Abstract
Peptides and peptidomimetics can function as immunomodulating agents by either blocking the immune response or stimulating the immune response to generate tolerance. Knowledge of B- or T-cell epitopes along with conformational constraints is important in the design of peptide-based immunomodulating agents. Work on the conformational aspects of peptides, synthesis and modified amino acid side chains have contributed to the development of a new generation of therapeutic agents for autoimmune diseases and cancer. The design of peptides/peptidomimetics for immunomodulation in autoimmune diseases such as multiple sclerosis, rheumatoid arthritis, systemic lupus and HIV infection is reviewed. In cancer therapy, peptide epitopes are used in such a way that the body is trained to recognize and fight the cancer cells locally as well as systemically.
Keywords: β-amino acid, cyclotide, immunomodulation, peptide-based vaccine, peptidomimetics, T-cell epitope
Immunomodulation
T cells of the immune system recognize invasive antigens or foreign materials and neutralize the invaders while sparing the body’s own tissues. Thus, T cells should be able to distinguish between ‘self’ and ‘non-self’ when neutralizing or destroying cells [1]. One of the earliest models proposed for T-cell activation of naive T cells to effector T cells requires several protein molecules that interact with one another at the junction of T cells and antigen-presenting cells (APCs) and consists of two signals. The first signal is provided by the interaction between a polymorphic receptor expressed on T cells and its ligand on the target cells or the APC as a MHC. The specificity of the immune response is determined by engagement of the T-cell receptors by peptide antigens bound within the groove of MHC proteins expressed on the surface of APCs. These APCs generally include dendritic cells, macrophages and B-lymphocytes. The second signal is provided by adhesion molecules and/or costimulatory ligands on the APC through corresponding counter-receptors on the T cells. Once the T cells are activated, they undergo clonal expansion to produce the immune response. During the immune response generation, the costimulatory signal (signal 2) is delivered by cell adhesion molecules, including CD2-CD58, LFA-1-ICAM-1 (CD11a-CD18-CD54) and CD28-B7 (CD28-CD80). The activation of effector T cells occurs through a multistep process activated by signal 1 and 2 with different adhesion molecules to generate the immune response [2,3].
The immune system can also cause pathological consequences due to various reasons. One of the first consequences occurs in the normal immune system when a healthy immune response to a transplant leads to transplant rejection. A second case is when tolerance to self is deregulated, leading to autoimmune diseases. Although the immune system is well regulated, autoimmunity occurs when autoreactive immune cells are triggered to activate their responses against self-tissues. This happens due to a lack of immunotolerance or to a breakdown of the mechanism that controls immune tolerance, resulting in failure of the host system to distinguish self from nonself cells. Autoimmune diseases may affect a single organ or multiple organs. Organ-specific diseases include celiac disease, Type 1 diabetes mellitus, multiple sclerosis (MS) and myasthenia gravis. Systemic diseases include rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) [4–6]. Another case occurs when the immune system becomes overreactive to harmless antigens, leading to allergy or hypersensitivity. In all of the above pathological cases, immunomodulation is necessary to control the consequences of a deregulated immune system. Immunomodulating agents can be separated into different categories, depending on their actions. For example, agents that suppress or block the immune system as in the case of autoimmune diseases, allergy/asthma [5], inflammation and transplantation; agents that stimulate or activate the immune system as in the case of viral infections and cancer; and agents that remove unwanted cellular subtypes of the immune system via specific antigens as in the case of autoimmune diseases and cancer. These modulating agents can be small organic molecules, antibodies, or peptides and peptidomimetics. In the present review, our focus is on peptides and peptidomimetics that modulate the immune response. We have covered the roles of peptides and peptidomimetics in immunomodulation and their possible therapeutic effects on autoimmune diseases and cancer. Since the list of autoimmune diseases is long (more than 100), we have concentrated our review on a few major autoimmune diseases and have tried to include the research efforts in this area for the past 5–7 years.
Peptides & peptidomimetics
The use of peptides as drugs began as early as the 1950s with the discovery of hormones and neurotransmitters and treatment with peptide-based drugs for hormonal therapy [7,8]. Peptide-based drug design gained momentum as 3D structures of proteins and their functions on cell surfaces as well as in cells were delineated. Particularly for the immune response, several proteins are involved on cell surfaces that interact with one another (Figure 1), forming an immunological synapse [9,10]. Structural and functional studies of the proteins have suggested that protein–protein interaction (PPI) is required for any cell signaling process. Protein–protein complexes are transient and reversible. In the immune response, these interactions are dynamic in nature and the signal depends on the strength and duration of interaction. The amino acids that are present on the surface of proteins provide them with high specificity and affinity yet dynamic binding character. Since PPI surfaces are made up of epitopes of amino acids, peptides are a relevant choice to modulate such interactions. Peptides act as a mimicking surface of one of the proteins, interfere with PPI and modulate the signaling. This is particularly important in immune response, since these molecules will not completely shut down the signaling process but, rather, modulate the signaling. Traditionally, PPIs are modulated by antibodies and there are several antibody drugs for immunomodulation in the market [11]. However, antibodies have limitations in terms of stability, delivery and, more importantly, immunogenicity. Even the humanized versions of antibodies elicit an immune response. In some cases, long-term administration of antibodies results in chronic problems [12,13]. Peptide-based drugs have advantages in terms of specificity and, generally, are nonimmunogenic and can be synthesized in large amounts. Most of the peptides will not have tertiary and quaternary structures, making them more stable compared with antibodies. Peptides combine the favorable properties of small-molecule drugs and protein therapeutics [14–16]. However, peptides have limitations in terms of in vivo enzymatic stability, short half-life, fast renal clearance and formulation challenges [17]. To overcome short half-life and low bioavailability, several strategies have been investigated that can be adopted in the design of peptide-based drugs [18]. In vivo stability of peptides can be enhanced by peptide backbone modification; this can be accomplished by introduction of unnatural amino acids or D-amino acids, peptide-bond modification, N- and C-termini modifications and constraining the backbone by introducing cyclization, resulting in molecules that are stable against enzymatic degradation [19–21]. Bioavailability and renal clearance problems can be overcome by PEGylation of the peptides. Modification of the backbone or side chain of peptides produces peptidomimetics. Peptidomimetics are compounds whose pharmacophore mimics a natural peptide or protein in 3D space with the ability to interact with the biological target and produce the same biological effect [8]. The idea behind this design is that proteins exert their biological effects through small regions on their surface called epitopes. A short sequence of peptides or functional groups that are close together can be reproduced in smaller, conformationally similar fragments that can bind to the receptor and provide steric hindrance between the receptor and the native protein ligand. Peptidomimetics have advantages over peptides in terms of stability and bioavailability associated with a natural peptide. Therefore, peptidomimetics have great potential in drug discovery. Peptidomimetics can have main- or side-chain modifications of the parent peptide designed for biological function (Figure 2A–2D) [22–25]. Some examples of peptidomimetics structures that are therapeutically useful and that are already in the market for cardiovascular disorder are shown in Figure 2E [26]. In terms of design considerations, peptidomimetics can be designed from protein epitopes with global or local conformational restrictions. Global conformational restrictions impose a particular shape or secondary structure on the peptide and also provide stability against enzymatic degradation. Examples of global conformational constraints include cyclization of the peptide using nonpeptide moieties, lactam bridges or inclusion of penicillamine (dimethyl cysteine) to form disulfide bonds. Local conformational restrictions can be applied using backbone modifications at particular amino acid residues or between two amino acid residues in the peptide. Backbone amides can be replaced by amide bond-like surrogates and isosteric substituents (Figure 2B) [27]. These backbone-modified mimetics can have regular amino acids. Side chains of amino acids in the peptides can be replaced with analogs of amino acids that have functional properties similar to those of amino acid side chains but with conformational restrictions of χ angles for side-chain rotation (Figure 2C). The side chain-modified peptidomimetics can expose the proper functional groups to bind with the targeted receptors with high affinity compared with normal side chains of amino acids. Another tactic to design the peptidomimetics is a minimalistic approach [28] where the secondary structure of the peptide epitope is mimicked using α-helical, β-turn or β-strand constraints to introduce organic functional groups (Figure 2D). The entire peptide backbone can be modified to mimic turn or helical structures using organic functional groups without any peptide bonds. The design of helical or turn mimetics provided by Hamilton et al. [29] and Hirschmann et al. [30] provides such peptidomimetics. However, synthesis of such mimetics requires extensive expertise in synthesis to achieve the desired product for biological investigation. In recent years, peptides and peptidomimetics have gained significant importance in various clinical areas such as immunology, endocrinology, urology and oncology. Most of the diseases in the body occur as a result of either overexpression or underexpression of certain proteins or PPIs. Since the epitope of a PPI is a peptide, strategies to design peptidomimetics to modulate this interaction are utilized in many pathological conditions. In this review, we will be focusing on the use of peptides and peptidomimetics as immunomodulators in the pathology of several autoimmune disorders, cancer and HIV. Furthermore, we will give a brief overview of cyclotides [31], which are used as templates to translate the pharmacophore designed in the peptide design strategy to multicyclic structures of naturally occurring, enzymatically stable peptides or miniproteins.
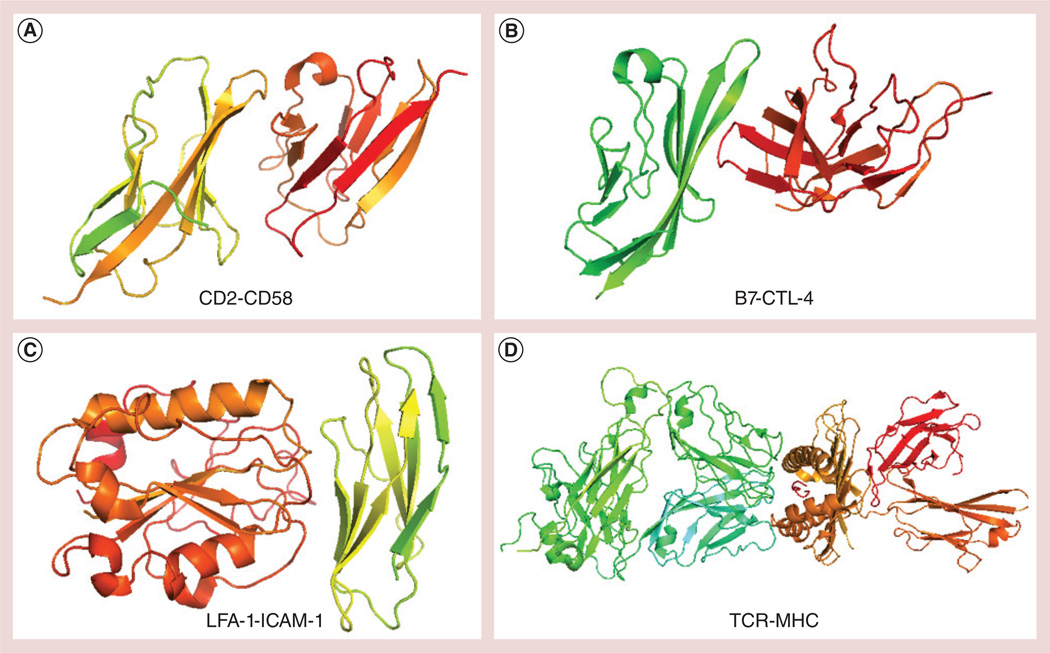
Figure 1. Crystal structures of protein complexes that are involved in adhesion or costimulation during immune response.
An array of these molecules on the T cell and antigen-presenting cell facilitates the contact between the cells apart from TCR-MHC molecules. (A) CD2-CD58 (Protein Data Bank ID: 1AQ9), (B) B7-CTL-4 (Protein Data Bank ID: 1I8L), (C) LFA-1-ICAM-1 (Protein Data Bank ID: 1MQ8) and (D) TCR-MHC (Protein Data Bank ID: 1G6R). CTL: Cytotoxic T lymphocyte; LFA: Leukocyte function-associated antigen; TCR: T-cell receptor.
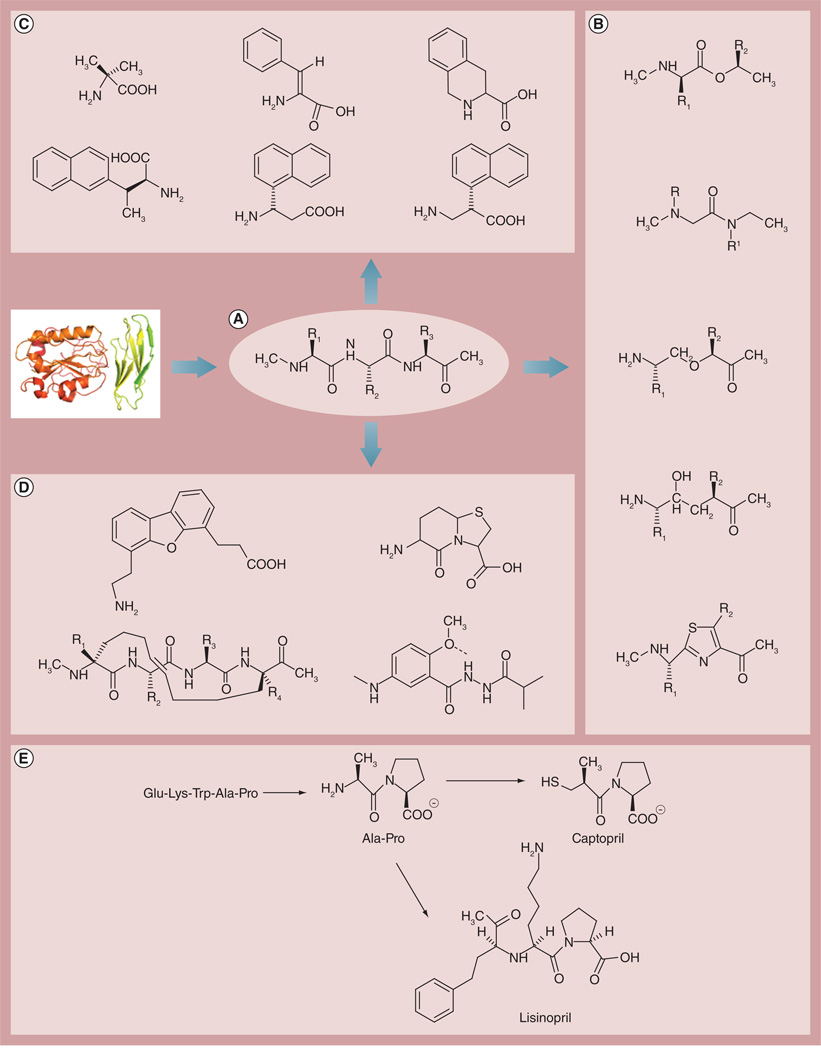
Figure 2. Design strategy for peptidomimetics.
