Abstract
The retinoid X receptor (RXR) partners with numerous nuclear receptors, such as the peroxisome proliferator activated receptor (PPAR) family, liver X receptors (LXRs), and farnesoid X receptor (FXR). Although each heterodimer can be activated by specific ligands, a subset of these receptors, defined as permissive nuclear receptors, can also be activated by RXR agonists known as rexinoids. Many individual RXR heterodimers have beneficial effects in vascular smooth muscle cells (SMCs). Because rexinoids can potently activate multiple RXR pathways, we hypothesized that treating SMCs with rexinoids would more effectively reverse the pathophysiologic effects of angiotensin II than an individual heterodimer agonist. Cultured rat aortic SMCs were pretreated with either an RXR agonist (bexarotene or 9-cis retinoic acid) or vehicle (dimethylsulfoxide) for 24 hours before stimulation with angiotensin II. Compared with dimethylsulfoxide, bexarotene blocked angiotensin II-induced SM contractile gene induction (calponin and smooth muscle-α-actin) and protein synthesis ([3H]leucine incorporation). Bexarotene also decreased angiotensin II-mediated inflammation, as measured by decreased expression of monocyte chemoattractant protein-1 (MCP-1). Activation of p38 mitogen-activated protein (MAP) kinase but not extracellular signal-related kinase (ERK) or protein kinase B (Akt) was also blunted by bexarotene. We compared bexarotene to five agonists of nuclear receptors (PPARα, PPARγ, PPARδ, LXR, and FXR). Bexarotene had a greater effect on calponin reduction, MCP-1 inhibition, and p38 MAP kinase inhibition than any individual agonist. PPARγ knockout cells demonstrated blunted responses to bexarotene, indicating that PPARγ is necessary for the effects of bexarotene. These data demonstrate that RXR is a potent modulator of angiotensin II-mediated responses in the vasculature, partially through inhibition of p38.
Introduction
As a dominant cell type within the vasculature, vascular smooth muscle cells (SMCs) are important to maintaining vascular tone and are key initiators of vascular disease. In a healthy adult artery, SMCs are quiescent and express a family of SM-specific proteins, including calponin, SM-α-actin, and SM myosin heavy chain. In response to multiple environmental cues, such as mechanical injury or growth factors, SMCs can undergo tremendous phenotypic changes (Owens et al., 2004). For example, in the setting of angioplasty or after stimulation with platelet-derived growth factor-BB (PDGF-BB), SMCs lose expression of differentiation markers, proliferate, and migrate from the media, leading to neointima formation. In contrast, in the setting of hypertension induced by angiotensin II (AII), SMCs undergo hypertrophy and increase expression of SMC-specific markers; eventually, this process will lead to thickening of the arterial media and increased vascular resistance (Mack, 2011). AII is an octapeptide that is closely linked to a variety of cardiovascular diseases. After binding to the angiotensin type 1 receptor (AT1R) on SMCs, AII causes an acute increase in intracellular calcium leading to SMC contraction. Over hours to days, via activation of multiple downstream pathways including extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinase, AII signaling results in increased expression of SM-specific genes, hypertrophy, and expression of inflammatory cytokines (Mehta and Griendling, 2007). Chronically, AII-induced vascular remodeling increases vascular resistance and promotes chronic hypertension. In many clinical trials, angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) have significant cardiovascular benefits that may be partially due to the effects on vascular remodeling (Mancia et al., 2013).
Retinoid X receptors (RXRs), members of the nuclear receptor superfamily, are common heterodimer partners with other nuclear receptors, such as retinoic acid receptor (RAR), peroxisome proliferator activated receptors (PPARs), and liver X receptor (LXR) (Lefebvre et al., 2010). These heterodimers act as ligand-activated transcription factors. Two examples of specific nuclear receptor agonists are thiazolidinediones, selective PPARγ-RXR agonists, and fibrates, selective PPARα-RXR agonists. In contrast, specific RXR agonists (rexinoids) have the potential to activate a large subset of heterodimers, including all PPAR isoforms, LXR, and farnesoid X receptor (FXR) (Altucci et al., 2007). Heterodimers activated by both rexinoids and nuclear receptor-specific ligands are termed permissive heterodimers. Other RXR heterodimers, such as RAR-RXR and vitamin D receptor (VDR)-RXR, cannot be activated by rexinoids alone and are termed nonpermissive heterodimers. In addition to activating RXR heterodimers, in cancer cells and monocytes, rexinoids have been shown to activate pathways regulating growth and inflammation via RXR-RXR homodimers (Tanaka et al., 2007; Szeles et al., 2010). Rexinoids have been shown to improve insulin sensitivity in animal models of diabetes, likely through activation of PPARγ (Mukherjee et al., 1997). Multiple preclinical studies have shown rexinoids have antitumor efficacy; bexarotene, a selective RXR agonist, is FDA-approved for cutaneous T-cell lymphoma (Lansigan and Foss, 2010). Recently, bexarotene was shown to be protective in a mouse model of Alzheimer’s disease by increasing clearance of amyloid plaques (Cramer et al., 2012).
There are accumulating data that permissive nuclear receptor agonists may activate potent vasculoprotective pathways. In SMCs, individual nuclear receptors have been shown to exert important biologic effects. For instance, activation of PPARγ by rosiglitazone has been shown to decrease AII-induced SMC hypertrophy (Benkirane et al., 2006). In contrast, PPARδ agonists decrease SMC senescence associated with AII (Kim et al., 2011), and LXR agonists decrease expression of the AT1R receptor (Imayama et al., 2008). The effects of rexinoids on SMCs have not been studied; however, in endothelial cells, bexarotene inhibits tumor necrosis factor α–induced adhesion molecule expression and decreases mononuclear cell recruitment (Sanz et al., 2012).
Activating multiple nuclear receptors may represent a potent approach for modulating the SMC phenotype. By activating multiple nuclear receptors, rexinoids may be able to engage distinct pathways (i.e., PPARγ- and LXR-induced pathways), which cooperate to promote enhanced responses compared with activation of a single nuclear receptor. In macrophages, PPARγ and LXR control overlapping and nonoverlapping anti-inflammatory pathways (Ogawa et al., 2005). In hepatocytes, gene profiling has revealed that rexinoids, PPARα agonists, and LXR agonists activate both common pathways and unique targets (Boergesen et al., 2012). We therefore hypothesized that rexinoids would more potently inhibit the biologic effects of AII on SMCs compared with an individual nuclear receptor agonist.
Materials and Methods
Materials.
Eagle’s minimal essential medium (EMEM) (Mediatech, Mannassas, VA) and fetal calf serum (FCS) (Hyclone, South Logan, UT) were used for culturing SMCs. For transient transfections, cells were placed in Opti-MEM (Life Technologies, Carlsbad, CA). Bexarotene (Biovision, Milpitas, CA), 9-cis retinoic acid (Sigma-Aldrich, St. Louis, MO), pioglitazone (Cayman Chemical, Ann Arbor, MI), fenofibrate (Cayman Chemical), T0901317 [N-[4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl]-N-(2,2,2-trifluoroethyl)benzenesulfonamide] (Cayman Chemical), GW501516 (2-[2-methyl-4-[[4-methyl-2-[4-(trifluoromethyl)phenyl]-1,3-thiazol-5-yl]methylsulfanyl]phenoxy]acetic acid) (Enzo, Farmingdale, NY), 6-ECDCA [6α-ethyl-chenodeoxycholic acid] (Cayman Chemical), PD98059 [2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one] (EMD Millipore, Billerica, MA), and SB 203580 [4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole] (EMD Millipore) were all dissolved in dimethylsulfoxide (DMSO; Sigma-Aldrich).
