Abstract
Background
There is a dual need for (1) innovative theory-based smartphone applications for smoking cessation and (2) controlled trials to evaluate their efficacy. Accordingly, this study tested the feasibility, acceptability, preliminary efficacy, and mechanism of behavioral change of an innovative smartphone-delivered Acceptance and Commitment Therapy (ACT) application for smoking cessation versus an application following US Clinical Practice Guidelines.
Method
Adult participants were recruited nationally into the double-blind randomized controlled pilot trial (N = 196) that compared smartphone-delivered ACT for smoking cessation application (SmartQuit) with the National Cancer Institute's application for smoking cessation (QuitGuide).
Results
We recruited 196 participants in two months. SmartQuit participants opened their application an average of 37.2 times, as compared to 15.2 times for QuitGuide participants (p <.0001). The overall quit rates were 13% in SmartQuit vs. 8% in QuitGuide (OR=2.7; 95% CI=0.8-10.3). Consistent with ACT's theory of change, among those scoring low (below the median) on acceptance of cravings at baseline (n = 88), the quit rates were 15% in SmartQuit vs. 8% in QuitGuide (OR=2.9; 95% CI=0.6-20.7).
Conclusions
ACT is feasible to deliver by smartphone application and shows higher engagement and promising quit rates compared to an application that follows US Clinical Practice Guidelines. As results were limited by the pilot design (e.g., small sample), a full-scale efficacy trial is now needed.
Keywords: smoking cessation, nicotine dependence, mHealth, telephone, acceptance, mindfulness
1. INTRODUCTION
On the fiftieth anniversary of the landmark 1964 US Surgeon General's Report on Smoking and Health (U.S. Department of Health, 1964), the 2014 Surgeon General's report concludes that, while all forms of tobacco use are unsafe, cigarette smoking: (1) accounts for 480,000 deaths; (2) remains the number one preventable cause of premature death; (3) causes diabetes and multiple cancers including colorectal and liver cancers; and (4) leads to $289 billion in healthcare and lost productivity costs annually in the US alone (CDC, 2014). The decline in the smoking prevalence has slowed in recent years, with 42 million Americans still smoking. States’ funding for population-level smoking cessation programs (e.g., quitlines) remains far below CDC-recommended levels. Consequently, there is a tremendous need for interventions with strong potential population-level impact at the lowest possible cost (CDC, 2014).
1.1 New technology: Smoking cessation apps
That potential can be found in the newest technological innovation in quit smoking interventions: smartphone-based smoking cessation software applications (“apps”; Abroms et al., 2013, 2011; Buller et al., 2013). Smartphones apps have all of the beneficial features of websites and text messaging interventions, but without their limitations (Abroms et al., 2013; Buller et al., 2013; Chen et al., 2012). Specifically, smartphone apps can have these important features: (1) available at arm's reach, (2) visually-engaging design, (3) video and audio capabilities, (4) unrestricted text capabilities, (5) access without cellular or internet connection, (6) immediate access to intervention content, (7) optimized to smartphone screen size, (8) content sharable via social media, and (9) tracking progress anywhere and anytime. Indeed, apps are an important technological advance over web sites and text messaging programs because of their high potential to boost user engagement—a consistently strong predictor of smoking cessation (Civljak et al., 2010; Shahab and McEwen, 2009; Webb, 2009; Whittaker et al., 2012).
1.2. Apps’ reach and effectiveness
Smoking interventions’ population-level impact is driven by the number of smokers they reach and their effectiveness. Smartphone apps for smoking cessation now have enormous reach. In 2013, there were over 400 smoking cessation apps (Abroms et al., 2013). In March 2014, using the xyo.net app search engine, we found a total of 546 English language smoking cessation apps in the Apple Store and Google Play that were downloaded to smartphones an estimated 3.2 million times in the United States and 20 million times worldwide. By contrast, during 2012-2013, there were an estimated 1 million enrollments to US tobacco quitlines (Consortium, 2013; Leischow et al., 2012) and an estimated 140,000 total subscriptions to US text messaging programs (L. Abroms, personal communication, March 4, 2014; E. Augustson, personal communication, March 4, 2014).
The reach of smoking cessation apps is climbing rapidly, greatly aided by the growing ownership of smartphones. The majority (58%) of US adults now own smartphones (Smith, 2013), and ownership is projected to reach at least 90% by 2020 (Dediu, 2013; Statista, 2014). Importantly, minority ownership is strong, with 64% of African Americans and 60% of Hispanics now owning smartphones, as compared to 53% for Caucasians (Smith, 2013). The greatest ownership growth rate is among those with low incomes (Nielsen, 2013; Smith, 2013). The current and projected demographics of smartphone ownership suggest that this treatment modality could address known tobacco-related health disparities associated with race/ethnicity and socioeconomic status (Fagan et al., 2004).
The effectiveness of smartphone apps for smoking cessation is largely unknown. Except for a pilot trial of young adults (Buller et al., 2013), no randomized trials of their effectiveness for general adult cessation have been published. The contrast between smoking cessation apps’ high usage and their lack of effectiveness data is a serious scientific gap that could stifle their population-level impact.
The current standard in smoking cessation interventions is the US Clinical Practice Guidelines (USCPG). The USCPG have the following essential content: tracking smoking status, offering quit planning, advice on pharmacotherapy, tools to enhance motivation, and social support for quitting (Fiore et al., 2008). Of the apps now available, a small minority follow the USCPG (Abroms et al., 2013, 2011). However, just following the USCPG is likely insufficient. For example, multiple recent meta-analyses of websites and of text messaging interventions that follow USCPG report that their average intent-to-treat 30-day point prevalence quit rates at 12 months post-randomization are remarkably similar, ranging from 7% to 10% (Civljak et al., 2010; Hutton et al., 2011; Shahab and McEwen, 2009; Whittaker et al., 2012). Consequently, an app that goes a key step beyond the USCPG through innovative theory-based intervention content has promise to produce higher quit rates.
