Abstract
Metabotropic glutamate receptors (mGluRs) function as dimers. Recent work suggests that mGluR1 and mGluR5 may physically interact, but the nature and functional consequences of this relationship have not been addressed. In this study, the functional and pharmacological consequences of this interaction were investigated. Using heterologous expression of mGluR cDNA in rat sympathetic neurons from the superior cervical ganglion and inhibition of the native calcium currents as an assay for receptor activation, a functional interdependence between mGluR1 and mGluR5 was demonstrated. In neurons coexpressing these receptors, combining a selective mGluR1 competitive antagonist with either an mGluR1- or mGluR5-selective negative allosteric modulator (NAM) BAY36-7620 [(3aS,6aS)-hexahydro-5-methylene-6a-(2-naphthalenylmethyl)-1H-cyclopenta[c]furan-1-one] or MPEP [2-methyl-6-(phenylethynyl)pyridine hydrochloride], respectively, strongly occluded signaling by both receptors to an approximately equal degree. By contrast, in cells coexpressing mGluR1 and mGluR2, combining the same mGluR1 competitive inhibitor with an mGluR1 or mGluR2 NAM yielded partial and full inhibition of the response, respectively, as expected for independently acting receptors. In neurons expressing mGluR1 and mGluR5, the selective NAMs each strongly inhibited the response to glutamate, suggesting that these receptors do not interact as heterodimers, which would not be inhibited by selective NAMs. Finally, evidence for a similar mGluR1/mGluR5 functional dependence is shown in medium spiny striatal neurons. Together, these data demonstrate cooperative signaling between mGluR1 and mGluR5 in a manner inconsistent with heterodimerization, and thus suggest an interaction between homodimers.
Introduction
Metabotropic glutamate receptors (mGluRs) are class C G protein–coupled receptors with widespread expression in the nervous system. There are three groups of mGluRs (I–III) categorized by homology, pharmacology, and G protein coupling (Schoepp, 2001). Until recently, most mGluRs, which form stable, covalently linked dimers (Romano et al., 1996; Ray and Hauschild, 2000; Tsuji et al., 2000; Kunishima et al., 2000; Romano et al., 2001), were thought to primarily function as homodimers. Recent work has shown that some combinations of mGluRs can form heteromeric receptors (Doumazane et al., 2011), including some (e.g., mGluR2 and mGluR4) that form heterodimers in heterologous systems (Doumazane et al., 2011) and in neurons (Kammermeier, 2012a; Yin et al., 2014).
It is becoming increasingly apparent that a careful characterization of the pharmacology of heteromeric mGluRs is necessary if their potential as therapeutic targets is to be fully realized. Although some mGluR heteromers seem to clearly form heterodimers (Doumazane et al., 2011), this may not necessarily be the case for every mGluR heteromer pair. Since the pharmacological consequences of heteromer formation are largely a result of the nature of the interaction, it will be important to know the stoichiometry of each heteromeric mGluR.
In a recent article, a physical association between the group I mGluRs, mGluR1 and mGluR5, was implied using a fluorescence energy transfer assay (Doumazane et al., 2011), but neither the functional consequences of this interaction, nor its pharmacological impact, have been determined. Furthermore, heterodimerization of wild-type mGluR1 and mGluR5 has not been demonstrated, and has been ruled out in at least one study (Romano et al., 1996). Assessing the functional role of heteromerization between highly homologous receptors such as mGluR1 and mGluR5 has been hampered by the lack of selective agonists (Kammermeier, 2012b). In a previous study, we circumvented this problem by constructing an mGluR1 point mutant, mGluR1 Y74A, with an alanine for tyrosine substitution near the glutamate binding pocket (Kammermeier and Yun, 2005). This mutation shifts the sensitivity of the receptor for glutamate by approximately 100-fold, rendering glutamate a selective agonist for coexpressed wild-type mGluRs. In that study, mGluR1 Y74A was coexpressed with wild-type mGluR1 in rat sympathetic neurons from the superior cervical ganglion (SCG) to assess the functional consequences of homodimerization. The “heteromeric” receptor (the mGluR1 mutant/mGluR1 wild-type dimer) was found to exhibit a dose response nearly identical to the mutant homodimer, which demonstrated that mGluR dimers require ligand in both subunits for strong activation. Fortuitously, this theoretically provided a functional assay for heterodimerization between mGluR1 Y74A and other mGluR subtypes. As with the mGluR1 Y74A/mGluR1 wild-type combination, a heterodimer between mGluR1 Y74A and another mGluR should exhibit a concentration response nearly identical to the Y74A mutant alone. Thus, in our study, the same mutant was coexpressed with mGluR5, a closely related receptor that resides in close proximity with mGluR1 (Doumazane et al., 2011). Beginning with this strategy, we show that these receptors do appear to interact functionally, and that this interaction gives rise to a functional receptor pool with a unique pharmacological profile with respect to its sensitivity to selective mGluR1 and mGluR5 compounds. However, the functional profile of the mGluR1/mGluR5 receptor pair appears distinct from that expected of a putative mGluR1/mGluR5 heterodimer in this functional assay. Thus, we interpret these results as evidence for an interaction between mGluR1 and mGluR5 homodimers. This is the first demonstration that mGluR1 and mGluR5, when coexpressed in the same neuron, exhibit functional interdependence. Finally, we present evidence that a similar pharmacological profile in cultured medium spiny neurons (MSNs) of the rat striatum, suggesting that the receptor interaction bears physiologic relevance, possibly giving rise to a unique therapeutic target.
Materials and Methods
SCG Neuron Isolation.
Male Wistar rats were euthanized with CO2 and decapitated, in accordance with the University Committee on Animal Research methods. All SCG dissections were performed in chilled Hanks’ balanced salt solution. The ganglia were removed and incubated for 60 minutes at 37°C in Earle’s balanced salt solution (Invitrogen, Carlsbad, CA) containing 0.5 mg/ml trypsin (Worthington Biochemical, Freehold, NJ) and 11 mg/ml collagenase D (Roche Diagnostics, Indianapolis, IN). After the incubation, cells were centrifuged twice for 6 minutes at 700 rpm, and resuspended in minimal essential medium (Thermo Fisher Scientific, Waltham, MA) containing 10% fetal bovine serum, penicillin/streptomycin, and GlutaMax (Invitrogen). Resuspended cells were plated onto 35-mm tissue culture dishes coated with poly-l-lysine) (Sigma-Aldrich, St. Louis, MO), and stored at 5% CO2 at 37°C for 3–5 hours.
cDNA Microinjection of SCG.
Nuclear microinjection of plasmids was performed 2–4 hours after SCG neuron isolation with an Eppendorf 5247 microinjector and an Injectman NI2 micromanipulator (Eppendorf North America, Hauppauge, NY). Injections were made with a Sutter (Novato, CA) P-97 horizontal electrode puller using thin-walled, borosilicate glass (World Precision Instruments, Inc., Sarasota, FL). The plasmids used for the injections were stored at −20°C varying from 0.9 to 1.5 μg/μl stock solution in TE buffer (10 mM Tris, 1 mM EDTA, pH 8). Each receptor (e.g., mGluR1) was injected into cells at 100–150 ng/µl with the enhanced green fluorescent protein pEGFPC1 reporter gene (0.02 μg/μl; Clontech Laboratories, Mountain View, CA). The injected concentration of each receptor's cDNA was fixed whether that receptor was injected alone or with another receptor. Depending on the quality of the SCG cell culture, 30–200 cells were injected in each 35-mm cell culture dish. The injected cells were placed back in the 37°C incubator and recordings were made the following day.
Electrophysiological Recordings and Data Analysis.
All patch-clamp experiments were performed at approximately 22°C (room temperature). Recording electrodes were made from 8250 glass (World Precision Instruments, Inc.) using a Sutter P-97 horizontal puller. Pipette resistances were 0.8–3.0 MΩ, which resulted in uncompensated series resistances of 1–5 MΩ. For each recording, series resistance compensation of 80% was used. Patch-clamp data were obtained using an EPC-7 (HEKA Elektronic, Lambrecht, Germany), or an Axon 200B (Molecular Devices, Sunnyvale, CA). Custom data acquisition software (courtesy of Stephen R. Ikeda; National Institutes of Health National Institute on Alcohol Abuse and Alcoholism, Rockville, MD) was used on a Macintosh G4 computer (Apple Computer, Cupertino, CA) with an InstruTech A/D board (ITC-16 or ITC-18; Heka Elektronik, Port Washington, NY) used for data acquisition. Data were sampled at 10 microseconds, low-pass filtered at 5 kHz, digitized, and stored for later analysis using IgorPro software (WaveMetrics, Lake Oswego, OR).
Recording Solutions and Pharmacological Agents.
The cell bath solution (external) contained 155 mM tris hydroxymethyl aminomethane, 20 mM HEPES, 10 mM glucose, 10 mM CaCl2, and 0.0003 mM tetrodotoxin, pH 7.4. The pipette solution (internal) contained 120 mM N-methyl-d-glucamine methanesulfonate, 20 mM TEA, 11 mM EGTA, 10 mM HEPES, 10 mM sucrose, 1 mM CaCl2, 4 mM MgATP, 0.3 mM Na2GTP, and 14 mM Tris creatine phosphate, pH 7.2. l-Glutamate, the endogenous ligand for mGluRs, was obtained from Sigma-Aldrich. Other mGluR-selective compounds including 3-MATIDA [α-amino-5-carboxy-3-methyl-2-thiopheneacetic acid], BAY36-7620 [(3aS,6aS)-hexahydro-5-methylene-6a-(2-naphthalenylmethyl)-1H-cyclopenta[c]furan-1-one], MPEP [2-methyl-6-(phenylethynyl)pyridine hydrochloride], and MNI-137 [4-(8-bromo-2,3-dihydro-2-oxo-1H-1,5-benzodiazepin-4-yl)-2-pyridinecarbonitrile] were obtained from Tocris Bioscience (Ellisville, MO). Application of the drugs was achieved by a custom, gravity-driven perfusion system positioned 50–150 μm from the cell, allowing rapid solution exchange (≤250 milliseconds). Maximum current inhibition was defined as an equivalent of the degree of calcium current inhibition after addition of each drug compared with the last current measurement prior to the addition.
