Abstract
Since approval of rituximab for treatment of B cell non-Hodgkin lymphoma, development of monoclonal antibodies (mAbs) for cancer treatment and elucidation of their cytotoxic mechanisms have been subject to intense investigations. Compelling evidence indicates that rituximab and another CD20 mAb, ofatumumab, must use the body’s cellular and humoral immune effector functions to kill malignant cells. Other U.S. Food and Drug Administration–approved mAbs, including obinutuzumab, cetuximab, and trastuzumab, require, in part, these effector mechanisms to eliminate tumor cells. Although gram quantities of mAbs can be administered to patients, our investigations of CD20 mAb-based therapies for chronic lymphocytic leukemia (CLL), including correlative measurements in clinical trials and studies with primary cells and cell lines, indicate that effector mechanisms necessary for mAb activity can be saturated or exhausted if tumor burdens are high, thus substantially compromising the efficacy of high-dose mAb therapy. Under these conditions, another reaction (trogocytosis) predominates in which bound CD20 mAb and CD20 are removed from targeted cells by effector cells that express Fcγ receptors, thereby allowing malignant cells to escape unharmed and continue to promote disease pathology. To address this problem, we propose that a low-dose strategy, based on administering 30–50 mg of CD20 mAb three times per week, may be far more effective for CLL than standard dosing because it will minimize effector function saturation and reduce trogocytosis. This approach may have general applicability to other mAbs that use immune effector functions, and could be formulated into a subcutaneous treatment strategy that would be more accessible and possibly more efficacious for patients.
Introduction
There is a voluminous literature that documents the successful use of monoclonal antibodies (mAbs) in the immunotherapy of cancer (Scott et al., 2012; Mahalingam and Curiel, 2013; Sliwkowski and Mellman, 2013; Zigler et al., 2013). However, although numerous clinical investigations have demonstrated varying degrees of efficacy of a given mAb (alone or in combination with chemotherapy), considerable uncertainty remains with respect to which mechanisms promote tumor cell elimination in humans (Glennie et al., 2007; Boross and Leusen, 2012; Sliwkowski and Mellman, 2013; Zigler et al., 2013). Studies in mouse models have provided insight but may be model dependent, favoring one mechanism over another, based simply on the details of the model design (Taylor and Lindorfer, 2014). Perhaps the greatest controversy centers on distinguishing between direct cytotoxic effects of a mAb on tumor cells and/or their environment versus establishing an absolute requirement of the mAb to harness one or more of the body’s immune effector mechanisms to kill tumor cells. For example, based only on in vitro experiments with cell lines, binding of the CD20 mAbs rituximab (RTX), ofatumumab (OFA), and obinutuzumab (OBZ) to B cells may initiate signaling cascades that mediate cell killing directly by pathways that include apoptosis as well as, in the case of OBZ, a noncaspase-dependent lysosomal reaction pathway (Glennie et al., 2007; Mössner et al., 2010; Alduaij et al., 2011). However, increasing evidence, based on rigorous experiments with primary tumor cells, carefully controlled murine model studies, and correlative measurements in clinical trials, has clearly demonstrated that the most important cytotoxic mechanisms of these mAbs require immune effector functions (Gong et al., 2005; Glennie et al., 2007; Wilson et al., 2011; Beurskens et al., 2012; Golay and Introna, 2012; Bologna et al., 2013; Golay et al., 2013a; Montalvao et al., 2013). That is, tumor cells that are opsonized with CD20 mAbs are killed by cellular effector reactions which include antibody-dependent cell-mediated cytotoxicity (ADCC), phagocytosis by macrophages and possibly neutrophils, or by complement-dependent cytotoxicity.
Because these effector functions are absolutely required for CD20 mAb efficacy, we submit that the usual pharmacological concepts of maximum tolerated dose and dose-limiting toxicity, axiomatic for evaluation of chemotherapeutic agents for cancer treatment, are not applicable for use of these mAbs. Indeed, although the pharmacokinetics and pharmacodynamics of RTX and of OFA for high mAb doses have been intensively studied (Berinstein et al., 1998; Coiffier et al., 2010; Golay et al., 2013b), several lines of evidence indicate that, particularly for chronic lymphocytic leukemia (CLL), the most effective doses and their timing require critical re-evaluation (Lindorfer et al., 2012; Baig et al., 2014; Zent et al., 2014).
RTX, the first mAb approved for treatment of cancer, has proven quite successful in the treatment of B cell lymphomas (McLaughlin et al., 1998; Davis et al., 2000; Cheson and Leonard, 2008; Weiner, 2010). Indeed, when combined with chemotherapy, the usual 375 mg/m2 dose of RTX was found to provide substantial therapeutic benefit for a number of indications, including CLL (Hallek et al., 2010; Furman et al., 2014). Therefore, a considerable research effort has been devoted to understanding the cytotoxic mechanisms of RTX as well as its limitations to develop second- and third-generation CD20 mAbs designed to have enhanced clinical activity (Teeling et al., 2004; Cheson, 2010; Mössner et al., 2010; Alduaij et al., 2011; Peipp et al., 2011). Recent provocative evidence indicates that other U.S. Food and Drug Administration–approved mAbs, including cetuximab (antiepidermal growth factor receptor), ipilimumab (anticytotoxic T lymphocyte associated antigen 4), and trastuzumab (antihuman growth factor receptor 2), also directly or indirectly make use of effector mechanisms mediated by cells that express Fcγ receptors (Zhang et al., 2007; Musolino et al., 2008; Taylor et al., 2009; Botta et al., 2012; Bulliard et al., 2013; Kim and Ashkenazi, 2013; Simpson et al., 2013; Bianchini and Gianni, 2014). Therefore, the lessons learned based on analyses of CD20 mAbs may have general implications for these mAbs as well.