1.3. ACT: An emerging theory-based intervention
To start a smartphone research paradigm focusing on innovative intervention content, the current study will compare an app that follows USCPG with one that adds novel content based on a behavior change model called Acceptance and Commitment Therapy (ACT; Hayes et al., 2006). ACT focuses on increasing willingness to experience physical cravings, emotions, and thoughts while making values-guided committed behavior changes. In ACT, acceptance means making room for intense physical cravings (e.g., urges to smoke), emotions (e.g., sadness that triggers smoking), and thoughts (e.g., thoughts that trigger smoking) while allowing them to come and go. Commitment in ACT means articulating what is deeply meaningful to individuals—i.e., their values—to motivate and guide specific plans of action (e.g., stopping smoking). Numerous studies have supported the effectiveness of ACT for a wide variety of problems including depression and drug addiction (Hayes et al., 2013, 2006).
1.4. This study
This study addressed the dual needs for (1) innovative theory-based intervention content and (2) controlled trials to evaluate the efficacy of apps for smoking cessation. Accordingly, we developed the first smartphone app-delivered ACT intervention for smoking cessation, called “SmartQuit.” We then conducted a nationally-recruited randomized, controlled pilot trial comparing SmartQuit with the National Cancer Institute's QuitGuide app. The aims were to determine (1) trial design feasibility, (2) participant receptivity and satisfaction, (3) preliminary cessation outcomes overall and for two key subgroups (those reporting: (a) heavy smoking; (b) low acceptance of cravings), and (4) potential impact on acceptance of cravings to smoke—ACT's theory-based process of change.
2. METHODS
2.1. Participants
Eligibility criteria: (1) age 18 or older; (2) smokes at least five cigarettes daily for at least past 12 months, as consistent with cessation trials (Civljak et al., 2010); (3) wants to quit in the next 30 days; (4) interested in learning skills to quit smoking; (5) willing to be randomly assigned to either smartphone application; (6) resides in US; (7) knows how to download a smartphone application from Apple's App Store; (8) willing and able to read English; (9) not using other smoking cessation interventions (including apps and our other intervention studies); and (10) has at least daily access to their own Apple iPhone 4, 4s, or 5. The iPhone was chosen as the singular smartphone platform for the study because it provided the technical simplicity of only one type of hardware to run the app, and thereby freed up limited resources for programming. Participants not eligible for or interested in randomization were given information on alternative treatment to help them quit.
2.2. Procedures
All study procedures were reviewed and approved by the Fred Hutchinson Cancer Research Center Institutional Review Board.
2.3. Sample size
As Aim 1's focus was on feasibility, the study's sample size was powered to show differences in engagement, operationalized as the number of times participants opened their assigned app. Extrapolating from our web-delivered intervention trial results on ACT vs. Smokefree.gov for smoking cessation (Bricker et al., 2013), we estimated that the mean number of times participants opened SmartQuit would be 9.02 for ACT and 5.46 for QuitGuide. Based on these estimates, there was 80% power to detect a significant difference in number of app openings with a sample size of 196. In contrast, the study was not powered to show differences in quit rates, but rather to provide an estimate of the effect size for smartphone-delivered ACT.
2.4. Recruitment
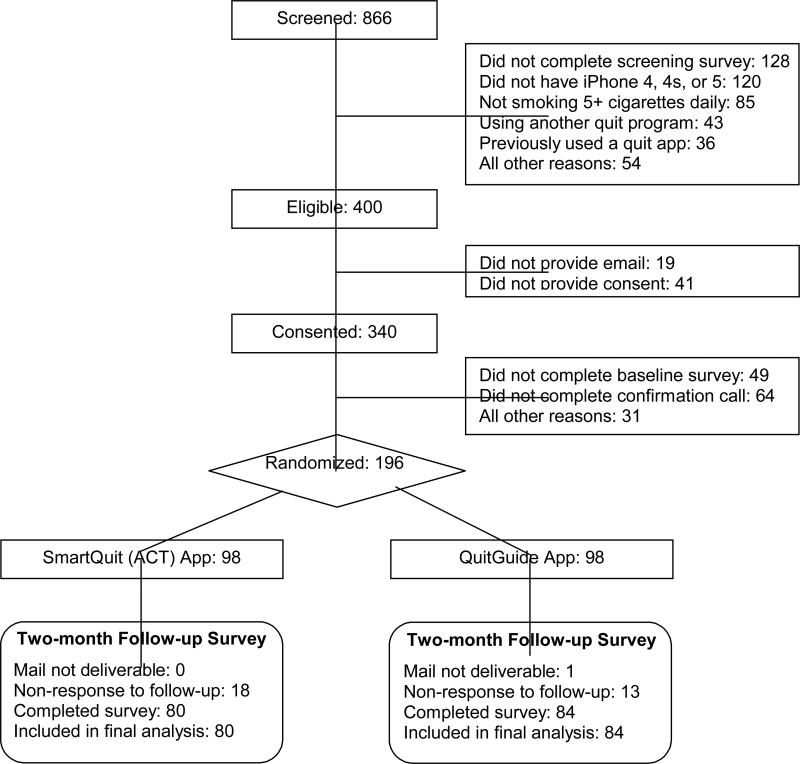
Recruitment occurred from March to May 2013. Based on prior success in national recruitment for a web-delivered trial (Heffner et al., 2013), we used both web-based and traditional methods of recruitment. Recruitment sources were: 39% from Facebook ads, 38% from press-release generated media (13% TV, 10% radio, 8% websites, 7% newspaper), and 23% from other sources (7% Google ads, 5% search engine results, 4% fhcrc.org, 7% other). Participants were directed to the study's recruitment webpage. Repeat logins from the same IP address were recorded and excluded. As shown in Figure 1, 738 completed the online screening survey, of whom 400 were eligible and 340 consented online. Of those, 205 completed the baseline survey and phone call to confirm study interest. These 205 participants were emailed a link allowing them to enroll in the study. All 196 (98 per arm) who clicked on this link were randomized into the trial. They were provided instructions for downloading and opening their randomly assigned app. The proportions of self- and criterion-driven exclusions from initial screening through randomization (see Figure 1) were very similar to our web-delivered intervention trial (Bricker et al., 2013), and a large web-based smoking cessation trial that used recruitment methods similar to the current study (Muñoz et al., 2006).
