Abstract
Remarkable methodological advances in the past decade have expanded the application of liquid chromatography coupled with mass spectrometry (LC/MS) analysis of biotherapeutics. Currently, LC/MS represents a promising alternative or supplement to the traditional ligand binding assay (LBA) in the pharmacokinetic, pharmacodynamic, and toxicokinetic studies of protein drugs, owing to the rapid and cost-effective method development, high specificity and reproducibility, low sample consumption, the capacity of analyzing multiple targets in one analysis, and the fact that a validated method can be readily adapted across various matrices and species. While promising, technical challenges associated with sensitivity, sample preparation, method development, and quantitative accuracy need to be addressed to enable full utilization of LC/MS. This article introduces the rationale and technical challenges of LC/MS techniques in biotherapeutics analysis and summarizes recently developed strategies to alleviate these challenges. Applications of LC/MS techniques on quantification and characterization of antibody biotherapeutics are also discussed. We speculate that despite the highly attractive features of LC/MS, it will not fully replace traditional assays such as LBA in the foreseeable future; instead, the forthcoming trend is likely the conjunction of biochemical techniques with versatile LC/MS approaches to achieve accurate, sensitive, and unbiased characterization of biotherapeutics in highly complex pharmaceutical/biologic matrices. Such combinations will constitute powerful tools to tackle the challenges posed by the rapidly growing needs for biotherapeutics development.
Introduction
Biotherapeutics, especially therapeutic monoclonal antibodies (mAb), have become one of the primary focuses for the pharmaceutic industry worldwide (van den Broek et al., 2013). Sensitive, accurate, and high-throughput analytical methods that deliver high-quality quantitative data for pharmacokinetic (PK), pharmacodynamic (PD), and toxicokinetic studies, are critically important to the development of these agents (Nowatzke et al., 2011; Geist et al., 2013b). Traditionally, ligand-binding assays (LBA), such as enzyme-linked immunosorbent assay (ELISA), are the primary means for quantification of therapeutic proteins, which are often considered to afford sufficient sensitivity and throughput for PK, PD, and toxicokinetics studies (Urva et al., 2010; Shah and Balthasar, 2014). Nevertheless, LBA methods may fall short in that they are often matrix and species dependent (e.g., methods developed in one matrix/species cannot be readily transferred to another), and the quantitative accuracy and specificity may be compromised by interferences from biomatrices, mAb modification/degradation, and anti-mAb antibody, especially when highly specific critical reagents are not available (Damen et al., 2009; Hoofnagle and Wener, 2009). Moreover, the method development is often time consuming and costly, which is particularly problematic in the phases of discovery and early development (Savoie et al., 2010).
Liquid chromatography coupled with mass spectrometry (LC/MS) has emerged as a promising alternative to LBA for quantitative characterization of biotherapeutics (Heudi et al., 2008). Since the late 1990s, LC/MS has been a powerful tool for sensitive, accurate and rapid analysis of small-molecule drugs, metabolites and biomarkers (Trufelli et al., 2011). More recently, various LC/MS techniques have been developed for the quantification of proteins of interest in complex biologic matrices (Qu et al., 2006; Pan et al., 2009). Although it is possible to quantify proteins by LC/MS on both intact-protein and proteolytic-peptide levels (Kippen et al., 1997; Pan et al., 2009; Duan et al., 2012a; Rauh, 2012; van den Broek et al., 2013), the vast majority of LC/MS-based protein quantifications are performed at peptide levels for several important reasons. First, the sensitivity of MS is far superior at the peptide level than at the protein level (Blackburn, 2013); second, in a biologic system, intact proteins often carry a cohort of posttranslational modifications (PTM), which shift the masses of the proteins and introduce considerable analytical variability; per contra, when protein is being quantified at the peptide level, the quantification is usually based on the selected peptide domains where modifications are not likely to occur, and thus ensuring high reliability and reproducibility (Hopfgartner et al., 2013); third, the upper m/z limits of most MS analyzers are often too low to analyze the multiply charged precursor ions of a relatively large protein such as a therapeutic mAb, whereas the m/z of most peptide precursors can be readily detected by almost all MS analyzers (Blackburn, 2013).
For protein quantification at peptide level, selected-reaction monitoring (SRM) operated on a triple-quadrupole MS is by far the most commonly used technique. Briefly, the first quadrupole analyzer selects a specific peptide precursor ion from the complex matrix, which is then fragmented in a downstream fragmentation chamber filled with collision gas; the second quadrupole analyzer then monitors a specific fragment from the target peptide (Qu and Straubinger, 2005). Compared with other tandem MS techniques, SRM-MS exhibits higher sensitivity, better quantitative accuracy, and a wider dynamic range for targeted protein quantification, and can be easily multiplexed (i.e., quantification of multiple analytes in one LC/MS analysis) by quickly switching among different precursor/product transitions (Qu and Straubinger, 2005). When the excellent specificity of SRM is combined with sufficient LC separation, the LC/SRM-MS constitutes a versatile and powerful tool for the quantification of proteins in complex matrices. A typical procedure for LC/SRM-MS-based quantification includes sample treatment/cleanup, digestion using enzymes, and quantification of the target proteins based on selected signature peptides (SP) derived from the target. Stable isotope labeled (SIL) SP surrogate or SIL full-length-protein is used as the internal standard (IS). Extensive reviews on this technique can be found in Lange et al. (2008) and Liebler and Zimmerman (2013).
LC/SRM-MS has several attractive features over LBA for analysis of biotherapeutics, including fast-method development and validation, high specificity, and small sample consumption per analysis (Jemal and Xia, 2006; Fernandez Ocana et al., 2012; Liu et al., 2013; van den Broek et al., 2013). Furthermore, many proteins can be simultaneously quantified in one LC/SRM-MS analysis (Xiao et al., 2014), and a method developed in one matrix or species can often be readily transferred to another (Savoie et al., 2010; Pendley and Shankar, 2011; Bronsema et al., 2012; Duan et al., 2012a; Li et al., 2012; Geist et al., 2013b; Jiang et al., 2013). Finally, LC/MS techniques can be employed to obtain critical information on the molecules of biotherapeutics that pose a daunting challenge for LBA, such as chemical degradation (e.g., oxidation or deamidation of residues) (Huang et al., 2005).
With an emphasis on the LC/SRM-MS technique, this article will compare LC/MS techniques versus LBA for quantification of antibody biotherapeutics, and then discuss the technical challenges of LC/SRM-MS and emerging approaches to alleviate these challenges. Finally, recent applications of LC/MS techniques to the analysis of biotherapeutics are summarized.
Comparison of LC/SRM-MS versus LBA
For quantification of biotherapeutics, the sensitivity, specificity, and robustness achievable by LBA are heavily dependent on the quality of critical reagents (often antibodies) raised against specific epitopes of the target proteins (Lee and Kelley, 2011; O’Hara et al., 2012). By comparison, usually the LC/SRM-MS method does not require target-specific critical reagents, and thus may provide the following prominent advantages over LBA.
First, the process to develop a LBA method for quantification of therapeutic proteins in complex pharmaceutical matrixes (e.g., plasma and tissues) with sufficient sensitivity, selectivity, and accuracy is often both time consuming (e.g., 6 to 12 months; Savoie et al., 2010) and costly. This process typically includes the development of optimal critical reagents, the extensive examination of endogenous interferences, and rigorous method validation in samples from various sources (Nowatzke et al., 2011; Pendley and Shankar, 2011). In contrast, LC/SRM-MS-based approaches do not require critical reagents, so these methods can be developed more rapidly (e.g., in 2 to 3 weeks) at substantially lower cost (Pan et al., 2009).
Second, the critical reagents used for LBA are generally produced via biologic processes, which are inherently prone to variability arising from such factors as PTM, variations in different batches of reagents, and biologic interferences (Lee and Kelley, 2011; O’Hara et al., 2012); as LBA does not employ an internal standard to correct quantitative bias and variation, stringently controlled operations are required to prevent deterioration of assay performance (Pandya et al., 2010; Lee and Kelley, 2011). Consequently, it is often challenging to maintain high interbatch/interlaboratory consistency, which is necessary to correlate results among batches and to transfer validated methods between laboratories (Ezan and Bitsch, 2009). On the contrary, analytical variation is frequently much less a concern for LC/SRM-MS approaches because critical reagents are not required, and isotope-coded peptides or proteins are prevalently used as IS, which effectively corrects analytical variations introduced by LC/MS analysis and matrix effects (Pan et al., 2009; Bronsema et al., 2012; van den Broek et al., 2013; Nouri-Nigjeh et al., 2014). In practice, the performance of a developed and validated LC/SRM-MS method is quite robust so long as instrument maintenance and quality control are performed properly.