Correlative Studies Associated with CD20 mAb Treatment of CLL
Ten years ago, we first reported results of correlative studies based on analyses of blood samples drawn from patients with CLL who were being treated with the standard weekly doses of 375 mg/m2 RTX (Kennedy et al., 2004). These results have been replicated several times and thus provide a framework for understanding key issues that underlie use of unconjugated mAbs in cancer immunotherapy. We found that after infusion of only 30 mg RTX, approximately 70% of the circulating CLL cells present before infusion were removed from the circulation, principally due to clearance of the RTX-opsonized cells by fixed tissue macrophages in the liver and spleen (Schreiber and Frank, 1972; Atkinson and Frank, 1974; Montalvao et al., 2013). Surviving circulating CLL cells were also opsonized with inactive complement fragment C3d. Based on comparable studies with antibody-opsonized erythrocytes, the clearance mechanism may have been mediated in part synergistically by Fcγ receptors and complement receptors on macrophages (Schreiber and Frank, 1972; Atkinson and Frank, 1974; Lindorfer et al., 2014). Immediately after completion of the full RTX infusions (600–700 mg), circulating CLL cell levels had increased considerably (relative to the levels after infusion of only 30 mg) due to re-equilibration of a “second wave” of cells from other compartments, and these cells persisted in the bloodstream despite high plasma levels of RTX (approximately 100 µg/ml). A key clue to understanding why these cells were not cleared from the circulation was revealed when we found that CD20 expression on these “surviving” CLL cells was substantially reduced, approximately 20-fold in most cases. In addition, complement titers were also reduced 10-fold or more in several patients. This was the first observation of what we have characterized as the “perfect storm” that occurs when large doses of CD20 mAbs are infused in CLL patients with high burdens of circulating malignant cells. Under these conditions, after an initial very rapid clearance of a large fraction of circulating cells, the surviving CLL cells are no longer subject to attack or clearance, despite the presence of large amounts of the CD20 mAb in the bloodstream. These cells have very low levels of CD20, and the low CD20 levels persist for several days to weeks, due to the continued presence of the mAb in the circulation (Beurskens et al., 2012; Baig et al., 2014). In addition, for some period of time, an important effector function (complement) is exhausted (Kennedy et al., 2004; Beurskens et al., 2012). Moreover, as we recently reported in a second observational study of OFA immunotherapy, cells that are isolated from the bloodstream soon after mAb infusion are no longer subject to complement-dependent cytotoxicity, even in the presence of fresh serum and additional CD20 mAb, presumably because CD20 levels are so low (Baig et al., 2014). These observations have been replicated in more than 60 CLL patients in several clinical studies conducted at the University of Virginia, the National Institutes of Health, and the Mayo Clinic (Kennedy et al., 2004; Williams et al., 2006; Beurskens et al., 2012; Baig et al., 2014; Zent et al., 2014).
Trogocytosis of mAb-Opsonized Cells
CD20 is expressed at quite comparable levels on CLL cells in the bloodstream and in other compartments (Tam et al., 2008). We concluded, based on comprehensive in vitro experiments as well as a mouse model, that the second wave of cells that re-equilibrates into the bloodstream, as well as cells not cleared in the early phase of the CD20 mAb infusion, rapidly lose CD20 due to trogocytosis or “shaving” (Beum et al., 2006), which predominates after natural clearance mechanisms are saturated or exhausted.
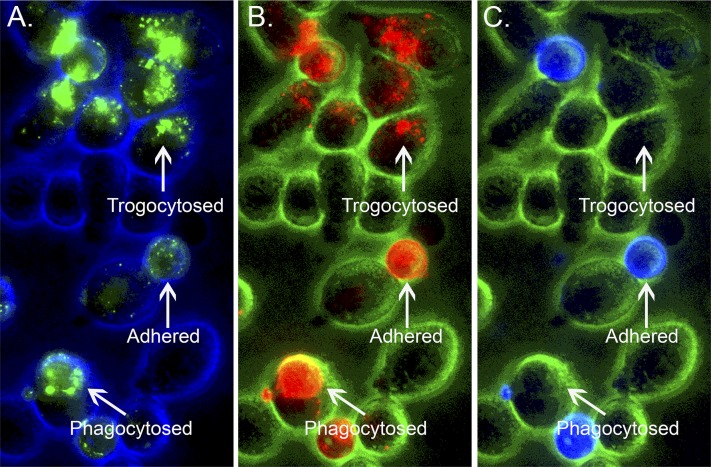
Trogocytosis is mediated by acceptor cells that express Fcγ receptors, including macrophages, monocytes, natural killer (NK) cells, and neutrophils (Beum et al., 2006, 2008a, 2011; Li et al., 2007). During trogocytosis the mAb-opsonized target donor cell and the acceptor cell first form an immunologic synapse, due to binding of Fcγ receptors on the acceptor cells to cognate Fc sites on the “immune-complexed” mAb bound to CD20 on the opsonized B cells (Joly and Hudrisier, 2003; Rossi et al., 2013; Taylor, 2013). The acceptor cell then removes the mAb/CD20 immune complex from the opsonized B cell along with a portion of the plasma membrane, and ultimately internalizes the immune complex. The reaction is rapid; the process goes to completion in less than 1 hour. At first examination, this reaction appears to be antithetical to Metchnikoff’s definition of macrophages as “big eaters,” which should engage in phagocytosis (Taylor, 2013). However, our in vitro experiments indicate that macrophages are capable of executing both processes when presented with RTX-opsonized cells (Fig. 1) (Daubeuf et al., 2010). That is, in certain cases, the macrophages are true to their phenotype and completely internalize opsonized B cells; however, in other cases, the macrophages simply remove and internalize CD20 and RTX. We confirmed, by flow cytometry, that the recovered B cells had indeed lost substantial amounts of bound RTX and CD20, but were otherwise intact (Daubeuf et al., 2010).
Fig. 1.
Both phagocytosis and trogocytosis occur simultaneously. 38C13-CD20+ B cells opsonized with Al488 RTX and dyed with PKH-26 were cocultured for 30 minutes with adhered J774 macrophages. After the 38C13-CD20+ B cells were removed, the adherent J774 cells were stained with Al647 anti-mouse IgM and then analyzed by fluorescence microscopy for Al488 RTX (A), PKH-26 (B), and Al647 anti-mouse IgM (C). Based on the images, selected regions in which the B cells were still adhered or in which the B cells had been either phagocytosed or trogocytosed by the J774 cells are identified. Original magnification, ×40. Reprinted with permission from Daubeuf et al. (2010). Copyright 2010, The American Association of Immunologists, Inc.
