Abstract
The liver is the largest organ in the body and is generally regarded by non-immunologists as not having lymphoid function. However, such is far from accurate. This review highlights the importance of the liver as a lymphoid organ. Firstly, we discuss experimental data surrounding the role of liver as a lymphoid organ. The liver facilitates a tolerance rather than immunoreactivity, which protects the host from antigenic overload of dietary components and drugs derived from the gut and is also instrumental to fetal immune tolerance. Loss of liver tolerance leads to autoaggressive phenomena which if are not controlled by regulatory lymphoid populations may lead to the induction of autoimmune liver diseases. Liver-related lymphoid subpopulations also act as critical antigen-presenting cells. The study of the immunological properties of liver and delineation of the microenvironment of the intrahepatic milieu in normal and diseased livers provides a platform to understand the hierarchy of a series of detrimental events which lead to immune-mediated destruction of the liver and the rejection of liver allografts. The majority of emphasis within this review will be on the normal mononuclear cell composition of the liver. However, within this context, we will discus select, but not all, immune mediated liver disease and attempt to place these data in the context of human autoimmunity.
Keywords: autoimmune disease, biliary epithelial cell, hepatocytes, immunity, liver, lymphocytes, tolerance
Introduction
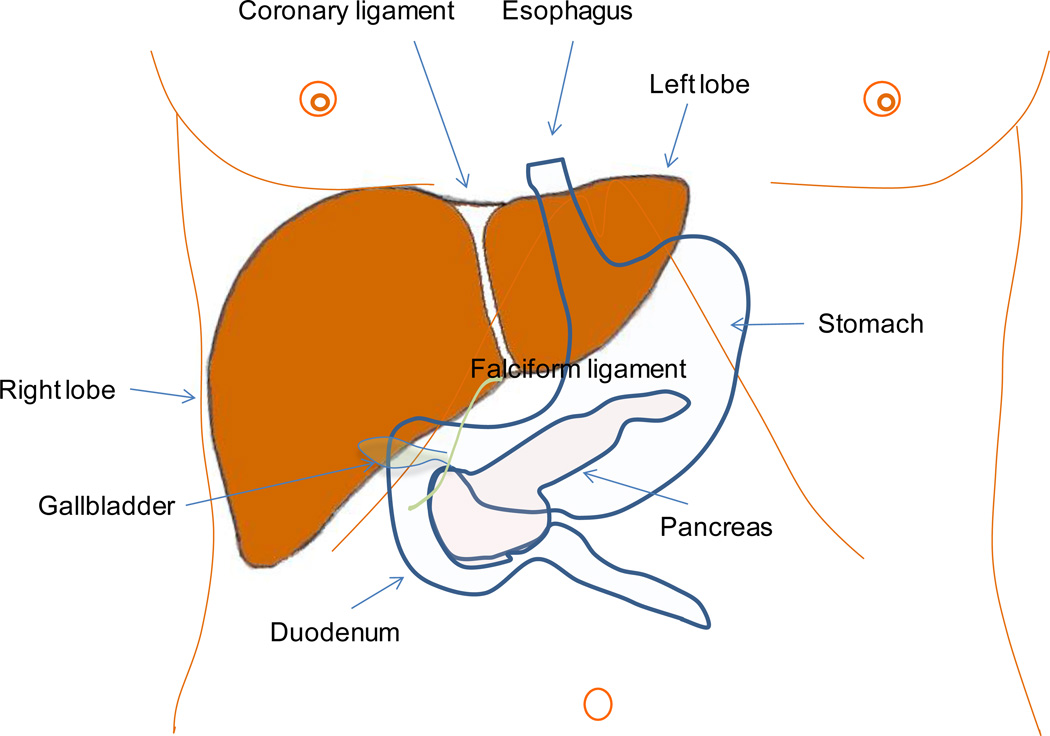
The liver is the largest solid organ of the human body, accounting for almost 2% of adult body weight and weighing approximately 1.5 kg; it performs an amazing number of tasks that support the function of other organs and impacts all physiologic systems. An essential function of the liver is protein synthesis and metabolism, including the metabolism of amino acids, carbohydrates, lipids and vitamins. However, the liver is also responsible for the removal of pathogens and exogenous antigens from the systemic circulation. The key position of the liver (Figure 1) and its unique vasculature allow it to carry out the degradation of toxins and waste products.
Figure 1.
Anatomical location and external appearance of the liver. The falciform ligament, on the surface of the diaphragm, splits the liver into right and left lobe. The anatomical relationship of the liver with organs such as the gallbladder, stomach, duodenum, and pancreas is illustrated.
The role of the liver as the main metabolic organ increases the rate of exposure to newly produced neo-antigens and enhances the inherited risk of overactivation of components of the immune system with potentially harmful consequences for cell homeostasis. Thus, the immune system developed dedicated mechanisms to be able to “switch” from a tolerant to a responsive state at any given time1. Early in the history of experimental transplantation, transplant surgeons were intrigued to note that while kidney, skin, pancreas and other allografts were rapidly rejected, allogeneic liver grafts were more tolerant. This prompted investigators to consider that the liver is predominantly an organ biased towards tolerance rather than a reactive state which would otherwise lead to rejections. The scientific basis for this tolerant state remained elusive for many years.
Historical Perspectives
The first human liver transplant was performed in 1963 by Starzl,2 with significant short-term success in 1967 when a recipient survived for more than a year. Subsequently, the systematic administration of cyclosporin by Calne and colleagues dramatically improved the outcome of the patients receiving a liver allograft3, 4. Further, it was noted in 1969 that liver allografts between unrelated pigs were not rejected in spite of MHC mismatch; the transplanted livers did not require high doses of immunosuppression to be sustained5. A seminal study, conducted two years before Calne’s report, from Cantor and Dumont demonstrated that administration of antigens to animals via the portal vein was tolerated better compared to systemic administration6. Subsequent studies confirmed the potential acceptance of MHC mismatched liver grafts in other species. Further, liver transplantation confers tolerance to heart and skin grafts from the same donors, while heart and skin grafts from other donors were immediately rejected. Interestingly, the rejection of other transplanted organs can be modulated by subsequent transplantation. Similarly, co-transplantation of human liver with another organ limits the likelihood of immediate rejection of the second organ and improves the survival of the allograft. The natural regenerative capacity of the liver parenchymal cells is significant; 25% of residual liver is sufficient for regeneration within a few weeks in rodents and a few months in humans. Because of its anatomical location, the liver is continuously exposed to an overload of antigenic stimuli which includes exogenous pathogens, dietary components and xenobiotics, including drugs and toxins.
Microanatomy of the Liver as an Immunological Organ
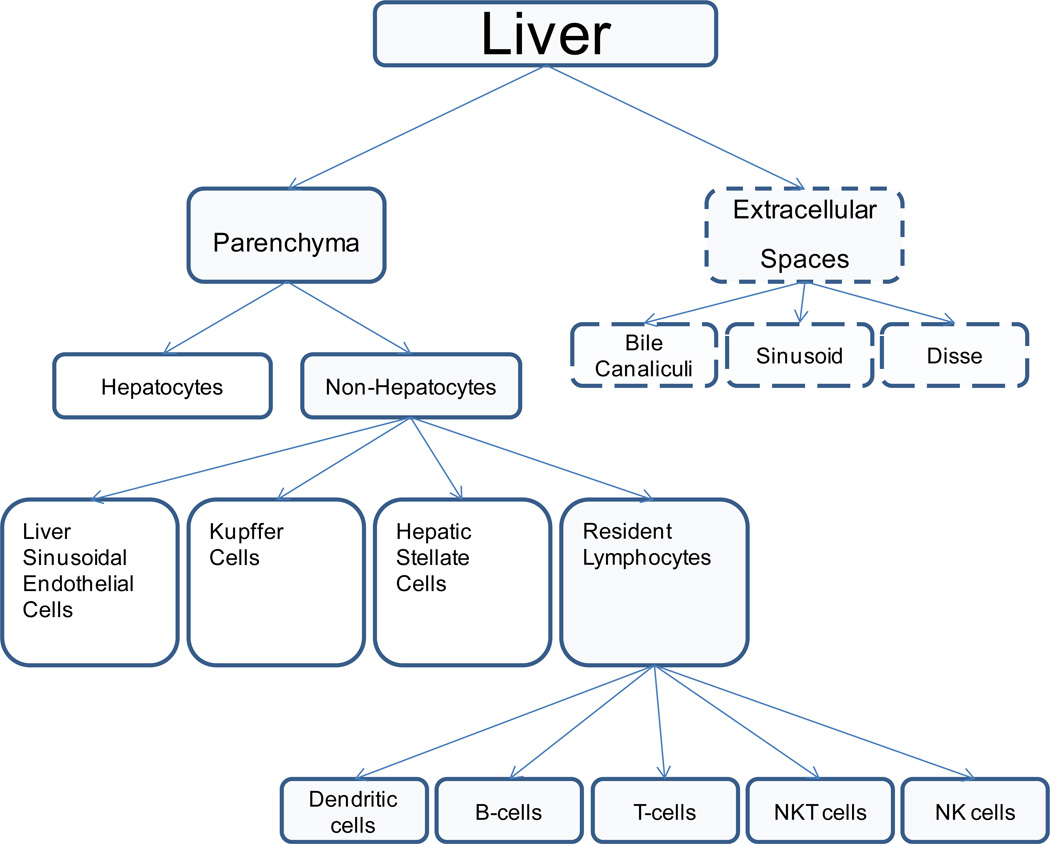
To achieve its multifaceted tasks, the liver is composed of a myriad of cell types, largely sub-divided in parenchymal and non-parenchymal cells (Table 1)7. Most of the liver volume is occupied by parenchymal cells (hepatocytes); these cells occupy approximately 78–80% of the total liver tissue, compared to just 5–6% of non-parenchymal cells7–11 (Table 1). The remaining 14–17% of the total liver tissue corresponds to cellular components of the extracellular space (Figure 2)7. The non-parenchymal cells consist of a diverse set of cells, including 45% liver sinusoidal endothelial cells (LSECs), 33% Kupffer cells (KCs), and 22% hepatic stellate cells (HSCs)12 (Table 1 and Figure 3). The liver can be considered to have two separate anatomic areas, the parenchyma and the portal tracts. Structurally, the liver can be further subdivided into five systems comprising the vascular system, the hepatic lobule, the hepatic sinusoidal system, the biliary system and the stroma. Each of these systems - directly or indirectly - plays an important role in the homeostasis of the innate and adaptive immune system.
Table 1.
Percentage of total volume of cellular and extracellular compartments in liver7.
| Mean % | ±SE | |
|---|---|---|
| Cells (84.1%) | ||
| Hepatocytes | 77.8 | 1.15 |
| Liver Sinusoidal Endothelial Cells | 2.8 | 0.2 |
| Kupffer Cells | 2.1 | 0.3 |
| Hepatic Stellate Cells | 1.4 | 0.2 |
| Extracellular Spaces (15.9%) | ||
| Sinusoidal lumen | 10.6 | 0.45 |
| Disse space | 4.9 | 0.35 |
| Biliary canaliculi | 0.4 | 0.05 |
| Total Sum (100%) | 100% |
Data are presented as mean% ± standard errors (SE) of the mean
Figure 2.
Cellular and extracellular composition of the liver
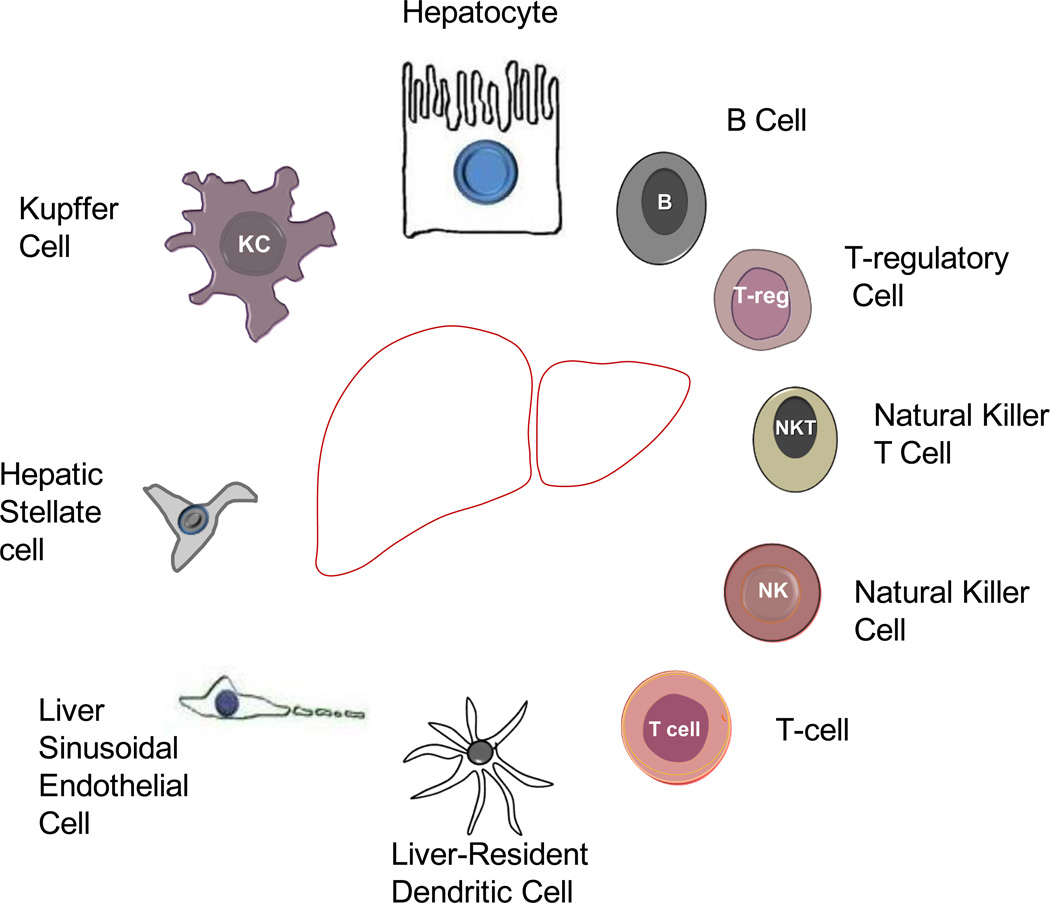
Figure 3.
The morphological appearance of cells within the liver.
Hepatic lobule
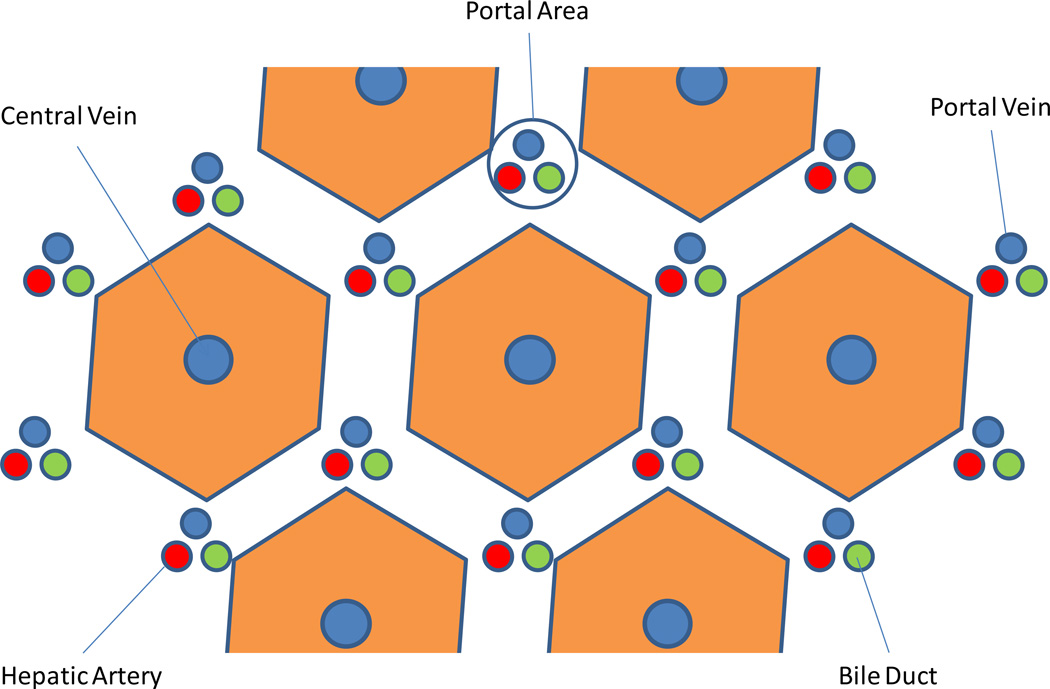
The simplest way to describe the cellular anatomy of the liver is by light microscopy. Thus, the hepatic lobule is not only the structural but also the functional unit of the liver13. These lobules are centered on central veins like spokes in wheel, and their periphery is demarcated by arbitrary lines joining each of the surrounding regions of portal tracts (Figure 4). Each portal tract consists of an intrahepatic bile duct and a collection of blood vessels including a branch of both the hepatic artery and portal vein. Such lining formulates a roughly hexagonal assembly of hepatocyte plates13, the extension of which forms the basis of the one-cell thick liver cell layers consisting of 15–25 cells each.
Figure 4.
The hepatic lobule is the structural unit of the liver. It consists of an hexagonal arrangement of hepatocyte plates with the central vein located in the center of the structure and the portal triads distributed at the vertices of the lobule. The portal triad consists of terminal branches of the portal vein and the hepatic artery and a bile duct.
The hepatic vasculature
The liver has a dual blood supply as it receives arterial blood from the right and left hepatic arteries and venous blood from the hepatic portal vein. The antigen-rich blood delivered through the portal vein accounts for more than 75–80% of the total blood. This blood originates from the stomach, alimentary tract, rectum and spleen, and contains large concentrations of antigens from dietary components and bacterial products from gut bacteria such as lipopolysaccharide endotoxin (LPS). This can be found at a concentration of up to 1 ng/ml14. Also, metastatic cells pass through the liver, which also has to deal with the load of detoxified byproducts with oncogenic potential. The remaining 20–25% is oxygenated blood delivered through the hepatic arteries which are branches of the celiac axis. The blood leaving the liver is drained into the inferior vena cava via the hepatic veins. The liver receives 1.5 L (30% of the total blood volume) each minute. Thus, the total volume of blood in the human body circulates through the liver approximately 360 times each day. The blood flows through the vascular sinusoids, and is drained into central veins or terminal hepatic venules, which are the branches of the hepatic veins.
Hepatic sinusoidal system
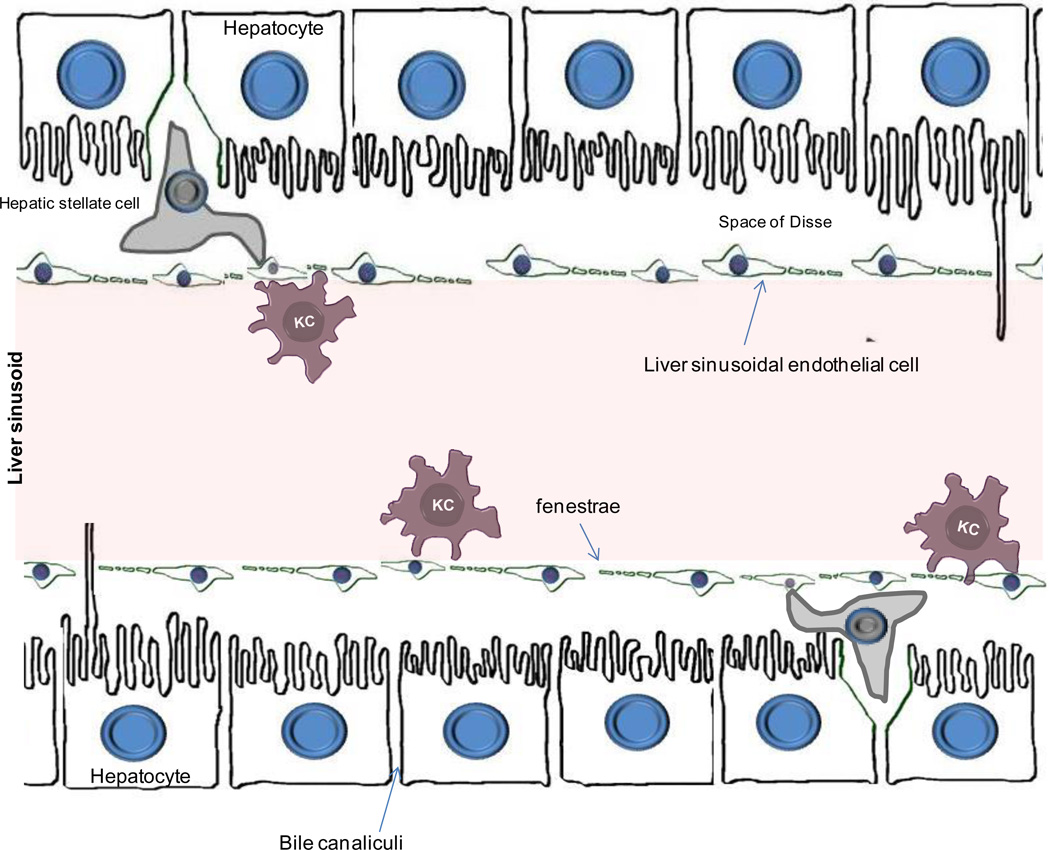
The hepatocytes form one cell thick plates and are separated from the bloodstream by a non-parenchymal, thin fenestrated (porous) barrier forming a labyrinth of specialized capillaries, termed liver sinusoids (Figure 5). These capillaries are primarily formed by liver sinusoidal endothelial cells (LSECs)15. The liver sinusoids are designed to allow easy transmission of molecules and cellular mediators between the sinusoids and the hepatocytes. To achieve such transfer, LSECs lack a basement membrane, making them permeable. Under normal conditions, the space of Disse formed between hepatocytes, LSECs and HSCs, allows exchange of cellular mediators without the need of a direct contact. A direct contact between hepatocytes and sinusoidal cells can be achieved from clusters of endothelial cells which contain small holes (or fenestrations)16 which perforate the cytoplasm and form ‘sieve plates’17–19 (Figure 5). Fenestrae manage the exchange of solutes and particles between the sinusoidal blood and the space of Disse18, 19. Blood also passes over a population of macrophages called Kupffer cells (KC). KCs account for approximately 80% of all macrophages in the body20. Their strategic position enables them to clear the blood from endotoxins and antigens and to eliminate microorganisms by phagocytosis. HSCs (also known as Ito cells) are located in close proximity to the vascular sinusoids and in particular in the vicinity of the terminal hepatic venules. Because of the small diameter of sinusoids, even minimal increases in systemic venous pressure can create stasis. Such stasis encourages lymphocyte extravasation and extends direct contact of APCs and lymphocyte populations.
Figure 5.
Illustration of the microanatomical localization of hepatocytes, liver sinusoidal endothelial cells, Kupffer cells, and hepatic stellate cells. The space of Disse separates hepatocytes from the liver sinusoids. The endothelium of liver sinusoids is discontinued (fenestrated) and is formed by a layer of liver sinusoidal endothelial cells16. These cells act as scavenger cells and form a physical filtering barrier between the sinusoidal blood and plasma69, 391. Kupffer cells are resident macrophages, that are attached to the layer of liver sinusoidal endothelial cells. The hepatic stellate cells are located in the sub-endothelial space of Disse and play vital role in fibrogenesis.
The liver as a lymphoid organ
Organs such as the thymus, lymph nodes, and spleen are "classical" lymphoid organs. However, other organs such as the gut and liver consist of cells whose primary function may not be immunological but nonetheless still perform essential immune tasks. Within these respective organs there are resident cells of the innate and adaptive immune system. Within a normal liver, the lymphocyte population is largely resident in the portal tract but can be also scattered throughout the parenchyma. The composition and localization of these lymphocyte populations changes dramatically when the liver architecture is altered as a consequence of acute or chronic inflammatory conditions. It is therefore not surprising, that hepatocytes and BECs are sites of immune-mediated destruction induced by a variety of infectious xenobiotic and tumor-originated sources1. The anatomic position of the liver and its distinctive vasculature accounts for its unique ability to continuously exchange immunological information. The antigen-rich blood passing through the liver sinusoids is "scanned" achieved by a complex network of conventional and non-conventional antigen presenting cells (APCs)21, 22.
