Abstract
Diacylglycerol (DAG) signaling relies on the presence of conserved domain 1 (C1) in its target proteins. Phospholipase C–dependent generation of DAG after T cell receptor (TCR) triggering is essential for the correct immune response onset. Accordingly, two C1-containing proteins expressed in T lymphocytes, Ras guanyl nucleotide-releasing protein1 (RasGRP1) and protein kinase Cθ (PKCθ), were shown to be fundamental for T-cell activation and proliferation. Although containing the same regulatory domain, they are proposed to relocate to distinct subcellular locations in response to TCR triggering. Here we studied intracellular localization of RasGRP1 and PKCθ C1 domains in living Jurkat T cells. The results demonstrate that, in the absence of significant primary sequence differences, the C1 domains of these proteins show specific localization within the cell and distinct responses to pharmacological stimulation and TCR triggering. These differences help explain the divergent localization and distinct functional roles of the full-length proteins, which contains them. The properties of these DAG-binding modules allow their characterization as functional markers that discriminate between DAG pools. Finally, we show that by binding to different diacylglycerol forms, overexpression of distinct C1 modules can attenuate DAG-dependent signals originating from the plasma or internal membranes. This is shown by analyzing the contribution of these two lipid pools to PLC-dependent Ras activation in response to TCR triggering.
INTRODUCTION
Generation of diacylglycerol (DAG) in the plasma membrane in response to T-cell receptor (TCR) triggering is a key signal in the initiation of T-cell activation (Downward et al., 1990). When the receptor is engaged, phospholipase C (PLC) enzymes hydrolyze phosphatidylinositol 4,5-bisphosphate (PI4,5P2) and produce DAG and inositol 1,4,5-triphosphate (IP3; Secrist et al., 1991). DAG is recognized by proteins that have conserved domain 1 (C1), whereas IP3 reaches the endoplasmic reticulum (ER), where it allows calcium release (Kane et al., 2000).
Appropriate TCR-dependent signal intensity and duration is essential for correct progression of the T-cell activation program. PLC-mediated DAG generation is a key signal initiated after TCR triggering, and DAG level modulation alters T-cell activation parameters (Sanjuan et al., 2001). As for other biological systems, protein kinase C (PKC) family members were believed to be the exclusive mediators of all DAG-dependent signals in T cells. In these cells, the novel isoform PKCθ, which is rapidly relocated to the plasma membrane during T-cell activation, is essential for TCR signaling (Monks et al., 1997; Bi et al., 2001; Villalba et al., 2002). PKCθ-deficient mice show normal thymic development but impaired T-cell activation (Sun et al., 2000). In response to increased DAG levels, and modulated by tyrosine phosphorylation, PKCθ translocates to the immune synapse (Diaz-Flores et al., 2003), where it activates c-Jun N-terminal kinase and initiates the nuclear factor-κB cascade (Isakov and Altman, 2002).
The presence in lymphocytes of other DAG-binding proteins such as Ras guanyl nucleotide-releasing protein (RasGRP1; Dower et al., 2000) has recently revealed the essential role of nonkinase DAG-binding proteins in the regulation of TCR-dependent signals. RasGRP are guanine exchange factors (GEF) for Ras family proteins; they contain one C1 domain (Ebinu et al., 1998) and provide a direct link between DAG generation and Ras/Raf/Mitogen-activated protein kinase (MAPK) activation (Ebinu et al., 2000). Analysis of RasGRP1-deficient mice demonstrated the fundamental role of this protein in thymocyte development (Dower et al., 2000) and correct T-cell expansion (Priatel et al., 2002). TCR triggering induces rapid, DAG-dependent RasGRP1 relocalization to internal membranes (Bivona et al., 2003). Membrane localization of PKCθ and RasGRP thus requires the presence of a C1 domain in their primary sequence; however, the reason for their apparent divergent localization in response to TCR triggering is unknown.
C1 domains were first described as necessary for phorbol ester (PMA, PDBu) binding (Ono et al., 1989; Burns and Bell, 1991); it was later established that they also bind DAG (Quest et al., 1994b) and many related compounds such as bryostatins, indolactams, or merezeins. The C1 domain consists of a conserved 50-amino-acid sequence bearing the motif HX11–12CX2CX12–14CX2CX4HX2CX6–7C (H, histidine, C, cysteine, X, any amino acid; Hubbard et al., 1991). Three nonconsecutive cysteines and one histidine are in close proximity in the ternary structure, forming two cavities to coordinate one Zn2+ ion in each (Hommel et al., 1994). The C1 domain folds independently of adjacent amino acid sequences, generating a well-characterized structure with a hydrophobic base that interacts with the membrane and is maintained by Zn2+ coordination. This base supports two parallel loops that generate a groove between them in which DAG binds, allowing C1 domain insertion in the membrane (Zhang et al., 1995). The residues implicated in DAG interaction have been studied extensively (Quest et al., 1994a; Kazanietz et al., 1995b; Zhang et al., 1995). C1 domains are classified according to these residues, which are conserved in typical C1 domains and are not found in atypical C1 (Hurley et al., 1997).
Eukaryotic cells contain five types of typical DAG-binding proteins, including certain isoforms of diacylglycerol kinases (DAGK; Kanoh et al., 1993), protein kinase D (PKD; Valverde et al., 1994), chimerins (Hall et al., 1990), RasGRP (Ebinu et al., 1998), and munc 13 (Betz et al., 1997). The increase in DAG-regulated protein number and functions suggests a high degree of complexity in the signaling pathways activated by this lipid. Many proteins have atypical C1 domains. The DAGK are the largest family of these proteins (Kanoh et al., 1993); others are vav (Coppola et al., 1991), raf (Ghosh et al., 1994), ROCK (Ishizaki et al., 1996), Citron (Madaule et al., 1995), Lfc (Whitehead et al., 1995), and the recently characterized C1-TEN (Hafizi et al., 2002). The exact role of atypical C1 domains in these proteins remains to be defined, although some studies suggest that they are essential for mediating membrane and/or protein interactions (Clark et al., 1997; Winkler et al., 1998; Williams et al., 2000; Booden et al., 2002).
Many laboratories have attempted to establish the affinity of C1 domains for DAG and its analogs (Quest et al., 1994b; Quest and Bell, 1994; Kazanietz et al., 1995a; Slater et al., 1996; Shindo et al., 2001; Irie et al., 2002), to determine the residues essential for binding (Burns and Bell, 1991; Quest et al., 1994a; Kazanietz et al., 1995b) and to evaluate GFP-fused C1 domain translocation in response to phorbol esters and other stimuli (Oancea et al., 1998). Nonetheless, the subcellular localization of C1 domains in response to physiological signals in T lymphocytes has not been studied, nor has the capacity of these domains to alter TCR-dependent signals by blocking DAG increases in distinct subcellular localizations. We isolated C1 domains from PKCθ, RasGRP1, and β2chimerin, typical C1-containing proteins expressed in T lymphocytes, fused them to GFP, and analyzed their intracellular localization in intact lymphocytes. We studied then their response to TCR triggering in the Jurkat cell model to compare it with the localization of the full-length proteins. Our studies reveal that the distinct C1 domains show very different subcellular localization, albeit always dependent on DAG binding. The subcellular distribution of C1 domains after TCR triggering compared with that of the full-length proteins indicate the need for additional signals to ensure correct PKCθ and RasGRP1 localization during T-cell activation. Moreover the study of the TCR-dependent MAPK pathway suggests that, by binding to distinct DAG pools, C1 domains contribute to modulate intensity and duration of Ras-dependent signals in T cells.
MATERIALS AND METHODS
Reagents
1,2-Dipalmitoyl-sn-glycerol-3-phosphocholine (DPPC), l-α-phosphatidylserine (PS; brain, porcine-sodium salt) and 1,2-dioleyl-sn-glycerol (DOG) were from Avanti (Alabaster, AL); 1-stearoyl-2 arachidonoyl-sn-glycerol (SAG) was from Biomol (Plymouth Meeting, PA). Orthovanadate, phenylmethylsulfonyl fluoride (PMSF), poly-dl-lysine, paraformaldehyde, Igepal CA-630 (NP40), propranolol, phorbol 12-myristate 13-acetate (PMA), and phorbol 12,13-dibutyrate (PDBu) were from Sigma (St. Louis, MO). Bovine serum albumin (BSA) fraction V, leupeptin, and aprotinin were from Roche (Basel, Switzerland), Tween-20 from Merck (Darmstadt, Germany) and U73122 and Triton X-100 from Calbiochem (San Diego, CA). Chamber slides were purchased from Nunc (Lab-Tek; Rochester, NY), and polystyrene microspheres (15.0 μm) from Polysciences (Eppelheim, Germany). Anti-CD3 and -CD28 antibodies were purchased from BD PharMingen (San Diego, CA), anti-MAPK from Zymed Laboratories (San Francisco, CA), antiphospho-p44/42 MAPK from Cell Signaling Technology (Boston, MA), and anti-PDI was from Stressgen (Victoria, BC, Canada). The anti-giantin-α antibody was a kind gift of Manfred Renz (Inst. Immunology and Molecular Genetics, Karlsruhe, Germany) and anti-lamin B was from Oncogene (Cambridge, MA). Anti-GFP mAb was from Clontech (Palo Alto, CA), horseradish peroxidase–conjugated goat anti-mouse and -rabbit immunoglobulin from Dako (Glostrup, Denmark), and Cy3-conjugated goat anti-mouse and Cy3-goat anti-rabbit were from Jackson ImmunoResearch (West Grove, PA).