Culturing of Striatal Neurons and Measurement of Intracellular Calcium.
MSN striatal neurons were cultured by a method derived for the culturing of hippocampal neurons reported previously (Kammermeier and Worley, 2007). Briefly, the striatum was dissected from P0-3 rats, enzymatically dissociated with about 25 U/ml Papain (Worthington Biochemical Corp., Lakewood, NJ) for 1 hour at 37°C, and then gently triturated, spun at 800 rpm for 5 minutes, and plated onto glass coverslips coated with poly-L-lysine in growth medium. Growth medium consisted of minimal essential medium (Gibco/Invitrogen, Carlsbad, CA) supplemented with fetal bovine serum (5%), penicillin/streptomycin, sodium pyruvate (1 mM), glucose (0.6%), B-27 (2%), mito-serum extender (0.1%), uridine (10 µM), and 5-fluorodeoxyuridine (2.5 µM) (Sigma-Aldrich). Cultures were used after 1 to 2 weeks in vitro, as indicated.
For ratiometric calcium imaging experiments, glass coverslips containing MSNs were loaded with Fura-2 AM for approximately 20 minutes and then transferred into a perfusion chamber. Cells were perfused in HEPES-buffered physiologic saline containing 137 mM NaCl, 0.56 mM MgCl2, 4.7 mM KCl, 1 mM Na2HPO4, 10 mM HEPES, 5.5 mM glucose, and 1.26 mM CaCl2, pH 7.4. Imaging was performed using an inverted Nikon microscope through a 40× oil immersion objective lens (numerical aperture, 1.3). Fura-2 AM–loaded cells were excited alternately with light at 340 and 380 nm by using a monochromator-based illumination system (TILL Photonics, Pleasanton, CA), and the emission at 510 nm was captured by using a digital frame transfer charge-coupled device camera. Images were captured every 2 seconds with an exposure of 40 milliseconds and 4 × 4 binning. Analysis was performed by TILL Vision software. Amplitudes of responses were defined as peak fluorescence induced by each drug application minus baseline preceding the application. For reporting of inhibitor effects in striatal neurons, decay of responses to dihydroxyphenylglycine (DHPG) (applied at the start and end of each experiment), were assumed to be linear and responses in the presence of inhibitors were compared with the predicted control response at the given time. DHPG, MATIDA, and BAY36 were obtained from Tocris.
Results
mGluR1 and mGluR5 Interact Functionally.
Neurons from the rat SCG provide a null-mGluR background upon which specific mGluRs can be expressed in a neuronal environment (Ikeda, 1997; Kammermeier and Ikeda, 1999). To assess the potential functional interaction between mGluR1 Y74A and mGluR5, each receptor (or both) was made to express in isolated rat SCG neurons by intranuclear cDNA injection (Lu et al., 2009), and the G protein–mediated inhibition of the native calcium currents was used as an assay for receptor signaling. Representative experiments are shown in Fig. 1A. Application of glutamate to neurons expressing mGluR5b (Fig. 1A, upper panel) results in a rapid and reversible inhibition of calcium currents (Fig. 1B, filled circles), when currents are measured using a 25-millisecond test pulse to +10 mV from a holding potential of −80 mV (Fig. 1A, upper right panel). Likewise, in cells injected with mGluR1 Y74A (Fig. 1, A and B, center and squares, respectively), glutamate induced a strong inhibition of the current, but with a shifted response, such that inhibition was not seen below 100 µM, and the maximal inhibition was stronger, peaking at >60%, consistent with results observed in previous work (Kammermeier and Yun, 2005). In that article, we showed that 1 mM glutamate produces a response that is about 90% saturating. These data are consistent with a recent report on group I mGluRs and their coupling to neuronal calcium channels (Kammermeier and Ikeda, 1999).
Fig. 1.
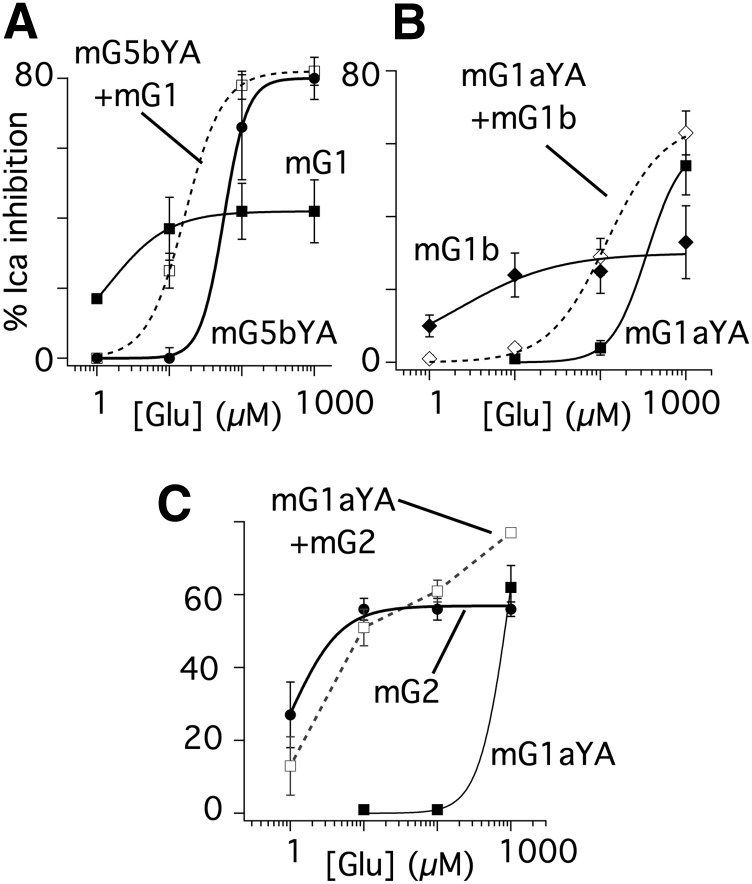
Functional interaction between mGluR5 and mGluR1 Y74A. (A) Calcium current amplitude time courses (left) and sample representative current traces (right) illustrating the effect of various concentrations of Glu (as indicated) in SCG neurons expressing mGluR5b (upper panel), mGluR1 Y74A (center panel), and both receptors (lower panel). The voltage protocol, a 25-millisecond step to +10 mV near the peak current from a holding potential of −80 mV, for the current traces is shown in (A) (upper right panel). (B and C) Average (± S.E.M.) responses at a range of [Glu]s from SCG neurons expressing mGluR5 alone (circles), mGluR1 Y74A alone (squares), or both receptors together (diamonds). Panel (B) shows a set of experiments using the mGluR5b splice variant, and (C) shows experiments using the mGluR5a splice variant. Numbers of cells for each point are given in the text. Solid and hatched lines show the results of fits to the Hill equation. Con, control.
When mGluR1 Y74A and mGluR5b were expressed together, the glutamate dose-response curve was surprising (Fig. 1, A and B, lower panel and diamonds, respectively). Coexpression resulted in a high-efficacy response, similar to the mGluR1 Y74A mutant, but with a potency that was intermediate to the receptors expressed separately. When similar experiments were conducted using a different mGluR5 splice variant (mGluR5a), the results were similar (Fig. 1C). In both cases, mGluR5 coexpression with mGluR1 Y74A resulted in an intermediate potency, high-efficacy glutamate response. These data are difficult to reconcile with a model in which the two receptors function independently.
To determine whether the functional interaction observed in these experiments was reciprocal, the corresponding mutation was made in mGluR5b. The resulting receptor (mGluR5b Y64A) was expressed alone or with mGluR1 (Fig. 2A). As with the Y-A mutation in mGluR1, the dose response of the mutant mGluR5 was right shifted, although less severely than the mGluR1 mutant. Responses peaked at nearly 80% inhibition with an apparent EC50 of about 56 µM. Cells expressing mGluR1 alone had responses as expected, peaking at approximately 40% inhibition and with an apparent EC50 of about 1 µM, consistent with previous reports (Kammermeier and Yun, 2005; Beqollari and Kammermeier, 2010). As with the mGluR1 Y74A/mGluR5 combination, when mGluR5 Y64A was coexpressed with mGluR1, responses showed strong efficacy and intermediate potency (EC50 of 16 µM) compared with each receptor alone. These data suggest that the interaction between mGluR1 and mGluR5 is at least qualitatively reciprocal.
Fig. 2.
The interaction between mGluR1 and mGluR5 is reciprocal, and mGluR1 does not interact functionally with mGluR2. (A) Average (± S.E.M.) Glu dose-response relationship of calcium current inhibition in SCG neurons in cells expressing mGluR1 alone (filled squares), mGluR5b Y64A alone (filled circles), or both receptors together (open squares). Fits to the Hill equation are shown as solid or hatched lines, yielding EC50 values of 1, 56, and 16 µM for mGluR1, mGluR5 Y64A, and both receptors together, respectively. (B) Average (± S.E.M.) Glu dose-response relationship of calcium current inhibition in SCG neurons in cells expressing mGluR1aY74A alone (filled squares), mGluR1b Y64A alone (filled diamonds), or both receptors together (open diamonds). Fits to the Hill equation are shown as solid or hatched lines, yielding EC50 values of 351, 2, and 118 µM for mGluR1aYA, mGluR1b, and both receptors together, respectively (assuming maximal responses of 60% for mGluR1aYA and 66% for both receptors together). (C) Average (± S.E.M.) Glu dose-response relationship of calcium current inhibition in SCG neurons in cells expressing mGluR1 Y64A alone (filled squares), mGluR2 alone (filled circles), or both receptors together (open squares). Fits to the Hill equation are shown as solid lines, yielding EC50 values of 5 and 980 µM for mGluR2 and mGluR1 Y74A, respectively. Due to the complex shape, the data from cells with both receptors coexpressed were not fit.