Figure 1.
Participant Flow Diagram
2.5. Randomization
We stratified randomization on two known predictors of cessation: nicotine dependence [Heaviness Smoking Index cut off score of 4 (Heatherton et al., 1989)] and motivation to quit [Commitment to Quitting Scale cutoff score of 4; (Kahler et al., 2007)]. The two conditions were balanced at baseline on all measured characteristics (p-value average: .59; all p-values > .05).
2.6. Interventions
The two interventions were evaluated over the eight weeks following randomization, during which participants could access their assigned app as often as they wished. All app content was available for participants to use at any time. Eight identical weekly email reminders were sent to all participants following randomization in order to prompt use of the app.
2.7. SmartQuit
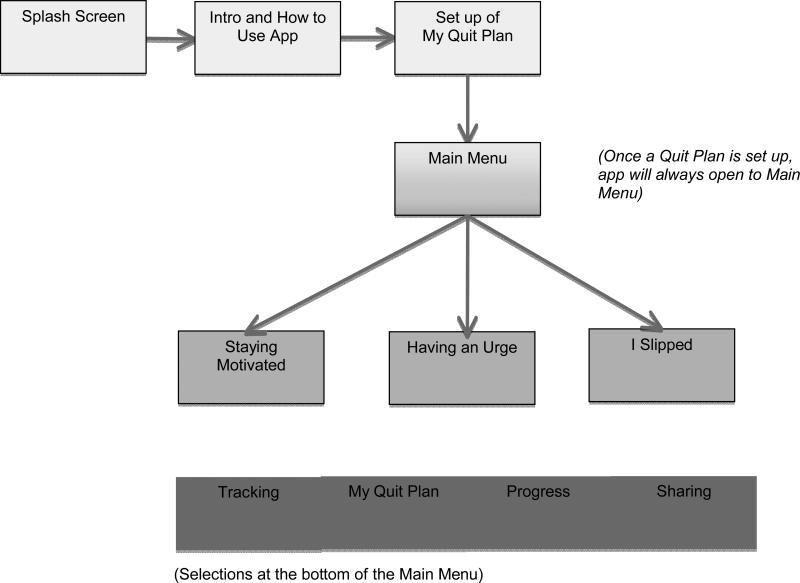
SmartQuit content was adapted from our web and telephone-based ACT interventions (Bricker et al., 2013, 2010) and developed into a self-paced intervention program. SmartQuit included the following features: (1) “Staying Motivated” focuses on ACT values-based motivations to quit via testimonials of former smokers describing how quitting smoking has helped them do things that deeply matter them (e.g., spend quality time with children) and also shows photos uploaded by users to remind them why they are quitting; (2) “My Quit Plan,” helps users develop a personalized quit plan, identify social support for quitting, and provides information on selecting FDA-approved medications for quitting smoking. Based on ACT, the plan includes a key step of naming values guiding quitting (e.g., quitting because I love my daughter) and uploading photos symbolizing that value (e.g., a picture of my daughter); (3) “Having an Urge” teaches a set of ACT audio and text-based acceptance skills presented as separate tools for coping with cravings to smoke; (4) “I Slipped” teaches ACT skills in self-compassion for recovering from smoking lapses and the self-judgments that often accompany them, and (5) “Tracking” lets users record the number of times they allowed an urge to pass without smoking (the key to learning acceptance) and the number of times they practice one of the program's exercises. Tracking also includes self-monitoring of each cigarette smoked and use of medications to aid cessation. On the bottom of the main menu, participants can select to view and update their quit plan, view their progress in the “Progress” section with simple graphs, receive graphic rewards for their progress (e.g., a badge), or share their progress via Facebook, Twitter, email, or text message.
When a functional version of SmartQuit was completed, we conducted evaluations for usability with eight participants. Participants were instructed to download SmartQuit onto their iPhone and complete a set of eight tasks that tested various aspects of the application's functionality (e.g., reviewing an exercise, using the tracking feature, reviewing their progress). The first four participants were internal team members who directly noted their identified errors in a log and suggested changes to improve usability.. The second set of four participants were adult, community-recruited smokers who wanted to quit. For the external users, their use of the app was video recorded, and were asked to use the think-aloud technique to verbalize their experience of the app in real time. Members of the research team viewed these videos and noted errors and suggested changes onto a spreadsheet that was emailed to the programming team. Results of these tests lead to the following three classes of corrections: (1) logic/flow errors (e.g., buttons lead to the wrong screen), (2) content (e.g., text too wordy), and (3) aesthetics (e.g., fonts too small). These corrections yielded the final version of SmartQuit used in the trial. A flow chart of SmartQuit (see Figure 2) shows the structure of the app and its specific features.
Figure 2.