Third, measurement of the distribution of biotherapeutics in different matrices (e.g., plasma or tissues) and across different species is important for the development and evaluation of these agents (Lin et al., 1999; Garg and Balthasar, 2009; Deng et al., 2011; Shah and Betts, 2013) but is difficult to achieve with a single LBA method. The specificity of LBA is profoundly affected by the matrix: in different matrices or species, the extents of interferences and cross-reactions by matrix components vary considerably (Pendley and Shankar, 2011), rendering it difficult to transfer a LBA method among matrices (e.g., from plasma to a tissue or among different tissues) (Damen et al., 2009; Ezan and Bitsch, 2009; Hoofnagle and Wener, 2009). Conversely, as LC/SRM-MS minimizes the matrix effect by employing isotope-coded IS and sufficient chromatographic separation (Qu and Straubinger, 2005), the methods are often readily transferrable among different matrices. For example, recently we applied the same LC/SRM-MS method for the quantification of therapeutic mAbs in mouse plasma and tissues, such as brain, heart, liver, spleen, kidney, and lung, with rapid and simple verification and revalidation in different matrices (Duan et al., 2012a).
Fourth, although it is very challenging to develop a LBA method capable of quantifying multiple proteins in one analysis, LC/SRM-MS approaches can be multiplexed for hundreds of targets in one run (Li et al., 2012; Shi et al., 2012; Xiao et al., 2014). Such multiplexing capacity has been widely used in biomarker and proteomewide mechanism studies (Sakamoto et al., 2011; Percy et al., 2014; Xiao et al., 2014). The multiplexing method is also highly valuable for the research and development of biotherapeutics such as simultaneous quantification of an mAb and its circulating or tissue-specific targets (Li et al., 2009; Kawakami et al., 2011; Ohtsuki et al., 2012; Chambers et al., 2014) or multiple drug candidates in the same sample obtained from administration strategies such as cassette dosing (Jiang et al., 2013; Li et al., 2013). The cassette-dosing strategy, which doses multiple drug candidates together to one subject to enable rapid screening, has long been employed in PK profiling and metabolite screening for small-molecule drugs (Bayliss and Frick, 1999; Korfmacher et al., 2001). This approach also substantially reduces the time and resources required for the initial screening and development of biotherapeutics (Liu et al., 2008). A multiplexed LC/SRM-MS approach enables the simultaneous albeit specific analysis of different mAb candidates—even the sequences are only slightly different (Geist et al., 2013a; Li et al., 2013)—and thus is the method of choice for cassette-dosing studies.
Other merits of LC/SRM-MS over LBA include low sample consumption and high operational robustness (Ouyang et al., 2012; Liu et al., 2013). For example, in our recent studies, each LC/SRM-MS analysis only used peptide digests from ∼0.1 µL of plasma or ∼0.1 mg of tissue (Duan et al., 2012a,b; Nouri-Nigjeh et al., 2014), which is much lower than the sample amount required by a typical LBA method.
The main disadvantage of LC/SRM-MS compared with LBA is that an expensive MS instrument is required. Moreover, for these well-established biotherapeutics with industry-grade LBA methods that have been already been developed and validated, sensitive and high-throughput quantification can be achieved following a relatively straightforward LBA procedure (Ezan et al., 2009). Consequently, LBA remains the preferred choice for these targets (Damen et al., 2009).
Challenges of LC/SRM-MS and Strategies
Despite of the merits of LC/SRM-MS and its rapidly growing utility in the quantification of biotherapeutics, a number of significant technical challenges still exist. This section discusses the challenges and recent efforts to address them.
Method Development.
Until now, the optimal strategy for the development of an LC/SRM-MS method has remained elusive (Pan et al., 2009; Duan et al., 2012a). The key aspect for method development is the discovery of optimal SP derived from the target biotherapeutics that ensures sensitive, specific, and robust quantification. Currently, in silico prediction approaches are popularly used that employ tools such as PeptideAtlas, Skyline, and MRMaid, to identify and validate the SRM transition with minimum wet laboratory labor (Cham Mead et al., 2010; Halquist and Thomas Karnes, 2011; Stergachis et al., 2011; Rauh, 2012). Nevertheless, this approach may not accurately predict the most sensitive, stable peptides or matrix-dependent parameters such as chemical interferences (Cao et al., 2010; Duan et al., 2012a). Discovery of optimal SP by experimentally evaluating many proteolytic candidates in the target matrix (e.g., plasma or tissue digest) is the most reliable approach (Cao et al., 2010; Duan et al., 2012a); however, to experimentally evaluate these candidates, it is necessary to obtain optimal SRM conditions (e.g., the optimal parent/product transitions and the declustering/collision energy) for each of the many candidates in a digest mixture, which is challenging. Moreover, it is important to choose stable peptides as the SP (Cao et al., 2010; Duan et al., 2012a) to prevent quantitative variation and bias arising from poor peptide stability, which has been often overlooked. Finally, most methods use a lone SP for the quantification of a mAb, which may carry a significant risk of error where the mAb could be truncated biologically outside the SP domain or certain residues within the SP domain could be biologically modified (Hoofnagle and Wener, 2009).
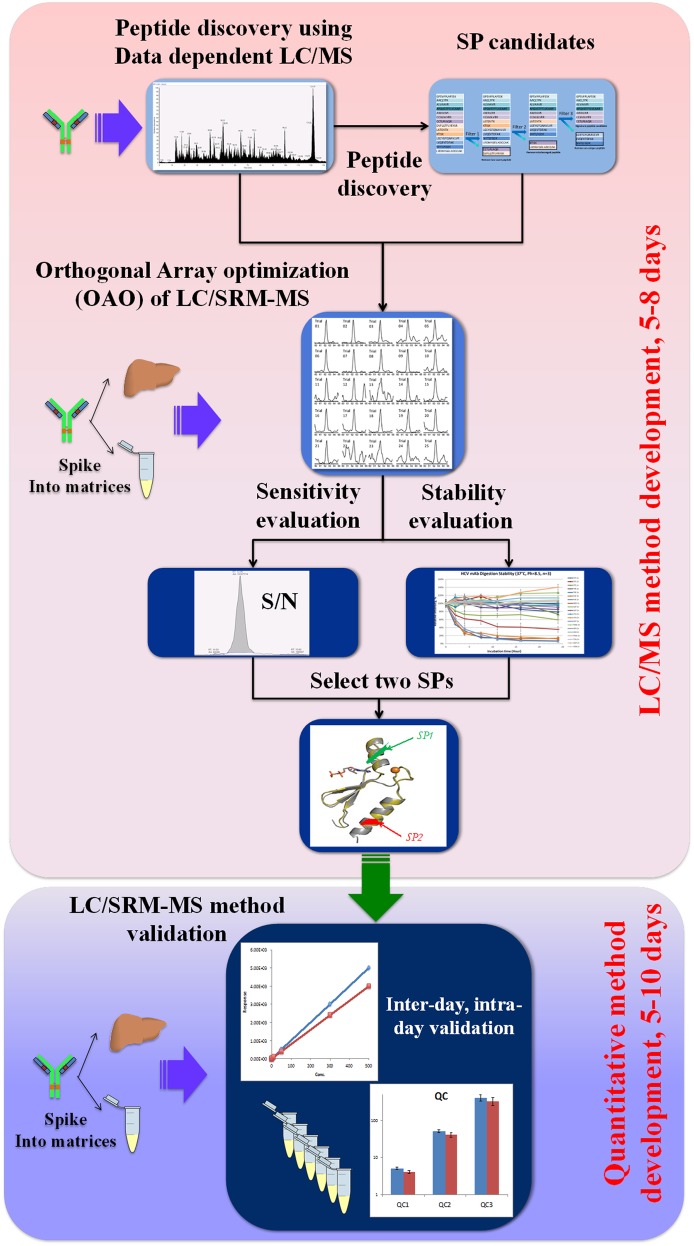
To address these issues, we devised a new pipeline to facilitate a high-throughput and accurate method development (shown in Fig. 1). Instead of using an in silico method to predict the best SP and optimal SRM conditions, we employed an experimental strategy to discover and optimize many SP candidates, and then evaluate these candidates in target matrices prior to SP selection (Cao et al., 2010; Duan et al., 2012a,b; Nouri-Nigjeh et al., 2014). Briefly, the pool of SP candidates was generated by a data-dependent LC/MS experiment following a stringent filtering step to remove peptides that are not unique to the target, containing labile amino acid (cysteine residues were not excluded as a number of studies showed cysteine-containing peptides may be used in a reliable quantification) (Keshishian et al., 2007; Picotti et al., 2009), known modification or miss cleavage. To evaluate these candidates, the target protein was spiked into the blank matrices (e.g., plasma or tissue extract) and then prepared and digested. The optimal LC/MS conditions of all SP candidates were accurately obtained by a high-throughput and on-the-fly orthogonal array optimization (OAO) procedure (Cao et al., 2010; Duan et al., 2012a,b), which has the capacity to develop the SRM conditions for >100 candidates within one single LC/MS run with high accuracy and reproducibility. Using the developed LC/MS conditions, all candidates were thoroughly assessed for stability and signal-to-noise ratios (S/N) in the matrix digest. Among the stable peptides, two peptides with the highest S/N were selected as the SPs. The use of two SP from different domains of the same protein provides a versatile gauge for the reliability of quantitative methods and results. Details can be found in the following references (Cao et al., 2010; Duan et al., 2012a,b).
