Abstract
Direct communication of neighboring cells by gap junction channels is essential for the development of tissues and organs in the body. Whereas vertebrate gap junctions are composed of members of the connexin family of transmembrane proteins, in invertebrates gap junctions consist of Innexin channel proteins. Innexins display very low sequence homology to connexins. In addition, very little is known about their cellular role during developmental processes. In this report, we examined the function and the distribution of Drosophila Innexin 2 protein in embryonic epithelia. Both loss-of-function and gain-of-function innexin 2 mutants display severe developmental defects due to cell death and a failure of proper epithelial morphogenesis. Furthermore, immunohistochemical analyses using antibodies against the Innexins 1 and 2 indicate that the distribution of Innexin gap junction proteins to specific membrane domains is regulated by tissue specific factors. Finally, biochemical interaction studies together with genetic loss- and gain-of-function experiments provide evidence that Innexin 2 interacts with core proteins of adherens and septate junctions. This is the first study, to our knowledge, of cellular distribution and protein–protein interactions of an Innexin gap junctional channel protein in the developing epithelia of Drosophila.
INTRODUCTION
In both vertebrates and invertebrates, the direct communication of cells is mediated by gap junctions, which consist of clusters of intercellular aqueous channels that allow the exchange of ions and small molecules between the cytoplasm of closely apposed cells (Goodenough et al., 1996; Kumar and Gilula, 1996). Single gap junction channels consist of two hemi-channel subunits, with one hemi-channel contributed by each participating cell. In vertebrates, each hemi-channel represents a hexameric protein complex, which is composed of members of the connexin family of transmembrane proteins (Willecke et al., 1991, 2002). Most mammalian cells express more than one connexin isoform and assemble homomeric or heteromeric hemi-channels (connexons), leading to the formation of homotypic or heterotypic gap junctions (Jiang and Goodenough, 1996; Diez et al., 1999; He et al., 1999; Locke et al., 2000, Cottrell et al., 2002). Consistent with a role in inter- and intracellular signaling processes, the connexins 32, 36, 43, and 46 are targeted to lipid rafts, which are cholesterol–sphingolipid-rich microdomains that function as membrane trafficking and signal transduction platforms (Schubert et al., 2002). Electron microscopy has revealed that gap junction channels, which are formed by head-to-head-docking of hemi-channels from neighboring cells, are arranged into tightly packed plaques that may consist of hundreds or thousands of channels; forming structures of several microns in diameter (Goodenough et al., 1996; Lauf et al., 2002). Studies in tissue culture have shown that connexin assembly and plaque formation is enabled by calcium-dependent core components of adherens junctions, the E-cadherins (Musil et al., 1990; Jongen et al., 1991; Meyer et al., 1992; Fujimoto et al., 1997; Xu et al., 2001; for review, see Yeager et al., 1998).
Connexin homologues have not been identified in invertebrates (Adams et al., 2000). Instead, a separate protein family, called Innexins, which are structurally and functionally analogous to connexins, were shown to be the key components of gap junction channels (for review, see Phelan and Starich, 2001). The analysis of the few innexin mutants have been as yet identified in Drosophila and Caenorhabditis elegans has demonstrated an essential role for these gap junctional channel proteins in electrical signal transmission. Mutations in the C. elegans unc-7 and unc-9 genes result in loss-of-coordination phenotypes (Starich et al., 1996; Barnes and Hekimi, 1997). In addition, eat-5 mutants display feeding defects that result from impaired of the electrical coupling between pharynx muscles (Starich et al., 1996). Similarly, mutations in the Drosophila shaking-B and ogre genes interfere with transmission at electrical synapses of the giant fiber system (Phelan et al., 1996, 1998) and reduce optical ganglia size (Watanabe and Krankel, 1990), respectively. The germline-specific Drosophila innexin 4 gene was recently shown to play a role in the survival of early germ cells during gametogenesis (Tazuke et al., 2002). We have previously shown that innexin 2 is required to control foregut morphogenesis in response to the Wingless/WNT signaling cascade (Bauer et al., 2002). Whereas the mRNA expression patterns of the Drosophila innexin genes have been extensively studied by in situ hybridization during oogenesis and embryogenesis, virtually no studies exist on protein distribution, trafficking, assembly, turnover, or interactions with other cellular proteins. Here, we present the first study of gap junction protein distribution in Drosophila epithelial cells. We demonstrate an essential function for Innexin 2 for epithelial morphogenesis, and we provide evidence of interaction between Innexin 2 and other junctional core proteins in polarized epithelial cells.
MATERIALS AND METHODS
Cloning and Transgene Production
An innexin 2 fragment corresponding to the full-length innexin 2cDNA was subcloned into the pP{UAST} transformation vector (Brand and Perrimon, 1993) to generate the P{UAS-innexin 2} transgene. To generate a inx 2-GFP fusion construct a polymerase chain reaction (PCR) fragment coding for full-length innexin 2 was cloned via EcoRI/BamHI in frame with the eGFP coding sequence of the pMJ Green vector (gift from K. Willecke, Universitat Bonn, Bonn, Germany). The innexin 2-GFP fusion fragment was excised using EcoRI/NotI and inserted in the pP{UAST} to get P{UAS-innexin 2-GFP} transgenic flies. The nucleotide sequence of both constructs was confirmed by sequencing. The pP{UAS-innexin 2} and pP{UAS-innexin 2-GFP} constructs were introduced into w- flies by P element-mediated transformation (Rubin and Spradling, 1982).
Genetic Manipulation of Flies
We used standard techniques for fly manipulation. The P allele kropf P16 that was used in this study is a transcript-null allele (Bauer et al., 2002). The amorphic alleles armYP35 and shg2 were a gift from E. Knust. cor 5 was a gift from A. Fehon, Duke University, Durham, NC. Homozygous mutant kropfP16 embryos were identified by the absence of green fluorescent protein (GFP) expression derived from the FM7, GAL4-Kr::UAS-GFP balancer. High levels of ectopic Innexin 2 were induced by crossing the UAS-innexin 2 lines with the ubiquitously driving maternal V32-Gal4 strain (gift from D. St. Johnston) or the paired-Gal4 effector strain (Xiao et al., 1996) that mediates expression in a pair-rule pattern in blastoderm embryos (Xiao et al., 1996). UAS-p35 was obtained from the Bloomington Stock Center, Indiana University, Bloomington, IN. To express p35 together with innexin 2, w; UAS-innexin 2/+; UAS-p35/+ males were crossed to w; V32-GAL4 virgin females. The effect of overexpression was analyzed in the progeny by performing anti-Innexin 2 staining and terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay. Germ line clones of kropf P16 mutants were induced as described by Chou and Perrimon (1996) by using BL#1844 as FRT line and BL#1813 as ovoD1 line (stocks were obtained from the Bloomington Stock Center).
Immunohistochemistry
For antibody staining, embryos were fixed in 4% formaldehyde, 100 mM phosphate buffer. Otherwise, antibody staining was performed as described previously (Bauer et al., 2002). Primary antibodies were as follows: anti-Innexin 1 (rabbit, 1:50), anti-Innexin 2 (rabbit, 1:50), anti-Coracle (guinea pig, 1:1000; a gift from R. Fehon), anti-Armadillo (1:10; Hybridoma Bank), anti-Crumbs (1:10; Developmental Studies Hybridoma Bank, Iowa City, IA), anti-DE-cadherin (1:50; Santa Cruz Biotechnology, Santa Cruz, CA), and anti-GFP (1:50; Santa Cruz Biotechnology). Secondary antibodies were as follows: Alexa Fluor 488 (1:200; MoBiTec, Goettingen, Germany), Alexa Fluor 546 (1:400; MoBiTec), Cy3 (1:200, mouse; Dianova, Hamburg, Germany), Cy2 (1:100, mouse; Dianova), Cy3 (1:200, rabbit; Dianova), and Cy5 (1:100, mouse; Dianova). Fluorescent images were recorded using a Leica TSP2 confocal microscope (Leica, Wetzlar, Germany), and images of multilabeled samples were acquired sequentially on separate channels. All images were processed with Adobe Photoshop software.
Antibody Generation
Polyclonal antisera were generated in rabbits (Davids Biotechnology, Regensburg, Germany) against Innexin 1– and Innexin 2–specific oligopeptides conjugated to KLH via a N-terminal cysteine. The peptides used were FAKQVEPSKHDRAK representing amino acids 348–362 of Innexin 1 and KLRHLLLRARSRLA representing amino acids 297–310 of Innexin 2. IgGs were purified from immune serum by affinity purification.
TUNEL Staining
Fixed embryos were subjected to TUNEL assay by using the in situ cell death detection kit (TM Red; Roche Diagnostics, Mannheim, Germany). The assay was performed as described previously (Booth et al., 2000).
