Abstract
The UDP-glucuronosyltransferase (UGT) enzymes are critical for regulating nutrients, hormones, and endobiotics, as well as for detoxifying xenobiotics. Human and murine fetuses are known to express glucuronidation enzymes, but there are currently no data prior to implantation. Here we addressed this gap in knowledge and tested whether Ugt enzymes are already present in preimplantation-stage embryos. Blastocysts were obtained after in vitro fertilization with gametes from B6D2F1 hybrid mice and from embryo culture. Protein expression and localization were determined using pan-specific UGT1A and UGT2B, as well as anti-human isoform-specific antibodies. Immunofluorescence analysis showed that blastocysts expressed Ugt1a globally, in the cytoplasm and nuclei of all of the cells. Western blots demonstrated the presence of Ugt1a6 but not Ugt1a1, Ugt1a3, Ugt1a4, or Ugt1a9. The Ugt2b proteins were not detected by either assay. The level of Ugt activity in murine blastocysts was comparable with that of the adult human liver (per milligram of protein), but the activity of β-glucuronidase, an Ugt-partnering enzyme responsible for substrate regeneration, was lower. Altogether, these data confirm that Ugt1a proteins are present and active in preimplantation murine embryos and point to a potential role for these proteins in implantation and early embryonic and fetal development.
Introduction
The UDP-glucuronosyltransferases (UGTs) are a superfamily of enzymes that catalyze the conjugation of glucuronic acid to molecules primarily to facilitate systemic elimination (Radominska-Pandya et al., 1999). These enzymes are critical for eliminating chemicals, steroid hormones, nutrients, and other endobiotics, thereby regulating systemic levels of compounds and maintaining homeostasis.
The first evidence for Ugts in mouse fetal development was in 1975, when Fyffe and Dutton reported on development of glucuronidation in murine tissues (Fyffe and Dutton, 1975). Shortly after this, it was discovered that the activities of hepatic UGTs (the primary site of metabolism) do not develop until close to term and/or after birth in humans (Onishi et al., 1979; Kawade and Onishi, 1981). Hence, during gestation, glucuronidation is performed largely by the maternal liver and placenta (Collier et al., 2002a,b). Genetic, tissue-specific, and environmental factors have all been inferred to influence UGT in development since the placenta is of fetal (not maternal) origin and expresses active UGTs, whereas the fetus does not.
We have been working toward elucidating the roles of UGT/Ugt in reproduction, gestation, and development. In the mouse, procedures commonly used in assisted reproduction, such as in vitro fertilization, intracytoplasmic sperm injection, and embryo biopsy, can dysregulate placental function and fetal development (Collier et al., 2009, 2012; Sugawara et al., 2012). These effects occur, in part, through interference with Ugt expression and function in murine placental and fetal tissues (Collier et al., 2009, 2012; Raunig et al., 2011a,b). Since these studies were performed in term tissues, a question remained as to when Ugt enzymes arise in mouse embryonic and fetal tissues. We hypothesized that Ugt expression and activity may occur as early as the blastocyst stage of embryonic development, when the precursors of the placenta begin to differentiate.
The mouse model offers the opportunity to study early developmental stages such as blastocysts and cleavage-stage embryos in culture, and researchers can perform manipulations that may not or cannot be attempted with human embryos. Thus, mouse models of assisted reproduction and development are common, particularly in the developmental and toxicological fields. Recently, mRNA microarray analysis demonstrated that Ugt transcripts in the murine maternal liver, kidney, small intestine, and placenta were stable across gestation in normal pregnancy (Shuster et al., 2013). The authors inferred that the pregnancy process itself did not substantially alter transcript levels of Ugt genes. Expanding and complementing these studies, here we show expression and activity of Ugt1a enzymes in preimplantation murine embryos, providing the earliest evidence for active Ugts in mammalian development.
Materials and Methods
Animals.
B6D2F1 mice (C57BL/6 crossed with DBA/2) were obtained from the National Cancer Institute (Raleigh, NC) at 6–8 weeks of age. Mice were fed ad libitum with a standard diet and were maintained in a temperature- and light-controlled room (22°C, 14-hour light/10-hour dark), in accordance with National Research Council guidelines. The protocol for animal handling and treatment procedures was reviewed and approved by the University of Hawaii Animal Care and Use Committee.