Composition of the Liver Immune System
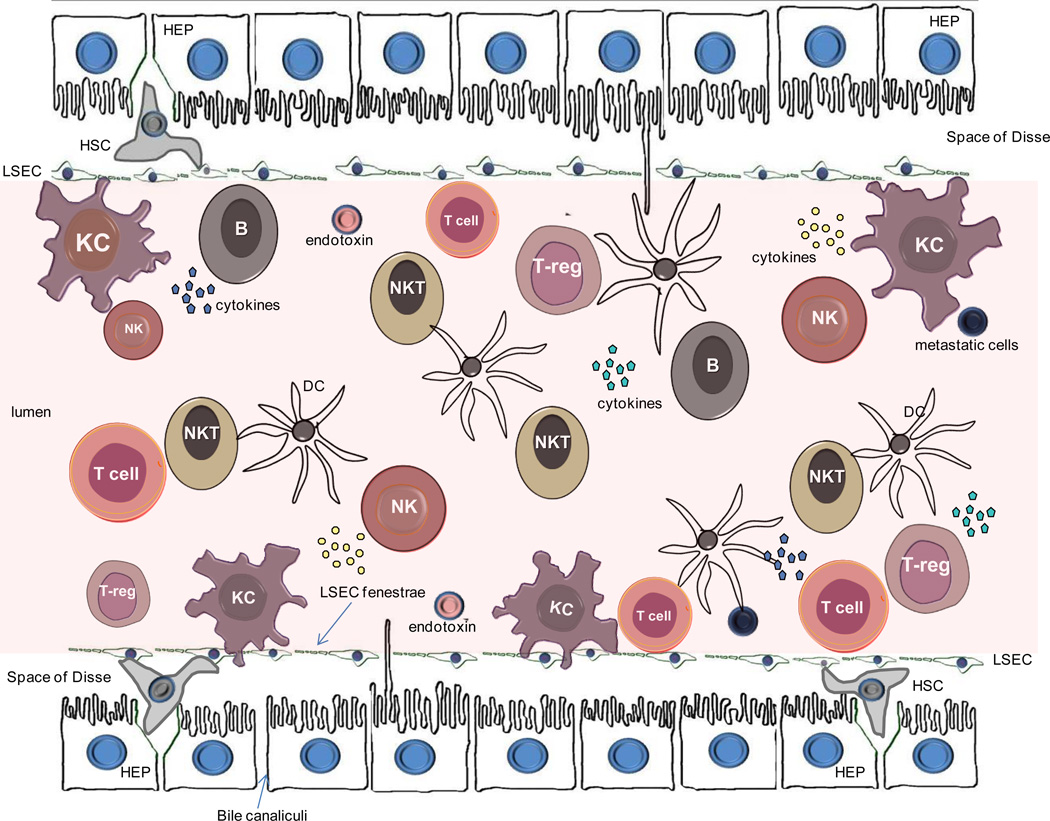
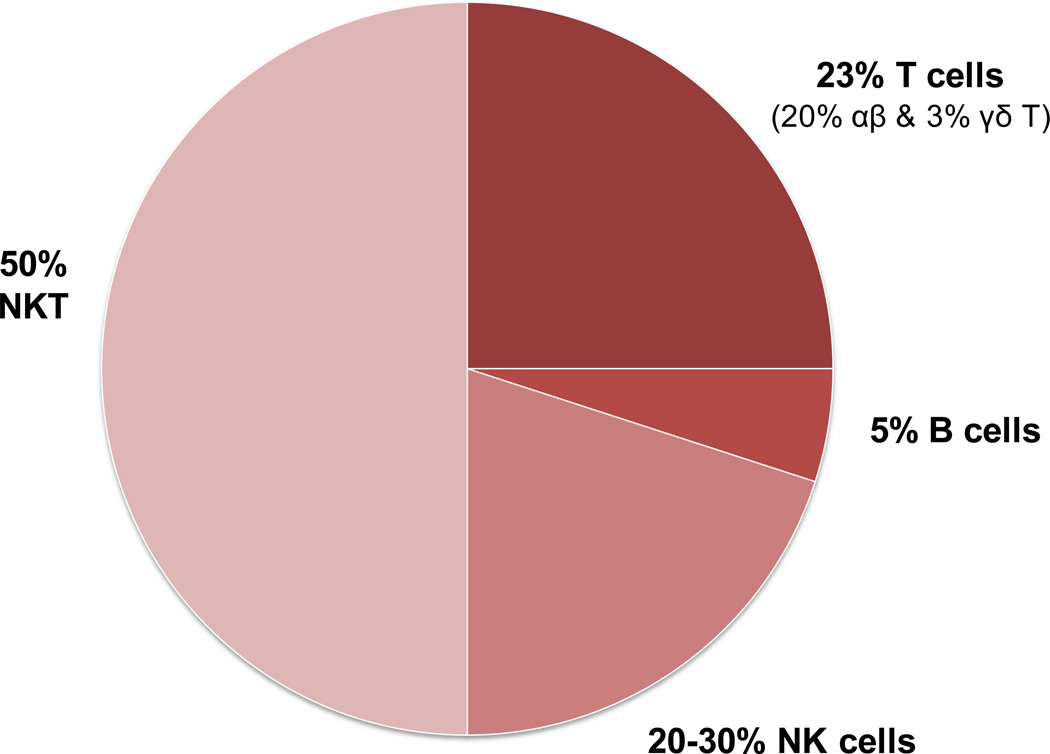
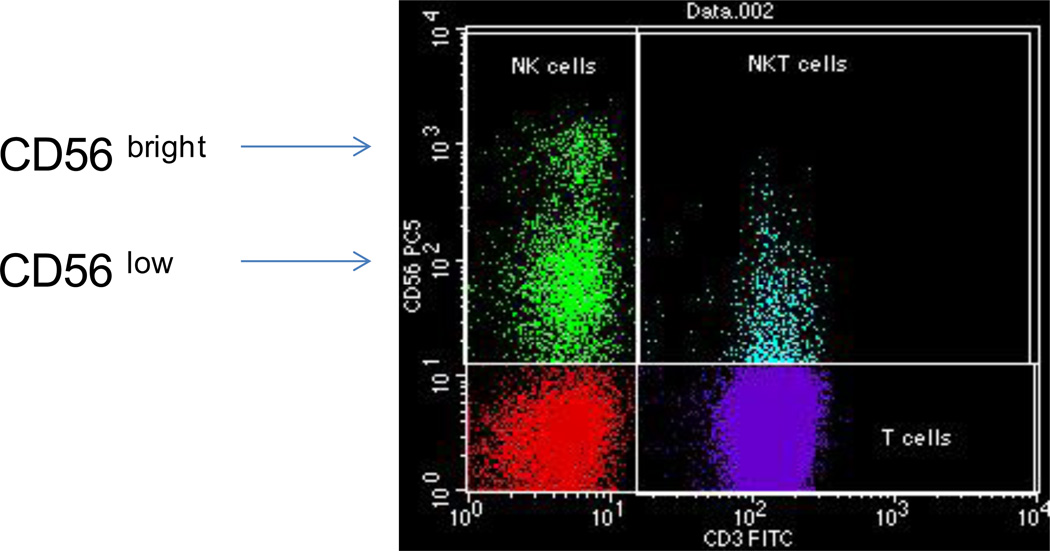
The human liver has a lymphocyte population normally resident in the portal tract, but also scattered throughout the parenchyma23–26. Approximately 500,000 to one million lymphocytes can be retrieved from 100 mg of normal human liver tissue, bringing the total number of these cells to approximately 0.75–1.5 1010 cells for a liver weighing 1.5 kg23. The lymphoid repertoire includes significant numbers of T-cells, B cells, natural killer (NK) and natural killer T (NKT) cells (Figure 6). The relative contribution1, 27 of these cells in the liver is illustrated in Figure 7. Using CD3 as a pan-T cell marker, these lymphoid populations can be subdivided into CD3+ and CD3−. CD3+ T lymphocytes outnumber CD3− lymphoid cells. The most widely used marker for NK cells is CD56; all three sub-populations CD3+CD56− T cells, CD3-CD56+ NK cells and CD3+CD56+ NKT cells have cytotoxic activity. B lymphocytes comprise only 5% of the total lymphocytes23–26. Many of the liver-resident lymphocytes differ phenotypically and functionally from circulating lymphocytes24, 25, 28–31, for reasons poorly understood27. The hepatic lymphocyte repertoire includes conventional and unconventional lymphocyte sub-populations of both the innate and the adaptive immune system.
Figure 6.
Cells comprising the liver including hepatocytes (HEP), liver sinusoidal ensothelial cells (LSEC), Kupffer cells (KC), hepatic stellate cells (HSC) and lymphoid cell sub-populations. NK, natural killer; NKT, natural killer T-cells; DC, dendritic cell; Treg, T-regulatory cell
Figure 7.
Distribution of cell sub-populations within intrahepatic lymphocytes
αβ- and γδ-T cells in liver immunity
The conventional T-cell population consists of CD8+ and CD4+ T cells exhibiting a diverse repertoire with αβ-chain T cell receptors (TCR). These receptors recognize short peptidyl sequences from antigens in the context of MHC class I and class II molecules for CD8 and CD4, respectively. More than 80% of the CD3+ T cells are αβ T-cells, with the remainder expressing the γδT-cell receptor in the liver1. The mean prevalence rate of 15% for γδTCR+ cells is 5 times higher in the liver compared to the periphery (range 1–5%)32, 33. Lymphocyte repertoires rich in γδTCR+ cells are also found in skin, the gut and the genitourinary tract. The role of γδTCR+ cells in the immune homeostasis of the liver remains elusive. Although a protective role of these cells in the concavalin A-induced animal model of acute liver failure has been reported34, the protective role is initiated by Vγ4 and not Vγ1, γδT cells. IL-17A deficient γδT cells are unable to protect from liver destruction, suggesting that IL-17A is an important mediator of such protection. Vγ4 and not Vγ1, γδT T cells are the major subsets of peripheral lymphoid γδT T cells. A different set of experiments have shown that the γδT cells protect from liver failure by targeting NKT cells34. An earlier report has suggested that IL-17A induced by γδT cells is expressed in the liver of mice infected with Listeria monocytogenes at an early stage of infection and plays an important role in the initiation of innate immune responses35.
The role of γδT cells is not limited to control of infection but appears to also play distinct immunoregulatory roles in tumor immunity36, 37. They have also been involved in the induction and maintenance of liver-specific autoimmunity, as they appear increased in patients with active autoimmune hepatitis and primary sclerosing cholangitis38–41. Some T-cells do not express CD4 or CD8 and are known as ‘double negative’ T cells. They have been studied mainly in mice, and appear to develop extrathymically and expresses specific V TCR β genes42, 43. They can be found in liver, expressing either the αβ or γδ TCR and are considered to be cells with primarily regulatory properties23, 44–46, they may be critical to induction of autoimmunity47.
The participation of conventional T-cells bearing αβ TCRs in the immune homeostasis of a normal liver is by far the best studied and is reviewed elsewhere1, 48, 49; the role played by these cells in the induction of immune-mediated liver injury has been extensively studied1, 48, 49.
NK and NKT cells in the liver
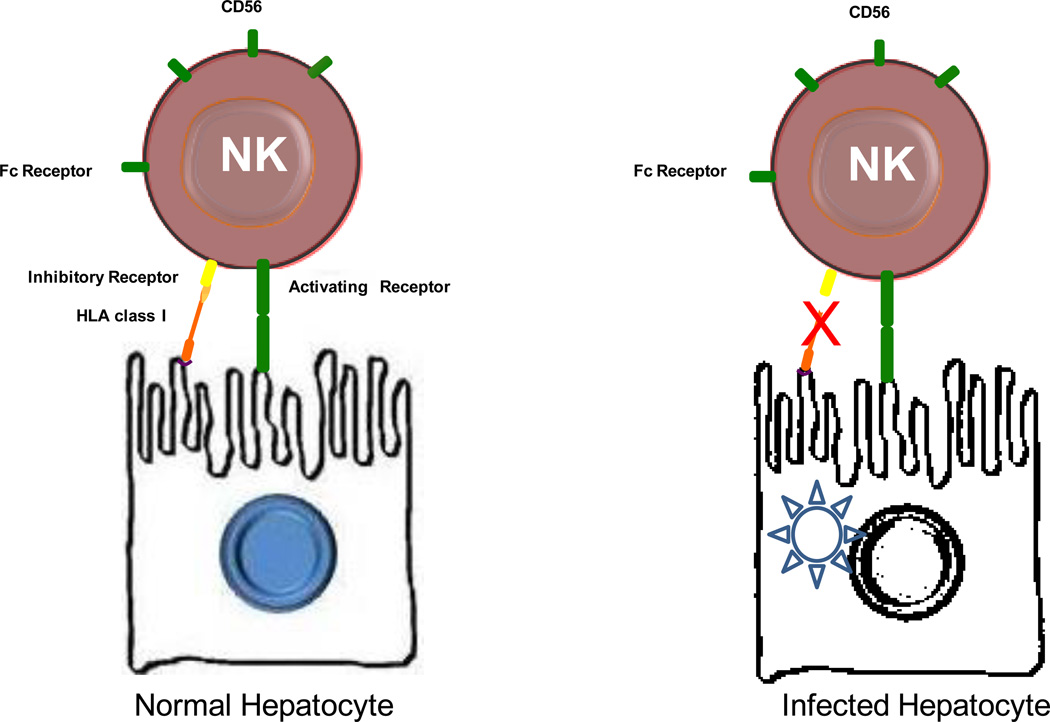
NK cells are bone-marrow derived large granular cells; their main task is to kill target cells. NK cells represent approximately 20–30% of the total number of liver-resident lymphocytes, a percentage unusually high compared to less than 5% seen in peripheral blood25. Their over-representation in human liver probably relates to their primary role, which is surveillance for infection, killing of infected hepatocytes (Figure 8) and possibly malignant transformation. They are conventional constituents of the innate immune system, but emerging evidence, both in mice and humans, indicates that NK cells are ‘educated’ throughout the development in a way similar to that seen in lymphoid cells of the adaptive immune system50. NK cells acquire antigen-specific receptors, go through clonal expansion during exposure to infectious agents and produce long-lived memory cells.
Figure 8.
Schematic illustration of NK cell receptors and killing of viral hepatitis infected cells. Under normal conditions, non-infected cells are not killed because inhibitory signals from HLA class I molecules prevail over activating signals. Virus-infected cells are characterized by altered expression of HLA class I molecules. This disrupts the inhibitory signals and allows activation of NK cells and subsequent lysis of the infected hepatocytes. NK-mediated killing of infected hepatocytes is not operated in viral hepatitides.
Liver lymphocytes are also enriched in NKT cells, accounting for 20–35% of mouse liver lymphocytes and 10–15% of rat and human liver lymphocytes (Gao et al., J Leukocyte Biology 2009, 86:513–528). NKT cells are a heterogeneous group of T lymphocytes that express both NK and T cell markers, and recognize the lipid antigens presented by the nonclassical MHC class I-like molecule CD1. The functions of NKT cells are mainly mediated via the production of a variety of cytokines (e.g. IFN-γ and IL-4), which play important roles in regulating innate and adaptive immunity. Accumulating evidence suggests that NKT cells play a diverse role in liver injury, inflammation, fibrosis, and regeneration (Gao et al., J Leukocyte Biology 2009, 86:513–528; Park et al., Hepatology 2009, 49:1683-94).
The hypothesis that lymphocyte sub-populations participate in routine immuno-surveillant functions in normal liver has largely been based on evidence demonstrating that they express mature/activated phenotypes24, 51. Early evidence suggested that these populations may have arisen locally, a finding that has implications not only in the maintenance of immune homeostasis but also in the induction of immune-mediated liver injury. Phenotypic and functional characterization of these liver-related lymphocytes has led to the appreciation of a role in the immunopathogenesis of liver diseases, including autoimmune liver diseases, chronic viral hepatitides, liver-related tumor immunology, alcoholic hepatitis, drug-induced immune-mediated liver disease and allograft rejection1, 49.
Liver-Related Antigen Presenting Cells in Immunity and Tolerance
The liver is unique in its ability to recruit distinct cell types to become APCs; LSECs, KCs and hepatic DCs may all be considered conventional or classical liver APCs. However, under pathophysiological circumstances and during persistent liver inflammation, hepatocytes and BECs can express MHC II antigens and act as non-conventional APCs. Hepatocytes and BECs as APCs play an important role in the initiation and maintenance of processes important for loss of immunological tolerance. All these cells come in continuous contact with naïve T cells recirculating through the blood, and under normal of pathological conditions may participate in their activation (Figure 8)52.
One working hypothesis to explain the ability of the liver to induce systemic tolerance21 is based on the assumption that liver-resident DCs have distinct properties which promote tolerance rather than an immune response. Another plausible explanation is the intrinsic tolerogenic capacity of liver-related APCs such as the LSECs or the KCs. Donor cell chimerism has also been postulated to account for the observed tolerance53. According to this scenario, donor-derived leukocytes including liver-resident APCs migrate to central lymphoid organs within 120 minutes post- transplantation, and persist for a long period, accounting for the hepatic tolerogenicity seen. Also, the systemic tolerance of the liver has been attributed to the induction of allospecific regulatory cells such as those with a CD4+, CD25+, FoxP3+ phenotype54. These mechanisms may act in isolation or in combination.
Liver sinusoidal endothelial cells
Wisse was the first to demonstrate that LSEC is a distinct cell type with a characteristic open fenestration without basement membrane or diaphragm16. Aging, liver disease and various stimuli co-cultured with LSECs (such as vascular endothelial growth factor) alter the number, size and localization of the fenestrations55–58. Wisse was also the first to suggest that because of the unusually high amounts of endocytic vehicles that LSECs contain, these cells are probably involved in the uptake of proteins circulating in sinusoidal blood16. This notion has been proven correct 15 years later when hyaluronic acid was identified as the first physiological macromolecule cleared from the blood by rat LSECs59.
The role of LSECs in hepatic tolerance and the generation of immunity has been investigated and several surface markers have been found to be expressed by resting and activated LSECs (Table 2). The LSEC is an efficient APC with multiple functions21, 22. The capacity of LSECs to possess several of the properties seen in DCs, such as the expression of MHC class II, various co-stimulatory molecules, and CD11c and their ability to activate naive T cells has been reported60–68. LSECs can take up antigen using a multitude of receptors69. The question as to whether a) LSECs are efficient APCs and b) can induce T-cell tolerance has also been addressed systematically. Lohse et al60 were among the first to study the ability of LSECs to act as APC and demonstrated that LSECs are efficient APC, carrying functional B7-2 molecules60. The ability of LSECs to present soluble antigens to CD4+ T cells is down-regulated by IL-1060. Subsequent work63 reflects that murine LSECs are efficient APCs63. However, levels of MHC class II63 are relatively low and stimulation of toll like receptors is unable to induce IL-12 expression63. Also, treatment with endotoxin down-regulates the surface expression of constitutively expressed MHC class II, and CD80/CD86 co-stimulatory molecules63. These data suggest that although endotoxin does not alter the ability of LSEC to remove gut-derived peptides from circulation, it affects antigen processing and expression of accessory molecules63.
Table 2.
Phenotypic markers of liver sinusoidal endothelial cells as reported by various studies. Liver sinusoidal endothelial cells express CD54, vWF, CD31 in more than three studies
| Molecule | References |
|---|---|
| CD4 | 62, 382, 383 |
| CD14 | 382 |
| CD16 | 382 |
| CD31 | 67, 384–386 |
| CD32 | 67, 382 |
| CD34 | 384, 386, 387 |
| CD54 (ICAM-1) | 62, 382, 383, 385–388 |
| CD106 (VCAM-1) | 387 |
| AcLDL | 388, 389 |
| vWF | 67, 382, 384, 386, 390 |
| VIII | 388, 390 |
| MHC-I | 388 |
| MHC-II | 70, 382 |
| CD11b | 388 |
| CD11c | 388 |
| CD40 | 70 |
| CD80 | 62, 70 |
| CD86 | 62, 70 |
| CD105 | 70, 388 |
| L-SIGN | 388 |
The relative expression of these markers varies amongst studies. Methodology used for the assessment of their expression included immuno-histochemical analysis, PCR and flowcytometry using mouse, rat or human cultured LSECs (reviewed in 15); CD, cluster of differentiation; ICAM-1, intercellular adhesion molecule 1; VCAM-1, vascular cell adhesion molecule 1; AcLDL, acetylated low-density lipoprotein; vWF, von Willebrant factor; L-SIGN, liver/lymph node-specific ICAM-3-grabbing nonintegrin
Contrary to these results, LSECs isolated from primed mice can present antigen and induce antigen-specific CD8+ T cell tolerance65. Also, adaptive transfer of LSECs isolated from ovalbumin-fed animals can induce antigen-specific T-cell tolerance to unfed animals, clearly indicating an involvement of LSECs in the induction of CD8 T cell tolerance against oral antigens66. The ability of murine LSECs to tolerize T cells across MHC barriers has been studied70; LSECs can regulate a polyclonal population of T cells with direct allospecificity. Data have demonstrated the Fas/Fas ligand pathway as important in the tolerizing capacity of LSECs towards alloreactive T cells70. Though the molecular mechanisms responsible for LSEC-induced T cell anergy are poorly understood, reported data suggests that B7-H1 signaling on LSECs is a prerequisite for the induction of CD8+ T cell tolerance via programmed death (PD)-1 ligation71. Also, the contact of LSECs with DCs suppress neighboring antigen-presenting DCs to fully activate naive CD8 T cells72.
The capacity of LSECs as efficient APCs ex vivo and in vivo has been the focus of heated debate, as the results obtained have not been always supportive of their APC role21. Thus, in contrast to the findings presented above, Katz et al67 challenged the dogma that LSECs have DC properties expressing MHC class II antigens, CD40, CD80, and CD86 co-stimulatory molecules, and CD11c and are able to effectively stimulate naïve T-cells. Using isolated LSECs with the phenotypic markers of endothelial cells (CD45−, CD31+, vWF+) (Table 2), it was reported that such cells lacked the expression of CD11c, the most widely used marker of murine DC. Additionally, they had minimal expression of MHC class II and undetectable levels of CD40, CD80, and CD86. Such LSECs were able to capture antigen but could not induce IFN-γ production or significant CD4+ or CD8+ T-cell proliferation. The possibility that the tolerogenic effect of the liver may be due to the continuous exposure of LSECs to gut bacterial products has been tested experimentally. Physiological concentrations of entotoxin contained within the blood drained from the portal veins to the liver can induce the secretion of interleukin (IL)-10 from LSECs73; physiological concentrations of endotoxin appear to be able to down-regulate LSEC-mediated CD4+ T-cell activation via the modulatory effects they exert in the expression of MHC class II, CD80 and CD8662.
More recent studies have addressed the potential of diseased liver to provide a microenvironment which reverses the function of LSECs from tolerogenic to pro-inflammatory and highly immunogenic. LSECs from fibrotic livers induce T-cells to produce pro-inflammatory cytokines such as IFN-γ, IL-6, and TNF-α74. Such data clearly implicate LSECs in intrahepatic immune-mediated inflammation seen in hepatic fibrosis and possibly to liver allograft tolerance70, 75, 76.
Kupffer cells
The tolerogenic capacity of KCs has been demonstrated in the induction of tolerance to allergic and drug-induced reactions77, 78. KCs have also been implicated in the induction of liver tolerance caused by preoperative donor-specific blood transfusion after liver transplantation79. Their important role in tolerance has been suggested because gadolinium chloride-induced blockade of KCs prevents the induction of tolerance by the portal venous route in rat cardiac allograft transplantation80, 81. KCs can also produce prostaglandin E2, which in turn can inhibit antigen (ovalbumin)-specific T-cell activation by DCs82.
The close interplay of KCs with LSECs or Tregs may be of relevance to the tolerogenic capacity of the liver. Early studies on human KCs challenged with LPS have suggested that these cells can also release IL-1073. In vitro experiments have also demonstrated that exogenous IL-10 down-regulates the secretion of IL-6 and tumor necrosis factor (TNF)-α by LPS-stimulated human KCs73. Kupffer cell-derived IL-10 may decrease the expression of both MHC class II and co-stimulatory molecules expressed by LSECs64. Kupffer cell-produced prostaglandin E2 abrogates the capacity of LSEC to activate cloned, antigen-specific CD4+ T cells64. Interaction of KCs with Tregs provoke the secretion of IL-10 by Tregs and facilitates the induction of systemic tolerance to hepatocyte-derived antigens, such as human α-1 antitrypsin83. Earlier data have demonstrated in a concavalin A-induced model of liver injury the ability of both Tregs and KCs to provoke liver tolerance to concanavalin A through the induction of IL-1084. KCs can freely transcytose LSECs and can secrete cytokines and chemokines to eliminate pathogens.
Hepatic dendritic cells
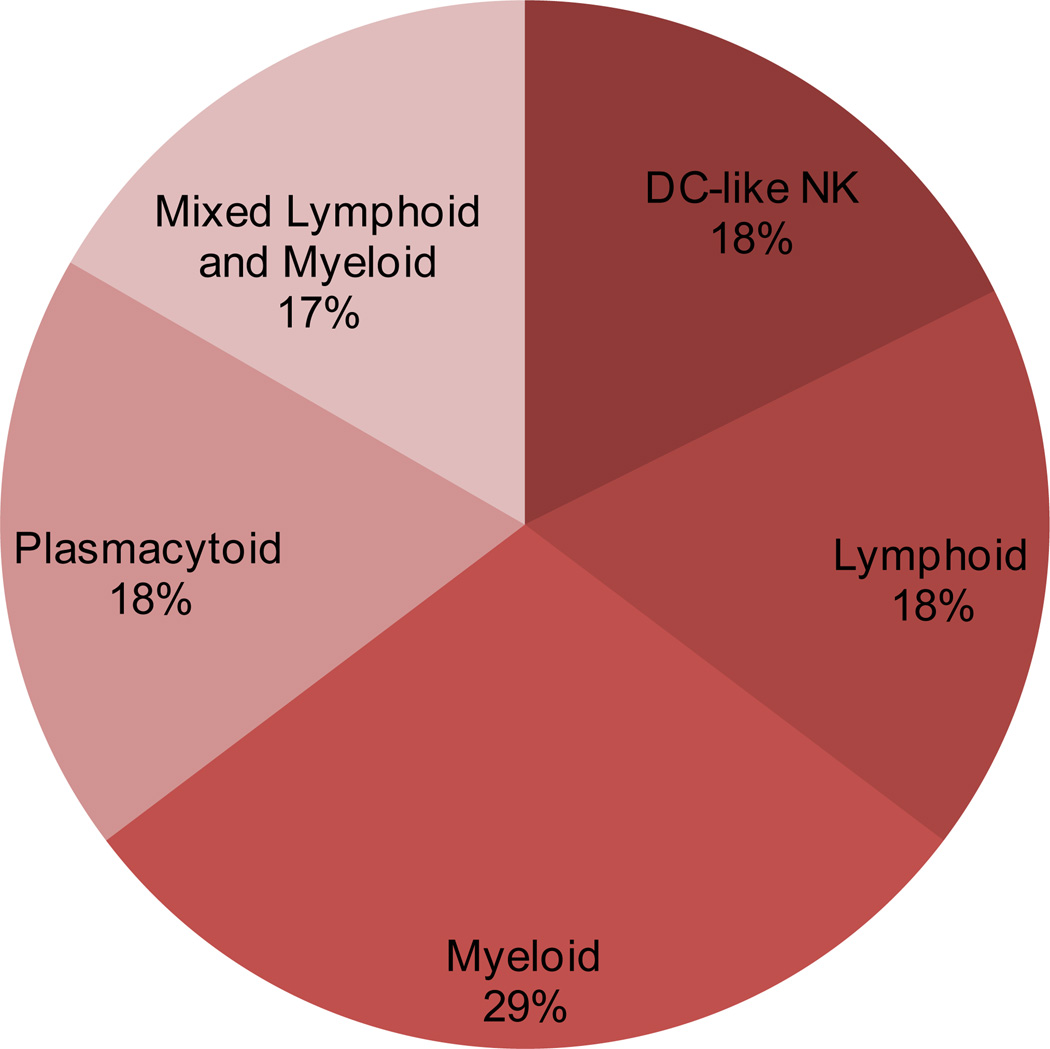
Hepatic DCs appear in high numbers throughout the portal triad, surrounding the central vein85. A smaller number of DCs can also be found scattered throughout the parenchyma86. These DCs are largely sub-divided into five sub-sets on the basis of their phenotypic characteristics, including classical myeloid DCs (mDCs) and plasmacytoid DCs (pDCs). They also consist of a unique mixture of mDCs and pDCs, a group of lymphoid DCs and a group of NK DCs (or DC-like NKs). A simplified phenotypic subdivision based on specific surface markers for these sub-populations is as follows: lymphoid (CD8α+, B220−, CD11b−); plasmacytoid (CD8α−, B220+); myeloid (CD8α−, B220−, CD11b+); myeloid & plasmacytoid mixture (B220−, CD11b−); and NK DCs (B220−,CD11cint, CD69+, 2B4++, DX5+)21, 87, 88. Several other markers have been used in the recent past to better identify and investigate these subsets of dendritic cells in mouse and human liver. Myeloid and lymphoid DCs are the main sub-populations22, 89, 90. Hepatic DCs are less immunogenic compared to their counterparts in spleen or other tissues89. The low immunogenicity of hepatic DCs has been attributed to differences in subtype composition between the liver and spleen, which reflects the lack of expression of constitutive costimulatory molecules89. However, hepatic DCs produce significantly higher amounts of cytokines and have a greater phagocytic capacity than the lymphoid organ counterparts91, 92. The relative contribution of liver dendritic cell subsets in mouse liver91 is illustrated in Figure 5. Comparative analysis and morphometrics estimation of DCs in normal mice demonstrates that the normal liver contains more interstitial DCs compared to any other parenchymal organ93.
It has been suggested - but not yet been proven - that the large number of DCs within the liver may result from the large number of pathogen-associated molecular patterns (PAMPs) contained in portal blood22. PAMPs are responsible for the activation of pattern recognition patterns (PRPs) which activates hepatic scavenger cells and induces the production of IL-6 or other cytokines, subsequently inducing the over-expression of complement C and C-reactive protein. This sequence of events is important for the induction of innate immunity, as acute phase proteins bind to pathogens and accelerate phagocytosis but abrogate the production of tumor necrosis factor (TNF)-α by Kupffer cells (KCs)94. The constitutive expression of PPRs initiates the expression of chemokines and adhesion molecules and promotes immune recruitment to liver. This recruitment drives a series of processes which control the fine balance between recruited effector and regulatory cells and their cytokine milieu, subsequently leading to either hepatic tolerance or immunity20. The cytokine milieu can cause hepatic DCs to become tolerogenic; these cytokines include - but are not limited to - IL-10, TGF-β and are induced by the complex interplay of KCs, LSECs, HSCs and other cell composites22, 95–100.
Hepatic stellate cells
HSCs have a dual role. Under normal conditions they control blood flow through the sinusoidal system, while in pathological conditions and upon exposure to various inflammatory stimuli appear to differentiate into myofibroblasts. They then secrete inhibitors of tissue matrix metalloproteinases, deposit collagen and generate fibrous tissue leading to liver fibrosis. Experimental data in support of the capacity of HSCs to act as APCs are limited101–103, in comparison to that of HSECs. Their tolerogenic capability has been indicated by studies demonstrating that transplanted HSCs effectively protect islet allografts from rejection in an islet transplantation mouse model104. HSC-exerted immunomodulation is regulated by the inducible expression of B7-H1, an inhibitory molecule of B7 family104; depletion of activated HSCs with gliotoxin decreases transplanted hepatocyte engraftment in rats105.