In Vitro Translation, Immunoprecipitation, and Immunoblotting
Full-length Armadillo and epitope-tagged Innexin 2 fusion protein (6 copies of myc epitope recognized by c-Myc 9E10 mAb) were independently transcribed and translated in the presence of [35S]methionine at 30°C for 2 h in a 50-μl mixture by using a TNTR-coupled rabbit reticulocyte lysate (Promega, Madison, WI) according the manufacturer's instructions. To analyze the interaction of Armadillo and Innexin 2, the in vitro-translated reactions were mixed and immunoprecipitated. Immunoprecipitation was performed using the immunoprecipitation starter pack (Amersham Biosciences, Piscataway, NJ) according to the manufacturer's instruction. To prevent nonspecific binding of proteins to protein G-Sepharose, preclearing of the in vitro lysate was performed in the presence of protein G-Sepharose according the manufacturer's instructions. Subsequently, antibody incubation and precipitation of the immune complexes from precleared in vitro lysate was done in phosphate-buffered saline (PBS) containing 5% bovine serum albumin in a final volume of 400 μl. The immunoprecipitation samples were incubated for 2 h either with anti-Arm monoclonal antibody (N-27 A1), or with mouse monoclonal anti-c-Myc 9E10 (c-Myc 9E10; Santa Cruz Biotechnology). Protein G-Sepharose (40 μl) was added to each mixture and incubated for 90 min. Beads were washed five times with excess PBS and two times with wash buffer (50 mM Tris, pH 8), resuspended in SDS-PAGE sample buffer, and boiled for 3 min. Samples were run on SDS-gel. The gel was dried and analyzed by autoradiography. To perform coimmunoprecipitation analysis using embryonic extracts, Drosophila embryos (0–24 h) were collected, rinsed with water, dechorionated with 50% bleach for 3 min, and rinsed with 0.1% Triton X-100. For the following immunoprecipitation, the Drosophila embryos were homogenized either in radioimmunoprecipitation (RIPA) buffer (150 mM NaCl, 1% IGEPAL CA-630, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris [pH 8.0]) or immunoprecipitation (IP) buffer (CHAPS-buffer; Laing et al., 2001) containing PBS, 1% Triton X-100, 0.5% CHAPS, 0.1% SDS, and protease inhibitors (Roche Diagnostics). Cleared supernatant was incubated for 4 h with anti-Innexin 2 antibody, preimmune serum, and control IgG or with the control anti-c-Myc antibody followed by the addition of Sepharose beads as described above. The beads were washed three times with excess IP buffer (RIPA- or CHAPS-buffer) and three times with excess PBS. Immunoblots were blocked with nonfat dry milk in Tris-buffered saline plus 0.05% Tween. Washes were done in Tris-buffered saline plus 0.05% Tween without milk. Bound antigen was detected by enhanced chemiluminescence (Amersham Biosciences). The antibodies for immunoblotting were used at the following concentrations: anti-Armadillo (1:300; Hybridoma Bank), anti-DE-cadherin (1: 300l; Santa Cruz Biotechnology), and anti-Innexin 2 (1:300).
Yeast Two-Hybrid Analysis and Plasmid Construction
For the construction of the bait vector, the full-length cytoplasmic loop sequence of Innexin 2 (DNA fragment corresponding to amino acids 131–179) was cloned into the CytoTrap bait vector pSOS (Stratagene, LA Jolla, CA). An innexin 2 PCR product was generated using the LD 11658 clone as template together with the following primers: 5′-ggg atc ccg aag tcc tgg gaa ggc gga-3′ (5′ primer), and 5′-cga gct cgt cgg aag gcg tag aaa tt-3′ (3′ primer). Subsequently, the Innexin 2 PCR amplification product was cloned in frame with hSOS into the bait vector via the 5′-BamHI and 3′-SacI restriction sites.
The CytoTrap Screen was performed according the CytoTrap manual (Stratagene) instructions. After the testing of the Innexin 2CL/hSOS fusion protein for autoactivation, 100 μg of an embryonic Drosophila melanogaster cDNA library (catalog no. 200444; Stratagene) together with 100 μg of the bait construct were transformed into the yeast Saccharomyces cerevisiae temperature-sensitive mutant strain cdc25H. This strain contains a point mutation at amino acid residue 1328 of the CDC25-gene, which prevents growth at 37°C, but allows normal growth at a permissive temperature (25°C). The CytoTrap system is based on the ability of the hSos, to complement the cdc25 defect and to activate the yeast Ras signaling pathway (for details, see Broder et al., 1998). The localization of hSos to the plasma membrane occurs through the interaction with the target proteins, which are localized to the plasma membrane by a fused myristylation sequence. After first screening procedures and incubation at 37°C for 5 d, 17 potential interacting target clones were isolated. Five of them were confirmed in a rescreen, among them a clone coding for the intracellular part of the adhesion protein DE-cadherin (aa 1350–1570). Target clone 2 was tested for lack of autoactivation by retransformation of clone 2 together with the pMyr Vector into the cdc25H strain.
RESULTS
A Specific Innexin 2 Antibody
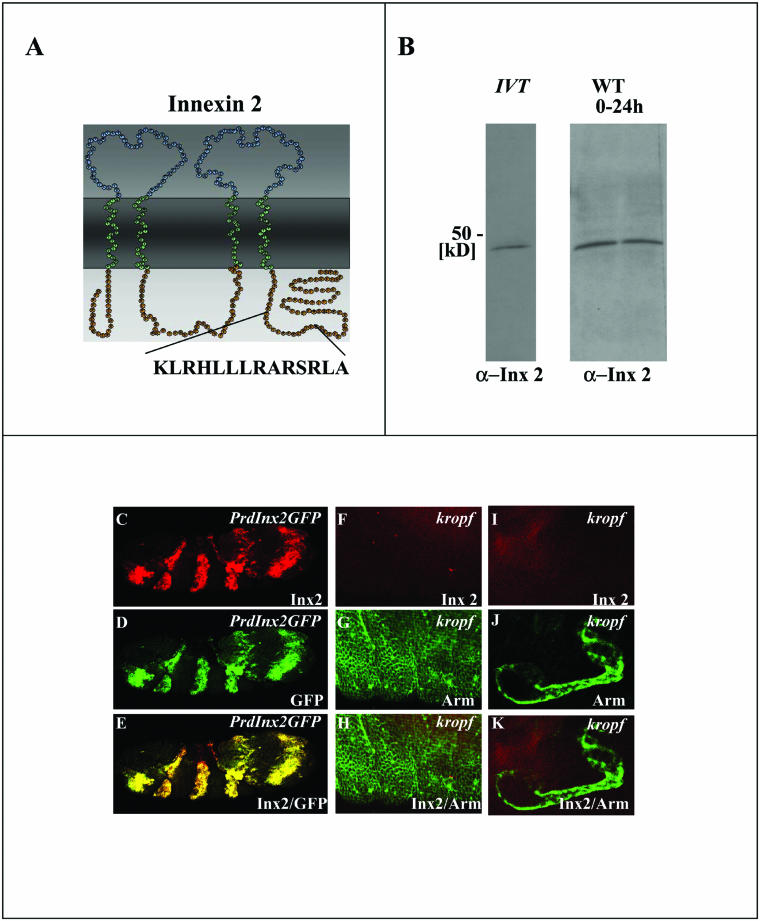
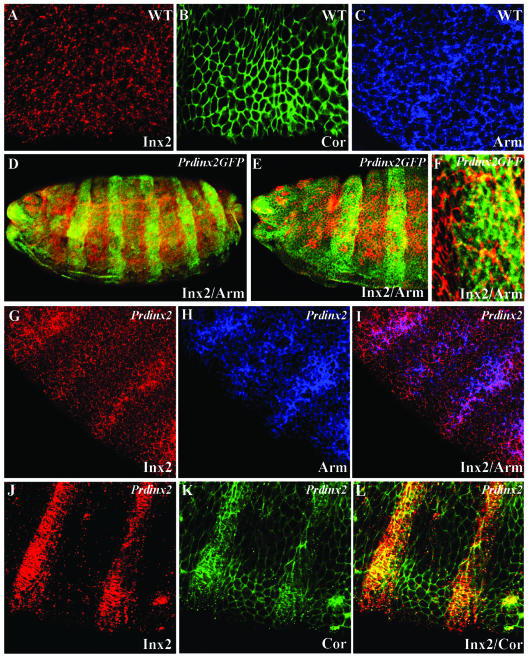
As a first step toward a functional analysis of Innexin gap junction proteins in the development of tissues and organs in the Drosophila embryo, we generated an anti-Innexin 2 antibody against a peptide containing the C-terminal amino acids 297–310 of Innexin 2 (see MATERIALS AND METHODS; Figure 1A). This antibody recognized the 43-kDa protein band of in vitro-translated Innexin 2 protein and a band of the same size in embryonic extracts of wild-type embryos (Figure 1B). To test the specificity of the antibody, we overexpressed an Innexin 2-GFP construct in a pair rule pattern in every other segment of the epidermis by using paired-Gal4/UAS-innexin 2-GFP transgenic flies and performed immunostaining by using the Innexin 2 antibody. As shown in Figure 1, C–E, the antibody detects the stripe pattern in the ectopic expression experiment. In contrast, anti-Innexin 2 antibody staining was not detected in the epidermis (Figure 1, F–H) nor in other epithelial organs such as the salivary glands (Figure 1, I–K) of late zygotic kropf mutants, whereas other proteins such as the β-catenin homolog Armadillo are expressed. Consistent with these in vivo results, protein extracts of zygotic kropf mutants, which are innexin 2 transcript null (Bauer et al., 2002), reveal a successive decay of maternal Innexin 2 protein from stage 12 onwards. Finally, no protein could be detected in late zygotic mutants (our unpublished data), further demonstrating the specificity of the antibody.