SMC Isolation and Culture.
Rat SMCs were isolated and cultured as described previously elsewhere (Furgeson et al., 2010). Briefly, thoracic aortas were dissected from male Harlan Sprague-Dawley rats (250–300 g) and incubated in EMEM containing 2 mg/ml collagenase for 1 hour at 37°C. The adventitia was removed, and the aortas were minced and incubated in the MEM collagenase solution at 37°C for 2 hours. The isolated cells were plated at a density of 1 × 104 cells/ml culture medium (EMEM) containing 100 units/ml penicillin, 100 μg/ml streptomycin, and 10% FCS in 35-mm culture dishes. Cells were passed by trypsinization and used between passage numbers 7 and 15. For all experiments, cells were growth arrested in EMEM with 0.1% calf serum the day after plating.
Quantitative Reverse-Transcription Polymerase Chain Reaction.
Total RNA was isolated from SMCs using the QIAshredder and RNeasy plus kits (Qiagen, Valencia, CA), and first-strand cDNA was made using the iScript cDNA synthesis kit (BioRad, Hercules, CA). The quantitative reverse-transcription polymerase chain reaction (qRT-PCR) reaction was performed with Power SYBR Green PCR master mix (Applied Biosystems, Carlsbad, CA). Sequence-specific primers are as follows: calponin forward (5′-CACCAATCATACACAAGTTCA-3′), reverse (5′-CTTCTCAGGCTCAAATCTCC-3′); glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward (5′-CGTGGAGTCTACTGGCGTCTTCAC-3′), reverse (5′-CGGAGATGATGACCCTTTTGG-3′); MCP-1 forward (5′-TGCTGTCTCAGCCAGATGCAGTTA-3′), reverse (5′-TACAGCTTCTTTGGGACACCT-3′); dual-specificity phosphatase 10 (DUSP10) forward (5′-TGCCCTTCCTGTTTCTTGGCAATG-3′), reverse (5′-CGCAAGTTCTGCTTGTTGCTGTCT-3′); DUSP4 forward (5′-TGCCTGCTTAAAGGTGGCTAT-3′), reverse (5′-CGGCCAGGGCCTTAGTTTTA-3′); DUSP5 forward (5′-GTGGATGCGAAGCCCATTTCA-3′), reverse (5′-TGCATGGTAGGCACTTCCAAG-3′); DUSP6 forward (5′-TGTACGAGTCGTCGCACATCGAAT-3′), reverse (5′-ACCTGTGTTCTCATTCCAGTCGCT-3′); and DUSP1 forward (5′-CTGGAGCATATCGTGCCG AA-3′), reverse (5′-GAAAACGCTTCATATCCTCCTTG-3′).
Western Analysis and Enzyme-Linked Immunosorbent Assay.
At the indicated times, SMCs were lysed with ice-cold RIPA buffer, pH 7.4 (50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 1% Na deoxycholate, 0.1% SDS, 50 mM NaF, 2 mM EDTA 200 μM Na3VO4, and protease inhibitors). Solubilized proteins were centrifuged at 14,000g in a microcentrifuge (4°C) for 10 minutes. Supernatants were separated using 10% SDS-polyacrylamide gel electrophoresis and transferred to Immobilon P membranes (Millipore, Billerica, MA). Membranes were blocked for 1 hour at room temperature in Tris-buffered saline (10 mM Tris- HCl, pH 7.4, 140 mM NaCl) containing 0.1% Tween-20 (TTBS) and 2.5% bovine serum albumin (Sigma-Aldrich), and then incubated with 5% bovine serum albumin in TTBS solution containing primary antibodies for 16 hours at 4°C. Membranes were washed in TTBS, and bound antibodies were visualized either with horseradish peroxidase-coupled secondary antibodies and enhanced chemiluminescence reagent (Fisher/Thermo Scientific, Pittsburgh, PA) or alkaline phosphatase-coupled secondary antibodies and Lumi-Phos WB (Thermo Scientific, Rockford IL) according to the manufacturer’s directions. Antibodies used were calponin (Abcam, Cambridge, MA), phospho-p38, total p38, phospho-ERK, total ERK, phospho-protein kinase B (p-Akt), total Akt, phospho-4EBP1 (Cell Signaling, Danvers, MA), and Sm-α-actin and β-actin (Sigma-Aldrich). Anti-rabbit and anti-mouse secondary antibodies were used (Santa Cruz, Dallas, TX). Enzyme-linked immunosorbent assay (ELISA) was performed as previously described elsewhere (Nemenoff et al., 2008). To detect secreted MCP-1, conditioned medium from SMCs 48 hours after stimulation with AII was assayed using a mouse/rat MCP-1 specific solid-phase ELISA kit according to the protocols provided (R&D Systems, Minneapolis, MN). Values were normalized to total cell protein from individual cell cultures.
Plasmids and Transient Promoter Assays.
For transient transfections measuring promoter activity, a plasmid encoding 549nt upstream of the calponin initiation site ligated into a luciferase vector (PA3-Luc) was used (a gift from Joseph Miano, University of Rochester). SMCs were transiently transfected with Lipofectamine Plus (Life Technologies) in 60-mm dishes using 2 μg of the calponin promoter-luciferase construct together with 2 μg of a plasmid encoding cytomegalovirus-β-galactosidase vector (Clontech, Mountain View, CA) for normalization of transfection efficiency. Some experiments also included 2 μg of CA-MKK6 (MAP kinase kinase 6) or empty vector/pCDNA (Life Technologies). Cells were incubated for 16 hours in EMEM with 0.2% FCS and then placed in EMEM with 0.1% FCS with or without AII and bexarotene for 48 hours. For PPAR-RE (response element) and LXR-RE reporter assays, 2 μg of reporter plasmid and 2 μg of β-galactosidase were added to SMCs in the presence of Lipofectamine Plus. After 5 hours, EMEM with 10% calf serum was added, and the medium was changed at 24 hours. Agonists were added for an additional 24 hours. Transiently transfected SMCs were then washed twice with ice-cold phosphate-buffered saline and harvested in luciferase reporter lysis buffer (Promega, Madison, WI). Cell lysates were centrifuged, and the supernatants were assayed for luciferase and β-galactosidase activities, as previously described elsewhere (Horita et al., 2011).
[3H]Leucine Incorporation.
SMCs were serum restricted in 0.1%-FCS-EMEM for 24 hours then treated with bexarotene or DMSO for an additional 24 hours. Fresh drugs plus or minus AII (10−6 M) were then added to serum-free medium. [3H]Leucine (1 mCi/ml) was added to the media at the same time, and cells were incubated for 24 hours. Cells were rinsed extensively on ice with ice-cold phosphate-buffered saline to remove free [3H]leucine, and then precipitated with 10% (w/v) trichloroacetic acid (Sigma-Aldrich) for 30 minutes on ice. Cells were then rinsed with 10% trichloroacetic acid, followed by three washes with 95% ice-cold ethanol. Ethanol was removed, and samples were air-dried on ice. Precipitated material was solubilized with 300 μl of SDS/NaOH solution. [3H]leucine incorporation was quantified via scintillation (Beckman Coulter, Brea, CA).
Short Hairpin RNA Infection of SMCs.