Mice were used as oocyte and sperm donors for in vitro fertilization, performed as reported by Sugawara et al., (2012). Briefly, oocytes were collected from females induced to superovulate and epididymal sperm were collected from males by release from caudae epididymides directly into T6 medium and capacitated for 1.5 hours at 37°C in a humidified atmosphere of 5% CO2. Gametes were coincubated for 4 hours, and then oocytes were washed with HEPES–Chatot, Ziomek, and Bavister (CZB) medium followed by at least one wash with CZB medium. Fertilized oocytes with two well developed pronuclei and extruded second polar bodies were cultured in 50-μl drops of CZB for 120 hours, when they reached the expanded blastocyst stage and were used for experimentation.
Ugt Protein Expression and Localization In Situ with Fluorescence Confocal Microscopy.
Immunofluorescence analysis of blastocysts was performed as previously described (Alarcon, 2010). The primary antibodies and concentrations were as follows: pan-specific UGT1A and UGT2B (1:200; Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibodies were Biotin-SP–conjugated AffiniPure donkey anti-rabbit IgG for UGT1A and donkey anti-goat IgG for UGT2B (both 1:200; Jackson ImmunoResearch Laboratories Inc., West Grove, PA). Streptavidin-fluorescein RPN 1232 (Amersham Biosciences, Pittsburgh, PA) was used to bind to biotin, and then stained embryos were mounted on slides in Vectashield with 4′,6′-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA). Embryos were analyzed with an FV1000-IX81 confocal fluorescence microscope with MetaMorph Microscopy Automation & Image Analysis Software and Fluoview software (version 2.1; Olympus America, Center Valley, PA). The same settings (exposure time, pinhole size, and gain) were used to record images of embryos that were stained in the same batch and used for comparison of fluorescence intensities. Fluorescence intensities of embryos were compared with ImageJ software (http://imagej.nih.gov/ij/; National Institutes of Health, Bethesda, MD).
Western Blot Analysis of Ugt Protein Expression in Mouse Blastocysts.
Western blotting was performed for Ugt protein expression analysis as previously described (Miyagi and Collier, 2011), using 20 µg pooled blastocyst protein in each well. Antibody incubations were as follows: UGT1A and UGT2B (1:1000), UGT1A1 and UGT1A6 (1:2000; Santa Cruz Biotechnology), UGT1A3 (mouse anti-human; Abcam, Cambridge, UK), and UGT1A4 and UGT1A9 (sheep anti-human; gifts from Professor M.W.H. Coughtrie, University of British Columbia, Vancouver, BC, Canada). The UGT antibodies used were all to human proteins. UGT1A and UGT2B have been demonstrated to crossreact with mouse proteins, but the specificity of the UGT1A6 antibody for Ugt1a6 has not been confirmed in the mouse. The secondary antibody was donkey anti-species biotin (1:8000; (Jackson ImmunoResearch Laboratories Inc.), and biotin detection was done with streptavidin/horseradish peroxidase/biotin (1:10,000; GE Healthcare, Piscataway, NJ). Detection was done with ECL Plus (GE Healthcare). Proteins were sized by comparison with a Rainbow Marker (GE Healthcare) and recombinant controls. Each membrane contained a positive control human tissue (microsomes derived from 200 pooled human livers; Xenotech, Lenexa, KS) and a recombinant positive control (GE Healthcare). All antibodies were blotted three times, and each gel contained three individual lanes representing different pools of blastocysts. Exposures for each antibody were performed for the same amount of time, but exposure time between antibodies differed (UGT1A and UGT2B, 45 minutes; UGT1A1, UGT1A3, UGT1A4, and UGT1A9, 20 minutes; and UGT1A6, 90 minutes).
Biochemical Detection and Quantification of UGT and β-Glucuronidase Activity.
The assay for Ugt activity with 4-methylumbelliferone (4-MU) was carried out as previously described, with 0.5 mg/ml blastocyst protein and alamethicin 50 μg/mg protein as the activator (Collier et al., 2000). The intra- and inter-assay coefficients of variation for the slopes of the standard curves were 4.1 and 9%, respectively, and the intra- and inter-assay variability for pooled adult human liver microsomes (n = 200, positive control) was 12.2 and 14.1%, respectively.
The assay for β-glucuronidase activity was performed with 4-MU glucuronide according to the method of Trubetskoy and Shaw (1999). The accuracy and precision of the standard curves were the same as reported above for 4-MU activity.
Statistical Analyses.
Data were analyzed using one-way analysis of variance, with Bonferroni multiple comparison post hoc analysis. Bars are presented as means ± S.D. or S.E.M., as indicated in the figure legends. Statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad, San Diego, CA) with ∝ ≤ 0.05.
Results
Immunofluorescence of Ugt Expression and Localization in Blastocysts.
