for LDL cholesterol reduction, taking into account safety considerations.6,7 In hyper triglyceridaemia, because LDL cholesterol often cannot be established, achievement of non-HDL cholesterol or APOB targets should also be a goal of treatment.56
Fibrates
With a triglyceride-lowering effect of 40%,57 dependent on baseline triglyceride concentrations, fibrates are the first-line treatment to decrease risk of pancreatitis for patients with triglyceride concentrations of more than 10 mmol/L. Although controversial, findings from a meta-analysis28 including more than 45 000 individuals suggested that fibrates could reduce non-fatal acute coronary events and revascularisation by about 9% (together with an absence of overall effect for total and cardiovascular mortality and a non-significant increase in non-cardiovascular deaths), particularly in people with triglyceride concentrations of more than 2·3 mmol/L and HDL cholesterol concentrations of less than 1·0 mmol/L.29 Therefore, fibrates can be used as additional therapy for individuals with high triglyceride and low HDL cholesterol.3 However, in monogenic hypertriglyceridaemia due to LPL deficiency and triglyceride concentrations of more than 20 mmol/L, fibrates have little to no clinical benefit.
Nicotinic acid
Treatment with nicotinic acid (also known as niacin) at a dose of 2–3 g/day is associated with up to 30% reduction in triglyceride concentration, 20% increase in HDL cholesterol concentration, up to 20% lowering of LDL cholesterol concentration, and up to 25% reduction in lipoprotein(a) concentration. Findings from studies of the cardiovascular disease benefits of nicotinic acid are conflicting. The 2011 Consensus Panel recommendations from the European Atherosclerosis Society3 supported the addition of nicotinic acid to statin therapy for individuals not at target concentrations of LDL cholesterol or non-HDL cholesterol, particularly if triglyceride remains high and HDL cholesterol is low. Combination therapy that includes nicotinic acid is a therapeutic option for statin-intolerant patients. However, nicotinic acid is no longer a therapeutic option in Europe, because of the withdrawal of extended-release nicotinic acid combined with laropiprant after the announcement of negative results from the HPS-2 THRIVE study.58 Extended-release nicotinic acid remains available in North America under the trade name Niaspan. This formulation was used in the AIM-HIGH study,30 findings from which also showed no clinical benefit in reducing primary endpoints of death from coronary heart disease, non-fatal myocardial infarction, and ischaemic stroke.
Bile acid sequestrants
In patients with hypertriglyceridaemia, bile acid sequestrants can often cause a further increase in triglyceride concentrations, so these drugs should be used with caution in this patient group. Colesevelam can reduce LDL cholesterol concentrations by 15–20% in addition to the reduction achieved with statin therapy,59 and might be an option in the context of very mild hypertriglyceridaemia for individuals whose LDL cholesterol, APOB, or non-HDL cholesterol are not at target concentrations, or in statin-intolerant people.
Omega-3 fatty acids
Omega-3 polyunsaturated fatty acids at doses of up to 4 g daily reduce triglyceride concentrations by up to 30%, dependent on baseline concentrations, and might therefore be useful for prevention of pancreatitis.4 Findings from a meta-analysis31 showed that omega-3 supplementation was not significantly associated with reductions in all-cause mortality, myocardial infarction, or stroke.
Future research directions for hypertriglyceridaemia
Recent meta-analyses of gene-centric genome-wide association studies, and resequencing studies, have begun to further expand and elucidate the genetic underpinnings of different forms of hyper triglyceridaemia.60 Incorporation of this knowledge into future exome and genome sequencing studies might enable identification of new candidate genes. For example, patients with hypertriglyceridaemia and families with a high genetic risk score could be sequenced for known triglyceride loci to identify the full range of hypertriglyceridaemia-associated variants in these regions, whereas patients with hypertriglyceridaemia and families with a low hypertriglyceridaemia genetic risk score could be sequenced at the exome or genome level to identify new variants and genes for hypertriglyceridaemia.
Such approaches might offer the possibility of personalised medicine, in which individuals with hypertriglyceridaemia are assessed, diagnosed, and treated according to their individual genetic composition and molecular phenotype.60 To address the complexity of this task, systems approaches—integrating genomic, transcriptomic, proteomic, and epigenomic data with metabolic and clinical phenotypes—are under development.61 One example is weighted coexpression network analysis that correlates gene expression and methylation networks with variants and phenotypes.62 This analysis could provide a functionally oriented method to identify additional new hypertriglyceridaemia-associated genes and pathways in tissues relevant to lipid metabolism. However, for many individuals with hypertriglyceridaemia, the usual treatment options will probably be equally effective, irrespective of the underlying combinations of predisposing alleles; this hypothesis needs to be formally studied.