(A) Epitope of a protein that has relevant biological function identified from functional studies. Peptidomimetic design based on (B) backbone modifications, (C) side-chain modifications using amino acid analogs and (D) secondary structures such as α-helix or β-turn mimetics [8,27–29]. (E) Examples of peptidomimetics that are therapeutically useful for cardiovascular disease [26].
Peptides/peptidomimetics as immunomodulators in autoimmune diseases
Historically, therapies for autoimmune diseases reduce the symptoms and, in many cases, include the suppression of the immune system. Most of the autoimmune diseases are inflammatory in nature and involve a T-cell response and cytokine production [32–34]. Hence, targeted therapies using biologics target particular proteins and PPIs. In recent years, many of the protein molecules that are overexpressed in certain disease pathologies have been studied in detail, and parts of the molecular mechanisms of biochemical pathways that lead to the disease have been elucidated. Hence, peptides and peptidomimetics are targeted to bind to these proteins and modulate the immune response. Several costimulatory molecules that are involved in inflammation and T-cell response have been targeted for autoimmune disease therapy [3]. In this review article, we will cover therapies for major autoimmune diseases such as RA, MS and SLE employing peptides and peptidomimetics. There are reports of opioid peptidomimetics that can be used for immunomodulation [35].
Rheumatoid arthritis
RA is a chronic, systemic inflammatory disease in which the immune and inflammatory systems are closely linked, resulting in the destruction of cartilage and bone. Apart from targeting the synovial lining of the joints, other organs such as the lungs, heart and blood vessels can also be affected. If the disease is untreated, it leads to permanent damage of the joints and significant impairment to the quality of life [36–38]. Current treatments for RA include NSAIDs, glucocorticoids and disease-modifying antirheumatic drugs; biological agents targeting TNF-α; IL-1; B cell-targeted therapy; and costimulation blockers [36,39]. It is well known that costimulatory molecules or cell adhesion molecules play a pivotal role in the recruitment of T cells to inflammatory sites. Molecular pairs such as ICAM-1-LFA-1, CD2-LFA3 (CD58) and CD28/CD152-CD80/CD86 bring the T cell and the APC into contact and help in the immunogical synapse, which results in signaling for inflammatory cytokines. Hence, modulation or blocking of adhesion and costimulatory molecules reduces the inflammatory cell accumulation and modifies the process of inflammation. Based on the PPIs of these molecules, peptides and peptidomimetics have been designed as possible therapeutic agents for RA [40–42].
The CD80/CD86–CD28/CD152 costimulatory pathways transmit signals for CD4+ T-cell activation and suppression and are critically involved in the pathogenesis of RA. Interactions between the B7 ligands on APC and the CD28/CD152 costimulatory receptors on T cells are also essential to the initiation and expansion of autoreactive T cells and survival in the target tissue. A significant number of CD4+ T cells and macrophages in the rheumatoid synovium express elevated levels of CD80. Using a rational design approach, Srinivasan et al. have designed polyproline types of peptides to inhibit CD80-CD28/CD152 interaction [43]. Using an ELISA assay, the authors have examined the binding of these peptides to CD80. In vivo studies using a collagen-induced arthritis (CIA) model suggested that a single administration of select CD80-competitive antagonist peptides significantly reduced the clinical, radiologic and histologic disease severity in CIA. Importantly, administration of CD80-competitive antagonist peptides during activated immune response significantly suppressed disease development by reducing mononuclear cell infiltration in the joints and mediating peripheral deletion of activated CD4+ T cells.
In our laboratory, we have developed peptides and peptidomimetics for immunomodulation by targeting CD2-CD58/CD48 costimulatory molecules. CD2-CD58 molecular pairs are important molecules that participate in cell adhesion between T cells and APCs in the early stages of immune response. The extracellular domain D1 of CD2 binds to CD58 in humans and to CD48 in rodents. The interaction between CD2 and CD58 is known to enhance the T-cell recognition of antigens and involves adhesion and signaling mechanisms. The structural details of the PPIs of CD2 and CD58 have been studied in detail (Figure 3) [44]. It is known that, in RA synovitis, upregulation of CD2 occurs in T cells [32]. CD58 expression is also known to be upregulated in inflammatory lesions in RA. CD2 is expressed on all T lymphocytes and CD58 on target cells or APCs. CD2 and CD58/CD48 PPIs are unique because the affinity of the interactions is relatively low (kd = µM); however, the specificity is high. There is poor shape complementarity between CD2-CD58/CD48 interactions. Based on structural and mutagenesis studies, we have designed a peptide to modulate CD2-CD58/CD48 interactions. Using in vitro assays, it has been shown that the designed peptide inhibits the cell adhesion interaction with an IC50 value of 6 nM. The peptide was modified to a peptidomimetic using a dibenzofuran moiety. The basis for the design of the peptidomimetic was the adhesion domain of CD2, which has β-sheet structure. Mutational studies suggested that the important amino acid residues are in the F and C strands of CD2 protein and that a β-turn structure stabilizes these strands [44,45]. Based on this structural information and using F and C β-strands of CD2 protein, peptidomimetics were designed (Figure 4A & 4B). Among these, peptidomimetic 7 was stabilized by a dibenzofuran moiety (Figure 4C). The peptidomimetic retained cell adhesion inhibition activity in the nanomolar range with an IC50 value of 11 nM. The peptide and peptidomimetic molecules were evaluated for immunomodulation using a CIA model. In vivo studies provided encouraging results in suppressing RA in the CIA animal model. Furthermore, the molecules designed suppressed the T-cell immune response in a transgenic animal model and were nonimmunogenic [40,41].
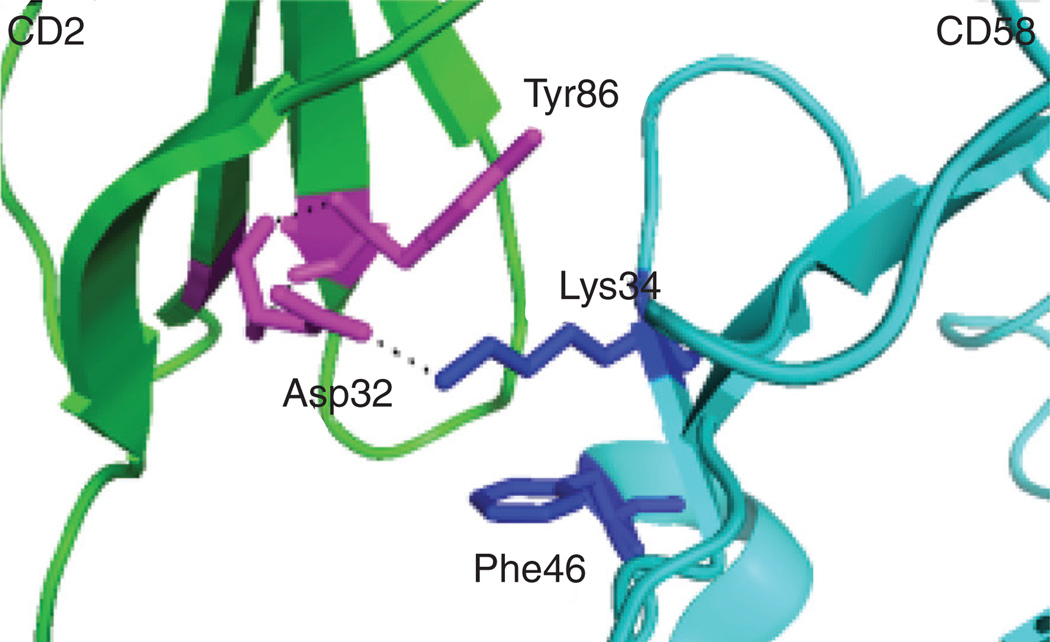
Figure 3. Hot spot in CD2-CD58 structure used for the design of CD2-based peptides for immunomodulation.
Tyr86 and Phe46 sandwich the Lys34 forming a hydrophobic interaction. Mutation of Tyr86 by Ala results in complete loss of binding of CD2 protein to CD58 suggesting hot-spot residues in the interaction of CD2 and CD58 [44].
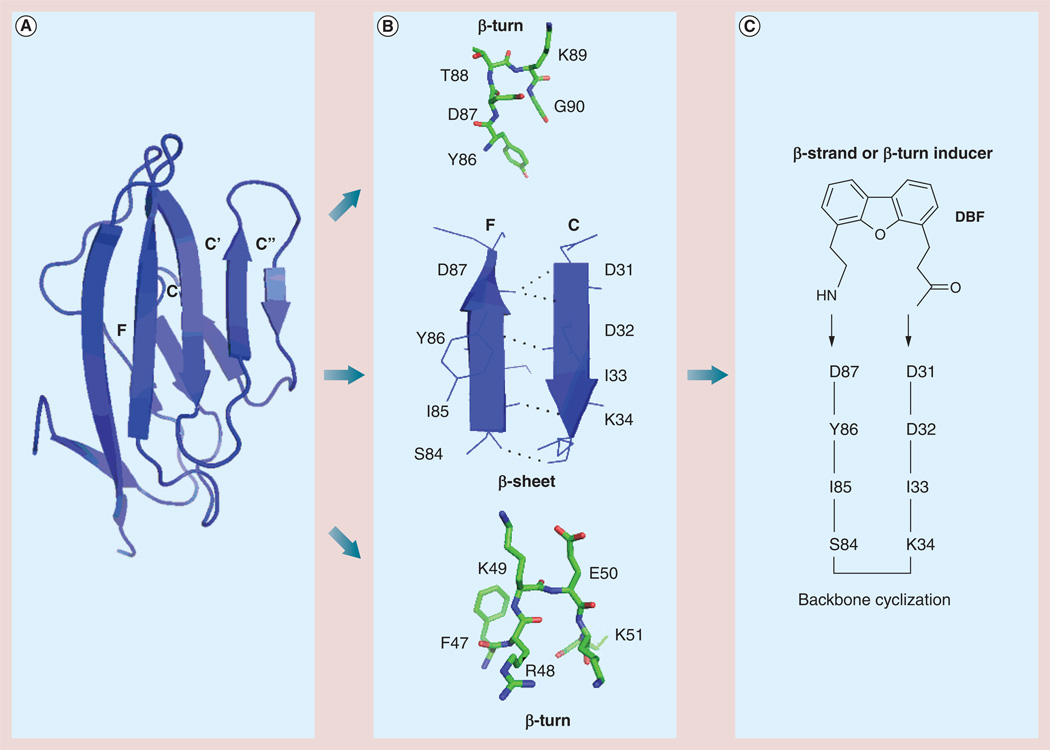
Figure 4. Design of a peptidomimetic for immunomodulation.
(A) Based on the hot-spot region of F and C strands of CD2 protein that binds to CD58, epitopes of secondary structure of CD2 protein were chosen. (B) Epitopes of CD2 protein from the adhesion domain that have β-strand and β-turn structures. (C) Based on F and C strands, a peptide was designed with backbone cyclization and insertion of β-hairpin, β-turn inducer DBF [3,40,46].
DBF: Dibenzofuran.