Plasmids and DNA Constructs
C1 domains were amplified by PCR. For human PKCθ C1a, C1b, and C1a+C1b, we used the PKCθ -EGFP plasmid from Clontech. For RasGRP1 C1, we used a rat RasGRP1 plasmid generously donated by J.C. Stone (Department of Biochemistry, University of Alberta, Canada). For human β2chimerin C1, we used a pEFbosGFPβ2chimerin plasmid, a kind gift of M.G. Kazanietz (Centre for Experimental Therapeutics, University of Pennsylvania, Philadelphia, PA). Oligonucleotides were designed to amplify the 50 consensus amino acids for C1 domains plus three additional residues each at the N and C termini as well as the restriction sites for subcloning into pEFbosEGFP (modified from pEGFP, Clontech), which were XhoI (N terminus) and EcoRI (C terminus). Each construct contained the following residues of the full-length proteins: C1aPKCθ: 156–212, C1bPKCθ: 229–284, C1a+C1bPKCθ: 156–284, C1RasGRP1: 540–596, and C1β2chimerin: 357–413. Plasmids with GFP were used for transfection of PKCθ (Clontech) and full-length RasGRP1 (rat RasGRP subcloned in pEGFP, Clontech).
Cell Lines and Transient Transfections
Jurkat cells were maintained in DMEM (BioWhittaker, Walkersville, MD) supplemented with 10% fetal bovine serum (Sigma) and 2 mM glutamine (BioWhittaker). Cells (1.2 × 107) in 400 μl of complete medium were transfected by electroporation with 20 μg DNA using a Gene Pulser (270V, 975 μF; Bio-Rad, Hercules, CA). Cells were immediately transferred to 10 ml of growth medium and assayed 24 h later.
Western Blot
Cells were suspended in lysis buffer (150 mM NaCl, 10 mM NaF, 10 mM Na4P2O7, 50 mM Tris-HCl, pH 7.4, 1% NP40, 1 mM orthovanadate, 1 mM PMSF, and 10 μg/ml each aprotinin and leupeptin) and incubated on ice (15 min). After centrifugation (15,000 × g, 15 min, 4°C), supernatants were assayed for total protein (DC protein assay, Bio-Rad) and an equivalent amount of protein for each sample was analyzed by SDS-PAGE. Proteins were transferred to nitrocellulose, and GFP-coupled construct expression was determined with an anti-GFP mAb and the ECL detection kit (Amersham Bioscience, Buckinghamshire, UK).
Vesicle-binding Assay
Lipid vesicles were prepared as described (Sanchez-Pinera et al., 1999), with slight modifications. Briefly, a lipid mixture in chloroform was dried under a nitrogen stream, followed by vacuum (2 h), then resuspended in binding buffer (20 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 200 μM CaCl2, 0.3 mg/ml BSA) to a final concentration of 400 μM lipid, and vortexed for 50 s. The composition of the multilamellar vesicles generated was DPPC, PS (4:1 mol:mol), and in the cases indicated DOG (2.5 μM) or SAG (2.5 μM).
Jurkat cells were transfected with different C1 constructs and collected 24 h later. Cells were suspended in ice-cold hypotonic buffer (10 mM Tris-HCl, pH 7.5, 10 mM NaF, 1 mM orthovanadate, 1 mM PMSF, and 10 μg/ml each aprotinin and leupeptin) and incubated on ice (20 min). Cells were lysed by 15 passages through a 30-gauge needle; lysates were centrifuged (15,000 × g, 15 min) to remove nuclei and cell debris. Supernatants were collected and centrifuged (100,000 × g, 30 min, 4°C) to remove all particles. Supernatants were assayed for total protein (DC protein assay, Bio-Rad), and 100 μg of total protein were incubated with lipid vesicles (15 min, room temperature). To isolate vesicle-bound proteins, the mixture was centrifuged (100,000 × g, 30 min), and pellets and supernatants were analyzed by SDS-PAGE. C1 domains levels were analyzed using anti-GFP antibody.
Stimulation with Soluble anti-CD3
Cells were transfected with pEFbosEGFP, EGFPC1a+C1bPKCθ, and EGFPC1RasGRP1 and sorted 24 h later to recover GFP-positive cells (Altra Hypersort, Beckman Coulter, Miami, FL). Cells were maintained 8 h in growth medium, then starved for 1 h in DMEM 1% BSA, and analyzed by flow cytometry (Epics XL-MCL, Beckman Coulter) to calculate the percentage of GFP-positive cells. For stimulation, cells expressing each construct were separated in six 1.5-ml vials at a final concentration of 1 × 106 cells/ml in HEPES-balanced salt solution (HBSS; 25 mM HEPES KOH, pH 7.4, 1 mM MgCl2, 1 mM CaCl2, 132 mM NaCl, 0.1% BSA) and stimulated with soluble anti-CD3 (1 μg/ml final concentration). Cells were collected at different times and processed for Western blot. PMAPK levels were analyzed using antiphospho-p44/42 MAPK and anti-MAPK antibody to control total protein levels.
Immunofluorescence
At 24 h after transfection, cells were transferred to poly-dl-lysine–coated chamber slides and allowed to attach (15 min), then fixed (10 min) with 2% PFA, washed at least three times with 150 mM Tris-HCl, pH 7.4, permeabilized (10 min) with PBS 0.2% Triton X-100, washed once with PBS, blocked in PBS 1% BSA, and washed three times with PBS. Antibodies were incubated (1 h, room temperature) and washed three times with PBS 0.1% Tween-20. Primary antibodies used were antigiantinα to detect Golgi and antiprotein disulfide isomerase (anti-PDI) for ER. Secondary antibodies were Cy3-anti-rabbit and Cy3-anti-mouse immunoglobulin. Cells were imaged with a laser scanning confocal microscope (TCS-NT, Leica, Wetzlar, Germany) and images processed using ImageJ software.
Confocal Microscopy Imaging
At 24 h after transfection, Jurkat cells were pelleted and suspended in HBSS, then transferred to poly-dl-lysine–coated chamber slides, allowed to attach at 37°C, and maintained at this temperature to record the image time series. Where appropriate, cells were preincubated with U73122 (1 μM, 30 min) before transfer to chamber slides; they were maintained in U73122 throughout the experiment. PMA or PDBu (200 nM) were added to HBSS after the first frame, and images were recorded every 11 s. Propranolol (250 μM) was added in the same conditions, and frames were recorded every 5 s. Cells were imaged with a laser scanning TCS-NT confocal microscope, and images were processed using ImageJ software.
Stimulation with Antibody-coated Microspheres
Antibodies were adsorbed to microspheres by mixing 1 μg antibody (1:1 CD3:CD28) in PBS with 0.5 × 106 microspheres in a final volume of 1 ml and incubated (1.5 h, room temperature) with continuous mixing; 1.5 ml 1% BSA in PBS was added and mixing continued (30 min). Microspheres were washed three times with PBS and suspended in PBS for addition to cells. For stimulation, cells were mixed with antibody-coated microspheres at a 2:1 cell/bead proportion and plated on poly-dl-lysine–coated chamber slides. Images were captured each 15 s by confocal microscopy and processed using ImageJ software.
Fluorescence Recovery after Photobleaching
At 24 h after transfection with GFPC1RasGRP1, Jurkat cells were suspended in HBBS, then transferred to poly-dl-lysine coated chamber slides, allowed to attach at 37°C and maintained at this temperature to record the image time series. For the fluorescence recovery after photobleaching (FRAP) experiment, the selected area was exposed to maximum laser power and recovery from bleaching was measured every 500 msec. Images were captured with a laser scanning confocal microscope (LSM510 META, Zeiss, Thornwood, NY) and processed with ImageJ software.
RESULTS
C1 Domain Expression and Localization in Jurkat Cells
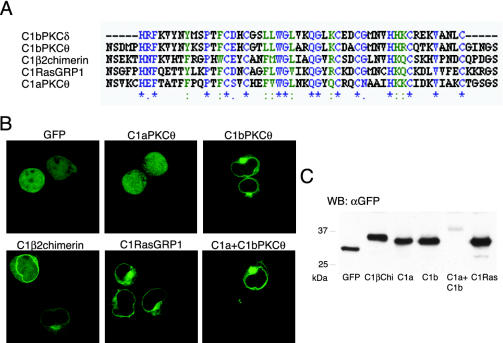
To study the role of C1 domains as potential DAG sensors, we cloned some of these domains tagged with GFP to trace them in in vivo time-lapse microscopy. PKCθ C1a and C1b were cloned independently or in tandem, in addition to RasGRP1 C1, and β2chimerin C1. These three proteins are expressed in T lymphocytes, in which DAG levels are under tight control (Sanjuan et al., 2001), and require C1 domains for function. Sequence alignment of C1 domains from these proteins with a well-characterized C1 domain (C1b PKCδ; Kazanietz et al., 1995b; Zhang et al., 1995) showed that all residues necessary for DAG binding are conserved (Kazanietz et al., 1995b; Figure 1A).
Figure 1.