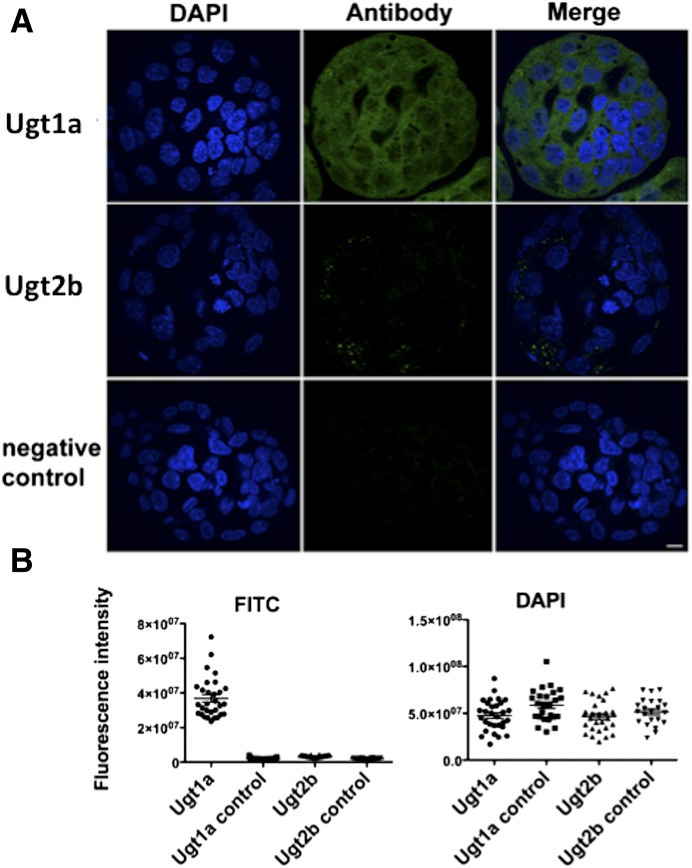
Blastocysts (n = 22–30 total per group, three experimental replicates) were examined for Ugt1a and Ugt2b expression using confocal microscopy immunofluorescence after staining with pan-specific antibodies, raised in humans for UGT1A and UGT2B, that were previously demonstrated to crossreact with mouse isoforms. Proteins from the Ugt1a family were present throughout the blastocysts, with both nuclear and cytoplasmic localization (Fig. 1A). Residual staining for Ugt2b proteins could be observed (Fig. 1A), but the quantitative analysis of fluorescence intensity revealed no differences from the background control (Fig. 1B). All examined groups showed similar levels of fluorescence intensity after 4′,6′-diamidino-2-phenylindole nuclear staining, demonstrating comparable cell numbers in the blastocysts (Fig. 1B).
Fig. 1.
Confocal immunofluorescence analysis of Ugt expression and localization in blastocysts. (A) Exemplary images of blastocysts stained with pan-specific antibodies against Ugt1a and Ugt2b, with DAPI staining to show the cell nuclei. Strong Ugt1a signal is present throughout the blastocysts, whereas Ugt2b shows only weak cytoplasmic staining. No signal is observed in negative (no antibody) control. Scale = 10 µm. (B) Quantitative analysis of fluorescence intensity. Levels of DAPI and antibody staining were measured in individual blastocysts. Three replicates were performed with n = 6–11 embryos per group in each triplicate. The Ugt1a and Ugt2b controls are embryos from the same pool as the antibody-stained embryos, but for which the antibody was omitted. The graphs show plotted data for individual blastocysts. There were no differences in average DAPI fluorescence intensity between Ugt1a and UGT2b and their respective controls, Ugt1a and Ugt2b, and two sets of controls (P = N.S.). Ugt1a staining was significantly higher than all other groups (P < 0.0001) and no differences were observed between Ugt2b and its control and between two controls (P = N.S.). Significance was defined by analysis of variance with Bonferroni post hoc comparison. All scatter dot plots show the mean and S.E.M. DAPI, 4′,6′-diamidino-2-phenylindole; FITC, fluorescein isothiocyanate.
Western Blot Analysis of Ugt Expression and Localization in Blastocysts.