During the pathogenesis of RA, pannus formation in the joint tissue is one of the hallmarks of increased expression of extracellular matrix proteins, immune cell infiltration and neovascularization. Fibroblast-like synoviocytes serve as one of the primary players in joint destruction by producing proteolytic enzymes, including matrix metalloproteinases (MMPs). TGF inducible gene h3 (IG-H3) is an extracellular matrix protein highly expressed in rheumatoid synovium [47]. The expression of MMP-1, which is produced by synovial lining cells, is increased in synovial tissue during the early stages of RA [48]. The active form of MMP-1 is also abundant, along with increased expression of MMP-3, which is an activator of pro-MMP-1 [49]. A composite peptide was developed [50] based on results showing that the dhfas-1 and RGD (Arg-Gly-Asp) motifs had therapeutic effects, and that MMPs, especially MMP-1, are abundantly expressed in inflamed synovial tissue. The peptide consisted of dhfas-1 and RGD motifs linked by a substrate of MMP-1. The amino acid sequence of MMP-1 substrate GPQGIAG, which is specifically cleavable by active MMP-1, was selected for the design. The peptide (MEK24) was effective in inhibiting cellular functions and suppressing arthritis severity in mice with CIA. It was shown that MFK24 was specifically cleaved by MMP-1 in vitro and showed improved therapeutic efficacy in mice with CIA. In addition, inflammatory mediators in joint tissue at both the mRNA and protein levels were quantified in the peptide-treated groups. Transcripts of mediators, including receptor activator of NF-κB ligand, vascular cell adhesion molecule 1, TNF-α, MMP-1, MMP-3, IL-1, IL-6 and monocyte chemotactic protein-1, were significantly reduced by MFK24, and immunoblotting also revealed a substantial decrease in the expression of ICAM-1 and receptor activator of NF-κB ligand in the treatment groups. As mentioned in the introduction, cyclic peptides tend to be more stable in vivo and exhibit stable conformation for biological activity. Ali et al. have designed a nine-amino acid residue peptide from the transmembrane region epitope of T-cell antigen receptor α-chain [51]. The peptide core peptide (CP) was able to inhibit IL-2 production in T cells following antigen recognition. To overcome the problem of delivery of this peptide in vivo, they designed peptides with alternating D- and L-amino acids. They have shown that a cyclic peptide C1 with alternating D- and L-amino acids exhibits better stability and biological activity. The ability of C1 to modulate the immune response was evaluated in adjuvant arthritis in rats. The cyclic peptide was able to suppress adjuvant-induced arthritis in rats, and the results were comparable to that of immunosuppressive drug cyclosporine. Stability studies of linear and cyclic peptides CP and C1 suggested that the cyclic peptide C1 had significant stability in human skin compared with linear peptide CP. These studies highlight the importance of peptide-based drug design in the treatment of RA.
Another related autoimmune disease is psoriasis, in which the skin cells grow rapidly, causing inflammation and dry skin. The effects of the disease can range from moderate to severe. In some people, psoriasis causes joints to become swollen, tender and painful (psoriatic arthritis). Hyperproliferation of keratinocytes of the skin is observed in psoriasis. Several cytokines, expressed in keratinocytes of patients with psoriasis, have been recognized as inducing epidermal proliferation and inflammatory cell chemotaxis [52,53]. TNF-α targeted therapies with antibodies have proven to be efficacious in several clinical aspects of psoriasis [54]. However, such therapies resulted in side effects such as the incidence of serious infections (tuberculosis and neoplasms). New biological treatments such as ustekinumab, a human monoclonal antibody against the p40 subunit of IL-12/IL-23, have also been used in the treatment of moderate-to-severe psoriasis as well as psoriatic arthritis [55]. In an attempt to suppress the cytokine production in keratinocytes, peptidomimetics were designed and evaluated in keratinocytes. Peptide T (PT), an octapeptide derived from the gp120 coating protein, has been shown to be useful for psoriasis treatment. However, owing to the limitations of PT peptide for in vivo studies, peptidomimetic analogs of PT were designed. Using computational and synthetic approaches, a natural product, amygdalin, was identified as a mimic of PT. Owing to the toxic effect of amygdalin, different analogs of amygdalin were synthesized and evaluated for the production of inflammatory cytokines in human keratinocytes [56,57]. These mimetics were shown to reduce the proinflammatory cytokines such as IL-6, IL-8 and IFN-γ in human keratinocytes. However, we have to note here that the molecules designed do not resemble the peptide PT; rather, they mimic the action of PT.
Multiple sclerosis
MS is a demyelinating disease that occurs in genetically susceptible individuals. The pathogenesis of MS is known to result from antigen-specific autoimmunity in which autoreactive cells, T- and B-lymphocytes, directed at myelin-related peptides cause the destruction of myelin within the CNS [58]. Activated CD4+ T cells are known to infiltrate and cause the inflammation and are responsible for direct and indirect demyelination [59,60]. The pathogenesis of MS is regulated by myelin epitope-specific IgE, which, when critically affixed to myelin, may elicit degranulation of mast cells, leading to progression of the disease [61]. Dimeric IgE, when coupled to unique myelin-surface epitopes on proteolipid protein (PLP), myelin oligodendrocyte glycoprotein (MOG) and myelin basic protein (MBP) causes site-specific mast cell degranulation [62]. The most widely used method for evaluation of MS in animal models is experimental autoimmune encephalomyelitis (EAE), which has characteristics similar to those of MS. Polyproline helical structure peptides are common in immune response and in immunomodulation. PPI interfaces have proline-rich domains, and nearly 60% of mouse and human proteomes exhibit at least one proline-rich motif [63]. In glucocorticoidinduced leucine zipper (GILZ) protein, a proline-rich segment is known to bind to the p65 subunit of NF-κB. GILZ produces anti-inflammatory effects upon binding to p65 by inhibiting transactivation of inflammatory cytokines. Using an in silico approach Srinivasan and Janardhanam have designed a peptide using the C-terminal proline-rich domain of GILZ [64]. This peptide, GILZ-P, adopts a polyproline II helical structure and binds to p65 to inhibit translocation of p65, thereby suppressing the T-cell response. To facilitate the delivery of the GILZ-P peptide into the cells, an amphipathic chariot was used. It was shown that mixing of a chariot peptide with GILZ-P formed stable nanoparticle complexes. When the peptide was evaluated for T-cell immune response using in vitro assays, a significant decrease in T-cell response was seen in CD4+ lymph node cells. The peptide was also evaluated for its ability to prevent relapse of EAE using a single dose given at the time of induction of EAE in an autoimmune disease model. GILZ-P significantly suppressed the symptoms of MS. Furthermore, the peptide treatment suppressed the clinical relapse of EAE. This method provides new ways of targeting MS by incorporating the mechanisms of action of glucocorticoids and NF-κB.
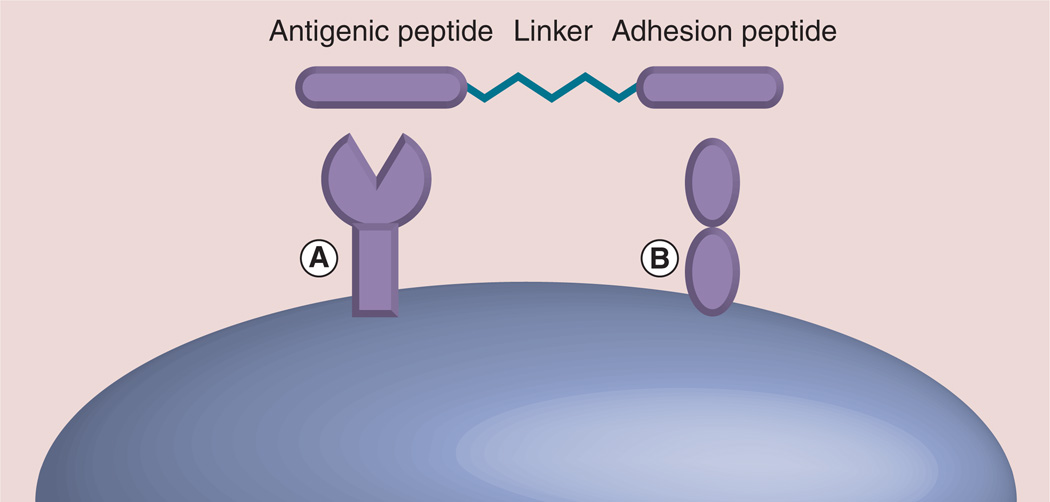
A new direction in the development of peptide-based drug design is targeting more than one binding site using bi- or multi-functional molecules. Bifunctional peptides have two functional epitopes joined by a linker with the two epitopes binding to different protein surfaces (Figure 5) or to two binding surfaces of the same protein to elicit a biological response of agonist or antagonist. The concept of multivalent or bivalent design is based on the fact that PPIs in biological responses are multivalent in nature [65–67]. Many receptor molecules are arranged in a cluster to generate their pharmacological action. This is particularly important in immune response because many adhesion and costimulatory molecules cluster on the cell surface and interact with one another to generate the immune response. This type of interaction provides optimum binding energy for the molecule, even though each individual interaction is weak (kd on the order of 1–20 µM) and dynamic. Thus, targeting multiple binding sites simultaneously improves the binding efficiency of the designed inhibitor [6,68,69]. Novel bifunctional peptide inhibitors (BPIs) have been designed to target APC and selectively inhibit immunological response toward a specific antigen [60,70–72]. These molecules have an antigenic peptide that binds to MHC II molecules and is covalently linked to an adhesion peptide that binds to an adhesion protein on the surface of APCs. The linker of the peptide consisted of aminocaproic acid or polyethylene glycol that facilitates the simultaneous binding of both pharmacophores to two binding sites. PLP is a major CNS-specific encephalitogenic antigenic peptide. CD4+ T cells are known to have reactivity to discrete PLP peptide determinants, resulting in development of acute, chronic relapsing and chronic progressive experimental autoimmune encephalomyelitis (EAE). Several cell adhesion peptide molecules that are derived from ICAM-1 or LFA-1 were linked to PLP139-151 and studied in an EAE model for the suppression of MS clinical symptoms. Badawi et al. have provided a proof of concept that BPI peptides could suppress EAE when injected subcutaneously [70]. These peptides can be administered for suppression of EAE before the induction of the disease or after the clinical signs appear. They have been shown to downregulate the production of proinflammatory cytokines and increase the production of regulatory cytokines. This same group of authors has conducted another interesting study in which a BPI peptide, namely Ac-PLP-BPI-NH2, was formulated for controlled release. The peptide was formulated into alginate-PLGA/chitosan-PLGA colloidal gels. One-time dosing of the peptide conjugate formulation using slow delivery acted as a vaccine-like administration. The results suggested that the formulation was effective in suppressing EAE as well as the relapse of EAE in the mouse model [71].
Figure 5. Design concept of bifunctional peptides.
The peptide sequence that binds to molecule (A) can be antigenic, and the peptide sequence that binds to (B) is specific for a surface receptor protein or cell adhesion molecule that is involved in immunomodulation. The two peptide sequences can be linked by glycine linker or by amino caproic or hexanoic acid or polyethylene glycol linkers. Using this strategy, both the peptide sequences bind simultaneously to two receptors. These peptide sequences can be modified to peptidomimetics for enzymatic stability [60,65,70].
Apart from its inflammatory effects during MS, it has recently been shown that myelin epitope-specific IgE, when critically affixed to myelin, could be eliciting degranulation of indrawn mast cells, thus causing and sustaining the MS condition [61,62]. Enzymes such as proteases released from the mast cells damage or disintegrate myelin and eventually damage the axon as well. It is postulated that mast cell degranulation is probably triggered by dimeric IgE coupled to unique myelin-surface epitopes on PLP, MOG and, possibly, myelin basic protein [61]. The unique structural epitope expressed on the myelin surface is a peptide sequence. PLP molecules are highly expressed on myelin. Based on these facts, a peptide epitope was designed to suppress the action of degranulation. Similarly, a strategy to block MOG-driven mast cell degranulation was undertaken with the help of IgE-neutralizing, mimotopic peptides HSYQE and KTGQF. This mimicking strategy was predicted to work since elimination of one of two dimer points would prevent adequate mast cell coupling, followed by degranulation. HSYQE was designed to suppress the outer-surface MOG dimers HSYQE–VTLRI and HSYQE–RNVRF. However, the solubility of HSYQE in aqueous media was found to be variable and, thus, a more soluble peptide substitute, DHSYQE, was synthesized. This peptide was found to retain the mimotopic structural uniqueness of HSYQE, but did not cause any immunogenicity [73]. The peptides were administered to a small group of patients in a pilot study. The results from the study demonstrated that peptide-based immunotherapy can eliminate myelin surface-directed, epitope-specific autoantibodies, especially IgE. The study also found that in the MS patients treated with peptides there was a suppression of myelin-directed mast cell degranulation and ongoing neuronal injury. The subjects also exhibited improved ambulatory function, as well as improved auditory discrimination and recovered tactile sensation. PLP peptides were also designed with the strategy of altered peptide ligand (APL) with thiopalmitoylation for therapeutic effect for MS. APLs are MHC presented, autoantigen-related peptides that are modified by amino acid substitution in the native self-peptides [74]. These peptides have modifications at the T-cell receptor interacting sites. PLP is normally thiopalmitoylated (attachment of palmitic acid via thioester bond to cysteine residue of a protein) and during demyelination, thiopalmitoylation is known to be increased. The enhanced demyelination due to thiopalmitoylation is known to be owing to increased uptake of these peptides into the MHC class II presentation pathway compared with nonpalmitoylated peptides. Cloake et al. used the altered PLP peptides that exhibit protective effect in EAE [75]. Conjugate peptides S-palm APLs exhibited enhanced protective effect against MS in the EAE model. They have shown that thiopalmitoylation of APL peptides increased the stability of peptides in serum, increased the uptake of peptide conjugates into MHC class II presentation pathway and increased the level of IL-10 enabling the peptide conjugate to inhibit encephalotogenecity in the model studied.