HSCs present lipids to CD4+, CD8+ T-cells and NKT cells. Cultured HSCs can perform fluid-phase and receptor-mediated endocytosis and retain their ability for phagocytosis101. They express MHC class I and II molecules and lipid-presenting CD1b and CD1c molecules, as well as CD86, CD40 and other co-stimulatory molecules101, 102. CD86 is also over-expressed when HSCs have been activated in vivo in a state of extended fibrosis and cirrhosis101. Exposure of HSCs to proinflammatory cytokines such as IFNγ markedly up-regulates CD80101 while CD40 activation leads to a 10-fold increase of the secretion of IL-8 by HSCs and a 2-fold increase of the monocyte chemoattractant protein-1 by the same cells102. Their potential tolerogenic capacity has been supported by their ability to produce vitamin A-derived retinoic acid and TGFβ. Activated HSCs express the negative co-stimulator programmed death (PD)-L1 and can inhibit T-cell responses via B7-H1-mediated apoptosis106.
Hepatocytes and biliary epithelial cells as APCs
A wealth of experimental data reflect the ability of hepatocytes to serve as APCs. Murine studies have shown that the fenestrations of LSECs allow direct hepatocyte-T lymphocytes interaction107. Hepatocytes constitutively express intercellular adhesion molecule-1 and addition of cytokines such as IFN-γ leads to moderate expression of HLA class I molecules. This cytokine leads to significant enhancement of HLA class II only if used at high doses within cultures108. Hepatocytes prime naïve CD8+ T cell, even when they express low levels of MHC class I molecules109. These hepatocyte-primed naive T-cells can expand, but in the absence of co-stimulatory signals they undergo apoptosis, leading to intrahepatic tolerance by clonal deletion109–111. Also, when allogeneic hepatocytes are exposed to T-cells, T-cells activate and apoptose112. In vivo experiments have also provided convincing evidence in support of the APC capacity of hepatocytes52, 113, 114.
The study of the immunogenicity of human BECs or human BEC lines has been studied115–120. BECs from normal human livers express HLA class I at a low frequency. HLA class II molecules are not expressed121. Infection with hepatotropic or hepatotrophic viruses enhances HLA class I expression115; cytomegalovirus infection leads to overexpression of HLA class leaves unaltered HLA class II115. Also, the same virus appears to reduce the rate of IFN-γ induced de novo expression of HLA class II, leaving unaffected HLA class I115. Data have shown that cytokine-induced expression of adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and lymphocyte function-associated antigen-3 (LFA-3) of human BECs is comparable to that seen in Epstein-Barr virus-transformed B-cell lines, a known antigen presenting cell117. The expression of these adhesion molecules is a prerequisite for effector cells to exert their cytolytic action118. In hepatocytes, the expression of HLA class II is increased in cultures performed in the presence of IFN-γ or when other pro-inflammatory cytokines are added117–120. The lack of costimulatory CD28 ligands renders cytokine-stimulated human intraepithelial BECs unable to induce effective T-cell activation119. In pathological conditions such as that of PBC, destructed BECs overexpress HLA class II, as well as CD80 and CD86 co-stimulatory molecules116. While antigen presentation by CD80/CD86-positive APCs induces T-cell activation, antigen presentation in the absence of sufficient CD80/CD86 costimulation may induce tolerance. However, the capacity of BECs to efficiently present antigens and to activate effector cells also depends on whether there is efficient control by regulatory cells.
Efficient antigen presentation and T-cell activation of cells not undergoing apoptosis is a complex process, the fate of which depends on the action exercised by regulatory T-cells in the microenvironment.
Immune Mediated Liver Injury
Viral or bacterial antigens that pass through the liver sinusoids generally induce an immune response; in most cases, an efficient immune response will remove the microorganism. However, this cannot always be achieved, and chronic infectivity may be the final outcome. Interestingly, neither hepatitis B nor hepatitis C are considered cytopathic. In fact, the destruction of the hepatocytes is the outcome of the host’s immune response to clear the virus. This scenario implies that the liver becomes the victim of a friendly fire targeting the virus rather than the hepatocyte. Similarly, autoimmune liver diseases are models for investigation of mechanisms which lead to loss of immunological tolerance.
Autoimmune liver diseases
Autoimmune diseases of the liver affect the hepatocytes in the case of AIH and biliary epithelial cells in the case of PBC. Although select individuals can have features of both diseases, the clinical phenotypes and the immunological characteristics of these two diseases differ significantly1, 122, 123.
AIH has a strong female preponderance, affects all ages, sexes and races and responds well to immunosuppressive treatment. Immunosuppression has no benefit in patients with PBC, a disease which is rarely seen in men and children and is currently treated with ursodeoxycholic acid, a bile constituent122.
Genetic susceptibility associations with HLA and non-HLA genes have been noted in AIH but large genome wide association studies are still lacking. Contrary to AIH, large genome wide studies have been conducted for PBC; a large number of loci have been found to be associated with disease, including HLA and non-HLA genes with immunological significance (such as that of IL12A, IL12RB, STAT4 and CTLA4)124–128. STAT4 has been identified in several previous studies, and is of interest given that it is closely involved with IL12 signalling and participates in the immunopathogenesis of autoimmune diseases.
Despite enormous efforts, the pathogenesis of both diseases remains poorly understood. Some progress has been made and major experimental findings in support of the involvement of the innate and the adaptive arm of immunity are presented123, 129–131. Translational research on the immunopathogenesis of AIH focused on the role played by humoral responses against specific antigens, the involvement of conventional and unconventional T-cells, and the immunomodulatory role of Tregs. Attempts have also been made to identify environmental triggers of the disease and to develop animal models resembling the human condition. In PBC, credible antigen-specific animals models of the disease have been developed132–144.
Primary biliary cirrhosis
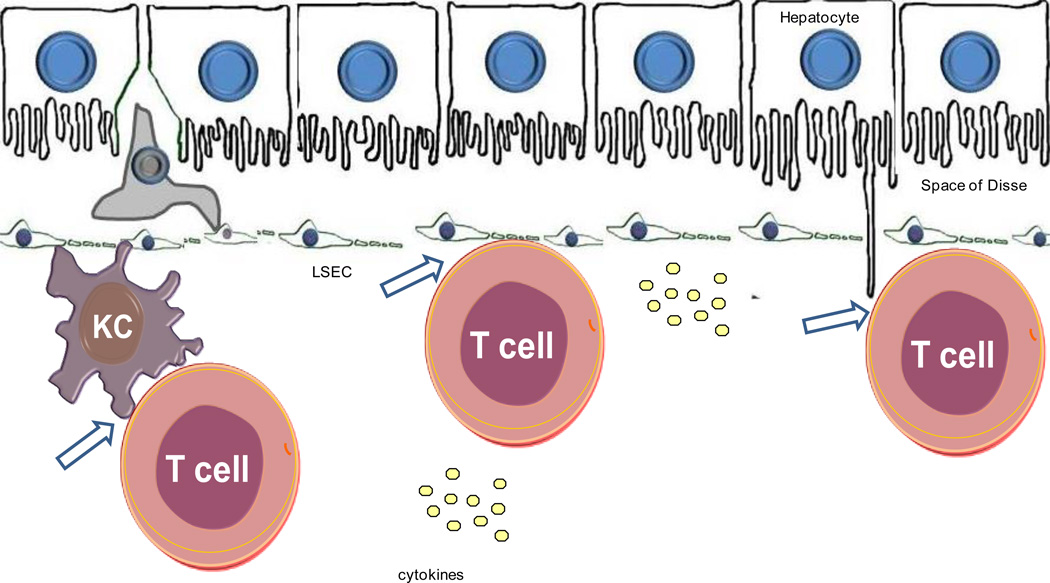
PBC is a chronic cholestatic liver disease characterized by an inflammatory destruction of the medium-size and small intrahepatic bile ducts (Figure 5), which eventually leads to fibrosis, cirrhosis and liver failure122.
PBC was first recognized by Addison and Gull in 1851 but its nature became better understood in 1958 when Mackay reported a case with high titers of complement-fixing antibodies to tissue homogenates145; this autoantibody could be absorbed out using a mitochondrial fraction of rat liver146, and was present in the majority of patients147. The target antigens of these anti-mitochondrial antibodies (AMA) localized to the inner membrane of the mitochondria148. A major step forward in the study of PBC was the cloning of the major AMA target, the E2 subunit of the pyruvate dehydrogenase complex149, 150. Subsequent studies identified as AMA antigens other E2 subunits of the 2-oxo-acid dehydrogenase multienzyme family such as the branched-chain 2-oxo acid dehydrogenase complex (BCOADC) and 2-oxoglutarate dehydrogenase complex (OGDC)151–156. Each of these three multifunctional complexes is involved in set of chain reactions and holds a key position in energy metabolism as PDC links glycolysis to the Krebs cycle, OGDC is essential to the Krebs cycle itself, and BCOADC is involved in the regulation of the oxidation of the branched-chain amino acids157, 158.
Anti-PDC-E2 antibodies belong primarily to the IgG3 subclass, but IgM and IgA autoantibodies targeting this antigen can also be found. Autoantibodies to the 2-OADC enzymes are also detected in bile, saliva, and urine of patients with PBC159–161. Notably, high-titer AMAs in PBC sera can block in vitro the catalytic function of the 2-OAD multienzyme complexes and the AMA bound to PDC-E2 with the greatest affinity are effective in inhibiting PDC-E2 enzymatic activity162. The fine specificity of AMA within their respective antigens has been delineated150, 163–169 and the core epitopic region on PDC-E2 has been mapped within the lipoyl domain of PDC-E2, though, at a 100-fold higher concentration, AMA also react with the outer domain170. AMA specific for BCOADC-E2 and OGDC-E2 are directed against conformational epitopes that include the inner lipoyl domain171.
Adaptive Immunity in PBC
There is evidence of antigen-specific T-cell responses in PBC172, 173. CD4 and CD8-T cell mapping studies have provided intriguing findings, as there is a major CD4 and CD8 epitope, which overlaps with the core B-cell epitope174–180. This overlap of the autoepitopic regions of B- and T-cells has promoted investigators to suggest that this bears a pathogenetic significance for the induction of PBC. This was especially the case after reports indicating that soluble PDC-E2 complexed with a PDC-E2-specific human monoclonal antibody promotes the generation of PDC-E2-specific cytotoxic cells, at a 100-fold lower concentration than otherwise required in the presence of the soluble antigen alone177.
Apotopes and PBC
The basis for the selective autoimmune attack of BECs lining the medium and small-size intrahepatic bile ducts remains elusive. However, it is interesting that specific destruction of small intrahepatic bile ducts is a consequence of the unique characteristics of human intrahepatic BECs during apoptosis and can be explained by exposure to the immune system of intact immunoreactive PDC-E2 within apoptotic blebs181, 182. After apoptosis, human intrahepatic biliary epithelial cells (HiBECs), but not other epithelial cells, translocate PDC-E2 immunologically intact into apoptotic bodies, forming apotopes182. This observation suggests that PDC-E2 is accessible to the immune system during apoptosis. Subsequent experiments demonstrated an intense inflammatory cytokine production in the presence of the unique triad of BEC apotopes, mature monocyte-derived macrophages macrophages from patients with PBC, and AMAs181; other PBC-specific autoantigens also appear to be contained intact into apoptotic bodies appear to contain183.
The role of innate immunity in PBC
The role of innate immunity in the immunopathogenesis of PBC has been supported by a plethora of experimental data demonstrating the intrinsic ability of cholangiocytes to express a variety TLRs, the cellular activators of innate immunity, or other PPRs184–188. Peroxisome proliferator-activated receptor γ (PPARγ) is constitutively expressed in BECs isolated from small intrahepatic bile ducts. They appear to be downregulated in PBC bile ducts185. PBC is also characterized by an upregulation of TLR4 and TLR9 in cholangiocytes and of TLR3 and type I interferon gamma signaling pathways in portal tracts and parenchymal cells92, 185.
A significant role for periductal Langerhans cells and biliary epithelial cell-derived macrophage inflammatory protein-3alpha184. Langerin-positive cells are predominantly within or scattered around biliary epithelial layers of bile ducts on liver biopsy specimens from PBC patients and may act as APC184. The close interaction of innate immunity cells with lymphoid cells with immunoregulatory importance has been strengthened by data demonstrating the induction and perpetuation of Th17 cells in the periductal area in cases of PBC and the differentiation into Th17 cells in periductal dendritic cells and macrophages189. It appears that in PBC, there is a characteristic periductal accumulation of IL-17-positive mononuclear cells189. This is of interest given the wealth of experimental data from murine models of the disease based on the regulatory T-cell defects190, 191.
Autoimmune hepatitis
Early data in patients with AIH192–195 suggested that the disease is characterized by disease-specific circulating lymphocytes ‘sensitized’ to liver constituents of the so called liver soluble protein which were able to kill target cells in vitro. Subsequent experiments using separated T- and non-T- cell subsets from the peripheral blood of AIH patients and xenogenic target cells implies that cytotoxic cells were present in the non-T-cell subpopulation. The participation of antibody dependent cell-mediated cytotoxicity has been considered the most likely mechanism to explain the damage. Subsequent work has shown that IgG bound to hepatocytes are prone to injury by lymphocytes from healthy individuals196. However, clonal analysis studies clearly demonstrated that cytotoxicity against specific antigens of the liver is due to T-cells197. Further, CD4 T-cells have the capacity to initiate autoantibody production by autologous B lymphocytes197. Neutralization with antibodies specific for HLA-DR, CD4 and IL-2R abolishes the ability to produce autoantibodies197.
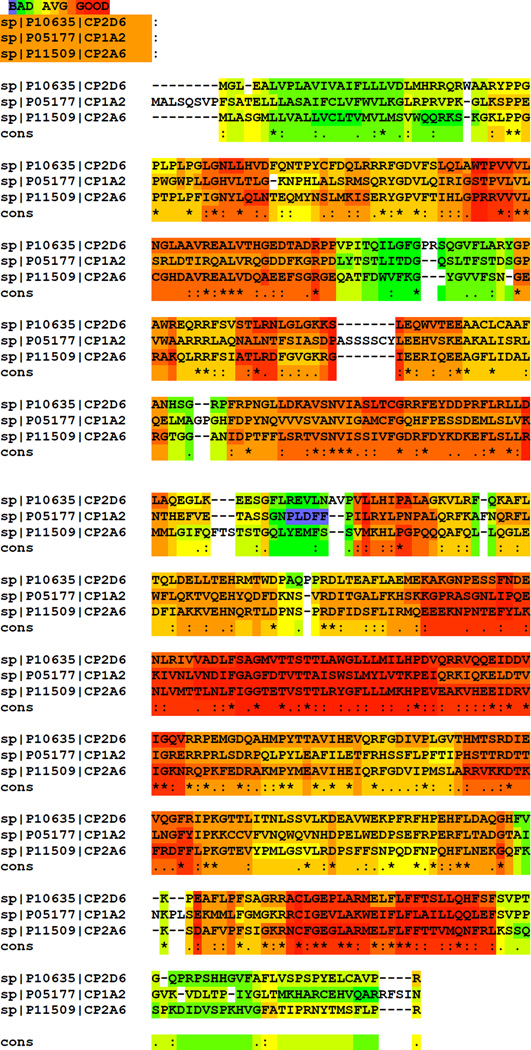
Although there are serotypes of the disease characterized by autoimmune responses against poorly defined nuclear and smooth muscle autoantigens198–200, there are others (albeit less frequent) where the immune responses target liver-specific antigens194, 201–206. Such an example is the immune response against cytochrome P450IID6 (CYP2D6), a member of the cytochrome P450 family of enzymes207. This is a family of exquisitely hepatic detoxifying enzymes, the most prevalent of those within the liver being the CYP3 and CYP2C families. It remains unclear as to why members of the CYP3 family are not target autoantigens in immune-mediated liver injuries. Also, peculiar to AIH is the fact that despite the high degree of homology between CYP2D6, CYP1A2 and CYP2A6 (Figure 12), antibodies against CYP2D6 do not cross-react with the two other cytochromes202, 208, 209. The overall homology of the three antigens is not restricted to amino acid similarity but is also present at the 3D level202 (Figure 12). The apparent lack of cross-recognition may be due to the fact that the epitopic regions recognized by individual anti-CYP antibodies are significantly dissimilar and can generate individual non cross-reactive humoral immune responses. This plausible explanation has been addressed experimentally for a limited number of peptides and proved to be valid210. The epitope specificity of antibodies against CYP2D6 has been delineated and the major linear epitopic regions appear to be exposed on the surface of the molecule (Figure 13).
Figure 12.
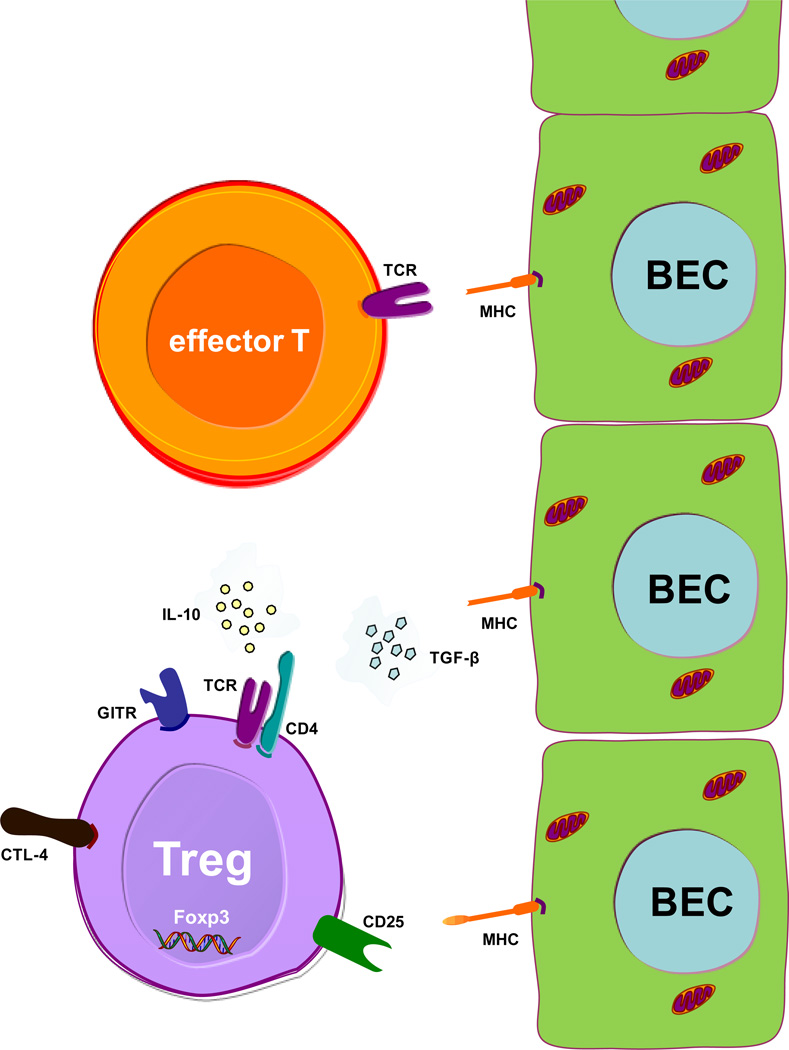
Cell-Cell interaction of biliary epithelial cells (BECs) as antigen presenting cells, effector and regulatory T-cells
Figure 13.
CYP2D6, CYP1A2, and CYP2D6 amino acid homology.
The three cytochromes appear highly conserved but autoantibody responses against the one does not invoke cross-reactive immunity targeting the other. Amino acid analysis has been performed using the T-coffee software. Highlights of red, yellow and green correspond to areas of good, average and bad degree of homology; cons, conservation of amino acids (* indicate identical amino acids, : indicate conserved and . semi-conservative substitutions).
Recent studies have identified the CD4 and CD8-T cell epitopes on CYP2D6 and have demonstrated a partial overlap of major B-and T-cell epitopic regions, a finding that has been described also before in studies for PBC211. T-cell clones generated from liver tissue and peripheral blood recognising a major B- and T-cell epitope (aa 262–285) appear to acquire a Th1 CD4 characterized by their ability to induce IFNγ production phenotype211. Recent work has demonstrated the presence of HLA class I-restricted, IFN-γ-producing CYP2D6-specific CD8 T-cells212.
This has followed earlier studies demonstrating the presence of CD8 T-cell responses specifically recognizing the asialoglycoprotein receptor (ASGPR), a hepatic lectin the antigen recognition of which appears to be associated more with AIH than any other autoimmune or non-autoimmune liver disease.
Early publications provided hints in support of an impairment of suppressory lymphocyte populations in AIH213, 214 These findings have been recapitulated by new evidence supporting a numerical and functional impairment of Tregs which characterizes patients with AIH215–217. This evidence warranties external validation. Nevertheless, functionally enhanced Tregs can be expanded and generated de novo in patients with AIH217. This has been achieved using a polyclonal T-cell stimulation approach which is based on the use of exogenous IL-2 and the engages the T-cell receptor using CD3 and the co-stimulatory molecule CD28217.
Alcoholic hepatitis (AH)
AH is a syndrome characterized by infiltration of the liver by inflammatory cells, including neutrophils, and hepatocellular injury. In addition to the alcohol-induced hepatotoxicity and oxidative stress, activation of innate immunity involving TLR4 and complement also plays an important role in initiating AH, but the role of adaptive immunity in the pathogenesis of AH remains largely unknown (Gao and Bataller, Gastroenterology, 2011,141:1572-85; Gao et al. Am J Physiol Gastrointest Liver Physiol. 2011;300:G516-25). The studies from last decade suggest that activation of a TLR4-mediated MyD88-independent (TRIF/IRF-3-dependent) signaling pathway and complement system in the liver play an important role in the initiation and progression of AH (Gao et al. Am J Physiol Gastrointest Liver Physiol. 2011;300:G516-25). In contrast to activation of TLR4 and complement, chronic alcohol consumption can attenuate NK cells, another important innate immunity component, and subsequently abolish the anti-viral and anti-fibrotic effect of NK cells, contributing to alcohol-mediated acceleration of viral infection and liver fibrosis in patients with chronic viral hepatitis (Jeong et al., Gastroenterology. 2008;134:248-58).
Pathogen-induced Liver Immunity
To better understand the mechanisms responsible for the initiation of an efficient immune response against bacteria, we need to be able to separate anti-bacterial defense in the sinusoidal compartment versus that noted in the parenchymal compartment. Under normal conditions, pathogens circulating in the sinusoidal blood will be eliminated by resident immune cells and will prevent them from accessing or entering hepatocytes.
Bacterial infection targeting the liver
Early sensing of pathogens is important for the initiation of efficient innate immunity and the successful elimination of pathogens218–220. The adaptive arm of immunity also appears to play an important role in the induction of early anti-bacterial immunity207, 208. Thus, KCs, NKT, NK and T cells collaborate in the eradication of pathogens in the sinusoidal blood219–222. Under normal conditions, KCs phagocytose bacteria leading to the rapid clearance of these bacteria from liver. KCs also present bacterial glycolipids on CD1 molecules to immune cells such as NKT218. Recent findings have demonstrated that KCs ingest Borrelia burgdorferi and induce chemokine receptor CXCR3-dependent clustering of invariant NKT cells218. KCs produce CXCL9 and other CXCR3 ligands in response to B. burgdorferi. The antigen-presenting molecule C1d allows for a stable contact of KCs and invariant NKTs in addition to activating NKTs. The attraction of NKT by KCs is achieved in a CXC-chemokine receptor 3-dependent manner218. Depletion of KCs prevents the formation of invariant NKT clusters. Also, the lack of KCS promotes a further increase of B. burgdorferi in liver parenchyma218. No internalization of spirochetes by LSECs has been noted, and the adherence spirochetes to LSECs is augmented when these cells are exposed to the pathogen and KCs are depleted218.
All together, these findings suggest that B. burgdorferi uses LSECs as a tool to escape resistance against pathogens provided by KCs in an attempt to gain access to the extravascular space218. Those Borrelia burgdorferi escaping KCs enter hepatocytes and survive despite initiation of immune responses by HSCs218. It seems that the cross-talk of KCs and NKT cells initiates an anti-bacterial immune response which prevents the host from persistent bacterial infection218. They also support the notion supported by data on Plasmodium yoelii, that KCs do not act as a portal for the entry of spirochetes into liver parenchyma223.
Granulomatous infections and liver immunity
Detection of granulomas in histological liver specimens is a relatively frequent finding, found in up to 4% of undivided samples analyzed224. In one of the largest studies conducted so far, evidence for granulomas was found in 442 (3.6%) of 12,161 liver biopsies analyzed. The presence of infectious organisms was document in only 15 samples (3.4%), with M. tuberculosis being detected in three of the 15 (20%). Other mycobacteria, Yersinia, Toxoplasma gondii, Bartonella henselae and Listeria monocytogenes have also been considered responsible for the formation hepatic granulomas224–226. The exact mechanisms by which these infectious agents induce the development of giant cells and subsequent hepatic granulomas remain unclear227. Granulomas mainly consist of focal accumulations of macrophages and the prevailing theory is that infection of macrophages plays an important role in the their formation227; the formation of granulomas is an attempt of the innate immune to control invasion of pathogens, especially when the adaptive arm of immunity is impaired. Listeria monocytogenes infection can induce the development of formatted by dendritic cells over-expressing indoleamine 2,3-dioxygenase227, 228. The study of granulomas induced by mycobacterial infection has also revealed an important role for matrix metalloproteinase 9 expression in their formation229. Expression of this matrix metalloproteinase is required for the recruitment of macrophages and the formation of granulomas229.
Parasitic infections of the liver
Malarial transmission to humans is achieved by sporozoite infection of hepatocytes230. Infection with malarial parasites is an ideal model to study host–parasite interactions and the mechanisms that allow a pathogen to avoid elimination from immune cells, resident in the sinusoids, and subsequent hepatocyte infection230–234. Work on animal models has provided a better understanding of the mechanisms responsible for parasite invasion. Sporozoites injected by mosquito bites enter the blood circulation, flow through liver sinusoids and subsequently infect hepatocytes,235–237 using poorly understood immune-mediated mechanisms.
Plasmodium spp. sporozoites appear to first target KCs238, 239. Pradel and Frevert239 were the first to provide conclusive data suggesting that sporozoites selectively target and actively invade KCs to avoid the sinusoidal barrier. However, experiments on KC-depleted rats have demonstrated that such a depletion does not abrogate the ability of sporozoites to infect the hepatocytes234. Contrary to the these findings, subsequent experiments conducted by Baer et al223 have shown that KC-deficient mice are resistant to sporozoite infection, probably because of the reduced number of portals to the liver parenchyma which prevents from direct access to hepatocytes. Recent data suggests that circumsporozoite protein, the sporozoite's major surface protein, has an adhesive confirmation in which the C-terminal cell-adhesive domain is exposed and a non-adhesive conformation in which the N terminus covers this domain, the former being important for hepatocyte invasion240. The state of hepatocytes also appears to play an important role for sporozoite infectivity; hepatocytes over-expressing the CD81, a tetraspanin which has been regarded as receptor for hepatitis C virus entry to hepatocytes, are susceptible to infection by Plasmodium falciparum and Plasmodium yoelli241, 242. Subsequent findings have indicated that CD81 may not interact directly with the ligand of Plasmodium yoelli during Plasmodium infection, but through an unidentified protein which is regulated by CD81241.
In endemic areas, repeated exposure to sporozoites during infection does not always lead to the clearance of the parasite from the circulation. This is probably due to the lack of protective immunity243, 244 manifested as antigen-specific antibody and CD8+ T cell responses targeting the circumsporozoite protein, which is one of the most immunodominant targets of malaria-specific immunity245. The number of Plasmodium-specific memory CD8 T cells required for immunity greatly exceeds the number required for resistance to other pathogens244, 246. Also, IL-4 secreting CD4+ T cells appear important for the development of CD8+ T-cell responses against malaria liver stages247. If CD4+ T cell are absent, CD8+ T cells responses are reduced by 90% compared to that seen in the presence of CD4+ T cells and the cell-cell interaction largely depends on the presence of IL-4247.
Viral Hepatitides
Hepatitis B and C viruses (HBV and HCV) are the most common infectious causes of chronic liver disease worldwide. More than 500 million people are infected with HBV and HCV worldwide, and a significant proportion of those develop chronic liver disease, with mortality predominantly from the complication of cirrhosis and from hepatocellular carcinoma248, 249. HBV and HCV are members of the hepadnaviridae and flaviridae families, respectively. The half-life of HBV is 2–3 days, while that of HCV is approximately 3 hours. An effective vaccine for the prevention of HBV has been available for years, but such a vaccine does not currently exist for HCV. Hepatitis A and E are food-borne RNA viruses, which infect the liver when they reach the blood through transversing gut epithelial cells250, 251. They are transmitted from person-to-person by ingestion of contaminated water or food or water or through direct contact with an infected individual. Their incidence is high in developing countries, and both can cause acute hepatitis.