We next tested whether mGluR1 Y74A (constructed from the mGluR1a variant), which appeared to dimerize with wild-type mGluR1a in our functional assay (Kammermeier and Yun, 2005), would interact with mGluR1b, which cannot form dimers with mGluR1a (Remelli et al., 2008). Thus, our model predicts that the glutamate concentration response of the mGluR1 Y74A/mGluR1b combination should mimic that of mGluR1 Y74A/mGluR5 (high efficacy, intermediate potency), rather than that of mGluR1 Y74A/mGluR1a (high efficacy, potency nearly identical to the YA mutant alone). Indeed, as shown in Fig. 2B when these receptors were expressed together, the partial glutamate-response curve indicates a high-efficacy response with a potency intermediate to that of mGluR1b and mGluR1 Y74A when expressed alone, validating our prediction. Thus, these data support the finding (Remelli et al., 2008) that mGluR1a and mGluR1b fail to dimerize.
It is possible that two overexpressed receptors with different potencies to the same agonist and sharing limited downstream signaling intermediates may yield a concentration response that lies between that of each receptor when expressed alone. This type of interaction is likely to produce a concentration response with a Hill slope observably less steep than the more potent receptor. This was not seen in the case of mGluR1 and mGluR5. Nevertheless, to rule out this interpretation, mGluR2, known to act independently of mGluR1 (Doumazane et al., 2011), was expressed in SCG neurons with mGluR1 Y74A as a negative control (Fig. 2C). The mGluR2 glutamate response has an EC50 in the low micromolar range with a maximal calcium current inhibition of about 50–60% (Fig. 2C, filled circles). The glutamate dose-response curve in the presence of both mGluR1 Y74A and mGluR2 was as expected if these receptors were functioning independently, and if intracellular signaling elements were not limiting. That is, an intermediate potency response was not observed, despite mGluR2 being a high-efficacy receptor that may be expected to deplete intracellular signaling intermediates more so than mGluR5. At lower glutamate concentrations, the responses were indistinguishable from those seen with mGluR2. At 1 mM, the response to the combined receptors was increased slightly to 82 ± 4% (n = 5), suggesting a partially additive response. Note that a fully additive response is not expected because the inhibitory mechanism of the two receptors is partially shared (Kammermeier and Ikeda, 1999), and because the assay measures inhibition, which does not sum linearly in the very high-efficacy range and which mechanistically cannot reach 100% (Bean, 1989). Thus, the functional interaction between mGluR1 and mGluR5 exhibits selectivity consistent with the demonstrated close association between these receptors, but not between mGluR1 and mGluR2 (Doumazane et al., 2011).
Pharmacological Assessment of the mGluR1/mGluR5 Interaction.
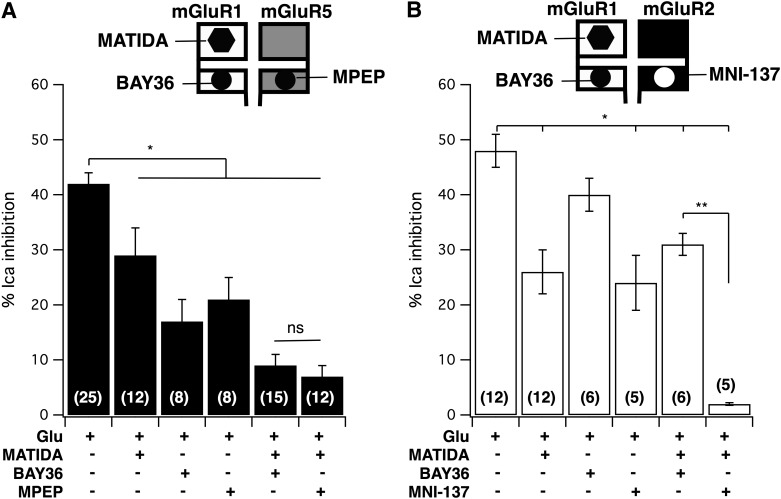
To begin to assess the nature of the cooperation between mGluR1 Y74A and mGluR5b (Fig. 1) or mGluR5 Y64A and mGluR1 (Fig. 2A) the effects of the mGluR1-selective negative allosteric modulator (NAM) BAY36-7620 (Carroll et al., 2001), and the mGluR5-selective NAM MPEP, a commonly used, highly selective and efficacious allosteric antagonist at mGluR5 (Gasparini et al., 1999) were examined (Fig. 3). A schematic of the experimental design illustrating the expressed receptors and compounds targeting them is shown above each panel in Fig. 3. Note that the upper, larger box used to illustrate each receptor represents the N-terminal orthosteric ligand binding site and the lower smaller box represents the heptahelical domain containing the allosteric site. Note also that these cartoons are not intended to convey receptor stoichiometry or even physical interaction, only to represent the receptors that were expressed in each experiment. Paired control experiments (recorded on the same days) for individually expressed receptors are shown in Supplemental Fig. 1.
Fig. 3.
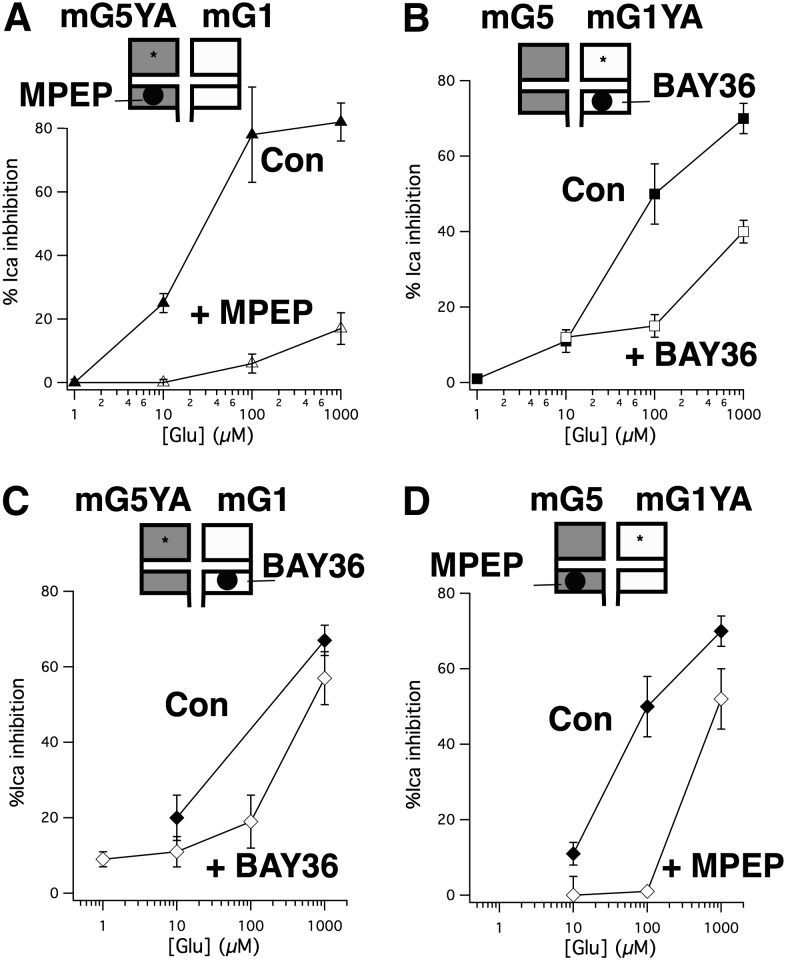
Selective NAMs strongly inhibit signaling of combined mGluR YA/mGluR wild-type signaling. (A) Neurons coexpressing mGluR5 Y64A and mGluR1 are strongly inhibited by 1 µM MPEP. Average (± S.E.M.) calcium current inhibition produced by indicated [glutamate] in cells coexpressing mGluR5YA with mGluR1 in the absence (filled triangles; n = 5) and presence (open triangles; n = 4) of 1 µM MPEP. (B) Average (± S.E.M.) calcium current inhibition produced by indicated [glutamate] in cells coexpressing mGluR5 with mGluR1YA in the absence (filled squares; n = 5) and presence (open squares; n = 6) of 1 µM BAY36. (C), Average (± S.E.M.) calcium current inhibition produced by indicated [glutamate] in cells coexpressing mGluR5YA with mGluR1 in the absence (filled diamonds; n = 12 at 1 mM, n = 7 at 100 µM) and presence (open diamonds; n = 12 at 10 µM, n = 5 at all other [Glu]s) of 1 µM BAY36. (D) Average (± S.E.M.) calcium current inhibition produced by indicated [glutamate] in cells coexpressing mGluR5 with mGluR1YA in the absence (filled diamonds; n = 6) and presence (open diamonds; n = 5 at 10 µM, n = 6 at all other [Glu]s) of 1 µM MPEP.