Lentiviral packaging vectors, control plasmid (pGIPZ), and PPARγ-specific short hairpin RNA (shRNA) plasmid (pGIPZ-PPARγ) were purchased from Open Biosystems (GE Dharmacon, Lafayette, CO). Human embryonic kidney 293T (HEK293T) cells were infected with lentiviral plasmids in the presence of EMEM with 10% FCS. The next day, the medium was removed, filtered through a 40-μm filter, and added to the target SMCs. On the next day, SMC medium containing 2 μg/ml puromycin was added for selection. Cells were used for experiments after two passages under puromycin selection.
Transfection with Small-Interfering RNA Oligonucleotides.
Nontargeting small-interfering RNA (siRNA) oligonucleotides and oligonucleotides for dual-specificity phosphatase (DUSP) isoforms were purchased from Dharmacon (GE Dharmacon, Lafayette, CO). Cells were transfected with 25 nM of oligonucleotides using Dharmafect 2 (Dharmacon) for 24 hours, following the manufacturer’s protocol.
Statistical Analysis.
All experiments were repeated a minimum of three times. Paired t tests were performed to compare the drug treatment effect to DMSO. For multiple condition comparisons, analysis of variance (ANOVA) with Tukey’s post-test was used. All data are presented as mean ± S.E.
Results
Rexinoids Inhibit AII-Induced SMC Differentiation and SMC Hypertrophy.
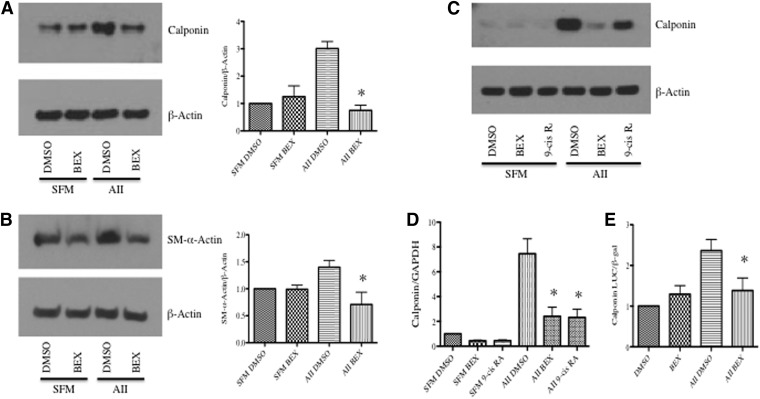
We first determined whether bexarotene (Altucci et al., 2007), a selective rexinoid, modulated the effects of AII on expression of the SMC-specific markers SM-α-actin (SMA) and calponin in cultured aortic SMCs. We treated SMCs with 1 μM bexarotene, a concentration achievable in humans using standard dosages (Rizvi et al., 1999). Because bexarotene most likely causes transcription of target genes in SMCs, we treated SMCs with bexarotene for 24 hours before adding other agonists. To induce expression of SMC genes over a 48-hour period, we treated cells with AII (10−6M). Because AII is degraded by proteases over a 48-hour period, we used the higher concentration of AII. Bexarotene alone had no effect on expression of SMC markers. However, pretreatment with bexarotene blocked the increase in SMC marker expression induced by AII (Fig. 1, A–B).
Fig. 1.
RXR activation inhibits AII-induced SM gene expression. SMCs were pretreated with either bexarotene (1 μM) or 9-cis retinoic acid (9-cis RA; 1 μM) for 24 hours and then stimulated with DMSO or AII (10−6 M) for 3 hours (RNA) or 48 hours (protein and luciferase assay). (A and B) Western blot demonstrating that bexarotene inhibits AII-mediated (A) calponin and (B) SM-α-actin (SMA) induction. (C) Western blot and (D) qRT-PCR showing that 9-cis retinoic acid and bexarotene inhibit induction of (C) calponin protein and (D) mRNA. For all Western blot analyses, β-actin was used as a loading control. Representative blots are shown; fold changes in densitometry measurements ± S.E.M. are shown in graphs. (E) Calponin-luciferase reporter assay demonstrating bexarotene blocks AII-induced transcription of calponin. *P < 0.05 versus serum-free medium DMSO.
As opposed to induction of SM marker genes, PDGF-BB is a known repressor of the SMC differentiation program. In contrast to effects mediated by AII, bexarotene had no effect of PDGF-BB–mediated down-regulation of SM markers (data not shown). To demonstrate that these biologic effects were not due to off-target effects of bexarotene, we repeated the experiments with a second chemically distinct rexinoid: 9-cis retinoic acid (alitretinoin) (Altucci et al., 2007). At a concentration of 1 μM, 9-cis retinoic acid is also similar to concentrations seen in humans (Lawrence et al., 2001). Both rexinoids inhibited the up-regulation of calponin protein and mRNA after stimulation with AII (Fig. 1, C and D). A calponin luciferase reporter assay was conducted to confirm that the effects of rexinoids occur at the transcriptional level. As shown in Fig. 1E, bexarotene pretreatment blocked AII-induced calponin promoter activity.
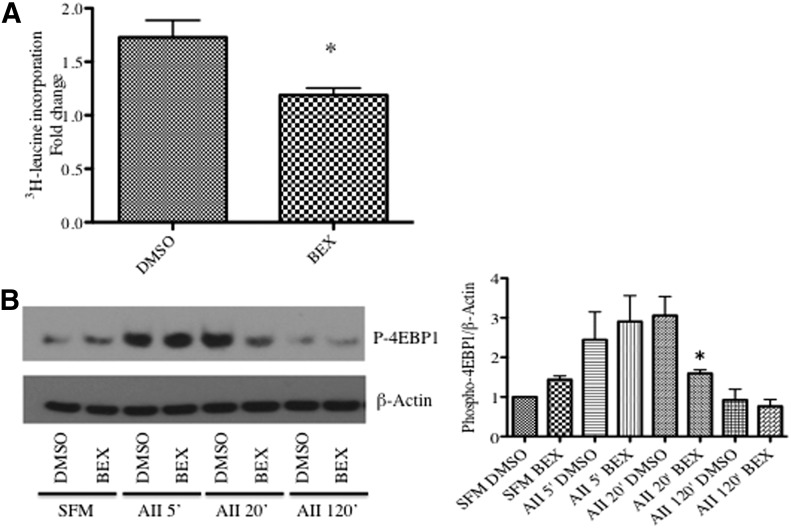
We next examined the effect of bexarotene on AII-induced SMC hypertrophy. Bexarotene inhibited AII-induced hypertrophy, as measured by protein synthesis using [3H]leucine incorporation (Fig. 2A). AII-induced hypertrophy is associated with phosphorylation and inactivation of the translation repressor protein eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) (Benkirane et al., 2006). Consistent with an effect on SMC hypertrophy, bexarotene pretreatment resulted in significantly less phosphorylated and therefore active 4E-BP1 at 20 minutes (Fig. 2B). These data suggest that modulation of 4E-BP1 is one possible mechanism mediating the antigrowth effects of bexarotene.
Fig. 2.
Bexarotene inhibits SM hypertrophy. (A) SMCs were pretreated with bexarotene for 24 hours, and [3H]leucine was added for an additional 24 hours. [3H]Leucine incorporation was measured as described in Materials and Methods. (B) After bexarotene pretreatment, cells were collected at indicated time points after AII treatment and subjected to Western blotting for p-4EBP1. β-Actin was used as a loading control. Representative blot shown; fold changes in densitometry measurements ± S.E.M. are shown in graph. *P < 0.05 versus DMSO.