C1 domain constructs and their expression in Jurkat cells. (A) alignment of C1bPKCθ, C1β2chimerin, C1RasGRP1, and C1aPKCθ domains with C1bPKCδ, as an example of a typical C1 domain. *, single, fully-conserved residue,:, highly conservative changes in side groups and., low conservative changes in side groups. Alignment was done with the Clustal W program using Biology Workbench 3.2 (San Diego Supercomputer Center, University of California, San Diego, CA). (B) In vivo expression of GFP-fused C1 domains in Jurkat cells. Cells were transfected and after 24 h, pelleted and suspended in HBSS for plating on poly-d,l-lysine–coated chamber slides. Slides were mounted on a 37°C plate on the confocal microscope and analyzed. Images are representative of the field observed for each condition. (C) Immunoblot analysis of Jurkat cells transfected with indicated C1 domain constructs. Cells were transfected and processed 24 h later for Western blot (see MATERIALS AND METHODS). Analysis with anti-GFP mAb showed proteins of the predicted molecular weight. C1a+C1bPKCθ was less abundant than the other constructs in all experiments. In flow cytometry analysis, C1a+C1bPKCθ–expressing cells were less numerous and less bright than cells expressing the other constructs.
We designed primers to amplify the 50 consensus residues described for C1 domains (Hubbard et al., 1991), plus an additional three N-terminal and three C-terminal residues to allow correct protein folding. For C1a+C1bPKCθ, we amplified both C1 domains as well as the residues between them. The GFP is fused at the N-terminus. Constructs with GFP fused at the C-terminus showed the same localization (unpublished data).
Cloned C1 domain expression was analyzed in living Jurkat cells. In Western blot, all constructs migrated according to the predicted molecular weight of the fusion protein and were expressed in large amounts, except the C1a+C1b construct (Figure 1C). Confocal images showed distinct C1 domain localization; C1aPKCθ was distributed homogeneously throughout the cytoplasm and the nucleus. C1bPKCθ localized in some perinuclear regions, as did C1β2chimerin, with a diffuse signal in cytoplasm and the nucleus. C1RasGRP1 appeared to have the same perinuclear spreading and was distributed in a network in cytoplasm. C1a+C1b was found predominantly in the plasma membrane and in the perinuclear region (Figure 1B). Expression in the PAE pig endothelial cell line showed similar localization (unpublished data).
The Internal Localization of the C1 Domains Correlates with ER and Golgi Staining
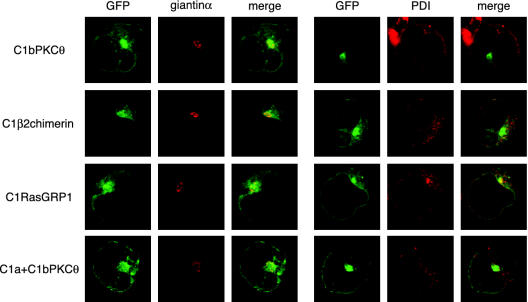
To assess C1 domain localization, we used anti-PDI (Ferrari and Soling, 1999) and -giantinα (Seelig et al., 1994) antibodies to detect ER and Golgi, respectively. Although complete colocalization was not observed, the principal accumulation of perinuclear GFP signaling localized to the region of giantinα staining for C1bPKCθ, C1β2chimerin, C1RasGRP1, and C1a+C1bPKCθ (Figure 2). Although PDI staining was perinuclear, GFP appeared to stain a more internal structure that also surrounded the nucleus. Possible explanations may be that PDI stains only the outer ER sacs, whereas the tubules proximal to the nucleus remain unstained, or the GFP constructs may be located in the nuclear membrane (Figure 2). We thus stained the nuclear membrane with an anti-lamin B antibody (Gerace et al., 1978), but again colocalization was not clear (unpublished data). For C1RasGRP1, the netlike arrangement in the cytosol coincided partially with PDI staining. All together, the results point to the association of these four constructs with internal membranes.
Figure 2.
Confocal microscopy analysis of C1 domain internal localization. Jurkat cells transfected with distinct GFP-fused C1 domains were fixed after 24 h and processed (see MATERIALS AND METHODS) to stain Golgi with anti-giantin-α (red, left panels) or ER with anti-PDI (red, right panels). Merged GFP and Cy3 fluorescence is shown.
Membrane Localization of C1 Domains Is DAG Dependent
Plasma membrane synthesis of new components takes place in ER and Golgi, and DAG is one of the principal substrates for new complex lipid generation (Henneberry et al., 2002). PLC activity can also contribute to DAG generation, hydrolyzing PI4,5P2 in internal membranes and in plasma membrane. We tested the DAG dependence of specific C1 localization using two inhibitors, U73122 and propranolol.
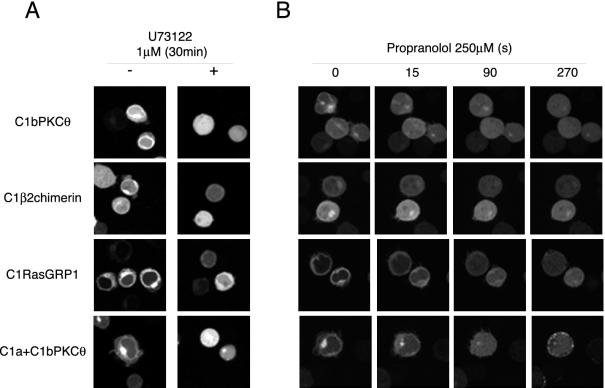
U73122 is a generic PLC inhibitor (Thompson et al., 1991) that blocks PI4,5P2 hydrolysis and decreases DAG levels in different subcellular localizations (Rebecchi and Pentyala, 2000). In U73122-treated cells (1 μM, 30 min), C1bPKCθ, C1β2chimerin, and C1a+C1bPKCθ lost their localized expression. C1RasGRP1 decreased its perinuclear signal, although it maintained residual internal localization (Figure 3A).
Figure 3.
C1 domains change their localization in U73122- or propranolol-treated cells. (A) Cells transfected with GFPC1 constructs alone (-) or treated (+) with U73122 (1 μM, 30 min), were plated and mounted for confocal microscopy as in Figure 1B. Images are representative of a field observed for each condition. (B) Cells transfected with GFPC1 constructs were plated and mounted for confocal microscopy as in Figure 1B. Propranolol (250 μM) was added immediately after the first frame, and images captured every 5 s. Time-lapse confocal recording is shown in Supplementary Videos 1–4. Images at times indicated are shown to illustrate GFPC1 domain subcellular localization.
Propranolol is a specific inhibitor of phosphatidate phosphohydrolase type 1 (PAP1), an enzyme responsible for phosphatidic acid dephosphorylation in cytosol and inner membranes (Roberts et al., 1998), which has been used to block DAG production (Kurscheid-Reich et al., 1995; Baron and Malhotra, 2002). Propranolol addition at a final concentration of 250 μM dissociated C1 domains from internal membranes in <1 min and relocalized them to cytoplasm, except for C1a+C1b, part of which remained in the plasma membrane (Figure 3B and Supplementary Videos 1–4).
Specific Binding of C1 Domains to Lipid Vesicles Is Dependent on DAG Composition
Previous experiments suggested that DAG is essential for C1 domain binding; however, targeting to specific membranes known to differ in their lipid composition (Zinser et al., 1991; Stahelin et al., 2003) suggests a requirement for other lipids. The plasma membrane is enriched in phosphatidylserine and cholesterol, whereas phosphatidylcholine is the principal component of inner membranes. To analyze whether lipid composition was responsible for C1 domain localization, we generated vesicles mimicking inner or plasma membrane (Das et al., 2003). We incubated lysates of transfected Jurkat cells with these vesicles and the protein bound was analyzed by Western blot. Under these conditions, we did not observe differences that would account for specific subcellular localization of C1 domains (unpublished data).
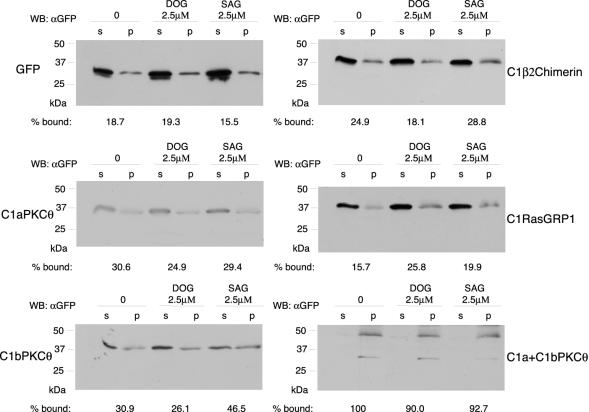
Another explanation for these distributions is C1 domain association with specific DAG pools. In inner membranes, an elevated percentage of DAG contains monounsaturated or saturated fatty acids, whereas in most cases, plasma membrane DAGs have a saturated fatty acid in position 1 of the glycerol chain and an polyunsaturated fatty acid in position 2 (Hodgkin et al., 1998). To simulate these two contexts, we generated multilamellar vesicles with DPPC and PS (4:1, mol:mol) as a platform in which we incorporated DOG (2.5 μM) as an example of inner DAG, and SAG (2.5 μM) as plasma membrane DAG. Lysates of Jurkat cells transfected with C1 domain constructs and GFP (control) were incubated with vesicles (15 min); bound protein was recovered by centrifugation and analyzed by Western blot (Figure 4). Although C1aPKCθ did not appear to associate with membranes in intact cells (Figure 1B), it bound vesicles nonspecifically in vitro. C1bPKCθ pelleted preferentially with plasma membrane DAG vesicles, as did C1β2chimerin, although not to the same extent. Nearly 100% of C1a+C1bPKCθ bound to all vesicle types, showing slightly less binding in the presence of DOG, as seen for C1bPKCθ and C1β2chimerin. In contrast, C1RasGRP1 was the only domain that bound to DOG vesicles with higher affinity than to those containing SAG. These in vitro binding differences could explain specific C1 domain localization due to its capacity to sense DAG pools in intact cells.