Virus-specific immunity has been considered responsible for the clearance of the virus and protective immunity but also for the immune-mediated destruction of hepatocytes and the development of cirrhosis252–254. The study of the immunobiology of these viruses has been hampered by the lack of well-defined small animal models that accurately resemble the human disease255–258. Virus-host interactions have been studied in the chimpanzee model (the only animal that can be infected with both viruses) and in translational research projects using biological material from infected individuals252, 253, 259–264. From these studies it has become apparent that HBV and HCV share in common various immunopathologic features, but are highly variable in their virologic properties and kinetics during acute and chronic infection. They also differ in their viral- immune evasion and survival tactics219, 253.
While perinatal HBV infection normally leads to chronic hepatitis, 90% or more of the acutely infected adults resolve from symptoms, develop virus-specific antibodies and preserve lifelong protective immunity. T-cell mediated liver destruction manifested as an increase of serum alanine aminotransferase is seen 10–15 weeks after infection. Humans and chimpanzees infected with HCV develop adaptive cellular and humoral responses only after at least 1 month and 2 months, respectively, after the infection265, 266. An increase of transaminases levels is noted 8–12 weeks after infection with HCV.
Innate immune responses in viral hepatitides
A wealth of experimental data in infected chimpanzees has provided a better understanding of the role of innate immunity in the early phases of the infection252, 267–270. The study of the chimpanzee model has shown that HBV DNA is cleared from the serum and the livers of the infected animals long before the development of significant anti-viral adaptive immunity252. Work on transgenic mice has shown that IFN-γ produced by NKT cells can significantly suppress HBV replication271, 272. Also, type I IFNs produced by KCs and other innate immune cells play a major role in the control of viral replication and spreading to neighbouring hepatocytes273–275; such IFNs also prevent the induction of severe immune-mediated hepatocyte destruction273–275. These data have prompted investigators to consider an important role for innate immunity in controlling virus replication271, 272. Genetic microarray analyses in sequential liver samples from HBV infected chimpanzees have failed to show remarkable changes in the expression of type I IFN268 leading to the assumption that the inability to provoke strong innate immune responses in the first weeks of infection is an evasion strategy used by the virus in order to persist. KCs appear to control, to some extent, the magnitude of innate responses, both in HBV and HCV. Capsid proteins of these viruses trigger the expression of TLR-1, TLR-2 and TLR-6 expression on KCs and the subsequent release of pro-inflammatory and regulatory cytokines276–278, involving various PPRs in cellular activation by viral proteins.
Despite the lack of strong innate responses, the recovery rate following HBV infection remains paradoxically very high. On the other hand, HCV infection provokes early changes in the expression of type I IFN and other genes269. The fact that HCV infected patients, who are homozygous for certain killer cell immunoglobulin-like receptor haplotypes are more likely to clear the virus and recover from HCV infection adds strength to the notion innate immunity is required for an efficient control of the virus279.
These data demonstrate that HBV and HCV are highly sensitive to type I IFN280–282 and this has been the basis for the treatment of chronically infected patients, which is based on the administration of IFN-α, alone or in combination with anti-viral agents. Specific genetic polymorphisms in the IL28B locus predict a favourable outcome and response to IFN-α283–288. It also appears that the induction of strong immune responses at early stages of the infection predicts a more favourable outcome and indicates subsequent clearance of HCV289. Against this background, it has become apparent that initiation of strong innate immune responses per se does not immediately preclude the clearance of HCV, as viral chronicity is established in more 50% of cases.
Hepatocyte receptors of hepatotropic viruses and innate immunity
The attachment of HBV and HCV to hepatocytes requires cross-linking with heparin sulphate proteoglycans. Clear demonstration of a molecule which can serve as a receptor for HBV has not been provided. None of the molecules reported so far as receptors of HCV are hepatocyte-specific. The tetraspin CD81 was amongst the first molecules to be identified as hepatocyte receptors of HCV290.
Other molecules which can bind to HCV and seem important for the internalization of HCV into hepatocytes include the low density lipoprotein receptor291 and the scavenger receptor B1292. Also, the envelope E2 protein of HCV can bind to the liver and lymph-node specific ICAM3 grabbing non-integrin (L-SIGN) which is also expressed by LSECs and the DC-specific ICAM3 grabbing non-integrin (D-SIGN) which is also expressed by KCs293–297. Claudin-1298, occludin299, the epidermal growth factor receptor and ephrin type A receptor 2300 are also co-receptors of HCV and play a role in the late steps of viral entry. It appears, that when HCV reaches the liver attached to heparin sulphate proteoglycans in liver sinusoids, it binds to low density lipoprotein, CD81 or scavenger receptor B1 and before endocytosis attaches to claudin and occludin. The receptor tyrosine kinases epidermal growth factor receptor and ephrin type A receptor mediate HCV entry by regulating CD81-claudin-1 co-receptor interactions.
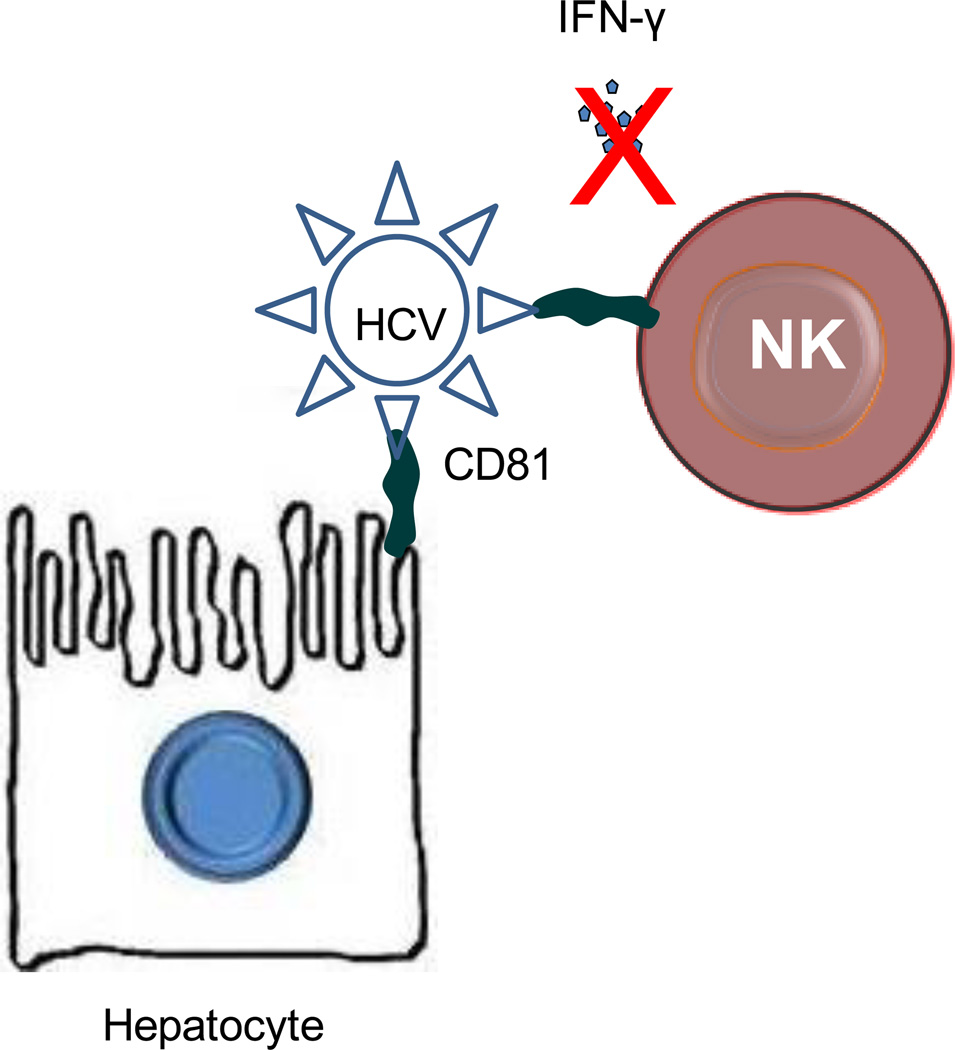
The role of the HCV receptors is not limited to the entry of the virus into hepatocytes. They appear to alter host-virus innate immunity responses with mechanisms poorly understood. Thus, binding of HCV envelope 2 protein with NKs expressing CD81 inhibits the production of IFN-γ and other pro-inflammatory cytokines and suppresses their cytotoxic effects innate immune cells expressing CD81 can alter host-virus innate immunity301302, 303 (Figure 14). Collectively, these data demonstrate that the cytotoxic potential of NK cells from healthy individuals is impaired in the presence of high concentrations of HCV envelope 2 protein, and that NKs from patients infected with HCV are unable to produce pro-inflammatory cytokines and to activate DCs301302, 303. Such findings may not be limited to CD81-mediated HCV/NK interactions but may include other innate immune cells.
Figure 14.
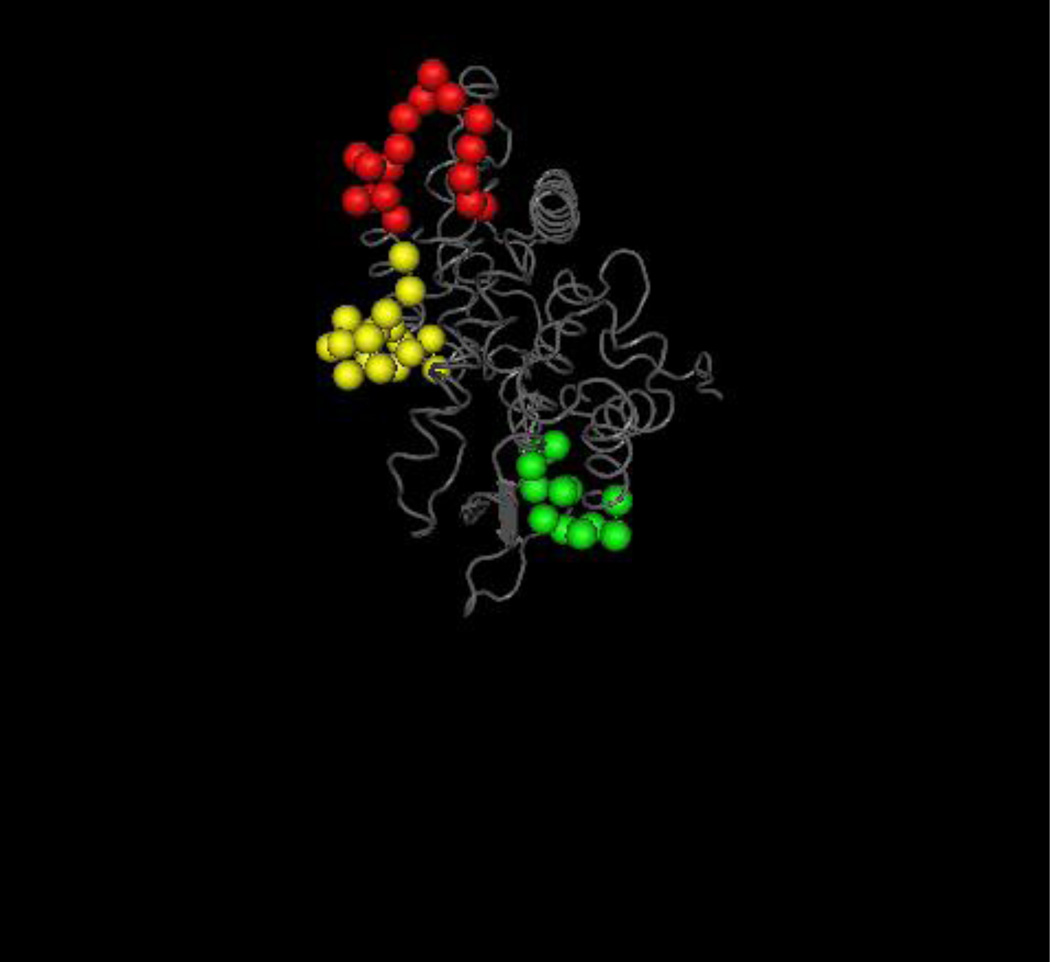
3D-prediction model of the three major linear epitopic regions of human cytochrome P450IID6 (CYP2D6).
The B-cell epitopes of anti-CYP2D6 antibodies (also known as anti-liver kidney microsomal type 1 antibodies- anti-LKM1)392 has been studied and the three main epitopic regions recognized span CYP2D6254–271, CYP2D6193–212 and CYP2D6321–351 sequences, being targeted by more than 55% of the patients with CYP2D6 autoantibodies393–396. The antigenicity of this area may in part been explained by the exposure of these sequences to the surface of the molecule as it is illustrated in Figure 7.
Aminoacids of the autoepitopic regions are presented in the form of space fill in different colours and the remaining in a wire worm backbone (grey); in red and yellow are the dominant CYP2D6254–271 and CYP2D6193–212 epitopes. Prediction analysis anticipates that the epitopes are exposed on the surface of the molecule. The structure was analyzed with the Cn3D visualization tool.
A mucin-like class I integral membrane glycoprotein has been considered the attachment receptor of HAV304, 305. Anti-HAV specific antibodies of the IgA class can carry the virus. Asialoglycoprotein receptor is a C-type lectin which clears IgA from the circulation306, 307. Anti-HAV specific IgA antibodies use asialoglycoprotein receptor to infect hepatocytes308. Dotzauer et al308 used a mouse hepatocyte model which, because it does not permit infection with HAV, can be used as a model to study carrier-mediated HAV entry into host cells without any interference by the HAV receptors. These authors have shown that HAV is taken up by hepatocytes in the form of HAV-specific IgA immunocomplexes and are endocytosed by ASGPR. This has led Protzer et al to hypothesize that KCs expressing the Fcα receptor309 may bind the IgA-HAV complexes219. They may then transfer these complexes to hepatocytes, where the virus can be internalized via the asialoglycoprotein receptor219.
Adaptive immune responses in viral hepatitis B and C
Neither HBV nor HCV clearance can be sustained through the development of strong innate immunity. It appears that the elimination of these viruses can only be achieved in the presence of strong antigen-specific CD4+ and CD8+ T cell responses, a finding that clearly supports the thesis that the adaptive arm of immunity is the most critical for the immune-mediated clearance of the virus281, 310, 311.
Humoral Immunity
The isotype and antigen-specificity of antibody responses in acute and chronic HBV indicates the stage of the disease. IgM antibodies against the hepatitis B core antigen are early markers of infection. Acutely infected patients who recover develop neutralizing antibodies against hepatitis surface antigen (and antigen-specific CD4+ and CD8+ T-cell responses against the core and other antigens of the DNA virus) that confer lifelong protection. The great majority of virus-free individuals who are vaccinated with the surface antigen develop high-titer neutralizing antibodies and are protected from exposure to HBV. Recent data has demonstrated reactivation of the disease following B-cell depletion, indicating the importance of these cells in controlling the virus312.
Chronic HBV infection is roughly subdivided in the immunotolerant, immunoreactive, low replicative and high replicative phases. The duration of these stages varies amongst infected individuals. Antibodies against hepatitis e and surface antigens appear late and are signs of a favorable course while the presence of antibodies against the core and the surface antigens are present forever after the resolution of clinical symptoms.
The influence of anti-HCV antibodies over the course of the disease is far from clear. Infection with HCV does not initiate early humoral responses and the highest concentrations of anti-HCV antibody responses are typically present in patients with well-established chronic HCV infection. In contrast, patients who recovered from HCV do not have detectable anti-HCV antibodies, a finding that has prompted investigators to consider the emergence of HCV escape mutants. How HCV constantly evades neutralizing antibodies during chronic infection remains unclear, though it is most likely due to its ability to ‘creep’ from one hepatocyte to another (cell-to-cell transmission) and to generate a selection of escape mutants are the most likely scenarios.313, 314.
Cellular immunity against hepatitis B and C
The study of the immunobiology of HBV and HCV has led to the appreciation that persistent infection is largely due to the inability of the immune system to clear the virus. Depletion and exhaustion of cytotoxic T lymphocytes specific for viral antigens is the main cause for the inability to control the virus. Antigen-specific cytotoxic lymphocytes are prone to apoptosis315. Also, HCV-specific CD8+ T cells undergo substantial apoptosis in the periphery during acute HCV infection and in the liver during chronic infection316. BCL-2-Interacting Mediator, a key pro-apoptotic mediator which is known as BIM, plays an important role in the apoptosis of virus-specific CD8+ T cells315, 317. Consistent exposure to antigenic overload during infection leads to the ‘exhaustion’ of cytotoxic T lymphocytes318, 319. This phenomenon is largely mediated through the programmed death PD-1/PD-L1 T cell co-inhibitory pathway. PD-1 is a co-inhibitory molecule that controls T-cell reactivity. PD-L1 and PD-L2 binding to the PD-1 receptor negatively regulates T cells, leading to decreased proliferation and down-regulation of IL-2 and IFN-γ. This ‘exhaustion’ operates at several levels and allows the virus to escape immune recognition and to establish persistent infection320. Blocking PD-1 restores intrahepatic HBV-specific T-cell responses321, 322. This may explain why HBV patients have over-expressed PD-1 compared to healthy individuals322. Also, HCV-specific cytotoxic T lymphocytes that are phenotypically exhausted are characterized by PD-1 over-expression323, 324. Patients with chronic HBV and HCV infection over-express co-inhibitory molecules such as PD-1, CTL4, T cell immunoglobulin domain protein 3 and 2B4325. An imbalance on the operation of co-inhibitory pathways in viral hepatitides B and C appears not to be limited at the lymphocyte level, as it includes KCs LSECs and HSCs. These cells appear to express (at least) PD-L1, and their induction has been demonstrated during viral infection71, 106, 326–331. The role of CD4+ T cells has been extensively studied in patients and chimpanzees infected with HBV or HCV. These studies have shown that induction of vigorous, antigen-specific CD4 T cell responses positively correlates with the recovery from the infection332. Epitope mapping studies have delineated the disease-specific autopepitopic regions recognized by acutely and chronically infected patients at various phases of the diseases254. Despite the low frequency of CD4+ T cells in the periphery, flow cytometric analyses have shown that in the early phase of the infection with HCV CD4+ T cell become detectable at the time CD8+ T cell recover266. The close interplay between CD4+ and CD8+ T cells has been shown in studies indicating that CD4 depletion from a chimpanzee recovered from HCV infection leads to the loss of protective immunity upon re-exposure to the virus311. These studies have provided data to suggest that CD4+ T cells confer protective immunity but are also participating the induction of immune-mediated hepatocyte destruction.
Extrahepatic immune-mediated manifestations are frequently seen in chronic HBV and HCV. In the great majority of the cases are manifested as low to medium titer autoantibodies against nuclear and smooth muscle antibodies333. Whether these autoantibodies are induced as a result of polyclonal B-expansion during hepatocyte destruction or they are the outcome of antigen-driven mechanisms such as molecular mimicry and immunological cross-reactivity remains elusive202, 208, 333–338. Immune complex-mediated rheumatic manifestations, mixed cryoglobulinemia, and non-Hodgkin lymphomas are more frequently seen in chronic HCV infected patients than HBV339. Viral-induced autoimmune liver diseases are rarely noted, and a link between the virus and the autoimmune condition is difficult to make340–342.
The immunopathology of hepatocellular carcinoma
It has become apparent that while a vigorous antigen-specific immune response against HBV and HCV leads to viral clearance, the depletion and exhaustion of cytotoxic T lymphocytes specific for viral antigens induces chronic hepatitis and subsequent cirrhosis. In an attempt to respond to the process of necroinflammation, the liver regenerates, and in this process activates macrophages which release free radical with carcinogenic potential. The concert action of mitogenic and mutagenic stimuli causing cellular and DNA damage and the dysregulation of cellular growth leads to the development of hepatocellular carcinoma343, 344. HBV vaccination programs prevented the development of newly infected cases and indirectly decreased the number of new hepatocellular carcinoma cases345. In this respect, the hepatitis B vaccine can be considered one of the very first successful immunotherapy cancer vaccines.
Tumor markers such as alpha fetoprotein are increased during the course of hepatocarcinogenesis and T-cell responses specific for this antigen are present in the peripheral blood of patients with cancer346–349. Several other antigens have been identified as potential tumor markers and target autoantigens but none of them is highly disease-specific. Nevertheless, strong tumor-specific CD8+ T cell responses control tumor progression and prevent from the recurrence of hepatocellular carcinoma350, 351. Several studies have shown that tumors have adopted numerous immune escape mechanisms, that include the induction of immunosuppressory cells. These include regulatory T cells and myeloid-derived suppressor cells. Such cells have the potential to mask tumor-specific immune responses in patients with hepatocellular carcinoma.
The frequency of circulating CD4(+)CD25(+)FoxP3(+) Treg is increased significantly in patients with hepatocellular carcinoma compared to controls and correlated with disease progression352, 353. Clustering of Tregs and reduction rates of infiltration CD8+ T cells are characteristically found in tumor regions compared with nontumor regions and these Tregs suppress the anti-CD3/CD28 induced cytolytic activity of CD8+ T cells352. Current attempts concentrate in targeting regulatory T cells as this will potentially enhance tumor-specific CD8+ T cells354, 355.
Conclusions
Historically, the immune system was divided into mucosal versus systemic immunity. While this division is still accurate, it is equally important to note specific immunological contributions of specific organs; this is illustrated not only by the liver as discussed herein, but also with respect to skin, lung and other tissues. We have not attempted to discuss loss of tolerance in the detail deserved in a paper devoted to liver and the immune response and we refer to a number of recent publications which deal specifically with genetics, environment and immunity356–375. However, it is noteworthy that the liver is anatomically unique and its function is essential for fetal tolerance and host protection from gut flora and the enormous repertoire of materials that pass through the portal circulation. It is indeed ironic that the liver, which is so critical for immune tolerance, can itself become a victim in diseases such as autoimmune hepatitis and primary biliary cirrhosis. We have not discussed primary sclerosing cholangitis herein because the scope is far beyond that of the thesis herein. However, it also brings to mind great voids that exist with respect to our knowledge on chemokines and their cognate receptors with respect to lymphoid homing and we refer to a number of seminal publications by Adams and colleagues376–380. Finally, there is also the hope that the liver as a facilitator of tolerance can be used as a tolerizing vehicle to restore immune homeostasis in other examples of human autoimmune disease381.
Figure 9.
Cell-cell interaction which can lead to activation of naïve T lymphocytes within the liver include contact with Kupffer cells (KC), liver sinusoidal endothelial cells (LSEC) or hepatocytes (indicated by the respective arrows).
Figure 10.
Flow cytometric analysis of peripheral blood reflects the relative proportion of bright and low NK (CD36+) cells, NKT (CD3+CD56+) and T-cells (CD3+CD56−) in a representative donor. Bright and low NK cells can be seen.
Figure 11.
Hepatic dendritic subsets in mouse
Figure 15.
The tetraspin CD81 is used by hepatitis C virus to entry the hepatocyte. Expression of CD81 by NK suppresses the induction of pro-inflammatory cytokines such as interferon-γ and inhibits the cytotoxic capability of these cells allowing for the persistence of the virus. Other HCV co-receptors (for further details, see main text), expressed by cells of the innate immune system resident within the sinusoids, may participate in a similar fashion facilitating the inability of the host to clear the virus.
Abbreviations
- AIH
Autoimmune hepatitis
- AMA
Antimitochondrial antibody
- ANA
Antinuclear antibody
- APC
Antigen presenting cell
- DC
Dendritic cell
- HSC
Hepatic stellate cell
- IFN
Interferon
- IL
Interleukin
- MHC
Major Histocompatibility Complex
- KC
Kupffer cell
- LPS
Lipopolysaccharide endotoxin
- LSEC
Liver sinusoidal endothelial cell
- mDC
myeloid DC
- NK
Natural Killer
- NKT
Natural Killer T-cell
- OADC
oxo-acid dehydrogenase complex
- OGDC
2-oxoglutarate dehydrogenase complex
- PAMP
pathogen-associated molecular pattern
- PBC
primary biliary cirrhosis
- PD
programmed death
- pDC
plasmacytoid DC
- PD-L1
programmed death ligand 1
- PPR
Pattern recognition patterns
- TLR
Toll-like receptor
Footnotes
Conflict of Interest: None of the authors has a conflict of interest to declare.