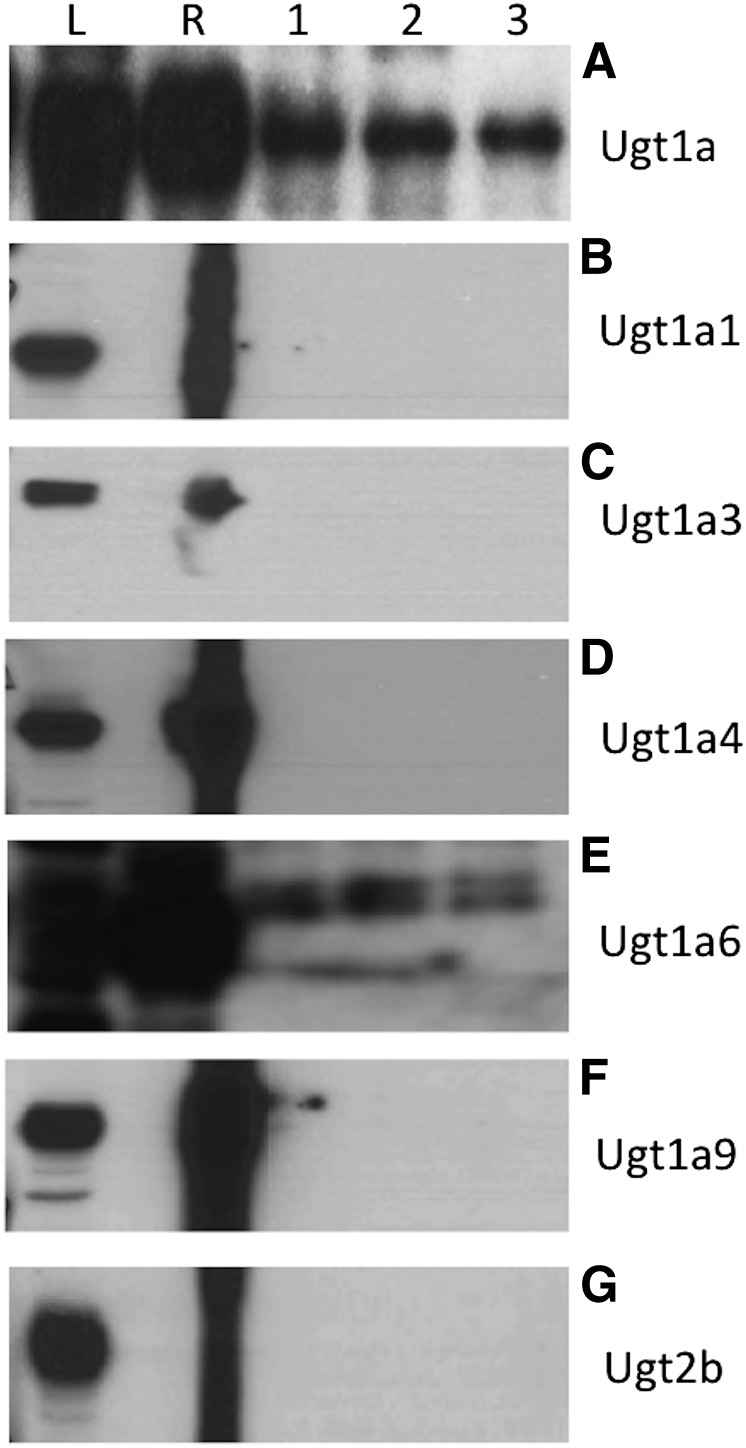
Western blot analysis for the general Ugt subfamilies demonstrated that murine Ugt1a proteins were present and crossreactive to UGT1A antibodies (Fig. 2A). Crossimmunoreactivity to anti-human UGT1A1, UGT1A3, UGT1A4, and UGT1A9 antibodies was not observed (Fig. 2, B–D and F). Proteins reactive to a human UGT1A6 antibody were observed (Fig. 2E). We do not know whether murine Ugt1a6a and/or Ugt1a6b were detected, since Ugt1a6 protein appeared as a doublet (Fig. 2E). It is not uncommon for UGT/Ugt proteins to blot as doublets, and it was speculated that this is due to dimerization of proteins to form an active unit (Meech and Mackenzie, 1997) and/or antibodies detecting both endoplasmic reticulum–anchored proteins and cytosolic proteins that are being chaperoned to the endoplasmic reticulum and have not yet cleaved their homing sequence (Radominska-Pandya et al., 1999). Crossreactivity to UGT2B antibodies was not detected (Fig. 2G). Hence, UGT2B/Ugt2b isoform-specific antibodies were not further used.
Fig. 2.