Figure 4.
C1 domains bind selectivity to vesicles containing distinct DAG species. DPPC and PS (4:1, mol:mol) vesicles containing no DAG (0), 1,2-dioleyl-sn-glycerol (DOG) or 1-stearoyl-2-arachidonoyl-sn-glycerol (SAG) were incubated with lysates from Jurkat cells transfected with different GFPC1 constructs or with GFP (control). Bound protein was recovered by centrifugation and detected by SDS-PAGE and western blot, (p, pellet fraction; s, supernatant fraction). Bands were quantified by densitometry; these values were used to calculate the percentage of membrane-bound protein using the formula %bound = band intensity in p/(band intensity in p + band intensity in s).
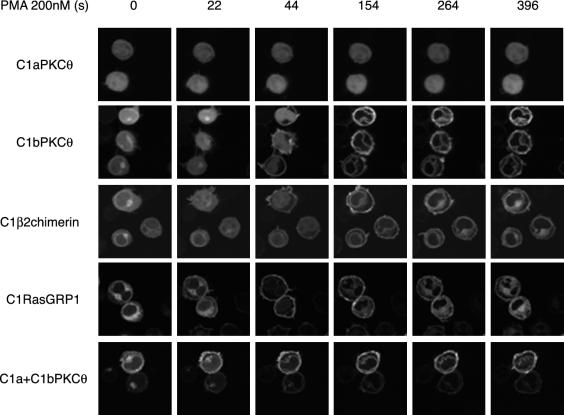
C1bPKCθ, C1a+C1bPKCθ, C1RasGRP1, and C1β2chimerin Translocate to the Plasma Membrane in Response to PMA; C1aPKCθ Does Not
To confirm that the cloned C1 are typical C1 domains and respond to PMA by changing their localization, we analyzed their response to extracellular addition of PMA (200 nM) by time-lapse videomicroscopy (Supplementary Videos 5–9; Figure 5 shows selected frames from these videos). C1a did not translocate after PMA addition, even at a concentration of 1 mM, much higher than that normally used (unpublished data). Cytoplasm- and inner membrane-associated fractions of C1bPKCθ and C1β2chimerin translocated with similar kinetics to the plasma membrane in <1 min; 1.5 min after PMA addition, the nuclear fraction translocated to nuclear membrane. C1 domains were associated only with cell membranes after 6-min stimulation, with no visible signal in nucleus or cytoplasm. C1RasGRP1 was dissociated from its localization in <1 min and associated with plasma membrane; it later gradually recovered its initial intracellular position. C1a+C1bPKCθ was partially localized in plasma membrane before stimulation; after PMA addition, the remaining cytosolic and Golgi fractions translocated rapidly to plasma membrane, where they were maintained for at least 15 min (unpublished data). The remaining nuclear membrane signal was probably due to the small proportion of the construct in the nucleoplasm that was able to translocate.
Figure 5.
C1β2chimerin, C1bPKCθ, C1a+C1bPKCθ, and C1RasGRP1, but not C1aPKCθ translocate in response to PMA. Cells transfected with GFPC1 constructs were plated and mounted for confocal microscopy as in Figure 1B. PMA (200 nM) was added immediately after the first frame, and images captured every 11 s. Time-lapse confocal recording is shown in Supplementary Videos 5–9. Images corresponding to times indicated show GFPC1 domain translocation.
When cells were PDBu-stimulated (Supplementary Videos 10–14), C1a did not translocate; the C1bPKCθ, C1β2chimerin, and C1a+C1bPKCθ soluble fractions translocated simultaneously to internal and plasma membranes, with no preference for one or the other. C1RasGRP1 appeared to be unaffected, as it remained in its initial localization in internal membranes and no signal was detected in the plasma membrane.
These differences between PMA and PDBu stimulation are probably due to the less hydrophobic nature and smaller size of PDBu (Oancea et al., 1998), which permits more rapid cell entry and internal membrane localization than PMA. PMA remains trapped in the plasma membrane (Ananthanarayanan et al., 2003), explaining the lack of signal in nuclear membrane until 2 min poststimulation. With time, the inner membranes are saturated with PMA, and equilibrium is established between inner and plasma membranes by 6 min poststimulation.
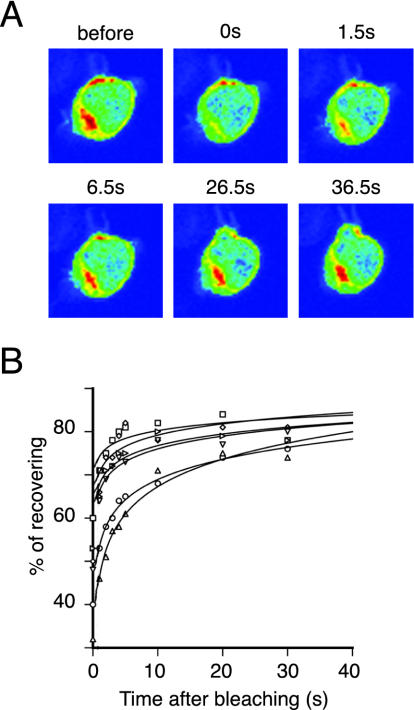
Although C1RasGRP1 appeared to be tightly associated with inner membranes and no signal was detected in cytosol or nucleoplasm (Figure 1B), experiments with PMA suggest continuous association and disassociation at this site. FRAP studies showed rapid recovery of fluorescence in photobleached perinuclear areas (Figure 6A). We detected new signal 500 msec after photobleaching, which nearly reached initial fluorescence levels in <30 s (Figure 6B). These results show that C1RasGRP1 is not membrane-anchored, but is continuously bound and released from internal membranes.
Figure 6.
C1RasGRP1 associates and dissociates continuously from internal membranes. Cells transfected with GFPC1RasGRP1 were plated and mounted for confocal microscopy. (A) Perinuclear region was selected and exposed to maximum laser power. Images before and after (at indicated times) photobleaching were captured. Color range: red: maximum GFP fluorescence, blue: minimum GFP fluorescence. (B) Data from six representative experiments were plotted.
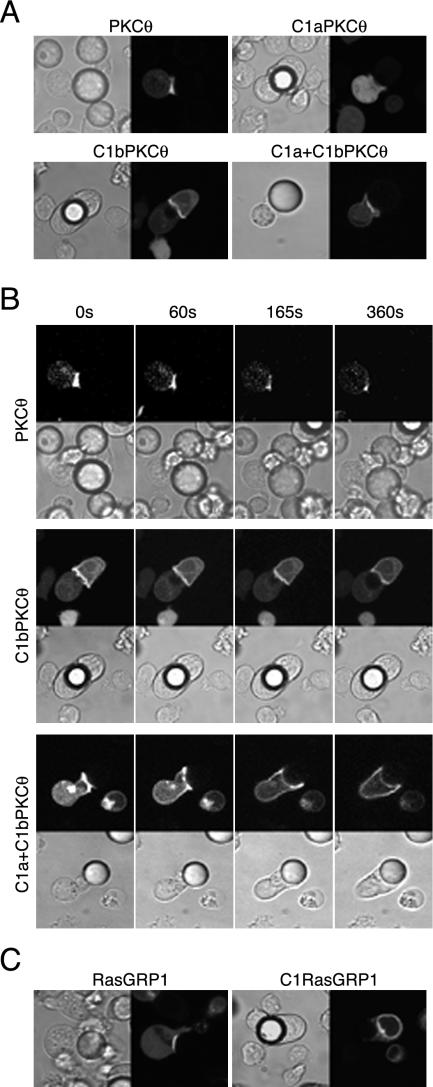
C1bPKCθ and C1a+C1bPKCθ But Not C1RasGRP1 Translocate to Plasma Membrane in Response to TCR Triggering
Transfected cells were incubated with anti-CD3+CD28–coated microspheres to study C1 domain relocation to the contact site. Cells expressing C1a+C1bPKCθ or C1bPKCθ accumulated GFP signal in the microsphere contact zone, as did the full-length protein (Figure 7A). Recruitment of these C1 domains to the synapse was not sustained, and they dispersed over the cell membrane after a short time; nonetheless, the complete protein was consistently localized at the contact site (Supplementary Videos 15–17; Figure 7B shows selected frames). As for PMA stimulation, C1aPKCθ did not translocate. C1bPKCθ and C1a+C1bPKCθ translocation in response to CD3+CD28 was DAG production dependent, as when cells were preincubated with U73122, C1 domains did not translocate (unpublished data). These results are consistent with previous studies of PKCθ translocation that showed the requirement for DAG, which is detected through its C1 domains, and for other signals that probably maintain correct PKCθ localization by interacting with synapse complex proteins (Villalba et al., 2002; Diaz-Flores et al., 2003).
Figure 7.