Fig. 1.
Flowchart of novel LC-SRM-MS method development process based on orthogonal array optimization. The detailed procedure can be found in previous publications.
Recently, Furlong et al. (2012) described the use of a “universal surrogate peptide” derived from the constant Fc region of human antibody for quantification of human antibodies in nonhuman animal models. This method may greatly simplify and expedite the method development for the study of human antibodies in preclinical animal models.
Sample Preparation.
To achieve a sensitive and accurate quantification with LC/SRM-MS, it is critically important to achieve efficient and reproducible sample preparation, including effective sample cleanup, high and quantitative recovery of protein and efficient, and reproducible peptide recovery (Qu et al., 2006; Pan et al., 2009). So far, a universal and optimal preparation procedure for the quantification of biotherapeutics in pharmaceutic matrices has yet to be established, largely because the tissue and plasma samples are highly complex with numerous proteins and small-molecule compounds and the structure of a typical mAb renders it resistant to enzymatic digestion (Ouyang et al., 2012; Yuan et al., 2012). Moreover, it is challenging to prepare tissue samples for quantification of biotherapeutics, owing to the generally low drug concentrations in tissues and the lack of a quantitative and high-throughput protein extraction procedure that is compatible with LC/SRM-MS analysis (Duan et al., 2012a).
Recently, we developed a gel- and filter-free procedure that achieved effective protein denaturation and sample cleanup, and high, quantitative peptide yields from plasma and tissue samples (Duan et al., 2009, 2012a). Briefly, plasma or tissue samples were treated or extracted with high concentrations of detergent cocktail, which not only effectively solubilized proteins in the samples to ensure a high recovery but also completely denatured the proteins to achieve an efficient reduction, alkylation, and digestion (Duan et al., 2009; Tu et al., 2013); the mixture was then cleaned up by precipitation with cold organic solvents, which effectively removed the detergents and significantly reduced nonprotein matrix components such as lipids and fragmented or small-molecule nucleic acids that may negatively affect the robustness and consistency of LC/SRM-MS analysis (Qu et al., 2014). After precipitation, an on-pellet-digestion approach was employed without dissolving the protein pellet. This approach consists of two phases: under active agitation, the short phase-I digestion brings the pellets into solution by cleaving the pelleted proteins into soluble albeit large tryptic peptides; these incompletely cleaved peptides were then subjected to an overnight phase-II digestion for complete cleavage. Compared with other preparation methods used for LC/SRM-MS-based protein quantification, the detergent-aid precipitation/on-pellet-digestion provided higher digestion efficiency and much cleaner sample than in-solution digestion (Duan et al., 2009; Cao et al., 2010; Yuan et al., 2012) while affording higher and more reproducible peptide yields than in-gel digestion (Olsen et al., 2006) and filter-aided sample preparation (FASP) methods (Manza et al., 2005; Wisniewski et al., 2009). Thus, this procedure can be used for high-throughput quantification of mAb in plasma and tissues with excellent analytical sensitivity and robustness.
Recently, a number of techniques were developed to enable rapid digestion of proteins, such as digestion assisted by microwave (Lesur et al., 2010), ultrasound (Priego-Capote and de Castro, 2007), and infrared radiation (Wang et al., 2008a), and accelerated digestion with immobilized trypsin (Krenkova et al., 2009; Yamaguchi et al., 2009; Yuan et al., 2009). Some products using immobilized trypsin have been commercially available, such as Perfinity Flash Digest Kit (Perfinity Biosciences, West Lafayette , IN) for rapid and efficient digestion (Rivera-Burgos and Regnier, 2012). The performance of these newly emerged techniques for quantification of biotherapeutics remains to be extensively evaluated.
Sensitivity.
Although LC/SRM-MS is considered a highly sensitive technique, insufficient sensitivity is often a prominent concern for quantification of therapeutic mAb, largely for two reasons: 1) the signal response of LC/MS depends on the molar rather than mass amounts of the analyte, so the large molecular weights of mAb pose a considerable disadvantage; 2) owing to the very high protein contents in plasma or tissue samples (Tu et al., 2011), it is often necessary to dilute the samples to a large extent before analysis (Dams et al., 2003; Chambers et al., 2007; Cao et al., 2010; Duan et al., 2012b; Yuan et al., 2012).
To improve sensitivity for targeted protein analysis, we have developed a robust nano-flow LC/SRM-MS strategy (Cao et al., 2010; Duan et al., 2012a,b) that typically lowers the limit of quantification (LOQ) by ∼30- to 50-fold compared with a conventional-flow LC/SRM-MS. Another approach to improve sensitivity is to enrich target proteins or peptides before LC/SRM-MS analysis. For instance, Dubois et al. (2008) achieved a LOQ at 0.02 µg/ml for quantification of a chimeric mAb in human serum samples with an enrichment procedure; Lin et al. (2013) used immunoprecipitation enrichment before LC/SRM-MS analysis to achieve a LOQ of 10 ng/ml for mAb analysis.
A variety of other techniques have been developed to increase the sensitivity for LC/SRM-MS-based protein quantification, although these have yet been applied in quantification of biotherapeutics. To give several examples, the stable isotope standards and capture by antipeptide antibodies (SISCAPA) technique was developed to enrich signature peptides using polyclonal antibodies (Anderson et al., 2004). More recently, Neubert et al. developed a series of affinity-based methods for quantitative enrichment of target proteins and/or SPs in plasma, achieving ultrasensitive quantification of circulating biomarkers in plasma (Ocaña and Neubert, 2010; Neubert et al., 2013; Palandra et al., 2013). Furthermore, except to increase sensitivity, the affinity capture–based method can quantify specific targets, such as free or total mAb (Fernandez Ocana et al., 2012), which will be reviewed in the application section. Other approaches to improve the sensitivity of targeted quantification include strong cation exchange fractionation (Keshishian et al., 2009), high-pH fractionation before LC/MS analysis (Shi et al., 2012), and the use of long columns to obtain high S/N of target peptides (Shi et al., 2013).
Quantitative Accuracy.
The correct measurement of drug concentrations in plasma and tissues is essential for the research and development of biotherapeutics; consequently, highly accurate quantification methods are important (Wang et al., 2008b). SIL-IS is prevalently used for LC/SRM-MS quantification of therapeutic proteins, which greatly enhances the analytical reproducibility (Pan et al., 2009; Li et al., 2012; Nouri-Nigjeh et al., 2014). However, insufficient quantitative accuracy frequently represents a daunting problem. Most current works on LC/MS-based protein quantification employ synthesized peptides as the calibrator and SIL peptides as IS (spiked after digestion) (Bronsema et al., 2012; van den Broek et al., 2013). Such peptide-level calibration approaches enable straightforward development of quantitative methods, and both the calibrators and SIL-IS are readily available from commercial sources. However, the use of a SIL peptide IS only addresses variations/biases caused by LC/MS analysis but not the upstream steps such as sample preparation and digestion (van den Broek et al., 2013); furthermore, these approaches assume nearly 100% efficiency of the preparation and digestion procedures, which may not be true (Cao et al., 2010); for example, tryptic digestion is rarely complete and can be partially nonspecific (Picotti et al., 2007). Our recent investigations showed severe negative biases by the peptide calibration approaches (Duan et al., 2012b; Nouri-Nigjeh et al., 2014), and the quantification using two different SP from distinct domains of the same mAb resulted in profoundly discordant quantitative results.
To address problems related to digestion efficiency, the extended-peptide calibration approaches, which use synthesized extended-peptide containing the SP sequence and (typically) three to six flanking residues extended from both the N- and C- termini, were introduced. A SIL extended-peptide is used as the IS, which is spiked before digestion (Ocaña and Neubert, 2010; Rauh, 2012; Neubert et al., 2013). This approach may help to compensate for the bias and variation introduced in the digestion step (e.g., missed cleavage or peptide degradation; Ocaña and Neubert, 2010). Neubert et al. recently demonstrated that the extended-peptide calibration approach enabled accurate and sensitive quantification of a small protein biomarkers (e.g., nerve growth factors) in plasma (Neubert et al., 2013; Palandra et al., 2013). Our recent study showed that the extended-peptide calibration method still resulted in considerable negative bias when quantifying a much larger protein (mAb) (Nouri-Nigjeh et al., 2014).