Additionally, gene therapy is being studied in individuals with familial hyperchylomicronaemia. Specifically, expression of a recombinant virus containing the human hyperfunctional LPL*S447X variant showed promise in animals,63 and early clinical trials in people with intramuscular injections of alipogene tiparvovec (an adeno-associated virus carrying LPL) mediated local LPL expression, and was associated with a transient reduction in plasma triglyceride concentrations.64 This treatment, which is also known by the trade name Glybera, was recently approved by the European Medicines Agency for the treatment of classic hyperlipoproteinaemia type 1 (LPL deficiency).
Finally, new treatments for hypertriglyceridaemia have been developed that are based on genetic studies that identified rare causative mutations in families with phenotypes of severely diminished triglyceride concentrations. For example, lomitapide, an inhibitor of MTTP that reduces triglyceride in addition to all APOB-containing lipoproteins, was developed because individuals with homozygous mutations in MTTP causing abetalipoproteinaemia have depressed triglyceride concentrations.65 Similarly, low triglyceride concentrations in other families with monogenic triglyceride deficiency prompted the development of new biological agents targeting APOB (the recently approved drug mipomersen),66 APOC3,67 and ANGPTL3.68
Conclusions
Diagnosis of hypertriglyceridaemia is relevant because even slight increases in triglyceride concentrations are usually associated with increased risk of cardiovascular disease, severely increased triglyceride is associated with increased risk of pancreatitis, and hyper tri glyceridaemia often coexists with other metabolic disturbances that are associated with increased cardiometabolic risk. Epidemiological, genetic, and clinical trial evidence has led us to recommend a simplified definition of hypertriglyceridaemia (panel 1), with severe hypertriglyceridaemia (triglyceride concentrations of more than 10 mmol/L, especially in the paediatric age group) more likely to be related to monogenic causes, and mild-to-moderate hyper triglyceridaemia (triglyceride concentrations of 2–10 mmol/L) more likely to have a polygenic basis with secondary factors. The presence of concomitant lipid disturbances depends on additional genetic factors. Knowledge of the precise molecular defect might be helpful to guide therapy for monogenic hyper triglyceridaemia disorders, particularly in children and adolescents with severe hyper triglyceridaemia due to LPL deficiency and related disorders. However, in polygenic hypertriglyceridaemia, no evidence suggests that genotyping improves diagnosis or management. Non-fasting lipid measurements might improve the efficiency of screening and diagnosis of hyper-triglyceridaemia, whereas related variables (eg, non-HDL cholesterol and APOB) can provide guidance for therapy, especially when hypertriglyceridaemia is moderate to severe. The present mainstay of treatment for all types of hypertriglyceridaemia focuses on risk-factor control, diet, and lifestyle choice to ensure greatest health for individuals with hyper tri glyceridaemia. Pharmaco therapy can also be useful in selected subgroups, provided that it is in line with guideline recommendations. Finally, research in progress, both genetic and non-genetic, might identify new therapeutic targets that could lead to optimisation of clinical management in individuals with hypertriglyceridaemia.
Supplementary Material
Panel 1: Secondary causes of hypertriglyceridaemia
Obesity
Metabolic syndrome
Diet with high positive energy-intake balance, and high fat or high glycaemic index
Increased alcohol consumption*
Diabetes (mainly type 2 diabetes)
Hypothyroidism
Renal disease (proteinuria, uraemia, or glomerulonephritis)
Pregnancy (particularly in the third trimester)
Paraproteinaemia
Systemic lupus erythematosus
Drugs including corticosteroids, oral oestrogen, tamoxifen, thiazides, non-cardioselective β blockers and bile acid sequestrants, cyclophosphamide, asparaginase, protease inhibitors, and second-generation antipsychotic drugs (eg, clozapine and olanzapine)
*Although the range is variable, clinically the risk of hypertriglyceridaemia is generally thought to increase with more than two units daily for men, and more than one unit daily for women.