Figure 1.
Innexin 2 antibody production. (A) Predicted structure of Innexin 2 with four transmembrane domains, two extracellular loops, an intracellular cytoplasmic loop, and the intracellular N- and C-terminal domains. The amino acid sequence and the localization of the Innexin 2 peptide used for antibody production is indicated. (B) Western blot using anti-Innexin 2 antibody; in vitro translated (IVT) Innexin 2 and embryonic protein extract of 0- to 24-h wild-type embryos. (C–E) Expression of UAS-innexin 2-GFP by using a paired-Gal4 driver line. Embryos were stained with anti-Innexin 2 in red and anti-GFP in green. The staining pattern is completely overlapping further confirming the specificity of the Innexin 2 antibody. (F–K) Staining of a stage 16 kropf P16 mutant embryo by using anti-Innexin 2 (red) and anti-Armadillo (green). Confocal images in F–H and I–K depict epidermal and salivary gland tissue, respectively.
Dynamics of Innexin 2 Protein Localization in Epithelial Cells of Developing Embryos
Previous studies have shown that in the early embryonic stages when the segmentation pattern is determined, innexin 2 mRNA is ubiquitously distributed in the embryo (Curtin et al., 1999; Stebbings et al., 2000; Bauer et al., 2002; Stebbings et al., 2002). With the beginning of gastrulation, innexin 2 transcripts become refined to a segmental pattern. In contrast, Innexin 2 was distributed rather ubiquitously during germband extension stage in epidermal epithelial cells, most likely due to the stability of maternally deposited protein compared with the innexin 2 RNA. Using confocal microscopy, we were able to determine that Innexin 2 is found in a punctate pattern in the plasma membrane and to some extent also in the cytoplasm of the epithelial cells (Figure 2, C and F). Notably, this kind of protein distribution is characteristic of the vertebrate connexin gap junction proteins; they oligomerize to hexameric units that traffic in vesicles through the cytoplasm to the plasma membrane where they integrate and display mobility as hemi-channels (for reviews, see Evans and Martin, 2002a; Goodenough and Paul, 2003).
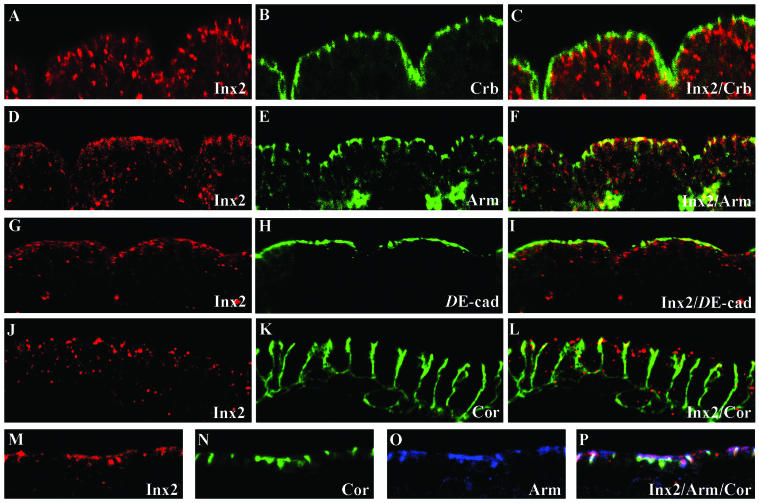
Figure 2.
Innexin 2 localization in the epidermis of Drosophila wild-type embryos. (A–C) Coimmunostaining of Innexin 2 (red) and Crumbs (green) in a stage 14 wild-type embryo. (D–F) Double staining of a stage 14 embryo monitoring expression of Innexin 2 (red) and Armadillo (green). Note the overlap of Armadillo with Innexin 2. (G–I) Innexin 2 (red) and DE-cadherin (green) double staining of a stage 14 embryo. Note the overlap of DE-cadherin with Innexin 2. (J–L) Coimmunostaining of Innexin 2 (red) and Coracle (green) in a stage 14 wild-type embryo. Shown is a magnification of the epidermal cells as compared with the perspective in F and C to better visualize the cellular distribution of Innexin 2 compared with the Coracle domain (see text for further details). (M–P) Triple staining of an early stage 15 embryo by using anti-Innexin 2 (red), anti-Coracle (green), and anti-Armadillo (blue) antibodies. Note the distribution of Innexin 2 apical to the Coracle domain, but overlapping with Armadillo in the epidermal cells.
To more precisely determine the localization of Innexin 2 channel proteins along the apico-basal axis of the epithelial cells of the embryo, we performed coimmunostaining with membrane and cell polarity markers. Anti-Crumbs antibody (Figure 2, A–C) was used to visualize apical membrane localization; anti-Armadillo (Figure 2, D–F) and anti-DE-cadherin antibodies (Figure 2, G–I) were used to mark the adherens junctions on the apico-lateral side of epithelial cells. The DE-cadherin transmembrane protein and the cytoplasmic protein Armadillo, which encodes the Drosophila homolog of β-catenin, are structural components of adherens junctions that mediate cell-cell adhesion and communication (Peifer, 1993; Cox et al., 1996, 1999; Pai et al., 1996; Peifer and Polakis 2000). Anti-Coracle antibody (Figure 2, J–L) was used to label the septate junctions on the lateral region of epithelial cells (Tepass and Hartenstein, 1994; for review, see Tepass et al., 2001). Coracle is a septate junction-associated protein and a member of the Protein 4.1 super-family of cytoplasmic proteins (Fehon et al., 1994) that includes Protein 4.1, and the Ezrin, Radixin, and Moesin proteins (Takeuchi et al., 1994).
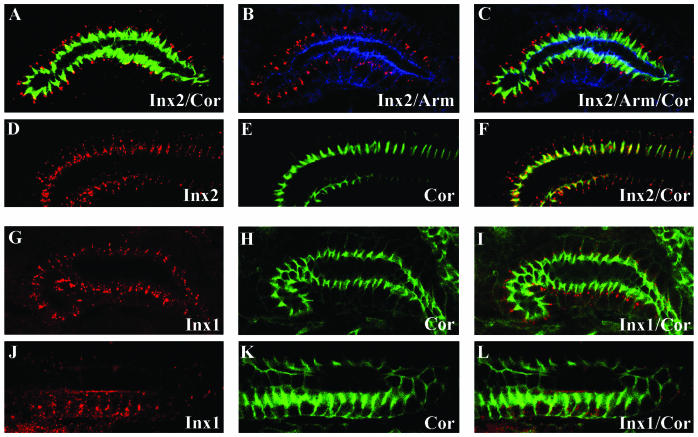
Investigating the distribution of Innexin 2 proteins along the apico-basal axis of epidermal cells revealed an accumulation of Innexin 2 signals in the apico-lateral membrane domain. The expression domain was apical to that of Coracle (Figure 2, J–L), but colocalized with Armadillo and DE-cadherin (Figure 2, D–F, G–I; triple staining in M–P). It is of note, however, that we also found Innexin 2 signals in lateral and baso-lateral positions, which may reflect the mobility of the protein in the plasma membrane as suggested for vertebrate connexin hemi-channels (Figure 2C; F; Lauf et al., 2002). In contrast to the apico-lateral accumulation of Innexin 2 in the epidermis, in salivary gland cells the protein accumulated in the baso-lateral region, below the septate junctions as shown by anti-Innexin 2/anti-Coracle/anti-Armadillo/triple staining (Figure 3, A–C). Finally, in hindgut epithelial cells we found an accumulation of Innexin 2 in the Coracle expression domain in the lateral membrane region of the cells (Figure 3, D–F). In contrast to the variable tissue/organ positioning of Innexin 2 along the apico-basal axis of epithelial cells, we found an accumulation of Innexin 1 in the baso-lateral region for both the salivary gland and the hind-gut (Figure 3, G–I and J–L, respectively).
