Abstract
Bupropion is used clinically to treat depression and to promote smoking cessation. It is metabolized by CYP2B6 to its active metabolite hydroxybupropion, yet alterations in CYP2B6 activity have little impact on bupropion plasma levels. Furthermore, less than 10% of a bupropion dose is excreted as urinary bupropion and its characterized metabolites hydroxybupropion, threohydrobupropion, and erythrohydrobupropion, suggesting that alternative metabolic pathways may exist. In vitro data suggested CYP2C19 could metabolize bupropion. The current study investigated the impact of functional CYP2C19 genetic variants on bupropion pharmacokinetics and treatment outcomes. In 42 healthy volunteers, CYP2C19*2 (a reduced activity allele) was associated with higher bupropion area under the plasma concentration–time curve (AUC), but similar hydroxybupropion AUC. The mean bupropion AUC was 771 versus 670 hours⋅ng/ml in individuals with and without CYP2C19*2, respectively (P = 0.017). CYP2C19*2 was also associated with higher threohydrobupropion and erythrohydrobupropion AUC (P < 0.005). Adjusting for CYP2B6 genotype did not alter these associations, and CYP2C19 variants did not alter the utility of the hydroxybupropion/bupropion ratio as a measure of CYP2B6 activity. Finally, in a clinical trial of 540 smokers, CYP2C19 genotype was not associated with smoking cessation outcomes, supporting the hypothesis that bupropion response is mediated by hydroxybupropion, which is not altered by CYP2C19. In conclusion, our study reports the first in vivo evidence that reduced CYP2C19 activity significantly increases the steady-state exposure to bupropion and its reductive metabolites threohydrobupropion and erythrohydrobupropion. These pharmacokinetic changes were not associated with differences in bupropion’s ability to promote smoking cessation in smokers, but may influence the side effects and toxicity associated with bupropion.
Introduction
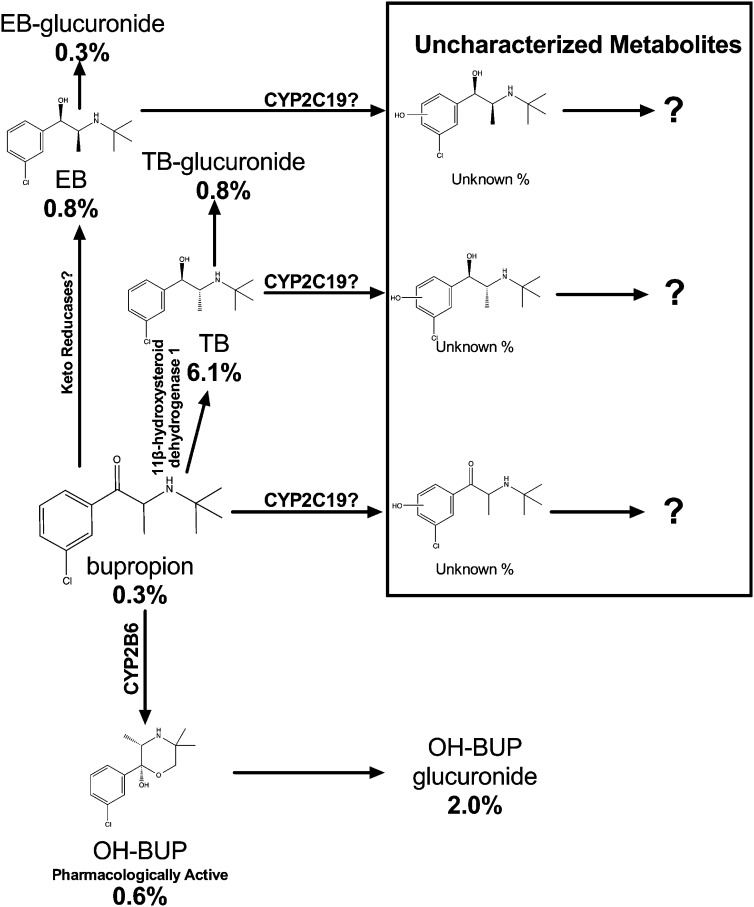
Bupropion (Wellbutrin and Zyban) is used clinically to treat depression, to promote smoking cessation, and to treat obesity (Hurt et al., 1997; Greenway et al., 2010; Moreira, 2011). Bupropion is extensively metabolized in humans, with less than 10% of the dose recovered in urine as the parent compound (Lai and Schroeder, 1983; Laizure et al., 1985; Benowitz et al., 2013). In vivo pharmacokinetic studies have indicated the existence of multiple metabolites, with three identified in plasma as hydroxybupropion (OH-BUP), erythrohydrobupropion (EB), and threohydrobupropion (TB) (Laizure and DeVane, 1985). Among these metabolites, OH-BUP had the highest steady-state plasma levels, and has been shown to be pharmacologically active (Fig. 1) (Damaj et al., 2004, 2010; Zhu et al., 2012; Benowitz et al., 2013). Higher levels of OH-BUP, but not bupropion, were associated with greater rates of smoking cessation in smokers receiving bupropion treatment (Zhu et al., 2012). Previous in vitro studies, using cDNA-expressed recombinant cytochrome P450 enzymes and human liver microsomes, demonstrated that CYP2B6 mediates the metabolism of bupropion to OH-BUP (Fig. 1) (Faucette et al., 2001). Consistent with this, an in vivo association between CYP2B6 genotype and OH-BUP levels and the ratio of OH-BUP to bupropion (OH-BUP/BUP ratio, an indicator of bupropion hydroxylation activity) has been shown. However, no associations between CYP2B6 genotype and the levels of the parent drug bupropion were observed (Kirchheiner et al., 2003; Zhu et al., 2012; Benowitz et al., 2013). Furthermore, when the potent CYP2B6 inhibitors clopidogrel and ticlopidine were given to healthy volunteers, their OH-BUP formation decreased dramatically (>80%), but there was little alteration in bupropion levels (Turpeinen et al., 2004, 2005). Together, these data have suggested that alternative bupropion clearance pathways exist, which may be able to compensate for the reduction in CYP2B6-mediated bupropion metabolism.