Translocation of full-length RasGRP1, PKCθ, and their C1 domains in response to CD3+CD28 stimulation. Jurkat cells were transfected with (A) GFP-fused PKCθ, C1aPKCθ, C1bPKCθ or C1a+C1bPKCθ or (C) with GFPRasGRP1 or GFPC1RasGRP1 constructs. At 24 h post-transfection, cells were suspended in HBSS and CD3+CD28-coated microspheres were added at a 2:1 cell:bead proportion, mounted as in Figure 1B for confocal microscopy, and representative cells captured for each condition. (B) Images at times indicated are shown to illustrate GFPC1 domain subcellular localization; full time-lapse confocal recording is shown in Supplementary Videos 15–17.
RasGRP1 is a cytosolic protein that also moved from cytosol to the membrane:microsphere contact region (Figure 7C); we observed no Golgi signal in response to anti-CD3+CD28, in contrast to previous reports (Bivona et al., 2003). Under the same conditions, C1RasGRP1 did not translocate. This suggests that, although the isolated RasGRP1 C1 domain has a higher affinity for Golgi, the full-length protein receives additional signals, which render it capable of overriding C1 domain fate.
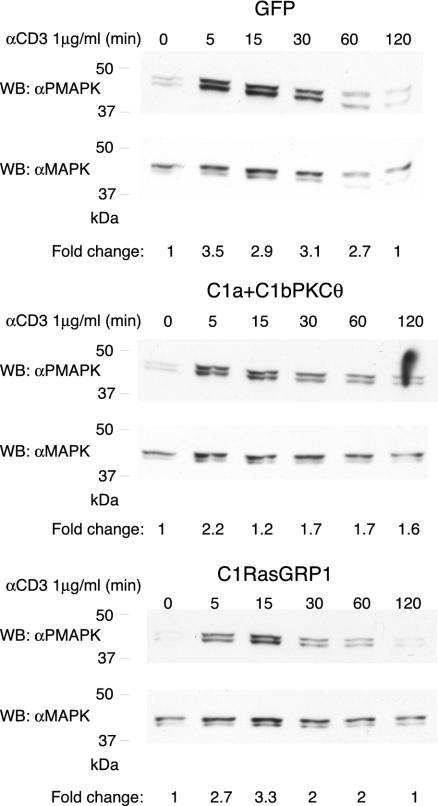
C1a+C1bPKCθ and C1RasGRP1 Modify Anti-CD3–dependent Ras Signaling
TCR triggering increases DAG levels, promoting Ras activation and subsequent MAPK phosphorylation (Marshall, 1995). Expression of an inactive DAGKα form that does not permit DAG transformation into PA, renders increasing DAG levels and as a consequence, more activated Ras (Jones et al., 2002). We thus considered that C1 domains might modify Ras signaling. We tested whether C1a+C1bPKCθ in plasma membrane or C1RasGRP in internal localizations block the DAG essential for Ras signaling (Bivona and Philips, 2003). Cells transfected with GFP (control), with C1a+C1bPKCθ or C1RasGRP1 were sorted to obtain a 100% GFP-positive population, cultured (8 h), then analyzed by flow cytometry to assess GFP expression and the percentage of positive cells. Both GFP- and C1RasGRP1-transfected cells were 100% GFP positive; however, C1a+C1bPKCθ cells lost construct expression, and the percentage of positive cells varied from 60–80% in distinct experiments. Cells were subsequently starved (1 h) in DMEM with 1% BSA and then stimulated with anti-CD3 (1 μg/ml) for a maximum of 2 h. Cells were recovered at different times and CD3-induced Ras activation was analyzed by PMAPK Western blot (Figure 8). C1a+C1bPKCθ expression decreased the MAPK phosphorylation level, probably because of C1a+C1bPKCθ interaction with DAG produced in response to anti-CD3, causing blockade of DAG connection with effectors as RasGRP1. C1RasGRP1 expression also modulated Ras signaling, although the construct did not translocate to the synapse. Maximum phosphorylation was delayed to 15 min and the signal was decreased at all time points. These results may confirm the recently reported role for RasGRP1 in Ras activation in Golgi (Bivona et al., 2003), where C1RasGRP1 localizes.
Figure 8.
C1RasGRP and C1a+C1bPKCθ modify Ras activation in response to soluble CD3 activation. Cells transfected with GFP, C1RasGRP or C1a+C1bPKCθ were sorted to recover GFP-positive cells. GFP expression was assessed by flow cytometry after 8 h culture; cells were then serum-starved (1 h), stimulated with soluble CD3 (1 μg/ml) for the times indicated, then collected and processed (see MATERIALS AND METHODS) for Western blot. Analysis with PMAPK antibody showed Jurkat cell activation differences according to the construct transfected. Anti-MAPK antibody was used as a protein loading control. Results are representative of four independent experiments. GFP and GFPC1RasGRP cells were 95% GFP-positive; GFPC1a+C1bPKCθ cells were 78% GFP-positive. The x-fold change in activation for each condition was estimated by densitometric analysis of filters. Values (arbitrary units) were normalized taking into consideration the protein levels of each sample as determined by Western blot analysis.
DISCUSSION
Although C1 domains have been studied extensively, their response to physiological signals in T lymphocytes has not been established. We used Jurkat T cells transfected with various GFP-fused C1 domains to follow their response to TCR triggering. We selected typical C1 domains of two proteins that are expressed in Jurkat cells, PKCθ (C1aPKCθ, C1bPKCθ, C1a+C1bPKCθ) and RasGRP1 (C1RasGRP1), which control NFκB activation (Isakov and Altman, 2002) and the Ras/Raf/MAPK pathway (Ebinu et al., 2000), respectively. We also cloned the β2chimerin C1 domain (C1β2chimerin) as an example of a C1 domain responsible for ER localization of the full-length protein (Wang and Kazanietz, 2002). β2chimerin is a lymphocytes-expressed (Siliceo, M. and Merida, I., unpublished results) GTPase-activating protein (GAP) for Rac (Diekmann et al., 1991), although its exact role remains unknown.
C1aPKCθ was the only construct that did not behave as predicted for a typical C1 domain, as it did not translocate after PMA or PDBu stimulation. PKC C1a domains are known to have lower affinity for phorbol esters than C1b (Shindo et al., 2001); indeed, the C1a from PKCθ and PKCδ have been described as domains that do not bind DAG (Shindo et al., 2001; Irie et al., 2002). Sequence analysis shows that all residues considered essential for binding are conserved (Kazanietz et al., 1995b). A proline at C1aPKCθ position 9 (considering the first histidine in the domain as position 1) and a glycine at the same position in C1aPKCδ nonetheless appear to be essential, because their mutation to lysine, the amino acid at this position in other C1a domains, renders them able to bind DAG (Shindo et al., 2001). The importance of this residue lies in its position at the base of one of the loops that constitutes the DAG binding groove; a proline or a glycine would modify loop conformation, blocking its correct folding.
Although the C1aPKCθ domain did not bind PMA, as shown here, it increased C1bPKCθ affinity for the plasma membrane, and the combined construct (C1a+C1bPKCθ) was expressed constitutively in plasma membrane. C1a domains are essential for anchoring classical PKC to the cell membrane. In the presence of Ca2+, conserved domain 2 (C2) direct the protein to the membrane, where phosphatidylserine disrupts C1a:C2 domain contacts. This exposes C1a residues for interaction with DAG, bringing C1b into closer proximity to the plasma membrane (Medkova and Cho, 1999; Bittova et al., 2001). For the novel PKC such as PKCθ, the role of the C1a domain is not well established, although its folding may allow greater PKCθ interaction with the specific lipid environment of the plasma membrane, as reported for other C1 domain combinations (Szallasi et al., 1996; Hunn and Quest, 1997). Accordingly, in vesicle binding experiments, the GFPC1aPKCθ construct interacts with vesicles with no specificity for distinct DAG forms. This domain appears to recognize membranes and may assist the C1bPKCθ in GFPC1a+C1bPKCθ construct to bind vesicles with high affinity, as shown by the percentage of membrane-bound GFPC1a+C1bPKCθ (Figure 4).
Another possibility is C1a domain interaction with membrane proteins, as described for other C1 domains (Oancea et al., 2003). C1b domain affinity for DAG would direct PKCθ to the membrane, which would be stabilized by C1a interaction with plasma membrane protein(s). Further studies should address the exact nature of the role of C1a in membrane interaction. Notwithstanding, the rapid diffusion of C1a+C1bPKCθ over the membrane after anti-CD3+CD28-polarized stimulation suggests that membrane interaction of this domain is lipid driven. It is important to note the difference in membrane localization of C1b or C1a+C1bPKCθ domains compared with that of full-length PKCθ, which remains at the contact zone for a longer period of time. These results concur with previous observations showing that vav-regulated cytoskeletal reorganization participates in PKCθ localization (Villalba et al., 2002).
The combination of confocal microscopy, pharmacological inhibition of the different DAG-generating pathways, and in vitro vesicle binding demonstrates that C1 domains are specific for different DAG species. The C1a+C1bPKCθ domain behaves as a sensor of polyunsaturated DAG in the plasma membrane, whereas RasGRP1 C1 binds strongly to saturated DAG. We nonetheless cannot rule out the influence of other lipids or proteins (Slater et al., 1994; Johnson et al., 1998; Ho et al., 2001). For instance, C1β2chimerin did not show high specificity for inner membranes in binding assays, but its interaction with Tmp21-I, an ER protein (Wang and Kazanietz, 2002) may explain the internal localization.