The results of these experiments place into context our pilot clinical study in which lower doses of RTX were infused in CLL patients (Williams et al., 2006). We reasoned that thrice-weekly intravenous RTX doses of only 20 mg/m2 should provide enough mAb to target and clear circulating cells, but that the small dose of RTX would minimize its concentration in the bloodstream afterward. Therefore, these low doses should better preserve effector functions, reduce CD20 loss via trogocytosis, and allow for more rapid re-expression of CD20 on CLL cells, thus making possible additional targeting and clearance of cells after subsequent low-dose RTX infusions. The general paradigm was validated in that study and was recently confirmed (Zent et al., 2014). During each infusion, targeted CLL cells are cleared very quickly, supporting the concept that the clearance mechanism follows the same pattern reported by Frank et al. for clearance of IgG-opsonized erythrocytes (Schreiber and Frank, 1972; Atkinson and Frank, 1974). We also found that B cell clearance and trogocytosis of CD20 occurred simultaneously. The most reasonable explanation is that as RTX-opsonized cells come into contact with fixed tissue macrophages in the liver and spleen, some cells are removed and phagocytosed, whereas others are partially shaved and return back into the bloodstream. However, because these cells still have bound IgG RTX, they can be cleared in the second or third or even later passes through these organs.
The Importance of Exhaustion
It is clear that very large quantities (approximately 2 g) of immunotherapeutic mAbs such as RTX or OFA can be infused intravenously in patients, because for the most part there is no dose-limiting toxicity. However, at high B cell burdens in CLL, high mAb doses generate very large quantities of “immune complexes” (mAb-opsonized cells) that can not only activate and exhaust complement, but also can overwhelm and saturate cell-mediated effector functions. One of these is phagocytosis and/or direct killing of CD20 mAb-opsonized cells by macrophages. Several well designed mouse models have clearly demonstrated the importance of this cytotoxic mechanism, and have provided evidence for saturation or exhaustion. Boross et al. (2011) examined how low and high tumor burdens are handled in a peritoneal syngeneic mouse model. They found that at low cell burdens, complement is adequate to clear the cells; however, at 10-fold higher cell burdens, both complement and macrophage-mediated killing and clearance are required. However, even though 10-fold more mAb is administered at the higher cell burdens, thus maintaining the same mAb/tumor ratio, the percentage of cells cleared drops from 95% to 70%. That is, there is adequate mAb to easily saturate the cells with anti-CD20 mAb for both challenges, but the effector mechanisms simply cannot adequately process and destroy the large number of immune-complexed, mAb-opsonized cells at the higher tumor burdens. These observations are reinforced by in vitro studies that indicate that a monocyte-derived human macrophage can phagocytose no more than 10 RTX-opsonized CLL cells (C. Zent, personal communication). The macrophage cannot take up any more RTX-opsonized cells for at least 24 hours, until the ingested cells are processed and degraded. The human liver has approximately 3 × 1010 Kupffer cells (macrophages) (Boyer, 2003). Given the high circulating cell burdens common in CLL (100,000 cells/µl), thus corresponding to about 4 × 1011 malignant B cells, clearance of 80%–90% of these mAb-opsonized cells by liver macrophages presents a real challenge. In addition, malignant cells will rapidly re-equilibrate from other compartments. Therefore, it is not surprising that after infusions of even large quantities of RTX or OFA, the cell counts drop precipitously but then increase over 24 hours, even though the mAb remains at high concentrations in the bloodstream.
Similarly, ADCC of CD20 mAb-opsonized cells mediated by NK cells can also be exhausted at high cell burdens. Berdeja et al. (2007) reported that 1 hour after treatment of lymphoma patients with large doses of RTX, the ADCC activity of their NK cells against RTX-opsonized targets was substantially reduced, but was partially restored after 24 hours. This clinical observation of NK cell exhaustion is complemented by several in vitro investigations. Bhat and Watzl (2007) reported that after NK cells had killed two to four substrates, the levels of perforin and granzyme in the cells had decreased, and the killing capacity of the cells was substantially reduced for at least 24 hours; indeed, they designated these cells as “exhausted NK cells.” Comprehensive in vitro investigations reported by Weiner et al. indicate that levels of CD16 are reduced considerably when NK cells mediate ADCC of RTX-opsonized cells, and these reductions in CD16 correlate with ADCC (Bowles and Weiner, 2005; Veeramani et al., 2011). In the absence of CD16, the NK cells cannot mediate ADCC and would clearly have an “exhausted” phenotype. Finally, Zent et al. (2014) recently reported that CD16 is also rapidly reduced on circulating NK cells when CLL patients are treated with low doses of RTX, providing powerful in vivo evidence for reaction of NK cells with RTX-opsonized circulating CLL cells. Presumably, due to the low-dose treatment, levels of CD16 would be expected to return in a few days, but this issue has not yet been directly addressed.
Possible Generalization to Other Immunotherapeutic mAbs
OBZ (GA101; Genentech, South San Francisco, CA) is a glycoengineered type II CD20 mAb that was recently approved for the treatment of CLL. A phase 3 study demonstrated substantial efficacy for OBZ plus chlorambucil in the treatment of CLL (Goede et al., 2014). The cytotoxic mechanisms used by OBZ in killing CLL cells are still not completely defined, but considerable evidence indicates that, in common with RTX and OFA, effector functions likely play major roles in its cytotoxic action (Bologna et al., 2011). We are unaware of any correlative studies for OBZ to date that are comparable with the studies we have cited for RTX and OFA, but other type II CD20 mAbs are capable of promoting trogocytosis of CD20 in vitro (Pedersen et al., 2011).
Many of our findings of trogocytosis and effector function exhaustion with respect to CD20 mAbs in CLL have been replicated and extended, in some cases to other mAb-antigen pairs, in the clinic and in the laboratory (Boross et al., 2012; Jones et al., 2012; Masuda et al., 2013; Rossi et al., 2013; Baig et al., 2014). Therefore, the implications of these studies with respect to use of CD20 mAbs may also pertain to other mAbs currently used to treat cancer. Indeed, it was first thought that the principal mechanisms of action of both cetuximab and trastuzumab were based on direct cell killing via signaling and downstream apoptotic mechanisms. However, several recent reports, both in preclinical models and based on correlative studies, strongly suggest that a substantial component of their cytotoxic mechanisms is derived from cellular effector functions mediated by Fcγ receptors on monocytes, macrophages, and NK cells.