Rexinoids Negatively Regulate AII-Induced Inflammation.
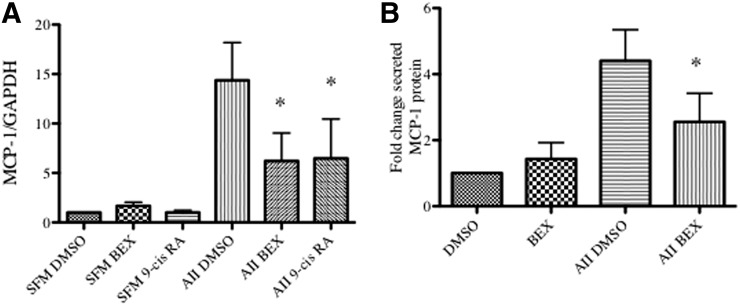
As AII is also a potent SMC proinflammatory agent, we tested whether bexarotene affected transcription of inflammatory cytokines. As shown in Fig. 3A, AII significantly increased monocyte chemoattractant protein (MCP-1/CCL-2) mRNA at 3 hours. Bexarotene and 9-cis retinoic acid alone had no effect on MCP-1 expression, but pretreatment with either agent blunted the increase in MCP-1 induced by AII. Furthermore, to confirm a change in MCP-1 protein, levels of MCP-1 in conditioned media were measured. AII increased MCP-1 protein levels at 48 hours whereas bexarotene pretreatment attenuated the increase in MCP-1 (Fig. 3B).
Fig. 3.
Rexinoids inhibit cytokine expression after stimulation with AII. Cells were pretreated for 24 hours with bexarotene or 9-cis retinoic acid and stimulated with AII for 3 hours (mRNA) or 48 hours (protein). (A) Total RNA was isolated and qRT-PCR conducted for MCP-1 mRNA. Shown are mRNA absolute copy number normalized to GAPDH. Mean ± S.E.M., n = 3 independent experiments. (B) Conditioned media was collected and assayed for MCP-1 protein levels normalized to total protein. Shown are mean ± S.E. *P < 0.05 versus DMSO.
Activation of p38 MAP Kinase Is Blunted by Rexinoids.
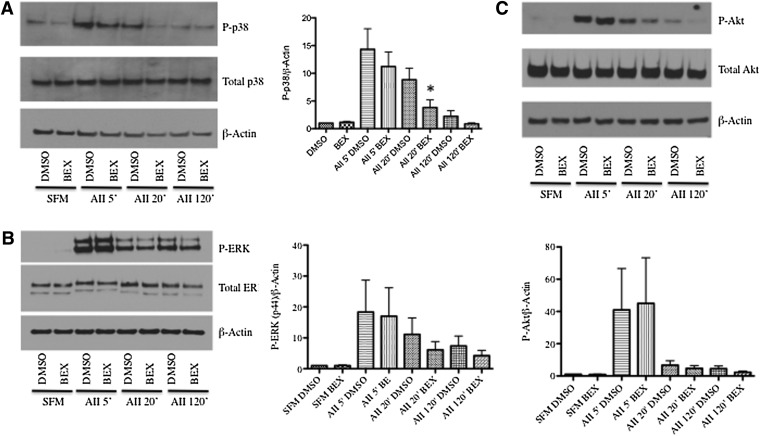
Because p38 and ERK MAP kinases have been shown to drive SM gene expression and MCP-1 production, we next studied whether rexinoids modulated short-term signaling events. As with the previous experiments, SMCs received 24 hours of bexarotene treatment before stimulation with AII to allow transcription and translation of RXR targets. Cells were stimulated with AII (10−6M) to correlate the short-term experiments with the 48-hour experiments. As shown in Fig. 4A, AII led to a rapid induction of phospho-p38 MAP kinase, which returned to basal levels by 120 minutes after stimulation. Bexarotene pretreatment had no effect on maximal activation of p38 at 5 minutes, but there was a significant reduction after 20 minutes of AII stimulation. This same pattern was seen using a lower concentration of angiotensin (10−7 M, not shown). In contrast, although AII stimulation also activated ERK and Akt, bexarotene did not inhibit either (Fig. 4, B and C), suggesting bexarotene selectively blocks AII-mediated p38 signaling.
Fig. 4.
Bexarotene inhibits p38 activation after stimulation with AII. Cells were treated for 24 hours with bexarotene before stimulation with AII (10−6 M) for the indicated times. (A–C). Left panels: Representative Western blots for phospho- and total p38 (A), phospho- and total ERK (B), and phospho- and total Akt (C). β-actin was used as a loading control. N = 3 independent experiments. Right: Fold changes in densitometry measurements ± S.E.M. are shown in the graphs. *P < 0.05 versus AII DMSO at 20 minutes.
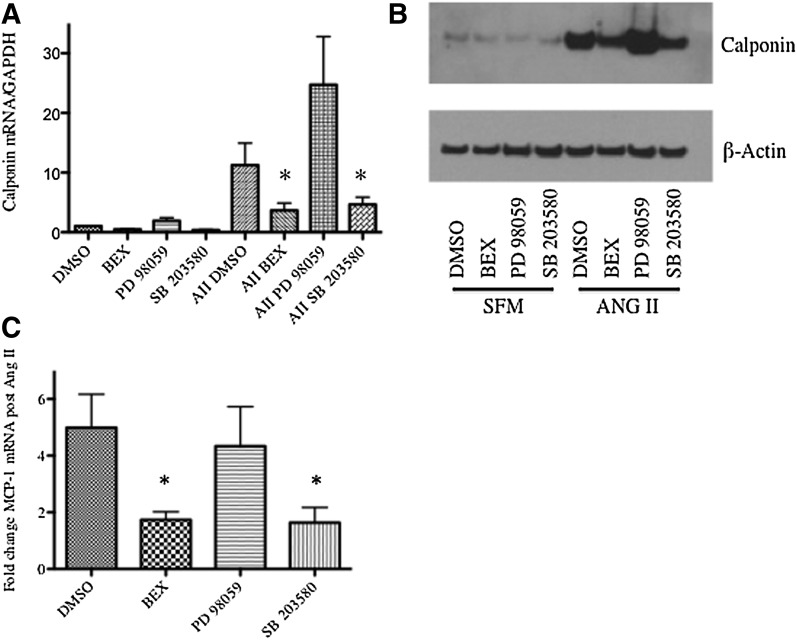
To determine whether the rexinoids’ effect on calponin induction was mediated via p38 inhibition, we compared bexarotene pretreatment to pretreatment with either a MEK inhibitor (PD98059; upstream ERK inhibitor) or a p38 inhibitor (SB203580). As shown in Fig. 5, A and B, bexarotene and SB203580 both inhibited calponin induction at the mRNA and protein levels, but PD98059 had no effect. Induction of p38 also, in part, mediates MCP-1 induction after AII (Takahashi et al., 2008; Ebrahimian et al., 2011). Bexarotene and SB203580, but not PD98059, inhibited induction of MCP-1 mRNA (Fig. 5C). Collectively, these data indicate that effects of RXR agonists on AII-induced SMC gene and MCP-1 expression are possibly mediated via negative regulation of p38.
Fig. 5.