Panel 2: Proposed simplified redefinition of hypertriglyceridaemia
Normal: triglyceride concentration less than 2·0 mmol/L (175 mg/dL)
Mild-to-moderate: triglyceride concentration between 2·0 and 10·0 mmol/L (175–885 mg/dL)
Severe: triglyceride concentration more than 10·0 mmol/L (885 mg/dL)
Panel 3: Desirable concentrations of lipids and APOB in patients at high risk of cardiovascular disease
Triglycerides: concentration less than 1·7 mmol/L (150 mg/dL)
Non-HDL cholesterol: concentration less than 2·6 mmol/L (100 mg/dL)
APOB: concentration less than 0·8 g/L in high-risk patients, and less than 0·7 g/L in very high-risk patients
Search strategy and selection criteria.
We searched Medline, Current Contents, PubMed, and relevant references with the terms “triglyceride”, “hypertriglyceridaemia”, “hyperlipidaemia”, “familial”, “monogenic”, “polygenic”, “polymorphism”, “mutation”, and “pharmacogenetics”. Articles published in English between 2000 and 2013 were included. This Review was based on discussions at two meetings of the European Atherosclerosis Society Consensus Panel organised and chaired by MJC and HNG, where the search results and drafts of the Review were critically appraised; most of the Review results from a consensus of expert opinions.
Acknowledgments
The European Atherosclerosis Society is supported by unrestricted educational grants from Amgen, Aegerion, AstraZeneca, Genzyme, Hoffman-La Roche, Kowa Europe, Novartis, and Sanofi-Aventis/ Regeneron. These companies were not present at the Consensus Panel meetings, had no role in the design or content of the Review, and had no right to approve or disapprove the final document. RAH is supported by the Jacob J Wolfe Distinguished Medical Research Chair at the Western University, the Edith Schulich Vinet Canada Research Chair in Human Genetics (Tier I), the Martha G Blackburn Chair in Cardiovascular Research, and operating grants from the CIHR (MOP-13430, MOP-79523, CTP-79853), and the Heart and Stroke Foundation of Ontario (NA-6059, T-6018, PRG-4854). We thank Jane Stock (European Atherosclerosis Society Consensus Panel Administration Office, London, UK) for editorial and administrative support.
Footnotes
Contributors
MJC and HNG are cochairs of the European Atherosclerosis Society Consensus Panel. MA, JB, EB, ALC, MJC, HNG, RAH, GKH, JAK, PP, KKR, AFHS, ES, M-RT, and AT-H are members of the Consensus Panel writing committee. OSD, SEH, PTK, LM, BGN, KGP, FJR, RDS, GFW, and OW are members of the Consensus Panel. The Panel met twice in Paris and London at meetings organised and chaired by MJC and HNG. The first meeting critically reviewed the literature whereas the second meeting scrutinised the first draft of the Review. RAH, MA, JB, EB, ALC, JAK, PP, KKR, AFHS, ES, M-RT, AT-H, MJC, and HNG each drafted sections or outlines for the first version, and the complete draft was revised by RAH, MJC, and HNG. All Panel members agreed to the conception and design, contributed to interpretation of available data, and suggested revisions to this Review. All Panel members approved the final document before submission.