Structural analyses have been used to attempt to explain C1 DAG specificity. Studies of C1bPKCγ indicated that interaction with micelles takes place only in the loops and in the second Zn2+ coordination region (Xu et al., 1997). Analysis of membrane-interacting residues to study the cause of distribution variation showed that C1RasGRP1 has a negative charge (glutamic acid) at position five, whereas C1β2chimerin and C1bPKCθ have a valine; this position is involved in stabilizing the residues implicated in second Zn2+ coordination (Zhang et al., 1995). As other regions related to membrane binding (Wang et al., 2001) show no clear differences, position five may be important in defining C1 domain specificity and affinity for inner DAG pools. To test this possibility, we generated mutants in position five, replacing glutamic acid with valine in C1RasGRP1, GFPC1RasGRP1(E_V), and valine with glutamic acid in C1bPKCθ, GFPC1bPKCθ(V_E). Localization of the mutated constructs did not differ from the original forms, and they responded equally to PMA stimuli (unpublished data). These residues thus do not appear to be implicated in specificity.
C1RasGRP1 has been modeled using a docking approach (Rong et al., 2002); the resulting structure shows that its binding pocket is shallower than that of C1bPKCδ. This may explain its better recognition of saturated DAG, which is more compact and has less headgroup spacing than polyunsaturated DAG. DOG might insert more easily into the binding pocket than SAG, explaining C1RasGRP1 differential specificity for inner or plasma membrane DAG. Similar observations have been made for the DAG analog specificity of other C1-containing proteins (Wang et al., 2003). Binding groove differences may also explain C1 domain conformational flexibility, which may account for ligand affinity and membrane penetration (Ananthanarayanan et al., 2003)
RasGRP1 translocation to Golgi in response to soluble anti-CD3 and -CD28 was recently described (Bivona et al., 2003), although here we show clear localization to the site in contact with anti-CD3+CD28–coated microspheres. This discrepancy could be due to the activation protocol, because Golgi localization is observed after stimulation with soluble antibodies that induce less sustained responses than that triggered by surface-coupled antibodies (Huppa et al., 2003). Accordingly, in response to anti-CD3–coated plates, RasGRP1 translocates to plasma membrane and accumulates in an internal localization at later times (60 min; Sanjuan et al., 2003). All together, the data suggest that although sustained and polarized signals drive RasGRP1 to the plasma membrane, Golgi localization takes place after transient signaling, or at later times. RasGRP1 localization at the immune synapse would have additional requirements, such as the described vav-dependent cytoskeleton regulation (Caloca et al., 2003b).
The PLCγ-induced SAG increase in the contact zone with anti-CD3+CD28–coated microspheres did not dissociate the C1 domain from its basal internal membrane localization, because C1RasGRP1 has lower affinity for this lipid than for inner membrane DOG. This suggests that RasGRP1 must be in cytosol to be recruited to the plasma membrane. This observation is important, because we find cytoplasmic RasGRP1 only in lymphocytes. When transfected in adherent cells such as COS (Caloca et al., 2003a) or PAE (unpublished data), RasGRP1 is found only in internal structures. This probably renders the protein unable to sense receptor-derived DAG and suggests that RasGRP1 only activates Ras at internal localizations in nonlymphoid cells (Caloca et al., 2003a).
In this study, we characterized various DAG-binding domains that can act at different subcellular sites in T lymphocytes and reached several significant conclusions. First, the C1a+C1bPKCθ domain behaves as a DAG sensor at the plasma membrane, whereas the C1RasGRP1 domain is responsive to DAG mostly at internal localizations. Second, we show that both domains can act not only as lipid sensors, but also perturb the lipid-dependent signal at these sites, altering the kinetics of MAPK phosphorylation in response to T-cell activation. Third, our results showing differential blockade of Ras-dependent signals indicate that RasGRP1 is activated at distinct subcellular sites. Experiments are currently under way to determine the nature of the signals triggered by differential RasGRP1 localization and whether this localization is related to the intensity of the TCR-derived signals. This is of paramount importance, because processes such as positive and negative selection or activation vs. anergy depend on signal intensity (Hogquist, 2001; Appleman and Boussiotis, 2003). The recent description of the deficient mouse phenotype highlighted the complexity role of this protein. Its presence is indispensable for thymocyte development, specifically for positive selection (Dower et al., 2000). Although RasGRP1 is apparently not essential for peripheral T-lymphocyte activation, it is required to maintain their continued proliferation (Priatel et al., 2002).
The combination of C1 domain specificity for different DAG pools, the presence in a protein of one or more C1 domains, and their possible interactions with other proteins indicate the importance of these domains in determining the spatio-temporal localization of the proteins that contain them. The C1 domains characterized here can thus serve as DAG biosensors, specific for distinct DAG pools in the cell. As we show for plasma membrane, Golgi and ER, they can be used to block stimulation-induced DAG increases at these sites. Finally, characterization of C1RasGRP1 has allowed us to define a possible role for RasGRP1 activation of the Ras pathway in Golgi, opening new directions for study of the activation of this and other members of the small GTPase family in internal cell localizations.
Acknowledgments
We thank Drs. J.C. Stone and M.G. Kazanietz for RasGRP1 and β2chimerin plasmids, respectively; Dr. M. Renz for antigiantinα antibody; IM group members for stimulating discussion; C. Moreno-Ortiz and A. Moreno for flow cytometry and sorting; S. Gutiérrez and C. Sánchez for confocal and FRAP studies; and C. Mark for excellent editorial assistance. This work was supported in part by Grants BMC2001-1066 from the Spanish Ministry of Science and Technology, 08.3/0022/20002 from Comunidad deMadrid and G03/179 from Instituto deSalud Carlos III. S.C. receives a fellowship from the Spanish Ministry of Education. The Department of Immunology and Oncology was founded and is supported by the Spanish Council for Scientific Research (CSIC) and by Pfizer.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-11-0844. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-11-0844.
Online version of this article contains supporting material.
Online version is available at www.molbiolcell.org.
References
- Ananthanarayanan, B., Stahelin, R.V., Digman, M.A., and Cho, W. (2003). Activation mechanisms of conventional protein kinase C isoforms are determined by the ligand affinity and conformational flexibility of their C1 domains. J. Biol. Chem. 278, 46886-46894. [DOI] [PubMed] [Google Scholar]
- Appleman, L.J., and Boussiotis, V.A. (2003). T cell anergy and costimulation. Immunol. Rev. 192, 161-180. [DOI] [PubMed] [Google Scholar]
- Baron, C.L., and Malhotra, V. (2002). Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science 295, 325-328. [DOI] [PubMed] [Google Scholar]
- Betz, A., Okamoto, M., Benseler, F., and Brose, N. (1997). Direct interaction of the rat unc-13 homologue Munc13–1 with the N terminus of syntaxin. J. Biol. Chem. 272, 2520-2526. [DOI] [PubMed] [Google Scholar]
- Bi, K., Tanaka, Y., Coudronniere, N., Sugie, K., Hong, S., van Stipdonk, M.J., and Altman, A. (2001). Antigen-induced translocation of PKC-theta to membrane rafts is required for T cell activation. Nat. Immunol. 2, 556-563. [DOI] [PubMed] [Google Scholar]
- Bittova, L., Stahelin, R.V., and Cho, W. (2001). Roles of ionic residues of the C1 domain in protein kinase C-alpha activation and the origin of phosphatidylserine specificity. J. Biol. Chem. 276, 4218-4226. [DOI] [PubMed] [Google Scholar]
- Bivona, T.G. et al. (2003). Phospholipase Cgamma activates Ras on the Golgi apparatus by means of RasGRP1. Nature 424, 694-698. [DOI] [PubMed] [Google Scholar]
- Bivona, T.G., and Philips, M.R. (2003). Ras pathway signaling on endomembranes. Curr. Opin. Cell Biol. 15, 136-142. [DOI] [PubMed] [Google Scholar]
- Booden, M.A., Campbell, S.L., and Der, C.J. (2002). Critical but distinct roles for the pleckstrin homology and cysteine-rich domains as positive modulators of Vav2 signaling and transformation. Mol. Cell. Biol. 22, 2487-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns, D.J., and Bell, R.M. (1991). Protein kinase C contains two phorbol ester binding domains. J. Biol. Chem. 266, 18330-18338. [PubMed] [Google Scholar]