Fig. 1.
A quantitative scheme of bupropion metabolism profile in humans. The percentage below each metabolite represents the percentages of the total oral bupropion dose (150 mg in this case) excreted in urine as a percentage of daily dose of bupropion as the compound at steady state. These numbers were estimated using a 24-hour urine collection. The quantitative aspects were estimated using the study published by Benowitz et al. (2013). The metabolic pathway data were compiled from Butz et al. (1981), Laizure et al. (1985), DeVane et al. (1990), Sweet et al. (1995), Hsyu et al. (1997), Faucette et al. (2000), (Johnston et al. (2002), Chen et al. (2010), and Meyer et al. (2013). More than 20 bupropion metabolites have been identified; some poorly characterized secondary and tertiary metabolites are not shown on this figure. After 7 days of 150 mg/day bupropion treatment, the steady-state plasma level of bupropion is 29 ng/ml, OH-BUP is 400 ng/ml, EB is 32 ng/ml, and TB is 175 ng/ml (Benowitz et al., 2013). However, these metabolites account for a very small percentage of the total bupropion dose in urine; this suggests that other uncharacterized bupropion metabolites may still be quantitatively important in bupropion clearance.
The best characterized alternative bupropion metabolic routes are the reduction of bupropion to EB and TB. Despite their substantial plasma levels (Fig. 1) (Laizure and DeVane, 1985; Zhu et al., 2012), a very small percentage of bupropion was recovered in urine as EB, TB, and their glucuronides (Fig. 1) (Benowitz et al., 2013), and differences in the CYP2B6-mediated bupropion hydroxylation pathway did not result in any compensatory changes in EB or TB levels (Zhu et al., 2012; Benowitz et al., 2013). Together, these data suggest that other poorly characterized metabolic routes may play an important role in bupropion clearance (Fig. 1).
Recently, an in vitro study using human liver microsomes and cDNA-expressed recombinant cytochrome P450 enzymes suggested the existence of additional hydroxybupropion metabolites which were likely formed by CYP2C19 (Fig. 1) (Chen et al., 2010). Their existence could explain the relatively small amount of bupropion dose recovered as OH-BUP, EB, TB, and their metabolites (Fig. 1). However, the role of CYP2C19 in bupropion pharmacokinetics has not been explicitly investigated in humans.
The human CYP2C19 gene is polymorphic with more than 25 known variant alleles (http://www.cypalleles.ki.se/cyp2c19.htm). The most common loss-of-function allele is CYP2C19*2 (c.681G>A; rs4244285), which has a 15% allele frequency in Caucasians and African Americans and a 30% allele frequency in Asians (Scott et al., 2011, 2013). Other reduced or loss-of-function CYP2C19 alleles have also been identified, but their allele frequencies are below 1% in Caucasians and African Americans (Scott et al., 2011, 2013). CYP2C19*3 (c.636G>A; rs4986893) is generally found in Asians, with an allele frequency of 2–9%. A gain-of-function allele, CYP2C19*17 (c.-806C>T; rs12248560), has recently been identified, resulting in higher activity as a consequence of enhanced CYP2C19 transcription (Sim et al., 2006). It has an allele frequency of 21% in Caucasians, 16% in African Americans, and 3% in Asians (Sim et al., 2006; Scott et al., 2011, 2013).
In this study, we investigated the influence of these CYP2C19 genetic variants on steady-state in vivo bupropion pharmacokinetics. We hypothesized that the loss-of-function CYP2C19 genetic variants, CYP2C19*2 and CYP2C19*3, would result in lower bupropion oral clearance and higher steady-state bupropion exposure [i.e., area under the plasma concentration–time curve (AUC)], whereas the gain-of-function CYP2C19 genetic variant, CYP2C19*17, would result in faster bupropion oral clearance and lower steady-state bupropion exposure. Furthermore, we explored the influence of CYP2C19 genetic variation on the plasma levels of bupropion metabolites OH-BUP, EB, and TB. Last, we sought to evaluate the pharmacodynamic impact of CYP2C19 genotype on bupropion’s ability to promote smoking cessation in smokers.
Materials and Methods
Participants and Procedures
Study 1: Steady-State Bupropion Pharmacokinetics in Healthy Volunteers.
Healthy volunteers (n = 42, 62% men) were recruited for the pharmacokinetic study (Benowitz et al., 2013), which included 21 Caucasians, 14 African Americans, and 7 Asians. The exclusion criteria included being younger than 18 years of age, taking regular medications (including oral contraceptives), using alcohol or illicit drugs, or having a history of seizures, head trauma, or eating disorders. Eligible and consenting participants were given 150 mg of bupropion XL (once per day, sustained release) daily for 7 days, allowing bupropion and its metabolites to reach plasma steady state. The participants were asked to call the clinic daily to confirm their bupropion adherence. The participants were admitted into a clinical research inpatient unit on day 7, and their plasma samples were taken every 4 hours for a 24-hour period. A complete 24-hour urine sample was also collected. Supplemental Table 1 summarizes the baseline demographics and CYP2C19 genotype frequencies in study 1. All participants provided written informed consent in accordance with the principles expressed in the Declaration of Helsinki, and the study protocol was approved by the Institutional Review Boards at the University of Toronto and the University of California, San Francisco.
Study 2: Bupropion Treatment Efficacy in Smokers.
The association between CYP2C19 variants and the treatment efficacy of bupropion was investigated in a randomized, double-blind, and placebo-controlled clinical trial of 540 African American smokers (DNA was available for 535 individuals) (Cox et al., 2012). This study consisted of a placebo arm (n = 270) and a bupropion arm (n = 270, 150 mg/day for 3 days, then 150 mg BID for 7 weeks). The inclusion criteria included being ≥18 years of age, having smoked an average of 10 or fewer cigarettes per day for at least 6 months before enrollment, and having smoked at least 25 of the past 30 days. The exclusion criteria were consistent with the contraindications of bupropion use (Cox et al., 2012). Cotinine-verified (≤15 ng/ml) smoking cessation was assessed at week 3 during treatment, at the end of the 7 weeks of treatment, and at week 26 follow-up consistent with accepted practice (SRNT Subcommittee on Biochemical Verification, 2002). The baseline demographics and CYP2C19 genotype frequencies in study 2 can be found in Supplemental Table 3.