For example, trastuzumab had considerable efficacy in suppressing human tumor cell growth in a xenograft mouse model; however, the mAb had only modest activity in a comparable study in mice in which the common γ chain was knocked out, thus eliminating Fcγ receptor-mediated activity on effector cells (Clynes et al., 2000). Fcγ receptor IIIA polymorphisms correlated with response to trastuzumab in breast cancer patients, a correlation that also has been reported for RTX therapy in patients with non-Hodgkin lymphoma (Cartron et al., 2002; Weng and Levy, 2003; Musolino et al., 2008). Indeed, Varchetta et al. (2007) found that CD16 is severely reduced on NK cells when they promote ADCC of trastuzumab-opsonized cells, suggesting that NK cell exhaustion may also be associated with trastuzumab therapy of breast cancer. Finally, stimulation of CD137 on NK cells can increase ADCC of both trastuzumab-opsonized breast cancer cells as well as of RTX-opsonized B cells, again implying that cell killing mechanisms mediated by Fcγ receptors on effector cells are also important for trastuzumab (Kohrt et al., 2011, 2012).
Evidence that cetuximab makes use of cell-based effector mechanisms to eliminate cancer cells derives from clinical correlative studies and in vitro investigations. Polymorphisms in Fcγ receptors IIA and IIIA correlate with increases in progression-free survival for colorectal cancer patients treated with either single-agent cetuximab, or with cetuximab plus irinotecan (Zhang et al., 2007; Taylor et al., 2009; Botta et al., 2012). CD20 is also decreased on targeted cells in tissues after RTX infusion (Laurent et al., 2007; Teng et al., 2007), but there is little evidence to indicate whether the analogous reaction occurs in vivo after infusion of cetuximab or trastuzumab. We have demonstrated that both cetuximab and trastuzumab can promote the shaving reaction in vitro (Beum et al., 2008b). In view of the substantial tumor burdens associated with cancers that are being treated with these mAbs, it is likely that effector functions will be saturated in these cases as well.
Quantitative Considerations
The amount of mAb required to saturate the antigenic sites on a tumor can be far less than the quantities routinely administered. In the case of CLL, just 10 mg of an infused CD20 mAb will saturate 100,000 antigenic sites/cell on circulating CLL cells at levels of 100,000 cells/μl blood (Lindorfer et al., 2012). More cells will re-equilibrate from other compartments, and the question of delivery to and penetration of liquid or solid tumors by the mAb constitutes an additional uncertainty (Jain and Baxter, 1988). These considerations provide reasonable justification for treating patients with large amounts of mAb. However, if the mAb requires effector functions to eliminate tumor cells, then treatment of a large tumor burden with a high mAb dose is likely to result in saturation of effector functions. The consequence, in the case of CD20 mAbs, is that opsonized cells are subject to trogocytosis; ironically, the excess CD20 mAb remaining in the circulation actually helps the circulating malignant B cells “escape” by promoting trogocytosis of mAb/CD20 complexes (Beum et al., 2006). The pharmacokinetics and pharmacodynamics of RTX and OFA have been comprehensively studied (Berinstein et al., 1998; Coiffier et al., 2010; Golay et al., 2013b). At high doses, RTX (and OFA) can persist in the circulation for several months, and we found that CD20 levels on circulating CLL cells remained depressed over extended periods for up to 1 month or longer after infusions of large doses of these mAbs (Kennedy et al., 2004; Beurskens et al., 2012).
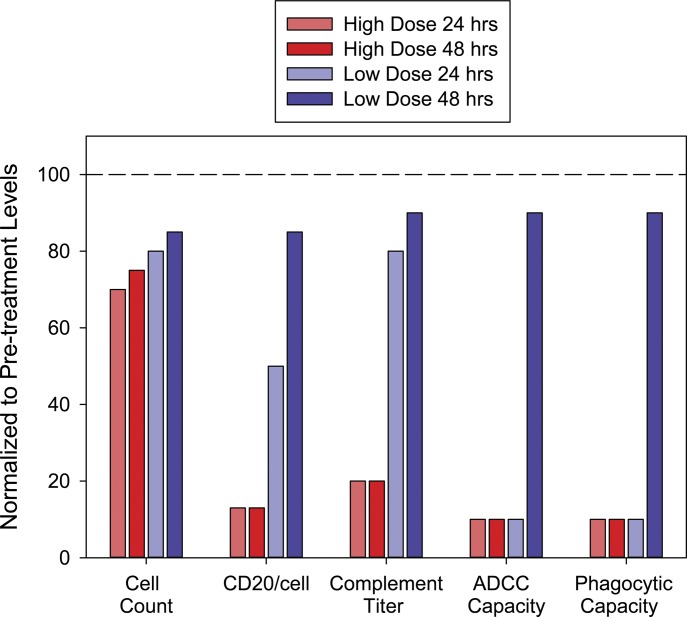
Based on our correlative observations in the clinical trials, Fig. 2 provides an idealized summary that compares and approximates the changes in the key measureable parameters that are evaluated under conditions of either high-dose or low-dose CD20 mAb therapy in CLL (Beum et al., 2004; Kennedy et al., 2004; Williams et al., 2006; Beurskens et al., 2012; Baig et al., 2014; Zent et al., 2014). There have been exceptions to these patterns, but the overall trends have been replicated in numerous studies. The key point (Fig. 2) is that 48 hours after an injection of only 20 mg/m2 mAb, CD20 is expressed at relatively high levels on CLL B cells (compared with pretreatment) in the low-dose group. In addition, the body’s immune effector functions have recovered and reset from the first infusion, and thus are capable of clearing the next round of cells when more mAb is infused. A similar pattern for the low-dose group is seen for subsequent infusions at 48-hour intervals.
Fig. 2.