Figure 3.
Cellular distribution of Innexin 1 and 2 in Drosophila wild-type embryos. Innexin 2 (red, A–F) and Innexin 1 (red, G–L) distribution. Confocal images of stage 16 salivary glands (sg) (A–C and G–I) and of stage 16 hindgut (hg) epithelia (D–F and J–L). Anti-Coracle (green) was used as a marker for septate junctions and anti-Armadillo (blue) for adherens junctions. For further information, see the text.
Innexin 2 Is Required for Epithelial Morphogenesis
To investigate whether Innexin 2 plays an essential role during epithelial development, we analyzed the phenotype of kropf mutants. As shown previously, kropf is required for zygotic viability because approximately one-third of the homozygous kropf mutant animals die during the early larval stages due to a malformation of the proventriculus organ (Bauer et al., 2002). The other two-thirds of the kropf mutants are embryonic lethal. Cuticle preparations demonstrated that these embryos often displayed large holes in the epidermis, predominantly in the head region with occasional segment defects accompanied by a reduction in the size of the embryos (Bauer et al., 2002; our unpublished data). Monitoring the expression of the segment polarity gene engrailed in stage 11 wild-type and kropf mutant embryos reveals that the formation of epidermal segments seems to be initiated normally in a large fraction of the mutant embryos (Figure 4, A–D). However, we observed strong morphogenetic defects during germband retraction and at later stages of embryonic development. In extreme cases, we found an almost complete loss of the foregut epithelium and a strong reduction of the hindgut and Malpighian tubule tissues as revealed by anti-Crumbs antibody staining (Figure 4, E–H).
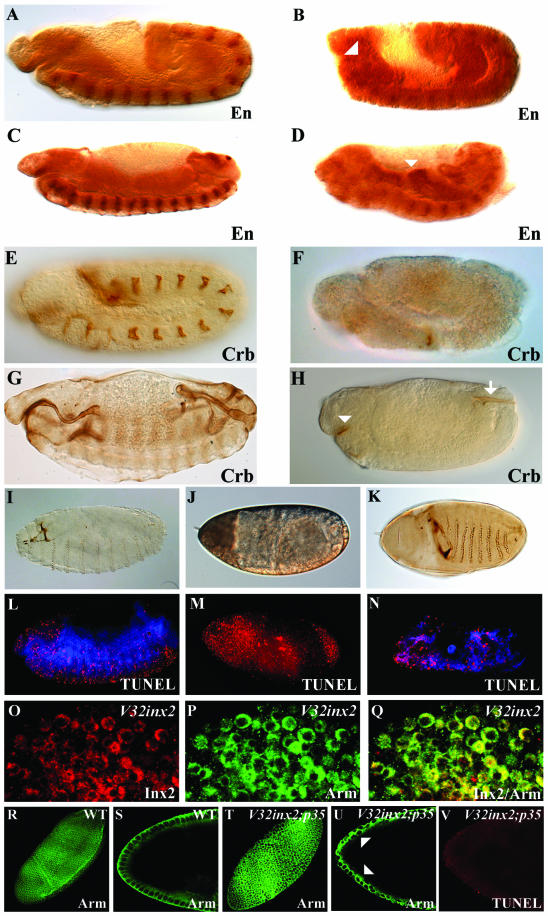
Figure 4.
Epithelial defects and cell death in kropf mutants. Anti-Engrailed antibody staining of stage 10 (A and B) and stage 13 (C and D) wild-type (A and C) and kropf P16 mutant embryos (B and D). The segmental pattern of engrailed is not dramatically altered; however, note the prominent foregut (B, arrowhead) and the midgut defects (D, arrowhead). kropf P16 mutant embryos are much smaller than wild-type embryos. Anti-Crumbs antibody staining of stage 10 (E and F) and stage 13 (G and H) wild-type (E and G) and kropf P16 mutant embryos (F and H). Note that many epithelial tissues that invaginate during embryonic development, including the foregut (arrowhead), the hindgut (arrow), the Malpighian tubules, the salivary glands, and the tracheal system, are strongly reduced in size (H compared with G). Cuticle preparation and TUNEL staining of a wild-type embryo (I and L), a maternal and zygotic kropf null mutant (J and M), a maternal null, zygotic heterozygous kropf mutant (K and N). Armadillo (blue) was used in L and N to outline the embryos' morphology. For further details, see text. (O–Q) Ubiquitous expression of innexin 2 by using the maternal V32Gal4 driver and the UAS-innexin 2 effector strains. Innexin 2 in red and Armadillo in green. (R–V) Comparison of wild-type embryos and embryos expressing ubiquitously innexin 2 and p35 by applying the maternal V32Gal4 driver together with the UAS-p35 and the UAS-innexin 2 effector lines. (R–U) Confocal images showing immunostaining of Armadillo (green) in wild-type stage 7 embryos (R and S) and stage 7 embryos overexpressing innexin 2 and p35 (T and U). Note the multilayering and an irregular cell shape (U, arrowheads; compare with S). For further information, see text. (V) TUNEL assay of the same embryo as described in U. Note that apoptosis is absent indicating p35 activity.
The variability of the phenotype and the mRNA expression pattern during oogenesis suggested a maternal contribution of innexin 2. We therefore produced germline mosaics to generate mutants lacking both the maternal and zygotic innexin 2 expression (see MATERIALS AND METHODS; Chou and Perrimon, 1996). We found that in these embryos, epithelial tissues and organs were severely affected and the cuticle failed to form (Figure 4J, compare with 4I). TUNEL staining indicated that extensive cell death occurred in these embryos (Figure 4M, compare with 4L). This phenotype is consistent with the role of gap junctions in adhesion and cell-to-cell communication, both of which are required for the integrity of cell layers and the morphogenesis of tissues and organs (for reviews, see Starich and Phelan, 2001; Evans and Martin, 2002a). The epithelial defects and cell lethality were rescued to a large extent when one paternal copy of innexin 2 was added back to the maternal null background (maternal null, zygotically heterozygous; Figure 4, K and N). The latter embryos showed a mutant phenotype very similar to the zygotic one, displaying large holes in the head region and showing a variable spectrum of segment defects (Figure 4K). Misexpression of Innexin 2 by using the maternal driver line V32-Gal4, which is known to mediate very high levels of ubiquitous expression in early blastoderm embryos, also resulted in massive cell death in all epithelia of the embryo (Figure 4, O–Q); all epithelial tissues disintegrated, the cells rounded up, and the cell polarity markers lost their membrane localization. V32Gal4/UAS-innexin 2 misexpression in the background of ubiquitous p35 activity, which results in the absence of cell death, produced multilayering and a variation of cell size in the embryos (Figure 4, T–V, compare with WT in R and S). In summary, the loss and gain-of-function data strongly suggest that correct levels of Innexin 2 are essential for both the survival of epithelial cells and to ensure proper morphogenesis of epithelial tissues.