Genotyping
CYP2C19*2, CYP2C19*3, and CYP2C19*17 were genotyped using an ABI Viia 7 Real-Time PCR System (Applied Biosystems, Foster City, CA). The genotyping reaction was performed with 5 μl of TaqMan GTXpress master mix and 5 μl of water containing 10 ng of DNA and 0.5 μl of 20× TaqMan drug metabolism analysis probes (CYP2C19*2: rs4244285, C__25986767_70; CYP2C19*3: rs4986893, C__27861809_10; and CYP2C19*17: rs12248560, C____469857_10; Applied Biosystems). The allele discrimination data were analyzed using Viia 7 software version 1.2. The CYP2B6 genotyping was described and performed previously (Zhu et al., 2012; Benowitz et al., 2013).
Analytical Chemistry
Plasma and urine concentration of bupropion and its metabolites were measured using liquid chromatography–tandem mass spectroscopy as described previously (Haas et al., 2004; Benowitz et al., 2013).
In brief, all plasma samples (500 μl), standards, and controls were first spiked with a deuterium-labeled internal standard (100 μl), then deproteinized with 3% aqueous perchloric acid and extracted with 0.5 ml of 50% w/v aqueous tripotassium phosphate and 4 ml of pentane:ethyl acetate:isopropanol (80:15:5). The organic layer was evaporated to dryness and reconstituted with 200 μl of 0.1% formic acid and 10 mM ammonium formate in water:methanol (75:25). The samples were analyzed by Agilent 1200 liquid chromatograph (Agilent Technologies, Santa Clara, CA) interfaced to a Thermo-Finnigan TSQ Quantum Ultra mass spectrometer using a BDS Hypersil Phenyl column (150 × 4.6 mm; Phenomenex, Torrance, CA) with a gradient mobile phase of mobile phase A (10 mM ammonium formate, 0.1% formic acid in high-performance liquid chromtography–grade water) and mobile phase B (10 mM ammonium formate, 0.1% formic acid in methanol) under a 0.8 ml/min flow rate (75% mobile phase A at 0 minute, 55% mobile phase A at 1.5 minutes, 50% mobile phase A at 7 minutes, 0% mobile phase A at 8 minutes, 0% mobile phase A at 11 minutes, and 75% mobile phase A at 11.1 minutes). The limit of quantitation for bupropion, OH-BUP, EB, and TB was 1 ng/ml. Data on within-run and between-run accuracy and precision have been published previously (see Supplemental Table 1 of Benowitz et al., 2013). Urine samples were measured as previously described, with and without glucuronide deconjugation. The deconjugation procedure was conducted by incubating 125 μl of urine with 125 μl of β-glucuronidase (containing 750 units) in 0.9% saline and 250 μl of 0.1 M (pH 5.0) sodium acetate solution at 37°C overnight (Petsalo et al., 2007). Concentrations of conjugated bupropion or metabolites were determined as the difference between concentrations measured with and without deconjugation.
Pharmacokinetic and Statistical Analyses
The steady-state exposure to bupropion and its metabolites was measured using the AUC for 24 hours on day 7. Urinary excretion data were presented both as absolute concentrations and the molar percentage of each metabolite in relation to the total steady-state bupropion dose. Average steady-state concentration was computed as AUC for 24 hours/24 hours. A linear regression approach was used to examine the effects of having zero, one, or two copies of variant CYP2C19 alleles on bupropion and metabolites exposure with and without adjusting for CYP2B6 genotype. The statistical impact (i.e., P values) of the CYP2C19 variant alleles was derived from linear regression analyses. This approach gives an estimation of the effect size of each CYP2C19 allele while controlling for the effect of other CYP2C19 alleles and the CYP2B6 genotype. The CYP2C19*2 genotype was coded in the regression model as *1/*1 = 0, *1/*2 = 1, and *2/*2 = 2. The CYP2C19*17 genotype was coded in the regression model as *1/*1 = 0, *1/*17 = 1, and *17/*17 = 2. The CYP2B6 genotype was coded in the regression model as normal metabolizers (*1/*1) = 0, intermediate metabolizers (*1/*6 or *1/*18) = 1, and slow metabolizers (*6/*18, *6/*6, and *18/*18) = 2 (Zhu et al., 2012). The regression residuals were normally distributed, thus the pharmacokinetic parameters were not log transformed. Chi square tests were used to evaluate the association between CYP2C19 genotype and smoking abstinence in study 2. The likelihood ratio test (the “lrtest” procedure in Stata) was used to evaluate the impact of adjusting CYP2C19 genotype on the association between CYP2B6 genotype and the OH-BUP/BUP ratio. Statistical analyses were performed using R v2.5.3 (The R Project for Statistical Computing) and Stata version 12 (StataCorp, College Station, TX).
Results
Study 1: CYP2C19*2 Was Associated with Significantly Higher Steady-State Bupropion, EB, and TB Exposure.