In the first set of experiments (Fig. 3, A and B) a partial glutamate dose-response curve was constructed in the absence and presence of a NAM targeting the mutant (YA) subunit. Interestingly, strong inhibition was observed by both 1 µM MPEP (Fig. 3A) and 1 µM BAY36 (Fig. 3B) at glutamate concentrations below the range at which the targeted receptors (mGluR5 YA and mGluR1 YA, respectively) are active. In addition, the overall shape of the curves in the presence of the inhibitors more closely resembled that of the mutant receptor when expressed alone, although with decreased efficacy. This was surprising because in each case, the mutant receptor was the target of the inhibitor. As a control, we tested whether cells expressing mGluR1 Y74A and mGluR2, as in Fig. 2C were susceptible to inhibition by BAY36 at a glutamate concentration below that which the mGluR1 mutant was expected to be active. As expected, BAY36 failed to reduce the response to 10 µM glutamate in these cells. Calcium current was inhibited in these cells by 41 ± 4% when 10 µM glutamate was applied alone and 40 ± 4% when glutamate was applied in the presence of BAY36 (n = 6). Next, similar experiments were performed but this time targeting the wild-type receptors with their respective NAMs (Fig. 3, C and D). The results of these experiments were perhaps less surprising, with each inhibitor leaving a response quite similar to that of the untargeted (mutant) receptor when expressed alone (see Supplemental Fig. 1 for comparison). To some extent, these data should be interpreted with caution, particularly because BAY36 was a poor inhibitor of mGluR1 at [glutamate] above 100 µM, but the data with MPEP, a more reliable inhibitor, were similar. It is intriguing that regardless of the receptor targeted by the NAM (mutant or wild type), the potency of the resulting response was similar. However, at this point it is difficult to conceptualize a model for the interaction between these receptors without knowing to what extent the YA mutation alters the behavior of the receptors, or the functional consequences of their interaction. Thus, a series of experiments were conducted using selective antagonists in SCG neurons expressing wild-type mGluR1, mGluR5, or both receptors together.
Effects of Selective NAMs on the Function of Wild-Type mGluR1 and mGluR5 when Coexpressed.
To determine whether coexpression of wild-type mGluR1 and mGluR5 in SCG neurons results in a receptor pool that interacts functionally, responses to a single concentration of glutamate (10 µM) were examined when applied alone or in the presence of saturating concentrations of 1 µM BAY36 or 1 µM MPEP in the same cells (Fig. 4). Control experiments illustrating the effects of these drugs on SCG neurons expressing mGluR1 or mGluR5 alone are shown in Supplemental Fig. 2. In eight cells examined, the average (± S.E.M.) calcium current inhibition observed upon application of 10 µM glutamate was 44 ± 4%. On average, the responses in the presence of BAY36 and MPEP were 17 ± 4% and 21 ± 4%, respectively. The average responses under each condition are shown in Fig. 4A. Consistent with the results described above (Fig. 3), each inhibitor alone produced a relatively strong inhibition of the basal glutamate response that was surprisingly superadditive compared with the responses in the presence of each antagonist, which summed to only about 86% of the control response. This suggests that the two receptors are not acting in a strictly independent manner.
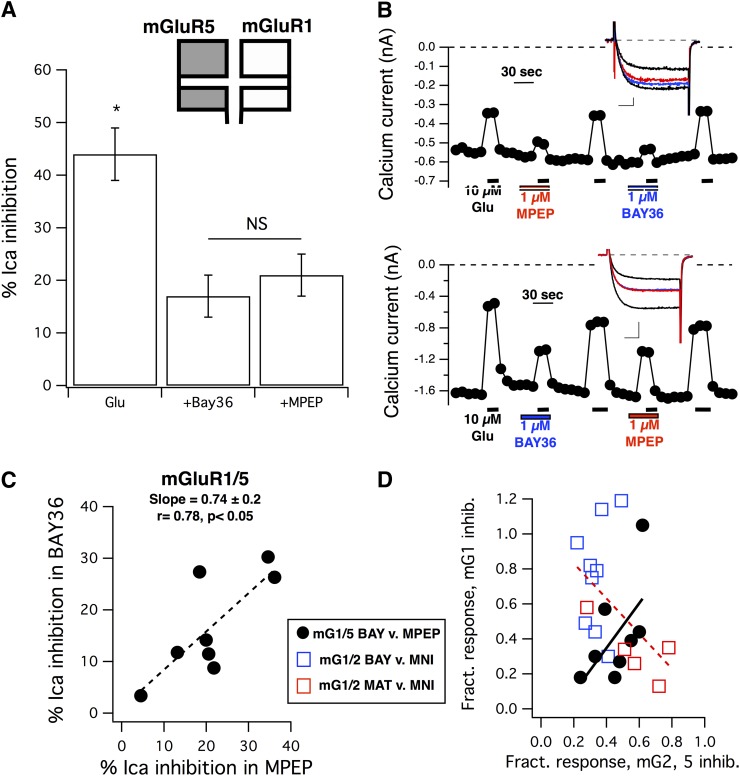
Fig. 4.
Inhibition of the glutamate response by mGluR1 and mGluR5 selective NAMs in SCG neurons expressing mGluR1 and mGluR5. (A) Average (± S.E.M.) calcium current inhibition by 10 µM glutamate in the absence of inhibitors (Glu) and in the presence of either 1 µM BAY36 (+BAY36) or 1 µM MPEP (+MPEP). Asterisk indicates that the control response is statistically distinguishable from the responses in either inhibitor (P < 0.05, analysis of variance). NS indicates that the responses in BAY36 and MPEP are not significantly different. (B) Calcium current amplitude time courses for sample cells showing strong inhibition by both BAY36 and MPEP (upper panel) and weak inhibition by both inhibitors (lower panel). Graphs indicate the time and duration of each drug application. The order of application of the inhibitors (BAY36 and MPEP) was alternated from cell to cell to avoid systematic errors, although responses did not appear to consistently desensitize. Insets show sample control current traces (larger black traces), currents inhibited by 10 µM glutamate alone (smaller black traces), and glutamate inhibited currents in the presence of BAY36 (blue) or MPEP (red). Scale bars in (B) represent 0.1 nA and 5 milliseconds (upper panel) and 0.5 nA and 5 milliseconds (lower panel). (C) Correlation between the magnitude of glutamate responses in the presence of BAY36 and in MPEP in SCG neurons coexpressing mGluR1 and mGluR5, as in (A) and (B). Data were fit to a line function (y = mx + b), with a slope of +0.74 and an r value of 0.78 (n = 8), yielding a statistically significant positive correlation (P < 0.05, Pearson correlation). (D) Positive correlation of the fractional inhibition (inhibition in each drug divided by that of 10 µM glutamate alone in the same cell) in the presence of BAY36 versus that in MPEP in mGluR1/mGluR5-expressing cells [black circles, dotted line, from the same data illustrated in (C)] and in the presence of BAY36 versus that in MNI-137 (blue open squares) or MATIDA versus that in MNI-137 (red squares) in cells expressing mGluR1 and mGluR2. Fit to a line of these combined data illustrating the expected negative correlation is shown as a red dotted line. BAY, BAY36; MNI, MNI-137.
Perhaps more surprising was the relationship between the specific responses in each cell compared with each other and with the paired control responses in each cell. Fig. 4B illustrates calcium current time courses and sample current traces from two sample cells. One example (Fig. 4B, upper panel) shows a cell in which the responses in the presence of each NAM were quite small compared with the control responses such that the sum of the responses was smaller than the control response. Another shows a cell in which the sum of the inhibited responses were larger than the control response (Fig. 4B, lower panel). These data are interesting because if mGluR1 and mGluR5 acted in a strictly independent manner, one would expect that the responses in each selective inhibitor be inversely proportional. That is, the larger the contribution of one receptor to the total response in a given cell, the smaller the expected contribution is of the other. In fact, the relationship between responses in the presence of BAY36 and MPEP in these cells was the opposite. Fig. 4C shows the absolute response (percentage of calcium current inhibition) of 10 µM glutamate in the presence of BAY36 plotted versus the response in the presence of MPEP in the same cell. The data show a clear, statistically significant positive correlation (slope = +0.74 ± 0.2 from a fit to a simple line function; dotted line, y = mx + b; r = 0.78; P < 0.05 from Pearson correlation).
To verify the prediction that responses in the presence of selective inhibitors should be inversely correlated, similar experiments were analyzed in which mGluR1 and mGluR2 were coexpressed in SCG neurons and 10 µM glutamate was applied alone and with a selective mGluR1 inhibitor [either the competitive antagonist MATIDA (Moroni et al., 2002) at 100 or 1 µM BAY36] as well as a selective mGluR2 inhibitor [the mGluR2-selective NAM MNI-137 (Hemstapat et al., 2007) at 3 µM]. Since the maximal responses varied more widely in these cells, each response was normalized to that in 10 µM glutamate alone and was plotted as a ‘fractional’ response in the presence of the mGluR1 inhibitor (as indicated in Fig. 4D) versus the response in the presence of MNI-137. These data were also fit to a line function and as predicted demonstrated a negative correlation (Fig. 4D, dotted red line). The data from Fig. 4C were also recalculated as fractional inhibition in each cell (BAY36 versus MPEP) to illustrate that the positive correlation persisted (Fig. 4D, solid line). Control responses to these drugs in SCG neurons individually expressing each receptor are shown in Supplemental Fig. 2. These data combined with those presented above suggest that mGluR1 and mGluR5, when coexpressed in SCG neurons, gives rise to a pool of receptors that appear to function in an interdependent manner. To further elucidate the nature of this interaction, additional experiments are necessary.
Effect of Combining Selective Competitive and Noncompetitive Antagonists.
To gain further insight into the functional interaction between mGluR1 and mGluR5 and to determine whether this interaction was similar between wild-type receptors and the interaction we observed between wild-type and YA mutant mGluRs (Figs. 1–3), responses to glutamate in the presence of the selective NAMs BAY36 and MPEP were examined in the presence of the mGluR1-selective competitive antagonist MATIDA. In theory, these data should be somewhat comparable to those in Fig. 3, since MATIDA as a competitive antagonist, should have a similar effect as the YA mutation in that it should shift potency of mGluR1 rightward. Indeed, Supplemental Fig. 3 shows that MATIDA does selectively right-shift mGluR1 potency compared with mGluR5. Interestingly, combining MATIDA with either BAY36 or MPEP (Fig. 5) results in a glutamate concentration response curve that appears quite similar to the NAM inhibited curves using the YA mutants (Fig. 3). These data suggest that the mGluR1/mGluR5 receptor complex responds in a qualitatively similar way to the mGluR1 YA/mGluR5 or the mGluR5 YA/mGluR1 complexes. Perhaps more revealing is that the responses were nearly identical when MATIDA was combined with either BAY36, an mGluR1 NAM, or MPEP, an mGluR5 NAM. Clearly, if mGluR5 was acting independently from mGluR1, coapplication of BAY36 and MATIDA should not hinder its activity, and much larger responses should be seen at 10 and 100 µM glutamate. Together, these data suggest that the mGluR YA mutants appear to accurately mimic the interactions between wild-type receptors, and provide additional support for the conclusion that mGluR1 and mGluR5 function in a mutually dependent manner.