Conflicts of interest
RAH has received lecture honoraria, consultancy fees, or research funding from Aegerion, Amgen, Merck/Schering Plough, and Valeant. HNG has received lecture honoraria, consultancy fees, or research funding from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Genzyme, Hoffman-La Roche, Janssen, Kowa, Merck/Schering Plough, Novartis, Pfizer, and Sanofi-Aventis/Regeneron. MJC has received lecture honoraria, consultancy fees, or research funding from Aegerion, Amgen, AstraZeneca, Danone, Genzyme, Hoffman-La Roche, Kowa, Merck/Schering Plough, Pfizer, and Sanofi-Aventis/Regeneron. BGN has received lecture honoraria, consultancy fees, or research funding from Aegerion, AstraZeneca, ISIS Pharmaceuticals, Merck/Schering Plough, Pfizer, and Sanofi-Aventis/Regeneron. MA has received lecture honoraria, consultancy fees, or research funding from Aegerion, Genzyme, Hoffman-La Roche, Merck/Schering Plough, Pfizer, and Sanofi-Aventis/Regeneron. JB has received lecture honoraria, consultancy fees, or research funding from AstraZeneca, Merck/Schering Plough, Pfizer, and Sanofi-Aventis/Regeneron. EB has received lecture honoraria, consultancy fees, or research funding from Aegerion, AstraZeneca, Danone, Genfit, Genzyme, Hoffman-La Roche, Kraft, Merck/Schering Plough, Sanofi-Aventis/Regeneron, and Unilever. ALC has received lecture honoraria, consultancy fees, or research funding from Aegerion, Amgen, AstraZeneca, Genzyme, Kowa, Lilly, Merck/Schering Plough, Pfizer, and Sanofi-Aventis/Regeneron. OSD has received lecture honoraria, consultancy fees, or research funding from AstraZeneca, Merck/Schering Plough, Pfizer, Sanofi-Aventis/Regeneron, and Solvay. GKH has received lecture honoraria, consultancy fees, or research funding from Genzyme, Merck/Schering Plough, and Pfizer. SEH has received lecture honoraria, consultancy fees, or research funding from Genzyme. LM has received lecture honoraria, consultancy fees, or research funding from Amgen, AstraZeneca, Danone, Kowa, Merck/Schering Plough, Novartis, and Sanofi-Aventis/Regeneron. KGP has received lecture honoraria, consultancy fees, or research funding from Abbott, Aegerion, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Genzyme, ISIS Pharmaceuticals, Lilly, Merck/Schering Plough, Novartis, and Sanofi-Aventis/Regeneron. FJR has received lecture honoraria, consultancy fees, or research funding from Amgen, ISIS Pharmaceuticals, and Sanofi-Aventis/Regeneron. KKR has received lecture honoraria, consultancy fees, or research funding from Abbott, Aegerion, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Genzyme, Hoffman-La Roche, Kowa, Merck/Schering Plough, Novartis, Novo-Nordisk, Pfizer, Sanofi-Aventis/Regeneron, Solvay, and Takeda. RDS has received lecture honoraria, consultancy fees, or research funding from Aegerion, Amgen, AstraZeneca, Bristol-Myers Squibb, Genzyme, ISIS Pharmaceuticals, Merck/Schering Plough, Novo-Nordisk, Pfizer, and Sanofi-Aventis/Regeneron. AFHS has received lecture honoraria, consultancy fees, or research funding from Genzyme and Hoffman-La Roche. ES has received lecture honoraria, consultancy fees, or research funding from Bristol-Myers Squibb, Genzyme, ISIS Pharmaceuticals, and Sanofi-Aventis/Regeneron. M-RT has received lecture honoraria, consultancy fees, or research funding from AstraZeneca, Boehringer Ingelheim, Genzyme, Hoffman-La Roche, Kowa, Lilly, Merck/Schering Plough, Novartis, Novo-Nordisk, Pfizer, and Sanofi-Aventis/Regeneron. GFW has received lecture honoraria, consultancy fees, or research funding from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Genfit, Merck/Schering Plough, Pfizer, and Sanofi-Aventis/Regeneron. OW has received lecture honoraria, consultancy fees, or research funding from AstraZeneca, Merck/Schering Plough, Pfizer, and Sanofi-Aventis/Regeneron. JAK, PTK, PP, and AT-H declare that they have no conflicts of interest.
Contributor Information
Robert A Hegele, Department of Medicine, Western University, London, ON, Canada.
Henry N Ginsberg, Irving Institute for Clinical and Translational Research, Columbia University, New York, NY, USA.
M John Chapman, Dyslipidaemia and Atherosclerosis Research Unit, INSERM U939, Pitié-Salpêtrière University Hospital, Paris, France.
Børge G Nordestgaard, Department of Diagnostic Sciences, Herlev Hospital, University of Copenhagen, Denmark.
Jan Albert Kuivenhoven, Department of Molecular Genetics, University Medical Center Groningen, University of Groningen, Netherlands.
Maurizio Averna, Department of Internal Medicine, University of Palermo, Palermo, Italy.
Jan Borén, Strategic Research Center, Sahlgrenska Center for Cardiovascular and Metabolic Research, University of Gothenburg, Gothenburg, Sweden.
Eric Bruckert, Department of Endocrinology and Metabolism, Endocrinology and Cardiovascular Disease Prevention, Hôpital Pitié-Salpêtrière, Paris, France.