Detection of Ugt proteins in mouse blastocysts. (A) Proteins of the Ugt1a family were present as detected by a UGT1A/Ugt1a pan-specific antibody (55 kDa). (B) Murine Ugt1a1 was not detected (52 kDa). (C) Murine Ugt1a3 was not detected (37 kDa). (D) Murine Ugt1a4 was not detected (60 kDa). (E) Murine Ugt1a6 was detected (61 kDa). (F) Murine Ugt1a9 was not detected (52 kDa). (G) Proteins of the UGT2b family were not detected using a UGT2B pan-specific antibody (55 kDa). L indicates the human liver positive control. R indicates the recombinant protein positive control, which was a human recombinant the same as the antibody (except UGT1A, in which recombinant UGT1A1 was used, and UGT2B, in which recombinant UGT2B7 was used). Lanes 1, 2, and 3 represent three different pools of blastocyst protein lysates. Blots were performed three times for each antibody and representative blots are shown.
Ugt and β-Glucuronidase Activities.
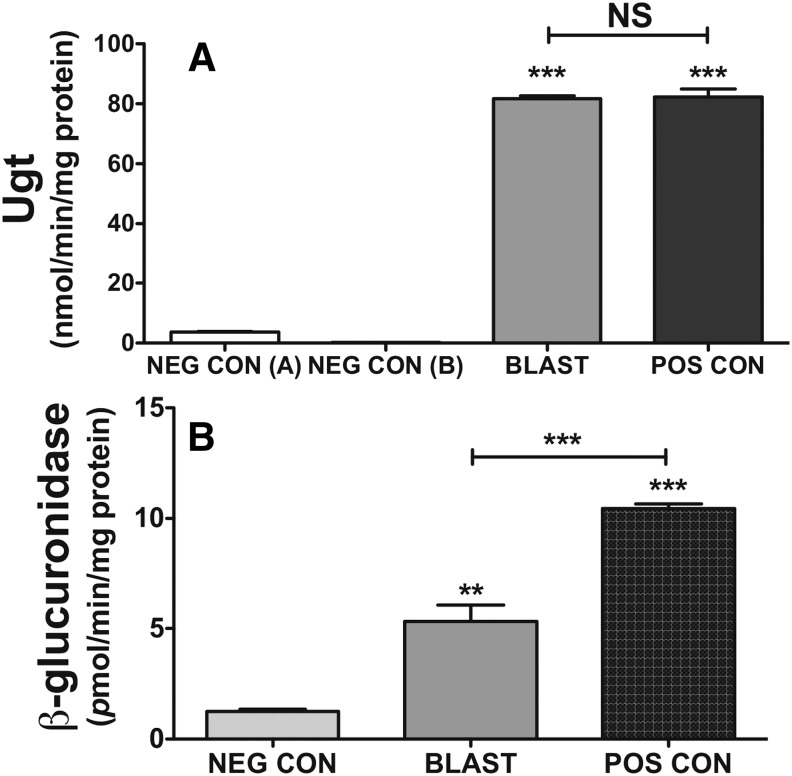
Blastocysts showed similar levels of Ugt activity toward the general substrate 4-MU (per milligram of protein) as the pooled adult human liver positive control. (Fig. 3A; P < 0.001 versus negative control). Although 4-MU is metabolized by multiple UGT/Ugt isoforms from both the UGT1A/Ugt1a and UGT2B/Ugt2b families (Uchaipichat et al., 2004), UGT1A4 does not metabolize 4-MU. This is, however, moot for the mouse, in which Ugt1a4 is a pseudogene. In addition, UGT1A1 and UGT1A6 are the most active toward 4-MU, with rates up to 10 times higher than UGT2B and other UGT1A isoforms (Jin et al., 1993; Green et al., 1994; Burchell et al., 2005). Therefore, because neither Ugt1a1, Ugt1a9, nor any Ugt2b proteins were detected, it is plausible that the activity measured is primarily Ugt1a6, although contributions from Ugt1a1, Ugt1a2, Ugt1a5, Ugt1a7c, Ugt1a8, Ugt1a9, and Ugt1a10 cannot be ruled out. For β-glucuronidase, although activities were significantly (Fig. 3B; P < 0.01) above background, they were also significantly lower than for the adult human liver (P < 0.001).
Fig. 3.
Total Ugt activity measured using 4-MU, and activity of β-glucuronidase in blastocyst lysates. (A) Blastocyst lysates demonstrate measurable Ugt activity that was significantly higher than negative control A (boiled blastocysts, P < 0.001) and negative control B (reaction with no cofactor, P < 0.001). Ugt activities in blastocysts were not significantly different (N.S., P = 0.91) than those of the human liver positive control. (B) Blastocysts show significantly higher β-glucuronidase activity than the negative control (5 mM sacchrolactone, P < 0.01), but significantly less than human liver microsomes positive control (P < 0.001). Significance was defined by analysis of variance with Bonferroni post hoc comparisons as indicated by bars. **P < 0.01; ***P < 0.001. All bars are means ± S.D. BLAST, blastocyst lysate; NEG CON, negative control; POS CON, positive control.