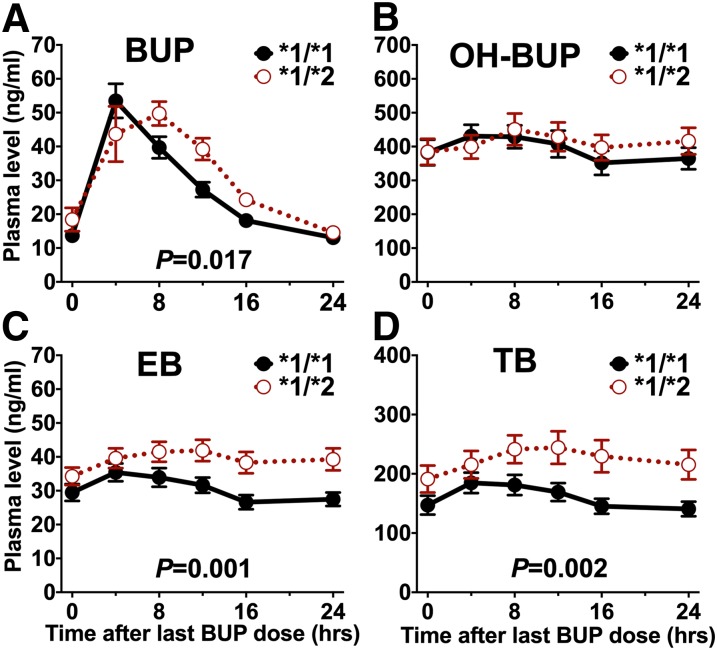
In the pharmacokinetic study, there were 17 individuals with CYP2C19*1/*1 genotype (i.e., without CYP2C19*2, CYP2C19*3, or CYP2C19*17), 10 individuals with CYP2C19*1/*2 genotype, 9 individuals with *1/*17 genotype, 5 individuals with *2/*17 genotype, and 1 individual with *17/*17 genotype (Supplemental Table 1). Individuals with a CYP2C19*2 allele had a significantly higher steady-state plasma bupropion AUC compared with those without any variants (771 and 670 hours⋅ng/ml, respectively; P = 0.017) (Fig. 2A; Table 1). Steady-state plasma OH-BUP exposure did not differ between those with the CYP2C19*2 allele and those without any variants (Fig. 2B; Table 1). Steady-state EB and TB plasma AUCs were significantly higher in individuals with the CYP2C19*2 allele compared with those without any variants [EB: 947 and 732 hours⋅ng/ml, respectively; P = 0.001 (Fig. 2C; Table 1); TB: 5427 and 3867 hours⋅ng/ml, respectively; P = 0.002 (Fig. 2D; Table 1)]. Adjusting for CYP2B6 genotypes did not statistically alter the association between CYP2C19*2 and plasma BUP, EB, and TB AUC (Table 2; statistical inference was derived by the likelihood ratio tests in Table 3).
Fig. 2.
Steady-state plasma concentrations (mean ± S.E.M.) of bupropion (BUP) (A) and its metabolites OH-BUP (B), EB (C), and TB (D) over 24 hours by CYP2C19*2 genotype. The *1/*1 group (n = 17) represents individuals without any CYP2C19*2, *3, or *17 allele. The *1/*2 group represents individuals with one copy of CYP2C19*2 (n = 15). The P values were derived from the linear regression analyses presented in Table 1, which include five people with CYP2C19*2/*17 genotype.
TABLE 1.
Regression analysis of the association between CYP2C19 genotype and plasma bupropion or its metabolites AUC (study 1)
| BUP AUC24 (r2 = 0.146) |
OH-BUP AUC24 (r2 = 0.007) |
EB AUC24 (r2 = 0.277) |
TB AUC24 (r2 = 0.274) |
|||||
|---|---|---|---|---|---|---|---|---|
| B (95% CI) | P | B (95% CI) | P | B (95% CI) | P | B (95% CI) | P | |
| CYP2C19 *2 | 132 (25,239) | 0.017* | 613 (-1966,3191) | 0.634 | 266 (113.3,418.3) | 0.001* | 1838 (744,2933) | 0.002* |
| CYP2C19 *17 | −23 (−118.6,73.5) | 0.638 | −236 (−2557,2085) | 0.838 | −91 (−228,47) | 0.189 | −756 (−1741,229) | 0.129 |
AUC24, AUC for 24 hours; CI, confidence interval.
Indicates statistical significance.
TABLE 2.
Regression analysis of the association between CYP2C19 genotype and plasma bupropion or its metabolites AUC after adjusting for CYP2B6 genotype (study 1)
| BUP AUC24 (r2 = 0.151) |
OH-BUP AUC24 (r2 = 0.313) |
EB AUC24 (r2 = 0.333) |
TB AUC24 (r2 = 0.317) |
|||||
|---|---|---|---|---|---|---|---|---|
| B (95% CI) | P | B (95% CI) | P | B (95% CI) | P | B (95% CI) | P | |
| CYP2C19 *2 | 134 (26, 243) | 0.016* | 1080 (-11023, 263) | 0.323 | 284 (135, 433) | 0.001* | 1955 (875, 3035) | 0.001* |
| CYP2C19 *17 | −24 (-137, 88) | 0.664 | 1468 (-8093, 744) | 0.200 | −36 (-191, 120) | 0.646 | −493 (-1620, 633) | 0.381 |
| CYP2B6 NM>IM>SM | −13 (-90, 64) | 0.733 | −3144 (-4688, -1600) | 0.001* | −103 (-208, 3) | 0.06 | −630 (-1394, 134) | 0.103 |
AUC24, AUC for 24 hours; CI, confidence interval; IM, intermediate metabolizers; NM, normal metabolizers; SM, Slow metabolizers (Zhu et al., 2012; Benowitz et al., 2013).
Indicates statistical significance.
TABLE 3.
| BUP AUC24 | OH-BUP AUC24 | EB AUC24 | TB AUC24 | |
|---|---|---|---|---|
| Likelihood Ratio χ2 | 0.16 | 14.53 | 3.84 | 2.91 |
| P | 0.6902 | 0.0001* | 0.0501 | 0.0881 |
AUC24, AUC for 24 hours.
Indicates statistical significance.