Alberico L Catapano, Department of Pharmacological Sciences, University of Milan and Multimedica IRCSS, Milan, Italy.
Olivier S Descamps, Centre de Recherche Médicale, Lipid Clinic, Hopital de Jolimont, Haine Saint-Paul, Belgium.
G Kees Hovingh, Department of Vascular Medicine, Academic Medical Center, University of Amsterdam, Amsterdam, Netherlands.
Steve E Humphries, Centre for Cardiovascular Genetics, Institute of Cardiovascular Science, University College London, London, UK.
Petri T Kovanen, Wihuri Research Institute, Helsinki, Finland.
Luis Masana, Vascular Medicine and Metabolism Unit, Sant Joan University Hospital, Universitat Rovira & Virgili, IISPV, CIBERDEM, Reus, Spain.
Päivi Pajukanta, Department of Human Genetics, David Geffen School of Medicine, University of California, Los Angeles, CA, USA.
Klaus G Parhofer, Department of Endocrinology and Metabolism, University of Munich, Munich, Germany.
Frederick J Raal, Division of Endocrinology and Metabolism, Director of the Carbohydrate and Lipid Metabolism Research Unit, University of the Witwatersrand, Johannesburg, South Africa.
Kausik K Ray, Cardiovascular Sciences Research Centre, St George's Hospital NHS Trust, London, UK.
Raul D Santos, Lipid Clinic Heart Institute (InCor), University of São Paulo Medical School Hospital, São Paulo, Brazil.
Anton F H Stalenhoef, Department of Internal Medicine, Radboud University Medical Center, Nijmegen, Netherlands.
Erik Stroes, Department of Vascular Medicine, Academic Medical Center, University of Amsterdam, Amsterdam, Netherlands.
Marja-Riitta Taskinen, Cardiovascular Research Group, Heart and Lung Centre, Helsinki University Central Hospital and Research Programs Unit, Diabetes and Obesity, University of Helsinki, Helsinki, Finland.
Anne Tybjærg-Hansen, Department of Clinical Biochemistry, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark.
Gerald F Watts, School of Medicine and Pharmacology, Royal Perth Hospital Unit, The University of Western Australia, Perth, WA, Australia.
Olov Wiklund, Department of Cardiology, Wallenberg Laboratory, Sahlgrenska University Hospital, Gothenburg, Sweden.
References
- 1.Berglund L, Brunzell JD, Goldberg AC, et al. the Endocrine Society Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2969–89. doi: 10.1210/jc.2011-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boullart AC, de Graaf J, Stalenhoef AF. Serum triglycerides and risk of cardiovascular disease. Biochim Biophys Acta. 2012;1821:867–75. doi: 10.1016/j.bbalip.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Chapman MJ, Ginsberg HN, Amarenco P, et al. the European Atherosclerosis Society Consensus Panel Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. 2011;32:1345–61. doi: 10.1093/eurheartj/ehr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuan G, Al-Shali KZ, Hegele RA. Hypertriglyceridemia: its etiology, effects and treatment. CMAJ. 2007;176:1113–20. doi: 10.1503/cmaj.060963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 6.Catapano AL, Reiner Z, De Backer G, et al. the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS), and the ESC Committee for Practice Guidelines 2008–2010 and 2010–2012 Committees ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Atherosclerosis 2011. 217(suppl 1):S1–44. doi: 10.1016/j.atherosclerosis.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Reiner Z, Catapano AL, De Backer G, et al. the European Association for Cardiovascular Prevention & Rehabilitation, and the ESC Committee for Practice Guidelines (CPG) 2008–2010 and 2010–2012 Committees ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011. 32:1769–818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 8.Sarwar N, Danesh J, Eiriksdottir G, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262 525 participants in 29 Western prospective studies. Circulation. 2007;115:450–58. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 9.Sarwar N, Sattar N. Triglycerides and coronary heart disease: have recent insights yielded conclusive answers? Curr Opin Lipidol. 2009;20:275–81. doi: 10.1097/MOL.0b013e32832dd4dc. [DOI] [PubMed] [Google Scholar]