In another study, the myelin peptides were used as a vaccine against development of MS [76]. The immunogenic peptides were proposed to produce immunotolerance by modifying or altering them and administering at low concentration. Three myelin peptides, MBP85-99, PLP139-151 and MOG35-55, were designed. Since skin is the first line of immune defense, the immune system of skin possesses large numbers of immune cells that control the induction of immune response and tolerance. In this study, a mixture of three myelin peptides was administered as a skin patch to 30 patients with relapsing MS. Using MRI and clinical outcomes, the efficacy of the peptide treatment as a vaccine was evaluated. The results suggested that these peptides can be used to decrease lesions and also that the peptides were safe and without significant side effects. This therapy, which uses a skin patch, offers a very selective treatment for relapsing MS patients because it targets the immune response rather than blocking or shutting down the entire immune system. Overall, peptide-based therapies have seen some success in the case of MS, and a few candidates are in clinical trial.
Systemic lupus erythematosus
SLE is a chronic autoimmune disease characterized by the presence of pathogenic autoantibodies directed against double-stranded DNA (dsDNA) [77,78]. These dsDNA mediate both systemic and local inflammation and can also be used as diagnostic agents in SLE. Studies have shown that the production of anti-dsDNA antibodies affects the kidney and contributes to kidney disease in the majority of lupus patients [78]. N-methyl-D-aspartate receptor (NMDAR) is a glutamate receptor that controls synaptic plasticity and memory function. NMDAR is a heteromeric protein with subunits NR1 and NR2. Bloom et al. have shown that a nephritogenic mouse monoclonal anti-dsDNA antibody R4A binds to NR2A and NR2B subunits of mouse and human NMDAR [79]. The peptide epitope that recognizes the antibody consists of a pentapeptide sequence DWEYS [79]. The levels of crossreactive anti-dsDNA/NMDAR antibodies in human serum are found to be elevated and act as a biomarker for SLE [80–82]. An idea that has been developed for drug design is to inhibit the anti-dsDNA/NMDAR interaction that prevents antibodies from mediating tissue damage in SLE. It has been shown previously that in vivo administration of the D-isoform of the DWEYS peptide protects the tissue against anti-dsDNA/NMDAR antibody binding, thus blocking renal and brain deposition of antibodies and ameliorating ongoing disease in mouse lupus model. As indicated earlier, administration of peptide DWEYS in vivo presents problems such as bioavailability and stability. Bloom and his group have designed small molecules or peptidomimetics that contain the structural features of DWEYS peptide that neutralize anti-dsDNA/NMDAR antibodies. The molecular topologies of the DWEYS structure were mimicked using modifications of amino acid side chains such as replacement of the tryptophan and tyrosine residues, respectively, by 1,2,3,4-tetrahydroquinolin-3-ol and phenyl moieties immobilized on a polyamine scaffold [79,83]. The resulting peptidomimetic was FISLE-412, which inhibited the binding of dsDNA to NMDAR in vitro and in situ. The peptidomimetic, with its stable structure, will serve as a good candidate for the development of therapeutic agents for SLE treatment. The study clearly demonstrates the translation of a peptide scaffold to a peptidomimetic that is biologically active but stable against enzymatic degradation.
A phosphopeptide that modulates the immune response of CD4-expressing T cells has made advancement to Phase III development (Lupuzor™; ImmuPharma, London, UK) and is a promising peptide-based drug for the treatment for SLE [84]. Peptide P140 is a 21-mer linear peptide from the small nuclear ribonucleoprotein U1-70K and is phosphorylated at the Ser140 position [85]. Studies in the MRL/lpr lupus-prone murine model and using peripheral blood mononuclear cells from patients with SLE have shown that the peptide displays tolerogenic and immunomodulatory effects leading to the inhibition of T-cell reactivity with MHCpresented self-peptides [86]. The sequence of peptide (residues 131–151 of 70 K spliceosomal protein) is conserved in mice and human. Studies related to this peptide in mouse models of SLE (MRL/lpr mice) showed reduction in proteinuria, vasculitis and dermatitis and prevented the production of antibodies to dsDNA. In an open-label study of 20 patients with moderately active SLE, patients who received a low dose of peptide (200 µg) showed significant improvement in SLE, and the drug was generally well tolerated [87]. A study by Zimmer et al. showed that administration of 200 µg peptide via subcutaneous injection every 4 weeks significantly reduced disease activity in patients with SLE who were receiving standard of care [88]. Since these peptides are phosphopeptides, synthesis of these peptides for therapeutic purposes is challenging. Pertrillo et al. have developed a one-pot process of synthesis of phosphopeptide and phosphoamino acids [89]. The method described is less expensive compared with the previous methods available and uses readily available starting material for the synthesis of such modified peptides.
Cancer
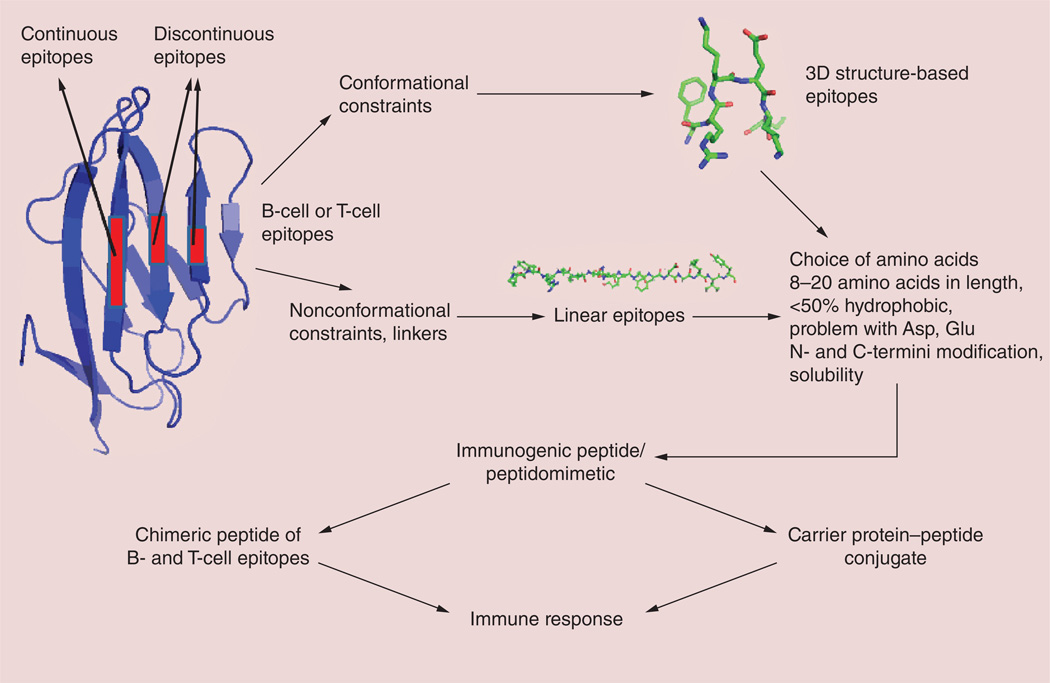
Immunotherapy has an advantage over radiation and chemotherapies in that it can act specifically against the tumor without damaging normal tissue. Most anticancer drugs have high toxicities or generate immune response and resistance, often resulting in relapse and metastasis [90–93]. The approach of immunomodulation in immunotherapy is a necessary new trend for cancer therapy. The idea is to train the immune system to recognize the tumor cells and kill them [94]. Antigen-specific cancer immunotherapy can achieve this goal by targeting systemic tumors. Although preventive vaccination has been known for a long time, therapeutic vaccination has not progressed like targeted biologics such as antibodies. This approach requires induction of cell-mediated immunity that is capable of attacking and eliminating antigen-bearing cells. There has been some success in cancer therapy using therapeutic vaccines [95–97], but the use of peptides and peptidomimetics for immunotherapy of cancer is a novel method that is in progress. Immunization with antigenic peptides containing B- or T-cell epitopes or both B- and T-cell epitopes can stimulate a patient’s own immune system to develop specific high-affinity antibodies. Once the antibodies are developed against a cancer antigen, the treatment can continue without frequent dosing of the peptide-based drugs. These peptides must be designed based on epitopes of antibodies or antigens [98]. The advantages of these peptide-based therapies are that they can be easily modified and, in most cases, they are nontoxic, do not develop resistance and are cost effective. Most of the development in this area is related to HER2-positive cancers or VEGF-related cancers; these have been reviewed extensively by Kaumaya et al. [98–101]. Here, we briefly describe some of these approaches. In the case of known proteins that can be used as B- or T-cell epitopes, the design is based on the binding region of the antibody. In the case of an unknown protein, prediction of the epitope has to be carried out with the available data on antigenicity, hydrophilicity and secondary structure propensity (Figure 6). Computer algorithms are available to predict the epitopes and several peptides need to be synthesized before achieving a suitable immunogenic epitope. 3D-structure templates such as helical structures or turn or loop structures can be used in the design to generate immunogenicity. Alternatively, epitopes may be eluted from the MHC proteins of antigen-fed APCs [16]. Some of the peptide- and peptidomimetic-based vaccines against HER2 and VEGF targets are now in clinical trials [98,102].
Figure 6. Design of an immunogenic peptide.
Linear or conformationally constrained peptides can be designed to generate an immune response for a particular antigenic sequence [94,98].
Immunotherapeutic approaches to pancreatic cancer have included the use of monoclonal antibodies, cytokine vaccines and lymphokine-activated killer cells [103]. The efficacy of a MUC1-peptide-pulsed dendritic cells in advanced pancreatic cancer patients has been reported [104]. In one study, approximately 75% of mice with palpable human papillomavirus (HPV)16-positive tumors were permanently cured by vaccination with a long peptide containing the immunodominant cytotoxic T lymphocyte epitope and an overlapping helper peptide in combination with the TLR ligand CpG [105,106]. The same group embarked on a study of the clinical aspects of these vaccine peptides. Overlapping peptides with 27–35 amino acids long of the complete sequence of the HPV16 E6 and E7 oncogenic proteins were tested in end-stage cervical cancer. The vaccine was well-tolerated and induced robust T-cell responses against multiple CD4 and CD8 epitopes of HPV16 E6 and E7 [105,107]. Recently, an attempt to design antigenic peptides highly specific against the cancer tumor was investigated using mutational analysis of tumors [23,108]. This is based on peptides encoded by mutated genes in cancer that are generated by analyzing the mutant proteins in a cancer patient and can act as T-cell epitopes. These peptides, called ‘m-peptides’, can be designed as peptide vaccines. At present, these approaches are still being evaluated in animal models by different researchers [109,110]. However, the described mutanome technology will help to design personalized medicines for cancer patients with cancers that are difficult to treat with existing approaches.
HIV
The design of peptidomimetics targeting HIV infection is not new. In fact, the original discovery of HIV protease inhibitors started with symmetric peptides that were later converted to peptidomimetics [111,112]. There are different targets for the design of drugs for HIV infection; the main ones are proteases, reverse transcriptase and integrase, and surface proteins gp41 and coreceptors. Since currently available drugs that target HIV infection develop resistance due to viral strains with multidrug resistance, new pathways are sought to stop the viral spread. During the transfer of viral DNA from the cytoplasm to nucleus, preintegration proteins play a major role. Inhibition of these preintegration complexes provides new ways of stopping HIV viral replication. The integrase C-terminal domain is known to interact with Vpr during the preintegration process. Vpr is one of the proteins of the HIV-1 preintegration complex, which is required in virus replication of nondividing cells such as macrophages [113]. Based on the original Vpr fragment, an octa-arginine conjugate was designed from the second region of the helix of the total Vpr protein. Arginine amino acid residue was incorporated in the peptide for cell membrane penetration. To retain the helical structure in the designed peptide, a stapling strategy was used. The designed molecule was a peptidomimetic with anti-HIV activity, reduced cytotoxicity and stability against enzymatic degradation. These peptidomimetics were efficient in modulating the immune responses occurring due to the integrase activity. In one of the other studies, α-helical peptidomimetics were designed from Rev protein, which is known to interact with HIV-1 Rev response element (RRE) RNA. The peptidomimetic was generated based upon a highly specific RRE-binding peptide, R6QR7, which was identified in a genetic selection experiment. The modified Rev peptide with α-helix constraint inserted bound to the RRE RNA with higher affinity and specificity [114]. Recently, an immunosuppressive motif-based peptide from the gp41 fusion protein was reported. This peptide termed ‘ISLAD CM’ was shown to have similar cell proliferation inhibition activity when the L-amino acids in the peptides were replaced with D-amino acids [115]. These peptides were also shown to inhibit proinflammatory cytokine secretion of pathogenic T cells. Such peptides can be used for treating T cell-mediated pathogenesis.
The PPI inhibition strategy was also used in the design of peptides and peptidomimetics to inhibit HIV viral replication. Particular domains of viral proteins interact with host-cell receptors to fuse and complete the viral replication cycle [116–118]. Hayouka et al. have designed peptides to inhibit the interaction of the integrase binding loop with protein LEDGF/p75 [119–121]. By using combinatorial library screening, they designed an epitope (amino acids 361–370) from the loops of LEDGF/p75. They used conformational constraints such as backbone cyclization or side-chain cyclization to make the linear epitope of LEDGF peptide stable against enzymatic degradation. Comparison of backbone versus side-chain cyclization suggested that side-chain-cyclized peptides exhibited comparable activities of integrase inhibition and HIV-1 replication inhibition in infected cells. From these studies, the authors concluded that, although backbone-cyclized peptides showed slightly higher potency of inhibition of integrase proteins compared with side-chain-cyclized peptides, in terms of difficulty, yield and overall cost of synthesis, the side-chain-cyclization strategy may be more efficient. The studies related to this type of cyclic peptides presented here can be used to design new peptides to inhibit PPIs of different receptors in viral and host cells [119].