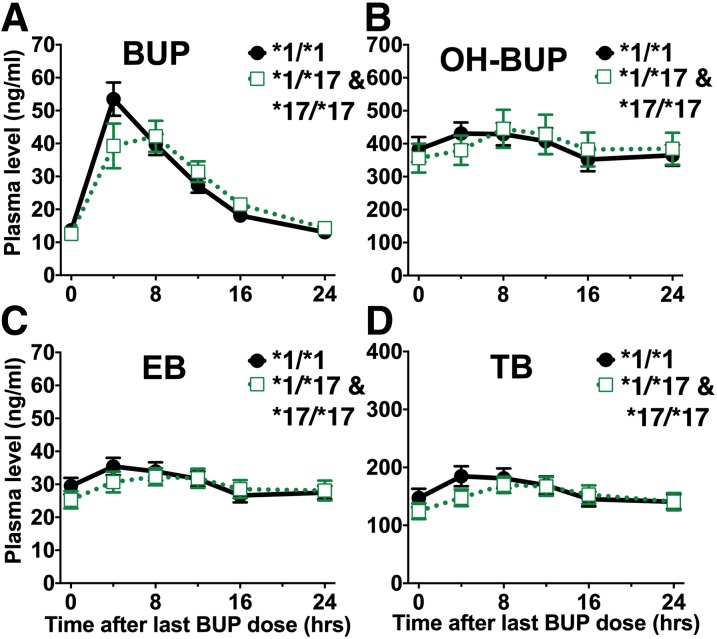
The direction of the CYP2C19*17 effect was consistent with the expected gain of function associated with this allele, but the effect size of CYP2C19*17 was small and did not reach statistical significance in the regression analyses (Fig. 3; Table 1). Individuals with the gain-of-function CYP2C19*17 allele had similar plasma bupropion (with CYP2C19*17: 663 hours⋅ng/ml; without any CYP2C19 variants: 670 hours⋅ng/ml), EB (with CYP2C19*17: 714 hours⋅ng/m;; without any CYP2C19 variants: 732 hours⋅ng/ml), and TB (with CYP2C19*17: 3669 hours⋅ng/ml; without any CYP2C19 variants: 3867 hours⋅ng/ml) exposure. The absolute and relative amounts of bupropion excreted as bupropion, OH-BUP, EB, and TB in urine did not significantly differ between CYP2C19 genotypes (Supplemental Table 2). CYP2C19*3 was genotyped in this study, but none of the participants had a CYP2C19*3 allele.
Fig. 3.
Steady-state plasma concentrations (mean ± S.E.M.) of bupropion (BUP) (A) and its metabolites OH-BUP (B), EB (C), and TB (D) over 24 hours by CYP2C19*17 genotype. The *1/*1 group (n = 17) represents individuals without any known CYP2C19*2, *3, or *17 alleles. The *1/*17 and *17/*17 groups represent individuals with one or two copies of CYP2C19*17 (n = 15; there was one individual with the *17/*17 genotype). There were no significant effects as described by the linear regression analyses presented in Table 1, which include five people with CYP2C19*2/*17 genotype.
The ratio of plasma OH-BUP levels to plasma bupropion levels (the OH-BUP/BUP ratio) following an acute bupropion administration or at steady state is often used in humans as an indicator of in vivo CYP2B6 activity (Kirchheiner et al., 2003; Fradette et al., 2004; Hesse et al., 2004). We investigated whether variation in CYP2C19 would have a meaningful impact on the utility of the OH-BUP/BUP ratio as an indicator of CYP2B6 activity. Controlling for CYP2C19 genotype did not meaningfully alter the association between CYP2B6 genotype and the steady-state OH-BUP/BUP plasma AUC ratio [CYP2B6 genotype’s effect without controlling for CYP2C19 genotype: β = −0.38, P = 0.01 (Table 4, top); CYP2B6 genotype’s effect after controlling for CYP2C19 genotype: β = −0.40, P = 0.01 (Table 4, middle); likelihood ratio test P = 0.49 (Table 4, bottom)]. Thus, CYP2C19 genotype is unlikely to alter the utility of the OH-BUP/BUP ratio as a measure of CYP2B6 activity.
TABLE 4.
Impact of CYP2C19 genetic variation on the association between CYP2B6 genotype and plasma steady-state OH-BUP to bupropion ratio (study 1)
| B | β | 95% CI | P | |
|---|---|---|---|---|
| OH-BUP/BUP model without CYP2C19a | ||||
| CYP2B6 NM>IM>SM | −6.48 | −0.38 | −11.5 to -1.43 | 0.01* |
| OH-BUP/BUP model with CYP2C19b | ||||
| CYP2B6 NM>IM>SM | −6.88 | −0.40 | −12.3 to −1.51 | 0.01* |
| CYP2C19*2 | −2.13 | −0.09 | −9.78 to 5.51 | 0.58 |
| CYP2C19*17 | 2.50 | 0.11 | −4.64 to 9.64 | 0.48 |
| Likelihood Ratio χ2c | 1.44 | |||
| P | 0.49 | |||
IM, intermediate metabolizers; NM, normal metabolizers; SM, slow metabolizers (Zhu et al., 2012; Benowitz et al., 2013).
The association between CYP2B6 genotype and plasma steady state OH-BUP/BUP ratio.
The association between CYP2B6 genotype and plasma steady state OH-BUP/BUP ratio after controlling for CYP2C19 genotype.
Comparing the likelihood of the model without CYP2C19 versus with CYP2C19, adjusting for CYP2C19 did not significantly alter the association between CYP2B6 and plasma steady state OH-BUP/BUP ratio.
Indicates statistical significance.
Study 2: CYP2C19 Genotype Was Not Associated with Bupropion’s Ability to Promote Smoking Cessation.
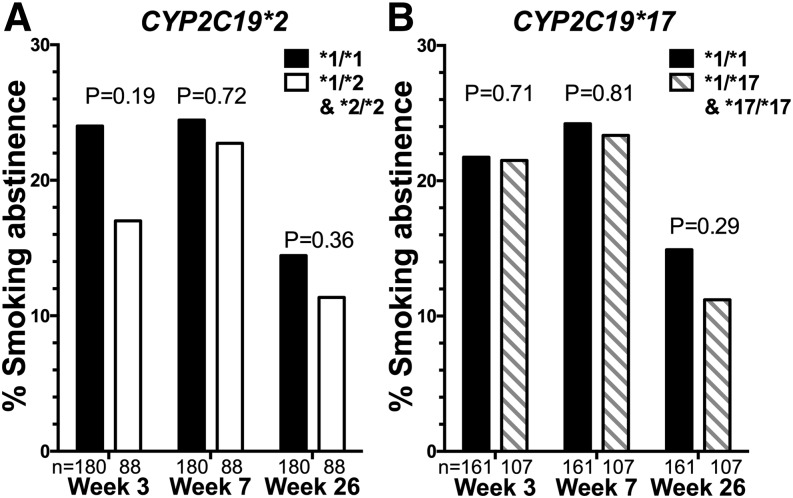
Next, we examined the clinical impact of CYP2C19 variants on bupropion’s ability to promote smoking cessation in smokers. There were 197 individuals with CYP2C19*1/*1 genotype (i.e., without CYP2C19*2 or CYP2C19*17), 120 individuals with CYP2C19*1/*2 genotype, 14 individuals with CYP2C19*2/*2 genotype, 137 individuals with the *1/*17 genotype, 47 individuals with *2/*17 genotype, and 20 individuals with *17/*17 genotype (Supplemental Table 3). In this double-blind, placebo-controlled, randomized clinical trial, CYP2C19 genotype was not significantly associated with smoking cessation outcomes (Fig. 4, A and B).