Interaction of Innexin 2 and Other Junctional Proteins in Epithelial Cells of the Embryo
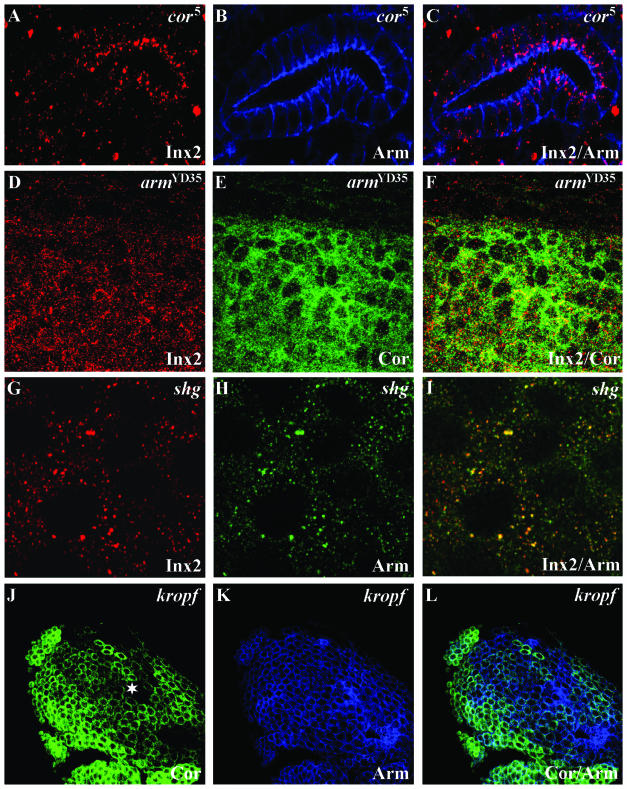
To further examine the role of Innexin 2 in epithelial cells, we determined its distribution in mutants for Coracle, DE-cadherin, and Armadillo. Coracle is a septate junction-associated protein required for dorsal closure, salivary gland morphogenesis, and cuticle formation during embryonic development (Fehon et al., 1994; Lamb et al., 1998). In the salivary glands of amorphic coracle5 mutants in which the septate junctions are disrupted but cell polarity is not affected (Lamb et al., 1998), Innexin 2 accumulated in the apical region compared with the baso-lateral region of wild-type stage 15 embryos (Figure 5, A–C, compare with Figure 3, A–C). In amorphic armadillo YD35 mutants, we found increased levels of Innexin 2 in the cytoplasm (Figure 5, D–F). Similarly, Innexin 2 accumulated in the cytoplasm (Figure 5, G–I) of zygotic shotgun (DE-cadherin) mutants, which show disorganization of epithelial tissues in the head and the ventral epidermis (Uemura et al., 1996; Tepass et al., 1996). It is known that in the latter mutants, the level of membrane-bound Armadillo is decreased and its cytoplasmic level is slightly increased (Tepass et al., 1996; Uemura et al., 1996). Consistent with this finding and with our previous observation that Innexin 2 and Armadillo colocalize in the epidermis, we observed an extensive overlap of Innexin 2 and Armadillo signals in the cytoplasm of shotgun mutant cells in the ventral epidermis (Figure 5I, compare with Figure 2F). In zygotic kropf mutants in which the head region is most heavily affected, we detected the loss of expression (Figure 5J, asterisk) or the mislocalization of Coracle, Armadillo, and DE-cadherin in many cells (Figure 5K; our unpublished data). The affected cells often showed a “rounded up” phenotype and seemed to have lost polarity (Figure 5, J–L), further supporting the essential role of Innexin 2 for the maintenance of epithelia. It is of note, however, that zygotic armadillo, shotgun, and kropf mutants contain significant amounts of respective maternal proteins that contributed substantially to the maintenance of the general epithelial architecture and of the apico-basal polarity in many cells of the embryo; it is known that mutant effects can only be observed in a subset of tissues (Tepass et al., 1996; Uemura et al., 1996).
Figure 5.
Defect mutants for innexin 2 and other junctional components. (A–C) Innexin 2 (red) and Armadillo (blue) staining in stage 16 coracle mutants. Note the mislocalization of Innexin 2 compared with WT salivary glands of the same stage (see Figure 3, A–C). (D–F) Confocal images showing coimmunostaining of Innexin 2 (red) and Coracle (green) in the epidermis of stage 10 armadillo mutants. (G–I) High magnification of a coimmunostaining of Innexin 2 (red) and Armadillo (green) of the ventral epidermis of stage 10 shotgun (DE-cadherin) mutants. Note the strong overlap of Innexin 2 and Armadillo signals. (J–L) Confocal images showing coimmunostaining of Coracle (green) and Armadillo (blue) in the head region of zygotic stage 10 kropf mutants. Asterisk (*) marks loss of Coracle expression. For further information, see text.
To further test the role of Innexin 2 for cell polarity, we performed misexpression experiments by using the UAS-Gal4 system (Brand and Perrimon, 1993) and examined the localization of Innexin 2 and of the various junction markers. Because we obtained massive cell death when using the maternal V32-Gal4 line to drive the UAS-innexin 2 effector construct, we switched to the use of the paired-Gal4 driver line that mediates misexpression from blastoderm stage onwards in a pair-rule pattern (Gutjahr et al., 1993; Xiao et al., 1996). For these experiments, we used UAS-innexin 2 or UAS-innexin 2-GFP effector lines to monitor misexpression.
In stage 14 wild-type embryos, Armadillo, DE-cadherin, and Coracle are rather uniformly expressed (Figure 6, A–C; our unpublished data). On misexpression of innexin 2 or innexin 2-GFP, we found an accumulation of Armadillo (Figure 6, D–F and G–I) or Coracle (Figure 6, J–L) in the paired domains in every other segment, whereas expression of these proteins in the interstripe regions was unchanged. In summary, our loss and gain-of-function analyses provide evidence for interactions between Innexin 2 and other junctional core components.
Figure 6.
Innexin 2 overexpression experiments. (A–C) Uniform localization of Innexin 2 (red), Coracle, (green), and Armadillo (blue) in the epidermis of stage 13 wild-type embryos. (D–F) stage 15 pairedGal4/UAS-innexin 2-GFP embryo. Anti-Innexin 2 is in red and anti-Armadillo is in green. Note the overlapping expression pattern of Innexin 2-GFP and Armadillo. (G–I) Stage 13 pairedGal4/UAS-innexin 2 embryo. Anti-Innexin 2 is depicted in red and anti-Armadillo is in blue. Note the overlapping expression of Innexin 2 and Armadillo. (J–L) Immunostaining of a stage 13 embryo by using a paired-Gal4 driver line to induce Innexin 2 expression. Innexin 2 (red); Coracle (green). Note the enrichment of Coracle in the ectopic Innexin 2 stripes, compared with wild-type (A–C).
Biochemical Interaction Studies with Innexin2 and Other Junctional Proteins
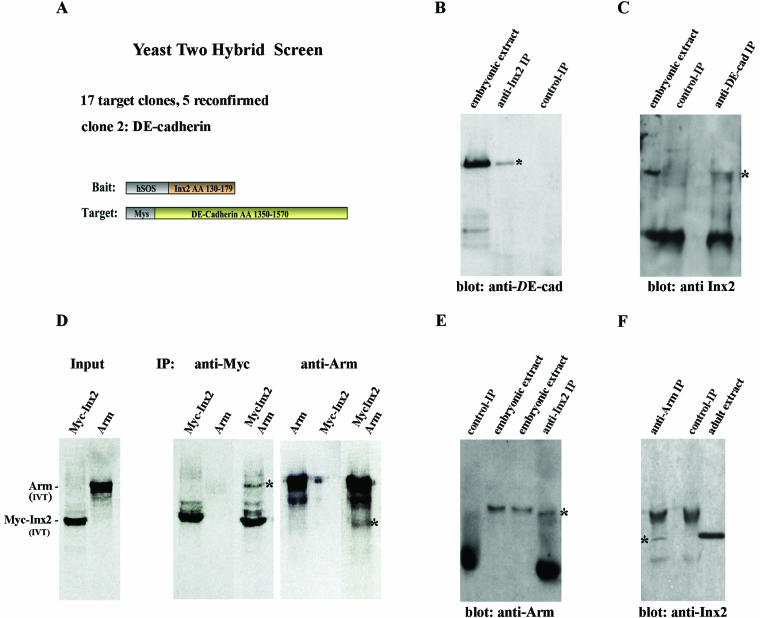
The genetic loss- and gain-of-function experiments suggest the possibility that Innexin 2 and the other junctional proteins may be part of multiprotein complexes required for epithelial development. To further examine this possibility we performed a molecular screen for interaction partners of Innexin 2 by using the yeast Ras-recruitment system (see MATERIALS AND METHODS; Broder et al., 1998; Köhler and Müller, 2002). This screening system is a modification of the canonical yeast two-hybrid system in that it enables the study of membrane-associated proteins interacting with cytoplasmic proteins fused to Ras (see MATERIALS AND METHODS; Broder et al., 1998). When using the cytoplasmic loop of Innexin 2 as bait to screen a 0- to 24-h embryonic Drosophila library, we isolated 17 putative target clones of which five were verified in the rescreen. Among the five clones isolated were a PDZ-domain protein, β-tubulin (homologues of both are known interaction partners for Connexin 43; Lehmann and Hoch, unpublished data; fore review, see Evans and Martin, 2002a) and one clone encoding the cytoplasmic domain of DE-cadherin (Figure 7A; aa 1350–1570). The intracellular domain of DE-cadherin is known to contain the binding site for Armadillo, which in turn binds to α-catenin linking the complex to the actin cytoskeleton (Kemler, 1993). To further test whether an Innexin 2/DE-cadherin–protein interaction occurs in vivo in the fly embryo, we prepared extracts of 0- to 24-h wild-type embryos and performed coimmunoprecipitation analysis. As shown in Figure 7B, DE-cadherin was specifically coimmunoprecipitated by anti-Innexin 2 antibody and vice versa (Figure 7C), indicating that the two proteins may also directly interact in vivo. We also tested for an interaction of Innexin 2 with Armadillo. Because we were unable to isolate Armadillo clones with yeast two-hybrid screens by using the cytoplasmic loop or the C-terminal domain of Innexin 2 as bait, we tested for protein–protein interactions in vitro. We performed in vitro translation reactions to generate full-length Armadillo and c-Myc–tagged Innexin 2 proteins, respectively. Both translation reactions were subsequently mixed, incubated and immunoprecipitated with either anti-Armadillo or anti-c-Myc antibodies (see MATERIALS AND METHODS). As shown in Figure 7D, anti-c-Myc antibody coprecipitated Armadillo and anti-Armadillo antibody coprecipitated Innexin 2. To examine whether an interaction between these proteins occurs in vivo in the fly embryo, we prepared extracts of 0- to 24-h wild-type embryos and assayed for protein interactions by coimmunoprecipitation. As shown in Figure 7E, Armadillo was specifically coimmunoprecipitated by anti-Innexin 2 antibody and vice versa (Figure 7F), indicating that the two proteins may directly interact both in vitro and in vivo. Similarly, anti-Innexin 2 antibody also specifically coimmunoprecipitated Coracle when extracts of 0- to 24-h wild-type embryos were used (our unpublished data). In summary, our immunohistochemical, biochemical, and genetic data provide evidence that the gap junctional channel protein Innexin 2 directly interacts with other junctional core proteins.