Host defense peptides
Another class of peptides that are nature’s chemical libraries are host defense peptides (HDPs); they are also called antimicrobial peptides and have been known for a long time [122]. HDPs are produced by the immune systems of all multicellular organisms and are diverse in terms of sequence and structure. They are cationic in nature, and more than 2000 natural peptides are found in eukaryotes and bacteria. Apart from their cell-penetrating ability, these peptides have immunomodulatory properties that include reduction of the level of proinflammatory kinase in response to microbial molecules, modulation of the expression of chemokines, stimulation of angiogenesis and leukocyte activation. Typically, HDPs contain 12–50 amino acid residues in the sequence, a net positive charge ranging from +2 to +7, and >30% hydrophobic residues. Structurally, these peptides possess different secondary structures and can be classified into amphipathic α-helical peptides, β-sheet peptides stabilized by disulfide bridges or peptides with extended structures and loop structures. With such diverse sequences, their membrane-penetrating nature, and all possible secondary structures of proteins and immunomodulating properties, they serve as good templates for the design of peptide-based therapies for immunomodulation (readers can refer to recent reviews) [123–126]. However, even with all these benefits, these peptides have limitations as therapeutic agents because they are not stable in vivo and some of them are toxic to eukaryotic cells. Modifications of these peptides for stability and lower toxicity are needed before they can be exploited for immunomodulatory therapeutic purposes.
Cyclotides as templates for immunomodulation
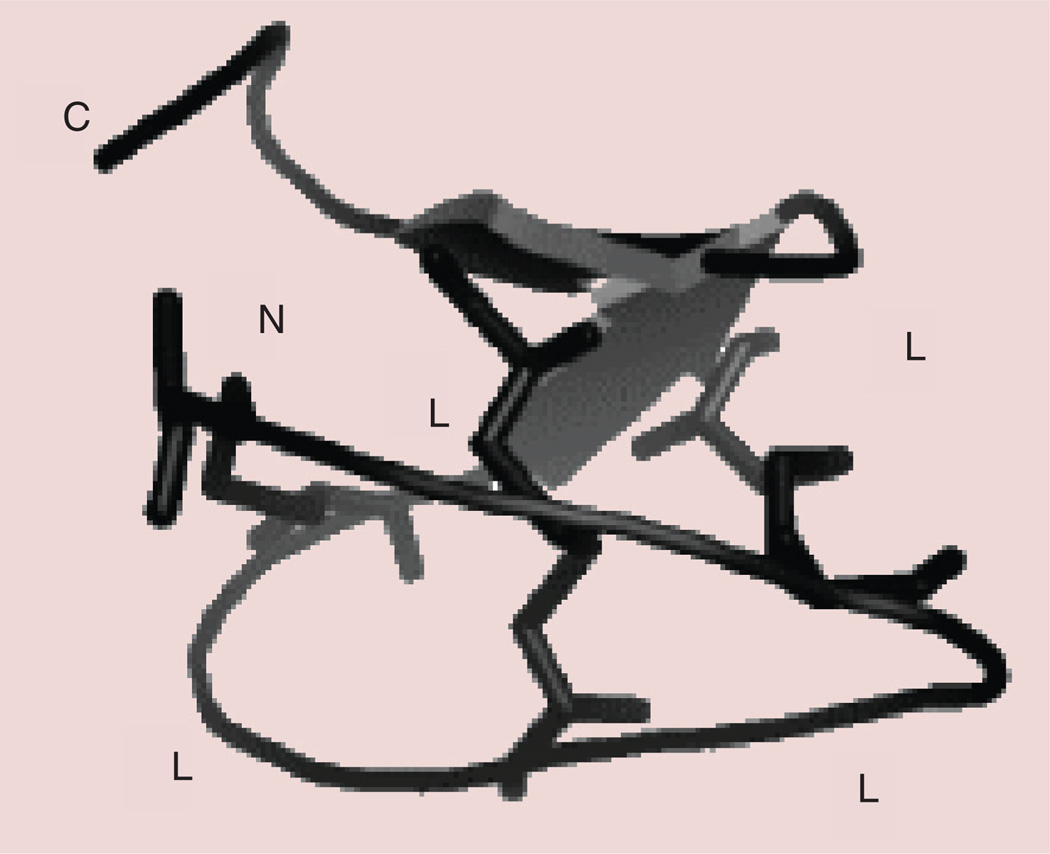
Cyclotides are plant-derived peptides (also called mini-proteins) consisting of 28–37 amino acids with a unique head-to-tail cyclized backbone stabilized by three disulfide bonds that form a cysteine-knot motif (Figure 7) [31]. Originally, these cyclotides were discovered in a tea ingredient that was used for medical purposes [127]. The idea that after boiling during tea preparation the peptide retained its activity led to the discovery of a therapeutically possible peptide template of these molecules. With their knotted structure that can be used as a template for drug design, these peptides are resistant to chemical, enzymatic and thermal treatment, making them a more versatile design for peptide-based therapeutics compared with other peptide-based therapeutics. Cyclotides are categorized into two subfamilies based on their structures. The Mobius strip structure has a twisted backbone structure whereas the bracelet structure has disulfide bridges without a twist, as the name suggests. Cyclotides are distinguished from most previously known, naturally occurring cyclic peptides in terms of their size, cysteine content and mechanism of biosynthesis [128]. Cyclotides are direct gene-coded products that are ribosomally synthesized and posttranslationally processed from linear precursor proteins. With their exceptional properties, they can be considered as natural combinatorial libraries that are intertwined within the constrained disulfide knots. One can insert any amino acid in the structure except the conserved cysteines that form the knot and, hence, biological epitopes can be grafted onto cyclotide structures.
Figure 7. Structure of a cyclotide that can be used for grafting an epitope.
L represents the loops that can be used for grafting peptide sequences. N and C refer to peptide N- and C-terminals, respectively. Disulfide bonds that form the knot are shown by sticks [128].
The immunomodulatory activity of cyclotides was investigated by several researchers [129,130], with the majority of the work reported by Craik et al. [128]. A cyclotide kalata B1 was used to suppress T-cell proliferation. Treatment of activated T cells with this compound decreased IL-2 surface expression and secretion. The peptide was also shown to decrease the effector function of the immune cells as indicated by reduced IFN-γ and TNF-α production [131]. Several chimeric molecules of cyclotides that contained a grafted sequence of MOG have been designed for immunosuppression [132]. A 21-amino-acid sequence of MOG with EAE activity was used to graft the pharmacophore onto the cyclotide. These studies illustrated an excellent structure–activity relationship of peptide sequences that can be grafted to cyclotides. It was found that long-sequence grafting was not tolerated in the molecule, and sequence composition rather than the length of the graft had a significant impact on the fold of the grafted cyclotide. Cyclotides have different loops and, from the structure–activity relationship studies, it was found that some loops could tolerate grafting while other loops did not result in good biological activity. Using the EAE model, the authors demonstrated that one of the grafted peptides, MOG3, displayed the ability to prevent the development of MS in mice.
Cyclotides have also been extensively studied for their anti-HIV activity. However, several of these studies demonstrated that there was no selectivity for HIV-infected and noninfected cells. Recently, cyclotides were used for the design of peptides targeting CXCR4, one out of 19 chemokine receptors. CXCR4 has several functions, such as promotion of chemotoxicity of leukocytes, cell migration and embryonic development of cardiovascular, hematopoietic and CNSs. It also acts as a coreceptor for viral entry into host cells during HIV replication. Several disulfide bonded peptides have been designed as CXCR4 antagonists to block HIV viral entry into host cells. However, they have limited stability and poor bioavailability. Aboye et al. have used the cyclotide MCoTI to graft a peptide antagonist for CXCR4 with an EC50 around 20 nM and HIV entry blocking around 2 nM (EC50) [129]. The designed molecule showed excellent serum stability. Such compounds will be lead compounds for therapeutic agents for HIV infection. The use of peptides for immune response is based on the fact that a small subunit of protein vaccine or antigen can be used; this has attracted a great deal of interest. The goal of vaccination design by peptides/peptidomimetics is to induce immunity by selectively stimulating antigen-specific T- and B-cell epitopes, using the smallest unit or chemical moiety that can be recognized by the immune system [94]. However, linear peptides are flexible and often do not mimic well conformational protein epitopes; they tend to have unfavorable in vivo stability due to proteolysis, and their ability to induce humoral immune responses (immunogenicity) is also very poor. Conformational constraints must be incorporated into the peptide epitopes from B cells in order to activate the B cells to generate a specific antibody response to a given antigen. This will assure that the peptide epitope possesses a conformation that is similar to that of the native antigen.
To design a peptide epitope to fold in a conformation that allows exposition of the correct epitope as the antigen, several approaches are available [133]. However, any approach in which conformational elements are incorporated requires some knowledge of the structure of the native antigen. The induction of cytotoxic T lymphocyte responses does not depend on the conformation of the epitope, and simple peptide epitopes composed of 8–10 amino acids are able to induce cytotoxic responses. Development in computational and bioinformatics tools has provided ways to design the peptide epitopes [134]. Conformational and nonconformational properties of the epitopes are included in the design of peptides for immunomodulation. In the case of peptides that block the immune response, peptide epitopes should be able to bind to the protein of interest and inhibit PPIs. In such cases, the peptide should be comparatively small in size, have high affinity, be stable against enzymatic degradation and, most importantly, be nonimmunogenic. In both the above cases, peptidomimetics are suitable choices. The incorporation of β-amino acids into epitopes can increase the binding affinity of the mimetic for the target molecule relative to the wild-type peptide [135]. The side chains of β-amino acids that replace the natural amino acid in the epitope should be identical to those of their parent α-amino acid to maintain the same physicochemical properties as the natural epitope. There are reports indicating that the introduction of a methylene moiety into peptides that are composed solely of β-amino acids results in complete resistance to proteolytic degradation [136,137]. In some cases, even a single amino acid substitution of the naturally occurring α-amino acid with the homologous β-amino acid residue can have dramatic effects on the overall stability of the entire peptide [135].
Conclusion & future perspective
Biologics or protein-/antibody-based therapeutics dominates the pharmaceutical market for treatment of immune and inflammatory diseases. More than 30% of the licensed pharmaceutical products are biologics that target autoimmune and inflammatory diseases and cancer. However, large portions of these come with immune-related safety warnings [13]. Peptide- and peptidomimetic-based therapy is gaining interest owing to the complications of unwanted side effects common in biologics. At present, there are more than 60 peptide-based approved drugs on the market and approximately 140 in clinical trials. Considering the fact that there are more than 100 autoimmune diseases and millions of people suffering from these diseases, immunomodulation by peptides and peptidomimetics is an area that has very high potential. Data from the literature suggest that there are two major areas of interest in which the size of the peptide molecule and its complex structure have to be investigated to make the druggable peptide molecule. In one case, the peptide molecule that is developed should block or modulate the immune response [138], and in another case, the peptide should induce an immune response for tolerance. Although there is abundant literature with respect to immunomodulation with peptides, translation of peptide immunotherapy into the clinical setting is slow. Most of these peptides and peptidomimetics are under clinical development for autoimmune diseases. The most notable of these are drugs for MS, Type 1 diabetes, and RA. There are also peptide molecules that are used for immunomodulation in disorders such as cat and ragweed allergies [139]. Overall, the optimization of peptides to peptidomimetics or peptides altered with D-amino acids or N- and C-termini modification as well as the chemistry developed for large-scale production of the desired active molecules will help the future of marketable peptide molecules. Major breakthroughs in peptide-based immunomodulation will come from the delivery of peptides. New methods are available for peptide-based delivery, including PEGylation, liposomal delivery and nasal delivery [18]. Apart from these, the technology allows identification of patient-specific epitopes based on their HLA haplotypes, which helps to tailor the peptides for personal medicine. Appropriate use of epitope engineering, chemical optimization and new delivery mechanisms will provide the next generation of peptide and peptidomimetic immunotherapeutics. Moreover, given the limitations of the therapeutic effects of current drugs for autoimmune disease, it will become necessary to design rational drugs based on peptidomimetics. In terms of cancer therapy, most of the available drugs develop resistance over a period of administration due to mutation. In such cases, training the body to fight cancer or injecting the tumor-associated antigen using peptide-based therapeutics that will result in an immune response to not only kill the tumor, but also provide a system response in case tumors develop in other areas, will be the ultimate therapy for cancer. In the next 10 years, peptidomimetic therapy will take a major step forward in cancer and immunotherapy. Two major challenges/barriers faced by the peptide-based drug design are oral delivery and cost of synthesis. These two barriers will be removed to a large extent in next 10 years. Chemical methods such as click chemistry [140–142] and peptide synthesis reactors that can handle large amounts of reaction material for solid-phase peptide synthesis have lowered the cost as well as improved the chemistry. The design of pseudo-peptides and the availability of β-amino acids as well as amino acid mimics will make the peptides stable against enzymatic degradation. In the future, peptidomimetic drugs that can be delivered via the mucosal route and skin patches will dominate, along with selected orally available peptidomimetic drugs. There will be challenges and competition from antibody drugs that can be produced in a relatively less expensive way due to the advancement in protein production in plant materials [143]. However, owing to the nonimmunogenic nature of peptidomimetics, these molecules will dominate the future pharmaceutical industry. Another major advancement that will help the field of peptidomimetic-based molecules as therapeutic agents is the available knowledge of PPIs. With the limitations of small molecules that can interact with flat surfaces of PPI sites, peptidomimetics will become an obvious choice. With more data available related to the 3D structure of protein complexes and PPIs and their importance in human diseases, peptide- and peptidomimetic-based therapeutic agents will become a major area of drug design, competing with natural products, synthetic small molecules and antibody-based therapies.