This idealized schematic summarizes the expected differences in key correlative parameters over 48 hours when CLL patients receive high doses [either 375 mg/m2 (approximately 700 mg) or 300 mg] or low doses [20 mg/m2 (approximately 35 mg)] of CD20 mAbs. The plasma concentrations of the mAbs immediately after completion of the respective first doses are approximately 100, 34, or 4 µg/ml. The results for individual patients are highly variable but the overall pattern displayed is representative of the general trends that have been observed. All values are relative to pretreatment levels. Cell count indicates absolute lymphocyte counts. Although levels of circulating CLL cells drop dramatically during infusion of the first 30 mg of mAb, re-equilibration from other compartments results in return to near pretreatment levels in a few days for both high and low doses. CD20/cell indicates that the default mechanism (trogocytosis) takes over after effector functions are saturated or exhausted with high doses of mAb. Regarding effector functions, the complement titer is sharply reduced with high doses, but is only slightly reduced with low doses. When high doses are administered, ADCC and phagocytosis are exhausted and/or rendered ineffective due to trogocytosis.
Concluding Remarks: The Way Forward
On this basis, we strongly suggest that analysis of the pharmacokinetics and pharmacodynamics of mAbs that require immune effector functions to eliminate cells may not provide the most important information with respect to efficacy and proper dosing, and that additional measurements should be made. In the case of CD20 mAb therapy in CLL, the level of CD20 on circulating B cells should be evaluated periodically to refine the treatment schedule. In addition, we propose that dynamic monitoring of a patient’s immune effector function status, including complement titer, and determination of the levels and fitness of circulating effector cells (expression of CD16 as well as of activation markers) to engage and kill mAb-opsonized cells (Bowles and Weiner, 2005; Berdeja et al., 2007; Bhat and Watzl, 2007) will better inform the design and implementation of dosing paradigms.
We propose that a more reasonable and generally applicable dosing paradigm would be to periodically treat cancer patients with much smaller mAb doses, either intravenously at 30–40 mg or subcutaneously at 50–60 mg to compensate for less efficient absorption (Golay et al., 2013b), and to repeat these doses approximately three times per week (Williams et al., 2006; Aue et al., 2010; Zent et al., 2014). The hypothesis is that each infusion will promote killing of a fraction of the tumor cells, and that trogocytosis will be minimized. Moreover, the effector systems will have time to recover based on this schedule, thereby allowing for a much higher degree of mAb efficacy. Recent evidence in support of the low-dose paradigm for RTX was reported by Zent et al. (2014), who examined the use of low but frequent doses of RTX in combination with pentostain and alemtuzumab in the treatment of progressive CLL. They found that this approach constituted an effective therapy that was able to activate effector mechanisms without causing substantial loss of CD20. Moreover, Goldenberg et al. reported that lower doses of the CD20 mAb veltuzumab, given either intravenously or subcutaneously, also have demonstrable activity in the treatment of lymphoma (Morschhauser et al., 2009; Negrea et al., 2011). There is also additional, historic precedence for a low-dose strategy. Alemtuzumab is a mAb specific for CD52 that is used in the treatment of CLL. This mAb also requires effector functions to promote CLL cell killing, and is given in either intravenous or subcutaneous doses of 30 mg, three times per week for extended periods (Zent et al., 2004).
The treatment strategy we envision should be most effective if careful correlative measurements that monitor the patients’ immune status are also conducted frequently, in effect allowing for more “personalized medicine” based on evaluation of laboratory parameters. Perhaps of most importance, if this approach were to prove successful and lead to equal or better outcomes compared with conventional high-dose therapies, it would be relatively straight-forward to refashion this low-dose paradigm into a subcutaneous injection strategy, which could make these treatments far more accessible and possibly more efficacious for patients.
Acknowledgments
The authors thank the CLL patients who volunteered to participate in the correlative studies.
Abbreviations
- ADCC
antibody-dependent cell-mediated cytotoxicity
- CLL
chronic lymphocytic leukemia
- mAb
monoclonal antibody
- NK
natural killer
- OBZ
obinutuzumab
- OFA
ofatumumab
- RTX
rituximab
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Taylor, Lindorfer.
Footnotes
The research was supported by the Commonwealth Foundation for Cancer Research, the National Institutes of Health National Cancer Institute [Grant P30-CA044579], Genmab, and GlaxoSmithKline.
References
- Alduaij W, Ivanov A, Honeychurch J, Cheadle EJ, Potluri S, Lim SH, Shimada K, Chan CHT, Tutt A, Beers SA, et al. (2011) Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosome-mediated cell death in B-cell malignancies. Blood 117:4519–4529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson JP, Frank MM. (1974) Complement-independent clearance of IgG-sensitized erythrocytes: inhibition by cortisone. Blood 44:629–637 [PubMed] [Google Scholar]