Fig. 5.
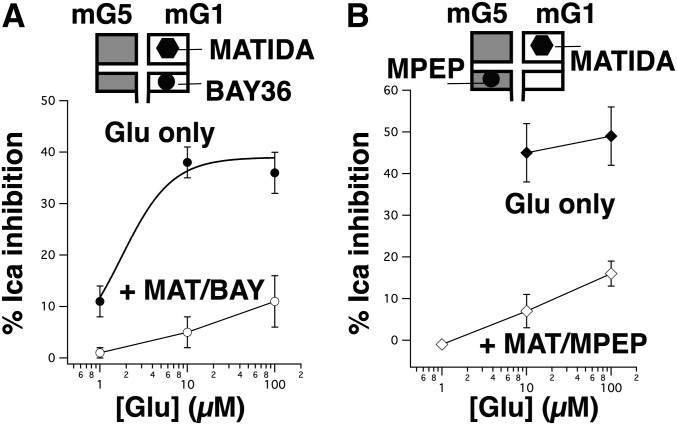
Effect of combined inhibition by MATIDA and selective NAMs on the partial glutamate concentration response of mGluR1/mGluR5. (A) Effect of glutamate (1–100 µM) applied alone (black circles) in SCG neurons expressing mGluR1 and mGluR5, and in the presence of 100 µM MATIDA and 1 µM BAY36 (open circles). All drug applications were paired in the same cells (n = 6 for all data points). (B) Effect of glutamate (10–100 µM) applied alone (black diamonds) in SCG neurons expressing mGluR1 and mGluR5, and 1–100 µM glutamate in the presence of 100 µM MATIDA and 1 µM BAY36 (open diamonds). All drug applications were paired in the same cells (n = 3 for all data points). BAY, BAY36; MAT, MATIDA.
To gain a better understanding of the mechanism underlying the mGluR1/mGluR5 interaction, it may be helpful to examine the pooled effects of various combinations of antagonists against a single concentration of glutamate. Thus, Fig. 6A illustrates average calcium current inhibitory responses in SCG neurons expressing both mGluR1 and mGluR5 to 10 µM glutamate applied alone or with the indicated combinations of the inhibitors BAY36 (1 µM), MPEP (1 µM), and MATIDA (100 µM). Effects of these compounds on SCG neurons expressing mGluR1 or mGluR5 separately are shown in Supplemental Fig. 2. As shown in Fig. 6A, all of the inhibitors significantly reduced the magnitude of calcium current inhibition by 10 µM glutamate. Combining the competitive antagonist MATIDA with either NAM produced particularly strong reductions in the glutamate response that were statistically indistinguishable. This strong reduction in the response was perhaps expected for the MATIDA + MPEP condition, since both expressed receptors were targeted with saturating concentrations of selective inhibitors (Supplemental Fig. 2), but the strong reduction in the response in the presence of MATIDA + BAY36 cannot be reconciled with a model in which mGluR1 and mGluR5 act independently since the inhibition of the glutamate response was almost completely occluded and was in fact comparable to the response of 5 ± 0.3% (n = 3) observed in the presence of the two NAMs (BAY36 and MPEP). Furthermore, the fact that each inhibitor when applied separately produced a significant but only partial reduction in the response demonstrates that both mGluR1 and mGluR5 are clearly expressed in these neurons. Combining both of the NAMs (BAY36 and MPEP) also strongly inhibited the responses to all receptor combinations as expected. The response to 10 µM glutamate in BAY36 and MPEP in cells expressing mGluR1, mGluR5, and both receptors were 3 ± 4% (n = 3), 0 ± 0.4% (n = 3), and 5 ± 0.3% (n = 3), respectively.
Fig. 6.
Average effects of selective inhibitors on coexpressed mGluRs versus 10 µM glutamate. (A) Average (± S.E.M.) response in SCG neurons expressing mGluR1 and mGluR5 to 10 µM glutamate applied alone or with the indicated inhibitors. The numbers of cells in each group are shown in parentheses. Drug concentrations were 100 µM MATIDA, 1 µM BAY36, and 1 µM MPEP. (B) Average (± S.E.M.) response in SCG neurons expressing mGluR1 and mGluR2 to 10 µM glutamate applied alone or with the indicated inhibitors. The number of cells in each group are shown in parentheses. Drug concentrations were 100 µM MATIDA, 1 µM BAY36, and 3 µM MNI-137. Asterisk denotes statistical difference from the control group (P < 0.05, analysis of variance). ns, not significant.
To validate the predictions about the behavior of independently acting receptors in this experiment, analogous experiments were performed in SCG neurons expressing mGluR1 and mGluR2. Here, the mGluR2-selective NAM MNI-137 was used (rather than MPEP) to inhibit that receptor specifically. The efficacy and selectivity of the compounds in cells expressing each receptor separately are shown in Supplemental Fig. 2. As expected, the selective inhibitors MATIDA and MNI-137 both significantly but partially reduced the glutamate response (Fig. 6B), confirming the expression of each receptor. In contrast with the results with mGluR1 and mGluR5, combining MATIDA with BAY36 only partially reduced the response, but a very strong reduction was seen when MATIDA was combined with MNI-137, precisely as expected for independently acting receptors. Together, these data support a model in which mGluR1 and mGluR5 function in a mutually dependent fashion when coexpressed in the same neuron, such that the heptahelical (7TM) domains of both receptors must be free of allosteric inhibitors for the receptor complex to produce substantive G protein activation.
Model for mGluR1/mGluR5 Interaction: Receptors Partially Activate in trans.
The data above strongly suggest that signaling by mGluR1 and mGluR5, when expressed in the same cell, is interdependent but the mechanistic details remain unclear. With these data however, some hypotheses can begin to be formed. Combining a wild-type group I mGluR subtype with a YA mutant is advantageous in that this mutation shifts glutamate sensitivity 50- to 100-fold, which renders glutamate a fairly selective agonist for the wild-type subunit. Another consistent effect of this mutation is that it causes the affected receptor to generate much higher efficacy responses, at least when inhibition of calcium currents in SCG neurons are used as the assay for signaling. The mechanistic basis for this increase in efficacy is unclear, but it may provide a fortuitous means to discriminate signaling between the two expressed receptor subtypes. A reexamination of the data in Fig. 1 could suggest a model in which two major effects are apparent. First, glutamate binding to the wild-type receptor (mGluR5 in Fig. 1) may produce G protein coupling via the YA mutant receptor. This hypothesis arises from the emergence of a high-efficacy response at lower glutamate concentrations than is apparent when the YA receptor is expressed alone. This hypothesis suggests that ligand binding in mGluR1 can induce some G protein activation in mGluR5, and vice versa, is not solidly supported with those data alone, but will be tested more thoroughly below. The second effect is that association with the YA mutant receptor seems to shift the potency of the wild-type receptor to the right. This is suggested by the lack of responses below 100 µM when the receptors are coexpressed. The nature of this effect is unclear and may be caused by either a reduced affinity for glutamate in the wild-type receptor or the inability to transactivate at lower glutamate levels, perhaps until at least some of the associated receptor (the YA mutant) is bound to ligand. Distinguishing between these possibilities may be difficult and is beyond the scope of this study, but this effect is likely not apparent when combining wild-type mGluR1 with wild-type mGluR5, since both receptors have similar affinities for glutamate, and indeed most agonists (Kammermeier, 2012b), and similar EC50s in this assay (see Supplemental Fig. 3).