Bexarotene modulates SMC phenotype via changes in p38. SMCs were pretreated with bexarotene, a p38 inhibitor (SB203580, 10 μM), or a MEK inhibitor (PD98059, 10 μM) followed by stimulation with AII. Total RNA and whole-cell lysates were isolated 3 hours and 48 hours after AII stimulation, respectively. (A) The qRT-PCR analysis for calponin mRNA. Shown are mRNA absolute copy numbers normalized to GAPDH. (B). Western blot analysis for calponin protein levels; shown is a representative Western blot from three independent experiments; β-actin was used as a loading control. (C) qRT-PCR for MCP-1 mRNA. Shown are mRNA absolute copy numbers normalized to GAPDH. *P < 0.05 versus AII DMSO.
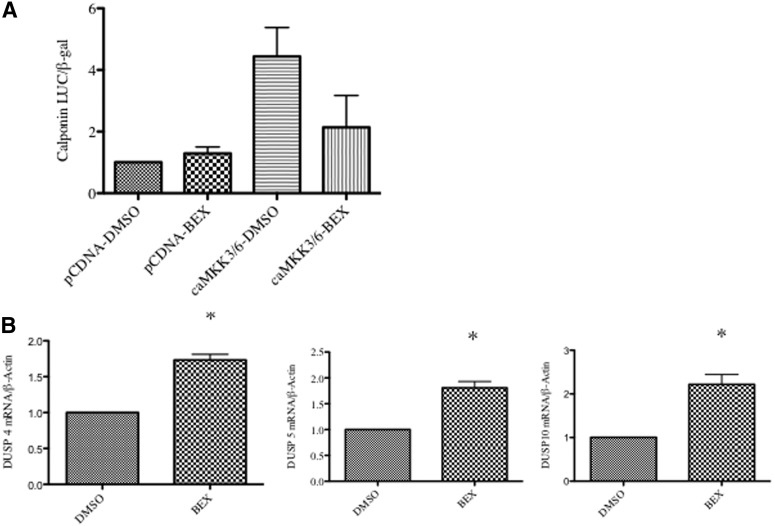
MKK3 and MKK6 are upstream kinases for p38, and we have previously shown that transfecting SMCs with a constitutively active MKK3/6 construct increases SMC gene expression (Garat et al., 2000). Because Mkk3/6 are downstream effectors of AT1R, it is unlikely that if bexarotene solely blocks AT1R activity, it would retain an inhibitory effect in the presence of constitutively activated MKK3/6. Given that bexarotene did not inhibit p38 activation at 5 minutes, we predicted it was not blocking upstream activators of p38. To confirm this possibility, SMCs were cotransfected with a calponin promoter-luciferase reporter construct and a constitutively active MKK3/6 plasmid. Consistent with an inhibitory role for bexarotene downstream of AT1R-MKK3/6-p38 signaling, bexarotene continued to block the effect of AII on SMC gene expression even in the presence of active MKK3/6 (Fig. 6A). These data raised the possibility that rexinoids up-regulate phosphatases that inactivate p38.
Fig. 6.
Bexarotene inhibits p38 activation downstream of MKK3/6. (A) SMCs were cotransfected with constitutively active MKK3/6 or control (pCDNA) and calponin-luciferase reporter. After pretreatment with bexarotene, cells were stimulated for 48 hours with AII. Luciferase activity normalized to β-galactosidase was determined. P = 0.06 ca-MKK3/6-DMSO versus caMKK3/6-BEX. (B) The qRT-PCR showing increased levels of DUSP4, DUSP5, and DUSP10. *P < 0.05 versus DMSO.
Inactivation of p38 is controlled by several members of the dual specificity phosphatase (DUSP) family. Multiple DUSPs have been described, which have different specificities for individual MAP kinase family members. Using qRT-PCR, we tested whether bexarotene increased levels of the DUSPs reported to dephosphorylate p38, DUSPs 1, 2, 4, 5, 6, 7, 8, 9, 10, 14, 16, and 26. We found that DUSP4, DUSP5, and DUSP10 were significantly increased with bexarotene (Fig. 6B). Knockdown experiments with siRNA constructs targeting each of these DUSPs did not reverse the effects of bexarotene (not shown). However, knockdown of DUSP4 was also associated with compensatory increases in other DUSPs (Supplemental Fig. 1).
Comparison of RXR Agonists to Individual RXR Heterodimer Agonists.
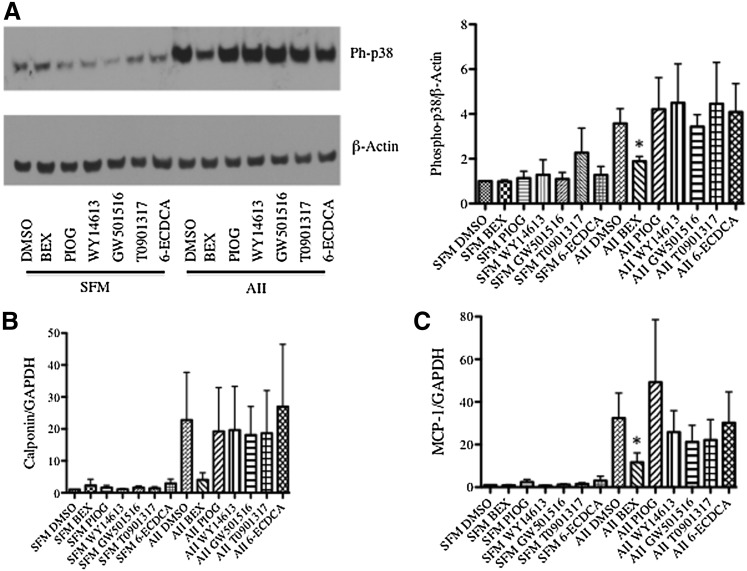
Because rexinoids activate multiple nuclear receptors, we next compared bexarotene with individual permissive nuclear receptor agonists to determine whether one or more receptors predominantly mediated the effects of bexarotene on SMCs. Bexarotene was compared with WY-14643 [4-chloro-6-(2,3-xylidino)-2-pyrimidinylthio]acetic acid] (PPARα agonist) (Forman et al., 1997), pioglitazone (PPARγ agonist) (Sakamoto et al., 2000), GW501516 (PPARδ agonist) (Oliver et al., 2001), T0901317 (LXR agonist) (Repa et al., 2000), and 6-α-ethyl-chenodeoxycholic acid (6-ECDCA, FXR agonist) (Pellicciari et al., 2002). We first confirmed that certain agonists activated target gene transcription using reporter constructs. Piogitazone (10 μM) activated a PPARγ response element to a similar extent as bexarotene (Supplemental Fig. 2A). T0901317 and bexarotene both activated LXR transcription at concentrations of 1 μM (Supplemental Fig. 2B). Bexarotene was much more effective at inhibiting p38 activation at 20 minutes than any individual agonist (Fig. 7A). Similarly, bexarotene inhibited MCP-1 and calponin induction significantly more than any individual agonist (Fig. 7, B and C), suggesting that bexarotene activates multiple nuclear receptor pathways.
Fig. 7.
Bexarotene inhibits AII-mediated calponin and MCP-1 induction by recruitment of multiple RXR heterodimers. SMCs were pretreated with bexarotene, pioglitazone (PPARγ agonist, 10 μM), WY-14643 (PPARα agonist, 1 μM), GW-501516 (PPARδ agonist, 1 μM), T0901317 (LXR agonist, 1 μM), and 6-ECDCA (FXR agonist, 1 μM). (A) Whole-cell lysates were collected 20 minutes after AII stimulation. Left: Western blot analysis for phospho-p38 protein levels; shown is a representative Western blot from three independent experiments; β-actin was used as a loading control. Right: Fold changes in densitometry measurements ± S.E.M. are shown in the graphs. (B and C) Total RNA was collected 3 hours after stimulation with AII. qRT-PCR analysis for (B) calponin and (C) MCP-1 mRNAs. Shown are mRNA absolute copy numbers normalized to GAPDH. *P < 0.05 versus AII DMSO.