Overall, peptide- and peptidomimetic-based therapy has reached maturity, particularly in immunomodulation and vaccine-based therapy for cancer. Development of new platforms for delivery of peptides and discovery of epitopes of B cells will push the boundaries of peptide-based therapeutics to another level. With the understanding of costimulatory molecules that participate in immune response and the epitopes that are important in MS and SLE, peptide-based therapy will become a major program for autoimmune disease. Combined with the knowledge of combinatorial chemistry and structural biology, mimics of amino acids and 3D structural conformational constraints, new peptide molecules can be designed that have high specificity yet enzymatic stability. HDPs and cyclotides can provide a platform for the design and clinical application of peptides and peptidomimetics.
Executive summary.
Autoimmunity
Autoimmunity occurs when autoreactive immune cells are triggered to activate their responses against self-tissues.
Autoimmune diseases are chronic in nature.
When the immune system becomes overreactive to harmless antigens, allergy or hypersensitivity can occur.
Immunomodulating agents
Immunomodulating agents are used as therapeutic agents to modulate the immune response in autoimmune diseases.
Immunomodulating agents can be divided into three categories depending on their actions, namely: agents that suppress or block the immune system; agents that stimulate or activate the immune system; and agents that remove unwanted cellular subtypes of the immune system via specific antigens as in the case of autoimmune diseases and cancer.
Antibody therapy
Although antibody drugs/biologics are used as therapeutic agents against autoimmune diseases, they have limitations due to immunogenicity, stability and long-term chronic effects.
Peptides & peptidomimetics
Peptides and peptidomimetics are being used as new-generation molecules for the treatment of autoimmune diseases and cancer.
In some cases, the immune response is blocked by peptides; in other cases, immunotolerence is generated using epitope peptides. Peptides and peptidomimetics are designed to modulate the immune response in rheumatoid arthritis, multiple sclerosis and systemic lupus erythematosus by targeting costimulatory molecules or the MHC.
Cancer immunotherapy
The approach of immunomodulation in immunotherapy is a necessary new trend for cancer therapy.
The idea is to train the immune system to recognize the tumor cells and kill them. Antigen-specific cancer immunotherapy can achieve this goal by targeting systemic tumors.
Using peptides and peptidomimetics for immunotherapy of cancer is a novel method that is in progress.
Limitation of peptides & challenges
Although there is an abundant literature with respect to immunomodulation with peptides, translation of peptide immunotherapy into the clinical setting is slow. Most of the peptides and peptidomimetics are under clinical development for autoimmune diseases.
The limitations of the peptides can be overcome by modification of side chains or backbone, producing peptidomimetics that are stable against enzymatic degradation.
Cyclotides
Cyclotides are plant-derived peptides (also called mini-proteins) and with their knotted structure, they can be used as template for drug design and these peptides are resistant to chemical, enzymatic and thermal treatment, making them a most versatile design for peptide-based therapeutics.
Future direction
Development of new platforms for delivery of peptides and finding epitopes of B cells will push the boundaries of peptide-based therapeutics to another level.
Combined with the knowledge of combinatorial chemistry and structural biology, mimics of amino acids and 3D structural conformational constraints, new molecules of peptides can be designed that have high specificity yet enzymatic stability.
Host defense peptides and cyclotides provide a platform for design and clinical applications of peptides and peptidomimetics.
Acknowledgements
The authors would like to thank Nancy Harmony for help with editing the manuscript.
Research reported in this publication was supported by an Institutional Development Award from the National Institute of General Medical Sciences of the NIH under grant number 8P20GM103424.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- 1.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 2. Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013;13(4):227–242. doi: 10.1038/nri3405. •• This review article provides overview of cell adhesion molecules and their importance in immune response.
- 3.Jois SD, Jining L, Nagarajarao LM. Targeting T-cell adhesion molecules for drug design. Curr. Pharm. Design. 2006;12(22):2797–2812. doi: 10.2174/138161206777947696. [DOI] [PubMed] [Google Scholar]
- 4. Balague C, Kunkel SL, Godessart N. Understanding autoimmune disease: new targets for drug discovery. Drug Discov. Today. 2009;14(19–20):926–934. doi: 10.1016/j.drudis.2009.07.002. • Provides a good background for the readers regarding immune response and strategy for drug design.
- 5. Larche M, Wraith DC. Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat. Med. 2005;11(4 Suppl.):S69–S76. doi: 10.1038/nm1226. • Provides the details of peptide-based therapeutics for autoimmune diseases.
- 6.Chittasupho C, Siahaan TJ, Vines CM, Berkland C. Autoimmune therapies targeting costimulation and emerging trends in multivalent therapeutics. Ther. Deliv. 2011;2(7):873–889. doi: 10.4155/tde.11.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hruby VJ, Cai M. Design of peptide and peptidomimetic ligands with novel pharmacological activity profiles. Ann. Rev. Pharmacol. Toxicol. 2013;53:557–580. doi: 10.1146/annurev-pharmtox-010510-100456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vagner J, Qu H, Hruby VJ. Peptidomimetics, a synthetic tool of drug discovery. Curr. Opin. Chem. Biol. 2008;12(3):292–296. doi: 10.1016/j.cbpa.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen SD, Rawale SV, Whitacre CC, Kaumaya PT. Therapeutic peptidomimetic strategies for autoimmune diseases: costimulation blockade. J. Pept. Res. 2005;65(6):591–604. doi: 10.1111/j.1399-3011.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 10.Teft WA, Madrenas J. The immunological synapse as a novel therapeutic target. Curr. Opin. Invest. Drugs. 2006;7(11):1008–1013. [PubMed] [Google Scholar]
- 11. Sperandio O, Reynes CH, Camproux AC, Villoutreix BO. Rationalizing the chemical space of protein–protein interaction inhibitors. Drug Discov. Today. 2010;15(5–6):220–229. doi: 10.1016/j.drudis.2009.11.007. • Interesting article that provides good background for protein–protein interactions and drug design.
- 12.Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. The safety and side effects of monoclonal antibodies. Nat. Rev. Drug Discov. 2010;9(4):325–338. doi: 10.1038/nrd3003. [DOI] [PubMed] [Google Scholar]
- 13.Sathish JG, Sethu S, Bielsky MC, et al. Challenges and approaches for the development of safer immunomodulatory biologics. Nat. Rev. Drug Discov. 2013;12(4):306–324. doi: 10.1038/nrd3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mcgregor DP. Discovering and improving novel peptide therapeutics. Curr. Opin. Pharmacol. 2008;8(5):616–619. doi: 10.1016/j.coph.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Benyamini H, Friedler A. Using peptides to study protein–protein interactions. Future Med. Chem. 2010;2(6):989–1003. doi: 10.4155/fmc.10.196. [DOI] [PubMed] [Google Scholar]
- 16.Wraith DC. Therapeutic peptide vaccines for treatment of autoimmune diseases. Immunol. Lett. 2009;122(2):134–136. doi: 10.1016/j.imlet.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamman JH, Enslin GM, Kotze AF. Oral delivery of peptide drugs: barriers and developments. BioDrugs. 2005;19(3):165–177. doi: 10.2165/00063030-200519030-00003. [DOI] [PubMed] [Google Scholar]
- 18. Pollaro L, Heinis C. Strategies to prolong the plasma residence time of peptide drugs. MedChemComm. 2010;1(5):319–324. • Strategies for different aspects of peptide-based drug design for in vivo efficacy.
- 19.Marschutz MK, Zauner W, Mattner F, Otava A, Buschle M, Bernkop-Schnurch A. Improvement of the enzymatic stability of a cytotoxic T-lymphocyte-epitope model peptide for its oral administration. Peptides. 2002;23(10):1727–1733. doi: 10.1016/s0196-9781(02)00148-1. [DOI] [PubMed] [Google Scholar]
- 20. Gentilucci L, De Marco R, Cerisoli L. Chemical modifications designed to improve peptide stability: incorporation of non-natural amino acids, pseudo-peptide bonds, and cyclization. Curr. Pharm. Design. 2010;16(28):3185–3203. doi: 10.2174/138161210793292555. • Provides good background for peptidomimetic design and modification of peptides.
- 21.Schnatbaum K, Locardi E, Scharn D, et al. Peptidomimetic C5a receptor antagonists with hydrophobic substitutions at the C-terminus: increased receptor specificity and in vivo activity. Bioorg. Med. Chem. Lett. 2006;16(19):5088–5092. doi: 10.1016/j.bmcl.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 22.Ko E, Liu J, Perez LM, Lu G, Schaefer A, Burgess K. Universal peptidomimetics. J. Am. Chem. Soc. 2011;133(3):462–477. doi: 10.1021/ja1071916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vence LM, Wang C, Pappu H, et al. Chemical castration of melanoma patients does not increase the frequency of tumor-specific CD4 and CD8 T cells after peptide vaccination. J. Immunother. 2013;36(4):276–286. doi: 10.1097/CJI.0b013e31829419f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gante J. Peptidomimetics – tailored enzyme inhibitors. Angew. Chem. Int. Ed. Engl. 1994;33(17):1699–1720. [Google Scholar]
- 25.Giannis A, Kolter T. Peptidomimetics for receptor ligands – discovery, development, and medical perspectives. Angew. Chem. Int. Ed. Engl. 1993;32:1244–1267. [Google Scholar]
- 26.Acharya KR, Sturrock ED, Riordan JF, Ehlers MR. Ace revisited: a new target for structure-based drug design. Nat. Rev. Drug Discov. 2003;2(11):891–902. doi: 10.1038/nrd1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cudic P, Stawikowski M. Peptidomimetics: Fmoc solid-phase pseudopeptide synthesis. Methods Mol. Biol. 2008;494:223–246. doi: 10.1007/978-1-59745-419-3_13. • Experimental aspects of synthesis of peptidomimetics.
- 28.Ko E, Liu J, Burgess K. Minimalist and universal peptidomimetics. Chem. Soc. Rev. 2011;40(8):4411–4421. doi: 10.1039/c0cs00218f. [DOI] [PubMed] [Google Scholar]
- 29.Jayatunga MK, Thompson S, Hamilton AD. Alpha-helix mimetics: outwards and upwards. Bioorg. Med. Chem. Lett. 2014;24(3):717–724. doi: 10.1016/j.bmcl.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 30. Hirschmann RF, Nicolaou KC, Angeles AR, Chen JS, Smith AB., 3rd The beta-D-glucose scaffold as a beta-turn mimetic. Acc. Chem. Res. 2009;42(10):1511–1520. doi: 10.1021/ar900020x. • A new type of turn mimetics and synthesis strategies for such molecules.
- 31. Poth AG, Chan LY, Craik DJ. Cyclotides as grafting frameworks for protein engineering and drug design applications. Biopolymers. 2013;100(5):480–491. doi: 10.1002/bip.22284. •• Introductory article for cyclotides and its design strategy for grafting of cyclotides.