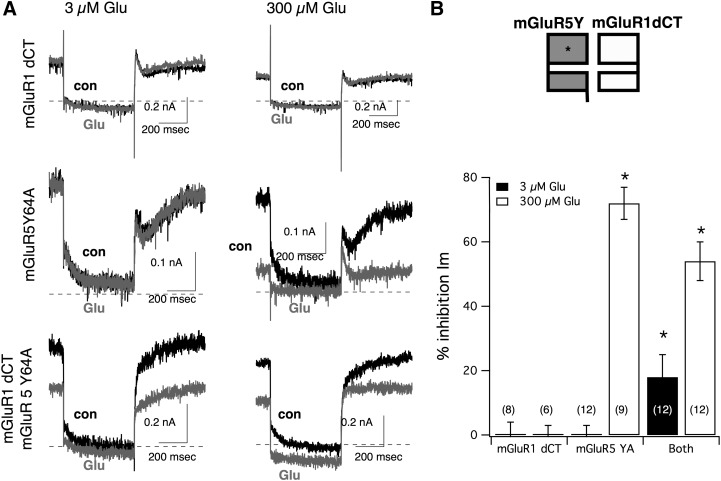
To begin to test whether activation can occur in trans between mGluR1 and mGluR5, inhibition of the M-type potassium current (Brown and Adams, 1980) in SCG neurons was used rather than calcium current inhibition as an assay. Inhibition of the M current occurs via depletion of PIP2 (phosphatidylinositol 4,5-bisphosphate) by phospholipase C upon Gq activation (Suh and Hille, 2002). This pathway is simpler than calcium channel inhibition in SCG neurons, which have both Gi/o (via Gβγ) and Gαq components (Kammermeier and Ikeda, 1999). For this experiment, two mutant receptors were used. The first was mGluR5 Y64A, which can activate Gαq and modulate the M current, but only at glutamate concentrations above the tens of µM range (Fig. 7). The second was a C-terminal deletion mutant of mGluR1 (termed mGluR1 dCT), which shows plasma membrane expression and function, and can activate Gαi/o, but not Gαq (Kammermeier, 2010), and cannot therefore modulate the M current (Fig. 7). As expected, in SCG neurons expressing mGluR1 dCT, application of glutamate up to 300 µM did not produce any detectable inhibition of the M current. At 3 and 300 µM glutamate, the M current was inhibited by −3 ± 5% (n = 6) and −7 ± 3% (n = 10), respectively (Fig. 7; negative inhibition indicates enhancement of the current, shown on the bar graph as 0% inhibition). In neurons expressing only mGluR5 Y64A, M-current inhibition was substantial, but only at the highest glutamate concentration tested. In these cells, inhibition by 3 and 300 µM glutamate was −4 ± 3% (n = 9) and 72 ± 5% (n = 12), respectively. Thus at 3 µM glutamate, neither receptor was capable of modulating the M current when expressed alone, although since mGluR1 dCT can induce calcium current inhibition at this concentration (Kammermeier, 2010), it can be assumed that glutamate binds this receptor when present at 3 µM. Finally, when mGluR1 dCT and mGluR5 Y64A were coexpressed in the same cells, inhibition was indeed observed at low glutamate concentrations. In these cells, inhibition by 3 and 300 µM glutamate was 18 ± 7% (n = 12) and 54 ± 6% (n = 12), respectively. Thus, when two mutant receptors that cannot separately induce M-current inhibition at 3 µM glutamate are coexpressed in SCG neurons, significant inhibition of the current is observed upon application of 3 µM glutamate. These data can be interpreted as supportive of the hypothesis predicting group I mGluR activation in trans. At 3 µM glutamate, ligand binds only to mGluR1 dCT, but the observed M-current inhibition is suggestive of G protein activation at the intracellular side of mGluR5 Y64A, the only mGluR present that can cause Gαq activation.
Fig. 7.
Activation of mGluR5 Y64A in trans by mGluR1 dCT at 3 µM glutamate. (A) Sample representative traces illustrating M current from SCG neurons expressing mGluR1 dCT alone (upper panel), mGluR5 Y64A alone (center panel), and both receptors together (lower panel). Each panel shows control, uninhibited currents (black) superimposed with current in the presence (gray) of 3 µM (left) or 300 µM (right) Glu, as indicated. (B) The upper panel illustrates receptor coexpression conditions. The lower panel shows the average (± S.E.M.) glutamate-induced M-current inhibition in SCG neurons expressing the indicated receptors. The number of cells in each group are shown in parentheses. Asterisks indicate P < 0.05 (analysis of variance) versus mGluR1 dCT only and mGluR5 Y64A only controls for the same [glutamate].
Evidence for an mGluR1/mGluR5 Interaction in Natively Expressed Receptors.
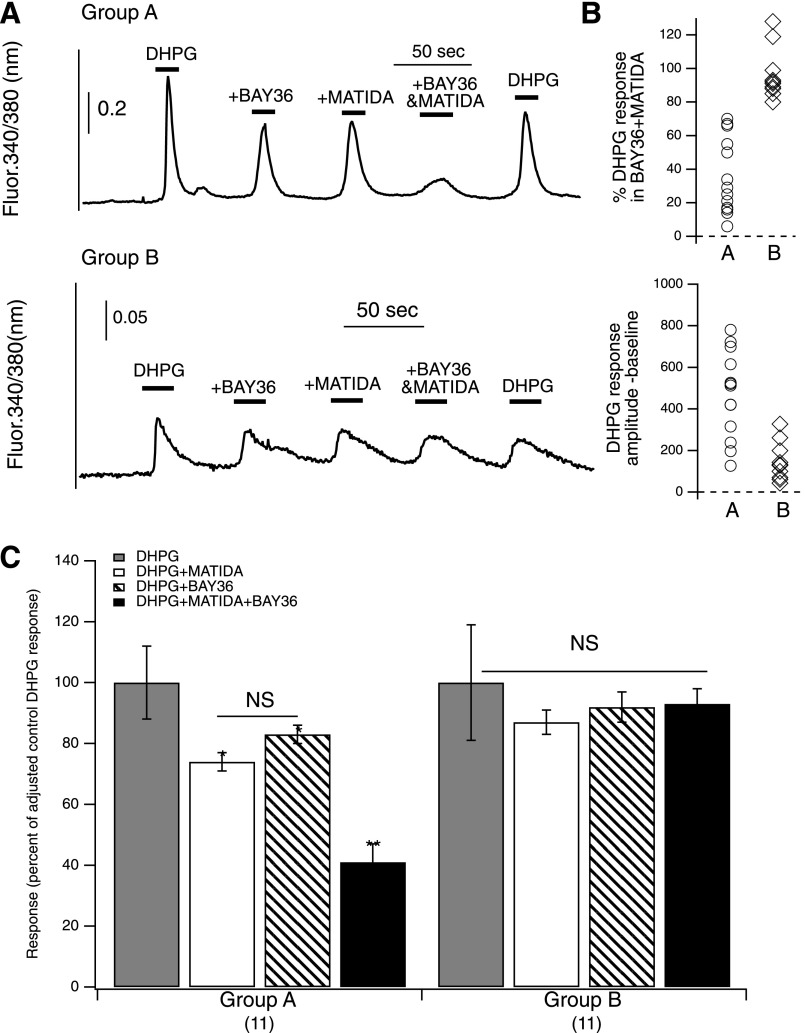
The data above provided compelling evidence that mGluR1 and mGluR5 interact in a mutually dependent manner when heterologously expressed in SCG neurons. To determine whether this interaction is physiologically relevant, it was necessary to observe it in neurons that natively express both receptors. To achieve this, MSNs from the striatum of neonatal rats were isolated and grown in culture for approximately 2 weeks. These cells were chosen because several studies have reported that they express both mGluR1 and mGluR5 (Shigemoto et al., 1992, 1993; Pisani et al., 2001; Gubellini et al., 2004). To maximize the throughput of the experiments, intracellular calcium responses were examined in Fura-2-AM–loaded cells in response to group I mGluR-selective compounds. The experimental protocol was similar to a subset of the experiments described in Fig. 6A (above), but utilizing the group I mGluR agonist DHPG to activate the receptors rather than glutamate to avoid activation of ionotropic glutamate receptors and group II and III mGluRs. Thus, 50 µM DPHG was applied alone (at the start and end of each experiment), and in the presence of BAY36, MATIDA, and BAY36 + MATIDA together (1 µM each). Notably, neurons grown in culture for less than a week failed to respond to DHPG (not shown). This was unsurprising because group I mGluR expression is developmentally regulated, and is not expected to be highly expressed before postnatal day 7 (Jokel et al., 2001). Fortuitously, many striatal neurons isolated from P0-3 rats did respond to DHPG when allowed to grow in culture for 2 weeks (Fig. 8). From these neurons, two distinct populations were evident: those that exhibited a clear reduction in the DHPG response in the combined presence of the two inhibitors BAY36 and 3-MATIDA (≤70% of the control response, n = 11 cells; group A), and those that did not (≥80% of the control response; n = 11 cells, group B; Fig. 8, A and B). Neurons in group B appeared to express only mGluR5, since they were insensitive to all combinations of the mGluR1 inhibitors. The magnitude of DHPG responses in these cells were weak compared with those in group A (Fig. 8A), which may indicate that expression of mGluR5 precedes mGluR1 in MSNs cultured from young rats. Interestingly, DHPG responses in neurons of group A were significantly, but not strongly, reduced by each inhibitor alone, but were potently reduced by coapplication of both (Fig. 8C). The weak effect of each mGluR1 inhibitor alone suggests that in group A cells, mGluR5 is present and responsible for much of the agonist-induced intracellular calcium response, but the strong inhibition of this effect with combined orthosteric and allosteric mGluR1 inhibitors is difficult to reconcile with any model in which mGluR1 and mGluR5 act independently. Furthermore, these results are consistent with the model in that occlusion of mGluR1 signaling appears to strongly blunt even signaling that is primarily mediated by mGluR5, as judged by the weak inhibition by each antagonist applied separately.
Fig. 8.
Mutually dependent signaling in striatal MSNs natively expressing both mGluR1 and mGluR5. (A) Sample time courses of Fura2 ratiometric fluorescence changes, indicative of cytoplasmic calcium changes, in an MSN that appeared to express both mGluR1 and mGluR5 (upper panel) and an MSN that appeared to express only mGluR5 (lower panel). DHPG (50 µM) was used as the agonist alone or in the presence of BAY36 (1 µM), MATIDA (100 µM), or both, as indicated. Note that the control response to DHPG alone did decrease somewhat during the course of the experiment, as indicated by the response to DHPG at the end of each experiment. (B) Distribution of MSN responses to MPEP (upper panel, degree of inhibition of the DHPG response), and magnitude of the initial DHPG response (lower panel) in the MSNs that responded to DHPG. Responses were taken as the peak amplitude minus baseline (absolute fluorescence ratio values). (C) Average calcium responses (± S.E.M.) expressed as a percentage of the DHPG responses in each cell (corrected for decay of the control response) in the two observed subtypes of MSNs, as indicated. NS, not significant.