Flow chart of SmartQuit's structure and features
2.8. QuitGuide
The National Cancer Institute's “QuitGuide” was the comparison app. QuitGuide was selected for the following reasons. First, it's one of the few apps with content that follows USCPG (Fiore et al., 2008). Second, its content and structure is directly based on Smokefree.gov, the most accessed cessation website in the world. Since 2006, it remains the number one website on Internet searches, with over 1.8 million US visitors in 2013 (E. Augustson, personal communication, March 4, 2014). Smokefree.gov has the highest user satisfaction rates of all non-profit websites for smoking cessation (Etter, 2006), and has 7-10% quit rates, consistent with other cessation websites not offering pharmacotherapy (Civljak et al., 2010; Hutton et al., 2011; Shahab and McEwen, 2009). Finally, QuitGuide's content is non-proprietary and free to the public, thereby providing maximal transparency, accessibility, and replicability.
QuitGuide has these features: (1) “Thinking about quitting” focuses on reason-based motivations to quit by encouraging users to list reasons for quitting and providing information on the health consequences of smoking and quitting; (2) “Preparing to Quit,” helps users develop a personalized quit plan, identify social support for quitting, and provides information on FDA-approved medications for quitting smoking; (3) “Quitting” teaches skills for avoiding cravings to smoke, such as finding replacement behaviors (e.g., chewing on carrot sticks) and staying busy; (4) “Staying Quit” presents skills for coping with slips via fighting cravings and trying to be positive. Users can share QuitGuide's content via Facebook, Twitter, or email.
2.9. Follow-up data collection
We administered a two-month post randomization follow-up survey. To maximize retention, we sequentially timed the administration of separate survey modalities until the surveys were completed: first via the web, then by telephone, and then by mail. Participants were compensated $25 for completing the survey. The achieved retention rate of 84% was much higher than the 54% typically obtained in web-based cessation trials (Civljak et al., 2010; Shahab and McEwen, 2009). Retention did not differ between study arms (p = .56).
2.10. Measures
2.10.1 Participant baseline characteristics
Participants reported at the baseline assessment a variety of demographics as well as smoking in the social environment. Nicotine dependence at baseline was measured with the two-item Heaviness Smoking Index from the Fagerström Test for Nicotine Dependence (Heatherton et al., 1991).
2.10.2 Treatment satisfaction
Treatment satisfaction at the two-month follow-up was measured with a brief survey. A sample item was: “How useful were your program's skills exercises for quitting smoking?” Response choices ranged from “Not at all” (1) to “Very much” (5).
2.10.3 Utilization
At the two-month follow-up, participants self-reported the number of times they opened their assigned app. Self-reports of utilization were necessary because, for technical reasons, automatic recording of this information was not possible for QuitGuide.
2.10.4 ACT theory-based acceptance process
The willingness to experience and not act on physical cravings to smoke (i.e., acceptance) was measured at baseline and two-month follow-up using a nine-item subscale of the Avoidance and Inflexibility Scale [adapted from (Gifford et al., 2004)]. The psychometric properties of the AIS have been previously reported in our research (Bricker et al., 2013). Response choices for each item ranged from “Not at all” (1) to “Very willing” (5). Scores were derived by averaging the items.
2.10.5 Thirty-day point prevalence cessation outcome at two-month follow-up
For scientific rigor and comparability with other low-intensity intervention trials (Hutton et al., 2011; Stead et al., 2013), the cessation outcome was thirty-day point prevalence abstinence (i.e., no smoking at all in the past thirty days). Smoking status was self-reported. Due to cost and low demand characteristics for false reporting, the SRNT Subcommittee on Biochemical Verification recommends biochemical confirmation is unnecessary in population-based studies with limited face-to-face contact and studies where the optimal data collection methods are through the mail or telephone (Benowitz et al., 2002). Self-reported smoking is a standard method for assessing the efficacy of low-intensity interventions (Hutton et al., 2011; Stead et al., 2013).
2.11. Statistical analyses
Demographic characteristics, smoking behavior, mental health measures (e.g., depression) and ACT theory-based process measures at baseline were compared between study groups using two sample t-tests for continuous variables and Fisher's exact test for binary variables. We used univariate logistic regression to test whether any of these factors predicted two-month retention. All participants were analyzed in the study arm to which they were randomized.
Logistic and linear regression was used to compare the two treatments on participant satisfaction, utilization of the app, and smoking cessation outcomes. Analyses of follow-up data were restricted to the population of participants who provided two-month follow-up data (i.e., complete cases). We did not use the worst-case scenario assumption that non-respondents were still smoking (i.e., missing = smoking) because such imputation has been shown to result in biases in effect size estimates, and may lead either to (1) reduced statistical power and increased type II error, or (2) underestimation of variance and increased type I error, depending on the rate of non-response in each study group and the smoking cessation rate of respondents (Hallgren and Witkiewitz, 2013; Nelson et al., 2009). ACT theory-based acceptance outcomes were compared using linear regression models and t-tests. Logistic regression was also used to examine whether the acceptance score at follow-up was associated with 30-day PPA at two-months. All regression models included as covariates any variables that differed between study arms at baseline or that predicted follow-up retention (p < 0.10). Statistical analyses were completed using R 2.15.3. (R Core Team, 2013).
3. RESULTS
3.1. Baseline balance and follow-up retention
Demographic characteristics, smoking behavior, and ACT theory-based measures were balanced between treatment groups at baseline, with the exception of race (Table 1). More participants identified themselves as Caucasian in the QuitGuide arm than in the ACT arm (94% vs. 85%, p = 0.06). The overall retention rate at two-month follow-up was 84% and did not differ between study groups (p = 0.56). Some baseline characteristics were predictive of two-month retention, including higher educational attainment, lower nicotine dependence score, light smoking, not smoking within five minutes of waking, and living with a partner who smokes (all p < 0.10).
Table 1.