- 10.Goussault Y, Turpin E, Neel D, et al. ‘Pseudohypertriglyceridemia’ caused by hyperglycerolemia due to congenital enzyme deficiency. Clin Chim Acta. 1982;123:269–74. doi: 10.1016/0009-8981(82)90171-1. [DOI] [PubMed] [Google Scholar]
- 11.Langsted A, Nordestgaard BG. Nonfasting lipids, lipoproteins, and apolipoproteins in individuals with and without diabetes: 58 434 individuals from the Copenhagen General Population Study. Clin Chem. 2011;57:482–89. doi: 10.1373/clinchem.2010.157164. [DOI] [PubMed] [Google Scholar]
- 12.Guerin M, Egger P, Soudant C, et al. Cholesteryl ester flux from HDL to VLDL-1 is preferentially enhanced in type IIB hyperlipidemia in the postprandial state. J Lipid Res. 2002;43:1652–60. doi: 10.1194/jlr.m200135-jlr200. [DOI] [PubMed] [Google Scholar]
- 13.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 14.Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, Nordestgaard BG. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA. 2008;300:2142–52. doi: 10.1001/jama.2008.621. [DOI] [PubMed] [Google Scholar]
- 15.Varbo A, Benn M, Tybjærg-Hansen A, Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61:427–36. doi: 10.1016/j.jacc.2012.08.1026. [DOI] [PubMed] [Google Scholar]
- 16.Jørgensen AB, Frikke-Schmidt R, West AS, Grande P, Nordestgaard BG, Tybjærg-Hansen A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J. 2013;34:1826–33. doi: 10.1093/eurheartj/ehs431. [DOI] [PubMed] [Google Scholar]
- 17.Nordestgaard BG, Benn M. Fasting and nonfasting LDL cholesterol: to measure or calculate? Clin Chem. 2009;55:845–47. doi: 10.1373/clinchem.2008.123083. [DOI] [PubMed] [Google Scholar]
- 18.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–16. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 19.Zilversmit DB. Atherogenesis: a postprandial phenomenon. Circulation. 1979;60:473–85. doi: 10.1161/01.cir.60.3.473. [DOI] [PubMed] [Google Scholar]
- 20.Nordestgaard BG, Wootton R, Lewis B. Selective retention of VLDL, IDL, and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo. Molecular size as a determinant of fractional loss from the intima-inner media. Arterioscler Thromb Vasc Biol. 1995;15:534–42. doi: 10.1161/01.atv.15.4.534. [DOI] [PubMed] [Google Scholar]
- 21.Norata GD, Grigore L, Raselli S, et al. Post-prandial endothelial dysfunction in hypertriglyceridemic subjects: molecular mechanisms and gene expression studies. Atherosclerosis. 2007;193:321–27. doi: 10.1016/j.atherosclerosis.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Malloy MJ, Kane JP. A risk factor for atherosclerosis: triglyceride-rich lipoproteins. Adv Intern Med. 2001;47:111–36. [PubMed] [Google Scholar]
- 23.Mamo JC, Proctor SD, Smith D. Retention of chylomicron remnants by arterial tissue; importance of an efficient clearance mechanism from plasma. Atherosclerosis. 1998;141(suppl 1):S63–69. doi: 10.1016/s0021-9150(98)00220-2. [DOI] [PubMed] [Google Scholar]
- 24.Di Angelantonio E, Sarwar N, Perry P, et al. the Emerging Risk Factors Collaboration Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng DS, Wong NC, Hegele RA. HDL—is it too big to fail? Nat Rev Endocrinol. 2013;9:308–12. doi: 10.1038/nrendo.2012.238. [DOI] [PubMed] [Google Scholar]
- 26.The Triglyceride Coronary Disease Genetics Consortium and Emerging Risk Factors Collaboration. Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet. 2010;375:1634–39. doi: 10.1016/S0140-6736(10)60545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Do R, Willer CJ, Schmidt EM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45:1345–52. doi: 10.1038/ng.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet. 2010;375:1875–84. doi: 10.1016/S0140-6736(10)60656-3. [DOI] [PubMed] [Google Scholar]
- 29.Sacks FM, Carey VJ, Fruchart JC. Combination lipid therapy in type 2 diabetes. N Engl J Med. 2010;363:692–94. doi: 10.1056/NEJMc1006407. [DOI] [PubMed] [Google Scholar]