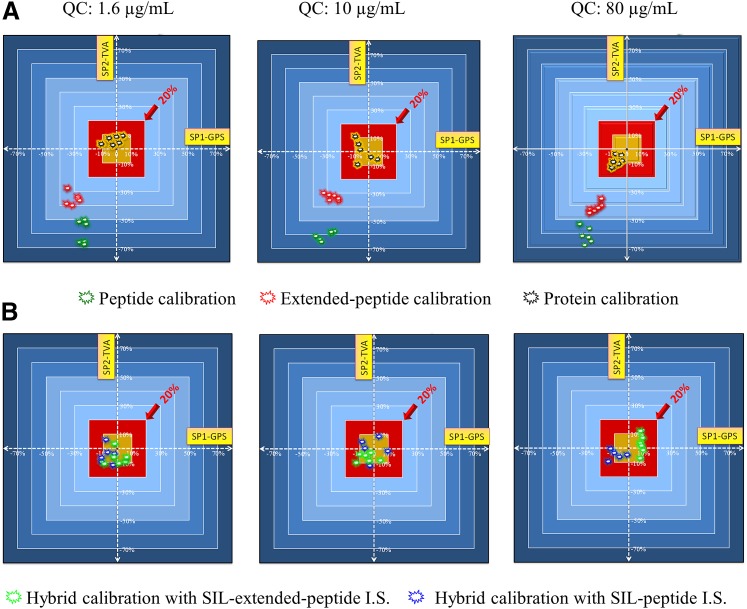
Protein-level calibration methods that employ full-length protein calibrator with SIL protein IS can correct errors and variations in all preparation and analytical steps, so they are considered the gold standard for accurate quantification (Heudi et al., 2008; Li et al., 2012). However, SIL proteins are costly to produce and may be impractical for many classes of proteins. Our laboratory and others demonstrated accurate quantification of regulatory proteins and protein drugs in plasma and tissues using “hybrid” calibration strategies (e.g., protein calibrator with SIL peptide or SIL extended peptide IS) (Cao et al., 2010; Duan et al., 2012a,b; Jiang et al., 2013), provided that reproducible sample preparation and digestion are achieved. Our recent study suggested the hybrid strategies may provide a cost-effective means for accurate quantification without the costly SIL protein (Nouri-Nigjeh et al., 2014). The quantitative biases by protein, extended-peptide, and peptide level calibrations and hybrid methods for the quantification of the same mAb in plasma are shown in Fig. 2.
Fig. 2.
Two-dimensional representations of the quantitative biases by (A) peptide, extended-peptide, and protein level calibration approaches and (B) the two “hybrid” calibration approaches (protein calibrator with SIL peptide/SIL extended-peptide IS). Quality control samples were prepared by spiking blank plasma with pure protein at three levels: 1.6, 10, and 80 µg/mL. The purities of all standards were accurately measured by quantitative amino acid analysis method to eliminate bias arising from possible inaccurate purity. Five aliquots of each quality control sample were individually prepared and analyzed in replicates by the five calibration approaches. Each sample was analyzed three times on each of two different days (day 1 and day 14, N = 6, shown as individual data points). For every calibration method, the quantitative values were obtained independently using the two signature peptides (SP): the GPS and TVA peptides. The two axes represent the quantitative biases by the two SPs. The red box in the center of each panel denotes the zone of <20% bias, and the golden box signifies the zone of <10% bias. Reprinted from (Nouri-Nigjeh et al.) Copyright 2014, American Chemical Society.
Applications of LC/MS in the Analysis of Biotherapeutics
Quantification of mAb in Plasma and Tissues.
LC/SRM-MS-based strategies have been widely applied to the quantification of mAb in plasma to support PK studies. Some representative works are exemplified here. Heudi et al. (2008) developed and validated an accurate quantitative method for a candidate mAb in marmoset serum by use of postdigestion Solid Phase Extraction (SPE) cleanup and SIL full-length-protein as IS. The method was applied to PK analysis in marmosets at a dosing level of 150 mg/kg. It was discovered that the concentrations by LC/SRM-MS were higher than those obtained via a parallel ELISA assay, which might reflect the fact that ELISA measures the free form of mAb whereas LC/SRM-MS measures the total mAb (Heudi et al., 2008). Li et al. (2012) developed a universal LC-SRM/MS approach for quantification of a variety of therapeutic mAb based on the use of a full-length SIL mAb as the common IS; such a method is valuable for preclinical studies.
Hagman et al. (2008) developed an LC/SRM-MS method to quantify a human mAb in the serum of cynomolgus monkeys. The study showed that the analytical sensitivity was significantly increased by an albumin-depletion procedure before LC/MS analysis. Ouyang et al. (2012) described the combination of on-pellet digestion with LC/SRM-MS for reproducible analysis of a mAb drug candidate in monkey plasma. Through an extensive comparison with other preparation methods, the investigators showed that on-pellet digestion was the optimal technique as it permitted a straightforward and efficient preparation of mAb.
Yang et al. (2007) established a quantification method for somatropin and a therapeutic human mAb in human and rat serum samples that employed a solid-phase extraction for cleanup and bovine fetuin as IS; the method was applied in a rat PK study at a dosage of 10 mg/kg. Lu et al. (2009) employed albumin depletion, protein A capture, and antibody capture coupled with LC/SRM-MS for sensitive quantification of a mAb candidate (CNTO736); the investigators concluded that all LC/SRM-MS-based methods evaluated in the study provided adequate sensitivity for PK study.
Fernandez Ocana et al. (2012) established a LC/MS strategy to quantify free and total anti-MadCAM mAb (PF-00547,659) in human serum, which captured free mAb with a biotinylated anti-idiotypic antibody followed by enrichment with streptavidin magnetic beads; the total target mAb was enriched by protein G magnetic beads. The strategy was successfully applied in a clinical PK study.
Our laboratory described a sensitive nano-LC/SRM-MS method for quantification of a chimeric mAb (cT84.66) in mouse serum (Duan et al., 2012b). Owing to the high sensitivity and selectivity achieved, the method was successfully applied to the preclinical PK study with a subcutaneous dosing at 1 mg/kg. The high sensitivity achieved in the work made it feasible for PK study at even lower dosages and/or over a longer period after administration.
Although the determination of the levels of biotherapeutics in tissues is critical for PK studies, such works have rarely been reported due to technical challenges such as the low drug concentrations and problems associated with tissue matrices, as discussed earlier. Using a sensitive nano-LC/SRM-MS, effective sample preparation, and a high-throughput method optimization strategy, we described sensitive quantification of two mAb (8c2 and cT84.66) in seven tissues with lower LOQ in the range of 0.156∼0.312 µg/g tissue (Duan et al., 2012a). The method was applied to the investigation of steady-state tissue distributions of 8c2 in various mouse models. Similar distribution characteristics were observed among the wild-type animals and those deficient in FcγRIIb and FcγRI/RIII; by comparison, the 8c2 tissue levels in the FcRn α-chain deficient group were significantly lower, as expected due to the absence of FcRn-mediated protection of antibody from catabolism. The work demonstrated that LC/SRM-MS is a promising alternative to radiolabeling strategies, which may fall short in problems related to assay accuracy and specificity, degradation of labeled protein, and radiation exposure to investigators and animals (Duan et al., 2012a).
Application in Cassette-Dosing.
As discussed previously, LC/SRM-MS is capable of simultaneous quantification of multiple targets, and therefore enabling cassette-dosing study of drug candidates. Though cassette-dosing has been prevalently used to screen small molecule candidates (White and Manitpisitkul, 2001; Smith et al., 2007), it was found to be even more suitable for preliminary investigation of mAb candidates (Li et al., 2013), because 1) studies of multiple mAb usually do not carry the risk of drug-drug interaction, and the PK of proteins is not affected by CYP450 and transporters (Zhou and Mascelli, 2011; Li et al., 2013), and 2) it is fairly straightforward to find an optimal, common formulation for multiple mAb (Dani et al., 2007; Spencer et al., 2012). Jiang et al. (2013) developed and validated an LC/SRM-MS method for simultaneous quantitation of two coadministrated mAb, which showed good sensitivity, reproducibility and accuracy for both targets. The method was successfully applied to toxicokinetic study in monkeys. Li et al. (2013) reported an analytical method to quantify four mAb after subcutaneous cassette-administration with LOQ of 0.1∼0.5 µg/ml in plasma.
Characterization of Intact mAb.
The recent technical advances and increasing availability of high-resolution MS instruments have resulted in rapid growth in using LC/MS to characterize intact mAb. Although most of these studies do not directly quantify antibodies, such works greatly facilitate assay development and/or PK/PD studies by affording detailed physicochemical information on these agents. In most cases, a top-down strategy employs a high-resolution analyzer to directly analyze intact proteins. Despite the top-down techniques generally being less sensitive than the bottom-up methods, they can provide overall information and accurate PTM mapping of the target protein (Peng et al., 2013).