- Aue G, Lindorfer MA, Beum PV, Pawluczkowycz AW, Vire B, Hughes T, Taylor RP, Wiestner A. (2010) Fractionated subcutaneous rituximab is well-tolerated and preserves CD20 expression on tumor cells in patients with chronic lymphocytic leukemia. Haematologica 95:329–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig NA, Taylor RP, Lindorfer MA, Church AK, LaPlant BR, Pettinger AM, Shanafelt TD, Nowakowski GS, Zent CS. (2014) Induced resistance to ofatumumab-mediated cell clearance mechanisms, including complement-dependent cytotoxicity, in chronic lymphocytic leukemia. J Immunol 192:1620–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdeja JG, Hess A, Lucas DM, O’Donnell P, Ambinder RF, Diehl LF, Carter-Brookins D, Newton S, Flinn IW. (2007) Systemic interleukin-2 and adoptive transfer of lymphokine-activated killer cells improves antibody-dependent cellular cytotoxicity in patients with relapsed B-cell lymphoma treated with rituximab. Clin Cancer Res 13:2392–2399 [DOI] [PubMed] [Google Scholar]
- Berinstein NL, Grillo-López AJ, White CA, Bence-Bruckler I, Maloney D, Czuczman M, Green D, Rosenberg J, McLaughlin P, Shen D. (1998) Association of serum rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin’s lymphoma. Ann Oncol 9:995–1001 [DOI] [PubMed] [Google Scholar]
- Beum PV, Kennedy AD, Taylor RP. (2004) Three new assays for rituximab based on its immunological activity or antigenic properties: analyses of sera and plasmas of RTX-treated patients with chronic lymphocytic leukemia and other B cell lymphomas. J Immunol Methods 289:97–109 [DOI] [PubMed] [Google Scholar]
- Beum PV, Kennedy AD, Williams ME, Lindorfer MA, Taylor RP. (2006) The shaving reaction: rituximab/CD20 complexes are removed from mantle cell lymphoma and chronic lymphocytic leukemia cells by THP-1 monocytes. J Immunol 176:2600–2609 [DOI] [PubMed] [Google Scholar]
- Beum PV, Lindorfer MA, Taylor RP. (2008a) Within peripheral blood mononuclear cells, antibody-dependent cellular cytotoxicity of rituximab-opsonized Daudi cells is promoted by NK cells and inhibited by monocytes due to shaving. J Immunol 181:2916–2924 [DOI] [PubMed] [Google Scholar]
- Beum PV, Mack DA, Pawluczkowycz AW, Lindorfer MA, Taylor RP. (2008b) Binding of rituximab, trastuzumab, cetuximab, or mAb T101 to cancer cells promotes trogocytosis mediated by THP-1 cells and monocytes. J Immunol 181:8120–8132 [DOI] [PubMed] [Google Scholar]
- Beum PV, Peek EM, Lindorfer MA, Beurskens FJ, Engelberts PJ, Parren PW, van de Winkel JGJ, Taylor RP. (2011) Loss of CD20 and bound CD20 antibody from opsonized B cells occurs more rapidly because of trogocytosis mediated by Fc receptor-expressing effector cells than direct internalization by the B cells. J Immunol 187:3438–3447 [DOI] [PubMed] [Google Scholar]
- Beurskens FJ, Lindorfer MA, Farooqui M, Beum PV, Engelberts P, Mackus WJM, Parren PWHI, Wiestner A, Taylor RP. (2012) Exhaustion of cytotoxic effector systems may limit monoclonal antibody-based immunotherapy in cancer patients. J Immunol 188:3532–3541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R, Watzl C. (2007) Serial killing of tumor cells by human natural killer cells—enhancement by therapeutic antibodies. PLoS ONE 2:e326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchini G, Gianni L. (2014) The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol 15:e58–e68 [DOI] [PubMed] [Google Scholar]
- Bologna L, Gotti E, Da Roit F, Intermesoli T, Rambaldi A, Introna M, Golay J. (2013) Ofatumumab is more efficient than rituximab in lysing B chronic lymphocytic leukemia cells in whole blood and in combination with chemotherapy. J Immunol 190:231–239 [DOI] [PubMed] [Google Scholar]
- Bologna L, Gotti E, Manganini M, Rambaldi A, Intermesoli T, Introna M, Golay J. (2011) Mechanism of action of type II, glycoengineered, anti-CD20 monoclonal antibody GA101 in B-chronic lymphocytic leukemia whole blood assays in comparison with rituximab and alemtuzumab. J Immunol 186:3762–3769 [DOI] [PubMed] [Google Scholar]
- Boross P, Jansen JH, Pastula A, van der Poel CE, Leusen JHW. (2012) Both activating and inhibitory Fc γ receptors mediate rituximab-induced trogocytosis of CD20 in mice. Immunol Lett 143:44–52 [DOI] [PubMed] [Google Scholar]
- Boross P, Jansen JHM, de Haij S, Beurskens FJ, van der Poel CE, Bevaart L, Nederend M, Golay J, van de Winkel JGJ, Parren PWHI, et al. (2011) The in vivo mechanism of action of CD20 monoclonal antibodies depends on local tumor burden. Haematologica 96:1822–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boross P, Leusen JHW. (2012) Mechanisms of action of CD20 antibodies. Am J Cancer Res 2:676–690 [PMC free article] [PubMed] [Google Scholar]
- Botta C, Bestoso E, Apollinari S, Cusi MG, Pastina P, Abbruzzese A, Sperlongano P, Misso G, Caraglia M, Tassone P, et al. (2012) Immune-modulating effects of the newest cetuximab-based chemoimmunotherapy regimen in advanced colorectal cancer patients. J Immunother 35:440–447 [DOI] [PubMed] [Google Scholar]
- Bowles JA, Weiner GJ. (2005) CD16 polymorphisms and NK activation induced by monoclonal antibody-coated target cells. J Immunol Methods 304:88–99 [DOI] [PubMed] [Google Scholar]
- Boyer TD. (2003) Zakim and Boyer's Hepatology: A Textbook of Liver Disease, Saunders, Philadelphia [Google Scholar]
- Bulliard Y, Jolicoeur R, Windman M, Rue SM, Ettenberg S, Knee DA, Wilson NS, Dranoff G, Brogdon JL. (2013) Activating Fc γ receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J Exp Med 210:1685–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. (2002) Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 99:754–758 [DOI] [PubMed] [Google Scholar]
- Cheson BD. (2010) Ofatumumab, a novel anti-CD20 monoclonal antibody for the treatment of B-cell malignancies. J Clin Oncol 28:3525–3530 [DOI] [PubMed] [Google Scholar]
- Cheson BD, Leonard JP. (2008) Monoclonal antibody therapy for B-cell non-Hodgkin’s lymphoma. N Engl J Med 359:613–626 [DOI] [PubMed] [Google Scholar]
- Clynes RA, Towers TL, Presta LG, Ravetch JV. (2000) Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med 6:443–446 [DOI] [PubMed] [Google Scholar]
- Coiffier B, Losic N, Rønn BB, Lepretre S, Pedersen LM, Gadeberg O, Frederiksen H, van Oers MHJ, Wooldridge J, Kloczko J, et al. (2010) Pharmacokinetics and pharmacokinetic/pharmacodynamic associations of ofatumumab, a human monoclonal CD20 antibody, in patients with relapsed or refractory chronic lymphocytic leukaemia: a phase 1-2 study. Br J Haematol 150:58–71 [DOI] [PubMed] [Google Scholar]