- Caloca, M.J., Zugaza, J.L., and Bustelo, X.R. (2003a). Exchange factors of the RasGRP family mediate Ras activation in the Golgi. J. Biol. Chem. 278, 33465-33473. [DOI] [PubMed] [Google Scholar]
- Caloca, M.J., Zugaza, J.L., Matallanas, D., Crespo, P., and Bustelo, X.R. (2003b). Vav mediates Ras stimulation by direct activation of the GDP/GTP exchange factor Ras GRP1. EMBO J. 22, 3326-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, G.J., Drugan, J.K., Rossman, K.L., Carpenter, J.W., Rogers-Graham, K., Fu, H., Der, C.J., and Campbell, S.L. (1997). 14–3-3 zeta negatively regulates raf-1 activity by interactions with the Raf-1 cysteine-rich domain. J. Biol. Chem. 272, 20990-20993. [DOI] [PubMed] [Google Scholar]
- Coppola, J., Bryant, S., Koda, T., Conway, D., and Barbacid, M. (1991). Mechanism of activation of the vav protooncogene. Cell Growth Differ. 2, 95-105. [PubMed] [Google Scholar]
- Das, S., Dixon, J.E., and Cho, W. (2003). Membrane-binding and activation mechanism of PTEN. Proc. Natl. Acad. Sci. USA 100, 7491-7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Flores, E., Siliceo, M., Martinez, A.C., and Merida, I. (2003). Membrane translocation of protein kinase Ctheta during T lymphocyte activation requires phospholipase C-gamma-generated diacylglycerol. J. Biol. Chem. 278, 29208-29215. [DOI] [PubMed] [Google Scholar]
- Diekmann, D., Brill, S., Garrett, M.D., Totty, N., Hsuan, J., Monfries, C., Hall, C., Lim, L., and Hall, A. (1991). Bcr encodes a GTPase-activating protein for p21rac. Nature 351, 400-402. [DOI] [PubMed] [Google Scholar]
- Dower, N.A., Stang, S.L., Bottorff, D.A., Ebinu, J.O., Dickie, P., Ostergaard, H.L., and Stone, J.C. (2000). RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat. Immunol. 1, 317-321. [DOI] [PubMed] [Google Scholar]
- Downward, J., Graves, J.D., Warne, P.H., Rayter, S., and Cantrell, D.A. (1990). Stimulation of p21ras upon T-cell activation. Nature 346, 719-723. [DOI] [PubMed] [Google Scholar]
- Ebinu, J.O., Bottorff, D.A., Chan, E.Y., Stang, S.L., Dunn, R.J., and Stone, J.C. (1998). RasGRP, a Ras guanyl nucleotide-releasing protein with calcium- and diacylglycerol-binding motifs. Science 280, 1082-1086. [DOI] [PubMed] [Google Scholar]
- Ebinu, J.O. et al. (2000). RasGRP links T-cell receptor signaling to Ras. Blood 95, 3199-3203. [PubMed] [Google Scholar]
- Ferrari, D.M., and Soling, H.D. (1999). The protein disulphide-isomerase family: unravelling a string of folds. Biochem. J. 339(Pt 1), 1-10. [PMC free article] [PubMed] [Google Scholar]
- Gerace, L., Blum, A., and Blobel, G. (1978). Immunocytochemical localization of the major polypeptides of the nuclear pore complex-lamina fraction. Interphase and mitotic distribution. J. Cell Biol. 79, 546-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, S., Xie, W.Q., Quest, A.F., Mabrouk, G.M., Strum, J.C., and Bell, R.M. (1994). The cysteine-rich region of raf-1 kinase contains zinc, translocates to liposomes, and is adjacent to a segment that binds GTP-ras. J. Biol. Chem. 269, 10000-10007. [PubMed] [Google Scholar]
- Hafizi, S., Alindri, F., Karlsson, R., and Dahlback, B. (2002). Interaction of Axl receptor tyrosine kinase with C1-TEN, a novel C1 domain-containing protein with homology to tensin. Biochem. Biophys. Res. Commun. 299, 793-800. [DOI] [PubMed] [Google Scholar]
- Hall, C., Monfries, C., Smith, P., Lim, H.H., Kozma, R., Ahmed, S., Vanniasingham, V., Leung, T., and Lim, L. (1990). Novel human brain cDNA encoding a 34,000 Mr protein n-chimaerin, related to both the regulatory domain of protein kinase C and BCR, the product of the breakpoint cluster region gene. J. Mol. Biol. 211, 11-16. [DOI] [PubMed] [Google Scholar]
- Henneberry, A.L., Wright, M.M., and McMaster, C.R. (2002). The major sites of cellular phospholipid synthesis and molecular determinants of fatty acid and lipid head group specificity. Mol. Biol. Cell 13, 3148-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, C., Slater, S.J., Stagliano, B., and Stubbs, C.D. (2001). The C1 domain of protein kinase C as a lipid bilayer surface sensing module. Biochemistry 40, 10334-10341. [DOI] [PubMed] [Google Scholar]
- Hodgkin, M.N., Pettitt, T.R., Martin, A., Michell, R.H., Pemberton, A.J., and Wakelam, M.J. (1998). Diacylglycerols and phosphatidates: which molecular species are intracellular messengers? Trends Biochem. Sci. 23, 200-204. [DOI] [PubMed] [Google Scholar]
- Hogquist, K.A. (2001). Signal strength in thymic selection and lineage commitment. Curr. Opin. Immunol. 13, 225-231. [DOI] [PubMed] [Google Scholar]
- Hommel, U., Zurini, M., and Luyten, M. (1994). Solution structure of a cysteine rich domain of rat protein kinase C. Nat. Struct. Biol. 1, 383-387. [DOI] [PubMed] [Google Scholar]
- Hubbard, S.R., Bishop, W.R., Kirschmeier, P., George, S.J., Cramer, S.P., and Hendrickson, W.A. (1991). Identification and characterization of zinc binding sites in protein kinase C. Science 254, 1776-1779. [DOI] [PubMed] [Google Scholar]
- Hunn, M., and Quest, A.F. (1997). Cysteine-rich regions of protein kinase Cdelta are functionally non-equivalent. Differences between cysteine-rich regions of non-calcium-dependent protein kinase Cdelta and calcium-dependent protein kinase Cgamma. FEBS Lett. 400, 226-232. [DOI] [PubMed] [Google Scholar]
- Huppa, J.B., Gleimer, M., Sumen, C., and Davis, M.M. (2003). Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat. Immunol. 4, 749-755. [DOI] [PubMed] [Google Scholar]
- Hurley, J.H., Newton, A.C., Parker, P.J., Blumberg, P.M., and Nishizuka, Y. (1997). Taxonomy and function of C1 protein kinase C homology domains. Protein Sci. 6, 477-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie, K. et al. (2002). Establishment of a binding assay for protein kinase C isozymes using synthetic C1 peptides and development of new medicinal leads with protein kinase C isozyme and C1 domain selectivity. Pharmacol. Ther. 93, 271-281. [DOI] [PubMed] [Google Scholar]
- Isakov, N., and Altman, A. (2002). Protein kinase C(theta) in T cell activation. Annu. Rev. Immunol. 20, 761-794. [DOI] [PubMed] [Google Scholar]
- Ishizaki, T. et al. (1996). The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 15, 1885-1893. [PMC free article] [PubMed] [Google Scholar]
- Johnson, J.E., Zimmerman, M.L., Daleke, D.L., and Newton, A.C. (1998). Lipid structure and not membrane structure is the major determinant in the regulation of protein kinase C by phosphatidylserine. Biochemistry 37, 12020-12025. [DOI] [PubMed] [Google Scholar]
- Jones, D.R., Sanjuan, M.A., Stone, J.C., and Merida, I. (2002). Expression of a catalytically inactive form of diacylglycerol kinase alpha induces sustained signaling through RasGRP. FASEB J. 16, 595-597. [DOI] [PubMed] [Google Scholar]
- Kane, L.P., Lin, J., and Weiss, A. (2000). Signal transduction by the TCR for antigen. Curr. Opin. Immunol. 12, 242-249. [DOI] [PubMed] [Google Scholar]
- Kanoh, H., Sakane, F., Imai, S., and Wada, I. (1993). Diacylglycerol kinase and phosphatidic acid phosphatase— enzymes metabolizing lipid second messengers. Cell Signal. 5, 495-503. [DOI] [PubMed] [Google Scholar]
- Kazanietz, M.G., Barchi, J.J., Jr., Omichinski, J.G., and Blumberg, P.M. (1995a). Low affinity binding of phorbol esters to protein kinase C and its recombinant cysteine-rich region in the absence of phospholipids. J. Biol. Chem. 270, 14679-14684. [DOI] [PubMed] [Google Scholar]
- Kazanietz, M.G., Wang, S., Milne, G.W., Lewin, N.E., Liu, H.L., and Blumberg, P.M. (1995b). Residues in the second cysteine-rich region of protein kinase C delta relevant to phorbol ester binding as revealed by site-directed mutagenesis. J. Biol. Chem. 270, 21852-21859. [DOI] [PubMed] [Google Scholar]
- Kurscheid-Reich, D., Throckmorton, D.C., and Rasmussen, H. (1995). Serotonin activates phospholipase D in rat mesangial cells. Am. J. Physiol. 268, F997-F1003. [DOI] [PubMed] [Google Scholar]
- Madaule, P., Furuyashiki, T., Reid, T., Ishizaki, T., Watanabe, G., Morii, N., and Narumiya, S. (1995). A novel partner for the GTP-bound forms of rho and rac. FEBS Lett. 377, 243-248. [DOI] [PubMed] [Google Scholar]
- Marshall, M.S. (1995). Ras target proteins in eukaryotic cells. FASEB J. 9, 1311-1318. [DOI] [PubMed] [Google Scholar]
- Medkova, M., and Cho, W. (1999). Interplay of C1 and C2 domains of protein kinase C-alpha in its membrane binding and activation. J. Biol. Chem. 274, 19852-19861. [DOI] [PubMed] [Google Scholar]