One important paradigm is the characterization of the critical reagent for LBA (Geist et al., 2013b). Such studies can substantially improve the management of critical reagents and contribute to the development of a robust LBA method by providing essential information such as Fab/Fc sequence, enzymatically produced PTM, and charge state. Details on the use of LC/MS approaches for troubleshooting LBA methods and critical reagent quality control can be found in a previous publication (Geist et al., 2013b).
Another prominent utility of top-down technique is the quality control of mAb products. Thompson et al. (2014) performed a top-down profiling of glycosylation on mAb. Wang et al. (2005) established a series of top-down and bottom-up electrospray ionization time-of-flight MS (ESI-TOF-MS) methods to comprehensively investigate N-terminal pyroglutamate formation, cleavage of C-terminal lysine, glucosylation, and deamidation of a recombinant mAb. Dillon et al. (2006) developed a LC/MS method with a TOF analyzer to obtain accurate mass and unique terminal ladder sequences of a recombinant antibody, which provided the glycosylation profile and heterogeneity information of the molecule. In another study, a LC-ESI-TOF method was developed to determine glycosylation pattern of a therapeutic mAb (siltuximab) after immunoaffinity purification (Geist et al., 2013a). The work quantified therapeutic mAb produced in two different host cell lines. Xie et al. (2010) developed a LC/TOF-MS method for comprehensive comparison between innovator mAb versus a biosimilar, in terms of intact protein mass, sequence, and peptide mapping, which provided valuable information for assessment of biosimilars.
Characterization of Antibody-Drug Conjugates (ADC).
Antibody-drug conjugates (ADC), which use mAb for targeted delivery of highly potent small-molecule drugs, constitute a new class of biotherapeutics for effective targeted therapy (Doronina et al., 2003). Drug-to-antibody ratios (DAR) and drug-load distribution are critical parameters that profoundly determine the in vivo efficacy and toxicity of an ADC (Ducry, 2013; Kaur et al., 2013). For example, even a small decrease in DAR (e.g., loss of conjugated drug in circulation) will lead to a significant change of exposure at the targeted site (Xu et al., 2011); thus, determination of DAR is essential for ADC development and PK/PD studies.
LC/MS with a high-resolution analyzer has become a promising technology for characterization of ADC in recent years. An affinity capture capillary LC coupled to quadrupole-TOF MS has been employed to analyze anti-MUC16 THIOMAB–drug conjugate (TDC), which obtained both in vitro and in vivo DAR information (Xu et al., 2011). Valliere-Douglass et al. (2012) developed a native LC/MS method for the determination of DAR via analysis of intact protein.
Recently, a more sensitive method was reported by Chen et al. (2013), which employed a native nano-ESI-TOF analysis in conjunction with limited digestion by cysteine protease to obtain DAR information. This method also demonstrated substantially higher sensitivity than the traditional hydrophobic interaction chromatography.
Conclusion and Future Perspectives
LC/MS represents a promising alternative to traditional LBA methods for the analysis of biotherapeutics because 1) it can be readily adapted to quantification in plasma and tissues and across various species; 2) it provides extraordinary specificity and high reproducibility with low sample consumption and low interlaboratory/batch variance; and 3) it is capable of simultaneous quantification of multiple proteins (e.g., biotherapeutics and/or their targets) in one analysis. Furthermore, the development and validation of a LC/MS method is rapid at a relatively low cost, which is a highly desirable feature that facilitates the rapidly growing developments of biotherapeutics. Nonetheless, LC/MS methods still face challenges associated with sensitivity, sample preparation, method development, and quantitative accuracy. Recent technical advances helped to overcome these problems; to name a few, the use of target-specific enrichment and low-flow-LC/MS have substantially improved analytical sensitivity; robust and efficient preparation methods are emerging; and hybrid calibration methods have been demonstrated to provide high quantitative accuracy without using expensive SIL protein IS.
In spite of the drastically increasing role of LC/MS in biotherapetuics analysis, most likely it will not fully replace traditional methods such as LBA in the foreseeable future. First, in the event that a well-developed LBA method is available for the target molecule, LBA may be advantageous over LC/MS because it may be more sensitive (de Dios et al., 2013) and does not require expensive LC/MS instruments; moreover, LBA can be developed to quantify free or total mAb whereas LC/MS only detects the total mAb unless specific affinity capture enrichment is employed (Lee et al., 2011). Second, the combination of LC/MS and traditional biochemical strategies provides a powerful tool to acquire critical information on biotherapeutics in highly complex pharmaceutic and biologic systems. For instance, immunocapture and immunodepletion have been demonstrated to greatly enhance the quantitative sensitivity of LC/MS; size-exclusion, immunoprecipitation, or native gel separation before LC/MS analysis will provide important information on the target protein, such as binding, aggregation, and degradation states.
In a broad sense, antibody capture of a target molecule from a biologic matrix followed by LC/MS analysis may be considered a new form of LBA method, with LC/MS as the means of detection. As LC/MS affords much higher selectivity and sensitivity than the spectrophotometers conventionally used for LBA, it will greatly facilitate and expedite methods development; for example, development of critical reagents may be markedly faster because the requirements for selectivity and sensitivity of the critical reagents are far less stringent when LC/MS is used as the downstream detection approach.
In summary, LC/MS is a versatile and powerful tool for analysis of biotherapeutics. It can be used either alone or as the detector downstream of various biochemical procedures. We expect its application in the research and development of biotherapeutics to continue to expand rapidly in the future.
Abbreviations
- ADC
antibody-drug conjugates
- DAR
drug-to-antibody ratio
- ELISA
enzyme-linked immunosorbent assay
- ESI
electrospray ionization
- IS
internal standards
- LBA
ligand-binding assay
- LC
liquid chromatograph
- LOQ
limit of quantification
- mAb
monoclonal antibodies
- MS
mass spectrometry
- PD
pharmacodynamic
- PK
pharmacokinetics
- PTM
posttranslational modifications
- SIL
stable isotope labeled
- S/N
signal-to-noise ratios
- SP
signature peptide
- SRM
selected-reaction monitoring
- TOF
time-of-flight
Authorship Contributions
Wrote or contributed to the writing:` An, Zhang, Qu.
Footnotes
This work was supported, in part, by National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grants U54 HD071594, HD075363], National Institute on Drug Abuse [Grant DA027528], National Institute of Allergy and Infectious Diseases [Grant AI060260], and National Heart, Lung, and Blood Institute [Grant HL103411], by the Center for Protein Therapeutics, and by American Heart Association (AHA) award [12SDG9450036] (all to J.Q.).