Figure 7.
Innexin 2 interacts biochemically with DE-cadherin and Armadillo. (A) CytoTrap Screen by using the full-length cytoplasmic loop of Innexin 2 as a bait. (B, C, E, and F) Embryonic extracts of 0- to 24-h wild-type embryos were used for immunoprecipitation with either Innexin 2-, DE-cadherin-, or Armadillo-antibody. Controls were performed either with preimmune serum (B), control IgG (E), or anti-c-Myc (C and F). (D) Immunoprecipitation with in vitro-translated (IVT) Myc-Innexin 2 (Myc-Inx2) or Armadillo. Input lanes depict one third of in vitro translated Myc-Innexin 2 and Armadillo used in the immunoprecipitation reactions. Immunoprecipitation was performed in the presence of anti-c-Myc- or anti-Armadillo-antibody. Asterisks (*) denote immunoprecipitated proteins.
DISCUSSION
The assembly and the cellular distribution of the junctional complement of a particular cell is a dynamic process that is essential for the proper differentiation of cells and tissues during development (Tepass and Hartenstein 1994; Nagafuchi 2001). The role of gap junctions for cellular communication and adhesion is well known from ultrastructural analysis in both vertebrates and invertebrates. Whereas extensive studies in the last decade have elucidated the role of connexin gap junction proteins in vertebrates (for review, see Evans and Martin, 2002a,b), it has only recently become clear that invertebrates gap junctions are composed of Innexins, which display very low sequence similarity to the connexins (for review, see Starich and Phelan, 2001). Here, we present the first study on the distribution of gap junction proteins in developing epithelia of the Drosophila embryo, and we demonstrate a function of Innexin 2 for the formation of epithelia. Furthermore, our immunhistochemical and biochemical studies provide evidence that Innexin 2 may interact with other junctional core proteins in polarized epithelial cells.
Innexin 2 Is Essential for Epithelial Morphogenesis
kropf mutant embryos lacking both the maternal and zygotic innexin contribution fail to develop epithelial tissues, and TUNEL staining demonstrates extensive cell death. Embryos, which are maternal null and contain one copy of zygotic innexin 2 mutants, show a milder phenotype in which many tissues go on to develop. However, in these embryos cells round up and loose polarity, which most likely produces the large cuticular holes and the variable spectrum of segment defects observed in these embryos (Figure 4). These embryos also display strong defects in epithelial tissue and organ formation. Similarly, we find severe morphogenesis defects of epithelial tissues and organs in zygotic kropf mutants (Figure 4, A–H). Embryos in which Innexin 2 has been extensively overexpressed (by using V32Gal4) are embryonic lethal and display defects such as multilayering and irregular cell shapes (Figure 4, R–V); as in the loss-of-function mutants, cells round up and loose polarity when the Innexin 2 level is increased in the cells. Similar, but not as drastic effects are obtained upon mild overexpression of Innexin 2 by using drivers such as daughterless-Gal4 (Bauer and Hoch, unpublished data); the epidermal phenotype of these embryos is similar to the one of kropf mutant embryos. Our genetic loss- and gain-of-function experiments thus clearly demonstrate that establishing and maintaining a proper Innexin 2 level is critical for the survival of epithelial cells and for proper morphogenesis of tissues and organs in the body. This is consistent with our earlier findings of Innexin 2 controlling morphogenesis of the foregut during proventriculus development (Bauer et al., 2002). Cells in tissues and organs have to coordinate their activities by communicating directly with each other during development. Gap junctions that link the cytoplasm of neighboring cells ensure the integration of metabolic activities in interacting cellular networks. Furthermore, ultrastrucutral analysis has shown an important role of gap junctions in cell adhesion. In neighboring attached cells each gap junction channel is formed by head-to-head docking of two partner hemi-channels, producing gap junction channels that associate to form large clusters of plaques. It is thus likely that the deleterious effects observed in the Innexin 2 loss- and gain-of-function situations occur through changes in cell adhesion and cell-to-cell communication.
Innexin 2 Distribution in Epithelial Cells
Our immunhistochemical data determining the distribution of Innexins 1 and 2 show that both proteins are localized in a punctate pattern in the plasma membrane and to some extent in the cytoplasm of the epithelial cells (Figures 2 and 3). A fraction of both proteins is distributed along the entire apico-basal axis of epithelial cells. However, in the case of each Innexin, we observed an accumulation of the proteins in specific membrane domains, depending on the Innexin and the type of tissue. Innexin 2 accumulates in the apico-lateral membrane domain in the epidermis, in the lateral domain in the hindgut, and in a baso-lateral position in the salivary glands (Figures 2 and 3). In contrast, we find an accumulation of Innexin 1 in the baso-lateral region for both the salivary gland and the hindgut (Figure 3). We conclude from these findings that the accumulation of Innexins in specific membrane domains of epithelial cells is likely to be regulated by tissue-specific factors during the development of epithelia in the fly embryo. A punctate pattern and localization in the cytoplasm and the membrane also occurs in vertebrate connexins. They are cotranslationally integrated into the endoplasmic reticulum and pass through the Golgi apparatus via vesicles to the plasma membrane (Falk, 2000). Live imaging analysis in vertebrate tissue culture cells has shown that unopposed hemi-channels dynamically move within the plasma membrane until they dock end to end with partners in neighboring attached cells to generate gap junction channels. Gap junction channels laterally associate with each other to form large gap junction plaques (Lauf et al., 2002; Schubert et al., 2002). It is therefore possible that the punctate distribution of Innexins 1 and 2 in the membranes may reflect the mobility of hemi-channels in the plasma membrane and that accumulation of Innexin signals may reflect the association of gap junctions into plaques.
Interaction of Innexin 2 with Other Junctional Proteins
In the epidermis, Innexin 2 protein accumulates in the apicolateral membrane domain and colocalizes with Armadillo and DE-cadherin. It is of note that this applies only to a fraction of the Innexin 2 protein pool. We find that in mutants for both zygotic armadillo and DE-cadherin, the Innexin 2 localization is altered, and in Innexin 2 overexpression experiments, Armadillo and DE-cadherin are organized into Innexin 2 pattern. Further evidence for an interaction of the proteins is provided by the yeast two-hybrid analysis and the immunoprecipitation both in vitro-translated and embryonic extracts. Armadillo and DE-cadherin are core components of adherens junctions, which are multiprotein complexes mediating cell-cell adhesion and communication (fore reviews, Tepass, 1999; Nagafuchi, 2001). DE-cadherin belongs to a large family of membrane proteins that mediate Ca2+-dependent homophilic adhesion (Tepass, 1999). It possesses a highly conserved cytoplasmic domain that binds to the cytoplasmic protein β-catenin/Armadillo, thereby forming a cadherin–catenin complex. β-Catenin is bound, in turn, by α-catenin to link the actin cytoskeleton to the (D)E-cadherin/β–catenin complex (Tepass, 1999; Nagafuchi, 2001). Whether the interaction of Innexin 2 with DE-cadherin and Armadillo plays a major or minor role in epithelial cells (given that only a portion of Innexin 2 protein colocalizes with the adherens junction proteins) and at which step of the biogenesis of Innexin 2-containing gap junctions this interaction may occur is not known at this point. However, it has been demonstrated that the spatial association between Gap and adherens junctions is an important factor in the maturation of vertebrate and invertebrate tissues (Tepass and Hartenstein, 1994; Angst et al., 1997). Furthermore, adherens junction assembly was demonstrated to be a prerequisite for the formation of gap junction plaques in fibroblasts (Ko et al., 2000). A direct interaction of the Armadillo homologue β-catenin with connexin 43 was shown to occur in cardiac myocytes (Ai et al., 2000). Tissue culture studies and the analysis of neural crest cell migration in the mouse embryo have shown that the assembly and function of gap junctions is directly controlled by E- and N-cadherins, respectively (Musil et al., 1990; Jongen et al., 1991; Meyer et al., 1992; Fujimoto et al., 1997; Xu et al., 2001). Recent analysis of connexin trafficking has suggested that adherens junction formation at high Ca2-concentration leads to the formation of actin cables that directly or indirectly transport connexins from the cytoplasm to the cell-cell contact membranes via the Golgi apparatus (Hernandez-Blazquez et al., 2001). Our results are thus consistent with findings in vertebrates that suggest a close link between gap junction and adherens junction biogenesis.