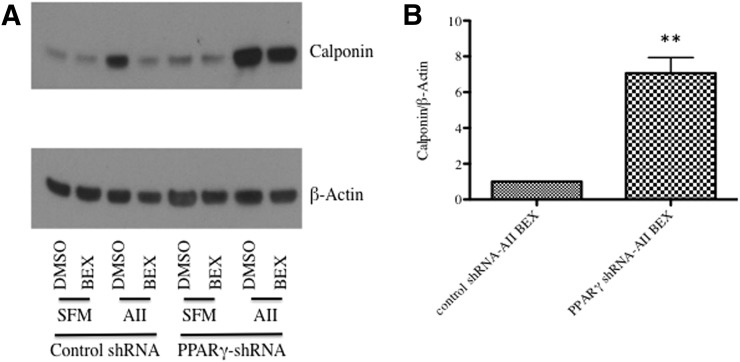
Because PPARγ has been shown to mediate widespread effects on the SMC phenotype, we tested whether PPARγ was a necessary nuclear receptor for rexinoids. We generated stable PPARγ knockdown SMCs using lentiviral infection. PPARγ-depleted SMCs displayed augmented induction of calponin after treatment with AII compared with control cells. In cells expressing control shRNA, bexarotene completely inhibited AII-mediated calponin induction; cells with PPARγ shRNA demonstrated a blunted response to bexarotene (Fig. 8).
Fig. 8.
Bexarotene inhibits AII-mediated calponin induction partly through activation of PPARγ. Cells expressing control shRNA or PPARγ shRNA were pretreated for 24 hours with bexarotene before the 48-hour treatment with AII. Shown is a representative Western blot for calponin. Densitometry measurements of both cell types treated with bexarotene and AII is shown on the right. **P < 0.01.
Discussion
We have demonstrated that specific RXR agonists can have selective effects on the SMC phenotype. Our data indicate that bexarotene blocks many of the responses of cultured SMC to AII, including hypertrophy, induction of SM-specific genes, and production of proinflammatory cytokines. These effects are mediated at least in part through blocking AII-mediated activation of p38 MAP kinase. Rexinoids were able to inhibit induction of SM genes in response to expression of a constitutively active form of the upstream p38 kinase MKK6, indicating that the inhibitory effects do not directly target the AII receptor but rather act downstream at the level of MAP kinase signaling. Because rexinoids target nuclear receptors, which are ligand-activated transcription factors, this suggests the possibility that the inhibitory effects on p38 activation are mediated through increased expression of counterregulatory phosphatases.
The DUSP family of phosphatases has been shown to dephosphorylate and inactivate MAP kinase family members. We found that three separate DUSPs were increased in response to bexarotene. Although silencing individual DUSPs using siRNA approaches did not reverse the inhibitory effects of bexarotene, multiple DUSPs target p38, and there is significant redundancy in these proteins. As our data suggest, silencing an individual DUSP may lead to compensation by other isoforms. This effect has been reported in cardiomyocytes, where redundancy of DUSP1 and DUSP4 has been demonstrated (Auger-Messier et al., 2013). Interestingly, bexarotene did not affect the responses of SMC to PDGF. This suggests that AII and PDGF regulate different signaling pathways to mediate effects on SM gene expression.
Importantly, the ability of bexarotene to block the effects of AII on SMC was greater than activators of individual nuclear receptors. One explanation could be that rexinoids are more potent activators of target gene pathways. It is possible that higher doses of pioglitazone, for instance, may have similar effects as low-dose bexarotene. However, our promoter assays did not demonstrate a greater activation with bexarotene. Given the data in other cell types, it is likely that rexinoids activate multiple nuclear receptors that cooperate to mediate the inhibitory effects observed. Consistent with this model, PPARγ agonists have been shown to repress AII-induced protein synthesis (Benkirane et al., 2006) but do not appear to regulate SM gene expression. Thus, the ability of bexarotene to inhibit both responses suggests that additional nuclear receptors may mediate the effects on SM gene expression. Because both overall protein synthesis and SM gene induction are important in vascular hypertrophy, rexinoids may be potent antihypertrophic agents. Combinatorial studies using agonists for specific receptors will be required, although the number of permissive receptors makes these studies difficult and specific agonists are not available for all the permissive receptors. In addition, bexarotene may mediate some biologic effects via activation of RXR homodimers in the vasculature. Finally, because RXR binds to other orphan nuclear receptors (such as Nur77 and pregnane X receptor/PXR), it is possible that rexinoids activate these receptors as well. No data exist demonstrating that bexarotene activates Nur77-RXR heterodimers, but other rexinoids have been shown to activate Nur77 (Ishizawa et al., 2012). The relationship between bexarotene and PXR is complex; a recent study indicated that bexarotene acts as an antagonist to PXR transcription (Pettersson et al., 2008).
These data raise numerous therapeutic possibilities for RXR agonists in vascular disease. Because the effects of rexinoids on angiotensin-mediated responses lies downstream of the angiotensin receptor, specifically at the level of MAP kinase signaling, it is possible that rexinoids could negatively regulate the biologic effects of other cytokines and growth factors in the vasculature by acting on a common downstream pathway. AII is clearly a key mediator of many vascular diseases, such as hypertensive vascular remodeling, postangioplasty restenosis, transplant vasculopathy, and aortic aneurysm (Richter et al., 2003; Heeneman et al., 2007; Valente et al., 2012). However, in all of those conditions, other vasoconstrictors, growth factors, cytokines, and mechanical forces also contribute to the disease process. Therefore, our study has potential important implications in that rexinoids may possess increased efficacy to inhibit a broader spectrum of agents compared with specific angiotensin receptor blockers.
The likelihood that rexinoids will modulate the phenotype of several cell types may also predict a potent therapeutic effect in many vascular diseases. Our study focused on the effects of RXR agonists in SMCs, but there are data that rexinoids may exert beneficial effects in other cardiovascular cell types. In endothelial cells, rexinoids reduce expression of adhesion molecules and decrease inflammatory cell recruitment in atherosclerosis. PPARγ appears to be the predominant target of rexinoids in endothelial cells (Sanz et al., 2012). Due to LXR activation in macrophages, rexinoids also alleviate atherosclerosis (Lalloyer et al., 2006). Other cell types in the cardiovascular system such as fibroblasts and cardiomyocytes have not been comprehensively examined. Given the variety of nuclear receptors and different cell types, rexinoids may be more effective than individual agonists in a variety of vascular diseases. Vascular disease in humans often involves multiple pathologic events; for example, restenosis usually occurs in the background of an atherosclerotic vessel. Therefore, successfully treating vascular remodeling in humans may require therapies that inhibit multiple extracellular signals in many cell types.
The large number of targets raises the possibility that rexinoid therapy may increase the likelihood of adverse effects. For example, bexarotene is associated with hypertriglyceridemia, possibly via activation of LXR-RXR heterodimers in the liver (Lalloyer et al., 2009). Consequently, further studies may also test whether a combination of two agonists (such as PPARα and PPARγ) may effectively mediate positive effects without negative effects (LXR-induced hypertriglyceridemia). Identifying cellular and molecular targets of rexinoids may enable activation of vasculoprotective pathways while avoiding targets that raise triglycerides.