- 30.Boden WE, Probstfield JL, Anderson T, et al. the AIM-HIGH Investigators Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–67. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 31.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308:1024–33. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 32.Fredrickson DS, Lees RS. A system for phenotyping hyperlipoproteinemia. Circulation. 1965;31:321–27. doi: 10.1161/01.cir.31.3.321. [DOI] [PubMed] [Google Scholar]
- 33.Hegele RA. Plasma lipoproteins: genetic influences and clinical implications. Nat Rev Genet. 2009;10:109–21. doi: 10.1038/nrg2481. [DOI] [PubMed] [Google Scholar]
- 34.Soutar AK, Naoumova RP. Mechanisms of disease: genetic causes of familial hypercholesterolemia. Nat Clin Pract Cardiovasc Med. 2007;4:214–25. doi: 10.1038/ncpcardio0836. [DOI] [PubMed] [Google Scholar]
- 35.Hegele RA, Ban MR, Hsueh N, et al. A polygenic basis for four classical Fredrickson hyperlipoproteinemia phenotypes that are characterized by hypertriglyceridemia. Hum Mol Genet. 2009;18:4189–94. doi: 10.1093/hmg/ddp361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johansen CT, Wang J, Lanktree MB, et al. An increased burden of common and rare lipid-associated risk alleles contributes to the phenotypic spectrum of hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2011;31:1916–26. doi: 10.1161/ATVBAHA.111.226365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johansen CT, Kathiresan S, Hegele RA. Genetic determinants of plasma triglycerides. J Lipid Res. 2011;52:189–206. doi: 10.1194/jlr.R009720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johansen CT, Hegele RA. Genetic bases of hypertriglyceridemic phenotypes. Curr Opin Lipidol. 2011;22:247–53. doi: 10.1097/MOL.0b013e3283471972. [DOI] [PubMed] [Google Scholar]
- 39.Johansen CT, Hegele RA. Allelic and phenotypic spectrum of plasma triglycerides. Biochim Biophys Acta. 2012;1821:833–42. doi: 10.1016/j.bbalip.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 40.Nordestgaard BG, Freiberg JJ. Clinical relevance of non-fasting and postprandial hypertriglyceridemia and remnant cholesterol. Curr Vasc Pharmacol. 2011;9:281–86. doi: 10.2174/157016111795495585. [DOI] [PubMed] [Google Scholar]
- 41.Talmud PJ, Shah S, Whittall R, et al. Use of low-density lipoprotein cholesterol gene score to distinguish patients with polygenic and monogenic familial hypercholesterolaemia: a case-control study. Lancet. 2013;381:1293–301. doi: 10.1016/S0140-6736(12)62127-8. [DOI] [PubMed] [Google Scholar]
- 42.Beil U, Grundy SM, Crouse JR, Zech L. Triglyceride and cholesterol metabolism in primary hypertriglyceridemia. Arteriosclerosis. 1982;2:44–57. doi: 10.1161/01.atv.2.1.44. [DOI] [PubMed] [Google Scholar]
- 43.Rahalkar AR, Giffen F, Har B, et al. Novel LPL mutations associated with lipoprotein lipase deficiency: two case reports and a literature review. Can J Physiol Pharmacol. 2009;87:151–60. doi: 10.1139/y09-005. [DOI] [PubMed] [Google Scholar]
- 44.Babirak SP, Iverius PH, Fujimoto WY, Brunzell JD. Detection and characterization of the heterozygote state for lipoprotein lipase deficiency. Arteriosclerosis. 1989;9:326–34. doi: 10.1161/01.atv.9.3.326. [DOI] [PubMed] [Google Scholar]
- 45.Hegele RA, Breckenridge WC, Cox DW, Maguire GF, Little JA, Connelly PW. Interaction between variant apolipoproteins C-II and E that affects plasma lipoprotein concentrations. Arterioscler Thromb. 1991;11:1303–09. doi: 10.1161/01.atv.11.5.1303. [DOI] [PubMed] [Google Scholar]
- 46.Nordestgaard BG, Abildgaard S, Wittrup HH, Steffensen R, Jensen G, Tybjaerg-Hansen A. Heterozygous lipoprotein lipase deficiency: frequency in the general population, effect on plasma lipid levels, and risk of ischemic heart disease. Circulation. 1997;96:1737–44. doi: 10.1161/01.cir.96.6.1737. [DOI] [PubMed] [Google Scholar]
- 47.Priore Oliva C, Pisciotta L, Li Volti G, et al. Inherited apolipoprotein A-V deficiency in severe hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2005;25:411–17. doi: 10.1161/01.ATV.0000153087.36428.dd. [DOI] [PubMed] [Google Scholar]