References
- 1.Kita H, Mackay IR, Van De Water J, Gershwin ME. The lymphoid liver: considerations on pathways to autoimmune injury. Gastroenterology. 2001;120:1485–1501. doi: 10.1053/gast.2001.22441. [DOI] [PubMed] [Google Scholar]
- 2.Starzl TE, Marchioro TL, Vonkaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the Liver in Humans. Surg Gynecol Obstet. 1963;117:659–676. [PMC free article] [PubMed] [Google Scholar]
- 3.Calne RY, Rolles K, White DJ, Thiru S, Evans DB, McMaster P, Dunn DC, Craddock GN, Henderson RG, Aziz S, Lewis P. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet. 1979;2:1033–1036. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- 4.Zimmermann FA, White DJ, Gokel JM, Calne RY. Orthotopic liver transplantation in rats. Prolonging of survival time of allotransplants using cyclosporin A in an acute rejection model. Chir Forum Exp Klin Forsch. 1979:339–344. [PubMed] [Google Scholar]
- 5.Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM, Binns RM, Davies DA. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472–476. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 6.Cantor HM, Dumont AE. Hepatic suppression of sensitization to antigen absorbed into the portal system. Nature. 1967;215:744–745. doi: 10.1038/215744a0. [DOI] [PubMed] [Google Scholar]
- 7.Blouin A, Bolender RP, Weibel ER. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol. 1977;72:441–455. doi: 10.1083/jcb.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolender RP, Weibel ER. A morphometric study of the removal of phenobarbital-induced membranes from hepatocytes after cessation of threatment. J Cell Biol. 1973;56:746–761. doi: 10.1083/jcb.56.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loud AV. A quantitative stereological description of the ultrastructure of normal rat liver parenchymal cells. J Cell Biol. 1968;37:27–46. doi: 10.1083/jcb.37.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weibel ER, Staubli W, Gnagi HR, Hess FA. Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol. 1969;42:68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staubli W, Hess R, Weibel ER. Correlated morphometric and biochemical studies on the liver cell. II. Effects of phenobarbital on rat hepatocytes. J Cell Biol. 1969;42:92–112. doi: 10.1083/jcb.42.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gumucio JJ, Berkovitz CM, Webster ST, Thornton AJ. Structural and functional organization of the liver. In: Kaplowitz N, editor. Liver and biliary diseases. II. Baltimore: Williams & Wilkins; 1996. pp. 3–19. [Google Scholar]
- 13.Rappaport AM, Borowy ZJ, Lougheed WM, Lotto WN. Subdivision of hexagonal liver lobules into a structural and functional unit; role in hepatic physiology and pathology. Anat Rec. 1954;119:11–33. doi: 10.1002/ar.1091190103. [DOI] [PubMed] [Google Scholar]
- 14.Freudenberg MA, Freudenberg N, Galanos C. Time course of cellular distribution of endotoxin in liver, lungs and kidneys of rats. Br J Exp Pathol. 1982;63:56–65. [PMC free article] [PubMed] [Google Scholar]
- 15.Elvevold K, Smedsrod B, Martinez I. The liver sinusoidal endothelial cell: a cell type of controversial and confusing identity. Am J Physiol Gastrointest Liver Physiol. 2008;294:G391–G400. doi: 10.1152/ajpgi.00167.2007. [DOI] [PubMed] [Google Scholar]
- 16.Wisse E. An electron microscopic study of the fenestrated endothelial lining of rat liver sinusoids. J Ultrastruct Res. 1970;31:125–150. doi: 10.1016/s0022-5320(70)90150-4. [DOI] [PubMed] [Google Scholar]
- 17.Braet F, de Zanger R, Seynaeve C, Baekeland M, Wisse E. A comparative atomic force microscopy study on living skin fibroblasts and liver endothelial cells. J Electron Microsc (Tokyo) 2001;50:283–290. doi: 10.1093/jmicro/50.4.283. [DOI] [PubMed] [Google Scholar]
- 18.Braet F, Kalle WH, De Zanger RB, De Grooth BG, Raap AK, Tanke HJ, Wisse E. Comparative atomic force and scanning electron microscopy: an investigation on fenestrated endothelial cells in vitro. J Microsc. 1996;181:10–17. doi: 10.1046/j.1365-2818.1996.72348.x. [DOI] [PubMed] [Google Scholar]
- 19.Fraser R, Dobbs BR, Rogers GW. Lipoproteins and the liver sieve: the role of the fenestrated sinusoidal endothelium in lipoprotein metabolism, atherosclerosis, and cirrhosis. Hepatology. 1995;21:863–874. [PubMed] [Google Scholar]
- 20.Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21–34. doi: 10.1034/j.1600-0528.2002.017408.x. [DOI] [PubMed] [Google Scholar]
- 21.Crispe IN. Liver antigen-presenting cells. J Hepatol. 2011;54:357–365. doi: 10.1016/j.jhep.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753–766. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 23.Norris S, Collins C, Doherty DG, Smith F, McEntee G, Traynor O, Nolan N, Hegarty J, O'Farrelly C. Resident human hepatic lymphocytes are phenotypically different from circulating lymphocytes. J Hepatol. 1998;28:84–90. doi: 10.1016/s0168-8278(98)80206-7. [DOI] [PubMed] [Google Scholar]
- 24.Norris S, Doherty DG, Collins C, McEntee G, Traynor O, Hegarty JE, O'Farrelly C. Natural T cells in the human liver: cytotoxic lymphocytes with dual T cell and natural killer cell phenotype and function are phenotypically heterogenous and include Valpha24-JalphaQ and gammadelta T cell receptor bearing cells. Hum Immunol. 1999;60:20–31. doi: 10.1016/s0198-8859(98)00098-6. [DOI] [PubMed] [Google Scholar]
- 25.Doherty DG, Norris S, Madrigal-Estebas L, McEntee G, Traynor O, Hegarty JE, O'Farrelly C. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol. 1999;163:2314–2321. [PubMed] [Google Scholar]
- 26.Hata K, Zhang XR, Iwatsuki S, Van Thiel DH, Herberman RB, Whiteside TL. Isolation, phenotyping, and functional analysis of lymphocytes from human liver. Clin Immunol Immunopathol. 1990;56:401–419. doi: 10.1016/0090-1229(90)90160-r. [DOI] [PubMed] [Google Scholar]
- 27.Doherty DG, O'Farrelly C. Innate and adaptive lymphoid cells in the human liver. Immunol Rev. 2000;174:5–20. doi: 10.1034/j.1600-0528.2002.017416.x. [DOI] [PubMed] [Google Scholar]
- 28.Ohteki T, Abo T, Seki S, Kobata T, Yagita H, Okumura K, Kumagai K. Predominant appearance of gamma/delta T lymphocytes in the liver of mice after birth. Eur J Immunol. 1991;21:1733–1740. doi: 10.1002/eji.1830210722. [DOI] [PubMed] [Google Scholar]
- 29.Abo T, Watanabe H, Iiai T, Kimura M, Ohtsuka K, Sato K, Ogawa M, Hirahara H, Hashimoto S, Sekikawa H, et al. Extrathymic pathways of T-cell differentiation in the liver and other organs. Int Rev Immunol. 1994;11:61–102. doi: 10.3109/08830189409061717. [DOI] [PubMed] [Google Scholar]
- 30.Hayakawa S, Saito S, Nemoto N, Chishima F, Akiyama K, Shiraishi H, Hayakawa J, Karasaki-Suzuki M, Fujii KT, Ichijo M, et al. Expression of recombinase-activating genes (RAG-1 and 2) in human decidual mononuclear cells. J Immunol. 1994;153:4934–4939. [PubMed] [Google Scholar]
- 31.Rocha B, Vassalli P, Guy-Grand D. Thymic and extrathymic origins of gut intraepithelial lymphocyte populations in mice. J Exp Med. 1994;180:681–686. doi: 10.1084/jem.180.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science. 2005;309:264–268. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 33.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 34.Zhao N, Hao J, Ni Y, Luo W, Liang R, Cao G, Zhao Y, Wang P, Zhao L, Tian Z, Flavell R, Hong Z, Han J, Yao Z, Wu Z, Yin Z. Vgamma4 gammadelta T cell-derived IL-17A negatively regulates NKT cell function in Con A-induced fulminant hepatitis. J Immunol. 2011;187:5007–5014. doi: 10.4049/jimmunol.1101315. [DOI] [PubMed] [Google Scholar]
- 35.Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD, Oshiro K, Okamoto Y, Watanabe H, Kawakami K, Roark C, Born WK, O'Brien R, Ikuta K, Ishikawa H, Nakae S, Iwakura Y, Ohta T, Matsuzaki G. IL-17A produced by gammadelta T cells plays a critical role in innate immunity against listeria monocytogenes infection in the liver. J Immunol. 2008;181:3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He W, Hao J, Dong S, Gao Y, Tao J, Chi H, Flavell R, O'Brien RL, Born WK, Craft J, Han J, Wang P, Zhao L, Wu J, Yin Z. Naturally activated V gamma 4 gamma delta T cells play a protective role in tumor immunity through expression of eomesodermin. J Immunol. 2010;185:126–133. doi: 10.4049/jimmunol.0903767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hao J, Dong S, Xia S, He W, Jia H, Zhang S, Wei J, O'Brien RL, Born WK, Wu Z, Wang P, Han J, Hong Z, Zhao L, Yin Z. Regulatory role of Vgamma1 gammadelta T cells in tumor immunity through IL-4 production. J Immunol. 2011;187:4979–4986. doi: 10.4049/jimmunol.1101389. [DOI] [PubMed] [Google Scholar]
- 38.Martins EB, Graham AK, Chapman RW, Fleming KA. Elevation of gamma delta T lymphocytes in peripheral blood and livers of patients with primary sclerosing cholangitis and other autoimmune liver diseases. Hepatology. 1996;23:988–993. doi: 10.1002/hep.510230508. [DOI] [PubMed] [Google Scholar]
- 39.Wen L, Ma Y, Bogdanos DP, Wong FS, Demaine A, Mieli-Vergani G, Vergani D. Pediatric autoimmune liver diseases: the molecular basis of humoral and cellular immunity. Curr Mol Med. 2001;1:379–389. doi: 10.2174/1566524013363672. [DOI] [PubMed] [Google Scholar]
- 40.Wen L, Peakman M, Mieli-Vergani G, Vergani D. Elevation of activated gamma delta T cell receptor bearing T lymphocytes in patients with autoimmune chronic liver disease. Clin Exp Immunol. 1992;89:78–82. doi: 10.1111/j.1365-2249.1992.tb06881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferri S, Longhi MS, De Molo C, Lalanne C, Muratori P, Granito A, Hussain MJ, Ma Y, Lenzi M, Mieli-Vergani G, Bianchi FB, Vergani D, Muratori L. A multifaceted imbalance of T cells with regulatory function characterizes type 1 autoimmune hepatitis. Hepatology. 2010;52:999–1007. doi: 10.1002/hep.23792. [DOI] [PubMed] [Google Scholar]
- 42.Murison JG, Quaratino S, Kahan M, Verhoef A, Londei M. Definition of unique traits of human CD4−CD8− alpha beta T cells. Clin Exp Immunol. 1993;93:464–470. doi: 10.1111/j.1365-2249.1993.tb08202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niehues T, Gulwani-Akolkar B, Akolkar PN, Tax W, Silver J. Unique phenotype and distinct TCR V beta repertoire in human peripheral blood alpha beta TCR+, CD4−, and CD8− double negative T cells. J Immunol. 1994;152:1072–1081. [PubMed] [Google Scholar]
- 44.Thomson CW, Lee BP, Zhang L. Double-negative regulatory T cells: non-conventional regulators. Immunol Res. 2006;35:163–178. doi: 10.1385/IR:35:1:163. [DOI] [PubMed] [Google Scholar]
- 45.Thomson CW, Teft WA, Chen W, Lee BP, Madrenas J, Zhang L. FcR gamma presence in TCR complex of double-negative T cells is critical for their regulatory function. J Immunol. 2006;177:2250–2257. doi: 10.4049/jimmunol.177.4.2250. [DOI] [PubMed] [Google Scholar]
- 46.Masuda T, Ohteki T, Abo T, Seki S, Nose S, Nagura H, Kumagai K. Expansion of the population of double negative CD4−8− T alpha beta-cells in the liver is a common feature of autoimmune mice. J Immunol. 1991;147:2907–2912. [PubMed] [Google Scholar]
- 47.von Boehmer H, Kirberg J, Rocha B. An unusual lineage of alpha/beta T cells that contains autoreactive cells. J Exp Med. 1991;174:1001–1008. doi: 10.1084/jem.174.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Selmi C, Mackay IR, Gershwin ME. The immunological milieu of the liver. Semin Liver Dis. 2007;27:129–139. doi: 10.1055/s-2007-979466. [DOI] [PubMed] [Google Scholar]
- 49.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 50.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ishihara S, Nieda M, Kitayama J, Osada T, Yabe T, Ishikawa Y, Nagawa H, Muto T, Juji T. CD8(+)NKR-P1A (+)T cells preferentially accumulate in human liver. Eur J Immunol. 1999;29:2406–2413. doi: 10.1002/(SICI)1521-4141(199908)29:08<2406::AID-IMMU2406>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 52.Bertolino P, McCaughan GW, Bowen DG. Role of primary intrahepatic T-cell activation in the 'liver tolerance effect'. Immunol Cell Biol. 2002;80:84–92. doi: 10.1046/j.0818-9641.2001.01048.x. [DOI] [PubMed] [Google Scholar]
- 53.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19:916–924. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li W, Kuhr CS, Zheng XX, Carper K, Thomson AW, Reyes JD, Perkins JD. New insights into mechanisms of spontaneous liver transplant tolerance: the role of Foxp3-expressing CD25+CD4+ regulatory T cells. Am J Transplant. 2008;8:1639–1651. doi: 10.1111/j.1600-6143.2008.02300.x. [DOI] [PubMed] [Google Scholar]
- 55.Horn T, Christoffersen P, Henriksen JH. Alcoholic liver injury: defenestration in noncirrhotic livers--a scanning electron microscopic study. Hepatology. 1987;7:77–82. doi: 10.1002/hep.1840070117. [DOI] [PubMed] [Google Scholar]
- 56.Braet F, Wisse E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp Hepatol. 2002;1:1. doi: 10.1186/1476-5926-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Le Couteur DG, Fraser R, Hilmer S, Rivory LP, McLean AJ. The hepatic sinusoid in aging and cirrhosis: effects on hepatic substrate disposition and drug clearance. Clin Pharmacokinet. 2005;44:187–200. doi: 10.2165/00003088-200544020-00004. [DOI] [PubMed] [Google Scholar]
- 58.Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol. 1998;140:947–959. doi: 10.1083/jcb.140.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fraser JR, Alcorn D, Laurent TC, Robinson AD, Ryan GB. Uptake of circulating hyaluronic acid by the rat liver. Cellular localization in situ. Cell Tissue Res. 1985;242:505–510. doi: 10.1007/BF00225415. [DOI] [PubMed] [Google Scholar]
- 60.Lohse AW, Knolle PA, Bilo K, Uhrig A, Waldmann C, Ibe M, Schmitt E, Gerken G, Meyer Zum Buschenfelde KH. Antigen-presenting function and B7 expression of murine sinusoidal endothelial cells and Kupffer cells. Gastroenterology. 1996;110:1175–1181. doi: 10.1053/gast.1996.v110.pm8613007. [DOI] [PubMed] [Google Scholar]
- 61.Knolle PA, Gerken G, Loser E, Dienes HP, Gantner F, Tiegs G, Meyer zum Buschenfelde KH, Lohse AW. Role of sinusoidal endothelial cells of the liver in concanavalin A-induced hepatic injury in mice. Hepatology. 1996;24:824–829. doi: 10.1002/hep.510240413. [DOI] [PubMed] [Google Scholar]
- 62.Knolle PA, Germann T, Treichel U, Uhrig A, Schmitt E, Hegenbarth S, Lohse AW, Gerken G. Endotoxin down-regulates T cell activation by antigen-presenting liver sinusoidal endothelial cells. J Immunol. 1999;162:1401–1407. [PubMed] [Google Scholar]
- 63.Knolle PA, Schmitt E, Jin S, Germann T, Duchmann R, Hegenbarth S, Gerken G, Lohse AW. Induction of cytokine production in naive CD4(+) T cells by antigen-presenting murine liver sinusoidal endothelial cells but failure to induce differentiation toward Th1 cells. Gastroenterology. 1999;116:1428–1440. doi: 10.1016/s0016-5085(99)70508-1. [DOI] [PubMed] [Google Scholar]
- 64.Knolle PA, Uhrig A, Hegenbarth S, Loser E, Schmitt E, Gerken G, Lohse AW. IL-10 down-regulates T cell activation by antigen-presenting liver sinusoidal endothelial cells through decreased antigen uptake via the mannose receptor and lowered surface expression of accessory molecules. Clin Exp Immunol. 1998;114:427–433. doi: 10.1046/j.1365-2249.1998.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Limmer A, Ohl J, Kurts C, Ljunggren HG, Reiss Y, Groettrup M, Momburg F, Arnold B, Knolle PA. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med. 2000;6:1348–1354. doi: 10.1038/82161. [DOI] [PubMed] [Google Scholar]
- 66.Limmer A, Ohl J, Wingender G, Berg M, Jungerkes F, Schumak B, Djandji D, Scholz K, Klevenz A, Hegenbarth S, Momburg F, Hammerling GJ, Arnold B, Knolle PA. Cross-presentation of oral antigens by liver sinusoidal endothelial cells leads to CD8 T cell tolerance. Eur J Immunol. 2005;35:2970–2981. doi: 10.1002/eji.200526034. [DOI] [PubMed] [Google Scholar]
- 67.Katz SC, Pillarisetty VG, Bleier JI, Shah AB, DeMatteo RP. Liver sinusoidal endothelial cells are insufficient to activate T cells. J Immunol. 2004;173:230–235. doi: 10.4049/jimmunol.173.1.230. [DOI] [PubMed] [Google Scholar]
- 68.Berg M, Wingender G, Djandji D, Hegenbarth S, Momburg F, Hammerling G, Limmer A, Knolle P. Cross-presentation of antigens from apoptotic tumor cells by liver sinusoidal endothelial cells leads to tumor-specific CD8+ T cell tolerance. Eur J Immunol. 2006;36:2960–2970. doi: 10.1002/eji.200636033. [DOI] [PubMed] [Google Scholar]
- 69.Elvevold KH, Nedredal GI, Revhaug A, Smedsrod B. Scavenger properties of cultivated pig liver endothelial cells. Comp Hepatol. 2004;3:4. doi: 10.1186/1476-5926-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Onoe T, Ohdan H, Tokita D, Shishida M, Tanaka Y, Hara H, Zhou W, Ishiyama K, Mitsuta H, Ide K, Asahara T. Liver sinusoidal endothelial cells tolerize T cells across MHC barriers in mice. J Immunol. 2005;175:139–146. doi: 10.4049/jimmunol.175.1.139. [DOI] [PubMed] [Google Scholar]
- 71.Diehl L, Schurich A, Grochtmann R, Hegenbarth S, Chen L, Knolle PA. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T cell tolerance. Hepatology. 2008;47:296–305. doi: 10.1002/hep.21965. [DOI] [PubMed] [Google Scholar]
- 72.Schildberg FA, Hegenbarth SI, Schumak B, Scholz K, Limmer A, Knolle PA. Liver sinusoidal endothelial cells veto CD8 T cell activation by antigen-presenting dendritic cells. Eur J Immunol. 2008;38:957–967. doi: 10.1002/eji.200738060. [DOI] [PubMed] [Google Scholar]
- 73.Knolle P, Schlaak J, Uhrig A, Kempf P, Meyer zum Buschenfelde KH, Gerken G. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J Hepatol. 1995;22:226–229. doi: 10.1016/0168-8278(95)80433-1. [DOI] [PubMed] [Google Scholar]
- 74.Connolly MK, Bedrosian AS, Malhotra A, Henning JR, Ibrahim J, Vera V, Cieza-Rubio NE, Hassan BU, Pachter HL, Cohen S, Frey AB, Miller G. In hepatic fibrosis, liver sinusoidal endothelial cells acquire enhanced immunogenicity. J Immunol. 2010;185:2200–2208. doi: 10.4049/jimmunol.1000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ge X, Karrar A, Ericzon BG, Broome U, Sumitran-Holgersson S. Antibodies to liver sinusoidal endothelial cells modulate immune responses in liver transplantation. Transplant Proc. 2005;37:3335–3337. doi: 10.1016/j.transproceed.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 76.Sumitran-Holgersson S, Ge X, Karrar A, Xu B, Nava S, Broome U, Nowak G, Ericzon BG. A novel mechanism of liver allograft rejection facilitated by antibodies to liver sinusoidal endothelial cells. Hepatology. 2004;40:1211–1221. doi: 10.1002/hep.20434. [DOI] [PubMed] [Google Scholar]
- 77.Ju C, Pohl LR. Tolerogenic role of Kupffer cells in immune-mediated adverse drug reactions. Toxicology. 2005;209:109–112. doi: 10.1016/j.tox.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 78.Ju C, McCoy JP, Chung CJ, Graf ML, Pohl LR. Tolerogenic role of Kupffer cells in allergic reactions. Chem Res Toxicol. 2003;16:1514–1519. doi: 10.1021/tx0341761. [DOI] [PubMed] [Google Scholar]
- 79.Sato K, Yabuki K, Haba T, Maekawa T. Role of Kupffer cells in the induction of tolerance after liver transplantation. J Surg Res. 1996;63:433–438. doi: 10.1006/jsre.1996.0288. [DOI] [PubMed] [Google Scholar]
- 80.Kamei T, Callery MP, Flye MW. Kupffer cell blockade prevents induction of portal venous tolerance in rat cardiac allograft transplantation. J Surg Res. 1990;48:393–396. doi: 10.1016/0022-4804(90)90001-i. [DOI] [PubMed] [Google Scholar]
- 81.Callery MP, Kamei T, Flye MW. Kupffer cell blockade inhibits induction of tolerance by the portal venous route. Transplantation. 1989;47:1092–1094. [PubMed] [Google Scholar]
- 82.You Q, Cheng L, Kedl RM, Ju C. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology. 2008;48:978–990. doi: 10.1002/hep.22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Breous E, Somanathan S, Vandenberghe LH, Wilson JM. Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology. 2009;50:612–621. doi: 10.1002/hep.23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Erhardt A, Biburger M, Papadopoulos T, Tiegs G. IL-10, regulatory T cells, and Kupffer cells mediate tolerance in concanavalin A-induced liver injury in mice. Hepatology. 2007;45:475–485. doi: 10.1002/hep.21498. [DOI] [PubMed] [Google Scholar]
- 85.Prickett TC, McKenzie JL, Hart DN. Characterization of interstitial dendritic cells in human liver. Transplantation. 1988;46:754–761. doi: 10.1097/00007890-198811000-00024. [DOI] [PubMed] [Google Scholar]
- 86.Woo J, Lu L, Rao AS, Li Y, Subbotin V, Starzl TE, Thomson AW. Isolation, phenotype, and allostimulatory activity of mouse liver dendritic cells. Transplantation. 1994;58:484–491. doi: 10.1097/00007890-199408270-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lian ZX, Okada T, He XS, Kita H, Liu YJ, Ansari AA, Kikuchi K, Ikehara S, Gershwin ME. Heterogeneity of dendritic cells in the mouse liver: identification and characterization of four distinct populations. J Immunol. 2003;170:2323–2330. doi: 10.4049/jimmunol.170.5.2323. [DOI] [PubMed] [Google Scholar]
- 88.Chen L, Calomeni E, Wen J, Ozato K, Shen R, Gao JX. Natural killer dendritic cells are an intermediate of developing dendritic cells. J Leukoc Biol. 2007;81:1422–1433. doi: 10.1189/jlb.1106674. [DOI] [PubMed] [Google Scholar]
- 89.Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004;172:1009–1017. doi: 10.4049/jimmunol.172.2.1009. [DOI] [PubMed] [Google Scholar]
- 90.O'Connell PJ, Morelli AE, Logar AJ, Thomson AW. Phenotypic and functional characterization of mouse hepatic CD8 alpha+ lymphoid-related dendritic cells. J Immunol. 2000;165:795–803. doi: 10.4049/jimmunol.165.2.795. [DOI] [PubMed] [Google Scholar]
- 91.Jomantaite I, Dikopoulos N, Kroger A, Leithauser F, Hauser H, Schirmbeck R, Reimann J. Hepatic dendritic cell subsets in the mouse. Eur J Immunol. 2004;34:355–365. doi: 10.1002/eji.200324336. [DOI] [PubMed] [Google Scholar]
- 92.Hsu W, Shu SA, Gershwin E, Lian ZX. The current immune function of hepatic dendritic cells. Cell Mol Immunol. 2007;4:321–328. [PubMed] [Google Scholar]
- 93.Steptoe RJ, Patel RK, Subbotin VM, Thomson AW. Comparative analysis of dendritic cell density and total number in commonly transplanted organs: morphometric estimation in normal mice. Transpl Immunol. 2000;8:49–56. doi: 10.1016/s0966-3274(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 94.Inatsu A, Kinoshita M, Nakashima H, Shimizu J, Saitoh D, Tamai S, Seki S. Novel mechanism of C-reactive protein for enhancing mouse liver innate immunity. Hepatology. 2009;49:2044–2054. doi: 10.1002/hep.22888. [DOI] [PubMed] [Google Scholar]
- 95.Khanna A, Morelli AE, Zhong C, Takayama T, Lu L, Thomson AW. Effects of liver-derived dendritic cell progenitors on Th1- and Th2-like cytokine responses in vitro and in vivo. J Immunol. 2000;164:1346–1354. doi: 10.4049/jimmunol.164.3.1346. [DOI] [PubMed] [Google Scholar]
- 96.Buelens C, Verhasselt V, De Groote D, Thielemans K, Goldman M, Willems F. Human dendritic cell responses to lipopolysaccharide and CD40 ligation are differentially regulated by interleukin-10. Eur J Immunol. 1997;27:1848–1852. doi: 10.1002/eji.1830270805. [DOI] [PubMed] [Google Scholar]
- 97.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol. 2005;6:280–286. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 98.Probst HC, Lagnel J, Kollias G, van den Broek M. Inducible transgenic mice reveal resting dendritic cells as potent inducers of CD8+ T cell tolerance. Immunity. 2003;18:713–720. doi: 10.1016/s1074-7613(03)00120-1. [DOI] [PubMed] [Google Scholar]
- 99.Matsuno K, Ezaki T, Kudo S, Uehara Y. A life stage of particle-laden rat dendritic cells in vivo: their terminal division, active phagocytosis, and translocation from the liver to the draining lymph. J Exp Med. 1996;183:1865–1878. doi: 10.1084/jem.183.4.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kudo S, Matsuno K, Ezaki T, Ogawa M. A novel migration pathway for rat dendritic cells from the blood: hepatic sinusoids-lymph translocation. J Exp Med. 1997;185:777–784. doi: 10.1084/jem.185.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vinas O, Bataller R, Sancho-Bru P, Gines P, Berenguer C, Enrich C, Nicolas JM, Ercilla G, Gallart T, Vives J, Arroyo V, Rodes J. Human hepatic stellate cells show features of antigen-presenting cells and stimulate lymphocyte proliferation. Hepatology. 2003;38:919–929. doi: 10.1053/jhep.2003.50392. [DOI] [PubMed] [Google Scholar]
- 102.Schwabe RF, Schnabl B, Kweon YO, Brenner DA. CD40 activates NF-kappa B and c-Jun N-terminal kinase and enhances chemokine secretion on activated human hepatic stellate cells. J Immunol. 2001;166:6812–6819. doi: 10.4049/jimmunol.166.11.6812. [DOI] [PubMed] [Google Scholar]
- 103.Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, Modlin RL, Liblau RS, Gressner AM, Kaufmann SH. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–129. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 104.Chen CH, Kuo LM, Chang Y, Wu W, Goldbach C, Ross MA, Stolz DB, Chen L, Fung JJ, Lu L, Qian S. In vivo immune modulatory activity of hepatic stellate cells in mice. Hepatology. 2006;44:1171–1181. doi: 10.1002/hep.21379. [DOI] [PubMed] [Google Scholar]
- 105.Benten D, Kumaran V, Joseph B, Schattenberg J, Popov Y, Schuppan D, Gupta S. Hepatocyte transplantation activates hepatic stellate cells with beneficial modulation of cell engraftment in the rat. Hepatology. 2005;42:1072–1081. doi: 10.1002/hep.20889. [DOI] [PubMed] [Google Scholar]
- 106.Yu MC, Chen CH, Liang X, Wang L, Gandhi CR, Fung JJ, Lu L, Qian S. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology. 2004;40:1312–1321. doi: 10.1002/hep.20488. [DOI] [PubMed] [Google Scholar]
- 107.Warren A, Le Couteur DG, Fraser R, Bowen DG, McCaughan GW, Bertolino P. T lymphocytes interact with hepatocytes through fenestrations in murine liver sinusoidal endothelial cells. Hepatology. 2006;44:1182–1190. doi: 10.1002/hep.21378. [DOI] [PubMed] [Google Scholar]