- Daubeuf S, Lindorfer MA, Taylor RP, Joly E, Hudrisier D. (2010) The direction of plasma membrane exchange between lymphocytes and accessory cells by trogocytosis is influenced by the nature of the accessory cell. J Immunol 184:1897–1908 [DOI] [PubMed] [Google Scholar]
- Davis TA, Grillo-López AJ, White CA, McLaughlin P, Czuczman MS, Link BK, Maloney DG, Weaver RL, Rosenberg J, Levy R. (2000) Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin’s lymphoma: safety and efficacy of re-treatment. J Clin Oncol 18:3135–3143 [DOI] [PubMed] [Google Scholar]
- Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, Barrientos JC, Zelenetz AD, Kipps TJ, Flinn I, et al. (2014) Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 370:997–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennie MJ, French RR, Cragg MS, Taylor RP. (2007) Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol 44:3823–3837 [DOI] [PubMed] [Google Scholar]
- Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, Chagorova T, de la Serna J, Dilhuydy M-S, Illmer T, et al. (2014) Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 370:1101–1110 [DOI] [PubMed] [Google Scholar]
- Golay J, Da Roit F, Bologna L, Ferrara C, Leusen JH, Rambaldi A, Klein C, Introna M. (2013a) Glycoengineered CD20 antibody obinutuzumab activates neutrophils and mediates phagocytosis through CD16B more efficiently than rituximab. Blood 122:3482–3491 [DOI] [PubMed] [Google Scholar]
- Golay J, Introna M. (2012) Mechanism of action of therapeutic monoclonal antibodies: promises and pitfalls of in vitro and in vivo assays. Arch Biochem Biophys 526:146–153 [DOI] [PubMed] [Google Scholar]
- Golay J, Semenzato G, Rambaldi A, Foà R, Gaidano G, Gamba E, Pane F, Pinto A, Specchia G, Zaja F, et al. (2013b) Lessons for the clinic from rituximab pharmacokinetics and pharmacodynamics. MAbs 5:826–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q, Ou Q, Ye S, Lee WP, Cornelius J, Diehl L, Lin WY, Hu Z, Lu Y, Chen Y, et al. (2005) Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J Immunol 174:817–826 [DOI] [PubMed] [Google Scholar]
- Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, Hensel M, Hopfinger G, Hess G, von Grünhagen U, et al. International Group of Investigators. German Chronic Lymphocytic Leukaemia Study Group (2010) Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 376:1164–1174 [DOI] [PubMed] [Google Scholar]
- Jain RK, Baxter LT. (1988) Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: significance of elevated interstitial pressure. Cancer Res 48:7022–7032 [PubMed] [Google Scholar]
- Joly E, Hudrisier D. (2003) What is trogocytosis and what is its purpose? Nat Immunol 4:815. [DOI] [PubMed] [Google Scholar]
- Jones JD, Hamilton BJ, Rigby WFC. (2012) Rituximab mediates loss of CD19 on B cells in the absence of cell death. Arthritis Rheum 64:3111–3118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AD, Beum PV, Solga MD, DiLillo DJ, Lindorfer MA, Hess CE, Densmore JJ, Williams ME, Taylor RP. (2004) Rituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemia. J Immunol 172:3280–3288 [DOI] [PubMed] [Google Scholar]
- Kim JM, Ashkenazi A. (2013) Fcγ receptors enable anticancer action of proapoptotic and immune-modulatory antibodies. J Exp Med 210:1647–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrt HE, Houot R, Goldstein MJ, Weiskopf K, Alizadeh AA, Brody J, Müller A, Pachynski R, Czerwinski D, Coutre S, et al. (2011) CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood 117:2423–2432 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kohrt HE, Houot R, Weiskopf K, Goldstein MJ, Scheeren F, Czerwinski D, Colevas AD, Weng WK, Clarke MF, Carlson RW, et al. (2012) Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest 122:1066–1075 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Laurent C, de Paiva GR, Ysebaert L, Laurent G, March M, Delsol G, Brousset P. (2007) Characterization of bone marrow lymphoid infiltrates after immunochemotherapy for follicular lymphoma. Am J Clin Pathol 128:974–980 [DOI] [PubMed] [Google Scholar]
- Li Y, Williams ME, Cousar JB, Pawluczkowycz AW, Lindorfer MA, Taylor RP. (2007) Rituximab-CD20 complexes are shaved from Z138 mantle cell lymphoma cells in intravenous and subcutaneous SCID mouse models. J Immunol 179:4263–4271 [DOI] [PubMed] [Google Scholar]
- Lindorfer MA, Kohl J, Taylor RP. (2014) Interactions between the complement system and Fcγ receptors, in Antibody Fc: Linking Adaptive and Innate Immunity (Ackerman ME, Nimmerjahn F. eds) pp 49–74, Elsevier, Philadelphia [Google Scholar]
- Lindorfer MA, Wiestner A, Zent CS, Taylor RP. (2012) Monoclonal antibody (mAb)-based cancer therapy: Is it time to reevaluate dosing strategies? OncoImmunology 1:959–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam D, Curiel TJ. (2013) Antibodies as cancer immunotherapy, in Cancer Immunotherapy: Paradigms, Practice and Promise (Curiel TJ. ed) pp 335–376, Springer, New York [Google Scholar]
- Masuda S, Iwasaki S, Tomaru U, Baba T, Katsumata K, Ishizu A. (2013) Possible implication of Fc γ receptor-mediated trogocytosis in susceptibility to systemic autoimmune disease. Clin Dev Immunol 2013:345745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin P, Grillo-López AJ, Link BK, Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Bruckler I, White CA, Cabanillas F, et al. (1998) Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol 16:2825–2833 [DOI] [PubMed] [Google Scholar]
- Montalvao F, Garcia Z, Celli S, Breart B, Deguine J, Van Rooijen N, Bousso P. (2013) The mechanism of anti-CD20-mediated B cell depletion revealed by intravital imaging. J Clin Invest 123:5098–5103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morschhauser F, Leonard JP, Fayad L, Coiffier B, Petillon MO, Coleman M, Schuster SJ, Dyer MJS, Horne H, Teoh N, et al. (2009) Humanized anti-CD20 antibody, veltuzumab, in refractory/recurrent non-Hodgkin’s lymphoma: phase I/II results. J Clin Oncol 27:3346–3353 [DOI] [PubMed] [Google Scholar]