Baseline characteristics and their prediction of outcome data retention of trial participants randomized to each arm.
| SmartQuit (n=98) | QuitGuide (n=98) | p-value (Baseline)1 | p-value2 (Retention) | |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean (SD) | 41.5 (12.0) | 41.6 (13.9) | 0.95 | 0.65 |
| Male | 47% | 49% | 0.89 | 0.89 |
| Caucasian | 85% | 94% | 0.06 | 0.33 |
| Hispanic | 4% | 3% | > 0.99 | 0.99 |
| Married | 37% | 46% | 0.25 | 0.93 |
| Working | 58% | 62% | 0.66 | 0.92 |
| HS or less education | 14% | 12% | 0.83 | 0.01 |
| Smoking Behavior | ||||
| Nicotine Dependence, mean (SD) | 4.9 (2.5) | 4.7 (2.4) | 0.50 | 0.03 |
| Smokes at least a pack per day | 28% | 21% | 0.41 | 0.02 |
| Smoked for 10 or more years | 72% | 77% | 0.62 | 0.71 |
| Quit attempts in past 12M, mean (SD) | 4.0 (5.4) | 3.0 (2.0) | 0.26 | > 0.99 |
| Friend & Partner Smoking | ||||
| Close friends who smoke, mean (SD) | 1.7 (1.5) | 1.8 (1.6) | 0.81 | 0.31 |
| Living with partner who smokes | 24% | 21% | 0.73 | 0.07 |
| ACT Theory-Based Measure, mean (SD) | ||||
| Acceptance of cravings score3 | 1.87 (0.36) | 1.94 (0.43) | 0.24 | 0.96 |
P-values compare baseline variables between the SmartQuit and QuitGuide arms. The p-values were generated from two-sample t-tests for continuous variables and Fisher's exact test for categorical variables.
P-values assess whether or not baseline characteristics were predictive of 2-month retention. The p-values were generated for each variable using univariate logistic regression models predicting an indicator of 2-month retention.
Response choices for each item ranged from “Not at all” (1) to “Very willing” (5). Scores were derived by averaging the items.
3.2. Participant utilization and satisfaction
As shown in Table 2, SmartQuit participants self-reported opening their assigned app, on average, 37 times compared to 15 times for QuitGuide (p = 0.0001). SmartQuit participants reported generally higher satisfaction: 85% reported their app was organized (vs. 67% for QuitGuide; p =.006), 53% reported it was useful for quitting (vs. 38% for QuitGuide; p =.10), and 59% were satisfied overall (vs. 45% for QuitGuide; p =.14).
Table 2.
Comparison of SmartQuit and QuitGuide at two-month follow-up.
| SmartQuit | QuitGuide | ||||
|---|---|---|---|---|---|
| n | Summary | n | Summary | p-value1 | |
| Utilization of assigned application, mean (SD) | |||||
| Times application opened | 69 | 37.2 (46.1) | 78 | 15.2 (13.6) | < 0.001 |
| Satisfaction with assigned application, % (n) | |||||
| Satisfied overall2 | 75 | 59% (44) | 80 | 45% (36) | 0.144 |
| Recommend to friend | 79 | 52% (41) | 84 | 37% (31) | 0.118 |
| Application was organized2 | 74 | 85% (63) | 79 | 67% (53) | 0.006 |
| Application useful for quitting2 | 78 | 53% (41) | 84 | 38% (32) | 0.103 |
| Cessation rate, % (n) | |||||
| All participants | 80 | 13% (10) | 84 | 8% (7) | 0.123 |
| Low acceptance3, at baseline | 48 | 15% (7) | 40 | 8% (3) | 0.211 |
| Heavy smokers4, at baseline | 18 | 11% (2) | 17 | 6% (1) | 0.692 |
| Not enrolled in another program5 | 79 | 13% (10) | 74 | 5% (4) | 0.140 |
Two-sided p values calculated from logistic regression models adjusted for race, education, nicotine dependence, smoking one or more packs per day, and living with a partner who smokes. Unadjusted two-sided p values were very similar.
Responses dichotomized as “Somewhat”, “Mostly”, or “Very Much” vs. “Not at all” or “A little”
Scoring below the median on acceptance of cravings, at baseline.
Smoking at least a pack a day (20 or more cigarettes), at baseline.
Excluding all 12 participants (all were in QuitGuide arm) who enrolled in another smoking cessation program during the trial.
3.3. Smoking cessation
SmartQuit had 62% to 88% descriptively higher rates of quitting, though there were wide 95% confidence intervals for the quit rate point estimates. Specifically, for all randomized participants (N = 196), the quit rates were 13% (95% CI: 6%-22%) in SmartQuit vs. 8% (95% CI: 3%-16%) in QuitGuide (OR = 2.7; 95% CI = 0.8-10.3). Among those at baseline scoring below the median on acceptance of cravings (n = 88), the quit rates were 15% (95% CI: 6%-28%) in SmartQuit vs. 8% (95% CI: 2%-20%) in QuitGuide (OR = 2.9; 95% CI = 0.6-20.7). Among those at baseline who were heavy smokers (i.e., at least a pack a day; n = 35), the quit rates were 11% (95% CI: 1%-33%) in SmartQuit vs. 6% (95% CI: 0%-30%) in QuitGuide (OR = 1.8; 95% CI = 0.1-53.3). Excluding all 12 participants who enrolled in another smoking cessation program during the trial (all 12 were in the QuitGuide arm), the quit rates were 13% (95% CI: 6%-22%) in SmartQuit vs. 5% (95% CI: 1%-13%) in QuitGuide (OR = 2.7; 95% CI = 0.8-10.0).