Our experiments further indicate that Coracle, a homologue of the vertebrate membrane-skeleton Protein 4.1, may be another Innexin 2 interaction. Coracle is a key component of septate junctions in the lateral membrane domain of epithelial cells and tissues (Fehon et al., 1994; for review, see Tepass et al., 2001). It has been suggested that septate junctions provide some of the functions ascribed to tight junctions in vertebrate tissues (Tepass and Hartenstein, 1994). Interactions of connexins with the tight junction proteins ZO1 (Giepmans and Moolenaar, 1998; Toyofuku et al., 1998) and occludin (Kojima et al., 1999) have been described and occur in hepatocytes. Although we have no evidence that the interaction of Innexin 2 with Coracle is direct, we believe that Coracle and Innexin 2 are part of multiprotein complexes that can be immunoprecipitated from embryonic extracts. Tissue and cell type-specific interaction partners for gap junction channels that may modulate the distribution, trafficking, maturation, signaling activity, and other biological functions of gap junction proteins may thus be a common feature for vertebrates and invertebrates.
Acknowledgments
We thank E. Knust, M. Peifer, H. Jäckle, U. Schäfer, J. Campos-Ortega, T. Uemura, and D. St. Johnston for flies and antibodies; and Nancy Fossett and Okot Nyormoi for helpful comments. We also thank Frank Josten for assistance with the confocal microscope. The work was supported by a Deutsche Forschungsgemeinschaft grant to M.H. (FOR 425).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-01-0056. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-01-0056.
References
- Adams, M.D., et al. (2000). The genome sequence of Drosophila melanogaster. Science 287, 2185-2195. [DOI] [PubMed] [Google Scholar]
- Ai, Z., Fischer, A., Spray, D.C., Brown, A.M., and Fishman, G.I. (2000). Wnt-1 regulation of connexin43 in cardiac myocytes. J Clin Invest. 105, 161-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst, A.D., Khan, L.U., Severs, N.J., Whitely, K., Rothery, S., Thompson, R.P., Magee, A.I., and Gourdie, R.G. (1997). Dissociated spatial patterning of gap junctions and cell adhesion junctions during postnatal differentiation of ventricular myocardium. Circ. Res. 80, 88-94. [DOI] [PubMed] [Google Scholar]
- Bauer, R., Lehmann, C., Fuss, B., Eckardt, F., and Hoch, M. (2002). The Drosophila gap junction channel gene innexin 2 controls foregut development in response to Wingless signalling. J. Cell Sci. 115, 1859-1867. [DOI] [PubMed] [Google Scholar]
- Barnes, T.M., and Hekimi, S. (1997). The C. elegans avermectin resistance and anesthetic respose gene unc-9 encodes a member of a protein family implicated in electrical coupling of excitable cells. J. Neurochem. 69, 2251-2260. [DOI] [PubMed] [Google Scholar]
- Booth, G.E., Kinrade, E.F., and Hidalgo, A. (2000). Glia maintain follower neuron survival during Drosophila CNS development. Development 127, 237-244. [DOI] [PubMed] [Google Scholar]
- Brand, A., and Perrimon, N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401-415. [DOI] [PubMed] [Google Scholar]
- Broder, Y.C., Katz, S., and Aronheim, A. (1998). The ras recruitment system, a novel approach to the study of protein-protein interactions. Curr. Biol. 8, 1121-1124. [DOI] [PubMed] [Google Scholar]
- Chou, T.B., and Perrimon, N. (1996). The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics 144, 1673-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell, G.T., Wu, Y., and Burt, J.M. (2002). Cx40 and Cx43 expression ratio influences heteromeric/heterotypic gap junction channel properties. Am. J. Physiol. 282, C1469-C1482. [DOI] [PubMed] [Google Scholar]
- Cox, R.T., Kirkpatrick, C., and Peifer, M. (1996). Armadillo is required for adherens junction assembly, cell polarity, and morphogenesis during Drosophila embryogenesis. J. Cell Biol., 134, 133-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, R.T., Pai, L.M., Kirkpatrick, C., Stein, J., and Peifer, M. (1999). Roles of the C-terminus of Armadillo in Wingless signalling in Drosophila. Genetics 153, 319-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin, K.D., Zhang, Z., and Wyman, R.J. (1999). Drosophila has several genes for gap junction proteins. Gene 232, 191-201. [DOI] [PubMed] [Google Scholar]
- Diez, J.A., Ahmad, S., and Evans, W.H. (1999). Assembly of heteromeric connexons in guinea-pig live en route to the Golgi apparatus, plasma membrane and gap junctions. Eur. J. Biochem. 262, 142-148. [DOI] [PubMed] [Google Scholar]
- Evans, W.H., and Martin, P.E.M. (2002a). Gap junctions: structure and function. Mol. Membr. Biol. 19, 121-136. [DOI] [PubMed] [Google Scholar]
- Evans, W.H., and Martin, P.E.M. (2002b). Lighting up gap junction channels in a flash. Bioessays 24, 876-880. [DOI] [PubMed] [Google Scholar]
- Falk, M.M. (2000). Biosynthesis and structural composition of gap junction intercellular membrane channels. Eur. J. Cell Biol. 79, 564-574. [DOI] [PubMed] [Google Scholar]
- Fehon, R. G. Dawson, I.A., and Artavanis-Tsakonas, S. (1994). A Drosophila homologue of membrane skeleton protein 4.1 is associated with septate junctions and is encoded by the coracle gene. Development 120, 545-547. [DOI] [PubMed] [Google Scholar]
- Fujimoto, K., Nagafuchi, A., Tsukita, S.K.A., Ohokuma, A., and Shibata, Y. (1997). Dynamics of connexins, E-cadherin and α-catenin on cell membranes during gap junction formation. J. Cell Sci. 110, 311-322. [DOI] [PubMed] [Google Scholar]
- Giepmans, B.N., and Moolenaar, W.H. (1998). the gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr biol. 13, 931-934. [DOI] [PubMed] [Google Scholar]
- Goodenough, D.A., Goliger, J.A., and Paul, D.L. (1996). Connexins, connexons and intercellular communication. Annu. Rev. Biochem. 65, 475-602. [DOI] [PubMed] [Google Scholar]
- Goodenough, D.A., and Paul, D.L. (2003). Beyond the gap: functions of unpaired connexon channels. Nat. Rev. 4, 1-10. [DOI] [PubMed] [Google Scholar]
- Gutjahr, T., Frei, E., and Noll, M. (1993). Complex regulation of early paired expression: initial activation by gap genes and pattern modulation by pair-rule genes. Development 117, 609-623. [DOI] [PubMed] [Google Scholar]
- He, D.S., Jiang, J.X., Taffet, S.M., and Burt, J.M. (1999). Formation of heteromeric gap junction channels by connexins 40 and 43 in vascular smooth muscle cells. Proc. Natl. Acad. Sci. USA 96, 6595-6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Blazquez, F.J., Joazeiro, P.P., Omori, Y., and Yamasaki, H. (2001). Control of intracellular movement of connexins by E-cadherin in murine skin papilloma cells. Exp. Cell Res. 270, 235-247. [DOI] [PubMed] [Google Scholar]
- Jiang, J.X., and Goodenough, D.A. (1996). Heteromeric connexons in lens fap junction channels. Proc. Natl. Acad. Sci. USA 93, 1287-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen, W.M.F., Fithgerald, D., J., Asamoto, M., Piccoli, C., Slaga, T.J., Gros, D., Takeichi, M., and Yamasaki, H. (1991). Regulation of connexin 43-mediated gap junctional intercellular communication by Ca2+ in mouse epidermal cells is controlled by E-cadherin. J. Cell Biol. 114, 545-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler, R. (1993). From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Gen. 9, 317-321. [DOI] [PubMed] [Google Scholar]
- Ko, K., Arora, P., Lee, W., and McCulloch, C. (2000). Biochemical and functional characterization of intercellular adhesion and gap junctions in fibroblasts. Cell Physiol. 279, C147. [DOI] [PubMed] [Google Scholar]
- Köhler, F., and Müller, K.M. (2003). Adaptation of the Ras-recruitment system to the analysis of interactions between membrane-associated proteins. Nucleic Acids Res. 31, 28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, N.M., and Gilula, N.B. (1996). The gap junction communication channel. Cell 84, 381-388. [DOI] [PubMed] [Google Scholar]