The primary differences between the current results observed in cultured MSNs and those obtained from patch-clamp studies in SCG neurons are that 1) inhibition by MATIDA or BAY36 applied separately was weaker than was observed in SCG neurons, and 2) the response in the presence of both BAY36 and MATIDA appeared slightly larger (as a fraction of the total response) than in SCG neurons. These discrepancies can both be explained if these MSNs express higher levels of mGluR5 than mGluR1 such that a fraction of mGluR5 signaling is coordinated with mGluR1 signaling, as in SCG neurons, but another subset acts independently. Indeed, despite the evidence that MSNs express both mGluR1 and mGluR5 (Shigemoto et al., 1992, 1993; Pisani et al., 2001; Gubellini et al., 2004), some data indicate that the group I mGluR-mediated calcium responses in these cells are primarily mediated via mGluR5 (Voulalas et al., 2005). Note that if mGluR1 and mGluR5 signal entirely independently, no additional reduction in the DHPG response would be expected with these inhibitors applied together versus each applied alone, since both target mGluR1. In addition, since the doses of each inhibitor applied should be saturating, and completely block mGluR1 signaling when expressed alone in SCG neurons, application of both inhibitors is not expected to produce an additive inhibition. Indeed, in this experiment, the block seen with both drugs was far greater than even an additive effect. Thus, these data strongly suggest that mGluR1 and mGluR5, whether heterologously expressed in peripheral neurons or natively expressed in central neurons, function in a mutually dependent manner, which suggests that efficient signaling of mGluR1 or mGluR5 requires that the partner receptor be potentially activatable. Furthermore, our results imply that when these receptors are expressed at native, presumably lower, expression levels they still exhibit signs of mutually dependent interaction that was described in more detail using heterologously expressed receptors as described above.
Discussion
The data presented here demonstrate a novel functional interdependence between mGluR1 and mGluR5. Using mutagenesis combined with selective pharmacology, it was demonstrated that combining mGluR1 and mGluR5 gives rise to a receptor with a unique pharmacological profile that cannot be adequately explained by the two receptors acting independently, nor by a heterodimerization model as assessed by functional and pharmacological assays. The data in Figs. 4 and 6 are the strongest evidence that wild-type mGluR1 and mGluR5 can signal in a mutually dependent way. These data show that when the receptors are coexpressed, the observed reductions in glutamate signaling by the mGluR1-targeted NAM BAY36 and the mGluR5 targeted NAM MPEP are positively correlated, in contrast with expectations for independently acting receptors, such as that observed using selective antagonists in cells expressing mGluR1 and mGluR2. In addition, coapplication of the mGluR1-selective competitive antagonist MATIDA with each selective NAM resulted in a similarly strong inhibition of the glutamate signal. If these receptors function independently, the inhibitors BAY36 and MATIDA should not produce additive reductions in the glutamate response (since both are saturating concentrations targeting the same receptor), and a clear difference in the response should be evident in the presence of BAY36 + MATIDA versus MPEP + MATIDA. However, neither of these predictions were borne out. The fact that each antagonist partially, but not completely, inhibited glutamate signaling provides a positive control for expression of both receptors. Together, these data provide strong evidence that mGluR1 and mGluR5 can signal through an interdependent pathway to at least some degree when coexpressed in the same neuron. Finally, the data from cultured striatal MSNs (Fig. 8) provides compelling evidence that the described mGluR1/mGluR5 functional interaction is physiologically relevant when these receptors are coexpressed natively in a central neuron.
Due to the nature of the interaction and the lack of highly selective orthosteric agonists (Kammermeier, 2012b), it is not difficult to see why this functional interdependence has gone unnoticed. First, because the receptors share a high degree of homology, each has a similar efficacy and potency (to glutamate and DHPG) for coupling to various effectors when they are natively expressed. In addition, because of the nature of the interaction, a very specific combination of inhibitors was necessary to detect it and most studies using these inhibitors were aimed at identifying which group I mGluR subtypes are involved in various functions rather than at deciphering an interaction between receptors. However, there are some indications from the literature suggesting the validity of our model. For example, Volk et al. (2006) showed that although mGluR-mediated long-term depression of hippocampal excitatory postsynaptic potentials was prevented with coapplication of the mGluR5 allosteric inhibitor MPEP and the mGluR1 orthosteric inhibitor LY367385 (S)-(+)-α-Amino-4-carboxy-2-methylbenzeneacetic acid (similar to the data presented here in Fig. 4A), the transient short-term depression persisted. Similar results were reported by Hou and Klann (2004). These responses may be akin to the small effects we see in the presence of MATIDA and MPEP, which together strongly reduce mGluR1/mGluR5 signaling, but less strongly than when each receptor is expressed separately (Fig. 6; Supplemental Fig. 2). However, it should be noted that there are examples of neurons that appear to coexpress mGluR1 and mGluR5 but exhibit separable function. Examples include GABAergic neurons in the substantia nigra (Marino et al., 2001) and CA1 pyramidal neurons (Mannaioni et al., 2001). These discrepancies can be explained in a few ways. Although Marino et al. (2001) showed that mGluR1 and mGluR5 are both expressed postsynaptically in substantia nigral neurons, they did so with electron microscopy. It is possible that each receptor was mutually expressed in a different subset of these neurons. However, it is also possible that some neurons actively segregate these receptors by restricting their subcellular localization to separate compartments such that coupling to certain effectors is achieved primarily by one receptor. This could be done with specific association with scaffolds like Homer proteins (Brakeman et al., 1997), which interact with all known mGluR5 splice variants, but not with mGluR1b or mGluR1c (Tu et al., 1998).
Although we have shown a functional interaction between mGluR1 and mGluR5 signaling, and recent studies have shown that these receptors may interact physically (Doumazane et al., 2011), the precise nature of this interaction cannot be determined at this time. Doumazane et al. (2011) used a fluorescence energy transfer approach to examine proximity between several mGluRs and their data suggested that the group I mGluRs can likely physically interact, as can all of the group II and III mGluRs tested in their assay. However, the stoichiometry of only one pair, mGluR2 and mGluR4, were examined in more detail. These were determined to form heterodimers (Doumazane et al., 2011). Our more recent study (Kammermeier, 2012a) confirmed that the mGluR2/4 interaction is consistent with a heterodimer interaction. Furthermore, Yin et al. (2014) recently identified native mGluR2/mGluR4 heterodimers in prefrontal cortical neurons. When coexpressed in SCG neurons (Kammermeier, 2012a), mGluR2 and mGluR4 formed a pharmacologically unique receptor that could not be strongly activated by specific orthosteric agonists, but was fully activated by glutamate and by combining mGluR2- and mGluR4-targeted selective agonists DCG-IV [(2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine] and l-AP4 [l-(+)-2-amino-4-phosphonobutyric acid], respectively. This was consistent with a heterodimer model, because mGluR dimers can only be substantially activated by ligand binding in both subunits. Furthermore, mGluR2/mGluR4 appeared to be insensitive to inhibition by the mGluR2-selective NAM Ro64-5229 [(Z)-1-[2-cycloheptyloxy-2-(2,6-dichlorophenyl)ethenyl]-1H-1,2,4-triazole], consistent with previous findings showing that mGluR dimers cannot be inhibited by a NAM that can bind only one subunit in the dimer (Hlavackova et al., 2005). It should be stressed that the mGluR1/mGluR5 interaction described here appears inconsistent with the expected dogmatic mGluR dimer interaction for two reasons. First, in the SCG system, mGluR1/mGluR5 heterodimers are expected to be inactive unless ligand can activate both subunits. It is apparent that this is not the case in the mutant/wild-type experiments described in Figs. 1 and 2 as well as the MATIDA experiments in Fig. 6. In Figs. 1 and 2, the heterodimer model would predict that the concentration-response curve would closely resemble the lower potency receptor when both receptors were coexpressed (the mutant receptors in Figs. 1 and 2). In Fig. 6, MATIDA would be expected to produce very strong block of a putative mGluR1/mGluR5 heterodimer, since it should prevent ligand binding at one dimer subunit. Second, at least two studies have demonstrated that mGluR dimers are not inhibited by NAMs when the compound can interact with only one subunit (Hlavackova et al., 2005; Kammermeier, 2012a). Hlavackova et al. (2005) showed that chimeric mGluR1 dimers engineered to contain only one MPEP binding site per dimer were uninhibited by MPEP, and we showed that mGluR2/mGluR4 heterodimers expressed in SCG neurons were unaffected by the mGluR2-selective NAM Ro64-5229 (Kammermeier, 2012a).
It should also be noted that in one recent article (Goudet et al., 2005), heterodimers of mGluR1 and mGluR5 were observable. However, that study examined chimeric mGluRs in which mGluR1 and mGluR5 were fused to the C termini of GABAB receptors. Those receptors were specifically engineered to form heterodimers so that the authors could control receptor subunit composition, and the experiments were as such not designed to determine whether mGluR1 and mGluR5 heterodimerize. Indeed, our data with mGluR1a and mGluR1b, which differ only in their C-terminal tails, reveal that the identity of the C terminus of group I mGluRs may be an important determinant of dimerization. Although mGluR1a (wild type) appears to form dimers with mGluR1a Y74A in our assay (Kammermeier and Yun, 2005), mGluR1b does not. This conclusion is supported by a recent biochemical study demonstrating that mGluR1a and mGluR1b cannot efficiently dimerize (Remelli et al., 2008).
Thus, the data presented in this study suggest that mGluR1 and mGluR5 interact functionally, but likely do not at least predominantly form heterodimers. In this model, we postulate that mGluR1 homodimers interact with mGluR5 homodimers when coexpressed in the same cells, such that efficient G protein coupling requires that the heptahelical domains of both receptors be uninhibited by NAMs to function with full efficacy. At this time, however, our data can only rule out that the receptors are not primarily heterodimers. It remains unclear whether some portion of the mGluR1/mGluR5 interaction consists of bona fide heterodimers. Thus, additional work will be necessary to further elucidate the stoichiometry of these receptors. At this time, however, the best interpretation of our data are that mGluR1 homodimers exhibit functional cooperation with mGluR5 homodimers.
Supplementary Material
Acknowledgments
The authors thank D.I. Yule (University of Rochester) for assistance and guidance with the calcium imaging experiments (Fig. 8), and A.V. Smrcka and G.G. Tall (University of Rochester) for helpful discussions.