- 108.Schroder AJ, Blaheta RA, Scholz M, Kronenberger B, Encke A, Markus BH. Effects of proinflammatory cytokines on cultivated primary human hepatocytes. Fluorometric measurement of intercellular adhesion molecule-1 and human leukocyte antigen-A, -B, -C, and -DR expression. Transplantation. 1995;59:1023–1028. doi: 10.1097/00007890-199504150-00018. [DOI] [PubMed] [Google Scholar]
- 109.Bertolino P, Trescol-Biemont MC, Rabourdin-Combe C. Hepatocytes induce functional activation of naive CD8+ T lymphocytes but fail to promote survival. Eur J Immunol. 1998;28:221–236. doi: 10.1002/(SICI)1521-4141(199801)28:01<221::AID-IMMU221>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 110.Holz LE, Benseler V, Bowen DG, Bouillet P, Strasser A, O'Reilly L, d'Avigdor WM, Bishop AG, McCaughan GW, Bertolino P. Intrahepatic murine CD8 T-cell activation associates with a distinct phenotype leading to Bim-dependent death. Gastroenterology. 2008;135:989–997. doi: 10.1053/j.gastro.2008.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bowen DG, Zen M, Holz L, Davis T, McCaughan GW, Bertolino P. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J Clin Invest. 2004;114:701–712. doi: 10.1172/JCI21593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qian S, Wang Z, Lee Y, Chiang Y, Bonham C, Fung J, Lu L. Hepatocyte-induced apoptosis of activated T cells, a mechanism of liver transplant tolerance, is related to the expression of ICAM-1 and hepatic lectin. Transplant Proc. 2001;33:226. doi: 10.1016/s0041-1345(00)01985-0. [DOI] [PubMed] [Google Scholar]
- 113.Arnold B. Parenchymal cells in immune and tolerance induction. Immunol Lett. 2003;89:225–228. doi: 10.1016/s0165-2478(03)00150-0. [DOI] [PubMed] [Google Scholar]
- 114.Schonrich G, Momburg F, Malissen M, Schmitt-Verhulst AM, Malissen B, Hammerling GJ, Arnold B. Distinct mechanisms of extrathymic T cell tolerance due to differential expression of self antigen. Int Immunol. 1992;4:581–590. doi: 10.1093/intimm/4.5.581. [DOI] [PubMed] [Google Scholar]
- 115.Scholz M, Cinatl J, Blaheta RA, Kornhuber B, Markus BH, Doerr HW. Expression of human leukocyte antigens class I and class II on cultured biliary epithelial cells after cytomegalovirus infection. Tissue Antigens. 1997;49:640–643. doi: 10.1111/j.1399-0039.1997.tb02813.x. [DOI] [PubMed] [Google Scholar]
- 116.Tsuneyama K, Harada K, Yasoshima M, Kaji K, Gershwin ME, Nakanuma Y. Expression of co-stimulatory factor B7-2 on the intrahepatic bile ducts in primary biliary cirrhosis and primary sclerosing cholangitis: an immunohistochemical study. J Pathol. 1998;186:126–130. doi: 10.1002/(SICI)1096-9896(1998100)186:2<126::AID-PATH167>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 117.Leon MP, Bassendine MF, Gibbs P, Burt AD, Thick M, Kirby JA. Hepatic allograft rejection: regulation of the immunogenicity of human intrahepatic biliary epithelial cells. Liver Transpl Surg. 1996;2:37–45. doi: 10.1002/lt.500020107. [DOI] [PubMed] [Google Scholar]
- 118.Leon MP, Bassendine MF, Gibbs P, Thick M, Kirby JA. Immunogenicity of biliary epithelium: study of the adhesive interaction with lymphocytes. Gastroenterology. 1997;112:968–977. doi: 10.1053/gast.1997.v112.pm9041260. [DOI] [PubMed] [Google Scholar]
- 119.Leon MP, Bassendine MF, Wilson JL, Ali S, Thick M, Kirby JA. Immunogenicity of biliary epithelium: investigation of antigen presentation to CD4+ T cells. Hepatology. 1996;24:561–567. doi: 10.1002/hep.510240317. [DOI] [PubMed] [Google Scholar]
- 120.Leon MP, Kirby JA, Gibbs P, Burt AD, Bassendine MF. Immunogenicity of biliary epithelial cells: study of the expression of B7 molecules. J Hepatol. 1995;22:591–595. doi: 10.1016/0168-8278(95)80456-0. [DOI] [PubMed] [Google Scholar]
- 121.Dillon PW, Belchis D, Minnick K, Tracy T. Differential expression of the major histocompatibility antigens and ICAM-1 on bile duct epithelial cells in biliary atresia. Tohoku J Exp Med. 1997;181:33–40. doi: 10.1620/tjem.181.33. [DOI] [PubMed] [Google Scholar]
- 122.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. The New England journal of medicine. 2005;353:1261–1273. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 123.Gershwin ME, Mackay IR. The causes of primary biliary cirrhosis: Convenient and inconvenient truths. Hepatology. 2008;47:737–745. doi: 10.1002/hep.22042. [DOI] [PubMed] [Google Scholar]
- 124.Hirschfield GM, Liu X, Han Y, Gorlov IP, Lu Y, Xu C, Chen W, Juran BD, Coltescu C, Mason AL, Milkiewicz P, Myers RP, Odin JA, Luketic VA, Speiciene D, Vincent C, Levy C, Gregersen PK, Zhang J, Heathcote EJ, Lazaridis KN, Amos CI, Siminovitch KA. Variants at IRF5-TNPO3, 17q12-21 and MMEL1 are associated with primary biliary cirrhosis. Nat Genet. 2010;42:655–657. doi: 10.1038/ng.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hirschfield GM, Liu X, Xu C, Lu Y, Xie G, Gu X, Walker EJ, Jing K, Juran BD, Mason AL, Myers RP, Peltekian KM, Ghent CN, Coltescu C, Atkinson EJ, Heathcote EJ, Lazaridis KN, Amos CI, Siminovitch KA. Primary biliary cirrhosis associated with HLA, IL12A, and IL12RB2 variants. The New England journal of medicine. 2009;360:2544–2555. doi: 10.1056/NEJMoa0810440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Invernizzi P. Human leukocyte antigen in primary biliary cirrhosis: an old story now reviving. Hepatology. 2011;54:714–723. doi: 10.1002/hep.24414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu X, Invernizzi P, Lu Y, Kosoy R, Bianchi I, Podda M, Xu C, Xie G, Macciardi F, Selmi C, Lupoli S, Shigeta R, Ransom M, Lleo A, Lee AT, Mason AL, Myers RP, Peltekian KM, Ghent CN, Bernuzzi F, Zuin M, Rosina F, Borghesio E, Floreani A, Lazzari R, Niro G, Andriulli A, Muratori L, Muratori P, Almasio PL, Andreone P, Margotti M, Brunetto M, Coco B, Alvaro D, Bragazzi MC, Marra F, Pisano A, Rigamonti C, Colombo M, Marzioni M, Benedetti A, Fabris L, Strazzabosco M, Portincasa P, Palmieri VO, Tiribelli C, Croce L, Bruno S, Rossi S, Vinci M, Prisco C, Mattalia A, Toniutto P, Picciotto A, Galli A, Ferrari C, Colombo S, Casella G, Morini L, Caporaso N, Colli A, Spinzi G, Montanari R, Gregersen PK, Heathcote EJ, Hirschfield GM, Siminovitch KA, Amos CI, Gershwin ME, Seldin MF. Genome-wide meta-analyses identify three loci associated with primary biliary cirrhosis. Nat Genet. 2010;42:658–660. doi: 10.1038/ng.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mells GF, Floyd JA, Morley KI, Cordell HJ, Franklin CS, Shin SY, Heneghan MA, Neuberger JM, Donaldson PT, Day DB, Ducker SJ, Muriithi AW, Wheater EF, Hammond CJ, Dawwas MF, Jones DE, Peltonen L, Alexander GJ, Sandford RN, Anderson CA. Genome-wide association study identifies 12 new susceptibility loci for primary biliary cirrhosis. Nat Genet. 2011;43:329–332. doi: 10.1038/ng.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liberal R, Longhi MS, Mieli-Vergani G, Vergani D. Pathogenesis of autoimmune hepatitis. Best Pract Res Clin Gastroenterol. 2011;25:653–664. doi: 10.1016/j.bpg.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 130.Vergani D, Longhi MS, Bogdanos DP, Ma Y, Mieli-Vergani G. Autoimmune hepatitis. Semin Immunopathol. 2009;31:421–435. doi: 10.1007/s00281-009-0170-7. [DOI] [PubMed] [Google Scholar]
- 131.Vergani D, Choudhuri K, Bogdanos DP, Mieli-Vergani G. Pathogenesis of autoimmune hepatitis. Clin Liver Dis. 2002;6:727–737. doi: 10.1016/s1089-3261(02)00018-1. [DOI] [PubMed] [Google Scholar]
- 132.Wei HX, Chuang YH, Li B, Wei H, Sun R, Moritoki Y, Gershwin ME, Lian ZX, Tian Z. CD4+ CD25+ Foxp3+ regulatory T cells protect against T cell-mediated fulminant hepatitis in a TGF-beta-dependent manner in mice. J Immunol. 2008;181:7221–7229. doi: 10.4049/jimmunol.181.10.7221. [DOI] [PubMed] [Google Scholar]
- 133.Oertelt S, Lian ZX, Cheng CM, Chuang YH, Padgett KA, He XS, Ridgway WM, Ansari AA, Coppel RL, Li MO, Flavell RA, Kronenberg M, Mackay IR, Gershwin ME. Anti-mitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. J Immunol. 2006;177:1655–1660. doi: 10.4049/jimmunol.177.3.1655. [DOI] [PubMed] [Google Scholar]
- 134.Aoki CA, Borchers AT, Li M, Flavell RA, Bowlus CL, Ansari AA, Gershwin ME. Transforming growth factor beta (TGF-beta) and autoimmunity. Autoimmun Rev. 2005;4:450–459. doi: 10.1016/j.autrev.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 135.Wakabayashi K, Yoshida K, Leung PS, Moritoki Y, Yang GX, Tsuneyama K, Lian ZX, Hibi T, Ansari AA, Wicker LS, Ridgway WM, Coppel RL, Mackay IR, Gershwin ME. Induction of autoimmune cholangitis in non-obese diabetic (NOD).1101 mice following a chemical xenobiotic immunization. Clin Exp Immunol. 2009;155:577–586. doi: 10.1111/j.1365-2249.2008.03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wakabayashi K, Lian ZX, Leung PS, Moritoki Y, Tsuneyama K, Kurth MJ, Lam KS, Yoshida K, Yang GX, Hibi T, Ansari AA, Ridgway WM, Coppel RL, Mackay IR, Gershwin ME. Loss of tolerance in C57BL/6 mice to the autoantigen E2 subunit of pyruvate dehydrogenase by a xenobiotic with ensuing biliary ductular disease. Hepatology. 2008;48:531–540. doi: 10.1002/hep.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Leung PS, Park O, Tsuneyama K, Kurth MJ, Lam KS, Ansari AA, Coppel RL, Gershwin ME. Induction of primary biliary cirrhosis in guinea pigs following chemical xenobiotic immunization. J Immunol. 2007;179:2651–2657. doi: 10.4049/jimmunol.179.4.2651. [DOI] [PubMed] [Google Scholar]
- 138.Kimura Y, Selmi C, Leung PS, Mao TK, Schauer J, Watnik M, Kuriyama S, Nishioka M, Ansari AA, Coppel RL, Invernizzi P, Podda M, Gershwin ME. Genetic polymorphisms influencing xenobiotic metabolism and transport in patients with primary biliary cirrhosis. Hepatology. 2005;41:55–63. doi: 10.1002/hep.20516. [DOI] [PubMed] [Google Scholar]
- 139.Amano K, Leung PS, Xu Q, Marik J, Quan C, Kurth MJ, Nantz MH, Ansari AA, Lam KS, Zeniya M, Coppel RL, Gershwin ME. Xenobiotic-induced loss of tolerance in rabbits to the mitochondrial autoantigen of primary biliary cirrhosis is reversible. J Immunol. 2004;172:6444–6452. doi: 10.4049/jimmunol.172.10.6444. [DOI] [PubMed] [Google Scholar]
- 140.Selmi C, Balkwill DL, Invernizzi P, Ansari AA, Coppel RL, Podda M, Leung PS, Kenny TP, Van De Water J, Nantz MH, Kurth MJ, Gershwin ME. Patients with primary biliary cirrhosis react against a ubiquitous xenobiotic-metabolizing bacterium. Hepatology. 2003;38:1250–1257. doi: 10.1053/jhep.2003.50446. [DOI] [PubMed] [Google Scholar]
- 141.Leung PS, Quan C, Park O, Van de Water J, Kurth MJ, Nantz MH, Ansari AA, Coppel RL, Lam KS, Gershwin ME. Immunization with a xenobiotic 6-bromohexanoate bovine serum albumin conjugate induces antimitochondrial antibodies. J Immunol. 2003;170:5326–5332. doi: 10.4049/jimmunol.170.10.5326. [DOI] [PubMed] [Google Scholar]
- 142.He XS, Ansari AA, Gershwin ME. Xenobiotic considerations for the development of autoimmune liver diseases: bad genes and bad luck. Rev Environ Health. 2001;16:191–202. doi: 10.1515/reveh.2001.16.3.191. [DOI] [PubMed] [Google Scholar]
- 143.Amano K, Leung PS, Rieger R, Quan C, Wang X, Marik J, Suen YF, Kurth MJ, Nantz MH, Ansari AA, Lam KS, Zeniya M, Matsuura E, Coppel RL, Gershwin ME. Chemical xenobiotics and mitochondrial autoantigens in primary biliary cirrhosis: identification of antibodies against a common environmental, cosmetic, and food additive, 2-octynoic acid. J Immunol. 2005;174:5874–5883. doi: 10.4049/jimmunol.174.9.5874. [DOI] [PubMed] [Google Scholar]
- 144.Park O, Grishina I, Leung PS, Gershwin ME, Prindiville T. Analysis of the Foxp3/scurfin gene in Crohn's disease. Ann N Y Acad Sci. 2005;1051:218–228. doi: 10.1196/annals.1361.125. [DOI] [PubMed] [Google Scholar]
- 145.Mackay I. Primary biliary cirrhosis showing a high titre of autoantibody: report of a case of a study. N Engl J Med. 1958:185–188. doi: 10.1056/NEJM195801232580407. [DOI] [PubMed] [Google Scholar]
- 146.Asherson GR. Antibodies against nuclear and cytoplasmic cell constituents in systemic lupus erythematosus and other liver diseases. Br J Exp Pathol. 1959;40:209–213. [PMC free article] [PubMed] [Google Scholar]
- 147.Doniach D, Roitt I, Walker J, Sherlock S. Tissue antibodies in primary biliary cirrhosis, active chronic (lupoid) hepatitis, cryptogenic cirrhosis and other diseases and their clinical implications. Clin Exp Immunol. 1966;1:237–262. [PMC free article] [PubMed] [Google Scholar]
- 148.Berg PA, Doniach D, Roitt IM. Mitochondrial antibodies in primary biliary cirrhosis. I. Localization of the antigen to mitochondrial membranes. J Exp Med. 1967;126:277–290. doi: 10.1084/jem.126.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gershwin ME, Mackay IR, Sturgess A, Coppel RL. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol. 1987;138:3525–3531. [PubMed] [Google Scholar]
- 150.Van de Water J, Gershwin ME, Leung P, Ansari A, Coppel RL. The autoepitope of the 74-kD mitochondrial autoantigen of primary biliary cirrhosis corresponds to the functional site of dihydrolipoamide acetyltransferase. J Exp Med. 1988;167:1791–1799. doi: 10.1084/jem.167.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yeaman SJ, Fussey SP, Danner DJ, James OF, Mutimer DJ, Bassendine MF. Primary biliary cirrhosis: identification of two major M2 mitochondrial autoantigens. Lancet. 1988;1:1067–1070. doi: 10.1016/s0140-6736(88)91894-6. [DOI] [PubMed] [Google Scholar]
- 152.Fussey SP, Guest JR, James OF, Bassendine MF, Yeaman SJ. Identification and analysis of the major M2 autoantigens in primary biliary cirrhosis. Proc Natl Acad Sci U S A. 1988;85:8654–8658. doi: 10.1073/pnas.85.22.8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fussey SP, Bassendine MF, James OF, Yeaman SJ. Characterisation of the reactivity of autoantibodies in primary biliary cirrhosis. FEBS Lett. 1989;246:49–53. doi: 10.1016/0014-5793(89)80251-0. [DOI] [PubMed] [Google Scholar]
- 154.Fussey SP, Bassendine MF, Fittes D, Turner IB, James OF, Yeaman SJ. The E1 alpha and beta subunits of the pyruvate dehydrogenase complex are M2'd' and M2'e' autoantigens in primary biliary cirrhosis. Clin Sci (Lond) 1989;77:365–368. doi: 10.1042/cs0770365. [DOI] [PubMed] [Google Scholar]
- 155.De Marcucci O, Lindsay JG. Component X. An immunologically distinct polypeptide associated with mammalian pyruvate dehydrogenase multi-enzyme complex. Eur J Biochem. 1985;149:641–648. doi: 10.1111/j.1432-1033.1985.tb08972.x. [DOI] [PubMed] [Google Scholar]
- 156.Surh CD, Danner DJ, Ahmed A, Coppel RL, Mackay IR, Dickson ER, Gershwin ME. Reactivity of primary biliary cirrhosis sera with a human fetal liver cDNA clone of branched-chain alpha-keto acid dehydrogenase dihydrolipoamide acyltransferase, the 52 kD mitochondrial autoantigen. Hepatology. 1989;9:63–68. doi: 10.1002/hep.1840090110. [DOI] [PubMed] [Google Scholar]
- 157.Baum H. Nature of the mitochondrial antigens of primary biliary cirrhosis and their possible relationships to the etiology of the disease. Semin Liver Dis. 1989;9:117–123. doi: 10.1055/s-2008-1040502. [DOI] [PubMed] [Google Scholar]
- 158.Baum H, Berg PA. The complex nature of mitochondrial antibodies and their relation to primary biliary cirrhosis. Semin Liver Dis. 1981;1:309–321. doi: 10.1055/s-2008-1040734. [DOI] [PubMed] [Google Scholar]
- 159.Nishio A, Van de Water J, Leung PS, Joplin R, Neuberger JM, Lake J, Bjorkland A, Totterman TH, Peters M, Worman HJ, Ansari AA, Coppel RL, Gershwin ME. Comparative studies of antimitochondrial autoantibodies in sera and bile in primary biliary cirrhosis. Hepatology. 1997;25:1085–1089. doi: 10.1002/hep.510250506. [DOI] [PubMed] [Google Scholar]
- 160.Tanaka A, Nalbandian G, Leung PS, Benson GD, Munoz S, Findor JA, Branch AD, Coppel RL, Ansari AA, Gershwin ME. Mucosal immunity and primary biliary cirrhosis: presence of antimitochondrial antibodies in urine. Hepatology. 2000;32:910–915. doi: 10.1053/jhep.2000.19254. [DOI] [PubMed] [Google Scholar]
- 161.Reynoso-Paz S, Leung PS, Van De Water J, Tanaka A, Munoz S, Bass N, Lindor K, Donald PJ, Coppel RL, Ansari AA, Gershwin ME. Evidence for a locally driven mucosal response and the presence of mitochondrial antigens in saliva in primary biliary cirrhosis. Hepatology. 2000;31:24–29. doi: 10.1002/hep.510310106. [DOI] [PubMed] [Google Scholar]
- 162.Van de Water J, Fregeau D, Davis P, Ansari A, Danner D, Leung P, Coppel R, Gershwin ME. Autoantibodies of primary biliary cirrhosis recognize dihydrolipoamide acetyltransferase and inhibit enzyme function. J Immunol. 1988;141:2321–2324. [PubMed] [Google Scholar]
- 163.Leung PS, Chuang DT, Wynn RM, Cha S, Danner DJ, Ansari A, Coppel RL, Gershwin ME. Autoantibodies to BCOADC-E2 in patients with primary biliary cirrhosis recognize a conformational epitope. Hepatology. 1995;22:505–513. [PubMed] [Google Scholar]
- 164.Dubel L, Tanaka A, Leung PS, Van de Water J, Coppel R, Roche T, Johanet C, Motokawa Y, Ansari A, Gershwin ME. Autoepitope mapping and reactivity of autoantibodies to the dihydrolipoamide dehydrogenase-binding protein (E3BP) and the glycine cleavage proteins in primary biliary cirrhosis. Hepatology. 1999;29:1013–1018. doi: 10.1002/hep.510290403. [DOI] [PubMed] [Google Scholar]
- 165.Fregeau DR, Davis PA, Danner DJ, Ansari A, Coppel RL, Dickson ER, Gershwin ME. Antimitochondrial antibodies of primary biliary cirrhosis recognize dihydrolipoamide acyltransferase and inhibit enzyme function of the branched chain alpha-ketoacid dehydrogenase complex. J Immunol. 1989;142:3815–3820. [PubMed] [Google Scholar]
- 166.Fregeau DR, Leung PS, Coppel RL, McNeilage LJ, Medsger TA, Jr, Gershwin ME. Autoantibodies to mitochondria in systemic sclerosis. Frequency and characterization using recombinant cloned autoantigen. Arthritis Rheum. 1988;31:386–392. doi: 10.1002/art.1780310310. [DOI] [PubMed] [Google Scholar]
- 167.Fregeau DR, Prindiville T, Coppel RL, Kaplan M, Dickson ER, Gershwin ME. Inhibition of alpha-ketoglutarate dehydrogenase activity by a distinct population of autoantibodies recognizing dihydrolipoamide succinyltransferase in primary biliary cirrhosis. Hepatology. 1990;11:975–981. doi: 10.1002/hep.1840110611. [DOI] [PubMed] [Google Scholar]
- 168.Fregeau DR, Roche TE, Davis PA, Coppel R, Gershwin ME. Primary biliary cirrhosis. Inhibition of pyruvate dehydrogenase complex activity by autoantibodies specific for E1 alpha, a non-lipoic acid containing mitochondrial enzyme. J Immunol. 1990;144:1671–1676. [PubMed] [Google Scholar]
- 169.Surh CD, Roche TE, Danner DJ, Ansari A, Coppel RL, Prindiville T, Dickson ER, Gershwin ME. Antimitochondrial autoantibodies in primary biliary cirrhosis recognize cross-reactive epitope(s) on protein X and dihydrolipoamide acetyltransferase of pyruvate dehydrogenase complex. Hepatology. 1989;10:127–133. doi: 10.1002/hep.1840100202. [DOI] [PubMed] [Google Scholar]
- 170.Surh CD, Coppel R, Gershwin ME. Structural requirement for autoreactivity on human pyruvate dehydrogenase-E2, the major autoantigen of primary biliary cirrhosis. Implication for a conformational autoepitope. J Immunol. 1990;144:3367–3374. [PubMed] [Google Scholar]
- 171.Mackay IR, Whittingham S, Fida S, Myers M, Ikuno N, Gershwin ME, Rowley MJ. The peculiar autoimmunity of primary biliary cirrhosis. Immunol Rev. 2000;174:226–237. doi: 10.1034/j.1600-0528.2002.017410.x. [DOI] [PubMed] [Google Scholar]
- 172.Van de Water J, Ansari A, Prindiville T, Coppel RL, Ricalton N, Kotzin BL, Liu S, Roche TE, Krams SM, Munoz S, et al. Heterogeneity of autoreactive T cell clones specific for the E2 component of the pyruvate dehydrogenase complex in primary biliary cirrhosis. J Exp Med. 1995;181:723–733. doi: 10.1084/jem.181.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Van de Water J, Ansari AA, Surh CD, Coppel R, Roche T, Bonkovsky H, Kaplan M, Gershwin ME. Evidence for the targeting by 2-oxo-dehydrogenase enzymes in the T cell response of primary biliary cirrhosis. J Immunol. 1991;146:89–94. [PubMed] [Google Scholar]
- 174.Shimoda S, Nakamura M, Ishibashi H, Hayashida K, Niho Y. HLA DRB4 0101-restricted immunodominant T cell autoepitope of pyruvate dehydrogenase complex in primary biliary cirrhosis: evidence of molecular mimicry in human autoimmune diseases. J Exp Med. 1995;181:1835–1845. doi: 10.1084/jem.181.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shimoda S, Nakamura M, Shigematsu H, Tanimoto H, Gushima T, Gershwin ME, Ishibashi H. Mimicry peptides of human PDC-E2 163–176 peptide, the immunodominant T-cell epitope of primary biliary cirrhosis. Hepatology. 2000;31:1212–1216. doi: 10.1053/jhep.2000.8090. [DOI] [PubMed] [Google Scholar]
- 176.Shimoda S, Van de Water J, Ansari A, Nakamura M, Ishibashi H, Coppel RL, Lake J, Keeffe EB, Roche TE, Gershwin ME. Identification and precursor frequency analysis of a common T cell epitope motif in mitochondrial autoantigens in primary biliary cirrhosis. J Clin Invest. 1998;102:1831–1840. doi: 10.1172/JCI4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kita H, Lian ZX, Van de Water J, He XS, Matsumura S, Kaplan M, Luketic V, Coppel RL, Ansari AA, Gershwin ME. Identification of HLA-A2-restricted CD8(+) cytotoxic T cell responses in primary biliary cirrhosis: T cell activation is augmented by immune complexes cross-presented by dendritic cells. J Exp Med. 2002;195:113–123. doi: 10.1084/jem.20010956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kita H, Matsumura S, He XS, Ansari AA, Lian ZX, Van de Water J, Coppel RL, Kaplan MM, Gershwin ME. Quantitative and functional analysis of PDC-E2-specific autoreactive cytotoxic T lymphocytes in primary biliary cirrhosis. J Clin Invest. 2002;109:1231–1240. doi: 10.1172/JCI14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bogdanos DP, Baum H, Grasso A, Okamoto M, Butler P, Ma Y, Rigopoulou E, Montalto P, Davies ET, Burroughs AK, Vergani D. Microbial mimics are major targets of crossreactivity with human pyruvate dehydrogenase in primary biliary cirrhosis. J Hepatol. 2004;40:31–39. doi: 10.1016/s0168-8278(03)00501-4. [DOI] [PubMed] [Google Scholar]
- 180.Bogdanos DP, Baum H, Okamoto M, Montalto P, Sharma UC, Rigopoulou EI, Vlachogiannakos J, Ma Y, Burroughs AK, Vergani D. Primary biliary cirrhosis is characterized by IgG3 antibodies cross-reactive with the major mitochondrial autoepitope and its Lactobacillus mimic. Hepatology. 2005;42:458–465. doi: 10.1002/hep.20788. [DOI] [PubMed] [Google Scholar]
- 181.Lleo A, Bowlus CL, Yang GX, Invernizzi P, Podda M, Van de Water J, Ansari AA, Coppel RL, Worman HJ, Gores GJ, Gershwin ME. Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis. Hepatology. 2010;52:987–998. doi: 10.1002/hep.23783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lleo A, Selmi C, Invernizzi P, Podda M, Coppel RL, Mackay IR, Gores GJ, Ansari AA, Van de Water J, Gershwin ME. Apotopes and the biliary specificity of primary biliary cirrhosis. Hepatology. 2009;49:871–879. doi: 10.1002/hep.22736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rong G, Zhong R, Lleo A, Leung PS, Bowlus CL, Yang GX, Yang CY, Coppel RL, Ansari AA, Cuebas DA, Worman HJ, Invernizzi P, Gores GJ, Norman G, He XS, Gershwin ME. Epithelial cell specificity and apotope recognition by serum autoantibodies in primary biliary cirrhosis. Hepatology. 2011;54:196–203. doi: 10.1002/hep.24355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Harada K, Shimoda S, Ikeda H, Chiba M, Hsu M, Sato Y, Kobayashi M, Ren XS, Ohta H, Kasashima S, Kawashima A, Nakanuma Y. Significance of periductal Langerhans cells and biliary epithelial cell-derived macrophage inflammatory protein-3alpha in the pathogenesis of primary biliary cirrhosis. Liver Int. 2011;31:245–253. doi: 10.1111/j.1478-3231.2010.02367.x. [DOI] [PubMed] [Google Scholar]
- 185.Selmi C, Lleo A, Pasini S, Zuin M, Gershwin ME. Innate immunity and primary biliary cirrhosis. Curr Mol Med. 2009;9:45–51. doi: 10.2174/156652409787314525. [DOI] [PubMed] [Google Scholar]
- 186.Shimoda S, Harada K, Niiro H, Shirabe K, Taketomi A, Maehara Y, Tsuneyama K, Nakanuma Y, Leung P, Ansari AA, Gershwin ME, Akashi K. Interaction between Toll-like receptors and natural killer cells in the destruction of bile ducts in primary biliary cirrhosis. Hepatology. 2011;53:1270–1281. doi: 10.1002/hep.24194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ikeda H, Sasaki M, Ishikawa A, Sato Y, Harada K, Zen Y, Kazumori H, Nakanuma Y. Interaction of Toll-like receptors with bacterial components induces expression of CDX2 and MUC2 in rat biliary epithelium in vivo and in culture. Lab Invest. 2007;87:559–571. doi: 10.1038/labinvest.3700556. [DOI] [PubMed] [Google Scholar]
- 188.Harada K, Ohira S, Isse K, Ozaki S, Zen Y, Sato Y, Nakanuma Y. Lipopolysaccharide activates nuclear factor-kappaB through toll-like receptors and related molecules in cultured biliary epithelial cells. Lab Invest. 2003;83:1657–1667. doi: 10.1097/01.lab.0000097190.56734.fe. [DOI] [PubMed] [Google Scholar]
- 189.Harada K, Shimoda S, Sato Y, Isse K, Ikeda H, Nakanuma Y. Periductal interleukin-17 production in association with biliary innate immunity contributes to the pathogenesis of cholangiopathy in primary biliary cirrhosis. Clin Exp Immunol. 2009;157:261–270. doi: 10.1111/j.1365-2249.2009.03947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wakabayashi K, Lian ZX, Moritoki Y, Lan RY, Tsuneyama K, Chuang YH, Yang GX, Ridgway W, Ueno Y, Ansari AA, Coppel RL, Mackay IR, Gershwin ME. IL-2 receptor alpha(−/−) mice and the development of primary biliary cirrhosis. Hepatology. 2006;44:1240–1249. doi: 10.1002/hep.21385. [DOI] [PubMed] [Google Scholar]
- 191.Yang GX, Lian ZX, Chuang YH, Moritoki Y, Lan RY, Wakabayashi K, Ansari AA, Flavell RA, Ridgway WM, Coppel RL, Tsuneyama K, Mackay IR, Gershwin ME. Adoptive transfer of CD8(+) T cells from transforming growth factor beta receptor type II (dominant negative form) induces autoimmune cholangitis in mice. Hepatology. 2008;47:1974–1982. doi: 10.1002/hep.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mackay IR, Taft LI, Cowling DC. Lupoid hepatitis. Lancet. 1956;271:1323–1326. doi: 10.1016/s0140-6736(56)91483-0. [DOI] [PubMed] [Google Scholar]