Fig. 4.
CYP2C19*2 (A) and *17 (B) were not significantly associated with smoking cessation outcomes in smokers treated with bupropion (i.e., within the bupropion arm of study 2). The values below the bars represent the total number of smokers in each group. The P values were derived from chi square tests.
Discussion
We present the first evidence that CYP2C19 variants, particularly CYP2C19*2, can significantly alter the steady-state exposure (AUC) to bupropion and it metabolites EB and TB while having no impact on OH-BUP. Consistent with the limited pharmacological activity associated with BUP, EB, and TB, CYP2C19 genotype did not have a significant impact on bupropion’s efficacy in promoting smoking cessation in smokers.
CYP2C19, Bupropion Metabolism, and Pharmacokinetics.
The CYP2B6-mediated terminal hydroxylation of bupropion to OH-BUP was hypothesized to be a major bupropion metabolic clearance pathway (Hesse et al., 2000; Kirchheiner et al., 2003). However, a number of studies have reported that variation in CYP2B6 activity significantly altered OH-BUP pharmacokinetics, but not bupropion pharmacokinetics (Kirchheiner et al., 2003; Turpeinen et al., 2004, 2005; Zhu et al., 2012; Benowitz et al., 2013). Our study suggested that CYP2C19-mediated hydroxylation of bupropion is a quantitatively important bupropion metabolic clearance pathway since reduced CYP2C19 activity resulted in a significant increase in bupropion exposure (Chen et al., 2010). Our findings extend previous in vitro reports that CYP2C19 can metabolize bupropion (Chen et al., 2010), and suggest that the OH-BUP (made by CYP2B6) pathway is not a quantitatively important bupropion clearance pathway, hence explaining the lack of association between CYP2B6 activity and bupropion levels (Kirchheiner et al., 2003; Turpeinen et al., 2004, 2005). Since more than 20 different bupropion metabolites have been identified in urine after a bupropion administration, the quantitative and enzymatic aspects of additional bupropion pathways remain to be further clarified (Petsalo et al., 2007).
In the pharmacokinetic study, the direction of the CYP2C19 genotype effects on bupropion, EB, and TB pharmacokinetics were consistent with the known functional impact of these variants. For example, CYP2C19*2, a loss-of-function CYP2C19 allele (Scott et al., 2013), was associated with higher bupropion, EB, and TB exposure, suggesting a reduction in the metabolic clearance of each of these compounds. Interestingly, no metabolic rerouting toward OH-BUP was observed in CYP2C19 slow metabolizers, which might be because the CYP2B6 genotypes were not evenly balanced between CYP2C19 genotypes. Only 1 out of 17 CYP2C19 normal metabolizers was a CYP2B6 slow metabolizer, whereas 4 out of 15 CYP2C19 slow metabolizers were CYP2B6 slow metabolizers, reducing the ability to detect metabolic switching if it occurred.
A significant association between the gain-of-function CYP2C19*17 allele and bupropion pharmacokinetics was not observed. The lack of association between CYP2C19*17 and bupropion pharmacokinetics could be due to the low number of homozygous CYP2C19*17 individuals in our study (there was one) as CYP2C19*17 can act recessively toward some substrates (Gawronska-Szklarz et al., 2012). For example, individuals with CYP2C19*1/*17 genotypes had similar pantoprazole exposure compared with the CYP2C19*1/*1 individuals, whereas those with CYP2C19*17/*17 genotype had significantly lower pantoprazole exposure compared with the CYP2C19*1/*1 individuals (Gawronska-Szklarz et al., 2012).
CYP2C19 and Bupropion Treatment Efficacy.
Bupropion is used clinically to promote smoking cessation. Animal and human data suggest that bupropion’s ability to promote smoking cessation is at least partially mediated by its major plasma metabolite OH-BUP (Damaj et al., 2010; Zhu et al., 2012). At steady state, plasma OH-BUP levels are 10–20 times higher than plasma bupropion levels, and OH-BUP has higher affinity for the α4β2 nicotinic acetylcholine receptors than bupropion (Damaj et al., 2004). In study 2, no significant associations between CYP2C19 genotype and smoking cessation outcomes were found. This finding supports the hypothesis that bupropion’s smoking cessation pharmacology is primarily mediated by its CYP2B6-mediated active metabolite OH-BUP, which did not differ by CYP2C19 genotype (Zhu et al., 2012). Previously, we have found that there was no relationship between the levels of the parent drug bupropion and smoking cessation outcomes, whereas levels of OH-BUP were predictive (Zhu et al., 2012). Thus, the present observations of a difference in bupropion, but not OH-BUP levels, due to CYP2C19*2 are consistent with a lack of significant impact of parent bupropion levels on smoking cessation outcomes; our current sample size was statistically powered to reject a 17% difference in smoking cessation outcomes.
Implications.
Although not directly associated with bupropion treatment outcomes, genetic variation in CYP2C19 may still influence side effects, or toxicity, associated with bupropion treatment. For example, the cardiotoxicity associated with bupropion is likely mediated by bupropion itself rather than OH-BUP, and the levels of bupropion could be altered by CYP2C19 (Al-Abri et al., 2013). Furthermore, the reductive metabolites of bupropion, TB and EB, are potent CYP2D6 inhibitors (Parkinson et al., 2010). Therefore, it is possible that variation in CYP2C19 activity could modulate the drug-drug interaction between bupropion and CYP2D6 substrates.