- 48.Johansen CT, Wang J, Lanktree MB, et al. Excess of rare variants in genes identified by genome-wide association study of hypertriglyceridemia. Nat Genet. 2010;42:684–87. doi: 10.1038/ng.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johansen CT, Wang J, McIntyre AD, et al. Excess of rare variants in non-genome-wide association study candidate genes in patients with hypertriglyceridemia. Circ Cardiovasc Genet. 2012;5:66–72. doi: 10.1161/CIRCGENETICS.111.960864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JH, Giannikopoulos P, Duncan SA, et al. The transcription factor cyclic AMP-responsive element-binding protein H regulates triglyceride metabolism. Nat Med. 2011;17:812–15. doi: 10.1038/nm.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–13. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson TJ, Grégoire J, Hegele RA, et al. update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2013. 2012;29:151–67. doi: 10.1016/j.cjca.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 53.Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4:337–45. doi: 10.1161/CIRCOUTCOMES.110.959247. [DOI] [PubMed] [Google Scholar]
- 54.Baigent C, Keech A, Kearney PM, et al. the Cholesterol Treatment Trialists’ (CTT) Collaborators Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 55.Morrone D, Weintraub WS, Toth PP, et al. Lipid-altering efficacy of ezetimibe plus statin and statin monotherapy and identification of factors associated with treatment response: a pooled analysis of over 21,000 subjects from 27 clinical trials. Atherosclerosis. 2012;223:251–61. doi: 10.1016/j.atherosclerosis.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 56.Boekholdt SM, Arsenault BJ, Mora S, et al. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: a meta-analysis. JAMA. 2012;307:1302–09. doi: 10.1001/jama.2012.366. [DOI] [PubMed] [Google Scholar]
- 57.Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98:2088–93. doi: 10.1161/01.cir.98.19.2088. [DOI] [PubMed] [Google Scholar]
- 58.University of Oxford Clinical Trial Service Unit and Epidemiological Studies Unit [Nov 28, 2013];HPS2-THRIVE. http://www.ctsu.ox.ac.uk/research/mega-trials/hps2-thrive.
- 59.Huijgen R, Abbink EJ, Bruckert E, et al. the Triple Study Group Colesevelam added to combination therapy with a statin and ezetimibe in patients with familial hypercholesterolemia: a 12-week, multicenter, randomized, double-blind, controlled trial. Clin Ther. 2010;32:615–25. doi: 10.1016/j.clinthera.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 60.Asselbergs FW, Guo Y, van Iperen EP, et al. the LifeLines Cohort Study Large-scale gene-centric meta-analysis across 32 studies identifies multiple lipid loci. Am J Hum Genet. 2012;91:823–38. doi: 10.1016/j.ajhg.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen R, Mias GI, Li-Pook-Than J, et al. Personal omics profiling reveals dynamic molecular and medical phenotypes. Cell. 2012;148:1293–307. doi: 10.1016/j.cell.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–99. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gaudet D, de Wal J, Tremblay K, et al. Review of the clinical development of alipogene tiparvovec gene therapy for lipoprotein lipase deficiency. Atheroscler Suppl. 2010;11:55–60. doi: 10.1016/j.atherosclerosissup.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 64.Stroes ES, Nierman MC, Meulenberg JJ, et al. Intramuscular administration of AAV1-lipoprotein lipase S447X lowers triglycerides in lipoprotein lipase-deficient patients. Arterioscler Thromb Vasc Biol. 2008;28:2303–04. doi: 10.1161/ATVBAHA.108.175620. [DOI] [PubMed] [Google Scholar]
- 65.Cuchel M, Meagher EA, du Toit Theron H, et al. the Phase 3 HoFH Lomitapide Study investigators Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet. 2013;381:40–46. doi: 10.1016/S0140-6736(12)61731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raal FJ, Santos RD, Blom DJ, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:998–1006. doi: 10.1016/S0140-6736(10)60284-X. [DOI] [PubMed] [Google Scholar]
- 67.Pollin TI, Damcott CM, Shen H, et al. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322:1702–05. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Musunuru K, Pirruccello JP, Do R, et al. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N Engl J Med. 2010;363:2220–27. doi: 10.1056/NEJMoa1002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.