- 193.Johnson PJ, McFarlane IG. Meeting report: International Autoimmune Hepatitis Group. Hepatology. 1993;18:998–1005. doi: 10.1002/hep.1840180435. [DOI] [PubMed] [Google Scholar]
- 194.Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ, Donaldson PT, Eddleston AL, Fainboim L, Heathcote J, Homberg JC, Hoofnagle JH, Kakumu S, Krawitt EL, Mackay IR, MacSween RN, Maddrey WC, Manns MP, McFarlane IG, Meyer zum Buschenfelde KH, Zeniya M, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 195.Hennes EM, Zeniya M, Czaja AJ, Pares A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H, Bianchi FB, Shibata M, Schramm C, Eisenmann de Torres B, Galle PR, McFarlane I, Dienes HP, Lohse AW. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 196.Vergani D, Mieli-Vergani G, Mondelli M, Portmann B, Eddleston AL. Immunoglobulin on the surface of isolated hepatocytes is associated with antibody-dependent cell-mediated cytotoxicity and liver damage. Liver. 1987;7:307–315. doi: 10.1111/j.1600-0676.1987.tb00361.x. [DOI] [PubMed] [Google Scholar]
- 197.Wen L, Peakman M, Lobo-Yeo A, McFarlane BM, Mowat AP, Mieli-Vergani G, Vergani D. T-cell-directed hepatocyte damage in autoimmune chronic active hepatitis. Lancet. 1990;336:1527–1530. doi: 10.1016/0140-6736(90)93306-a. [DOI] [PubMed] [Google Scholar]
- 198.Gabbiani G, Ryan GB, Lamelin JP, Vassalli P, Majno G, Bouvier CA, Cruchaud A, Luscher EF. Human smooth muscle autoantibody. Its identification as antiactin antibody and a study of its binding to "nonmuscular" cells. Am J Pathol. 1973;72:473–488. [PMC free article] [PubMed] [Google Scholar]
- 199.Bottazzo GF, Florin-Christensen A, Fairfax A, Swana G, Doniach D, Groeschel-Stewart U. Classification of smooth muscle autoantibodies detected by immunofluorescence. J Clin Pathol. 1976;29:403–410. doi: 10.1136/jcp.29.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Czaja AJ, Morshed SA, Parveen S, Nishioka M. Antibodies to single-stranded and double-stranded DNA in antinuclear antibody-positive type 1-autoimmune hepatitis. Hepatology. 1997;26:567–572. doi: 10.1002/hep.510260306. [DOI] [PubMed] [Google Scholar]
- 201.Bogdanos DP, Invernizzi P, Mackay IR, Vergani D. Autoimmune liver serology: current diagnostic and clinical challenges. World J Gastroenterol. 2008;14:3374–3387. doi: 10.3748/wjg.14.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bogdanos DP, Mieli-Vergani G, Vergani D. Autoantibodies and their antigens in autoimmune hepatitis. Semin Liver Dis. 2009;29:241–253. doi: 10.1055/s-0029-1233533. [DOI] [PubMed] [Google Scholar]
- 203.Berg PA, Stechemesser E, Strienz J. Hypergammaglobulinamische chronisch aktive Hepatitis mit Nachweis von leberpankreas-spezifischen komplementbindenden Autoantikorpern. Verh Dtsch Ges Inn Med. 1981;87:921–927. [Google Scholar]
- 204.Gelpi C, Sontheimer EJ, Rodriguez-Sanchez JL. Autoantibodies against a serine tRNA-protein complex implicated in cotranslational selenocysteine insertion. Proc Natl Acad Sci U S A. 1992;89:9739–9743. doi: 10.1073/pnas.89.20.9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lapierre P, Hajoui O, Homberg JC, Alvarez F. Formiminotransferase cyclodeaminase is an organ-specific autoantigen recognized by sera of patients with autoimmune hepatitis. Gastroenterology. 1999;116:643–649. doi: 10.1016/s0016-5085(99)70186-1. [DOI] [PubMed] [Google Scholar]
- 206.Wies I, Brunner S, Henninger J, Herkel J, Kanzler S, Meyer zum Buschenfelde KH, Lohse AW. Identification of target antigen for SLA/LP autoantibodies in autoimmune hepatitis. Lancet. 2000;355:1510–1515. doi: 10.1016/s0140-6736(00)02166-8. [DOI] [PubMed] [Google Scholar]
- 207.Gueguen M, Meunier-Rotival M, Bernard O, Alvarez F. Anti-liver kidney microsome antibody recognizes a cytochrome P450 from the IID subfamily. J Exp Med. 1988;168:801–806. doi: 10.1084/jem.168.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bogdanos DP, Dalekos GN. Enzymes as target antigens of liver-specific autoimmunity: the case of cytochromes P450s. Curr Med Chem. 2008;15:2285–2292. doi: 10.2174/092986708785747508. [DOI] [PubMed] [Google Scholar]
- 209.Clemente MG, Meloni A, Obermayer-Straub P, Frau F, Manns MP, De Virgiliis S. Two cytochromes P450 are major hepatocellular autoantigens in autoimmune polyglandular syndrome type 1. Gastroenterology. 1998;114:324–328. doi: 10.1016/s0016-5085(98)70484-6. [DOI] [PubMed] [Google Scholar]
- 210.Bogdanos DP, Muratori L, Bianchi FB, Vergani D. Hepatitis C virus and autoimmunity. Hepatology. 2000;31:1380. doi: 10.1053/jhep.2000.8347. [DOI] [PubMed] [Google Scholar]
- 211.Ma Y, Bogdanos DP, Hussain MJ, Underhill J, Bansal S, Longhi MS, Cheeseman P, Mieli-Vergani G, Vergani D. Polyclonal T-cell responses to cytochrome P450IID6 are associated with disease activity in autoimmune hepatitis type 2. Gastroenterology. 2006;130:868–882. doi: 10.1053/j.gastro.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 212.Longhi MS, Hussain MJ, Bogdanos DP, Quaglia A, Mieli-Vergani G, Ma Y, Vergani D. Cytochrome P450IID6-specific CD8 T cell immune responses mirror disease activity in autoimmune hepatitis type 2. Hepatology. 2007;46:472–484. doi: 10.1002/hep.21658. [DOI] [PubMed] [Google Scholar]
- 213.Hodgson HJ, Wands JR, Isselbacher KJ. Alteration in suppressor cell activity in chronic active hepatitis. Proc Natl Acad Sci U S A. 1978;75:1549–1553. doi: 10.1073/pnas.75.3.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Vento S, Hegarty JE, Bottazzo G, Macchia E, Williams R, Eddleston AL. Antigen specific suppressor cell function in autoimmune chronic active hepatitis. Lancet. 1984;1:1200–1204. doi: 10.1016/s0140-6736(84)91691-x. [DOI] [PubMed] [Google Scholar]
- 215.Longhi MS, Ma Y, Bogdanos DP, Cheeseman P, Mieli-Vergani G, Vergani D. Impairment of CD4(+)CD25(+) regulatory T-cells in autoimmune liver disease. J Hepatol. 2004;41:31–37. doi: 10.1016/j.jhep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 216.Longhi MS, Ma Y, Mitry RR, Bogdanos DP, Heneghan M, Cheeseman P, Mieli-Vergani G, Vergani D. Effect of CD4+ CD25+ regulatory T-cells on CD8 T-cell function in patients with autoimmune hepatitis. J Autoimmun. 2005;25:63–71. doi: 10.1016/j.jaut.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 217.Longhi MS, Meda F, Wang P, Samyn M, Mieli-Vergani G, Vergani D, Ma Y. Expansion and de novo generation of potentially therapeutic regulatory T cells in patients with autoimmune hepatitis. Hepatology. 2008;47:581–591. doi: 10.1002/hep.22071. [DOI] [PubMed] [Google Scholar]
- 218.Lee WY, Moriarty TJ, Wong CH, Zhou H, Strieter RM, van Rooijen N, Chaconas G, Kubes P. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat Immunol. 2010;11:295–302. doi: 10.1038/ni.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Protzer U, Maini MK, Knolle PA. Living in the liver: hepatic infections. Nat Rev Immunol. 2012;12:201–213. doi: 10.1038/nri3169. [DOI] [PubMed] [Google Scholar]
- 220.Keating R, Yue W, Rutigliano JA, So J, Olivas E, Thomas PG, Doherty PC. Virus-specific CD8+ T cells in the liver: armed and ready to kill. J Immunol. 2007;178:2737–2745. doi: 10.4049/jimmunol.178.5.2737. [DOI] [PubMed] [Google Scholar]
- 221.Polakos NK, Klein I, Richter MV, Zaiss DM, Giannandrea M, Crispe IN, Topham DJ. Early intrahepatic accumulation of CD8+ T cells provides a source of effectors for nonhepatic immune responses. J Immunol. 2007;179:201–210. doi: 10.4049/jimmunol.179.1.201. [DOI] [PubMed] [Google Scholar]
- 222.Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, Szczepanik M, Telenti A, Askenase PW, Compans RW, von Andrian UH. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11:1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Baer K, Roosevelt M, Clarkson AB, Jr, van Rooijen N, Schnieder T, Frevert U. Kupffer cells are obligatory for Plasmodium yoelii sporozoite infection of the liver. Cell Microbiol. 2007;9:397–412. doi: 10.1111/j.1462-5822.2006.00798.x. [DOI] [PubMed] [Google Scholar]
- 224.Wainwright H. Hepatic granulomas. Eur J Gastroenterol Hepatol. 2007;19:93–95. doi: 10.1097/MEG.0b013e3280115523. [DOI] [PubMed] [Google Scholar]
- 225.Kleiner DE. Granulomas in the liver. Semin Diagn Pathol. 2006;23:161–169. doi: 10.1053/j.semdp.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 226.Zumla A, James DG. Granulomatous infections: etiology and classification. Clin Infect Dis. 1996;23:146–158. doi: 10.1093/clinids/23.1.146. [DOI] [PubMed] [Google Scholar]
- 227.Popov A, Abdullah Z, Wickenhauser C, Saric T, Driesen J, Hanisch FG, Domann E, Raven EL, Dehus O, Hermann C, Eggle D, Debey S, Chakraborty T, Kronke M, Utermohlen O, Schultze JL. Indoleamine 2,3-dioxygenase-expressing dendritic cells form suppurative granulomas following Listeria monocytogenes infection. J Clin Invest. 2006;116:3160–3170. doi: 10.1172/JCI28996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Egen JG, Rothfuchs AG, Feng CG, Winter N, Sher A, Germain RN. Macrophage and T cell dynamics during the development and disintegration of mycobacterial granulomas. Immunity. 2008;28:271–284. doi: 10.1016/j.immuni.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Taylor JL, Hattle JM, Dreitz SA, Troudt JM, Izzo LS, Basaraba RJ, Orme IM, Matrisian LM, Izzo AA. Role for matrix metalloproteinase 9 in granuloma formation during pulmonary Mycobacterium tuberculosis infection. Infect Immun. 2006;74:6135–6144. doi: 10.1128/IAI.02048-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sinnis P, Willnow TE, Briones MR, Herz J, Nussenzweig V. Remnant lipoproteins inhibit malaria sporozoite invasion of hepatocytes. J Exp Med. 1996;184:945–954. doi: 10.1084/jem.184.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ishino T, Chinzei Y, Yuda M. Two proteins with 6-cys motifs are required for malarial parasites to commit to infection of the hepatocyte. Mol Microbiol. 2005;58:1264–1275. doi: 10.1111/j.1365-2958.2005.04801.x. [DOI] [PubMed] [Google Scholar]
- 232.Menard R. The journey of the malaria sporozoite through its hosts: two parasite proteins lead the way. Microbes Infect. 2000;2:633–642. doi: 10.1016/s1286-4579(00)00362-2. [DOI] [PubMed] [Google Scholar]
- 233.Mota MM, Pradel G, Vanderberg JP, Hafalla JC, Frevert U, Nussenzweig RS, Nussenzweig V, Rodriguez A. Migration of Plasmodium sporozoites through cells before infection. Science. 2001;291:141–144. doi: 10.1126/science.291.5501.141. [DOI] [PubMed] [Google Scholar]
- 234.Ishino T, Yano K, Chinzei Y, Yuda M. Cell-passage activity is required for the malarial parasite to cross the liver sinusoidal cell layer. PLoS Biol. 2004;2:E4. doi: 10.1371/journal.pbio.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ishino T, Chinzei Y, Yuda M. A Plasmodium sporozoite protein with a membrane attack complex domain is required for breaching the liver sinusoidal cell layer prior to hepatocyte infection. Cell Microbiol. 2005;7:199–208. doi: 10.1111/j.1462-5822.2004.00447.x. [DOI] [PubMed] [Google Scholar]
- 236.Shin SC, Vanderberg JP, Terzakis JA. Direct infection of hepatocytes by sporozoites of Plasmodium berghei. J Protozool. 1982;29:448–454. doi: 10.1111/j.1550-7408.1982.tb05431.x. [DOI] [PubMed] [Google Scholar]
- 237.Mota MM, Hafalla JC, Rodriguez A. Migration through host cells activates Plasmodium sporozoites for infection. Nat Med. 2002;8:1318–1322. doi: 10.1038/nm785. [DOI] [PubMed] [Google Scholar]
- 238.Cerami C, Frevert U, Sinnis P, Takacs B, Clavijo P, Santos MJ, Nussenzweig V. The basolateral domain of the hepatocyte plasma membrane bears receptors for the circumsporozoite protein of Plasmodium falciparum sporozoites. Cell. 1992;70:1021–1033. doi: 10.1016/0092-8674(92)90251-7. [DOI] [PubMed] [Google Scholar]
- 239.Pradel G, Frevert U. Malaria sporozoites actively enter and pass through rat Kupffer cells prior to hepatocyte invasion. Hepatology. 2001;33:1154–1165. doi: 10.1053/jhep.2001.24237. [DOI] [PubMed] [Google Scholar]
- 240.Coppi A, Natarajan R, Pradel G, Bennett BL, James ER, Roggero MA, Corradin G, Persson C, Tewari R, Sinnis P. The malaria circumsporozoite protein has two functional domains, each with distinct roles as sporozoites journey from mosquito to mammalian host. J Exp Med. 2011;208:341–356. doi: 10.1084/jem.20101488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Yalaoui S, Zougbede S, Charrin S, Silvie O, Arduise C, Farhati K, Boucheix C, Mazier D, Rubinstein E, Froissard P. Hepatocyte permissiveness to Plasmodium infection is conveyed by a short and structurally conserved region of the CD81 large extracellular domain. PLoS Pathog. 2008;4:e1000010. doi: 10.1371/journal.ppat.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Silvie O, Rubinstein E, Franetich JF, Prenant M, Belnoue E, Renia L, Hannoun L, Eling W, Levy S, Boucheix C, Mazier D. Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nat Med. 2003;9:93–96. doi: 10.1038/nm808. [DOI] [PubMed] [Google Scholar]
- 243.Li S, Rodrigues M, Rodriguez D, Rodriguez JR, Esteban M, Palese P, Nussenzweig RS, Zavala F. Priming with recombinant influenza virus followed by administration of recombinant vaccinia virus induces CD8+ T-cell-mediated protective immunity against malaria. Proc Natl Acad Sci U S A. 1993;90:5214–5218. doi: 10.1073/pnas.90.11.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Anders RF. Multiple cross-reactivities amongst antigens of Plasmodium falciparum impair the development of protective immunity against malaria. Parasite Immunol. 1986;8:529–539. doi: 10.1111/j.1365-3024.1986.tb00867.x. [DOI] [PubMed] [Google Scholar]
- 245.Kumar KA, Sano G, Boscardin S, Nussenzweig RS, Nussenzweig MC, Zavala F, Nussenzweig V. The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature. 2006;444:937–940. doi: 10.1038/nature05361. [DOI] [PubMed] [Google Scholar]
- 246.Schmidt NW, Podyminogin RL, Butler NS, Badovinac VP, Tucker BJ, Bahjat KS, Lauer P, Reyes-Sandoval A, Hutchings CL, Moore AC, Gilbert SC, Hill AV, Bartholomay LC, Harty JT. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc Natl Acad Sci U S A. 2008;105:14017–14022. doi: 10.1073/pnas.0805452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Carvalho LH, Sano G, Hafalla JC, Morrot A, Curotto de Lafaille MA, Zavala F. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat Med. 2002;8:166–170. doi: 10.1038/nm0202-166. [DOI] [PubMed] [Google Scholar]
- 248.Fattovich G. Natural history and prognosis of hepatitis B. Semin Liver Dis. 2003;23:47–58. doi: 10.1055/s-2003-37590. [DOI] [PubMed] [Google Scholar]
- 249.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35–S46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 250.Khuroo MS. Discovery of hepatitis E: the epidemic non-A, non-B hepatitis 30 years down the memory lane. Virus Res. 2011;161:3–14. doi: 10.1016/j.virusres.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 251.Ciocca M. Clinical course and consequences of hepatitis A infection. Vaccine. 2000;18(Suppl 1):S71–S74. doi: 10.1016/s0264-410x(99)00470-3. [DOI] [PubMed] [Google Scholar]
- 252.Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 253.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 254.Bertoletti A, Ferrari C. Kinetics of the immune response during HBV and HCV infection. Hepatology. 2003;38:4–13. doi: 10.1053/jhep.2003.50310. [DOI] [PubMed] [Google Scholar]
- 255.Heise T, Guidotti LG, Cavanaugh VJ, Chisari FV. Hepatitis B virus RNA-binding proteins associated with cytokine-induced clearance of viral RNA from the liver of transgenic mice. J Virol. 1999;73:474–481. doi: 10.1128/jvi.73.1.474-481.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Lohmann V, Korner F, Koch J, Herian U, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 257.Blight KJ, Kolykhalov AA, Rice CM. Efficient initiation of HCV RNA replication in cell culture. Science. 2000;290:1972–1974. doi: 10.1126/science.290.5498.1972. [DOI] [PubMed] [Google Scholar]
- 258.Guidotti LG, Chisari FV. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol. 2001;19:65–91. doi: 10.1146/annurev.immunol.19.1.65. [DOI] [PubMed] [Google Scholar]
- 259.Kolykhalov AA, Agapov EV, Blight KJ, Mihalik K, Feinstone SM, Rice CM. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science. 1997;277:570–574. doi: 10.1126/science.277.5325.570. [DOI] [PubMed] [Google Scholar]
- 260.Bassett SE, Brasky KM, Lanford RE. Analysis of hepatitis C virus-inoculated chimpanzees reveals unexpected clinical profiles. J Virol. 1998;72:2589–2599. doi: 10.1128/jvi.72.4.2589-2599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Nascimbeni M, Mizukoshi E, Bosmann M, Major ME, Mihalik K, Rice CM, Feinstone SM, Rehermann B. Kinetics of CD4+ and CD8+ memory T-cell responses during hepatitis C virus rechallenge of previously recovered chimpanzees. J Virol. 2003;77:4781–4793. doi: 10.1128/JVI.77.8.4781-4793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Logvinoff C, Major ME, Oldach D, Heyward S, Talal A, Balfe P, Feinstone SM, Alter H, Rice CM, McKeating JA. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc Natl Acad Sci U S A. 2004;101:10149–10154. doi: 10.1073/pnas.0403519101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Badr G, Bedard N, Abdel-Hakeem MS, Trautmann L, Willems B, Villeneuve JP, Haddad EK, Sekaly RP, Bruneau J, Shoukry NH. Early interferon therapy for hepatitis C virus infection rescues polyfunctional, long-lived CD8+ memory T cells. J Virol. 2008;82:10017–10031. doi: 10.1128/JVI.01083-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Shoukry NH, Grakoui A, Houghton M, Chien DY, Ghrayeb J, Reimann KA, Walker CM. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197:1645–1655. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Thimme R, Bukh J, Spangenberg HC, Wieland S, Pemberton J, Steiger C, Govindarajan S, Purcell RH, Chisari FV. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci U S A. 2002;99:15661–15668. doi: 10.1073/pnas.202608299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–1406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Bigger CB, Brasky KM, Lanford RE. DNA microarray analysis of chimpanzee liver during acute resolving hepatitis C virus infection. J Virol. 2001;75:7059–7066. doi: 10.1128/JVI.75.15.7059-7066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Wieland S, Thimme R, Purcell RH, Chisari FV. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci U S A. 2004;101:6669–6674. doi: 10.1073/pnas.0401771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Su AI, Pezacki JP, Wodicka L, Brideau AD, Supekova L, Thimme R, Wieland S, Bukh J, Purcell RH, Schultz PG, Chisari FV. Genomic analysis of the host response to hepatitis C virus infection. Proc Natl Acad Sci U S A. 2002;99:15669–15674. doi: 10.1073/pnas.202608199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Egger D, Wolk B, Gosert R, Bianchi L, Blum HE, Moradpour D, Bienz K. Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex. J Virol. 2002;76:5974–5984. doi: 10.1128/JVI.76.12.5974-5984.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kakimi K, Lane TE, Chisari FV, Guidotti LG. Cutting edge: Inhibition of hepatitis B virus replication by activated NK T cells does not require inflammatory cell recruitment to the liver. J Immunol. 2001;167:6701–6705. doi: 10.4049/jimmunol.167.12.6701. [DOI] [PubMed] [Google Scholar]
- 272.Baron JL, Gardiner L, Nishimura S, Shinkai K, Locksley R, Ganem D. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity. 2002;16:583–594. doi: 10.1016/s1074-7613(02)00305-9. [DOI] [PubMed] [Google Scholar]
- 273.Wu J, Meng Z, Jiang M, Pei R, Trippler M, Broering R, Bucchi A, Sowa JP, Dittmer U, Yang D, Roggendorf M, Gerken G, Lu M, Schlaak JF. Hepatitis B virus suppresses toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology. 2009;49:1132–1140. doi: 10.1002/hep.22751. [DOI] [PubMed] [Google Scholar]
- 274.Wu J, Lu M, Meng Z, Trippler M, Broering R, Szczeponek A, Krux F, Dittmer U, Roggendorf M, Gerken G, Schlaak JF. Toll-like receptor-mediated control of HBV replication by nonparenchymal liver cells in mice. Hepatology. 2007;46:1769–1778. doi: 10.1002/hep.21897. [DOI] [PubMed] [Google Scholar]
- 275.Lang PA, Recher M, Honke N, Scheu S, Borkens S, Gailus N, Krings C, Meryk A, Kulawik A, Cervantes-Barragan L, Van Rooijen N, Kalinke U, Ludewig B, Hengartner H, Harris N, Haussinger D, Ohashi PS, Zinkernagel RM, Lang KS. Tissue macrophages suppress viral replication and prevent severe immunopathology in an interferon-I-dependent manner in mice. Hepatology. 2010;52:25–32. doi: 10.1002/hep.23640. [DOI] [PubMed] [Google Scholar]
- 276.Chang S, Dolganiuc A, Szabo G. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. J Leukoc Biol. 2007;82:479–487. doi: 10.1189/jlb.0207128. [DOI] [PubMed] [Google Scholar]
- 277.Dolganiuc A, Oak S, Kodys K, Golenbock DT, Finberg RW, Kurt-Jones E, Szabo G. Hepatitis C core and nonstructural 3 proteins trigger toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology. 2004;127:1513–1524. doi: 10.1053/j.gastro.2004.08.067. [DOI] [PubMed] [Google Scholar]
- 278.Cooper A, Tal G, Lider O, Shaul Y. Cytokine induction by the hepatitis B virus capsid in macrophages is facilitated by membrane heparan sulfate and involves TLR2. J Immunol. 2005;175:3165–3176. doi: 10.4049/jimmunol.175.5.3165. [DOI] [PubMed] [Google Scholar]
- 279.Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, Cheng J, Goedert JJ, Vlahov D, Hilgartner M, Cox S, Little AM, Alexander GJ, Cramp ME, O'Brien SJ, Rosenberg WM, Thomas DL, Carrington M. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 280.Guidotti LG, Borrow P, Hobbs MV, Matzke B, Gresser I, Oldstone MB, Chisari FV. Viral cross talk: intracellular inactivation of the hepatitis B virus during an unrelated viral infection of the liver. Proc Natl Acad Sci U S A. 1996;93:4589–4594. doi: 10.1073/pnas.93.10.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 282.Frese M, Pietschmann T, Moradpour D, Haller O, Bartenschlager R. Interferon-alpha inhibits hepatitis C virus subgenomic RNA replication by an MxA-independent pathway. J Gen Virol. 2001;82:723–733. doi: 10.1099/0022-1317-82-4-723. [DOI] [PubMed] [Google Scholar]
- 283.Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ, Sulkowski M, McHutchison JG, Goldstein DB. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 284.Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, Nakagawa M, Korenaga M, Hino K, Hige S, Ito Y, Mita E, Tanaka E, Mochida S, Murawaki Y, Honda M, Sakai A, Hiasa Y, Nishiguchi S, Koike A, Sakaida I, Imamura M, Ito K, Yano K, Masaki N, Sugauchi F, Izumi N, Tokunaga K, Mizokami M. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105–1109. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- 285.Fischer J, Bohm S, Scholz M, Muller T, Witt H, George J, Sarrazin C, Susser S, Schott E, Suppiah V, Booth D, Stewart G, van Bommel F, Brodzinski A, Fulop B, Migaud P, Berg T. Combined effects of different IL28B gene variants on the outcome of dual combination therapy in chronic HCV type 1 infection. Hepatology. 2012 doi: 10.1002/hep.25582. [DOI] [PubMed] [Google Scholar]
- 286.Suppiah V, Gaudieri S, Armstrong NJ, O'Connor KS, Berg T, Weltman M, Abate ML, Spengler U, Bassendine M, Dore GJ, Irving WL, Powell E, Hellard M, Riordan S, Matthews G, Sheridan D, Nattermann J, Smedile A, Muller T, Hammond E, Dunn D, Negro F, Bochud PY, Mallal S, Ahlenstiel G, Stewart GJ, George J, Booth DR. IL28B, HLA-C, and KIR variants additively predict response to therapy in chronic hepatitis C virus infection in a European Cohort: a cross-sectional study. PLoS Med. 2011;8:e1001092. doi: 10.1371/journal.pmed.1001092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Smith KR, Suppiah V, O'Connor K, Berg T, Weltman M, Abate ML, Spengler U, Bassendine M, Matthews G, Irving WL, Powell E, Riordan S, Ahlenstiel G, Stewart GJ, Bahlo M, George J, Booth DR. Identification of improved IL28B SNPs and haplotypes for prediction of drug response in treatment of hepatitis C using massively parallel sequencing in a cross-sectional European cohort. Genome Med. 2011;3:57. doi: 10.1186/gm273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, Bassendine M, Spengler U, Dore GJ, Powell E, Riordan S, Sheridan D, Smedile A, Fragomeli V, Muller T, Bahlo M, Stewart GJ, Booth DR, George J. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100–1104. doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- 289.Amadei B, Urbani S, Cazaly A, Fisicaro P, Zerbini A, Ahmed P, Missale G, Ferrari C, Khakoo SI. Activation of natural killer cells during acute infection with hepatitis C virus. Gastroenterology. 2010;138:1536–1545. doi: 10.1053/j.gastro.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Weiner AJ, Houghton M, Rosa D, Grandi G, Abrignani S. Binding of hepatitis C virus to CD81. Science. 1998;282:938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 291.Agnello V, Abel G, Elfahal M, Knight GB, Zhang QX. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1999;96:12766–12771. doi: 10.1073/pnas.96.22.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Scarselli E, Ansuini H, Cerino R, Roccasecca RM, Acali S, Filocamo G, Traboni C, Nicosia A, Cortese R, Vitelli A. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 2002;21:5017–5025. doi: 10.1093/emboj/cdf529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Pohlmann S, Zhang J, Baribaud F, Chen Z, Leslie GJ, Lin G, Granelli-Piperno A, Doms RW, Rice CM, McKeating JA. Hepatitis C virus glycoproteins interact with DC-SIGN and DC-SIGNR. J Virol. 2003;77:4070–4080. doi: 10.1128/JVI.77.7.4070-4080.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Lai WK, Sun PJ, Zhang J, Jennings A, Lalor PF, Hubscher S, McKeating JA, Adams DH. Expression of DC-SIGN and DC-SIGNR on human sinusoidal endothelium: a role for capturing hepatitis C virus particles. Am J Pathol. 2006;169:200–208. doi: 10.2353/ajpath.2006.051191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Falkowska E, Durso RJ, Gardner JP, Cormier EG, Arrigale RA, Ogawa RN, Donovan GP, Maddon PJ, Olson WC, Dragic T. L-SIGN (CD209L) isoforms differently mediate trans-infection of hepatoma cells by hepatitis C virus pseudoparticles. J Gen Virol. 2006;87:2571–2576. doi: 10.1099/vir.0.82034-0. [DOI] [PubMed] [Google Scholar]