Another potential implication of the involvement of CYP2C19 in bupropion metabolism is that CYP2C19 may alter the utility of the OH-BUP/BUP ratio as a marker of CYP2B6 activity, as CYP2C19 alters bupropion levels. However, data from the pharmacokinetic study suggests that CYP2C19 genotype did not affect the association between the steady-state OH-BUP/BUP ratio and CYP2B6 genotype. It is not clear whether this would also be the case in single-dosing paradigms, or among other ethnic groups with a higher frequency of reduced-function CYP2C19 alleles.
In conclusion, our study reports the first in vivo evidence that CYP2C19 is involved in the metabolism of bupropion, EB, and TB, and that loss-of-function CYP2C19 variants could significantly increase the steady-state exposure to bupropion and its reductive metabolites. These pharmacokinetic changes were not associated with differences in bupropion’s ability to promote smoking cessation in smokers, but may influence the side effects, and toxicity, associated with bupropion.
Supplementary Material
Acknowledgments
The authors thank Lisa Yu, Olivia Yturralde, Polly Cheung, and Peyton Jacob III for assay development and performing the bupropion analytical chemistry.
Abbreviations
- AUC
area under the plasma concentration–time curve
- EB
erythrohydrobupropion
- OH-BUP
hydroxybupropion
- OH-BUP/BUP
hydroxybupropion/bupropion
- TB
threohydrobupropion
Authorship Contributions
Participated in research design: Cox, Benowitz, Tyndale.
Conducted experiments: Zhu, Zhou, Cox, Ahluwalia, Benowitz.
Contributed new reagents or analytic tools: Benowitz.
Performed data analysis: Zhu.
Wrote or contributed to the writing of the manuscript: Zhu, Zhou, Cox, Ahluwalia, Benowitz, Tyndale.
Footnotes
This work was supported by the National Institutes of Health [Grants DA020830, CA78603, CA091912, DA12393], and National Institutes of Health grants through University of California, San Francisco Clinical and Translational Science Institute Grant Number UL1 RR024131, Canadian Institutes of Health Research [Grant MOP86471], the Endowed Chair in Addiction for the Department of Psychiatry University of Toronto, grants from the Centre for Addiction and Mental Health (CAMH) and the CAMH Foundation, grants from the Canada Foundation for Innovation [Grants 20289 and 16014], and a grant from the Ontario Ministry of Research and Innovation.
N.L.B. serves as a consultant to several pharmaceutical companies that market smoking cessation medications, and has been a paid expert witness in litigation against tobacco companies. R.F.T. has participated in one-day advisory meetings for Novartis and McNeil.
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Al-Abri SA, Orengo JP, Hayashi S, Thoren KL, Benowitz NL, Olson KR. (2013) Delayed bupropion cardiotoxicity associated with elevated serum concentrations of bupropion but not hydroxybupropion. Clin Toxicol (Phila) 51:1230–1234 [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Zhu AZ, Tyndale RF, Dempsey D, Jacob P., 3rd (2013) Influence of CYP2B6 genetic variants on plasma and urine concentrations of bupropion and metabolites at steady state. Pharmacogenet Genomics 23:135–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz RF, Schroeder DH, Welch RM, Mehta NB, Phillips AP, Findlay JW. (1981) Radioimmunoassay and pharmacokinetic profile of bupropion in the dog. J Pharmacol Exp Ther 217:602–610 [PubMed] [Google Scholar]
- Chen Y, Liu HF, Liu L, Nguyen K, Jones EB, Fretland AJ. (2010) The in vitro metabolism of bupropion revisited: concentration dependent involvement of cytochrome P450 2C19. Xenobiotica 40:536–546 [DOI] [PubMed] [Google Scholar]
- Cox LS, Nollen NL, Mayo MS, Choi WS, Faseru B, Benowitz NL, Tyndale RF, Okuyemi KS, Ahluwalia JS. (2012) Bupropion for smoking cessation in African American light smokers: a randomized controlled trial. J Natl Cancer Inst 104:290–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Carroll FI, Eaton JB, Navarro HA, Blough BE, Mirza S, Lukas RJ, Martin BR. (2004) Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Mol Pharmacol 66:675–682 [DOI] [PubMed] [Google Scholar]
- Damaj MI, Grabus SD, Navarro HA, Vann RE, Warner JA, King LS, Wiley JL, Blough BE, Lukas RJ, Carroll FI. (2010) Effects of hydroxymetabolites of bupropion on nicotine dependence behavior in mice. J Pharmacol Exp Ther 334:1087–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVane CL, Laizure SC, Stewart JT, Kolts BE, Ryerson EG, Miller RL, Lai AA. (1990) Disposition of bupropion in healthy volunteers and subjects with alcoholic liver disease. J Clin Psychopharmacol 10:328–332 [PubMed] [Google Scholar]
- Faucette SR, Hawke RL, Lecluyse EL, Shord SS, Yan B, Laethem RM, Lindley CM. (2000) Validation of bupropion hydroxylation as a selective marker of human cytochrome P450 2B6 catalytic activity. Drug Metab Dispos 28:1222–1230 [PubMed] [Google Scholar]
- Faucette SR, Hawke RL, Shord SS, Lecluyse EL, Lindley CM. (2001) Evaluation of the contribution of cytochrome P450 3A4 to human liver microsomal bupropion hydroxylation. Drug Metab Dispos 29:1123–1129 [PubMed] [Google Scholar]
- Fradette C, Yamaguchi N, Du Souich P. (2004) 5-Hydroxytryptamine is biotransformed by CYP2C9, 2C19 and 2B6 to hydroxylamine, which is converted into nitric oxide. Br J Pharmacol 141:407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawrońska-Szklarz B, Adamiak-Giera U, Wyska E, Kurzawski M, Gornik W, Kaldonska M, Drozdzik M. (2012) CYP2C19 polymorphism affects single-dose pharmacokinetics of oral pantoprazole in healthy volunteers. Eur J Clin Pharmacol 68:1267–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, Kim DD, Dunayevich E, COR-I Study Group (2010) Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 376:595–605 [DOI] [PubMed] [Google Scholar]