References
- Anderson NL, Anderson NG, Haines LR, Hardie DB, Olafson RW, Pearson TW. (2004) Mass spectrometric quantitation of peptides and proteins using Stable Isotope Standards and Capture by Anti-Peptide Antibodies (SISCAPA). J Proteome Res 3:235–244 [DOI] [PubMed] [Google Scholar]
- Bayliss MK, Frick LW. (1999) High-throughput pharmacokinetics: cassette dosing. Curr Opin Drug Discov Devel 2:20–25 [PubMed] [Google Scholar]
- Blackburn M. (2013) Advances in the quantitation of therapeutic insulin analogues by LC-MS/MS. Bioanalysis 5:2933–2946 [DOI] [PubMed] [Google Scholar]
- Bronsema KJ, Bischoff R, van de Merbel NC. (2012) Internal standards in the quantitative determination of protein biopharmaceuticals using liquid chromatography coupled to mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 893–894:1–14 [DOI] [PubMed] [Google Scholar]
- Cao J, Gonzalez-Covarrubias V, Straubinger RM, Wang H, Duan X, Yu H, Qu J, Blanco JG. (2010) A rapid, reproducible, on-the-fly orthogonal array optimization method for targeted protein quantification by LC/MS and its application for accurate and sensitive quantification of carbonyl reductases in human liver. Anal Chem 82:2680–2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cham Mead JA, Bianco L, Bessant C. (2010) Free computational resources for designing selected reaction monitoring transitions. Proteomics 10:1106–1126 [DOI] [PubMed] [Google Scholar]
- Chambers E, Wagrowski-Diehl DM, Lu Z, Mazzeo JR. (2007) Systematic and comprehensive strategy for reducing matrix effects in LC/MS/MS analyses. J Chromatogr B Analyt Technol Biomed Life Sci 852:22–34 [DOI] [PubMed] [Google Scholar]
- Chambers EE, Fountain KJ, Smith N, Ashraf L, Karalliedde J, Cowan D, Legido-Quigley C. (2014) Multidimensional LC-MS/MS enables simultaneous quantification of intact human insulin and five recombinant analogs in human plasma. Anal Chem 86:694–702 [DOI] [PubMed] [Google Scholar]
- Chen J, Yin S, Wu Y, Ouyang J. (2013) Development of a native nanoelectrospray mass spectrometry method for determination of the drug-to-antibody ratio of antibody-drug conjugates. Anal Chem 85:1699–1704 [DOI] [PubMed] [Google Scholar]
- Damen CW, Schellens JH, Beijnen JH. (2009) Bioanalytical methods for the quantification of therapeutic monoclonal antibodies and their application in clinical pharmacokinetic studies. Hum Antibodies 18:47–73 [DOI] [PubMed] [Google Scholar]
- Dams R, Huestis MA, Lambert WE, Murphy CM. (2003) Matrix effect in bio-analysis of illicit drugs with LC-MS/MS: influence of ionization type, sample preparation, and biofluid. J Am Soc Mass Spectrom 14:1290–1294 [DOI] [PubMed] [Google Scholar]
- Dani B, Platz R, Tzannis ST. (2007) High concentration formulation feasibility of human immunoglubulin G for subcutaneous administration. J Pharm Sci 96:1504–1517 [DOI] [PubMed] [Google Scholar]
- de Dios K, Manibusan A, Marsden R, Pinkstaff J. (2013) Comparison of bioanalytical methods for the quantitation of PEGylated human insulin. J Immunol Methods 396:1–7 [DOI] [PubMed] [Google Scholar]
- Deng R, Iyer S, Theil FP, Mortensen DL, Fielder PJ, Prabhu S. (2011) Projecting human pharmacokinetics of therapeutic antibodies from nonclinical data: what have we learned? MAbs 3:61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon TM, Bondarenko PV, Rehder DS, Pipes GD, Kleemann GR, Ricci MS. (2006) Optimization of a reversed-phase high-performance liquid chromatography/mass spectrometry method for characterizing recombinant antibody heterogeneity and stability. J Chromatogr A 1120:112–120 [DOI] [PubMed] [Google Scholar]
- Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, DeBlanc RL, Gearing RP, Bovee TD, Siegall CB, et al. (2003) Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol 21:778–784 [DOI] [PubMed] [Google Scholar]
- Duan X, Abuqayyas L, Dai L, Balthasar JP, Qu J. (2012a) High-throughput method development for sensitive, accurate, and reproducible quantification of therapeutic monoclonal antibodies in tissues using orthogonal array optimization and nano liquid chromatography/selected reaction monitoring mass spectrometry. Anal Chem 84:4373–4382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Dai L, Chen SC, Balthasar JP, Qu J. (2012b) Nano-scale liquid chromatography/mass spectrometry and on-the-fly orthogonal array optimization for quantification of therapeutic monoclonal antibodies and the application in preclinical analysis. J Chromatogr A 1251:63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Young R, Straubinger RM, Page B, Cao J, Wang H, Yu H, Canty JM, Qu J. (2009) A straightforward and highly efficient precipitation/on-pellet digestion procedure coupled with a long gradient nano-LC separation and Orbitrap mass spectrometry for label-free expression profiling of the swine heart mitochondrial proteome. J Proteome Res 8:2838–2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M, Fenaille F, Clement G, Lechmann M, Tabet JC, Ezan E, Becher F. (2008) Immunopurification and mass spectrometric quantification of the active form of a chimeric therapeutic antibody in human serum. Anal Chem 80:1737–1745 [DOI] [PubMed] [Google Scholar]
- Ducry L. (2013) Antibody-Drug Conjugates. Humana Press, New York [Google Scholar]
- Ezan E, Bitsch F. (2009) Critical comparison of MS and immunoassays for the bioanalysis of therapeutic antibodies. Bioanalysis 1:1375–1388 [DOI] [PubMed] [Google Scholar]
- Ezan E, Dubois M, Becher F. (2009) Bioanalysis of recombinant proteins and antibodies by mass spectrometry. Analyst (Lond) 134:825–834 [DOI] [PubMed] [Google Scholar]
- Fernández Ocaña M, James IT, Kabir M, Grace C, Yuan G, Martin SW, Neubert H. (2012) Clinical pharmacokinetic assessment of an anti-MAdCAM monoclonal antibody therapeutic by LC-MS/MS. Anal Chem 84:5959–5967 [DOI] [PubMed] [Google Scholar]
- Furlong MT, Ouyang Z, Wu S, Tamura J, Olah T, Tymiak A, Jemal M. (2012) A universal surrogate peptide to enable LC-MS/MS bioanalysis of a diversity of human monoclonal antibody and human Fc-fusion protein drug candidates in pre-clinical animal studies. Biomed Chromatogr 26:1024–1032 [DOI] [PubMed] [Google Scholar]
- Garg A, Balthasar JP. (2009) Investigation of the influence of FcRn on the distribution of IgG to the brain. AAPS J 11:553–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geist BJ, Davis D, McIntosh T, Yang TY, Goldberg K, Han C, Pendley C, Davis HM. (2013a) A novel approach for the simultaneous quantification of a therapeutic monoclonal antibody in serum produced from two distinct host cell lines. MAbs 5:150–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geist BJ, Egan AC, Yang TY, Dong Y, Shankar G. (2013b) Characterization of critical reagents in ligand-binding assays: enabling robust bioanalytical methods and lifecycle management. Bioanalysis 5:227–244 [DOI] [PubMed] [Google Scholar]
- Hagman C, Ricke D, Ewert S, Bek S, Falchetto R, Bitsch F. (2008) Absolute quantification of monoclonal antibodies in biofluids by liquid chromatography-tandem mass spectrometry. Anal Chem 80:1290–1296 [DOI] [PubMed] [Google Scholar]
- Halquist MS, Thomas Karnes H. (2011) Quantitative liquid chromatography tandem mass spectrometry analysis of macromolecules using signature peptides in biological fluids. Biomed Chromatogr 25:47–58 [DOI] [PubMed] [Google Scholar]
- Heudi O, Barteau S, Zimmer D, Schmidt J, Bill K, Lehmann N, Bauer C, Kretz O. (2008) Towards absolute quantification of therapeutic monoclonal antibody in serum by LC-MS/MS using isotope-labeled antibody standard and protein cleavage isotope dilution mass spectrometry. Anal Chem 80:4200–4207 [DOI] [PubMed] [Google Scholar]
- Hoofnagle AN, Wener MH. (2009) The fundamental flaws of immunoassays and potential solutions using tandem mass spectrometry. J Immunol Methods 347:3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfgartner G, Lesur A, Varesio E. (2013) Analysis of biopharmaceutical proteins in biological matrices by LC-MS/MS II. LC-MS/MS analysis. Trends Analyt Chem 48:52–61 DOI: 10.1016/j.trac.2013.03.008. [Google Scholar]
- Huang L, Lu J, Wroblewski VJ, Beals JM, Riggin RM. (2005) In vivo deamidation characterization of monoclonal antibody by LC/MS/MS. Anal Chem 77:1432–1439 [DOI] [PubMed] [Google Scholar]
- Jemal M, Xia YQ. (2006) LC-MS Development strategies for quantitative bioanalysis. Curr Drug Metab 7:491–502 [DOI] [PubMed] [Google Scholar]
- Jiang H, Zeng J, Titsch C, Voronin K, Akinsanya B, Luo L, Shen H, Desai DD, Allentoff A, Aubry AF, et al. (2013) Fully validated LC-MS/MS assay for the simultaneous quantitation of coadministered therapeutic antibodies in cynomolgus monkey serum. Anal Chem 85:9859–9867 [DOI] [PubMed] [Google Scholar]
- Kaur S, Xu K, Saad OM, Dere RC, Carrasco-Triguero M. (2013) Bioanalytical assay strategies for the development of antibody-drug conjugate biotherapeutics. Bioanalysis 5:201–226 [DOI] [PubMed] [Google Scholar]