Discussion
Here we demonstrate that Ugt1a, but not Ugt2b, proteins are expressed in murine preimplantation embryos, with potential positive identification of Ugt1a6. Activity toward the general substrate 4-MU was observed, and was as high as the human liver (per milligram of protein). This is the earliest that UGT proteins have been found in mammalian development.
There are currently four recognized mammalian families of UGTs (UGT1, UGT2, UGT3, and UGT8). Although the UGT1A genes share homology between humans and mice (i.e., the human UGT1A1 gene is homologous to mouse Ugt1a1), the UGT2B/Ugt2b isoforms are nonorthologous (Mackenzie et al., 2005). The lack of homology in UGT2B/Ugt2b genes does not explain our inability to detect UGT2B proteins using the pan-specific antibody, because it is raised to a consensus sequence and has been used successfully in mice, humans, rats, and dogs (Muller et al., 2008). The UGT2A/Ugt2a, UGT3A/Ugt3a, and UGT8A/Ugt8a families appear to be homologous between humans and mice, but we did not probe for them due to their restricted (UGT2A/Ugt2a, olfaction) and undefined (UGT3 and UGT8) roles.
The different subfamilies of UGT/Ugt enzymes show diverse expression and activity profiles. The liver, gastrointestinal tract, and kidney are the major sites of UGT1A/Ugt1a enzyme expression and activities in humans and mice, which are primarily responsible for glucuronidation of essential endobiotics (e.g., bile acids, bilirubin), dietary substances, and chemicals, with smaller roles in steroid hormone metabolism (Radominska-Pandya et al., 1999). By contrast, although human and rodent UGT2B/Ugt2b members are expressed in the digestive tract, they tend to play more critical roles in steroid-target tissues (Radominska-Pandya et al., 1999). Hence, our failure to find Ugt2b proteins in the blastocysts is surprising because of the association of steroids with establishment of pregnancy and embryogenesis. However, our findings in the mouse show a similar pattern as in humans, in which UGT1A6 is the earliest of the UGT isoforms to become present and active in the fetus, whereas UGT1A1 does not develop until after birth (Onishi et al., 1979; Kawade and Onishi, 1981; Burchell et al., 1989; Miyagi and Collier, 2011). We cannot definitively state that Ugt1a6 is the sole Ugt1a present in murine preimplantation blastocysts because we cannot confirm that the human UGT1A6 antibody is specific for murine Ugt1a6. Despite this, the lack of crossreactivity to anti-human UGT1A1, UGT1A3, UGT1A4, and UGT1A9 makes our case more compelling. We did not expect to observe reactivity to human UGT1A3 or UGT1A4 antibodies because these are pseudogenes in the mouse, so absence of reactivity to these antibodies is not surprising. In addition, we did not probe for Ugt1a2, Ugt1a5, Ugt1a7c, Ugt1a8, or Ugt1a10; hence, these isoforms may also be present.
Prior to this study, the earliest that UGT enzymes had been reported in development was the presence of UGT immunoreactive cells in human liver parenchyme 32 days postovulation and in nucleated embryonic red blood cells 47 days postovulation (Hume et al., 1996). Such early expression caused speculation that UGTs may play a role in human embryogenesis. This was further confirmed when the obligate cofactor transporter for UGTs (the UDPGA transporter) was shown to be encoded on the fringe connection frc gene and involved in the Wingless, Hedgehog, fibroblast, and Notch signaling pathways in mice (Goto et al., 2001; Selva et al., 2001). Others have also demonstrated that the nuclear receptors retinoic X receptor (Königsdorf et al., 2012), peroxisome proliferator–activated receptor, subtype δ (Kang et al., 2011), and hepatic nuclear factor 4 (Duncan et al., 1994) are present in preimplantation murine embryos. Speculatively, the actions of these receptors could be driving Ugt expression in mouse blastocysts, because Ugts/UGTs are responsive to these receptors.
Our data imply a role for Ugt proteins in early murine development and may provide insights into preimplantation conditions in humans. Since mice are the most common laboratory model for pregnancy and are entrenched in the preclinical safety and reproductive toxicology battery (US Food and Drug Administration, 1999), better understanding of murine pregnancy, including metabolizing enzymes such as Ugts, has direct benefit. The presence of Ugts at the preimplantation stage of development indicates an as-yet unidentified role of these proteins in mammalian embryonic development that may be critical for good pregnancy outcomes.
Acknowledgments
The authors thank Dr. M.W.H. Coughtrie (University of British Columbia) for the gifts of sheep anti-human UGT1A4 and UGT1A9 antibodies.