- Laing, J.G., Manley-Markowski, R.N., Koval, M., Civitelli, R., and Steinberg, T.H. (2001). Connexin 45 interacts with zonula occludens-1 and connexin 43 in osteoblastic cells. J. Biol. Chem. 22, 23051-23055. [DOI] [PubMed] [Google Scholar]
- Lamb, R.S., Ward, R.E., Schweizer, L., and Fehon, R.G. (1998). Drosophila coracle, a member of the protein 4.1 superfamily, has essential structural functions in the septate junctions and developmental functions in embryonic and adult epithelial cells. Mol. Biol. Cell 9, 3505-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauf, U., Giepmans, B.N.G., Lopez, P., Braconnot, S., Chen, S.-C., and Falk, M.M. (2002). Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc. Natl. Acad. Sci. USA 99, 10446-10451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke, D., Perusinghe, N., Newman, T., Jayatilake, H., Evans, W.H., and Monaghan, P. (2000). Development, expression and assembly of connexins into homomeric and heteromeric gap junction hemi-channels in the mouse mammary gland. J. Cell Physiol. 183, 228-237. [DOI] [PubMed] [Google Scholar]
- Meyer, R.A., Laird, D.W., Revel, J.-P., and Johnson, R.G. (1992). Inhibition of gap junction and adherens junction assembly by connexin and A-CAM antibodies. J. Cell Biol. 119, 179-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil, L.S., Cunningham, B.A., Edelman, G.M., and Goodenough, D.A. (1990). Differential phosphorylation of the gap junction protein connexin 43 in junctional communication-competent and -deficient cell lines. J. Cell Biol. 111, 2077-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi, A. (2001). Molecular architecture of adherens junctions. Curr. Opin. Cell Biol. 13, 600-603. [DOI] [PubMed] [Google Scholar]
- Pai, L.M., Kirkpatrick, C., Blanton, J., Oda, H., Takeichi, M., and Peifer, M. (1996). Drosophila α-catenin and E-cadherin bind to distinct regions of Drosophila Armadillo. J. Biol. Chem. 271, 32411-32420. [DOI] [PubMed] [Google Scholar]
- Peifer, M. (1993). The product of the Drosophila segment polarity gene armadillo is part of a multi-protein complex resembling the vertebrate adherens junction. J. Cell Sci. 105, 993-1000. [DOI] [PubMed] [Google Scholar]
- Peifer, M., and Polakis, P. (2000). Wnt Signaling in oncogenesis and embryogenesis – a look outside the nucleus. Science 287, 1606-1609. [DOI] [PubMed] [Google Scholar]
- Phelan, P., Nakagawa, M., Wilkin, M.B., Moffat, K.G., O'Kane, C.J., Davies, J.A., and Bacon, J.P. (1996). Mutations in shaking-B prevent electrical synapse formation in the Drosophila giant fibre system. J. Neurosci. 16, 1101-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan, P., and Starich, T.A. (2001). Innexins get into the gap. Bioessays 23, 388-396. [DOI] [PubMed] [Google Scholar]
- Phelan, P., Stebbings, L.A., Baines, R.A., Bacon, J.P., Davies, J.A., and Ford, C. (1998). Drosophila Shaking-B protein forms gap junctions in paired Xenopus oocytes. Nature 391, 181-184. [DOI] [PubMed] [Google Scholar]
- Rubin, G.M., and Spradling, A.C. (1982). Genetic transformation of Drosophila with transposable element vectors. Science 218, 348-353. [DOI] [PubMed] [Google Scholar]
- Schubert, A.-L., Schubert, W., Spray, D.C., and Lisanti, M.P. (2002). Connexin family members target to lipid raft domains and interact with caveolin-1. Biochemistry 41, 5754-5764. [DOI] [PubMed] [Google Scholar]
- Starich, T.A., Lee, R.Y.N., Panzarella, C., Avery, L., and Shaw, J.E. (1996). eat-5 and unc-7 represent a multigene family in C. elegans involved in cell-cell coupling. J. Cell Biol. 134, 537-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbings, L.A., Todman, M.G., Phelan, P., Bacon, J.P., and Davies, J.A. (2000) The Drosophila Innexins are expressed in overlapping domains and cooperate to form gap junction channels. Mol. Biol. Cell 11, 2459-2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbings, L.A., Todman, M.G., Phelan, Phillips, R., Greer, C.E., Tam, J., Phelan, P., Jacobs, K., Bacon, J.P., and Davies, J.A. (2002). The Drosophila Innexins are expressed in overlapping domains and cooperate to form gap junction channels. Mol. Biol. Cell 11, 2459-2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi, K., Kawashima, A., Nagafuchi, A., and Tsukita, S. (1994). Structural diversity of band 4.1 superfamily members. J. Cell Sci. 107, 1921-1928. [DOI] [PubMed] [Google Scholar]
- Tazuke, S.I., Schulz, C., Gilboa, L., Fogarty, M., Mahowald, A.P., Guichet, A., Ephrussi, A., Wood, C.G., Lehmann, R., and Fuller, M.T. (2002). A germline-specific gap junction protein required for survival of differentiating early germ cells. Development 129, 2529-2539. [DOI] [PubMed] [Google Scholar]
- Tepass, U. (1999). Genetic analysis of cadherin function in animal morphogenesis. Curr. Opin. Cell Biol. 11, 540-548. [DOI] [PubMed] [Google Scholar]
- Tepass, U., Gruszynski-DeFeo, E., Haag, T.A., Omatyar, L., Török, T., and Hartenstein, V. (1996). shotgun encodes Drosophila E-cadherin and is preferentially required during cell rearrangement in the neuroectoderm and other morphogenetically active epithelia. Genes Dev. 10, 672-685. [DOI] [PubMed] [Google Scholar]
- Tepass, U., and Hartenstein, V. (1994). The development of cellular junctions in the Drosophila embryo. Dev. Biol. 161, 563-596. [DOI] [PubMed] [Google Scholar]
- Tepass, U., Tanentzapf, G., Ward, R., and Fehon, R. (2001). Epithelial cell polarity and cell junctions in Drosophila. Annu. Rev. Genet. 35, 747-784. [DOI] [PubMed] [Google Scholar]
- Teubner, B., et al. (2001). Functional expression of the new gap junction gene connexin47 transcribed in mouse brain and spinal cord neurons. J Neurosci. 15, 1117-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku, T., Yabuki, M., Otsu, K., Kuzuya, T., Hori, M., and Tada, M. (1998). Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem 22, 12725-12731. [DOI] [PubMed] [Google Scholar]
- Uemura, T., Oda, H., Kraut, R., Hayashi, S., Kataoka, Y., and Takeichi, M. (1996). Zygotic Drosophila E-cadherin expression is required for processes of dynamic epithelial cell rearrangement in the Drosophila embryo. Genes Dev. 10, 659-671. [DOI] [PubMed] [Google Scholar]
- Unger, V.M., Kumar, N.M., Gilula, N.B., and Yeager, M. (1997). Projection structure of a gap junction membrane channel at 7A resolution. Nat. Struct. Biol. 4, 39-43. [DOI] [PubMed] [Google Scholar]
- Unger, V.M., Kumar, N.M., Gilula, N.B., and Yeager, M. (1999). Three-dimensional structure of a recombinant gap junction membrane channel. Science 283, 1176-1180. [DOI] [PubMed] [Google Scholar]
- Watanabe, T., and Krankel, D.R. (1990). Molecular cloning and analysis of l(1) ogre, a locus of Drosophila melanogaster with prominent effects on postembryonic development of the central nervous system. Genetics 126, 1033-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willecke, K., Eiberger, J., Degen, J., Echardt, D., Romualdi, A., Guldenagel, M., Deutsch, U., and Sohl, G. (2002). Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 383, 725-737. [DOI] [PubMed] [Google Scholar]
- Willecke, K., Hennemann, H., Dahl, E., Jungbluth, S., and Heynkes, R. (1991). The diversity of connexin genes encoding gap junction proteins. Eur. J. Cell Biol. 56, 1-7. [PubMed] [Google Scholar]
- Xiao, H., Hrdlicka, L.A., and Nambu, J.R. (1996). Alternate functions of the single-minded and rhomboid genes in development of the Drosophila ventral neuroectoderm. Mech. Dev. 58, 65-74. [DOI] [PubMed] [Google Scholar]
- Xu, X., et al. (2001). Modulation of mouse neural crest cell motility by N-cadherin and connexin 43 gap junctions. J. Cell Biol. 154, 217-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager, M., Unger, V.M., and Falk, M.M. (1998). Synthesis, assembly and structure of gap junction intercellular channels. Curr. Opin. Struct. Biol. 8, 517-524. [DOI] [PubMed] [Google Scholar]