- 32.Webber A, Hirose R, Vincenti F. Novel strategies in immunosuppression: issues in perspective. Transplantation. 2011;91(10):1057–1064. doi: 10.1097/TP.0b013e3182145306. [DOI] [PubMed] [Google Scholar]
- 33.Devarajan P, Chen Z. Autoimmune effector memory T cells: the bad and the good. Immunol. Res. 2013;57(1–3):12–22. doi: 10.1007/s12026-013-8448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krogsgaard-Larsen PSKMU. Textbook of Drug Design and Discovery. FL, USA: CRC Press/Taylor & Francis; 2010. [Google Scholar]
- 35.Narayan P, Singh VK, Agarwal SS, et al. Immunomodulation by opioid peptidomimetic compound. Neuroimmunomodulation. 2001;9(3):134–140. doi: 10.1159/000049017. [DOI] [PubMed] [Google Scholar]
- 36.Gaffo A, Saag KG, Curtis JR. Treatment of rheumatoid arthritis. Am. J. Health Syst. Pharm. 2006;63(24):2451–2465. doi: 10.2146/ajhp050514. [DOI] [PubMed] [Google Scholar]
- 37.Bluml S, Scheinecker C, Smolen JS, Redlich K. Targeting TNF receptors in rheumatoid arthritis. Int. Immunol. 2012;24(5):275–281. doi: 10.1093/intimm/dxs047. [DOI] [PubMed] [Google Scholar]
- 38.Kavanaugh A, Smolen JS. The when and how of biologic agent withdrawal in rheumatoid arthritis: learning from large randomised controlled trials. Clin. Exp. Rheumatol. 2013;31(4) Suppl. 78:S19–S21. [PubMed] [Google Scholar]
- 39.Bossaller L, Rothe A. Monoclonal antibody treatments for rheumatoid arthritis. Expert Opin. Biol. Ther. 2013;13(9):1257–1272. doi: 10.1517/14712598.2013.811230. [DOI] [PubMed] [Google Scholar]
- 40.Gokhale A, Weldeghiorghis TK, Taneja V, Satyanarayanajois SD. Conformationally constrained peptides from CD2 to modulate protein-protein interactions between CD2 and CD58. J. Med. Chem. 2011;54(15):5307–5319. doi: 10.1021/jm200004e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gokhale A, Kanthala S, Latendresse J, Taneja V, Satyanarayanajois S. Immunosuppression by co-stimulatory molecules: inhibition of CD2-CD48/CD58 interaction by peptides from CD2 to suppress progression of collagen-induced arthritis in mice. Chem. Biol. Drug Design. 2013;82(1):106–118. doi: 10.1111/cbdd.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ali M, Manolios N. Peptide-based therapies for arthritis. Future Rheumatol. 2007;2(3):287–296. [Google Scholar]
- 43.Srinivasan M, Eri R, Zunt SL, Summerlin DJ, Brand DD, Blum JS. Suppression of immune responses in collagen-induced arthritis by a rationally designed CD80-binding peptide agent. Arthritis Rheum. 2007;56(2):498–508. doi: 10.1002/art.22324. [DOI] [PubMed] [Google Scholar]
- 44.Kim M, Sun ZY, Byron O, et al. Molecular dissection of the CD2-CD58 counter-receptor interface identifies CD2 Tyr86 and CD58 Lys34 residues as the functional ‘hot spot’. J. Mol. Biol. 2001;312(4):711–720. doi: 10.1006/jmbi.2001.4980. [DOI] [PubMed] [Google Scholar]
- 45.Wang JH, Smolyar A, Tan K, et al. Structure of a heterophilic adhesion complex between the human CD2 and CD58 (LFA-3) counterreceptors. Cell. 1999;97(6):791–803. doi: 10.1016/s0092-8674(00)80790-4. [DOI] [PubMed] [Google Scholar]
- 46.Satyanarayanajois SD, Buyuktimkin B, Gokhale A, Ronald S, Siahaan TJ, Latendresse JR. A peptide from the beta-strand region of CD2 protein that inhibits cell adhesion and suppresses arthritis in a mouse model. Chem. Biol. Drug Design. 2010;76(3):234–244. doi: 10.1111/j.1747-0285.2010.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nam EJ, Sa KH, You DW, et al. Up-regulated transforming growth factor beta-inducible gene h3 in rheumatoid arthritis mediates adhesion and migration of synoviocytes through alpha v beta3 integrin: regulation by cytokines. Arthritis Rheum. 2006;54(9):2734–2744. doi: 10.1002/art.22076. [DOI] [PubMed] [Google Scholar]
- 48.Yoshihara Y, Nakamura H, Obata K, et al. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann. Rheum. Dis. 2000;59(6):455–461. doi: 10.1136/ard.59.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rannou F, Francois M, Corvol MT, Berenbaum F. Cartilage breakdown in rheumatoid arthritis. Joint Bone Spine. 2006;73(1):29–36. doi: 10.1016/j.jbspin.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 50.Nam EJ, Kang JH, Sung S, et al. A matrix metalloproteinase 1-cleavable composite peptide derived from transforming growth factor beta-inducible gene h3 potently inhibits collagen-induced arthritis. Arthritis Rheum. 2013;65(7):1753–1763. doi: 10.1002/art.37932. [DOI] [PubMed] [Google Scholar]
- 51.Ali M, Amon M, Bender V, et al. Cyclization enhances function of linear anti-arthritic peptides. Clin. Immunol. 2014;150(1):121–133. doi: 10.1016/j.clim.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Girolomoni G, Mrowietz U, Paul C. Psoriasis: rationale for targeting interleukin-17. Br. J. Dermatol. 2012;167(4):717–724. doi: 10.1111/j.1365-2133.2012.11099.x. [DOI] [PubMed] [Google Scholar]
- 53.Ghoreschi K, Weigert C, Rocken M. Immunopathogenesis and role of T cells in psoriasis. Clinics Dermatol. 2007;25(6):574–580. doi: 10.1016/j.clindermatol.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 54.Alwawi EA, Krulig E, Gordon KB. Long-term efficacy of biologics in the treatment of psoriasis: what do we really know? Dermatol. Ther. 2009;22(5):431–440. doi: 10.1111/j.1529-8019.2009.01259.x. [DOI] [PubMed] [Google Scholar]
- 55.Tak PP, Kalden JR. Advances in rheumatology: new targeted therapeutics. Arthritis Res. Ther. 2011;13(Suppl. 1):S5. doi: 10.1186/1478-6354-13-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baroni A, Paoletti I, Greco R, et al. Immunomodulatory effects of a set of amygdalin analogues on human keratinocyte cells. Exp. Dermatol. 2005;14(11):854–859. doi: 10.1111/j.1600-0625.2005.00368.x. [DOI] [PubMed] [Google Scholar]
- 57.Paoletti I, De Gregorio V, Baroni A, Tufano MA, Donnarumma G, Perez JJ. Amygdalin analogues inhibit IFN-gamma signalling and reduce the inflammatory response in human epidermal keratinocytes. Inflammation. 2013;36(6):1316–1326. doi: 10.1007/s10753-013-9670-7. [DOI] [PubMed] [Google Scholar]
- 58.Mcfarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat. Immunol. 2007;8(9):913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 59.Lassmann H. Classification of demyelinating diseases at the interface between etiology and pathogenesis. Curr. Opin. Neurol. 2001;14(3):253–258. doi: 10.1097/00019052-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 60.Badawi AH, Siahaan TJ. Immune modulating peptides for the treatment and suppression of multiple sclerosis. Clin. Immunol. 2012;144(2):127–138. doi: 10.1016/j.clim.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Calenoff E. Interplaying factors that effect multiple sclerosis causation and sustenance. ISRN Neurol. 2012;851541 doi: 10.5402/2012/851541. (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsai M, Grimbaldeston M, Galli SJ. Mast cells and immunoregulation/immunomodulation. Adv. Exp. Med. Biol. 2011;716:186–211. doi: 10.1007/978-1-4419-9533-9_11. [DOI] [PubMed] [Google Scholar]
- 63.Ravi Chandra B, Gowthaman R, Raj Akhouri R, Gupta D, Sharma A. Distribution of proline-rich (PxxP) motifs in distinct proteomes: functional and therapeutic implications for malaria and tuberculosis. Protein Eng. Des. Sel. 2004;17(2):175–182. doi: 10.1093/protein/gzh024. [DOI] [PubMed] [Google Scholar]
- 64.Srinivasan M, Janardhanam S. Novel p65 binding glucocorticoid-induced leucine zipper peptide suppresses experimental autoimmune encephalomyelitis. J. Biol. Chem. 2011;286(52):44799–44810. doi: 10.1074/jbc.M111.279257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu L, Vagner J, Josan J, et al. Enhanced targeting with heterobivalent ligands. Mol. Cancer Ther. 2009;8(8):2356–2365. doi: 10.1158/1535-7163.MCT-08-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu L, Josan JS, Vagner J, et al. Heterobivalent ligands target cell-surface receptor combinations in vivo. Proc. Natl Acad. Sci. USA. 2012;109(52):21295–21300. doi: 10.1073/pnas.1211762109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kiessling LL, Gestwicki JE, Strong LE. Synthetic multivalent ligands as probes of signal transduction. Angew. Chem. 2006;45(15):2348–2368. doi: 10.1002/anie.200502794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnson RN, Kopeckova P, Kopecek J. Synthesis and evaluation of multivalent branched HPMA copolymer-Fab’ conjugates targeted to the B-cell antigen CD20. Bioconjug. Chem. 2009;20(1):129–137. doi: 10.1021/bc800351m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caplan MR, Rosca EV. Targeting drugs to combinations of receptors: a modeling analysis of potential specificity. Ann. Biomed. Eng. 2005;33(8):1113–1124. doi: 10.1007/s10439-005-5779-1. [DOI] [PubMed] [Google Scholar]
- 70.Badawi AH, Kiptoo P, Wang WT, et al. Suppression of EAE and prevention of blood-brain barrier breakdown after vaccination with novel bifunctional peptide inhibitor. Neuropharmacology. 2012;62(4):1874–1881. doi: 10.1016/j.neuropharm.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buyuktimkin B, Wang Q, Kiptoo P, Stewart JM, Berkland C, Siahaan TJ. Vaccine-like controlled-release delivery of an immunomodulating peptide to treat experimental autoimmune encephalomyelitis. Mol. Pharm. 2012;9(4):979–985. doi: 10.1021/mp200614q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Badawi AH, Siahaan TJ. Suppression of MOG- and PLP-induced experimental autoimmune encephalomyelitis using a novel multivalent bifunctional peptide inhibitor. J. Neuroimmunol. 2013;263(1–2):20–27. doi: 10.1016/j.jneuroim.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Calenoff E. Mimotopic peptide immunotherapy for the treatment of multiple sclerosis, an inflammatory autoimmune disease. Am. J. Clin. Exp. Immunol. 2013;2(3):208–221. [PMC free article] [PubMed] [Google Scholar]
- 74.Sloan-Lancaster J, Allen PM. Altered peptide ligand-induced partial T cell activation: molecular mechanisms and role in T cell biology. Annu. Rev. Immunol. 1996;14:1–27. doi: 10.1146/annurev.immunol.14.1.1. [DOI] [PubMed] [Google Scholar]
- 75.Cloake NC, Beaino W, Trifilieff E, Greer JM. Thiopalmitoylation of altered peptide ligands enhances their protective effects in an animal model of multiple sclerosis. J. Immunol. 2014;192(5):2244–2251. doi: 10.4049/jimmunol.1301871. [DOI] [PubMed] [Google Scholar]
- 76.Walczak A, Siger M, Ciach A, Szczepanik M, Selmaj K. Transdermal application of myelin peptides in multiple sclerosis treatment. JAMA Neurol. 2013;70(9):1105–1109. doi: 10.1001/jamaneurol.2013.3022. [DOI] [PubMed] [Google Scholar]
- 77.Waters ST, Mcduffie M, Bagavant H, et al. Breaking tolerance to double stranded DNA, nucleosome, and other nuclear antigens is not required for the pathogenesis of lupus glomerulonephritis. J. Exp. Med. 2004;199(2):255–264. doi: 10.1084/jem.20031519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bertsias GK, Salmon JE, Boumpas DT. Therapeutic opportunities in systemic lupus erythematosus: state of the art and prospects for the new decade. Ann. Rheum. Dis. 2010;69(9):1603–1611. doi: 10.1136/ard.2010.135186. [DOI] [PubMed] [Google Scholar]
- 79.Bloom O, Cheng KF, He M, et al. Generation of a unique small molecule peptidomimetic that neutralizes lupus autoantibody activity. Proc. Natl Acad. Sci. USA. 2011;108(25):10255–10259. doi: 10.1073/pnas.1103555108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Degiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat. Med. 2001;7(11):1189–1193. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- 81.Aranow C, Diamond B, Mackay M. Glutamate receptor biology and its clinical significance in neuropsychiatric systemic lupus erythematosus. Rheum. Dis. Clinics North Am. 2010;36(1):187–201. doi: 10.1016/j.rdc.2009.12.007. x–xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fragoso-Loyo H, Cabiedes J, Orozco-Narvaez A, et al. Serum and cerebrospinal fluid autoantibodies in patients with neuropsychiatric lupus erythematosus. Implications for diagnosis and pathogenesis. PLoS One. 2008;3(10):e3347. doi: 10.1371/journal.pone.0003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kowal C, Degiorgio LA, Lee JY, et al. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc. Natl Acad. Sci. USA. 2006;103(52):19854–19859. doi: 10.1073/pnas.0608397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koutsokeras T, Healy T. Systemic lupus erythematosus and lupus nephritis. Nat. Rev. Drug Discov. 2014;13(3):173–174. doi: 10.1038/nrd4227. [DOI] [PubMed] [Google Scholar]
- 85.Monneaux F, Muller S. Peptide-based therapy in lupus: promising data. Adv. Exp. Med. Biol. 2007;601:105–112. doi: 10.1007/978-0-387-72005-0_11. [DOI] [PubMed] [Google Scholar]
- 86.Page N, Gros F, Schall N, et al. HSC70 blockade by the therapeutic peptide P140 affects autophagic processes and endogenous MHCII presentation in murine lupus. Ann. Rheum. Dis. 2011;70(5):837–843. doi: 10.1136/ard.2010.139832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Muller S, Monneaux F, Schall N, et al. Spliceosomal peptide P140 for immunotherapy of systemic lupus erythematosus: results of an early Phase II clinical trial. Arthritis Rheum. 2008;58(12):3873–3883. doi: 10.1002/art.24027. [DOI] [PubMed] [Google Scholar]
- 88.Zimmer R, Scherbarth HR, Rillo OL, Gomez-Reino JJ, Muller S. Lupuzor/P140 peptide in patients with systemic lupus erythematosus: a randomised, double-blind, placebo-controlled Phase IIb clinical trial. Ann. Rheum. Dis. 2013;72(11):1830–1835. doi: 10.1136/annrheumdis-2012-202460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Petrillo DE, Mowrey DR, Allwein SP, Bakale RP. A general preparation of protected phosphoamino acids. Org. Lett. 2012;14(5):1206–1209. doi: 10.1021/ol203332j. [DOI] [PubMed] [Google Scholar]
- 90.Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nat. Rev. Cancer. 2009;9(1):28–39. doi: 10.1038/nrc2559. [DOI] [PubMed] [Google Scholar]
- 91.Livshits Z, Rao RB, Smith SW. An approach to chemotherapy-associated toxicity. Emerg. Med. Clinics North Am. 2014;32(1):167–203. doi: 10.1016/j.emc.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 92.Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat. Rev. Clin. Oncol. 2012;9(1):16–32. doi: 10.1038/nrclinonc.2011.177. [DOI] [PubMed] [Google Scholar]
- 93.Eskens FA, Verweij J. The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors; a review. Eur. J. Cancer. 2006;42(18):3127–3139. doi: 10.1016/j.ejca.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 94. Flower DR. Designing immunogenic peptides. Nat. Chem. Biol. 2013;9(12):749–753. doi: 10.1038/nchembio.1383. •• Provides design strategy for immunogenic peptides.