- Monks, C.R., Kupfer, H., Tamir, I., Barlow, A., and Kupfer, A. (1997). Selective modulation of protein kinase C-theta during T-cell activation. Nature 385, 83-86. [DOI] [PubMed] [Google Scholar]
- Oancea, E., Bezzerides, V.J., Greka, A., and Clapham, D.E. (2003). Mechanism of persistent protein kinase D1 translocation and activation. Dev. Cell 4, 561-574. [DOI] [PubMed] [Google Scholar]
- Oancea, E., Teruel, M.N., Quest, A.F., and Meyer, T. (1998). Green fluorescent protein (GFP)-tagged cysteine-rich domains from protein kinase C as fluorescent indicators for diacylglycerol signaling in living cells. J. Cell Biol. 140, 485-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono, Y., Fujii, T., Igarashi, K., Kuno, T., Tanaka, C., Kikkawa, U., and Nishizuka, Y. (1989). Phorbol ester binding to protein kinase C requires a cysteine-rich zinc-finger-like sequence. Proc. Natl. Acad. Sci. USA 86, 4868-4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priatel, J.J., Teh, S.J., Dower, N.A., Stone, J.C., and Teh, H.S. (2002). RasGRP1 transduces low-grade TCR signals which are critical for T cell development, homeostasis, and differentiation. Immunity 17, 617-627. [DOI] [PubMed] [Google Scholar]
- Quest, A.F., Bardes, E.S., and Bell, R.M. (1994a). A phorbol ester binding domain of protein kinase C gamma. Deletion analysis of the Cys2 domain defines a minimal 43-amino acid peptide. J. Biol. Chem. 269, 2961-2970. [PubMed] [Google Scholar]
- Quest, A.F., Bardes, E.S., and Bell, R.M. (1994b). A phorbol ester binding domain of protein kinase C gamma. High affinity binding to a glutathione-S-transferase/Cys2 fusion protein. J. Biol. Chem. 269, 2953-2960. [PubMed] [Google Scholar]
- Quest, A.F., and Bell, R.M. (1994). The regulatory region of protein kinase C gamma. Studies of phorbol ester binding to individual and combined functional segments expressed as glutathione S-transferase fusion proteins indicate a complex mechanism of regulation by phospholipids, phorbol esters, and divalent cations. J. Biol. Chem. 269, 20000-20012. [PubMed] [Google Scholar]
- Rebecchi, M.J., and Pentyala, S.N. (2000). Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol. Rev. 80, 1291-1335. [DOI] [PubMed] [Google Scholar]
- Roberts, R., Sciorra, V.A., and Morris, A.J. (1998). Human type 2 phosphatidic acid phosphohydrolases. Substrate specificity of the type 2a, 2b, and 2c enzymes and cell surface activity of the 2a isoform. J. Biol. Chem. 273, 22059-22067. [DOI] [PubMed] [Google Scholar]
- Rong, S.B. et al. (2002). Structural basis of RasGRP binding to high-affinity PKC ligands. J. Med. Chem. 45, 853-860. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pinera, P., Micol, V., Corbalan-Garcia, S., and Gomez-Fernandez, J.C. (1999). A comparative study of the activation of protein kinase C alpha by different diacylglycerol isomers. Biochem. J. 337(Pt 3), 387-395. [PMC free article] [PubMed] [Google Scholar]
- Sanjuan, M.A., Jones, D.R., Izquierdo, M., and Merida, I. (2001). Role of diacylglycerol kinase alpha in the attenuation of receptor signaling. J. Cell Biol. 153, 207-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan, M.A., Pradet-Balade, B., Jones, D.R., Martinez, A.C., Stone, J.C., Garcia-Sanz, J.A., and Merida, I. (2003). T cell activation in vivo targets diacylglycerol kinase alpha to the membrane: a novel mechanism for Ras attenuation. J. Immunol. 170, 2877-2883. [DOI] [PubMed] [Google Scholar]
- Secrist, J.P., Karnitz, L., and Abraham, R.T. (1991). T-cell antigen receptor ligation induces tyrosine phosphorylation of phospholipase C-gamma 1. J. Biol. Chem. 266, 12135-12139. [PubMed] [Google Scholar]
- Seelig, H.P., Schranz, P., Schroter, H., Wiemann, C., Griffiths, G., and Renz, M. (1994). Molecular genetic analyses of a 376-kilodalton Golgi complex membrane protein (giantin). Mol. Cell. Biol. 14, 2564-2576. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Shindo, M., Irie, K., Nakahara, A., Ohigashi, H., Konishi, H., Kikkawa, U., Fukuda, H., and Wender, P.A. (2001). Toward the identification of selective modulators of protein kinase C (PKC) isozymes: establishment of a binding assay for PKC isozymes using synthetic C1 peptide receptors and identification of the critical residues involved in the phorbol ester binding. Bioorg. Med. Chem. 9, 2073-2081. [DOI] [PubMed] [Google Scholar]
- Slater, S.J., Ho, C., Kelly, M.B., Larkin, J.D., Taddeo, F.J., Yeager, M.D., and Stubbs, C.D. (1996). Protein kinase Calpha contains two activator binding sites that bind phorbol esters and diacylglycerols with opposite affinities. J. Biol. Chem. 271, 4627-4631. [DOI] [PubMed] [Google Scholar]
- Slater, S.J., Kelly, M.B., Taddeo, F.J., Ho, C., Rubin, E., and Stubbs, C.D. (1994). The modulation of protein kinase C activity by membrane lipid bilayer structure. J. Biol. Chem. 269, 4866-4871. [PubMed] [Google Scholar]
- Stahelin, R.V., Rafter, J.D., Das, S., and Cho, W. (2003). The molecular basis of differential subcellular localization of C2 domains of protein kinase C-alpha and group IVa cytosolic phospholipase A2. J. Biol. Chem. 278, 12452-12460. [DOI] [PubMed] [Google Scholar]
- Sun, Z. et al. (2000). PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature 404, 402-407. [DOI] [PubMed] [Google Scholar]
- Szallasi, Z., Bogi, K., Gohari, S., Biro, T., Acs, P., and Blumberg, P.M. (1996). Non-equivalent roles for the first and second zinc fingers of protein kinase Cdelta. Effect of their mutation on phorbol ester-induced translocation in NIH 3T3 cells. J. Biol. Chem. 271, 18299-18301. [DOI] [PubMed] [Google Scholar]
- Thompson, A.K., Mostafapour, S.P., Denlinger, L.C., Bleasdale, J.E., and Fisher, S.K. (1991). The aminosteroid U-73122 inhibits muscarinic receptor sequestration and phosphoinositide hydrolysis in SK-N-SH neuroblastoma cells. A role for Gp in receptor compartmentation. J. Biol. Chem. 266, 23856-23862. [PubMed] [Google Scholar]
- Valverde, A.M., Sinnett-Smith, J., Van Lint, J., and Rozengurt, E. (1994). Molecular cloning and characterization of protein kinase D: a target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proc. Natl. Acad. Sci. USA 91, 8572-8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba, M. et al. (2002). Translocation of P.K.C[theta] in T. cells is mediated by a nonconventional, P.I3-K- and Vav-dependent pathway, but does not absolutely require phospholipase C. J. Cell Biol. 157, 253-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H., and Kazanietz, M.G. (2002). Chimaerins, novel non-protein kinase C phorbol ester receptors, associate with Tmp21-I (p23): evidence for a novel anchoring mechanism involving the chimaerin C1 domain. J. Biol. Chem. 277, 4541-4550. [DOI] [PubMed] [Google Scholar]
- Wang, Q.J., Fang, T.W., Nacro, K., Marquez, V.E., Wang, S., and Blumberg, P.M. (2001). Role of hydrophobic residues in the C1b domain of protein kinase C delta on ligand and phospholipid interactions. J. Biol. Chem. 276, 19580-19587. [DOI] [PubMed] [Google Scholar]
- Wang, Q.J., Fang, T.W., Yang, D., Lewin, N.E., Van Lint, J., Marquez, V.E., and Blumberg, P.M. (2003). Ligand structure-activity requirements and phospholipid dependence for the binding of phorbol esters to protein kinase D. Mol. Pharmacol. 64, 1342-1348. [DOI] [PubMed] [Google Scholar]
- Whitehead, I., Kirk, H., Tognon, C., Trigo-Gonzalez, G., and Kay, R. (1995). Expression cloning of lfc, a novel oncogene with structural similarities to guanine nucleotide exchange factors and to the regulatory region of protein kinase C. J. Biol. Chem. 270, 18388-18395. [DOI] [PubMed] [Google Scholar]
- Williams, J.G., Drugan, J.K., Yi, G.S., Clark, G.J., Der, C.J., and Campbell, S.L. (2000). Elucidation of binding determinants and functional consequences of Ras/Raf-cysteine-rich domain interactions. J. Biol. Chem. 275, 22172-22179. [DOI] [PubMed] [Google Scholar]
- Winkler, D.G., Cutler, R.E., Jr., Drugan, J.K., Campbell, S., Morrison, D.K., and Cooper, J.A. (1998). Identification of residues in the cysteine-rich domain of Raf-1 that control Ras binding and Raf-1 activity. J. Biol. Chem. 273, 21578-21584. [DOI] [PubMed] [Google Scholar]
- Xu, R.X., Pawelczyk, T., Xia, T.H., and Brown, S.C. (1997). NMR structure of a protein kinase C-gamma phorbol-binding domain and study of proteinlipid micelle interactions. Biochemistry 36, 10709-10717. [DOI] [PubMed] [Google Scholar]
- Zhang, G., Kazanietz, M.G., Blumberg, P.M., and Hurley, J.H. (1995). Crystal structure of the cys2 activator-binding domain of protein kinase C delta in complex with phorbol ester. Cell 81, 917-924. [DOI] [PubMed] [Google Scholar]
- Zinser, E., Sperka-Gottlieb, C.D., Fasch, E.V., Kohlwein, S.D., Paltauf, F., and Daum, G. (1991). Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J. Bacteriol. 173, 2026-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]