- Kawakami H, Ohtsuki S, Kamiie J, Suzuki T, Abe T, Terasaki T. (2011) Simultaneous absolute quantification of 11 cytochrome P450 isoforms in human liver microsomes by liquid chromatography tandem mass spectrometry with in silico target peptide selection. J Pharm Sci 100:341–352 [DOI] [PubMed] [Google Scholar]
- Keshishian H, Addona T, Burgess M, Kuhn E, Carr SA. (2007) Quantitative, multiplexed assays for low abundance proteins in plasma by targeted mass spectrometry and stable isotope dilution. Mol Cell Proteomics 6:2212–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshishian H, Addona T, Burgess M, Mani DR, Shi X, Kuhn E, Sabatine MS, Gerszten RE, Carr SA. (2009) Quantification of cardiovascular biomarkers in patient plasma by targeted mass spectrometry and stable isotope dilution. Mol Cell Proteomics 8:2339–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippen AD, Cerini F, Vadas L, Stöcklin R, Vu L, Offord RE, Rose K. (1997) Development of an isotope dilution assay for precise determination of insulin, C-peptide, and proinsulin levels in non-diabetic and type II diabetic individuals with comparison to immunoassay. J Biol Chem 272:12513–12522 [DOI] [PubMed] [Google Scholar]
- Korfmacher WA, Cox KA, Ng KJ, Veals J, Hsieh Y, Wainhaus S, Broske L, Prelusky D, Nomeir A, White RE. (2001) Cassette-accelerated rapid rat screen: a systematic procedure for the dosing and liquid chromatography/atmospheric pressure ionization tandem mass spectrometric analysis of new chemical entities as part of new drug discovery. Rapid Commun Mass Spectrom 15:335–340 [DOI] [PubMed] [Google Scholar]
- Krenkova J, Lacher NA, Svec F. (2009) Highly efficient enzyme reactors containing trypsin and endoproteinase LysC immobilized on porous polymer monolith coupled to MS suitable for analysis of antibodies. Anal Chem 81:2004–2012 [DOI] [PubMed] [Google Scholar]
- Lange V, Picotti P, Domon B, Aebersold R. (2008) Selected reaction monitoring for quantitative proteomics: a tutorial. Mol Syst Biol 4:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Kelley M. (2011) Quality assessment of bioanalytical quantification of monoclonal antibody drugs. Ther Deliv 2:383–396 [DOI] [PubMed] [Google Scholar]
- Lee JW, Kelley M, King LE, Yang J, Salimi-Moosavi H, Tang MT, Lu JF, Kamerud J, Ahene A, Myler H, et al. (2011) Bioanalytical approaches to quantify “total” and “free” therapeutic antibodies and their targets: technical challenges and PK/PD applications over the course of drug development. AAPS J 13:99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesur A, Varesio E, Hopfgartner G. (2010) Accelerated tryptic digestion for the analysis of biopharmaceutical monoclonal antibodies in plasma by liquid chromatography with tandem mass spectrometric detection. J Chromatogr A 1217:57–64 [DOI] [PubMed] [Google Scholar]
- Li H, Ortiz R, Tran L, Hall M, Spahr C, Walker K, Laudemann J, Miller S, Salimi-Moosavi H, Lee JW. (2012) General LC-MS/MS method approach to quantify therapeutic monoclonal antibodies using a common whole antibody internal standard with application to preclinical studies. Anal Chem 84:1267–1273 [DOI] [PubMed] [Google Scholar]
- Li H, Ortiz R, Tran LT, Salimi-Moosavi H, Malella J, James CA, Lee JW. (2013) Simultaneous analysis of multiple monoclonal antibody biotherapeutics by LC-MS/MS method in rat plasma following cassette-dosing. AAPS J 15:337–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Palandra J, Nemirovskiy OV, Lai Y. (2009) LC-MS/MS mediated absolute quantification and comparison of bile salt export pump and breast cancer resistance protein in livers and hepatocytes across species. Anal Chem 81:2251–2259 [DOI] [PubMed] [Google Scholar]
- Liebler DC, Zimmerman LJ. (2013) Targeted quantitation of proteins by mass spectrometry. Biochemistry 52:3797–3806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Alborn WE, Slebos RJ, Liebler DC. (2013) Comparison of protein immunoprecipitation-multiple reaction monitoring with ELISA for assay of biomarker candidates in plasma. J Proteome Res 12:5996–6003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YS, Nguyen C, Mendoza JL, Escandon E, Fei D, Meng YG, Modi NB. (1999) Preclinical pharmacokinetics, interspecies scaling, and tissue distribution of a humanized monoclonal antibody against vascular endothelial growth factor. J Pharmacol Exp Ther 288:371–378 [PubMed] [Google Scholar]
- Liu B, Chang J, Gordon WP, Isbell J, Zhou Y, Tuntland T. (2008) Snapshot PK: a rapid rodent in vivo preclinical screening approach. Drug Discov Today 13:360–367 [DOI] [PubMed] [Google Scholar]
- Liu G, Ji QC, Dodge R, Sun H, Shuster D, Zhao Q, Arnold M. (2013) Liquid chromatography coupled with tandem mass spectrometry for the bioanalysis of proteins in drug development: practical considerations in assay development and validation. J Chromatogr A 1284:155–162 [DOI] [PubMed] [Google Scholar]
- Lu Q, Zheng X, McIntosh T, Davis H, Nemeth JF, Pendley C, Wu SL, Hancock WS. (2009) Development of different analysis platforms with LC-MS for pharmacokinetic studies of protein drugs. Anal Chem 81:8715–8723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manza LL, Stamer SL, Ham AJ, Codreanu SG, Liebler DC. (2005) Sample preparation and digestion for proteomic analyses using spin filters. Proteomics 5:1742–1745 [DOI] [PubMed] [Google Scholar]
- Neubert H, Muirhead D, Kabir M, Grace C, Cleton A, Arends R. (2013) Sequential protein and peptide immunoaffinity capture for mass spectrometry-based quantification of total human β-nerve growth factor. Anal Chem 85:1719–1726 [DOI] [PubMed] [Google Scholar]
- Nouri-Nigjeh E, Zhang M, Ji T, Yu H, An B, Duan X, Balthasar J, Johnson RW, Qu J. (2014) Effects of calibration approaches on the accuracy for LC-MS targeted quantification of therapeutic protein. Anal Chem 86:3575–3584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowatzke WL, Rogers K, Wells E, Bowsher RR, Ray C, Unger S. (2011) Unique challenges of providing bioanalytical support for biological therapeutic pharmacokinetic programs. Bioanalysis 3:509–521 [DOI] [PubMed] [Google Scholar]
- O’Hara DM, Theobald V, Egan AC, Usansky J, Krishna M, TerWee J, Maia M, Spriggs FP, Kenney J, Safavi A, et al. (2012) Ligand binding assays in the 21st century laboratory: recommendations for characterization and supply of critical reagents. AAPS J 14:316–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocaña MF, Neubert H. (2010) An immunoaffinity liquid chromatography-tandem mass spectrometry assay for the quantitation of matrix metalloproteinase 9 in mouse serum. Anal Biochem 399:202–210 [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Schaefer O, Kawakami H, Inoue T, Liehner S, Saito A, Ishiguro N, Kishimoto W, Ludwig-Schwellinger E, Ebner T, et al. (2012) Simultaneous absolute protein quantification of transporters, cytochromes P450, and UDP-glucuronosyltransferases as a novel approach for the characterization of individual human liver: comparison with mRNA levels and activities. Drug Metab Dispos 40:83–92 [DOI] [PubMed] [Google Scholar]
- Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. (2006) Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127:635–648 [DOI] [PubMed] [Google Scholar]
- Ouyang Z, Furlong MT, Wu S, Sleczka B, Tamura J, Wang H, Suchard S, Suri A, Olah T, Tymiak A, et al. (2012) Pellet digestion: a simple and efficient sample preparation technique for LC-MS/MS quantification of large therapeutic proteins in plasma. Bioanalysis 4:17–28 [DOI] [PubMed] [Google Scholar]
- Palandra J, Finelli A, Zhu M, Masferrer J, Neubert H. (2013) Highly specific and sensitive measurements of human and monkey interleukin 21 using sequential protein and tryptic peptide immunoaffinity LC-MS/MS. Anal Chem 85:5522–5529 [DOI] [PubMed] [Google Scholar]
- Pan S, Aebersold R, Chen R, Rush J, Goodlett DR, McIntosh MW, Zhang J, Brentnall TA. (2009) Mass spectrometry based targeted protein quantification: methods and applications. J Proteome Res 8:787–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya K, Ray CA, Brunner L, Wang J, Lee JW, DeSilva B. (2010) Strategies to minimize variability and bias associated with manual pipetting in ligand binding assays to assure data quality of protein therapeutic quantification. J Pharm Biomed Anal 53:623–630 [DOI] [PubMed] [Google Scholar]
- Pendley C, Shankar G. (2011) Bioanalytical interferences in immunoassays for antibody biotherapeutics. Bioanalysis 3:703–706 [DOI] [PubMed] [Google Scholar]
- Peng Y, Chen X, Zhang H, Xu Q, Hacker TA, Ge Y. (2013) Top-down targeted proteomics for deep sequencing of tropomyosin isoforms. J Proteome Res 12:187–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy AJ, Chambers AG, Yang J, Hardie DB, Borchers CH. (2014) Advances in multiplexed MRM-based protein biomarker quantitation toward clinical utility. Biochim Biophys Acta 1844:917–926 [DOI] [PubMed] [Google Scholar]