- Mössner E, Brünker P, Moser S, Püntener U, Schmidt C, Herter S, Grau R, Gerdes C, Nopora A, van Puijenbroek E, et al. (2010) Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 115:4393–4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, Laccabue D, Zerbini A, Camisa R, Bisagni G, et al. (2008) Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol 26:1789–1796 [DOI] [PubMed] [Google Scholar]
- Negrea GO, Elstrom R, Allen SL, Rai KR, Abbasi RM, Farber CM, Teoh N, Horne H, Wegener WA, Goldenberg DM. (2011) Subcutaneous injections of low-dose veltuzumab (humanized anti-CD20 antibody) are safe and active in patients with indolent non-Hodgkin’s lymphoma. Haematologica 96:567–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen AE, Jungersen MB, Pedersen CD. (2011) Monocytes mediate shaving of B-cell-bound anti-CD20 antibodies. Immunology 133:239–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peipp M, van de Winkel JGJ, Valerius T. (2011) Molecular engineering to improve antibodies’ anti-lymphoma activity. Best Pract Res Clin Haematol 24:217–229 [DOI] [PubMed] [Google Scholar]
- Rossi EA, Goldenberg DM, Michel R, Rossi DL, Wallace DJ, Chang CH. (2013) Trogocytosis of multiple B-cell surface markers by CD22 targeting with epratuzumab. Blood 122:3020–3029 [DOI] [PubMed] [Google Scholar]
- Schreiber AD, Frank MM. (1972) Role of antibody and complement in the immune clearance and destruction of erythrocytes. I. In vivo effects of IgG and IgM complement-fixing sites. J Clin Invest 51:575–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AM, Wolchok JD, Old LJ. (2012) Antibody therapy of cancer. Nat Rev Cancer 12:278–287 [DOI] [PubMed] [Google Scholar]
- Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, et al. (2013) Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med 210:1695–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwkowski MX, Mellman I. (2013) Antibody therapeutics in cancer. Science 341:1192–1198 [DOI] [PubMed] [Google Scholar]
- Tam CS, Otero-Palacios J, Abruzzo LV, Jorgensen JL, Ferrajoli A, Wierda WG, Lerner S, O’Brien S, Keating MJ. (2008) Chronic lymphocytic leukaemia CD20 expression is dependent on the genetic subtype: a study of quantitative flow cytometry and fluorescent in-situ hybridization in 510 patients. Br J Haematol 141:36–40 [DOI] [PubMed] [Google Scholar]
- Taylor RJ, Chan SL, Wood A, Voskens CJ, Wolf JS, Lin W, Chapoval A, Schulze DH, Tian G, Strome SE. (2009) FcgammaRIIIa polymorphisms and cetuximab induced cytotoxicity in squamous cell carcinoma of the head and neck. Cancer Immunol Immunother 58:997–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RP. (2013) Gnawing at Metchnikoff’s paradigm. Blood 122:2922–2924 [DOI] [PubMed] [Google Scholar]
- Taylor RP, Lindorfer MA. (2014) The role of complement in mAb-based therapies of cancer. Methods 65:18–27 [DOI] [PubMed] [Google Scholar]
- Teeling JL, French RR, Cragg MS, van den Brakel J, Pluyter M, Huang H, Chan C, Parren PW, Hack CE, Dechant M, et al. (2004) Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood 104:1793–1800 [DOI] [PubMed] [Google Scholar]
- Teng YKO, Levarht EWN, Hashemi M, Bajema IM, Toes REM, Huizinga TWJ, van Laar JM. (2007) Immunohistochemical analysis as a means to predict responsiveness to rituximab treatment. Arthritis Rheum 56:3909–3918 [DOI] [PubMed] [Google Scholar]
- Varchetta S, Gibelli N, Oliviero B, Nardini E, Gennari R, Gatti G, Silva LS, Villani L, Tagliabue E, Ménard S, et al. (2007) Elements related to heterogeneity of antibody-dependent cell cytotoxicity in patients under trastuzumab therapy for primary operable breast cancer overexpressing Her2. Cancer Res 67:11991–11999 [DOI] [PubMed] [Google Scholar]
- Veeramani S, Wang SY, Dahle C, Blackwell S, Jacobus L, Knutson T, Button A, Link BK, Weiner GJ. (2011) Rituximab infusion induces NK activation in lymphoma patients with the high-affinity CD16 polymorphism. Blood 118:3347–3349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner GJ. (2010) Rituximab: mechanism of action. Semin Hematol 47:115–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng WK, Levy R. (2003) Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol 21:3940–3947 [DOI] [PubMed] [Google Scholar]
- Williams ME, Densmore JJ, Pawluczkowycz AW, Beum PV, Kennedy AD, Lindorfer MA, Hamil SH, Eggleton JC, Taylor RP. (2006) Thrice-weekly low-dose rituximab decreases CD20 loss via shaving and promotes enhanced targeting in chronic lymphocytic leukemia. J Immunol 177:7435–7443 [DOI] [PubMed] [Google Scholar]
- Wilson NS, Yang B, Yang A, Loeser S, Marsters S, Lawrence D, Li Y, Pitti R, Totpal K, Yee S, et al. (2011) An Fcγ receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer Cell 19:101–113 [DOI] [PubMed] [Google Scholar]
- Zent CS, Chen JB, Kurten RC, Kaushal GP, Lacy HM, Schichman SA. (2004) Alemtuzumab (CAMPATH 1H) does not kill chronic lymphocytic leukemia cells in serum free medium. Leuk Res 28:495–507 [DOI] [PubMed] [Google Scholar]
- Zent CS, Taylor RP, Lindorfer MA, Beum PV, Laplant B, Wu W, Call TG, Bowen DA, Conte MJ, Frederick LA, et al. (2014) Chemoimmunotherapy for relapsed/refractory and progressive 17p13-deleted chronic lymphocytic leukemia (CLL) combining pentostatin, alemtuzumab, and low-dose rituximab is effective and tolerable and limits loss of CD20 expression by circulating CLL cells. Am J Hematol 89:757–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Gordon M, Schultheis AM, Yang DY, Nagashima F, Azuma M, Chang HM, Borucka E, Lurje G, Sherrod AE, et al. (2007) FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol 25:3712–3718 [DOI] [PubMed] [Google Scholar]
- Zigler M, Shir A, Levitzki A. (2013) Targeted cancer immunotherapy. Curr Opin Pharmacol 13:504–510 [DOI] [PubMed] [Google Scholar]