- 95.Bedke J, Stenzl A. IMA901: a peptide vaccine in renal cell carcinoma. Expert Opin. Invest. Drugs. 2013;22(10):1329–1336. doi: 10.1517/13543784.2013.822066. [DOI] [PubMed] [Google Scholar]
- 96.Arens R, Van Hall T, Van Der Burg SH, Ossendorp F, Melief CJ. Prospects of combinatorial synthetic peptide vaccine-based immunotherapy against cancer. Semin. Immunol. 2013;25(2):182–190. doi: 10.1016/j.smim.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 97.Perez SA, Von Hofe E, Kallinteris NL, et al. A new era in anticancer peptide vaccines. Cancer. 2010;116(9):2071–2080. doi: 10.1002/cncr.24988. [DOI] [PubMed] [Google Scholar]
- 98. Kaumaya PT, Foy KC. Peptide vaccines and targeting HER and VEGF proteins may offer a potentially new paradigm in cancer immunotherapy. Future Oncol. 2012;8(8):961–987. doi: 10.2217/fon.12.95. • Anticancer vaccine overview.
- 99.Foy KC, Miller MJ, Moldovan N, Bozanovic T, Carson WE, III, Kaumaya PT. Immunotherapy with HER-2 and VEGF peptide mimics plus metronomic paclitaxel causes superior antineoplastic effects in transplantable and transgenic mouse models of human breast cancer. Oncoimmunology. 2012;1(7):1004–1016. doi: 10.4161/onci.21057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kaumaya PT. Bridging oncology and immunology: expanding horizons with innovative peptide vaccines and peptidomimetics. Immunotherapy. 2013;5(11):1159–1163. doi: 10.2217/imt.13.128. [DOI] [PubMed] [Google Scholar]
- 101.Miller MJ, Foy KC, Kaumaya PT. Cancer immunotherapy: present status, future perspective, and a new paradigm of peptide immunotherapeutics. Discov. Med. 2013;15(82):166–176. [PubMed] [Google Scholar]
- 102.Vicari D, Foy KC, Liotta EM, Kaumaya PT. Engineered conformation-dependent VEGF peptide mimics are effective in inhibiting VEGF signaling pathways. J. Biol. Chem. 2011;286(15):13612–13625. doi: 10.1074/jbc.M110.216812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kondo H, Hazama S, Kawaoka T, et al. Adoptive immunotherapy for pancreatic cancer using MUC1 peptide-pulsed dendritic cells and activated T lymphocytes. Anticancer Res. 2008;28(1B):379–387. [PubMed] [Google Scholar]
- 104.Rong Y, Qin X, Jin D, et al. A Phase I pilot trial of MUC1-peptide-pulsed dendritic cells in the treatment of advanced pancreatic cancer. Clin. Exp. Med. 2012;12(3):173–180. doi: 10.1007/s10238-011-0159-0. [DOI] [PubMed] [Google Scholar]
- 105.Welters MJ, Kenter GG, Piersma SJ, et al. Induction of tumorspecific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin. Cancer Res. 2008;14(1):178–187. doi: 10.1158/1078-0432.CCR-07-1880. [DOI] [PubMed] [Google Scholar]
- 106.Zwaveling S, Ferreira Mota SC, Nouta J, et al. Established human papillomavirus type 16-expressing tumors are effectively eradicated following vaccination with long peptides. J. Immunol. 2002;169(1):350–358. doi: 10.4049/jimmunol.169.1.350. [DOI] [PubMed] [Google Scholar]
- 107.Kenter GG, Welters MJ, Valentijn AR, et al. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin. Cancer Res. 2008;14(1):169–177. doi: 10.1158/1078-0432.CCR-07-1881. [DOI] [PubMed] [Google Scholar]
- 108.Overwijk WW, Wang E, Marincola FM, Rammensee H-G, Restifo NP. Mining the mutanome: developing highly personalized immunotherapies based on mutational analysis of tumors. J. Immunother. Cancer. 2013;1(11):1–4. doi: 10.1186/2051-1426-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Matsushita H, Vesely MD, Koboldt DC, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482(7385):400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Robbins PF, Lu YC, El-Gamil M, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat. Med. 2013;19(6):747–752. doi: 10.1038/nm.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huff JR. HIV protease: a novel chemotherapeutic target for AIDS. J. Med. Chem. 1991;34(8):2305–2314. doi: 10.1021/jm00112a001. [DOI] [PubMed] [Google Scholar]
- 112.Erickson J, Neidhart DJ, Vandrie J, et al. Design, activity, and 2.8 A crystal structure of a C2 symmetric inhibitor complexed to HIV-1 protease. Science. 1990;249(4968):527–533. doi: 10.1126/science.2200122. [DOI] [PubMed] [Google Scholar]
- 113.Gleenberg IO, Herschhorn A, Hizi A. Inhibition of the activities of reverse transcriptase and integrase of human immunodeficiency virus type-1 by peptides derived from the homologous viral protein R (Vpr) J. Mol. Biol. 2007;369(5):1230–1243. doi: 10.1016/j.jmb.2007.03.073. [DOI] [PubMed] [Google Scholar]
- 114.Nomura W, Aikawa H, Ohashi N, et al. Cell-permeable stapled peptides based on HIV-1 integrase inhibitors derived from HIV-1 gene products. ACS Chem. Biol. 2013;8(10):2235–2244. doi: 10.1021/cb400495h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Faingold O, Ashkenazi A, Kaushansky N, Ben-Nun A, Shai Y. An immunomodulating motif of the HIV-1 fusion protein is chirality-independent: implications for its mode of action. J. Biol. Chem. 2013;288(46):32852–32860. doi: 10.1074/jbc.M113.512038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rosenbluh J, Hayouka Z, Loya S, et al. Interaction between HIV-1 Rev and integrase proteins: a basis for the development of anti-HIV peptides. J. Biol. Chem. 2007;282(21):15743–15753. doi: 10.1074/jbc.M609864200. [DOI] [PubMed] [Google Scholar]
- 117.Hayouka Z, Rosenbluh J, Levin A, et al. Inhibiting HIV-1 integrase by shifting its oligomerization equilibrium. Proc. Natl Acad. Sci. USA. 2007;104(20):8316–8321. doi: 10.1073/pnas.0700781104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gross A, Mobius K, Haussner C, Donhauser N, Schmidt B, Eichler J. Mimicking protein-protein interactions through peptide-peptide interactions: HIV-1 gp120 and CXCR4. Front. Immunol. 2013;4:257. doi: 10.3389/fimmu.2013.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hayouka Z, Hurevich M, Levin A, et al. Cyclic peptide inhibitors of HIV-1 integrase derived from the LEDGF/p75 protein. Bioorg. Med. Chem. 2010;18(23):8388–8395. doi: 10.1016/j.bmc.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 120.Hayouka Z, Levin A, Hurevich M, et al. A comparative study of backbone versus side chain peptide cyclization: application for HIV-1 integrase inhibitors. Bioorg. Med. Chem. 2012;20(10):3317–3322. doi: 10.1016/j.bmc.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 121.De Luca L, Ferro S, Morreale F, Chimirri A. Inhibition of the interaction between HIV-1 integrase and its cofactor LEDGF/p75: a promising approach in anti-retroviral therapy. Mini Rev. Med. Chem. 2011;11(8):714–727. doi: 10.2174/138955711796268787. [DOI] [PubMed] [Google Scholar]
- 122.Nijnik A, Hancock R. Host defence peptides: antimicrobial and immunomodulatory activity and potential applications for tackling antibiotic-resistant infections. Emerg. Health Threats J. 2009;2:e1. doi: 10.3134/ehtj.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hilchie AL, Wuerth K, Hancock RE. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat. Chem. Biol. 2013;9(12):761–768. doi: 10.1038/nchembio.1393. [DOI] [PubMed] [Google Scholar]
- 124.Easton DM, Nijnik A, Mayer ML, Hancock RE. Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends Biotechnol. 2009;27(10):582–590. doi: 10.1016/j.tibtech.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rajasekaran K, Cary Jeffrey W, Jaynes Jesse M, Montesinos E. Small Wonders: Peptides for Disease Control. Washington DC, USA: American Chemical Society; 2012. [Google Scholar]
- 126.Lohan S, Bisht GS. Small cationic antimicrobial peptidomimetics: emerging candidate for the development of potential anti-infective agents. Curr. Pharm. Design. 2013;19(32):5809–5823. doi: 10.2174/13816128113199990003. [DOI] [PubMed] [Google Scholar]
- 127.Saether O, Craik DJ, Campbell ID, Sletten K, Juul J, Norman DG. Elucidation of the primary and three-dimensional structure of the uterotonic polypeptide kalata B1. Biochemistry. 1995;34(13):4147–4158. doi: 10.1021/bi00013a002. [DOI] [PubMed] [Google Scholar]
- 128.Craik DJ, Clark RJ, Daly NL. Potential therapeutic applications of the cyclotides and related cystine knot mini-proteins. Expert Opin. Invest. Drugs. 2007;16(5):595–604. doi: 10.1517/13543784.16.5.595. [DOI] [PubMed] [Google Scholar]
- 129.Aboye TL, Ha H, Majumder S, et al. Design of a novel cyclotide-based CXCR4 antagonist with anti-human immunodeficiency virus (HIV)-1 activity. J. Med. Chem. 2012;55(23):10729–10734. doi: 10.1021/jm301468k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Grundemann C, Koehbach J, Huber R, Gruber CW. Do plant cyclotides have potential as immunosuppressant peptides? J. Natur. Prod. 2012;75(2):167–174. doi: 10.1021/np200722w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grundemann C, Thell K, Lengen K, et al. Cyclotides suppress human T-lymphocyte proliferation by an interleukin 2-dependent mechanism. PLoS One. 2013;8(6):e68016. doi: 10.1371/journal.pone.0068016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang CK, Gruber CW, Cemazar M, et al. Molecular grafting onto a stable framework yields novel cyclic peptides for the treatment of multiple sclerosis. ACS Chem. Biol. 2013 doi: 10.1021/cb400548s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Purcell AW, Zeng W, Mifsud NA, Ely LK, Macdonald WA, Jackson DC. Dissecting the role of peptides in the immune response: theory, practice and the application to vaccine design. J. Pept. Sci. 2003;9(5):255–281. doi: 10.1002/psc.456. [DOI] [PubMed] [Google Scholar]
- 134.Brusic V, Bajic VB, Petrovsky N. Computational methods for prediction of T-cell epitopes – a framework for modelling, testing, and applications. Methods. 2004;34(4):436–443. doi: 10.1016/j.ymeth.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 135.Webb AI, Dunstone MA, Williamson NA, et al. T cell determinants incorporating beta-amino acid residues are protease resistant and remain immunogenic in vivo. J. Immunol. 2005;175(6):3810–3818. doi: 10.4049/jimmunol.175.6.3810. [DOI] [PubMed] [Google Scholar]
- 136.Palena C, Abrams SI, Schlom J, Hodge JW. Cancer vaccines: preclinical studies and novel strategies. Adv. Cancer Res. 2006;95:115–145. doi: 10.1016/S0065-230X(06)95004-0. [DOI] [PubMed] [Google Scholar]
- 137.Steer DL, Lew RA, Perlmutter P, Smith AI, Aguilar MI. Beta-amino acids: versatile peptidomimetics. Curr. Med. Chem. 2002;9(8):811–822. doi: 10.2174/0929867024606759. [DOI] [PubMed] [Google Scholar]
- 138.Purcell AW, McCluskey J, Rossjohn J. More than one reason to rethink the use of peptides in vaccine design. Nat. Rev. Drug Discov. 2007;6(5):404–414. doi: 10.1038/nrd2224. [DOI] [PubMed] [Google Scholar]
- 139.Tanabe S. Epitope peptides and immunotherapy. Curr. Protein Pept. Sci. 2007;8(1):109–118. doi: 10.2174/138920307779941569. [DOI] [PubMed] [Google Scholar]
- 140.Fabbrizzi P, Menchi G, Guarna A, Trabocchi A. Use of click-chemistry in the development of peptidomimetic enzyme inhibitors. Curr. Med. Chem. 2013;21(13):1467–1477. doi: 10.2174/0929867321666131218093611. [DOI] [PubMed] [Google Scholar]
- 141.Manetsch R, Krasinski A, Radic Z, et al. In situ click chemistry: enzyme inhibitors made to their own specifications. J. Am. Chem. Soc. 2004;126(40):12809–12818. doi: 10.1021/ja046382g. [DOI] [PubMed] [Google Scholar]
- 142.Sohma Y, Sasaki M, Hayashi Y, Kimura T, Kiso Y. Novel and efficient synthesis of difficult sequence-containing peptides through O-N intramolecular acyl migration reaction of O-acyl isopeptides. Chem. Commun. (Camb) 2004;1:124–125. doi: 10.1039/b312129a. [DOI] [PubMed] [Google Scholar]
- 143.Williams SC. Drugmaker reaps what it sows with first plant-made biologic. Nat. Med. 2012;18(1):5. doi: 10.1038/nm0112-5. [DOI] [PubMed] [Google Scholar]