Abbreviations
- 3-MATIDA
α-amino-5-carboxy-3-methyl-2-thiopheneacetic acid
- BAY36-7620
(3aS,6aS)-hexahydro-5-methylene-6a-(2-naphthalenylmethyl)-1H-cyclopenta[c]furan-1-one
- DCG-IV
(2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine
- DHPG
dihydroxyphenylglycine
- l-AP4
l-(+)-2-amino-4-phosphonobutyric acid
- LY367385
(S)-(+)-α-Amino-4-carboxy-2-methylbenzeneacetic acid
- mGluR
metabotropic glutamate receptor
- MNI-137
4-(8-bromo-2,3-dihydro-2-oxo-1H-1,5-benzodiazepin-4-yl)-2-pyridinecarbonitrile
- MPEP
2-methyl-6-(phenylethynyl)pyridine hydrochloride
- NAM
negative allosteric modulator
- PAM
positive allosteric modulator
- Ro64-5229
(Z)-1-[2-cycloheptyloxy-2-(2,6-dichlorophenyl)ethenyl]-1H-1,2,4-triazole
- SCG
superior cervical ganglion
Authorship Contributions
Participated in research design: Sevastyanova, Kammermeier.
Conducted experiments: Sevastyanova, Kammermeier.
Contributed new reagents or analytic tools: Kammermeier.
Performed data analysis: Sevastyanova, Kammermeier.
Wrote or contributed to the writing of the manuscript: Kammermeier.
Footnotes
This research was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant R01-GM101023].
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Bean BP. (1989) Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature 340:153–156 [DOI] [PubMed] [Google Scholar]
- Beqollari D, Kammermeier PJ. (2010) Venus fly trap domain of mGluR1 functions as a dominant negative against group I mGluR signaling. J Neurophysiol 104:439–448 [DOI] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O’Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. (1997) Homer: a protein that selectively binds metabotropic glutamate receptors. Nature 386:284–288 [DOI] [PubMed] [Google Scholar]
- Brown DA, Adams PR. (1980) Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature 283:673–676 [DOI] [PubMed] [Google Scholar]
- Carroll FY, Stolle A, Beart PM, Voerste A, Brabet I, Mauler F, Joly C, Antonicek H, Bockaert J, Müller T, et al. (2001) BAY36-7620: a potent non-competitive mGlu1 receptor antagonist with inverse agonist activity. Mol Pharmacol 59:965–973 [PMC free article] [PubMed] [Google Scholar]
- Doumazane E, Scholler P, Zwier JM, Trinquet E, Rondard P, Pin JP. (2011) A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. FASEB J 25:66–77 [DOI] [PubMed] [Google Scholar]
- Gasparini F, Lingenhöhl K, Stoehr N, Flor PJ, Heinrich M, Vranesic I, Biollaz M, Allgeier H, Heckendorn R, Urwyler S, et al. (1999) 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology 38:1493–1503 [DOI] [PubMed] [Google Scholar]
- Goudet C, Kniazeff J, Hlavackova V, Malhaire F, Maurel D, Acher F, Blahos J, Prézeau L, Pin JP. (2005) Asymmetric functioning of dimeric metabotropic glutamate receptors disclosed by positive allosteric modulators. J Biol Chem 280:24380–24385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubellini P, Pisani A, Centonze D, Bernardi G, Calabresi P. (2004) Metabotropic glutamate receptors and striatal synaptic plasticity: implications for neurological diseases. Prog Neurobiol 74:271–300 [DOI] [PubMed] [Google Scholar]
- Hemstapat K, Da Costa H, Nong Y, Brady AE, Luo Q, Niswender CM, Tamagnan GD, Conn PJ. (2007) A novel family of potent negative allosteric modulators of group II metabotropic glutamate receptors. J Pharmacol Exp Ther 322:254–264 [DOI] [PubMed] [Google Scholar]
- Hlavackova V, Goudet C, Kniazeff J, Zikova A, Maurel D, Vol C, Trojanova J, Prézeau L, Pin JP, Blahos J. (2005) Evidence for a single heptahelical domain being turned on upon activation of a dimeric GPCR. EMBO J 24:499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Klann E. (2004) Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci 24:6352–6361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda SR. (1997) Heterologous expression of receptors and signaling proteins in adult mammalian sympathetic neurons by microinjection. Methods Mol Biol 83:191–202 [DOI] [PubMed] [Google Scholar]
- Jokel ES, Garduno ER, Ariano MA, Levine MS. (2001) Metabotropic glutamate receptors mGluR1alpha and mGluR2/3 display dynamic expression patterns in developing rat striatum. Dev Neurosci 23:1–6 [DOI] [PubMed] [Google Scholar]
- Kammermeier PJ. (2010) C-terminal deletion of metabotropic glutamate receptor 1 selectively abolishes coupling to Galphaq. Eur J Pharmacol 627:63–68 [DOI] [PubMed] [Google Scholar]
- Kammermeier PJ. (2012a) Functional and pharmacological characteristics of metabotropic glutamate receptors 2/4 heterodimers. Mol Pharmacol 82:438–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier PJ. (2012b) The orthosteric agonist 2-chloro-5-hydroxyphenylglycine activates mGluR5 and mGluR1 with similar efficacy and potency. BMC Pharmacol 12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier PJ, Ikeda SR. (1999) Expression of RGS2 alters the coupling of metabotropic glutamate receptor 1a to M-type K+ and N-type Ca2+ channels. Neuron 22:819–829 [DOI] [PubMed] [Google Scholar]
- Kammermeier PJ, Worley PF. (2007) Homer 1a uncouples metabotropic glutamate receptor 5 from postsynaptic effectors. Proc Natl Acad Sci USA 104:6055–6060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier PJ, Yun J. (2005) Activation of metabotropic glutamate receptor 1 dimers requires glutamate binding in both subunits. J Pharmacol Exp Ther 312:502–508 [DOI] [PubMed] [Google Scholar]
- Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K. (2000) Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407:971–977 [DOI] [PubMed] [Google Scholar]
- Lu VB, Williams DJ, Won YJ, Ikeda SR. (2009) Intranuclear microinjection of DNA into dissociated adult mammalian neurons. J Vis Exp 34:1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. (2001) Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J Neurosci 21:5925–5934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino MJ, Wittmann M, Bradley SR, Hubert GW, Smith Y, Conn PJ. (2001) Activation of group I metabotropic glutamate receptors produces a direct excitation and disinhibition of GABAergic projection neurons in the substantia nigra pars reticulata. J Neurosci 21:7001–7012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni F, Attucci S, Cozzi A, Meli E, Picca R, Scheideler MA, Pellicciari R, Noe C, Sarichelou I, Pellegrini-Giampietro DE. (2002) The novel and systemically active metabotropic glutamate 1 (mGlu1) receptor antagonist 3-MATIDA reduces post-ischemic neuronal death. Neuropharmacology 42:741–751 [DOI] [PubMed] [Google Scholar]
- Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, Bernardi G, Calabresi P. (2001) Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience 106:579–587 [DOI] [PubMed] [Google Scholar]
- Ray K, Hauschild BC. (2000) Cys-140 is critical for metabotropic glutamate receptor-1 dimerization. J Biol Chem 275:34245–34251 [DOI] [PubMed] [Google Scholar]
- Remelli R, Robbins MJ, McIlhinney RAJ. (2008) The C-terminus of the metabotropic glutamate receptor 1b regulates dimerization of the receptor. J Neurochem 104:1020–1031 [DOI] [PubMed] [Google Scholar]
- Romano C, Miller JK, Hyrc K, Dikranian S, Mennerick S, Takeuchi Y, Goldberg MP, O’Malley KL. (2001) Covalent and noncovalent interactions mediate metabotropic glutamate receptor mGlu5 dimerization. Mol Pharmacol 59:46–53 [PubMed] [Google Scholar]
- Romano C, Yang WL, O’Malley KL. (1996) Metabotropic glutamate receptor 5 is a disulfide-linked dimer. J Biol Chem 271:28612–28616 [DOI] [PubMed] [Google Scholar]
- Schoepp DD. (2001) Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther 299:12–20 [PubMed] [Google Scholar]
- Shigemoto R, Nakanishi S, Mizuno N. (1992) Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: an in situ hybridization study in adult and developing rat. J Comp Neurol 322:121–135 [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. (1993) Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett 163:53–57 [DOI] [PubMed] [Google Scholar]
- Suh B-C, Hille B. (2002) Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron 35:507–520 [DOI] [PubMed] [Google Scholar]
- Tsuji Y, Shimada Y, Takeshita T, Kajimura N, Nomura S, Sekiyama N, Otomo J, Usukura J, Nakanishi S, Jingami H. (2000) Cryptic dimer interface and domain organization of the extracellular region of metabotropic glutamate receptor subtype 1. J Biol Chem 275:28144–28151 [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. (1998) Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron 21:717–726 [DOI] [PubMed] [Google Scholar]
- Volk LJ, Daly CA, Huber KM. (2006) Differential roles for group 1 mGluR subtypes in induction and expression of chemically induced hippocampal long-term depression. J Neurophysiol 95:2427–2438 [DOI] [PubMed] [Google Scholar]
- Voulalas PJ, Holtzclaw L, Wolstenholme J, Russell JT, Hyman SE. (2005) Metabotropic glutamate receptors and dopamine receptors cooperate to enhance extracellular signal-regulated kinase phosphorylation in striatal neurons. J Neurosci 25:3763–3773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S, Noetzel MJ, Johnson KA, Zamorano R, Jalan-Sakrikar N, Gregory KJ, Conn PJ, Niswender CM. (2014) Selective actions of novel allosteric modulators reveal functional heteromers of metabotropic glutamate receptors in the CNS. J Neurosci 34:79–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.