3.4. Acceptance of cravings
From baseline to the two-month follow-up, there was an increase in acceptance of cravings in the SmartQuit arm (p <.04), but not in the QuitGuide arm (p =.15). Higher acceptance of cravings was strongly associated with 30-day point prevalence abstinence at the two-month follow-up (OR = 6.1; 95% CI = 3.0-15.2).
4. DISCUSSION
This study addressed the dual needs for innovative theory-based intervention content and controlled trials to evaluate the efficacy of apps for smoking cessation. Accordingly, the aims were to determine (1) trial design feasibility, (2) participant receptivity and satisfaction, (3) preliminary cessation outcomes overall and for two key subgroups (heavy smokers and low acceptors of cravings), and (4) potential impact on theory-based acceptance of cravings to smoke. In general, the results supported all four aims.
4.1. Design feasibility
The enrollment sources and eligibility criteria provided assurances that study enrollment was feasible and timely. The outcome data collection protocol yielded a strong overall retention rate of 84%, much higher than the 54% typically obtained in comparable web-delivered cessation trials (Hutton et al., 2011; Shahab and McEwen, 2009).
4.2. Participant receptivity and satisfaction
SmartQuit participants opened their app 2.5 times more often than QuitGuide participants. This result, consistent with our trial of web-delivered ACT for smoking cessation (Bricker et al., 2013), suggests that SmartQuit content was more engaging than QuitGuide content. The comparatively higher level of satisfaction for SmartQuit than for QuitGuide is consistent with the satisfaction results in our face-to-face, telephone, and web-based ACT intervention trials (Bricker et al., 2013, 2014, 2010; Hernandez-Lopez et al., 2009). At the same time, we see room for improvement in user satisfaction. For example, we are currently conducting research showing which features of SmartQuit were most popular and which were most predictive of the two-month smoking cessation outcome (Heffner, in review). These results will inform design changes for the next version of SmartQuit. During this process, we will conduct additional iterations of user-centered design tests in order to improve the user experience, and thereby their satisfaction with the app.
4.3. Quit rates overall and among key subgroups
Tests of the encouraging quit rates are naturally underpowered because this was a pilot trial. Indeed, the 95% confidence intervals for the point estimates of quit rates were wide. But if proven definitive, the overall effect size would have high public health significance. For example, if the quit rates were 13% vs. 8%, then for every 20 smokers, SmartQuit would yield one more person who quits as compared to using QuitGuide [number needed to treat [NNT] = 20; (West, 2007)]. When scaled to a population level, the impact is high. For example, for every 1,000,000 smokers reached, an additional 50,000 adults would quit smoking by using SmartQuit as compared to using QuitGuide. The quit rate differences between the arms were more striking among the two subgroups we explored—descriptively about 80% to 90% higher quit rates. The effects among the low acceptance subgroup fit the ACT theoretical model, which suggests the treatment would be most beneficial for people who lack skills in accepting their cravings to smoke. The result for heavy smokers is also important because this group has very low quit rates and, thus, their morbidity and mortality rates are strikingly high (Fagan et al., 2004).
4.4. Impact on acceptance of cravings
The results on SmartQuit's baseline to follow-up changes in acceptance of cravings are important for several reasons. First, from an intervention design perspective, they suggest that the SmartQuit intervention protocol impacted its intended clinical process target. Second, the results comport with the ACT theoretical model of acceptance as it applies to smoking cessation, namely that acceptance of cravings is an underlying process of smoking cessation. Finally, the results are highly consistent with all of the previous studies of ACT for smoking cessation (Bricker et al., 2013, 2014, 2010; Gifford et al., 2004, 2011; Hernandez-Lopez et al., 2009).
4.5. Limitations
The study has important limitations. As a pilot randomized trial, the study's sample size was not powered to detect statistically significant differences in quit rates or to conduct formal mediation analysis of hypothesized treatment effects. Moreover, there is substantial relapse that naturally occurs after a two-month follow-up (Hollis et al., 2007; Stead et al., 2013) and therefore a longer term follow-up (e.g., twelve months) is recommended. Utilization data were self-reported because, for technical reasons, automatic recording of this information was not possible for QuitGuide. A future full-scale trial should use automatic recording of utilization data. Finally, we relied exclusively on self-reported abstinence in our estimate of 30-day point prevalence abstinence. However, expert consensus suggests that biochemical verification of abstinence is impractical and unnecessary in studies similar to the current one (Benowitz et al., 2002).
4.6. Future directions
The study results suggest four lines of future research: (1) provide a definitive test of the effectiveness for smoking cessation of smartphone-delivered SmartQuit compared with QuitGuide—an app that follows US Clinical Practice Guidelines; (2) demonstrate that the smoking cessation outcomes of SmartQuit, but not QuitGuide, are mediated by acceptance of internal cues to smoke (sensations, emotions, and thoughts), a psychological measure that is central to the theoretical model underlying ACT; (3) determine the comparative cost-effectiveness of SmartQuit versus QuitGuide; and (4) further explore the effectiveness for smoking cessation of SmartQuit versus QuitGuide among these baseline subgroups, including those who avoid cravings and heavy smokers.
4.7. Conclusion
ACT is feasible to deliver by smartphone app, acceptable to the majority of users, and shows promising quit rates compared to an app that follows US Clinical Practice Guidelines. As results were limited by the pilot design (e.g., small sample), a full-scale efficacy trial is now needed to further stimulate research on smartphone apps for smoking cessation.
Acknowledgements
We are thankful to our entire study staff—especially, Eric Meier, Carolyn Ehret, Jessica Harris, and Madelon Bolling. We are grateful for the programming expertise provided by 2Morrow Inc. We gratefully appreciate the participants for volunteering for this study.