Abbreviations
- 4-MU
4-methylumbelliferone
- CZB
Chatot, Ziomek, and Bavister
- UGT
UDP-glucuronosyltransferase
Authorship Contributions:
Participated in research design: Collier, Ward.
Conducted experiments: Yamauchi, Sato, Rougée.
Performed data analysis: Collier, Yamauchi, Sato, Rougée, Ward.
Wrote or contributed to the writing of the manuscript: Collier, Yamauchi, Sato, Rougée, Ward.
Footnotes
This research was supported by the National Institutes of Health National Institute on Minority Health and Health Disparities [Grant G12-MD007601], the National Institutes of Health National Institute of General Medical Sciences [Grant P20-GM103457], the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grant R01-HD072380], and the Hawaii Community Foundation. The funding bodies had no input into the study design, interpretation, or decision to publish.
References
- Alarcon VB. (2010) Cell polarity regulator PARD6B is essential for trophectoderm formation in the preimplantation mouse embryo. Biol Reprod 83:347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchell B, Coughtrie M, Jackson M, Harding D, Fournel-Gigleux S, Leakey J, Hume R. (1989) Development of human liver UDP-glucuronosyltransferases. Dev Pharmacol Ther 13:70–77 [DOI] [PubMed] [Google Scholar]
- Burchell B, Lockley DJ, Staines A, Uesawa Y, Coughtrie MW. (2005) Substrate specificity of human hepatic udp-glucuronosyltransferases. Methods Enzymol 400:46–57 [DOI] [PubMed] [Google Scholar]
- Collier AC, Ganley NA, Tingle MD, Blumenstein M, Marvin KW, Paxton JW, Mitchell MD, Keelan JA. (2002a) UDP-glucuronosyltransferase activity, expression and cellular localization in human placenta at term. Biochem Pharmacol 63:409–419 [DOI] [PubMed] [Google Scholar]
- Collier AC, Milam KA, Rougée LR, Sugawara A, Yamauchi Y, Ward MA. (2012) Upregulation of Ugt1a genes in placentas and fetal livers in a murine model of assisted reproduction. Placenta 33:77–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier AC, Miyagi SJ, Yamauchi Y, Ward MA. (2009) Assisted reproduction technologies impair placental steroid metabolism. J Steroid Biochem Mol Biol 116:21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier AC, Tingle MD, Keelan JA, Paxton JW, Mitchell MD. (2000) A highly sensitive fluorescent microplate method for the determination of UDP-glucuronosyl transferase activity in tissues and placental cell lines. Drug Metab Dispos 28:1184–1186 [PubMed] [Google Scholar]
- Collier AC, Tingle MD, Paxton JW, Mitchell MD, Keelan JA. (2002b) Metabolizing enzyme localization and activities in the first trimester human placenta: the effect of maternal and gestational age, smoking and alcohol consumption. Hum Reprod 17:2564–2572 [DOI] [PubMed] [Google Scholar]
- Duncan SA, Manova K, Chen WS, Hoodless P, Weinstein DC, Bachvarova RF, Darnell JE., Jr (1994) Expression of transcription factor HNF-4 in the extraembryonic endoderm, gut, and nephrogenic tissue of the developing mouse embryo: HNF-4 is a marker for primary endoderm in the implanting blastocyst. Proc Natl Acad Sci USA 91:7598–7602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe J, Dutton GJ. (1975) Induction of UDPglucose dehydrogenase during development, organ culture, and exposure to phenobarbital. Its relation to levels of UDPglucuronic acid and overall glucuronidation in chicken and mouse. Biochim Biophys Acta 411:41–49 [DOI] [PubMed] [Google Scholar]
- Goto S, Taniguchi M, Muraoka M, Toyoda H, Sado Y, Kawakita M, Hayashi S. (2001) UDP-sugar transporter implicated in glycosylation and processing of Notch. Nat Cell Biol 3:816–822 [DOI] [PubMed] [Google Scholar]
- Green MD, Oturu EM, Tephly TR. (1994) Stable expression of a human liver UDP-glucuronosyltransferase (UGT2B15) with activity toward steroid and xenobiotic substrates. Drug Metab Dispos 22:799–805 [PubMed] [Google Scholar]
- Hume R, Burchell A, Allan BB, Wolf CR, Kelly RW, Hallas A, Burchell B. (1996) The ontogeny of key endoplasmic reticulum proteins in human embryonic and fetal red blood cells. Blood 87:762–770 [PubMed] [Google Scholar]