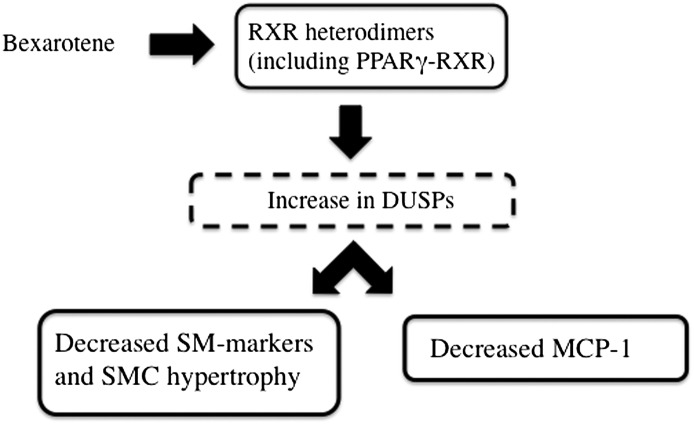
In conclusion, we have shown that RXR agonists significantly inhibit AII-induced phenotypic change in cultured SMCs. A summary of our hypothetical model is shown in Fig. 9. Our data suggest that rexinoids activate multiple nuclear receptors in SMCs, including PPARγ. By activating multiple nuclear receptors, rexinoids inhibit p38 activation, SMC hypertrophy, and SMC inflammation. Due to the large number of cellular and molecular RXR targets, identifying specific nuclear receptors and downstream pathways may reveal novel, potent pathways for treating a variety of vascular diseases.
Fig. 9.
Hypothetical model demonstrating proposed relationship between bexarotene treatment and effects on SMC phenotype. Bexarotene activates multiple nuclear receptors, including PPARγ. The combinatorial activation of multiple receptors increases expression of multiple DUSPs, which are negative regulators of p38, thereby inhibiting the prohypertrophic and proinflammatory effects of AII.
Supplementary Material
Abbreviations
- AII
angiotensin II
- Akt
protein kinase B
- AT1R
angiotensin type 1 receptor
- BEX
bexarotene
- DMSO
dimethylsulfoxide
- DUSP
dual-specificity phosphatase
- 4E-BP1
eukaryotic translation initiation factor 4E-binding protein 1
- 6-ECDCA
6α-ethyl-chenodeoxycholic acid
- ELISA
enzyme-linked immunosorbent assay
- EMEM
Eagle’s minimal essential medium
- ERK
extracellular signal-related kinase
- FCS
fetal calf serum
- FXR
farnesoid X receptor
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GW501516
2-[2-methyl-4-[[4-methyl-2-[4-(trifluoromethyl)phenyl]-1,3-thiazol-5-yl]methylsulfanyl]phenoxy]acetic acid
- LXR
liver X receptor
- MAP
mitogen-activated protein
- MCP-1
monocyte chemoattractant protein 1
- MKK6
MAP kinase kinase 6
- PD98059
2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one
- PDGF-BB
platelet-derived growth factor-BB
- PIO
pioglitazone
- PPAR
peroxisome proliferator activated receptor
- qRT-PCR
quantitative reverse-transcription polymerase chain reaction
- RE
response element
- RXR
retinoid X receptor
- SB203580
4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole
- shRNA
short hairpin RNA
- siRNA
small-interfering RNA
- SM
smooth muscle
- SMC
vascular smooth muscle cells
- T0901317
N-[4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl]-N-(2,2,2-trifluoroethyl)benzenesulfonamide
- TTBS
Tris-buffered saline containing 0.1% Tween-20
- WY-14643
4-chloro-6-(2,3-xylidino)-2-pyrimidinylthio]acetic acid
Authorship Contributions
Participated in research design: Lehman, Horita, Ostriker, Weiser-Evans, Nemenoff, Furgeson.
Conducted experiments: Lehman, Montford, Furgeson.
Performed data analysis: Lehman, Furgeson.
Wrote or contributed to the writing of the manuscript: Lehman, Weiser-Evans, Nemenoff, Furgeson.
Footnotes
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants 2P01-HL014985, 1R01-HL88643, K08-HL103774]; and National Institute of Diabetes and Digestive and Kidney Diseases [Grant T32-DK7135].
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. (2007) RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov 6:793–810 [DOI] [PubMed] [Google Scholar]
- Auger-Messier M, Accornero F, Goonasekera SA, Bueno OF, Lorenz JN, van Berlo JH, Willette RN, Molkentin JD. (2013) Unrestrained p38 MAPK activation in Dusp1/4 double-null mice induces cardiomyopathy. Circ Res 112:48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkirane K, Amiri F, Diep QN, El Mabrouk M, Schiffrin EL. (2006) PPAR-gamma inhibits ANG II-induced cell growth via SHIP2 and 4E-BP1. Am J Physiol Heart Circ Physiol 290:H390–H397 [DOI] [PubMed] [Google Scholar]
- Boergesen M, Pedersen TA, Gross B, van Heeringen SJ, Hagenbeek D, Bindesbøll C, Caron S, Lalloyer F, Steffensen KR, Nebb HI, et al. (2012) Genome-wide profiling of liver X receptor, retinoid X receptor, and peroxisome proliferator-activated receptor α in mouse liver reveals extensive sharing of binding sites. Mol Cell Biol 32:852–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE, Casali BT, Restivo JL, Goebel WD, James MJ, et al. (2012) ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science 335:1503–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimian T, Li MW, Lemarié CA, Simeone SM, Pagano PJ, Gaestel M, Paradis P, Wassmann S, Schiffrin EL. (2011) Mitogen-activated protein kinase-activated protein kinase 2 in angiotensin II-induced inflammation and hypertension: regulation of oxidative stress. Hypertension 57:245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman BM, Chen J, Evans RM. (1997) Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci USA 94:4312–4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furgeson SB, Simpson PA, Park I, Vanputten V, Horita H, Kontos CD, Nemenoff RA, Weiser-Evans MC. (2010) Inactivation of the tumour suppressor, PTEN, in smooth muscle promotes a pro-inflammatory phenotype and enhances neointima formation. Cardiovasc Res 86:274–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garat C, Van Putten V, Refaat ZA, Dessev C, Han SY, Nemenoff RA. (2000) Induction of smooth muscle alpha-actin in vascular smooth muscle cells by arginine vasopressin is mediated by c-Jun amino-terminal kinases and p38 mitogen-activated protein kinase. J Biol Chem 275:22537–22543 [DOI] [PubMed] [Google Scholar]
- Heeneman S, Sluimer JC, Daemen MJ. (2007) Angiotensin-converting enzyme and vascular remodeling. Circ Res 101:441–454 [DOI] [PubMed] [Google Scholar]
- Horita HN, Simpson PA, Ostriker A, Furgeson S, Van Putten V, Weiser-Evans MC, Nemenoff RA. (2011) Serum response factor regulates expression of phosphatase and tensin homolog through a microRNA network in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 31:2909–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayama I, Ichiki T, Patton D, Inanaga K, Miyazaki R, Ohtsubo H, Tian Q, Yano K, Sunagawa K. (2008) Liver X receptor activator downregulates angiotensin II type 1 receptor expression through dephosphorylation of Sp1. Hypertension 51:1631–1636 [DOI] [PubMed] [Google Scholar]