- Picotti P, Aebersold R, Domon B. (2007) The implications of proteolytic background for shotgun proteomics. Mol Cell Proteomics 6:1589–1598 [DOI] [PubMed] [Google Scholar]
- Picotti P, Bodenmiller B, Mueller LN, Domon B, Aebersold R. (2009) Full dynamic range proteome analysis of S. cerevisiae by targeted proteomics. Cell 138:795–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priego-Capote F, de Castro L. (2007) Ultrasound-assisted digestion: a useful alternative in sample preparation. J Biochem Biophys Methods 70:299–310 [DOI] [PubMed] [Google Scholar]
- Qu J, Jusko WJ, Straubinger RM. (2006) Utility of cleavable isotope-coded affinity-tagged reagents for quantification of low-copy proteins induced by methylprednisolone using liquid chromatography/tandem mass spectrometry. Anal Chem 78:4543–4552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J, Straubinger RM. (2005) Improved sensitivity for quantification of proteins using triply charged cleavable isotope-coded affinity tag peptides. Rapid Commun Mass Spectrom 19:2857–2864 [DOI] [PubMed] [Google Scholar]
- Qu J, Young R, Page BJ, Shen X, Tata N, Li J, Duan X, Fallavollita JA, Canty JM, Jr. (2014) Reproducible ion-current-based approach for 24-plex comparison of the tissue proteomes of hibernating versus normal myocardium in swine models. J Proteome Res 13:2571–2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh M. (2012) LC-MS/MS for protein and peptide quantification in clinical chemistry. J Chromatogr B Analyt Technol Biomed Life Sci 883-884:59–67 [DOI] [PubMed] [Google Scholar]
- Rivera-Burgos D, Regnier FE. (2012) Native protein proteolysis in an immobilized enzyme reactor as a function of temperature. Anal Chem 84:7021–7028 [DOI] [PubMed] [Google Scholar]
- Sakamoto A, Matsumaru T, Ishiguro N, Schaefer O, Ohtsuki S, Inoue T, Kawakami H, Terasaki T. (2011) Reliability and robustness of simultaneous absolute quantification of drug transporters, cytochrome P450 enzymes, and Udp-glucuronosyltransferases in human liver tissue by multiplexed MRM/selected reaction monitoring mode tandem mass spectrometry with nano-liquid chromatography. J Pharm Sci 100:4037–4043 [DOI] [PubMed] [Google Scholar]
- Savoie N, Garofolo F, van Amsterdam P, Bansal S, Beaver C, Bedford P, Booth BP, Evans C, Jemal M, Lefebvre M, et al. (2010) 2010 white paper on recent issues in regulated bioanalysis & global harmonization of bioanalytical guidance. Bioanalysis 2:1945–1960 [DOI] [PubMed] [Google Scholar]
- Shah DK, Balthasar JP. (2014) PK/TD modeling for prediction of the effects of 8C2, an anti-topotecan mAb, on topotecan-induced toxicity in mice. Int J Pharm 465:228–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah DK, Betts AM. (2013) Antibody biodistribution coefficients: inferring tissue concentrations of monoclonal antibodies based on the plasma concentrations in several preclinical species and human. MAbs 5:297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T, Fillmore TL, Gao Y, Zhao R, He J, Schepmoes AA, Nicora CD, Wu C, Chambers JL, Moore RJ, et al. (2013) Long-gradient separations coupled with selected reaction monitoring for highly sensitive, large scale targeted protein quantification in a single analysis. Anal Chem 85:9196–9203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T, Fillmore TL, Sun X, Zhao R, Schepmoes AA, Hossain M, Xie F, Wu S, Kim JS, Jones N, et al. (2012) Antibody-free, targeted mass-spectrometric approach for quantification of proteins at low picogram per milliliter levels in human plasma/serum. Proc Natl Acad Sci USA 109:15395–15400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NF, Raynaud FI, Workman P. (2007) The application of cassette dosing for pharmacokinetic screening in small-molecule cancer drug discovery. Mol Cancer Ther 6:428–440 [DOI] [PubMed] [Google Scholar]
- Spencer S, Bethea D, Raju TS, Giles-Komar J, Feng Y. (2012) Solubility evaluation of murine hybridoma antibodies. MAbs 4:319–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergachis AB, MacLean B, Lee K, Stamatoyannopoulos JA, MacCoss MJ. (2011) Rapid empirical discovery of optimal peptides for targeted proteomics. Nat Methods 8:1041–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson NJ, Rosati S, Heck AJ. (2014) Performing native mass spectrometry analysis on therapeutic antibodies. Methods 65:11–17 [DOI] [PubMed] [Google Scholar]
- Trufelli H, Palma P, Famiglini G, Cappiello A. (2011) An overview of matrix effects in liquid chromatography-mass spectrometry. Mass Spectrom Rev 30:491–509 [DOI] [PubMed] [Google Scholar]
- Tu C, Li J, Jiang X, Sheflin LG, Pfeffer BA, Behringer M, Fliesler SJ, Qu J. (2013) Ion-current-based proteomic profiling of the retina in a rat model of Smith-Lemli-Opitz syndrome. Mol Cell Proteomics 12:3583–3598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C, Li J, Young R, Page BJ, Engler F, Halfon MS, Canty JM, Jr, Qu J. (2011) Combinatorial peptide ligand library treatment followed by a dual-enzyme, dual-activation approach on a nanoflow liquid chromatography/orbitrap/electron transfer dissociation system for comprehensive analysis of swine plasma proteome. Anal Chem 83:4802–4813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urva SR, Yang VC, Balthasar JP. (2010) An ELISA for quantification of T84.66, a monoclonal anti-CEA antibody, in mouse plasma. J Immunoassay Immunochem 31:1–9 [DOI] [PubMed] [Google Scholar]
- Valliere-Douglass JF, McFee WA, Salas-Solano O. (2012) Native intact mass determination of antibodies conjugated with monomethyl Auristatin E and F at interchain cysteine residues. Anal Chem 84:2843–2849 [DOI] [PubMed] [Google Scholar]
- van den Broek I, Niessen WM, van Dongen WD. (2013) Bioanalytical LC-MS/MS of protein-based biopharmaceuticals. J Chromatogr B Analyt Technol Biomed Life Sci 929:161–179 [DOI] [PubMed] [Google Scholar]
- Wang L, Amphlett G, Lambert JM, Blättler W, Zhang W. (2005) Structural characterization of a recombinant monoclonal antibody by electrospray time-of-flight mass spectrometry. Pharm Res 22:1338–1349 [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang L, Yang P, Chen G. (2008a) Infrared-assisted tryptic proteolysis for peptide mapping. Proteomics 8:2579–2582 [DOI] [PubMed] [Google Scholar]
- Wang W, Wang EQ, Balthasar JP. (2008b) Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 84:548–558 [DOI] [PubMed] [Google Scholar]
- White RE, Manitpisitkul P. (2001) Pharmacokinetic theory of cassette dosing in drug discovery screening. Drug Metab Dispos 29:957–966 [PubMed] [Google Scholar]
- Wiśniewski JR, Zougman A, Nagaraj N, Mann M. (2009) Universal sample preparation method for proteome analysis. Nat Methods 6:359–362 [DOI] [PubMed] [Google Scholar]
- Xiao Y, Guo L, Wang Y. (2014) A targeted quantitative proteomics strategy for global kinome profiling of cancer cells and tissues. Mol Cell Proteomics 13:1065–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Chakraborty A, Ahn J, Yu YQ, Dakshinamoorthy DP, Gilar M, Chen W, Skilton SJ, Mazzeo JR. (2010) Rapid comparison of a candidate biosimilar to an innovator monoclonal antibody with advanced liquid chromatography and mass spectrometry technologies. MAbs 2:379–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Liu L, Saad OM, Baudys J, Williams L, Leipold D, Shen B, Raab H, Junutula JR, Kim A, et al. (2011) Characterization of intact antibody-drug conjugates from plasma/serum in vivo by affinity capture capillary liquid chromatography-mass spectrometry. Anal Biochem 412:56–66 [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Miyazaki M, Honda T, Briones-Nagata MP, Arima K, Maeda H. (2009) Rapid and efficient proteolysis for proteomic analysis by protease-immobilized microreactor. Electrophoresis 30:3257–3264 [DOI] [PubMed] [Google Scholar]
- Yang Z, Hayes M, Fang X, Daley MP, Ettenberg S, Tse FL. (2007) LC-MS/MS approach for quantification of therapeutic proteins in plasma using a protein internal standard and 2D-solid-phase extraction cleanup. Anal Chem 79:9294–9301 [DOI] [PubMed] [Google Scholar]
- Yuan H, Zhou Y, Zhang L, Liang Z, Zhang Y. (2009) Integrated protein analysis platform based on column switch recycling size exclusion chromatography, microenzymatic reactor and microRPLC-ESI-MS/MS. J Chromatogr A 1216:7478–7482 [DOI] [PubMed] [Google Scholar]
- Yuan L, Arnold ME, Aubry AF, Ji QC. (2012) Simple and efficient digestion of a monoclonal antibody in serum using pellet digestion: comparison with traditional digestion methods in LC-MS/MS bioanalysis. Bioanalysis 4:2887–2896 [DOI] [PubMed] [Google Scholar]
- Zhou H, Mascelli MA. (2011) Mechanisms of monoclonal antibody-drug interactions. Annu Rev Pharmacol Toxicol 51:359–372 [DOI] [PubMed] [Google Scholar]