- Jin CJ, Miners JO, Lillywhite KJ, Mackenzie PI. (1993) cDNA cloning and expression of two new members of the human liver UDP-glucuronosyltransferase 2B subfamily. Biochem Biophys Res Commun 194:496–503 [DOI] [PubMed] [Google Scholar]
- Kang HJ, Hwang SJ, Yoon JA, Jun JH, Lim HJ, Yoon TK, Song H. (2011) Activation of peroxisome proliferators-activated receptor δ (PPARδ) promotes blastocyst hatching in mice. Mol Hum Reprod 17:653–660 [DOI] [PubMed] [Google Scholar]
- Kawade N, Onishi S. (1981) The prenatal and postnatal development of UDP-glucuronyltransferase activity towards bilirubin and the effect of premature birth on this activity in the human liver. Biochem J 196:257–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Königsdorf CA, Navarrete Santos A, Schmidt JS, Fischer S, Fischer B. (2012) Expression profile of fatty acid metabolism genes in preimplantation blastocysts of obese and non-obese mice. Obes Facts 5:575–586 [DOI] [PubMed] [Google Scholar]
- Mackenzie PI, Bock KW, Burchell B, Guillemette C, Ikushiro S, Iyanagi T, Miners JO, Owens IS, Nebert DW. (2005) Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenet Genomics 15:677–685 [DOI] [PubMed] [Google Scholar]
- Meech R, Mackenzie PI. (1997) UDP-glucuronosyltransferase, the role of the amino terminus in dimerization. J Biol Chem 272:26913–26917 [DOI] [PubMed] [Google Scholar]
- Miyagi SJ, Collier AC. (2011) The development of UDP-glucuronosyltransferases 1A1 and 1A6 in the pediatric liver. Drug Metab Dispos 39:912–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A, Glattard E, Taleb O, Kemmel V, Laux A, Miehe M, Delalande F, Roussel G, Van Dorsselaer A, Metz-Boutigue M, et al. (2008) Endogenous morphine in SH-SY5Y cells and the mouse cerebellum. PLoS One 3:e1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi S, Kawade N, Itoh S, Isobe K, Sugiyama S. (1979) Postnatal development of uridine diphosphate glucuronyltransferase activity towards bilirubin and 2-aminophenol in human liver. Biochem J 184:705–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radominska-Pandya A, Czernik PJ, Little JM, Battaglia E, Mackenzie PI. (1999) Structural and functional studies of UDP-glucuronosyltransferases. Drug Metab Rev 31:817–899 [DOI] [PubMed] [Google Scholar]
- Raunig JM, Yamauchi Y, Ward MA, Collier AC. (2011a) Assisted reproduction technologies alter steroid delivery to the mouse fetus during pregnancy. J Steroid Biochem Mol Biol 126:26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raunig JM, Yamauchi Y, Ward MA, Collier AC. (2011b) Placental inflammation and oxidative stress in the mouse model of assisted reproduction. Placenta 32:852–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selva EM, Hong K, Baeg GH, Beverley SM, Turco SJ, Perrimon N, Häcker U. (2001) Dual role of the fringe connection gene in both heparan sulphate and fringe-dependent signalling events. Nat Cell Biol 3:809–815 [DOI] [PubMed] [Google Scholar]
- Shuster DL, Bammler TK, Beyer RP, Macdonald JW, Tsai JM, Farin FM, Hebert MF, Thummel KE, Mao Q. (2013) Gestational age-dependent changes in gene expression of metabolic enzymes and transporters in pregnant mice. Drug Metab Dispos 41:332–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara A, Sato B, Bal E, Collier AC, Ward MA. (2012) Blastomere removal from cleavage-stage mouse embryos alters steroid metabolism during pregnancy. Biol Reprod 87:4–, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trubetskoy OV, Shaw PM. (1999) A fluorescent assay amenable to measuring production of beta-D-glucuronides produced from recombinant UDP-glycosyl transferase enzymes. Drug Metab Dispos 27:555–557 [PubMed] [Google Scholar]
- Uchaipichat V, Mackenzie PI, Guo XH, Gardner-Stephen D, Galetin A, Houston JB, Miners JO. (2004) Human udp-glucuronosyltransferases: isoform selectivity and kinetics of 4-methylumbelliferone and 1-naphthol glucuronidation, effects of organic solvents, and inhibition by diclofenac and probenecid. Drug Metab Dispos 32:413–423 [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration (1999) International Conference on Harmonisation; Guidance on the duration of chronic toxicity testing in animals (rodent and nonrodent toxicity testing); availability. Fed Regist 64:34259–34260 [PubMed] [Google Scholar]