- Haas JS, Kaplan CP, Barenboim D, Jacob P, 3rd, Benowitz NL. (2004) Bupropion in breast milk: an exposure assessment for potential treatment to prevent post-partum tobacco use. Tob Control 13:52–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse LM, He P, Krishnaswamy S, Hao Q, Hogan K, von Moltke LL, Greenblatt DJ, Court MH. (2004) Pharmacogenetic determinants of interindividual variability in bupropion hydroxylation by cytochrome P450 2B6 in human liver microsomes. Pharmacogenetics 14:225–238 [DOI] [PubMed] [Google Scholar]
- Hesse LM, Venkatakrishnan K, Court MH, von Moltke LL, Duan SX, Shader RI, Greenblatt DJ. (2000) CYP2B6 mediates the in vitro hydroxylation of bupropion: potential drug interactions with other antidepressants. Drug Metab Dispos 28:1176–1183 [PubMed] [Google Scholar]
- Hsyu PH, Singh A, Giargiari TD, Dunn JA, Ascher JA, Johnston JA. (1997) Pharmacokinetics of bupropion and its metabolites in cigarette smokers versus nonsmokers. J Clin Pharmacol 37:737–743 [DOI] [PubMed] [Google Scholar]
- Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, Khayrallah MA, Schroeder DR, Glover PN, Sullivan CR, et al. (1997) A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med 337:1195–1202 [DOI] [PubMed] [Google Scholar]
- Johnston AJ, Ascher J, Leadbetter R, Schmith VD, Patel DK, Durcan M, Bentley B. (2002) Pharmacokinetic optimisation of sustained-release bupropion for smoking cessation. Drugs 62 (Suppl 2):11–24 [DOI] [PubMed] [Google Scholar]
- Kirchheiner J, Klein C, Meineke I, Sasse J, Zanger UM, Mürdter TE, Roots I, Brockmöller J. (2003) Bupropion and 4-OH-bupropion pharmacokinetics in relation to genetic polymorphisms in CYP2B6. Pharmacogenetics 13:619–626 [DOI] [PubMed] [Google Scholar]
- Lai AA, Schroeder DH. (1983) Clinical pharmacokinetics of bupropion: a review. J Clin Psychiatry 44:82–84 [PubMed] [Google Scholar]
- Laizure SC, DeVane CL. (1985) Stability of bupropion and its major metabolites in human plasma. Ther Drug Monit 7:447–450 [DOI] [PubMed] [Google Scholar]
- Laizure SC, DeVane CL, Stewart JT, Dommisse CS, Lai AA. (1985) Pharmacokinetics of bupropion and its major basic metabolites in normal subjects after a single dose. Clin Pharmacol Ther 38:586–589 [DOI] [PubMed] [Google Scholar]
- Meyer A, Vuorinen A, Zielinska AE, Strajhar P, Lavery GG, Schuster D, Odermatt A. (2013) Formation of threohydrobupropion from bupropion is dependent on 11β-hydroxysteroid dehydrogenase 1. Drug Metab Dispos 41:1671–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira R. (2011) The efficacy and tolerability of bupropion in the treatment of major depressive disorder. Clin Drug Investig 31 (Suppl 1):5–17 [DOI] [PubMed] [Google Scholar]
- Parkinson A, Kazmi F, Buckley DB, Yerino P, Ogilvie BW, Paris BL. (2010) System-dependent outcomes during the evaluation of drug candidates as inhibitors of cytochrome P450 (CYP) and uridine diphosphate glucuronosyltransferase (UGT) enzymes: human hepatocytes versus liver microsomes versus recombinant enzymes. Drug Metab Pharmacokinet 25:16–27 [DOI] [PubMed] [Google Scholar]
- Petsalo A, Turpeinen M, Tolonen A. (2007) Identification of bupropion urinary metabolites by liquid chromatography/mass spectrometry. Rapid Commun Mass Spectrom 21:2547–2554 [DOI] [PubMed] [Google Scholar]
- Scott SA, Sangkuhl K, Gardner EE, Stein CM, Hulot JS, Johnson JA, Roden DM, Klein TE, Shuldiner AR, Clinical Pharmacogenetics Implementation Consortium (2011) Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450-2C19 (CYP2C19) genotype and clopidogrel therapy. Clin Pharmacol Ther 90:328–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SA, Sangkuhl K, Stein CM, Hulot JS, Mega JL, Roden DM, Klein TE, Sabatine MS, Johnson JA, Shuldiner AR, Clinical Pharmacogenetics Implementation Consortium (2013) Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther 94:317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim SC, Risinger C, Dahl ML, Aklillu E, Christensen M, Bertilsson L, Ingelman-Sundberg M. (2006) A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther 79:103–113 [DOI] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification (2002) Biochemical verification of tobacco use and cessation. Nicotine Tob Res 4:149–159 [DOI] [PubMed] [Google Scholar]
- Sweet RA, Pollock BG, Kirshner M, Wright B, Altieri LP, DeVane CL. (1995) Pharmacokinetics of single- and multiple-dose bupropion in elderly patients with depression. J Clin Pharmacol 35:876–884 [DOI] [PubMed] [Google Scholar]
- Turpeinen M, Nieminen R, Juntunen T, Taavitsainen P, Raunio H, Pelkonen O. (2004) Selective inhibition of CYP2B6-catalyzed bupropion hydroxylation in human liver microsomes in vitro. Drug Metab Dispos 32:626–631 [DOI] [PubMed] [Google Scholar]
- Turpeinen M, Tolonen A, Uusitalo J, Jalonen J, Pelkonen O, Laine K. (2005) Effect of clopidogrel and ticlopidine on cytochrome P450 2B6 activity as measured by bupropion hydroxylation. Clin Pharmacol Ther 77:553–559 [DOI] [PubMed] [Google Scholar]
- Zhu AZ, Cox LS, Nollen N, Faseru B, Okuyemi KS, Ahluwalia JS, Benowitz NL, Tyndale RF. (2012) CYP2B6 and bupropion’s smoking-cessation pharmacology: the role of hydroxybupropion. Clin Pharmacol Ther 92:771–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.