Role of Funding Source. This study was funded by the Hartwell Innovation Fund of the Fred Hutchinson Cancer Research Center. Dr. Bricker's writing of this manuscript was partly supported by grants from the National Cancer Institute (#R01CA166646; #R01CA151251). Dr. Heffner's work on the project was supported by a grant from the National Institute on Drug Abuse (#K23DA026517). Dr. Vilardaga's contribution to this manuscript was supported by a training grant from the National Institute of Mental Health (T32MH082709). This paper does not necessarily express the views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors. Dr. Bricker conceived the study, led the entire conduct of the trial, contributed to the analysis planning, led the results interpretation, and was the primary manuscript writer. Ms. Mull led the analysis planning, conducted the analyses, and contributed to the manuscript writing. Dr. Vilardaga participated in the results interpretation and contributed to the manuscript writing. Dr. Kientz participated in the results interpretation and manuscript writing. Ms. Mercer participated in the analysis planning and interpretation of results. Ms. Akioka led the entire planning and management of the trial. Dr. Heffner participated in the trial conduct and contributed to the manuscript writing.
Author Disclosures Conflict of Interest. In 2011, Dr. Heffner served as a consultant for Pfizer. All of the authors declare that they have no other potential conflicts of interest. 2Morrow Inc. is developing SmartQuit under license from FHCRC, and all license proceeds will be used in support of the Fred Hutchinson Cancer Research Center and its research mission.
REFERENCES
- Abroms LC, Lee Westmaas J, Bontemps-Jones J, Ramani R, Mellerson J. A content analysis of popular smartphone apps for smoking cessation. Am. J. Prev. Med. 2013;45:732–736. doi: 10.1016/j.amepre.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abroms LC, Padmanabhan N, Thaweethai L, Phillips T. iPhone apps for smoking cessation: a content analysis. Am. J. Prev. Med. 2011;40:279–285. doi: 10.1016/j.amepre.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Jacob III P, Ahijevych K, Jarvis MJ, Hall S, LeHouezec J, Hansson A, Lichtenstein E, Henningfield J, Tsoh J, Hurt RD, Velicer W, (SRNT Subcommittee on Biochemical Verification) Biochemical verification of tobacco use and cessation. Nicotine Tob. Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Bricker J, Wyszynski C, Comstock B, Heffner JL. Pilot randomized controlled trial of web-based acceptance and commitment therapy for smoking cessation. Nicotine Tob. Res. 2013;15:1756–1764. doi: 10.1093/ntr/ntt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker JB, Bush T, Zbikowski SM, Mercer LD, Heffner JL. Randomized trial of telephone-delivered acceptance and commitment therapy versus cognitive behavioral therapy for smoking cessation: a pilot study. Nicotine Tob. Res. 2014 doi: 10.1093/ntr/ntu102. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker JB, Mann SL, Marek PM, Liu J, Peterson AV. Telephone-delivered acceptance and commitment therapy for adult smoking cessation: a feasibility study. Nicotine Tob. Res. 2010;12:454–458. doi: 10.1093/ntr/ntq002. [DOI] [PubMed] [Google Scholar]
- Buller DB, Borland R, Bettinghaus EP, Shane JH, Zimmerman DE. Randomized trial of a smartphone mobile application compared to text messaging to support smoking cessation. Telemed. J. E. Health. 2013;20:206–214. doi: 10.1089/tmj.2013.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. CDC; Atlanta: 2014. [Google Scholar]
- Chen YF, Madan J, Welton N, Yahaya I, Aveyard P, Bauld L, Wang D, Fry-Smith A, Munafo MR. Effectiveness and cost-effectiveness of computer and other electronic aids for smoking cessation: a systematic review and network meta-analysis. Health Technol. Assess. 2012;16:1–205. iii–v. doi: 10.3310/hta16380. [DOI] [PubMed] [Google Scholar]
- Civljak M, Sheikh A, Stead LF, Car J. Internet-based interventions for smoking cessation. Cochrane Database Syst. Rev. 2010:CD007078. doi: 10.1002/14651858.CD007078.pub3. [DOI] [PubMed] [Google Scholar]
- Consortium NAQ. Results From The 2012 NAQC Annual Survey Of Quitlines. North American Quitline Consortium; 2013. 2013. http://c.ymcdn.com/sites/www.naquitline.org/resource/resmgr/2012_annual_survey/oct23naqc_2012_final_report_.pdf. [Google Scholar]
- R Core Team . R: A language and environment for statistical computing. Foundation for Statistical Computing. Vienna: 2013. [Google Scholar]
- Dediu H. When Will The US Reach Smartphone Saturation? 2013 http://www.asymco.com/2013/10/07/when-will-the-us-reach-smartphone-saturation/
- Etter JF. A list of the most popular smoking cessation web sites and a comparison of their quality. Nicotine Tob. Res. Suppl. 2006;1:S27–34. doi: 10.1080/14622200601039923. [DOI] [PubMed] [Google Scholar]
- Fagan P, King G, Lawrence D, Petrucci SA, Robinson RG, Banks D, Marable S, Grana R. Eliminating tobacco-related health disparities: directions for future research. Am. J. Public Health. 2004;94:211–217. doi: 10.2105/ajph.94.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF, Froelicher ES, Goldstein MG, Healton CG, Lando HA, Mecklenburg RE, et al. Treating Tobacco Use And Dependence: 2008 Update., Clinical Practice Guideline. U.S. Department of Health and Human Services, Public Health Service; Rockville, MD: 2008. [Google Scholar]
- Gifford EV, Kohlenberg BS, Hayes SC, Antonuccio DO, Piasecki MM, Rasmussen-Hall ML, et al. Acceptance-based treatment for smoking cessation. Behav. Ther. 2004;35:689–705. [Google Scholar]