- 296.Cormier EG, Durso RJ, Tsamis F, Boussemart L, Manix C, Olson WC, Gardner JP, Dragic T. L-SIGN (CD209L) and DC-SIGN (CD209) mediate transinfection of liver cells by hepatitis C virus. Proc Natl Acad Sci U S A. 2004;101:14067–14072. doi: 10.1073/pnas.0405695101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Gardner JP, Durso RJ, Arrigale RR, Donovan GP, Maddon PJ, Dragic T, Olson WC. L-SIGN (CD 209L) is a liver-specific capture receptor for hepatitis C virus. Proc Natl Acad Sci U S A. 2003;100:4498–4503. doi: 10.1073/pnas.0831128100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wolk B, Hatziioannou T, McKeating JA, Bieniasz PD, Rice CM. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801–805. doi: 10.1038/nature05654. [DOI] [PubMed] [Google Scholar]
- 299.Ploss A, Evans MJ, Gaysinskaya VA, Panis M, You H, de Jong YP, Rice CM. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457:882–886. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Lupberger J, Zeisel MB, Xiao F, Thumann C, Fofana I, Zona L, Davis C, Mee CJ, Turek M, Gorke S, Royer C, Fischer B, Zahid MN, Lavillette D, Fresquet J, Cosset FL, Rothenberg SM, Pietschmann T, Patel AH, Pessaux P, Doffoel M, Raffelsberger W, Poch O, McKeating JA, Brino L, Baumert TF. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med. 2011;17:589–595. doi: 10.1038/nm.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Crotta S, Stilla A, Wack A, D'Andrea A, Nuti S, D'Oro U, Mosca M, Filliponi F, Brunetto RM, Bonino F, Abrignani S, Valiante NM. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J Exp Med. 2002;195:35–41. doi: 10.1084/jem.20011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Tseng CT, Klimpel GR. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J Exp Med. 2002;195:43–49. doi: 10.1084/jem.20011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Wack A, Soldaini E, Tseng C, Nuti S, Klimpel G, Abrignani S. Binding of the hepatitis C virus envelope protein E2 to CD81 provides a co-stimulatory signal for human T cells. Eur J Immunol. 2001;31:166–175. doi: 10.1002/1521-4141(200101)31:1<166::aid-immu166>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 304.Ashida M, Hamada C. Molecular cloning of the hepatitis A virus receptor from a simian cell line. J Gen Virol. 1997;78(Pt 7):1565–1569. doi: 10.1099/0022-1317-78-7-1565. [DOI] [PubMed] [Google Scholar]
- 305.Feigelstock D, Thompson P, Mattoo P, Zhang Y, Kaplan GG. The human homolog of HAVcr-1 codes for a hepatitis A virus cellular receptor. J Virol. 1998;72:6621–6628. doi: 10.1128/jvi.72.8.6621-6628.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Stockert RJ, Kressner MS, Collins JC, Sternlieb I, Morell AG. IgA interaction with the asialoglycoprotein receptor. Proc Natl Acad Sci U S A. 1982;79:6229–6231. doi: 10.1073/pnas.79.20.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Tomana M, Kulhavy R, Mestecky J. Receptor-mediated binding and uptake of immunoglobulin A by human liver. Gastroenterology. 1988;94:762–770. doi: 10.1016/0016-5085(88)90252-1. [DOI] [PubMed] [Google Scholar]
- 308.Dotzauer A, Gebhardt U, Bieback K, Gottke U, Kracke A, Mages J, Lemon SM, Vallbracht A. Hepatitis A virus-specific immunoglobulin A mediates infection of hepatocytes with hepatitis A virus via the asialoglycoprotein receptor. J Virol. 2000;74:10950–10957. doi: 10.1128/jvi.74.23.10950-10957.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.van Egmond M, van Garderen E, van Spriel AB, Damen CA, van Amersfoort ES, van Zandbergen G, van Hattum J, Kuiper J, van de Winkel JG. FcalphaRI-positive liver Kupffer cells: reappraisal of the function of immunoglobulin A in immunity. Nat Med. 2000;6:680–685. doi: 10.1038/76261. [DOI] [PubMed] [Google Scholar]
- 310.Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68–76. doi: 10.1128/JVI.77.1.68-76.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, Murthy KK, Rice CM, Walker CM. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 312.Garcia-Rodriguez MJ, Canales MA, Hernandez-Maraver D, Hernandez-Navarro F. Late reactivation of resolved hepatitis B virus infection: an increasing complication post rituximab-based regimens treatment? Am J Hematol. 2008;83:673–675. doi: 10.1002/ajh.21214. [DOI] [PubMed] [Google Scholar]
- 313.Brimacombe CL, Grove J, Meredith LW, Hu K, Syder AJ, Flores MV, Timpe JM, Krieger SE, Baumert TF, Tellinghuisen TL, Wong-Staal F, Balfe P, McKeating JA. Neutralizing antibody-resistant hepatitis C virus cell-to-cell transmission. J Virol. 2011;85:596–605. doi: 10.1128/JVI.01592-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.von Hahn T, Yoon JC, Alter H, Rice CM, Rehermann B, Balfe P, McKeating JA. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology. 2007;132:667–678. doi: 10.1053/j.gastro.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 315.Lopes AR, Kellam P, Das A, Dunn C, Kwan A, Turner J, Peppa D, Gilson RJ, Gehring A, Bertoletti A, Maini MK. Bim-mediated deletion of antigen-specific CD8 T cells in patients unable to control HBV infection. J Clin Invest. 2008;118:1835–1845. doi: 10.1172/JCI33402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Radziewicz H, Ibegbu CC, Hon H, Osborn MK, Obideen K, Wehbi M, Freeman GJ, Lennox JL, Workowski KA, Hanson HL, Grakoui A. Impaired hepatitis C virus (HCV)-specific effector CD8+ T cells undergo massive apoptosis in the peripheral blood during acute HCV infection and in the liver during the chronic phase of infection. J Virol. 2008;82:9808–9822. doi: 10.1128/JVI.01075-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Larrubia JR, Benito-Martinez S, Miquel J, Calvino M, Sanz-de-Villalobos E, Gonzalez-Praetorius A, Albertos S, Garcia-Garzon S, Lokhande M, Parra-Cid T. Bim-mediated apoptosis and PD-1/PD-L1 pathway impair reactivity of PD1(+)/CD127(−) HCV-specific CD8(+) cells targeting the virus in chronic hepatitis C virus infection. Cell Immunol. 2011;269:104–114. doi: 10.1016/j.cellimm.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 318.Kantzanou M, Lucas M, Barnes E, Komatsu H, Dusheiko G, Ward S, Harcourt G, Klenerman P. Viral escape and T cell exhaustion in hepatitis C virus infection analysed using Class I peptide tetramers. Immunol Lett. 2003;85:165–171. doi: 10.1016/s0165-2478(02)00224-9. [DOI] [PubMed] [Google Scholar]
- 319.Klenerman P, Lechner F, Kantzanou M, Ciurea A, Hengartner H, Zinkernagel R. Viral escape and the failure of cellular immune responses. Science. 2000;289:2003. doi: 10.1126/science.289.5487.2003a. [DOI] [PubMed] [Google Scholar]
- 320.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G, Bertoletti A, Ferrari C. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol. 2008;45:963–970. doi: 10.1016/j.molimm.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 323.Urbani S, Amadei B, Tola D, Pedrazzi G, Sacchelli L, Cavallo MC, Orlandini A, Missale G, Ferrari C. Restoration of HCV-specific T cell functions by PD-1/PD-L1 blockade in HCV infection: effect of viremia levels and antiviral treatment. J Hepatol. 2008;48:548–558. doi: 10.1016/j.jhep.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 324.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Bengsch B, Seigel B, Ruhl M, Timm J, Kuntz M, Blum HE, Pircher H, Thimme R. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 2010;6:e1000947. doi: 10.1371/journal.ppat.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp Med. 2003;198:39–50. doi: 10.1084/jem.20022235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Muhlbauer M, Fleck M, Schutz C, Weiss T, Froh M, Blank C, Scholmerich J, Hellerbrand C. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J Hepatol. 2006;45:520–528. doi: 10.1016/j.jhep.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 328.Zhang Z, Zhang JY, Wherry EJ, Jin B, Xu B, Zou ZS, Zhang SY, Li BS, Wang HF, Wu H, Lau GK, Fu YX, Wang FS. Dynamic programmed death 1 expression by virus-specific CD8 T cells correlates with the outcome of acute hepatitis B. Gastroenterology. 2008;134:1938–1949. 1949 e1–1949 e3. doi: 10.1053/j.gastro.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 329.Kassel R, Cruise MW, Iezzoni JC, Taylor NA, Pruett TL, Hahn YS. Chronically inflamed livers up-regulate expression of inhibitory B7 family members. Hepatology. 2009;50:1625–1637. doi: 10.1002/hep.23173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Mengshol JA, Golden-Mason L, Arikawa T, Smith M, Niki T, McWilliams R, Randall JA, McMahan R, Zimmerman MA, Rangachari M, Dobrinskikh E, Busson P, Polyak SJ, Hirashima M, Rosen HR. A crucial role for Kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection. PLoS One. 2010;5:e9504. doi: 10.1371/journal.pone.0009504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 332.Ferrari C, Penna A, Bertoletti A, Valli A, Antoni AD, Giuberti T, Cavalli A, Petit MA, Fiaccadori F. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J Immunol. 1990;145:3442–3449. [PubMed] [Google Scholar]
- 333.Bogdanos DP, Mieli-Vergani G, Vergani D. Virus, liver and autoimmunity. Dig Liver Dis. 2000;32:440–446. doi: 10.1016/s1590-8658(00)80266-2. [DOI] [PubMed] [Google Scholar]
- 334.Bogdanos DP, Choudhuri K, Vergani D. Molecular mimicry and autoimmune liver disease: virtuous intentions, malign consequences. Liver. 2001;21:225–232. doi: 10.1034/j.1600-0676.2001.021004225.x. [DOI] [PubMed] [Google Scholar]
- 335.Bogdanos DP, Mieli-Vergani G, Vergani D. Non-organ-specific autoantibodies in children with chronic hepatitis C virus infection. Clin Infect Dis. 2004;38:1505. doi: 10.1086/383578. author reply 1505–6. [DOI] [PubMed] [Google Scholar]
- 336.Bogdanos DP, Mieli-Vergani G, Vergani D. Non-organ-specific autoantibodies in hepatitis C virus infection: do they matter? Clin Infect Dis. 2005;40:508–510. doi: 10.1086/427293. [DOI] [PubMed] [Google Scholar]
- 337.Bogdanos DP, Smith H, Ma Y, Baum H, Mieli-Vergani G, Vergani D. A study of molecular mimicry and immunological cross-reactivity between hepatitis B surface antigen and myelin mimics. Clin Dev Immunol. 2005;12:217–224. doi: 10.1080/17402520500285247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Gregorio GV, Choudhuri K, Ma Y, Pensati P, Iorio R, Grant P, Garson J, Bogdanos DP, Vegnente A, Mieli-Vergani G, Vergani D. Mimicry between the hepatitis C virus polyprotein and antigenic targets of nuclear and smooth muscle antibodies in chronic hepatitis C virus infection. Clin Exp Immunol. 2003;133:404–413. doi: 10.1046/j.1365-2249.2003.02229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Dammacco F, Sansonno D, Piccoli C, Racanelli V, D'Amore FP, Lauletta G. The lymphoid system in hepatitis C virus infection: autoimmunity, mixed cryoglobulinemia, and Overt B-cell malignancy. Semin Liver Dis. 2000;20:143–157. doi: 10.1055/s-2000-9613. [DOI] [PubMed] [Google Scholar]
- 340.Vento S, Cainelli F. Is there a role for viruses in triggering autoimmune hepatitis? Autoimmun Rev. 2004;3:61–69. doi: 10.1016/S1568-9972(03)00053-3. [DOI] [PubMed] [Google Scholar]
- 341.Mackie FD, Peakman M, Yun M, Sallie R, Smith H, Davies ET, Mieli-Vergani G, Vergani D. Primary and secondary liver/kidney microsomal autoantibody response following infection with hepatitis C virus. Gastroenterology. 1994;106:1672–1675. doi: 10.1016/0016-5085(94)90426-x. [DOI] [PubMed] [Google Scholar]
- 342.Bogdanos DP, Lenzi M, Okamoto M, Rigopoulou EI, Muratori P, Ma Y, Muratori L, Tsantoulas D, Mieli- Vergani G, Bianchi FB, Vergani D. Multiple viral/self immunological cross-reactivity in liver kidney microsomal antibody positive hepatitis C virus infected patients is associated with the possession of HLA B51. Int J Immunopathol Pharmacol. 2004;17:83–92. doi: 10.1177/039463200401700112. [DOI] [PubMed] [Google Scholar]
- 343.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 345.Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. The New England journal of medicine. 1997;336:1855–1859. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 346.Butterfield LH, Ribas A, Meng WS, Dissette VB, Amarnani S, Vu HT, Seja E, Todd K, Glaspy JA, McBride WH, Economou JS. T-cell responses to HLA-A*0201 immunodominant peptides derived from alpha-fetoprotein in patients with hepatocellular cancer. Clin Cancer Res. 2003;9:5902–5908. [PubMed] [Google Scholar]
- 347.Butterfield LH, Meng WS, Koh A, Vollmer CM, Ribas A, Dissette VB, Faull K, Glaspy JA, McBride WH, Economou JS. T cell responses to HLA-A*0201-restricted peptides derived from human alpha fetoprotein. J Immunol. 2001;166:5300–5308. doi: 10.4049/jimmunol.166.8.5300. [DOI] [PubMed] [Google Scholar]
- 348.Butterfield LH, Koh A, Meng W, Vollmer CM, Ribas A, Dissette V, Lee E, Glaspy JA, McBride WH, Economou JS. Generation of human T-cell responses to an HLA-A2.1-restricted peptide epitope derived from alpha-fetoprotein. Cancer Res. 1999;59:3134–3142. [PubMed] [Google Scholar]
- 349.Thimme R, Neagu M, Boettler T, Neumann-Haefelin C, Kersting N, Geissler M, Makowiec F, Obermaier R, Hopt UT, Blum HE, Spangenberg HC. Comprehensive analysis of the alpha-fetoprotein-specific CD8+ T cell responses in patients with hepatocellular carcinoma. Hepatology. 2008;48:1821–1833. doi: 10.1002/hep.22535. [DOI] [PubMed] [Google Scholar]
- 350.Wada Y, Nakashima O, Kutami R, Yamamoto O, Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology. 1998;27:407–414. doi: 10.1002/hep.510270214. [DOI] [PubMed] [Google Scholar]
- 351.Hiroishi K, Eguchi J, Baba T, Shimazaki T, Ishii S, Hiraide A, Sakaki M, Doi H, Uozumi S, Omori R, Matsumura T, Yanagawa T, Ito T, Imawari M. Strong CD8(+) T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J Gastroenterol. 2010;45:451–458. doi: 10.1007/s00535-009-0155-2. [DOI] [PubMed] [Google Scholar]
- 352.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C, Yao J, Jin L, Wang H, Yang Y, Fu YX, Wang FS. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 353.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457–2464. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 354.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- 355.Golgher D, Jones E, Powrie F, Elliott T, Gallimore A. Depletion of CD25+ regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur J Immunol. 2002;32:3267–3275. doi: 10.1002/1521-4141(200211)32:11<3267::AID-IMMU3267>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 356.Aksoylar HI, Lampe K, Barnes MJ, Plas DR, Hoebe K. Loss of immunological tolerance in Gimap5-deficient mice is associated with loss of Foxo in CD4+ T cells. J Immunol. 2012;188:146–154. doi: 10.4049/jimmunol.1101206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Baughman EJ, Mendoza JP, Ortega SB, Ayers CL, Greenberg BM, Frohman EM, Karandikar NJ. Neuroantigen-specific CD8+ regulatory T-cell function is deficient during acute exacerbation of multiple sclerosis. J Autoimmun. 2011;36:115–124. doi: 10.1016/j.jaut.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Kerzerho J, Wunsch D, Szely N, Meyer HA, Lurz L, Rose L, Wahn U, Akbari O, Stock P. Effects of systemic versus local administration of corticosteroids on mucosal tolerance. J Immunol. 2012;188:470–476. doi: 10.4049/jimmunol.1101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Laakso SM, Laurinolli TT, Rossi LH, Lehtoviita A, Sairanen H, Perheentupa J, Kekalainen E, Arstila TP. Regulatory T cell defect in APECED patients is associated with loss of naive FOXP3(+) precursors and impaired activated population. J Autoimmun. 2010;35:351–357. doi: 10.1016/j.jaut.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 360.Li CR, Deiro MF, Godebu E, Bradley LM. IL-7 uniquely maintains FoxP3(+) adaptive Treg cells that reverse diabetes in NOD mice via integrin-beta7-dependent localization. J Autoimmun. 2011;37:217–227. doi: 10.1016/j.jaut.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Martinez RJ, Zhang N, Thomas SR, Nandiwada SL, Jenkins MK, Binstadt BA, Mueller DL. Arthritogenic self-reactive CD4+ T cells acquire an FR4hiCD73hi anergic state in the presence of Foxp3+ regulatory T cells. J Immunol. 2012;188:170–181. doi: 10.4049/jimmunol.1101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Miyagawa F, Gutermuth J, Zhang H, Katz SI. The use of mouse models to better understand mechanisms of autoimmunity and tolerance. J Autoimmun. 2010;35:192–198. doi: 10.1016/j.jaut.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Munoz-Suano A, Kallikourdis M, Sarris M, Betz AG. Regulatory T cells protect from autoimmune arthritis during pregnancy. J Autoimmun. 2011 doi: 10.1016/j.jaut.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Ray A, Basu S, Williams CB, Salzman NH, Dittel BN. A Novel IL-10-Independent Regulatory Role for B Cells in Suppressing Autoimmunity by Maintenance of Regulatory T Cells via GITR Ligand. J Immunol. 2012 doi: 10.4049/jimmunol.1103354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Santiuste I, Buelta L, Iglesias M, Genre F, Mazorra F, Izui S, Merino J, Merino R. B-cell overexpression of Bcl-2 cooperates with p21 deficiency for the induction of autoimmunity and lymphomas. J Autoimmun. 2010;35:316–324. doi: 10.1016/j.jaut.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 366.Wang YH, Yan Y, Rice JS, Volpe BT, Diamond B. Enforced expression of the apoptosis inhibitor Bcl-2 ablates tolerance induction in DNA-reactive B cells through a novel mechanism. J Autoimmun. 2011;37:18–27. doi: 10.1016/j.jaut.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Xiao X, Gong W, Demirci G, Liu W, Spoerl S, Chu X, Bishop DK, Turka LA, Li XC. New insights on OX40 in the control of T cell immunity and immune tolerance in vivo. J Immunol. 2012;188:892–901. doi: 10.4049/jimmunol.1101373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Zhang AH, Li X, Onabajo OO, Su Y, Skupsky J, Thomas JW, Scott DW. B-cell delivered gene therapy for tolerance induction: role of autoantigen-specific B cells. J Autoimmun. 2010;35:107–113. doi: 10.1016/j.jaut.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Zhao Q, Kuang DM, Wu Y, Xiao X, Li XF, Li TJ, Zheng L. Activated CD69+ T cells foster immune privilege by regulating IDO expression in tumor-associated macrophages. J Immunol. 2012;188:1117–1124. doi: 10.4049/jimmunol.1100164. [DOI] [PubMed] [Google Scholar]
- 370.Chen M, Felix K, Wang J. Immune regulation through mitochondrion-dependent dendritic cell death induced by T regulatory cells. J Immunol. 2011;187:5684–5692. doi: 10.4049/jimmunol.1101834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Chang C, Gershwin ME. Drugs and autoimmunity--a contemporary review and mechanistic approach. J Autoimmun. 2010;34:J266–J275. doi: 10.1016/j.jaut.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 372.Kim DH, Lee JC, Kim S, Oh SH, Lee MK, Kim KW, Lee MS. Inhibition of autoimmune diabetes by TLR2 tolerance. J Immunol. 2011;187:5211–5220. doi: 10.4049/jimmunol.1001388. [DOI] [PubMed] [Google Scholar]
- 373.Verkoczy L, Chen Y, Bouton-Verville H, Zhang J, Diaz M, Hutchinson J, Ouyang YB, Alam SM, Holl TM, Hwang KK, Kelsoe G, Haynes BF. Rescue of HIV-1 broad neutralizing antibody-expressing B cells in 2F5 VH × VL knockin mice reveals multiple tolerance controls. J Immunol. 2011;187:3785–3797. doi: 10.4049/jimmunol.1101633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Round JL, O'Connell RM, Mazmanian SK. Coordination of tolerogenic immune responses by the commensal microbiota. J Autoimmun. 2010;34:J220–J225. doi: 10.1016/j.jaut.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Christen U, Hintermann E, Holdener M, von Herrath MG. Viral triggers for autoimmunity: is the 'glass of molecular mimicry' half full or half empty? J Autoimmun. 2010;34:38–44. doi: 10.1016/j.jaut.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Goldstone AB, Bronster DJ, Anyanwu AC, Goldstein MA, Filsoufi F, Adams DH, Chikwe J. Predictors and outcomes of seizures after cardiac surgery: a multivariable analysis of 2,578 patients. Ann Thorac Surg. 2011;91:514–518. doi: 10.1016/j.athoracsur.2010.10.090. [DOI] [PubMed] [Google Scholar]
- 377.Humphreys EH, Williams KT, Adams DH, Afford SC. Primary and malignant cholangiocytes undergo CD40 mediated Fas dependent apoptosis, but are insensitive to direct activation with exogenous Fas ligand. PLoS One. 2010;5:e14037. doi: 10.1371/journal.pone.0014037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Oo YH, Shetty S, Adams DH. The role of chemokines in the recruitment of lymphocytes to the liver. Dig Dis. 2010;28:31–44. doi: 10.1159/000282062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Weston CJ, Adams DH. Hepatic consequences of vascular adhesion protein-1 expression. J Neural Transm. 2011;118:1055–1064. doi: 10.1007/s00702-011-0647-0. [DOI] [PubMed] [Google Scholar]
- 380.Adams DH, Ju C, Ramaiah SK, Uetrecht J, Jaeschke H. Mechanisms of immune-mediated liver injury. Toxicol Sci. 2010;115:307–321. doi: 10.1093/toxsci/kfq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Luth S, Huber S, Schramm C, Buch T, Zander S, Stadelmann C, Bruck W, Wraith DC, Herkel J, Lohse AW. Ectopic expression of neural autoantigen in mouse liver suppresses experimental autoimmune neuroinflammation by inducing antigen-specific Tregs. J Clin Invest. 2008;118:3403–3410. doi: 10.1172/JCI32132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Scoazec JY, Feldmann G. In situ immunophenotyping study of endothelial cells of the human hepatic sinusoid: results and functional implications. Hepatology. 1991;14:789–797. doi: 10.1002/hep.1840140508. [DOI] [PubMed] [Google Scholar]
- 383.Scoazec JY, Feldmann G. The cell adhesion molecules of hepatic sinusoidal endothelial cells. J Hepatol. 1994;20:296–300. doi: 10.1016/s0168-8278(05)80072-8. [DOI] [PubMed] [Google Scholar]
- 384.Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem. 2006;54:385–395. doi: 10.1369/jhc.4A6514.2005. [DOI] [PubMed] [Google Scholar]
- 385.Mousa SA. Expression of adhesion molecules during cadmium hepatotoxicity. Life Sci. 2004;75:93–105. doi: 10.1016/j.lfs.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 386.Daneker GW, Lund SA, Caughman SW, Swerlick RA, Fischer AH, Staley CA, Ades EW. Culture and characterization of sinusoidal endothelial cells isolated from human liver. In Vitro Cell Dev Biol Anim. 1998;34:370–377. doi: 10.1007/s11626-998-0018-9. [DOI] [PubMed] [Google Scholar]
- 387.Matsumura T, Takesue M, Westerman KA, Okitsu T, Sakaguchi M, Fukazawa T, Totsugawa T, Noguchi H, Yamamoto S, Stolz DB, Tanaka N, Leboulch P, Kobayashi N. Establishment of an immortalized human-liver endothelial cell line with SV40T and hTERT. Transplantation. 2004;77:1357–1365. doi: 10.1097/01.tp.0000124286.82961.7e. [DOI] [PubMed] [Google Scholar]
- 388.Karrar A, Broome U, Uzunel M, Qureshi AR, Sumitran-Holgersson S. Human liver sinusoidal endothelial cells induce apoptosis in activated T cells: a role in tolerance induction. Gut. 2007;56:243–252. doi: 10.1136/gut.2006.093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Irving MG, Roll FJ, Huang S, Bissell DM. Characterization and culture of sinusoidal endothelium from normal rat liver: lipoprotein uptake and collagen phenotype. Gastroenterology. 1984;87:1233–1247. [PubMed] [Google Scholar]
- 390.van der Kwast TH, Stel HV, Cristen E, Bertina RM, Veerman EC. Localization of factor VIII-procoagulant antigen: an immunohistological survey of the human body using monoclonal antibodies. Blood. 1986;67:222–227. [PubMed] [Google Scholar]
- 391.Smedsrod B, Pertoft H, Gustafson S, Laurent TC. Scavenger functions of the liver endothelial cell. Biochem J. 1990;266:313–327. doi: 10.1042/bj2660313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Homberg JC, Abuaf N, Bernard O, Islam S, Alvarez F, Khalil SH, Poupon R, Darnis F, Levy VG, Grippon P, et al. Chronic active hepatitis associated with antiliver/kidney microsome antibody type 1: a second type of "autoimmune" hepatitis. Hepatology. 1987;7:1333–1339. doi: 10.1002/hep.1840070626. [DOI] [PubMed] [Google Scholar]
- 393.Kerkar N, Choudhuri K, Ma Y, Mahmoud A, Bogdanos DP, Muratori L, Bianchi F, Williams R, Mieli-Vergani G, Vergani D. Cytochrome P4502D6(193–212): a new immunodominant epitope and target of virus/self cross-reactivity in liver kidney microsomal autoantibody type 1-positive liver disease. J Immunol. 2003;170:1481–1489. doi: 10.4049/jimmunol.170.3.1481. [DOI] [PubMed] [Google Scholar]
- 394.Dalekos GN, Wedemeyer H, Obermayer-Straub P, Kayser A, Barut A, Frank H, Manns MP. Epitope mapping of cytochrome P4502D6 autoantigen in patients with chronic hepatitis C during alpha-interferon treatment. J Hepatol. 1999;30:366–375. doi: 10.1016/s0168-8278(99)80092-0. [DOI] [PubMed] [Google Scholar]
- 395.Manns MP, Griffin KJ, Sullivan KF, Johnson EF. LKM-1 autoantibodies recognize a short linear sequence in P450IID6, a cytochrome P-450 monooxygenase. J Clin Invest. 1991;88:1370–1378. doi: 10.1172/JCI115443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Ma Y, Thomas MG, Okamoto M, Bogdanos DP, Nagl S, Kerkar N, Lopes AR, Muratori L, Lenzi M, Bianchi FB, Mieli-Vergani G, Vergani D. Key residues of a major cytochrome P4502D6 epitope are located on the surface of the molecule. J Immunol. 2002;169:277–285. doi: 10.4049/jimmunol.169.1.277. [DOI] [PubMed] [Google Scholar]