- Ishizawa M, Kagechika H, Makishima M. (2012) NR4A nuclear receptors mediate carnitine palmitoyltransferase 1A gene expression by the rexinoid HX600. Biochem Biophys Res Commun 418:780–785 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Ham SA, Kim MY, Hwang JS, Lee H, Kang ES, Yoo T, Woo IS, Yabe-Nishimura C, Paek KS, et al. (2011) PPARδ coordinates angiotensin II-induced senescence in vascular smooth muscle cells through PTEN-mediated inhibition of superoxide generation. J Biol Chem 286:44585–44593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalloyer F, Fiévet C, Lestavel S, Torpier G, van der Veen J, Touche V, Bultel S, Yous S, Kuipers F, Paumelle R, et al. (2006) The RXR agonist bexarotene improves cholesterol homeostasis and inhibits atherosclerosis progression in a mouse model of mixed dyslipidemia. Arterioscler Thromb Vasc Biol 26:2731–2737 [DOI] [PubMed] [Google Scholar]
- Lalloyer F, Pedersen TA, Gross B, Lestavel S, Yous S, Vallez E, Gustafsson JA, Mandrup S, Fiévet C, Staels B, et al. (2009) Rexinoid bexarotene modulates triglyceride but not cholesterol metabolism via gene-specific permissivity of the RXR/LXR heterodimer in the liver. Arterioscler Thromb Vasc Biol 29:1488–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansigan F, Foss FM. (2010) Current and emerging treatment strategies for cutaneous T-cell lymphoma. Drugs 70:273–286 [DOI] [PubMed] [Google Scholar]
- Lawrence JA, Adamson PC, Caruso R, Chow C, Kleiner D, Murphy RF, Venzon DJ, Shovlin M, Noone M, Merino M, et al. (2001) Phase I clinical trial of alitretinoin and tamoxifen in breast cancer patients: toxicity, pharmacokinetic, and biomarker evaluations. J Clin Oncol 19:2754–2763 [DOI] [PubMed] [Google Scholar]
- Lefebvre P, Benomar Y, Staels B. (2010) Retinoid X receptors: common heterodimerization partners with distinct functions. Trends Endocrinol Metab 21:676–683 [DOI] [PubMed] [Google Scholar]
- Mack CP. (2011) Signaling mechanisms that regulate smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol 31:1495–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, et al. (2013) 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 34:2159–2219 [DOI] [PubMed] [Google Scholar]
- Mehta PK, Griendling KK. (2007) Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292:C82–C97 [DOI] [PubMed] [Google Scholar]
- Mukherjee R, Davies PJ, Crombie DL, Bischoff ED, Cesario RM, Jow L, Hamann LG, Boehm MF, Mondon CE, Nadzan AM, et al. (1997) Sensitization of diabetic and obese mice to insulin by retinoid X receptor agonists. Nature 386:407–410 [DOI] [PubMed] [Google Scholar]
- Nemenoff RA, Simpson PA, Furgeson SB, Kaplan-Albuquerque N, Crossno J, Garl PJ, Cooper J, Weiser-Evans MC. (2008) Targeted deletion of PTEN in smooth muscle cells results in vascular remodeling and recruitment of progenitor cells through induction of stromal cell-derived factor-1alpha. Circ Res 102:1036–1045 [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lozach J, Benner C, Pascual G, Tangirala RK, Westin S, Hoffmann A, Subramaniam S, David M, Rosenfeld MG, et al. (2005) Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell 122:707–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver WR, Jr, Shenk JL, Snaith MR, Russell CS, Plunket KD, Bodkin NL, Lewis MC, Winegar DA, Sznaidman ML, Lambert MH, et al. (2001) A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci USA 98:5306–5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR. (2004) Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84:767–801 [DOI] [PubMed] [Google Scholar]
- Pellicciari R, Fiorucci S, Camaioni E, Clerici C, Costantino G, Maloney PR, Morelli A, Parks DJ, Willson TM. (2002) 6α-Ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem 45:3569–3572 [DOI] [PubMed] [Google Scholar]
- Pettersson F, Hanna N, Lagodich M, Dupéré-Richer D, Couture MC, Choi C, Miller WH., Jr (2008) Rexinoids modulate steroid and xenobiotic receptor activity by increasing its protein turnover in a calpain-dependent manner. J Biol Chem 283:21945–21952 [DOI] [PubMed] [Google Scholar]
- Repa JJ, Turley SD, Lobaccaro JA, Medina J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM, Mangelsdorf DJ. (2000) Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 289:1524–1529 [DOI] [PubMed] [Google Scholar]
- Richter MH, Richter HR, Olbrich HG, Mohr FW. (2003) Two good reasons for an angiotensin-II type 1 receptor blockade with losartan after cardiac transplantation: reduction of incidence and severity of transplant vasculopathy. Transpl Int 16:26–32 [DOI] [PubMed] [Google Scholar]
- Rizvi NA, Marshall JL, Dahut W, Ness E, Truglia JA, Loewen G, Gill GM, Ulm EH, Geiser R, Jaunakais D, et al. (1999) A phase I study of LGD1069 in adults with advanced cancer. Clin Cancer Res 5:1658–1664 [PubMed] [Google Scholar]
- Sakamoto J, Kimura H, Moriyama S, Odaka H, Momose Y, Sugiyama Y, Sawada H. (2000) Activation of human peroxisome proliferator-activated receptor (PPAR) subtypes by pioglitazone. Biochem Biophys Res Commun 278:704–711 [DOI] [PubMed] [Google Scholar]
- Sanz MJ, Albertos F, Otero E, Juez M, Morcillo EJ, Piqueras L. (2012) Retinoid X receptor agonists impair arterial mononuclear cell recruitment through peroxisome proliferator-activated receptor-γ activation. J Immunol 189:411–424 [DOI] [PubMed] [Google Scholar]
- Széles L, Póliska S, Nagy G, Szatmari I, Szanto A, Pap A, Lindstedt M, Santegoets SJ, Rühl R, Dezsö B, et al. (2010) Research resource: transcriptome profiling of genes regulated by RXR and its permissive and nonpermissive partners in differentiating monocyte-derived dendritic cells. Mol Endocrinol 24:2218–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Suzuki E, Takeda R, Oba S, Nishimatsu H, Kimura K, Nagano T, Nagai R, Hirata Y. (2008) Angiotensin II and tumor necrosis factor-alpha synergistically promote monocyte chemoattractant protein-1 expression: roles of NF-κB, p38, and reactive oxygen species. Am J Physiol Heart Circ Physiol 294:H2879–H2888 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Suh KS, Lo AM, De Luca LM. (2007) p21WAF1/CIP1 is a common transcriptional target of retinoid receptors: pleiotropic regulatory mechanism through retinoic acid receptor (RAR)/retinoid X receptor (RXR) heterodimer and RXR/RXR homodimer. J Biol Chem 282:29987–29997 [DOI] [PubMed] [Google Scholar]
- Valente AJ, Yoshida T, Murthy SN, Sakamuri SS, Katsuyama M, Clark RA, Delafontaine P, Chandrasekar B. (2012) Angiotensin II enhances AT1-Nox1 binding and stimulates arterial smooth muscle cell migration and proliferation through AT1, Nox1, and interleukin-18. Am J Physiol Heart Circ Physiol 303:H282–H296 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.