- Gifford EV, Kohlenberg BS, Hayes SC, Pierson HM, Piasecki MP, Antonuccio DO, Palm KM. Does acceptance and relationship focused behavior therapy contribute to bupropion outcomes? A randomized controlled trial of functional analytic psychotherapy and acceptance and commitment therapy for smoking cessation. Behav. Ther. 2011;42:700–715. doi: 10.1016/j.beth.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Hallgren KA, Witkiewitz K. Missing data in alcohol clinical trials: a comparison of methods. Alcohol. Clin. Exp. Res. 2013;37:2152–2160. doi: 10.1111/acer.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SC, Levin ME, Plumb-Vilardaga J, Villatte JL, Pistorello J. Acceptance and commitment therapy and contextual behavioral science: examining the progress of a distinctive model of behavioral and cognitive therapy. Behav. Ther. 2013;44:180–198. doi: 10.1016/j.beth.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SC, Luoma JB, Bond FW, Masuda A, Lillis J. Acceptance and commitment therapy: model, processes and outcomes. Behav. Res. Ther. 2006;44:1–25. doi: 10.1016/j.brat.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br. J. Addict. 1989;84:791–799. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- Heffner JL, Mull K, Kientz JL, Vilardaga R, Bricker JB. What is popular is not always right: feature-level analysis of the first Acceptance and Commitment Therapy smartphone application for smoking cessation. (in review)
- Heffner JL, Wyszynski CM, Comstock B, Mercer LD, Bricker J. Overcoming recruitment challenges of web-based interventions for tobacco use: the case of web-based acceptance and commitment therapy for smoking cessation. Addict. Behav. 2013;38:2473–2476. doi: 10.1016/j.addbeh.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Lopez M, Luciano MC, Bricker JB, Roales-Nieto JG, Montesinos F. Acceptance and Commitment Therapy for smoking cessation: a preliminary study of its effectiveness in comparison with cognitive behavioral therapy. Psychol. Addict. Behav. 2009;23:723–730. doi: 10.1037/a0017632. [DOI] [PubMed] [Google Scholar]
- Hollis JF, McAfee TA, Fellows JL, Zbikowski SM, Stark M, Riedlinger K. The effectiveness and cost effectiveness of telephone counselling and the nicotine patch in a state tobacco quitline. Tob. Control 16 Suppl. 2007;1:i53–59. doi: 10.1136/tc.2006.019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton HE, Wilson LM, Apelberg BJ, Tang EA, Odelola O, Bass EB, Chander G. A systematic review of randomized controlled trials: web-based interventions for smoking cessation among adolescents, college students, and adults. Nicotine Tob. Res. 2011;13:227–238. doi: 10.1093/ntr/ntq252. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Lachance HR, Strong DR, Ramsey SE, Monti PM, Brown RA. The commitment to quitting smoking scale: initial validation in a smoking cessation trial for heavy social drinkers. Addict. Behav. 2007;32:2420–2424. doi: 10.1016/j.addbeh.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leischow SJ, Provan K, Beagles J, Bonito J, Ruppel E, Moor G, Saul J. Mapping tobacco quitlines in North America: signaling pathways to improve treatment. Am. J. Public Health. 2012;102:2123–2128. doi: 10.2105/AJPH.2011.300529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz RF, Lenert LL, Delucchi K, Stoddard J, Perez JE, Penilla C, Perez-Stable EJ. Toward evidence-based Internet interventions: a Spanish/English Web site for international smoking cessation trials. Nicotine Tob. Res. 2006;8:77–87. doi: 10.1080/14622200500431940. [DOI] [PubMed] [Google Scholar]
- Nelson DB, Partin MR, Fu SS, Joseph AM, An LC. Why assigning ongoing tobacco use is not necessarily a conservative approach to handling missing tobacco cessation outcomes. Nicotine Tob. Res. 2009;11:77–83. doi: 10.1093/ntr/ntn013. [DOI] [PubMed] [Google Scholar]
- Nielsen Company Smartphone Switch: Three-Fourths Of Recent Acquirers Chose Smartphones. 2013 http://www.nielsen.com/us/en/insights/news/2013/smartphone-switch--three-fourths-of-recent-acquirers-chose-smart.html.
- Shahab L, McEwen A. Online support for smoking cessation: a systematic review of the literature. Addiction. 2009;104:1792–1804. doi: 10.1111/j.1360-0443.2009.02710.x. [DOI] [PubMed] [Google Scholar]
- Smith A. Smartphone Ownership--2013 Update. 2013 http://www.pewinternet.org/2013/06/05/smartphone-ownership-2013/
- Statista Smartphone Penetetration In The United States. 2014 http://www.statista.com/statistics/201183/forecast-of-smartphone-penetration-in-the-us/
- Stead LF, Hartmann-Boyce J, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database Syst. Rev. 2013;8:CD002850. doi: 10.1002/14651858.CD002850.pub3. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health, E., and Welfare . Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service. In: U.S. Department of Health, E., and Welfare, Public Health Service, Center for Disease Control, editor. Washington, DC.: 1964. [Google Scholar]
- Webb TL. Commentary on Shahab McEwen (2009): understanding and preventing attrition in online smoking cessation interventions: a self-regulatory perspective. Addiction. 2009;104:1805–1806. doi: 10.1111/j.1360-0443.2009.02751.x. [DOI] [PubMed] [Google Scholar]
- West R. The clinical significance of “small” effects of smoking cessation treatments. Addiction. 2007;102:506–509. doi: 10.1111/j.1360-0443.2007.01750.x. [DOI] [PubMed] [Google Scholar]
- Whittaker R, McRobbie H, Bullen C, Borland R, Rodgers A, Gu Y. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst. Rev. 2012;11:CD006611. doi: 10.1002/14651858.CD006611.pub3. [DOI] [PubMed] [Google Scholar]